Abstract
A wealth of research demonstrates attentional biases toward threat in the anxiety disorders. Several models have been advanced to explain these biases in anxiety, yet the mechanisms comprising and mediating the biases remain unclear. In the present article, we review evidence regarding the mechanisms of attentional biases through careful examination of the components of attentional bias, the mechanisms underlying these components, and the stage of information processing during which the biases occur. Facilitated attention, difficulty in disengagement, and attentional avoidance comprise the components of attentional bias. A threat detection mechanism likely underlies facilitated attention, a process that may be neurally centered around the amygdala. Attentional control ability likely underlies difficulty in disengagement, emotion regulation goals likely underly attentional avoidance, and both of these processes may be neurally centered around prefrontal cortex functioning. The threat detection mechanism may be a mostly automatic process, attentional avoidance may be a mostly strategic process, and difficulty in disengagement may be a mixture of automatic and strategic processing. Recommendations for future research are discussed.
Keywords: anxiety, attentional bias, information processing bias
Mechanisms of Attentional Biases towards Threat in the Anxiety Disorders: An Integrative Review
An attentional bias towards threat refers to differential attentional allocation towards threatening stimuli relative to neutral stimuli (Bar-Haim et al., 2007; MacLeod et al., 1986; Mogg & Bradley, 1998). A wealth of research demonstrates that anxious individuals display an attentional bias towards threatening sources of information, and this effect is less consistent or typically not observed in non-anxious individuals (Bar-Haim et al., 2007; Mogg & Bradley, 1998; Williams et al., 1996). While the attentional bias effect has been systematically demonstrated numerous times in anxious populations, the exact mechanisms that comprise and underlie attentional biases remain unexplained. Several theoretical models have been advanced to account for attentional biases towards threat in anxiety; however, these models have not been evaluated systematically in light of emerging empirical data. The purpose of this paper is to review the relevant theoretical models and empirical data in order to further illuminate the mechanisms of attentional biases towards threat in anxiety.
The conceptualization adapted in this paper is that the mechanisms of attentional biases can best be understood by examining three interrelated aspects of attentional bias: 1) the observed components of attentional bias, 2) the mechanisms that may mediate the expression of these components, and 3) the stage of information processing during which the mediating mechanisms operate. The components of attentional bias refer to the observable and measurable characteristics of attentional bias (i.e., what does an attentional bias ‘look’ like?). Observable characteristics of attentional bias that have been mentioned in the literature include facilitated attention (e.g., threat stimuli are detected faster than non-threat stimuli), difficulty in disengagement (e.g., it is harder to disengage attention from a threat stimulus relative to a neutral stimulus), and attentional avoidance (e.g., allocating attention towards locations opposite the location of threat; Cisler et al., 2009; Fox et al., 2001; 2002; Koster et al., 2004a; 2005; 2006; Mogg et al., 2004). The mediating mechanisms of attentional bias refer to the underlying mechanisms that may produce the observable characteristics of attentional bias. For example, the amygdala has been implicated as a neural mechanism that may mediate automatic vigilance for threat (Anderson & Phelps, 2001; Davis & Whalen, 2001; Ohman, 1996; 2005; Ohman & Wiens, 2004). Attentional control, the cognitive ability to regulate attentional allocation (Derryberry & Reed, 2002; Eysenck et al., 2007; Posner & Rothbart, 2000), has been mentioned as a possible mechanism that may mediate difficulties in disengaging attention from threat (Eysenck et al., 2007). Finally, the components of attentional bias and their mediating mechanisms may be tied to specific stages of information processing. Information processing is commonly divided into two stages: automatic and strategic (McNally, 1995; 1996; Moors & de Houwer, 2006; Schiffrin & Schneider, 1977), although the boundaries between these two stages are not well defined. Automatic processing generally refers to processing that is capacity-free and occurs without intent, control, or awareness, whereas strategic processing generally refers to processing that is intentional, controllable, capacity-limited, and dependent on awareness.
These three different domains are likely interrelated. Stage of information processing (e.g., automatic versus strategic) may constrain the functioning of an underlying mechanism (e.g., amygdala/threat detection mechanism may only be observed during automatic stages of processing), and the underlying mechanism may constrain the expression of the observable characteristic (e.g., facilitated attention to threat may occur due to activation of the amygdala/threat detection mechanism). Therefore, it is our contention that a theoretical explanation of attentional biases in anxiety must account for and explain observations in each domain.
We begin this review by summarizing the major sources of empirical evidence for attentional biases towards threat in anxiety. We then conduct a more detailed analysis as to the mechanisms that comprise and underlie this attentional bias effect, paying careful attention to the components, mediating mechanisms, and stages of information processing. This analysis includes a discussion of previous models, a review and integration of the empirical evidence, an evaluation of the prior models, and proposals for future research based on remaining ambiguities.
Brief Overview of Major Findings
Attentional bias towards threat among anxious populations is a relatively robust phenomenon (Bar-Haim et al., 2007; Cisler et al., 2009; Mogg & Bradley, 1998; Williams et al., 1996) , with a recent meta-analysis demonstrating an aggregate effect size of d = .45 (Bar-Haim et al., 2007). The following sections review 2 major findings. First, attentional biases are observed across several different experimental tasks. Second, attentional biases are observed across anxiety disorders.
Experimental Tasks
The observation that attentional biases have been observed in several different tasks is important because it suggests that the phenomenon is not an artifact of particular experimental procedures or task confounds. The general concept of an attentional bias predicts that attention will be preferentially allocated towards threatening compared to neutral stimuli. The demonstration that this effect occurs in several experimental settings suggests generalizability and ubiquitousness of the phenomenon. Thus, the wealth of data on attentional biases allows for a global prediction that if threatening and neutral stimuli occur together, the attention of an anxious individual will likely be biased towards the threat.
Modified Stroop task
The modified Stroop task (Stroop, 1935) displays different types of words (e.g., threatening and neutral) in varying colors. The participant is asked to report the color while ignoring the semantic content of the word. Heightened response times to report the color of threat words compared to neutral words are considered an indication of attentional bias. Numerous studies have demonstrated attentional biases in the Stroop task in anxious populations (see Bar-Haim et al., 2007; Mogg & Bradley, 1998; Williams et al., 1996). For example, McNally and colleagues (1990) compared Stroop response times towards PTSD related words, OCD related words, positive words, and neutral words between Vietnam combat veterans with and without PTSD. The results revealed that participants with PTSD had longer response times towards PTSD-related words relative to positive, OCD, and neutral words. Participants with PTSD had longer response times towards PTSD words compared to participants without PTSD, and the response times of participants without PTSD did not differ across word types.
Dot probe task
Although the modified stroop task is the most commonly used task to measure attentional bias in anxiety, it may not be an ideal measure of attention due to several interpretational difficulties. For instance, delayed responding to threat words can be due to enhanced attention as well as overall delayed responding to threat (Algom et al., 2004). Moreover, the modified Stroop task does not allow for the measurement of spatial attention allocation (e.g., MacLeod, Mathews, & Tata, 1986). To address these limitations, other tasks have been used to assess attentional biases towards threat. The dot probe task (MacLeod et al., 1986) displays two words on a computer screen with one at the top and one at the bottom (alternatively, the words may appear on the left and right side of the screen). Following a brief stimulus presentation duration (e.g., 500 ms), the stimuli disappear and a probe appears in a location previously occupied by one of the stimuli. The participant is asked to press a button indicating whether the top or bottom stimulus had been replaced by the probe. Attentional biases are inferred from different response times towards probes that replace threatening stimuli (i.e., congruent trials) compared to probes that replace neutral stimuli (i.e. incongruent trials). If an individual's attention is systematically drawn to the threat stimulus, response times will be shorter for probes that replace threatening stimuli compared to probes that replace neutral stimuli. A wealth of research has demonstrated attentional biases in the dot probe task (e.g., Bar-Haim et al., 2007; Mogg & Bradley, 1998). For example, Bradley and colleagues (1999) found that individuals with GAD demonstrated faster response times towards probes that replaced threatening faces relative to probes that replaced neutral faces, and this effect among GAD individuals was greater compared to non-anxious controls.
Visual search task
The visual search task (e.g., Öhman, Flykt, & Esteves, 2001; Rinck, Becker, Kellermann, & Roth, 2003) is another experimental task of attentional biases that allows for the assessment of spatial attentional allocation. Participants are asked to detect a target stimulus that is embedded in a matrix of distracting stimuli. For example, the target word “spider” might be displayed in a matrix (e.g., 3 row × 3 column pattern of stimulus presentation) of neutral distracter words. Conversely, a neutral target word may be embedded in a matrix of spider related words. Attentional biases are inferred from faster response times to detect a threatening stimulus in a matrix of neutral stimuli relative to response times to detect neutral stimuli in neutral matrices (i.e., the individual's attention is drawn to the threat stimulus). Attentional biases can also be inferred from slower response times to detect neutral stimuli in a matrix of threatening stimuli relative to response times to detect neutral stimuli in a matrix of neutral stimuli (i.e., the individual's attention is captured by the threat stimulus). The visual search task has also robustly demonstrated attentional biases (Cisler et al., 2009; Rinck, Becker, Kellermann, & Roth, 2003; Öhman et al., 2001; Miltner, Krieschel, Hecht, Tripp, & Weiss, 2004). For example, Rinck and colleagues (2005) found attentional biases towards spider stimuli relative to beetle and butterfly stimuli among spider fearful individuals in three experiments using visual search tasks, and these biases were greater relative to non-anxious controls.
Spatial cueing task
The spatial cueing task (Fox et al., 2001; Posner, 1980) also allows for the assessment of spatial attention allocation. Participants focus on a fixation point located between two rectangles. A cue is then presented (e.g., one of the rectangles brightens or a threatening stimulus appears in one of the rectangles), followed by the appearance of a target in one of the two rectangles. Participants are asked to press a key indicating the rectangle in which the target is located. Some of the trials are valid cues (the cue draws attention to the rectangle in which the target is located), some of the trials are invalid cues (the cue draws attention away from the rectangle in which the target is located). Attentional biases are indicated by faster responses on valid threat-cued trials relative to neutral-cued trials. Attentional biases are also indicated by slower responses on invalidly threat-cued trials relative to neutral-cued trials. The spatial cueing task has also revealed a robust attentional bias effect (Bar-Haim et al., 2007, Cisler et al., 2009).
Anxiety Diagnosis
A second main finding in attentional bias research is that the biases occur in all anxiety disorders. Attentional biases have been found in GAD (Bradley, Mogg, White, Groom, & de Bono, 1999; Bradley, Mogg, Millar, & White, 1995; Mogg et al., 1993; Rinck et al., 2003), social phobia (Amir et al., 2003; Becker, Rinck, Margraf, & Roth, 2001), PTSD (Bryant & Harvey, 1995; McNally et al., 1990a), specific phobia (Ohman et al., 2001; Rinck et al., 2005), panic disorder (Buckley, Blanchard, & Hickling, 2002; Horenstein & Segui, 1997), and OCD (Amir et al., in press; Cisler & Olatunji, in press; Foa et al., 1993; Tata et al., 1996). In the case of OCD, Moritz and colleagues (Moritz et al., 2008; Moritz & von Muhlenen, 2008) have recently found null results for an attentional bias effect in OCD. However, these null results are countered, and perhaps explained, by a recent study by Amir and colleagues (in press). These authors found that individuals with OCD display an attentional bias towards ideographically displayed stimuli in the dot probe task in the first third of the experimental trials. In the remainder of the trials the attentional bias effect diminishes. These findings, if replicated, suggest that the bias in OCD can be strategically overridden with sufficient practice and possibly implicate deficits in inhibition (cf. Chamberlain et al., 2005). In any case, a recent meta-analysis found that the attentional bias effect appears to occur in equal magnitude in all disorders (Bar-Haim et al., 2007). Accordingly, attentional biases do not appear to be a feature of any specific anxiety disorders, but likely appear to be a component of elevated trait anxiety per se. This finding is theoretically relevant because it necessitates an explanation as to why attentional biases are robustly related to elevated trait anxiety. That is, any theory of pathological anxiety must account for the observation that attentional biases towards threat co-occurs with chronic anxiety. Further, the observation that attentional biases are a component of elevated trait anxiety per se suggests that understanding attentional biases may strengthen our understanding of the processes of anxiety.
Summary
Numerous data demonstrate that attention is biased towards threatening information among anxious individuals. Bar-Haim and colleagues (2007) recently concluded from a meta-analysis of attentional biases that “with over 150 studies that have established the existence and typical magnitude of the threat-related bias in anxious individuals from different populations and with a variety of experimental conditions, it appears as if little will be gained from additional studies of threat-related bias unless these are strongly driven by theory” (pg. 18). Accordingly, the next step in attentional bias research is to elucidate how and why attention is biased towards threat in anxious individuals.
Previous Theoretical Models of Attentional Biases towards Threat in Anxiety
In the past 20 years several models have been put forward to account for the mechanisms underlying attentional bias in anxiety. The review of these models will focus on the mechanisms of attentional bias postulated by the models. It is important to note that these models have mainly focused on individual differences in trait anxiety. Spielberger, Gorsuch, Lushene, Vagg, and Jacobs (1983) described trait anxiety as a personality trait that predisposes an individual to respond with anxiety to stressful and novel situations. Provided that the magnitude of attentional bias does not differ between high trait anxious and clinically anxious individuals (Bar-Haim et al., 2007) and the observation that trait anxiety is an important predisposition to develop clinical anxiety (Barlow, 2002), it seems safe to assume that the mechanisms postulated in the context of trait anxiety are also useful in understanding effects related to clinical anxiety.
Beck and Clark's cognitive model
Beck and Clark's (1997) model suggests that anxiety is characterized by biases at (1) the initial registration of a threat stimulus; (2) the activation of a primal threat mode; and (3) the secondary activation of more elaborative and reflective modes of thinking. The first mode is involved with the automatic orienting to threat, which is largely stimulus-driven. Recognition of personally relevant, negative information subsequently leads to the activation of cognitive, affective, psycho-physiological, and behavioral responses that are innate and, in the case of anxiety, function to obtain safety and minimize threat (referred to as a “primal mode”). These responses tend to be rigid and inflexible because they were developed to maximize the chances of survival. At this stage, attention is captured by threat and actions involve coping with threat. The final stage in this model is the process of secondary elaboration where there is slow, detailed, effortful, and schema-driven processing of threat.
Williams, Watts, MacLeod & Mathews (1988) model
Williams et al. (1988) presented an influential account of information processing biases in anxiety and depression. In their model attentional biases are postulated at the preconscious level. They postulate that the threat value of incoming stimuli is determined by an affective decision mechanism (ADM). This system produces an initial decision whether information is high or low threatening and the output of this system is moderated by state anxiety. If stimulus input is appraised as highly threatening, a resource allocation mechanism (RAM) is activated. When the RAM is triggered, attentional resources will be allocated to threat. When stimulus input is determined as low threatening, attention will be maintained to the task at hand and the new stimulus input will not be attended to. According to Williams et al. (1988), trait anxiety modulates the RAM: HTA individuals will allocate attention to threat (which results in an attentional bias), whereas LTA individuals will ignore threatening information. This may lead to the favorable attentive processing of threat at preconscious and conscious levels in HTA individuals.
The Williams et al. model has inspired thorough empirical investigation into the nature of attentional bias. However, some of these ideas about threat processing in LTA individuals are problematic (Mogg & Bradley, 1998). Williams et al. propose that the RAM directs attentional resources away from threat in LTA individuals. This may be true for minor threat, but it seems implausible that severe threat will not attract attention regardless of an individual's anxiety level. Persistent attentional avoidance from severe threat would obviously interfere with adequate responding to threat. Indeed, Wilson and MacLeod (2003) recently demonstrated that LTA individuals display attentional biases for severely threatening, but not moderately threatening stimuli, whereas HTA individuals display attentional biases for both magnitudes of threat. Despite this limitation, the model has strongly influenced contemporary models of attentional bias to threat.
Öhman's (1996) feature detection model
The work on attention to threat by Arne Öhman (1996; 2005; Öhman & Wiens, 2004) has generally focused on attention to threat as an evolutionary adaptive process, with particular emphasis on the unconscious processing of threat. In his account, stimulus input is analyzed in a feature detection system. Biologically prepared or high intense stimuli can exert a direct influence on the arousal system through this feature detection system without any conscious mediation and facilitate attentional allocation to threat. It is worth noting that the feature detection system can explain biases towards pictorial stimuli, but not lexical stimuli, which do not have biologically relevant features. According to Öhman's model, when information has passed the feature detection system, it enters a significance evaluation system. Threatening or relevant information then enters the conscious perception system, which allows a slower, conscious appraisal of meaning through interaction with the emotional memories stored in the expectancy system. This slower, conscious processing route can also influence the arousal system if information is appraised as threatening. In this model, feedback loops are postulated between the autonomic arousal system and the significance evaluation system: heightened arousal further sensitizes the significance evaluation. Furthermore, the expectancy system may also sensitize the significance evaluation for specific stimuli due to prior learning.
Wells and Matthew's model
A markedly different view on attentional bias has been put forward by Wells and Matthews (1994). They strongly argued against the idea of an automatic attentional bias and the view that attentional bias is a computational accident. Wells and Matthews emphasized the role of top-down processes and framed their model within a general self-regulatory executive function model. In their view, attentional bias to threat is related to self-knowledge, with voluntary goals and beliefs of the individual guiding attention to threat. Wells and Mathews argue that anxiety is characterized by a consciously perceived threat to self-perseverance and that this motivational state is associated with the monitoring of threat. They propose that the attentional bias effect observed in cognitive-experimental tasks is caused by the belief that it is important to monitor threat. Indeed, Matthews and Wells (2000) discussed limitations of studies demonstrating attentional bias without conscious awareness and speculated that under several conditions attentional bias may occur without awareness due to the operations of voluntary strategies.
Mogg and Bradley's (1998) cognitive-motivational model
In the cognitive-motivational model, attention to threat is understood as a normal and adaptive mechanism. They draw strongly on the neurobiological work of LeDoux (1996) who demonstrated that threat can be processed through two neural pathways: (1) a fast and crude analysis of stimulus features related to previously encountered threat, and (2) a slower, more detailed analysis of stimulus input, contextual information, and information stored in memory. In Mogg and Bradley's model, attention to threat is determined by two systems. First, a valence evaluation system is responsible for the initial, preconscious appraisal of stimuli. Output from this system is also dependent upon contextual information, prior learning, and state anxiety level. Trait anxiety influences the reactivity of the valence evaluation system to threat, with a heightened sensitivity to threat in HTA individuals. In HTA individuals, mild threat cues are more readily appraised as high threat than in LTA individuals. Second, output from the valence evaluation system feeds into a goal-engagement system which determines the allocation of processing resources. If a stimulus is tagged as highly threatening, current behavior will be interrupted and attention will be allocated to the stimulus input. If stimulus input is tagged as low threatening, further processing of this stimulus will be inhibited, attention will be maintained at ongoing tasks, and current behavior will not be interrupted. According to this view, differential attention to threat in HTA and LTA individuals can be expected for mild threat but not for severe threat. Due to an oversensitive valence evaluation system, HTA individuals have the tendency to appraise mild and ambiguously threatening information as highly threatening and will attend to this information. In LTA individuals this information will be ignored as it will generally be appraised as low threatening. One initial difficulty with this theory was that there was little evidence for an overly sensitive valence evaluation mechanism apart from the observations the mechanism was used to explain.
Mathews and Mackintosh's (1998) model
Another model that shares many assumptions with the cognitive-motivational model (Mogg & Bradley, 1998), is the cognitive model developed by Mathews and colleagues (Mathews & Mackintosh, 1998; Mathews, Mackintosh, & Fulcher, 1997). In line with the ideas put forward by Williams et al. (1997), attentional bias is only predicted when threat has to compete with other stimuli or task-demands. A threat evaluation system (TES) is proposed that shares many characteristics with the ADM of the Williams et al (1997) model. Stimulus input is automatically evaluated and output of this system feeds into a distracter/threat representation system. The interference caused by the distraction representation is countered up to a certain level by voluntary effort aimed at attending targets from the task at hand and strengthening their representations. In accounting for attentional bias, it is postulated that the output of the TES is strengthened by anxiety level. More specifically, stimulus input needs to exceed a certain threshold before output will flow from the TES into the distraction representation. A heightened anxiety level lowers the threshold value from the TES and causes an increased output of this system. This model further proposes that strong danger cues will attract attention in everyone, whereas weak danger cues will only do so in individuals with a heightened anxiety level.
Eysenck and colleagues (2007) attentional control theory
Attentional control theory posits that anxiety disrupts two central executive functions related to attentional control: inhibition and shifting. Inhibition refers to the ability to inhibit or regulate dominant or automatic responses. Shifting refers to the adaptive ability to shift attention between tasks depending on context. Eysenck and colleagues discuss these functions in terms of top-down and bottom-up processing. Anxiety impairs inhibition in that anxiety weakens the degree to which inhibitory mechanisms can regulate automatic responses; that is, anxiety weakens top-down regulatory control. One manner in which this effect may manifest is in difficulty disengaging attention from distracting threat stimuli. Anxiety potentiates shifting in that anxiety heightens the degree to which attention is shifted from one task to another; that is, anxiety increases stimulus-driven bottom-up processing. One manner in which this effect manifests is in the facilitated detection of threat stimuli.
Bar-Haim and colleagues (2007) model
Bar-Haim and colleagues suggest a multidimensional model of attentional biases in which dysfunction of any sequence in the temporal chain may result in attentional bias. First, a preattentive threat evaluation system evaluates environmental stimuli. Threatening stimuli feeds into a resource allocation system and elicits physiological arousal and allocation of cognitive resources onto the stimuli. A guided threat evaluation system may then assess the context of the threat and assess available coping resources. If this system deems the threat low in significance, the input of the preattentive threat evaluation system may be overrided by a goal engagement system. If the guided threat evaluation system deems the threat high in significance, attention may be maintained on threat and a high state of anxiety may result.
Summary
It is obviously difficulty to evaluate these models in relation to the components of attentional bias, the mediating mechanisms, and stages of processing, given that the models were not all specifically developed to explain all of these aspects. Further, some of the models were developed prior to research being developed in certain attentional bias aspects (e.g., before difficulty in disengagement had been distinguished from facilitated attention). With that limitation explicitly stated, a summary of these models in regards to their likely predictions about the attentional bias components, mediating mechanisms, and stage of processing is provided in Table 1. These models all predict the component of vigilance/facilitated attention towards threat. The component of difficulty disengaging attention from threat is less consistent across the models, with only two models (Beck & Clark, 1997; Eysenck, 2007) accounting for and explaining this phenomenon. Attentional avoidance of threat is similarly less represented in the models and only accounted for and explained by one model (Williams et al., 1988).
Table 1.
Summary of theoretical models in regards to their predictions of the components, mediating mechanisms, and stage of processing in attentional biases towards threat among anxious individuals.
| Model | Attention Components | Mediating Mechanisms | Stage of Processing |
|---|---|---|---|
| Williams et al. 1988 | Facilitated attention to threat in HTA; Attentional avoidance in LTA | Affective Decision Mechanism; Resource Allocation Mechanism | Automatic |
| Wells & Mathews 1994 | Facilitated attention to threat | Voluntary goals (i.e., belief that threat monitoring is important) underlie facilitated attention to threat | Strategic |
| Ohman, 1996 | Facilitated attention to threat | Amygdala underlies threat detection Mechanism, which underlies facilitated attention, expectancy system is involved in conscious appraisal of stimulus | Primarily automatic but also strategic feedback loop |
| Beck & Clark, 1997 | Facilitated attention to threat; Difficulty in disengagement | Primal mode constricts attention onto threat; threat-relevant schemas maintains attention onto threat | Automatic facilitated attention; strategic difficulty in disengagement |
| Mogg & Bradley 1998 | Facilitated attention to threat | Valence evaluation mechanism appraises stimuli as threatening; goal-engagement System allocates attention to stimuli | Automatic |
| Mathews & mackintosh 1998 | Facilitated attention to threat | Threat evaluation system underlies facilitated attention; voluntary effort can inhibit facilitated attention | Automatic facilitated attention can be overridden by strategic effort |
| Bar-haim et al., 2007 | Facilitated attention to threat; Difficulty in disengagement | Threat detection mechanism appraises valence; resource allocation mechanism underlies facilitated attention; threat elaboration mechanism further appraises stimulus; Goal engagement mechanism maintains or disrupts on-going goal | Automatic threat detection and resource allocation; strategic threat elaboration and goal engagement |
| Eysenck et al. 2007 | Facilitated attention to threat; Difficulty in disengagement | Attentional Control underlies facilitated attention via augmenting stimulus-driven attention network, Attentional control underlies difficulty in disengagement via impairing the goal-directed attention network | automatic facilitated attention |
In regards to the mechanisms that mediate attentional biases, most models posit some sort of threat detection mechanism responsible for detecting and orienting attention towards threatening stimuli (Bar-Haim et al., 2007; Beck & Clark, 1997; Eysenck et al., 2007; Ohman, 1996; Mogg & Bradley, 1998; Mathews & Mackintosh, 1998; Williams et al., 1988). Only Wells and Mathews (1994) model does not posit an automatic threat detection mechanism. There is great theoretical discord in regard to other possible mechanisms. Some models posit a resource allocation mechanism that directs the use of available cognitive resources (Bar-Haim et al., 2007; Mogg & Bradley, 1998; Williams et al., 1988). Some models posit a threat elaboration mechanism, in which strategic processing evaluates the identified threat as either major or minor (Bar-Haim et al., 2007; Beck & Clark, 1997). Some models posit a strategic goal engagement mechanism, in which the individuals’ goals, beliefs, voluntary effort, or schematic processing can either maintain or override attention to threat (Bar-Haim et al., 2007; Eysenck et al., 2007; Mathews & Mackintosh, 1998; Wells & Mathews, 1994). The stage of information processing in which attentional biases occur is also inconsistently predicted across the models, with most models positing both stages (Bar-Haim et al., 2007; Beck & Clark, 1997; Ohman, 1996; Mathews & Mackintosh, 1998), some models positing only automatic processing (Mogg & Bradley, 1998; Williams et al., 1988), and one model positing only strategic processing (Wells & Matthews, 1994).
In regards to interrelations between the different aspects, most models generally link an automatic threat detection mechanism with facilitated attention for threat (Bar-Haim et al., 2007; Beck & Clark, 1997; Mathews & Mackintosh, 1998; Mogg & Bradley, 1998; Ohman, 1996; Williams et al., 1988). One model links difficulty in disengagement with a strategic threat elaboration mechanism (Beck & Clark, 1997), one model links difficulty in disengagement to a guided threat evaluation system (Bar-Haim et al., 2007), and one model links difficulty in disengagement to disruption of attentional control (Eysenck et al., 2007).
In sum, there is little theoretical agreement as to what components attentional biases have, what mechanisms mediate these components, and during what stage of processing the mechanisms operate. The only consistent prediction across the models is that a threat detection mechanism operates at the automatic stage of processing and underlies facilitated attention to threat. This general lack of agreement makes it difficult to understand why attention is biased towards threat in anxious individuals.
Review of Empirical Evidence for the Mechanisms of Attentional Bias
Given the theoretical discord, we now review the evidence in regards to the components of attentional bias, mediating mechanisms, and stages of processing.
Components of Attentional Bias
One of the emerging lines of inquiry in attentional bias in anxiety disorder research is the investigation of the components of attentional bias (Cisler et al., 2009; Fox et al., 2002; 2001; Koster et al., 2004a). Attentional biases towards threat may be comprised of facilitated attention to threat, difficulty disengaging attention away from threat, or attentional avoidance of threat. Facilitated attention refers to the relative ease or speed with which attention is drawn to a threat stimulus (i.e., attentional orienting). Difficulty in disengaging refers to the degree to which a threat stimulus captures attention and impairs switching attention from the threat to another stimulus. Attentional avoidance refers to a more recent empirical phenomenon in which attention is preferentially allocated towards locations opposite the location of the threat cue, thus indicating avoidance of the threat cue (e.g., Koster et al., 2005; Mogg et al., 2004). Measurement of these components necessitates a task that can differentiate the components. Three tasks that have been used in the literature for this purpose include the spatial cueing, visual search, and dot probe tasks.
Delayed disengagement from threat
Research using the spatial cueing task has invariably demonstrated difficulty in disengagement among anxious individuals (Amir, Elias, Klumpp, & Przeworski, 2003; Cisler & Olatunji, in press; Fox, Russo, Bowles, & Dutton, 2001; Fox, Russo, & Dutton, 2002, experiment 1; Koster, Crombez, Verschuere, Van Damme, & Wiersema, 2006; Koster et al., 2005; 2004; Van Damme et al., 2006; Yiend & Mathews, 2001). Research using the visual search task has almost invariably demonstrated difficulty in disengagement among anxious individuals (Byrne & Eysenck, 1995; Gilboa-Schechtman, Foa, & Amir, 1999; Juth, Lundqvist, Karlsson, & Öhman, 2005, experiment 5; Lipp & Waters, 2007; Miltner, Krieschel, Hecht, Tripp, & Weiss, 2004; Rinck, Becker, Kellermann, & Roth, 2003; Rinck, Reinecke, Ellwart, Heuer, & Becker, 2005), with only one exception (Pflugshaupt et al., 2005). The dot probe task methodology has only recently been improved to disentangle the effects of facilitated attention and difficulty in disengagement (Koster et al., 2004a), and the evidence thus far has almost invariably demonstrated difficulty in disengagement among anxious individuals (Koster et al., 2004a; Koster et al., 2006b; Salemnik et al., 2007), with one failure to find disengagement (Carlson & Reinke, 2008, though this study only used masked stimuli). Accordingly, there is considerably strong evidence that attentional biases towards threat are comprised of a difficulty in disengaging attention from threat stimuli. Mogg and colleagues (2008) have recently argued that the spatial cueing task may not provide unambiguous evidence for delayed disengagement, as there could be a confound between delayed disengagement and a generic slowdown effect caused by presentation of threat (e.g., Algom et al., 2004). Although this indeed is a problem in this task, the delayed disengagement hypothesis is also corroborated by results obtained in the dot probe and visual search tasks. Further, Cisler and Olatunji (in press) recently found that the relation between elevated contamination fear and difficulty in disengagement in the spatial cueing task remained when statistically controlling for generic response slowing, suggesting that this task confound does not explain the difficulty in disengagement effect. However, future research in this area is needed among other anxious populations.
Facilitated attention for threat
The evidence for facilitated attention among anxious individuals appears mixed at first glance, but important moderating variables may affect the observation of facilitated attention. Some studies using the spatial cueing task have failed to demonstrate evidence for facilitated attention among anxious individuals (Amir, Elias, Klumpp, & Przeworski, 2003; Fox, Russo, Bowles, & Dutton, 2001; Fox, Russo, & Dutton, 2002, experiment 1; Yiend & Mathews, 2001). However, Koster and colleagues (2006a) found that at 100 ms stimulus durations, facilitated attention was found towards highly, but not mildly, threatening pictures among high trait anxious individuals. At longer presentation times there was again no evidence of facilitated attention. Research using dot probe methodologies that disentangle facilitated attention from difficulty in disengagement have only found evidence for difficulty in disengagement towards supraliminally presented stimuli (Koster et al., 2004a; Koster et al., 2006b; Salemnik et al., 2007), while facilitated attention has been found towards subliminally presented stimuli (Carlson & Reinke, 2008). Accordingly, stimulus duration (i.e., quick stimulus presentation) may moderate the occurrence of facilitated attention among anxious individuals. One prior study (Fox et al., 2001; experiment 2) failed to find facilitated attention at 100 ms, but this study did not also manipulate threat intensity. Further demonstrating the importance of stimulus intensity, three studies demonstrate that neutral stimuli paired with aversive stimuli (e.g., loud noise bursts, shock) in classical conditioning paradigms elicit facilitated attention in the spatial cueing task (Koster et al., 2005; 2004b; Van Damme et al., 2006). These latter data suggest that stimuli predicting the occurrence of imminent threat elicit facilitated attention. Accordingly, it appears to be the case that facilitated attention towards threat is moderated by threat intensity (i.e., highly threatening stimuli) and stimulus duration (i.e., 100 ms or less).
Some studies using the visual search task have also failed to find evidence for facilitated attention (Rinck, Becker, Kellermann, & Roth, 2003; experiment 1; Rinck, Reinecke, Ellwart, Heuer, & Becker, 2005; experiment 1). However, these studies are contrasted by several other visual search studies that have documented facilitated attention among anxious individuals (Byrne & Eysenck, 1995; Gilboa-Schechtman, Foa, & Amir, 1999; Juth, Lundqvist, Karlsson, & Öhman, 2005, experiment 5; Miltner, Krieschel, Hecht, Tripp, & Weiss, 2004; Rinck et al., 2003; experiment 2; Rinck et al., 2005, experiments 2 and 3). Research using visual search tasks have not yet manipulated stimulus duration or threat intensity, so it remains to be seen whether these variables also explain the inconsistent results in visual search tasks.
Attentional avoidance
Finally, several studies have documented attentional avoidance among anxious individuals. Koster and colleagues (2005) found the standard congruency effect in the dot probe (i.e., reaction times on congruent trials were shorter than on incongruent trials) among high trait anxious participants when threat pictures were presented for 500 ms. However, at stimulus durations of 1250 ms, high trait anxious participants demonstrated attentional avoidance of the threat cues, indicated by longer reaction times on congruent trials compared to incongruent trials. This basic effect, that attentional avoidance is observed at long, but not short or intermediate stimulus presentation durations, has been replicated (Koster et al., 2006; Mogg et al., 2004). Moreover, attentional avoidance of threat has been found when directly measuring eye fixations (Garner et al., 2006; Calvo & Avero, 2005; Rohner, 2002; Pflugshaupt et al., 2005). For example, Pflugshaupt and colleagues (2005) found that spider phobic individuals initially demonstrated rapid eye movement fixations onto a spider stimulus (i.e., facilitated attention), but they subsequently demonstrated eye movement fixations away from the spider stimulus (i.e., attentional avoidance). Demonstrating robustness, the attentional avoidance effect at long stimulus durations has been found in the dot probe task (Garner et al., 2006; Koster et al., 2005; Mogg et al., 2004), exogeneous cueing task (Koster et al., 2006), and visual search tasks (Pflugshaupt et al., 2005). However, there have been some studies using similar methodologies that have failed to find attentional avoidance among anxious individuals at long (e.g., 1500 ms) stimulus durations (Bradley et al., 1998; Mogg et al., 1997). Future research is still needed in this area to clarify the inconsistencies and investigate potential moderators (e.g., threat intensity).
Mediating Mechanisms
Attentional control
One of the relevant areas of emerging research is investigating the role of attentional control in attentional biases (Derryberry & Reed, 2002; Eysenck et al., 2007; Mathews & Wells, 2000). Attentional control is an individual difference variable that refers to individuals’ ability to regulate their attentional allocation. This can be construed as a ‘top-down’ regulatory ability (Posner & Rothbart, 2000), such that it inhibits the ‘bottom-up’ influence of emotional distracters (Eysenck et al., 2007). Derryberry and Reed (2002) found that trait anxious individuals with poor attentional control, which was measured via self-report, displayed difficulty disengaging attention from threat at 250 ms stimulus duration as well as 500 ms stimulus durations. In contrast, trait anxious individuals with good attentional control demonstrated difficulty disengaging from threat at 250 ms stimulus duration but not at 500 ms stimulus duration. Peers and Lawrence (2009) also found that individuals with poor attentional control demonstrated difficulties disengaging attention from rapidly presented emotional faces (i.e., 100 ms) during a rapid serial visual presentation task (see Anderson, 2005; Raymond et al., 1992), whereas individuals with good attentional control displayed no disengagement difficulties.
Recent research has similarly demonstrated that effortful control moderates the relation between negative affectivity and attentional bias towards threat in the dot probe task among children and adolescents (Lonigan & Vasey, 2009), such that only youth high in negative affect and low in effortful control displayed attentional biases towards threat displayed for 1250 ms. However, this study used the original dot probe methodology (Macleod et al., 1986); thus, difficulty in disengagement could not specifically be demonstrated. Effortful control refers to individual differences in the ability to engage executive processes to override dominant responses (Posner & Rothbart, 2000), and is theorized to be linked with attentional control (Derryberry & Rothbart, 1997). This body of data converges in demonstrating that the ability to regulate attention allocation may moderate the degree to which attention can be disengaged from threatening stimuli. Accordingly, attentional control may be a higher order regulatory mechanism controlling the characteristics of attentional biases towards threat, specifically disengagement from threat.
Emotion regulation strategy/goals
Emerging research also demonstrates that emotion regulation strategies may moderate attentional biases towards threat. Emotion regulation refers to “the processes by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions” (Gross, 1998a, pg. 275). Attentional allocation has been strongly proposed as one mechanism of emotion regulation (Gross, 1998; 2001; 2007; Koole, 2009). For example, an individual may allocate attention onto a distracting poster on the wall while receiving an injection at the doctor's office in order to reduce negative affect during the injection. Purposeful attentional allocation towards neutral relative to unpleasant stimuli is akin to the concept of ‘distraction’ (van Dillen & Koole, 2007; Sheppes & Meiran, 2008).
Johnson (2009) engaged participants in a dot probe task displaying pairs of angry and happy faces and instructed participants to either focus attention onto the happy faces or provided no instruction. Stimuli were presented for 17 ms, 500 ms, and 1250 ms. Participants were also engaged in difficult anagram tasks before and after the dot probe task. Results demonstrated that participants given the goal to attend to happy faces demonstrated attentional biases towards the happy faces at only stimulus durations of 1250 ms, whereas this was not true of the participants given no instruction. Participants instructed to attend to happy faces demonstrated less frustration during the anagram tasks only after the dot probe tasks relative to participants given no instruction. Further, the bias towards attending to happy faces at 1250 ms predicted how many seconds participants engaged in the second anagram task before giving up only in participants instructed to attend to happy faces. Accordingly, this study demonstrates that a goal of attending to positive stimuli leads to more attention towards positive stimuli during strategic stages of processing, less frustration during difficult tasks, and better emotion regulation. Dunning and Hajack (2009) similarly found that participants displayed elevated late positive potential, a brain electrical activity indicator of increased attention to motivationally salient stimuli, while passively viewing unpleasant pictures. In contrast, participants directed to allocate their attention onto less distressing aspects of the unpleasant pictures displayed decreased late positive potential. Distraction has also been found to produce reduced memory for the threatening stimulus (Sheppes & Meiran, 2009). These data demonstrate that emotion regulation goals may moderate the components of attentional biases at late stages of processing and that attentional avoidance can indeed regulate emotion. These two observations suggest that attentional avoidance among anxious participants (Koster et al., 2005; 2006; Mogg et al., 2004; Pflugshaupt et al., 2005) may occur because these participants are attempting to strategically regulate negative affect via distraction.
Attentional control ability and emotion regulation strategies appear to both be moderators of attentional biases; however, they are likely separate mechanisms that moderate attentional biases in distinct manners. Attentional control can be construed as a regulatory ability: people with better attentional control can disengage attention from threatening stimuli, whereas people with poor attentional control may demonstrate difficulties disengaging attention from threatening stimuli. In contrast, emotion regulation may not be an ability per se, but may also reflect the individual's strategy for coping with negative emotion. Although research demonstrates that some strategies of emotion regulation (e.g., re-appraisal) are more acutely effective than others (e.g., suppression; Feldner et al., 2003; Gross & Levenson, 1993; 1997; Gross, 1998b; Moore et al., 2008), an individual's strategy for coping with negative emotion likely varies considerably given the context (Gross, 1998a). For example, an individual may express facial expressions of negative emotion while being yelled at by a loved one, but the same individual may suppress facial expressions of negative emotion while being yelled at by their boss. Accordingly, context may moderate an individual's online emotion regulation goals/strategies, which in turn moderates whether and to what degree attention will be deployed to a threatening stimulus. It is important to note that attentional control and emotion regulation are likely related, such that whether one can effectively regulate emotions may depend on higher-order regulatory mechanisms, such as attentional control. Thus, interactions between these mechanisms might be expected.
Neural mechanisms
A wealth of data demonstrate that the amygdala, a brain structure located in the temporal lobes, is critically involved in the processing of fear-related information and expression of fear-related behavior (Davis, 2006; Davis & Whalen, 2001; LeDoux, 2000; Myers & Davis, 2007; Rosen, 2004). Relevant to the current topic, enhanced amygdala activity is likely a neural mechanism involved in automatic vigilance/facilitated attention for threat. Carlson and colleagues (2009) recently demonstrated in the dot probe task that masked congruent trials were associated with increased amygdala activity, providing strong evidence that the amygdala is involved in automatic facilitated attention to threat. Similarly, research demonstrates correlations between the amygdala and attentional biases towards threat (e.g., Monk et al., 2004; Monk et al., 2008; van den heuvel et al., 2005; Anderson & Phelps, 2001). For example, van den Heuvel and colleagues (2005) found that attentional biases towards panic-related words among individuals with panic disorder predicted amygdala activity during fMRI scanning. Moreover, Anderson and Phelps (2001) found that an individual with bilateral amygdala lesions did not display attentional biases towards threat, whereas individuals with unilateral amygdala lesions did display attentional biases towards threat. Second, research demonstrates that masked and/or unattended stimuli elicit amygdala activation (Dolan & Vuilleumier, 2003; Morris et al., 1998; Vuilleumier, Armony, Driver, & Dolan, 2001; Whalen et al., 1998; 2004), which is analogous to the findings that masked stimuli elicit attentional biases (Bar-Haim et al., 2007; Mogg et al., 1993). For example, Whalen and colleagues (1998) found greater activation of the amygdala during masked presentations of fearful faces compared to masked happy faces. Further, Whalen and colleagues (2004) later found greater activation of the amygdala to masked images of fearful eyes (i.e., eyes with an enlarged whitened area) relative to masked images of normal eyes, demonstrating that the amygdala may respond automatically to specific fear-relevant features.
These lines of evidence converge in suggesting that the amygdala is critically involved in automatic vigilance for threat (Davis & Whalen, 2001; Ohman, 2005). However, it is important to note that automatic activation of the amygdala towards threat may depend on the availability of attentional resources (Pessoa, 2005), suggesting that this threat detection mechanism does not operate completely automatically and highlighting the fuzzy boundaries separating automatic from strategic processing. Further, it is unlikely that only the amygdala underlies the automatic detection of threat; rather, it is likely most accurate to conceptualize the amygdala as a central structure in a larger threat detection system.
A wealth of data is beginning to suggest that higher-order cortical structures, such as the prefrontal cortex (PFC) and its subunits and functionally-related structures (e.g., anterior cingulate cortex [ACC] , orbitofrontal cortex), may be neural mechanisms underlying difficulties disengaging attention from threat. These neural structures serve a regulatory purpose and can down-regulate emotion-relevant limbic structures, thus providing a ‘top-down’ processing influence (Miller & Cohen, 2001). Evidence that prefrontal structures down-regulate sub-cortical emotional systems comes from 1) studies demonstrating that the prefrontal cortex and related structures are critically involved in down-regulating amygdala processing during extinction learning (see Myers & Davis, 2007; Quirk, Garcia, & Gonzalez-Lima, 2006; Quirk et al., 2003; Sotres-Bayon, Cain, & LeDoux, 2006), and 2) studies demonstrating that employing the emotion regulation strategy of ‘re-appraisal’ (Gross, 1998b) results in increased PFC activity and reduced amygdala activity while watching aversive films (Eippert et al., 2007; Kim & Hamann, 2007; Urry et al., 2006; Ocshner et al., 2004). These data support the generic effect of top down regulatory control over sub-cortical fear circuits.
Emerging evidence suggests that prefrontal regulatory structures are involved in the disengagement of attention from threat. Derryberry and Reed (2002) found that trait anxious individuals with good attentional control, which is arguably a regulatory skill (also see Derryberry & Rothbart, 1997; Eysenck et al., 2007; Posner & Rothbart, 2000), are able to shift attention away from threatening stimuli at 500 ms, whereas trait anxious individuals with poor attentional control are not able to do so. Accordingly, higher-order attentional control ability appears to determine the degree to which an individual can disengage attention from threat. Bishop and colleagues (2004) found that state anxiety was significantly inversely correlated with PFC activity (r = −.60) during a task in which individuals had to ignore pictures of fearful facial expressions. These data suggest that regulatory ability (i.e., PFC activity) is reduced in anxious individuals attempting to ignore (i.e., disengage from) threatening information. Bishop and colleagues (2009) similarly found that elevated trait anxiety is associated with decreased PFC activity while performing a low demand non-threatening task, suggesting that the attentional system of trait anxious individuals generally (i.e., outside of the context of threat) involves ‘impoverished’ PFC control. Dolcos and McCarthy (2006) found that fear-related distracter pictures impaired working memory and that PFC activity was strongly inversely correlated with the degree to which the emotional pictures distracted the participants (r = −.74). These data suggest that the degree to which threatening stimuli distract is based on the magnitude of PFC: less PFC activity is linked with greater threat distraction, more PFC is linked with less threat distraction. These sources of data support the hypothesis that prefrontal regulatory structures may underlie difficulty in disengagement from threat. As is the case with the amygdala, though, it is likely most accurate to conceptualize the PFC as a central structure in a larger attentional control/regulatory system instead of making a 1:1 correspondence between PFC function and attentional control.
Stage of Information Processing
A wealth of data demonstrate that attentional biases are observed at varying stimulus duration presentations (e.g., 17 ms, 50 ms, 100 ms, 500 ms; Bar-Haim et al., 2007; Mogg et al., 1993; Koster et al., 2006). The observation that attentional biases are observed at varying stimulus presentation durations is important because it suggests that the effect is not dependent on, or only found in, certain stages of information processing. However, the nature of attentional bias may be dependent on the stage of information processing. Information processing is commonly conceptualized in two stages, automatic and strategic processing stages (Shiffrin & Schneider, 1977). Automatic processing generally refers to processing that is effortless, capacity free, unintentional, and outside of conscious control, whereas strategic processing generally refers to processing that is effortful, capacity-limited, intentional, and dependent on conscious control (Shiffrin & Schneider, 1977). Although the boundary conditions between automatic and strategic processing are blurry at best (see Moors & de Houwer, 2006), conceptualizing attentional biases in terms of automatic and strategic processing is considered theoretically meaningful. In particular, McNally (1995) has argued that the criterion of unintentionality is of importance in psychopathology as it relates to limited control over the processing biases.
First, there is substantial empirical evidence to suggest that attentional biases can occur under conditions of limited conscious awareness of the presence of threat. Evidence for this assertion comes from studies using masked stimuli: stimuli are presented briefly (e.g., 17 ms) and followed by a backwards mask that precludes conscious awareness of the stimulus. For example, in a Stroop task, the word ‘spider’ may occur for 17 ms and then be immediately replaced by ‘xxxx’ for 483 ms. Attentional biases towards masked stimuli have been demonstrated in the Stroop task (Bradley, Mogg, Millar, & White, 1995; Harvey, Bryant, & Rapee, 1996; Mogg et al., 1993; van den Hout, Tenney, Huygens, & de Jong, 1997; van Honk et al. 2001, experiment 2) and dot probe tasks (Carlson & Reinke, 2008; Mogg, Bradley, & Williams, 1995; Mogg, Bradley, & Hallowell, 1994). The Bar-Haim and colleague (2007) meta-analysis found an aggregate effect size of d = .32 for subliminally presented stimuli. This body of data is theoretically relevant because it suggests automatic pre-conscious biases towards threat. The observation that non-anxious controls do not tend to display automatic attentional biases (Bar-Haim et al., 2007) suggests that anxious individuals are uniquely characterized by an exaggerated threat detection mechanism. However, this research only manipulated awareness; thus, this research cannot specifically address intentionality or control. It will be important for future research to manipulate other aspects of automaticity to further test the degree to which attentional biases operate automatically.
Second, a substantial amount of empirical evidence also demonstrates an attentional bias towards supraliminally presented stimuli (Bar-Haim et al., 2007; Cisler et al., 2009). For example, Koster and colleagues (2006) found attentional biases for threat stimuli in the dot probe task among high trait anxious participants at stimulus presentation durations of 500 ms. It is important to note that an attentional bias towards supraliminally presented stimuli does not preclude automatic processing influences. Thus, demonstration of attentional biases towards supraliminal stimuli is not a “pure” indicator of strategic processing, but is instead likely a mixture of both automatic and strategic processing. Supraliminal attentional biases have been demonstrated in the Stroop task, dot probe task, spatial cueing task, and visual search task (see Bar-Haim et al., 2007; Cisler et al. 2009). The Bar-Haim and colleague (2007) meta-analysis found an aggregate effect size of d = .48 for consciously perceived stimuli, which did not significantly differ from the effect size of attentional biases towards masked stimuli. Attentional biases may then not only reflect automatic early warning threat detection mechanisms, but also reflect purposeful attempts to detect and cope with disorder-relevant sources of threat in the environment. These studies, however, only manipulate awareness, and thus cannot speak to other aspects of strategic processing, such as intentionality and control, thus necessitating future research using additional strategic processing manipulations. .
Summary and Integration
In regards to the components of attention biases, the evidence demonstrates that attentional biases are comprised of facilitated attention to threat at short stimulus durations and high threat intensities, delayed disengagement from threat, and attentional avoidance of threat at late stages of processing. In regards to the mechanisms that mediate attentional biases, attentional control appears to mediate difficulty in disengagement from threat, and emotion regulation goals may mediate attentional avoidance. Further, neurobehavioral research is beginning to illuminate that amygdala activity may mediate facilitated attention to threat. Higher-order cortical structures centered around PFC activity may mediate delayed disengagement from threat via individual differences in the ability to down-regulate the influence of sub-cortical fear structures and maintain attention on task-relevant stimuli. PFC-centered activity may mediate attentional avoidance, given that emotion regulation goals are linked with attentional avoidance and PFC-centered activity mediates emotion regulation. In regards to the stage of information processing during which attentional biases emerge, several lines of evidence demonstrate that attentional biases depend on both automatic and strategic processing.
From this review of the evidence, the postulates from previous models can be evaluated. Generally, no model (see Table 1) predicts all of the findings reviewed above. More specifically, difficulty in disengagement and attentional avoidance are particularly underrepresented characteristics in the models. Further, emotion regulation and attentional control are empirically supported underlying mechanisms that are generally unpredicted by the models. In contrast, several features of attentional biases that are common predictions across the models are indeed empirically supported. First, research demonstrates facilitated attention towards threat, which is consistent with several models. Second, neuroimaging research suggests a critical role of the amygdala in the automatic detection of threat, supporting the postulate of an automatic threat detection mechanism. Third, neuroimaging research also suggests a higher-order control mechanism, possibly centered around the PFC and functionally related structures, that regulates attentional allocation to threat. Some emerging research also suggests that this control mechanism may be related to difficulty in disengagement and attentional avoidance. Several models posit such a higher-order control mechanism underlying attentional biases towards threat. Fourth, research suggests that attentional biases are comprised of both automatic and strategic processing, which is consistent with the predictions of several models.
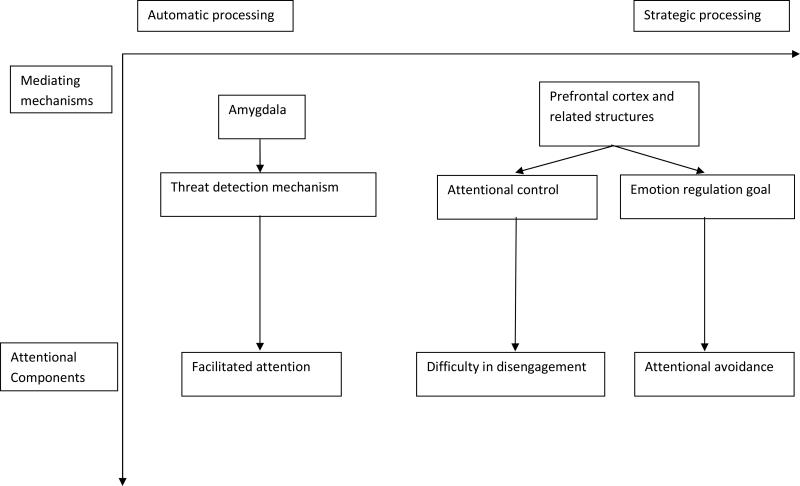
In a purely derivative manner, the research reviewed above on the mechanisms underling attentional bias can be arranged into one possible framework of attentional biases towards threat (see Figure 1). This framework is not intended to be an absolute explanation of attentional biases, but instead is a summary of what is currently known. Of particular relevance for the present topic are the interrelations between the different aspects implied in this framework. The threat detection mechanism is predicted to operate during automatic processing and underlie facilitated attention. While this is consistent with several lines of research (e.g., Koster et al., 2006; Whalen et al., 1998; 2004; van Damme et al., 2006), to our knowledge, only two studies have supported this prediction by using a methodology that concurrently assesses each of these aspects (Carlson et al., 2009; Monk et al., 2008). It will be important for future research to employ designs that allow for the concurrent assessment of stage of processing, neural activity, and component of attention in order to replicate these findings. Attentional avoidance and emotion regulation are predicted to operate in the strategic stage of processing and underlie attentional avoidance and difficulty in disengagement. Again, while this postulate is consistent with several lines of research (e.g., Derryberry & Reed, 2002; Johnson, 2009), to our knowledge, no study has concurrently demonstrated each of these levels of analyses in one methodology.
Figure 1.
Possible representation of the interrelations between the attentional components, mediating mechanisms, and stage of information processing in attentional biases towards threat among anxious individuals.
While the interactions between the mediating mechanisms and attentional components seem relatively well supported (i.e., across the vertical axis in Figure 1), what remains less clear are the interactions across stage of processing, mediating mechanisms, and attentional components (i.e., across the horizontal axis in Figure 1). For example, in what way are facilitated attention and difficulty in disengagement related? Research demonstrates that while difficulty in disengagement can be observed independently of facilitated attention (Fox, Russo, Bowles, & Dutton, 2001; Fox, Russo, & Dutton, 2002, experiment 1; Yiend & Mathews, 2001; Amir, Elias, Klumpp, & Przeworski, 2003; Rinck, Becker, Kellermann, & Roth, 2003; experiment 1; Rinck, Reinecke, Ellwart, Heuer, & Becker, 2005; experiment 1), facilitated attention almost never occurs without difficulty in disengagement also being observed (Byrne & Eysenck, 1995; Gilboa-Schechtman, Foa, & Amir, 1999; Koster et al., 2006a; 2005; 2004b; Miltner, Krieschel, Hecht, Tripp, & Weiss, 2004; Rinck et al., 2003; experiment 2; Rinck et al., 2005, experiments 2 and 3; Van Damme et al., 2006) with Pflugshaupt et al. (2005), and Carlson and Reinke (2008; though this study only used masked stimuli) as the two exceptions. Given the evidence linking facilitated attention with automatic stages of processing (Koster et al., 2006; Morris et al., 1998; Whalen et al., 1998), and difficulty in disengagement with higher-order control mechanisms (e.g., PFC activity; Bishop et al., 2004; 2009), it seems reasonable to conclude that facilitated attention precedes difficulty in disengagement when the two occur together. One possible explanation of the observed relation between these two characteristics may be that if stimulus properties in a given experiment are sufficient to fire the facilitated attention mechanism, then a feed-forward process is created whereby bottom-up affective processing (i.e., threat detection mechanism/amygdala) exerts too strong of an influence for prefrontal regulatory processes to control. Thus, stimuli capable of eliciting facilitated attention would necessarily also lead to difficulty in disengagement. Difficulty in disengagement may occur independently of facilitated attention because it is not dependent on initial absolute firing of the threat-detection mechanism. Instead, difficulty in disengagement may occur in situations where attention is allocated to a stimulus in a non-facilitated manner (e.g., if someone says ‘look at that spider!’) because of generally poor regulatory control when attention is allocated towards the threatening stimulus. This explanation seems plausible, but future research is necessary to clarify this seemingly complex relation between these characteristics of attentional bias.
The vigilance-avoidance hypothesis (e.g., Mogg et al., 2004) posits that anxious individuals first demonstrate facilitated attention to threat and then avoid attention away from threat. This hypothesis suggests a temporal relation between facilitated attention and attentional avoidance, but it is not clear how difficulty in disengagement fits into this description. Indeed, it would seem as though difficulty in disengagement and attentional avoidance are competing mechanisms: the avoidance of attention onto threat necessarily precludes simultaneous difficulty removing attention from threat. One possible resolution of the apparent conflict between these two characteristics comes from Wiereich and colleagues (2008), who argue that individuals may overtly avoid allocation attention onto threat, while concurrently covertly maintaining attention onto threat. For example, a socially phobic man may avert his eyes from disapproving faces (overt attentional avoidance), but his cognitive resources may still be allocated onto the disapproving face (covert difficulty disengaging). This explanation suggests a plausible manner by which these characteristics may co-occur. However, there is little empirical research investigating the relations between the components of attention, and future research along these lines is necessary to clarify the temporal dynamics of attentional biases.
Similarly, the relations between the candidate mediating mechanisms remain unclear. For example, to what degree are the threat detection mechanism and attentional control mechanism related? One interesting area for future research to explore is the relative weight of these mechanisms necessary to produce attentional biases. For example, attentional biases may occur due to overactivity of the threat detection mechanism, underactivity of the attentional control mechanism, or a combination of both. Consider the following illustrative example. The serotonin (5-HT) transporter (5-HTT) facilitates reuptake of 5-HT from the synaptic cleft. A polymorphism of the 5-HTT gene (5-HTTLPR) results in two variants: a short and long allele. The short allele results in a 50% reduction in 5-HTT availability, resulting in increased synaptic levels of 5-HT (Hariri et al., 2002). The short allele has been linked with attentional biases towards threat among psychiatric inpatients (Beevers et al., 2007) and healthy women (Osinsky, 2008). Further, the short allele has been linked specifically with difficulty in disengaging attention from threat and not with facilitated attention (Beevers et al., 2009). The short allele has also been linked with both amygdala hyperactivity towards threat (Harir et al., 2002; Munafo et al., 2008) and decreased functional connectivity between the amygdala and ACC (Pezawas et al., 2005). Accordingly, the route by which the short allele may affect attention for threat may be through enhanced amygdala activity, decreased regulatory control over the amygdala, or both. As this example illustrates, future research is needed to clarify how the different underlying mechanisms interact to produce the components of attentional biases. Research along these lines seems particularly important for understanding the processes underlying anxiety; that is, elucidation of the mechanisms and interactions among mechanisms responsible for attentional biases may shed light on the cognitive-emotional mechanisms underlying anxiety. Clarification of this issue may depend on the component of attention being measured (e.g., hyperactive threat detection may affect facilitated attention but not necessarily difficulty in disengagement) as well as the stage of processing being investigated.
Finally, the interaction between automatic and strategic processing also remains unclear. Whereas there is solid evidence that the amygdala responds to masked threat, suggesting (relative) automaticity, that attentional avoidance is mostly found during strategic processing, and that attentional control moderates difficulty in disengagement during strategic processing, there is little research suggesting that difficulty in disengagement operates mostly during any one stage. Studies have found difficulty in disengagement during a range of stimulus presentation durations (e.g., 100-600 ms; Amir et al., 2003; Fox et al., 2001; Koster et al., 2006), suggesting both stages of processing. Difficulty in disengagement's candidate underlying mechanism, attentional control, was found to affect biases at 500, but not 250 ms, stimulus duration in one study (Derryberry & Reed, 2002), but affect biases towards stimuli presented for 100 ms in another study (Peers & Lawrence, 2009). It is likely most accurate to conceptualize difficulty in disengagement and attentional control as reflecting a mixture of automatic and strategic processing. Further clarification of how stage of processing constrains the other domains in attentional biases is necessary. This research will likely entail stage of processing manipulations other than simply manipulating stimulus duration in order to assure that other aspects of processing (e.g., intentionality, control) are also manipulated.
The research on the stage of processing and mediating mechanisms of attentional bias is consistent with dual process models positing an automatic/associative/intuitive/emotional processing system and a planned/purposeful/verbal/cognitive processing system (Barrett, 2004; Carver, Johnson, & Joormann, 2008; Evans, 2008). That is, facilitated attention to threat and the threat detection mechanism seem related to an automatic affective mechanism linked with amygdala activity, and attentional avoidance, difficulty in disengagement, attentional control, and emotion regulation seem related to strategic cognitive-regulatory processes linked with higher-order cortical structures. Though a distinction is made between these dual modes of processing both conceptually and in the neural regions involved, the boundaries between these systems remain unclear. Pessoa (2008) recently commented that maintaining a distinction between these emotional and cognitive systems is problematic because the systems are interactive. Distinguishing between automatic versus strategic, or affective versus cognitive, seems theoretically important in that distinct mechanisms may be involved (see Figure 1), but a distinction between these systems should not entail the functional separation of these systems. That is, just as the keys of a piano are distinct from the piano's strings, neither alone is sufficient for the piano to function. Similarly, the question should not simply be ‘automatic or strategic?’, or ‘affective or cognitive?’, but a more nuanced investigation of how these systems interact to differentially impact attentional biases is needed.
Clearly, conceptualizing attentional biases as a dynamic system, in which interrelations between the domains are presumed and investigation of one component necessitates investigation of the other components, is necessary to fully understand relations between the components of attentional biases towards threat. This conceptualization may necessitate more complex, but more rigorous, methodological paradigms. Threat intensity may moderate facilitated attention (Carlson & Rienke, 2008; Koster et al., 2006; van Damme et al., 2006), and thus this manipulation may be necessary to observe facilitated attention. Stimulus duration manipulations have been a common means to manipulate stage of processing and, presumably, the underlying mechanisms. Another means that has only recently begun to be explored is the use of cognitive load (Van Dillen & Koole, in press). Embedding a cognitive load task into an attentional task may provide an interesting way to examine the automaticity of attentional biases. From the research reviewed above, it would be expected that the depletion of cognitive resources would potentiate difficulty in disengagement. Given that attentional avoidance may be linked with purposeful attempts to regulate affect, depletion of cognitive resources may attenuate attentional avoidance. Given that facilitated attention is presumed to be linked with an automatic threat detection mechanism, it would be expected that depletion of cognitive resources would have little effect on facilitated attention. Using cognitive load manipulations appears to be a critical next step in elucidating the mechanisms underlying attentional bias towards threat.
Future Directions
How do the biases relate to other aspects of anxious responding?
There is a surprising lack of research investigating how the different components of attentional bias correlate with other aspects of anxious responding. The research is limited to demonstrating correlations between emotional Stroop biases and self-reported distress during actual or imagined stressors (MacLeod & Hagan, 1992; Nay, Thorpe, Roberson-Nay, Hecker, & Sigmon, 2004; van den Hout, Tenney, Huygens, Merckelbach, & Kindt, 1995). It remains unclear whether facilitated attention, difficulty in disengagement, and attentional avoidance similarly predict self-reported distress. It is similarly unclear how the specific components of attentional biases predict avoidance, escape, success during exposure, etc. It is difficulty to determine how these aspects of attentional biases relate to a larger understanding of anxiety. Moreover, it is difficult to determine which cognitive/affective mechanisms underlie anxiety without knowing how the mechanisms of attentional bias predict aspects of anxious responding. Future research linking the specific aspects of attentional biases with aspects of anxious responding will be critical in elucidating the mechanisms of attentional bias as well as in understanding the processes underlying anxiety.
How do attention retraining methodologies affect the mechanisms of attentional biases?
Numerous studies are beginning to investigate whether training attentional biases affects anxiety. Studies have shown that training attention to be biased towards threat increases anxiety (MacLeod et al., 2002), and that training attention away from threat can actually reduce symptoms of social phobia (Amir et al., 2008; 2009) and GAD (Schmidt et al., 2009; Hazen et al., 2009). Although these emerging data are exciting, the mechanisms by which the training protocols work are not entirely clear. For example, protocols that train attention away from threat ostensibly are increasing attentional avoidance, which from this review would actually be contraindicated. However, an alternative explanation is that training attentional avoidance is necessarily also training increased ability to disengage from threat. As such, these attention training treatment protocols may work through bolstering the ability to disengage. Consistent with this explanation, the only attention training study that has investigated which components of attention bias change after the attention training (Amir, Beard, Taylor, et al., 2009) found decreases in difficulty in disengagement from pre-post attention training. Further, the current research on attention training has not examined how the training affects any of the candidate underlying mechanisms. For example, does attention training away from threat increase attentional control? It is clear that future studies of attention training need to assess how the training affects each component and underlying mechanism of attentional bias. Research in this area will have a large impact on understanding the mechanisms of attentional bias, the mechanisms of treatment success during attention retraining, as well as the overall mechanisms of pathological anxiety.
Concurrent assessment of each of the three domains involved in attentional biases
As noted above, conceptualizing attentional biases as a dynamic system in which these levels are presumed to interact will necessitate research concurrently measuring and/or manipulating aspects of each of the three levels. This will likely entail a stage of processing and mediating mechanism manipulation (e.g., stimulus duration, cognitive load, state anxiety, threat intensity) with concurrent measurement of the components of attentional bias. Translational research, in which cognitive, affective, and neuroscience each inform one another is likely to be of great help. Future research along these lines will help further elucidate the relations between these domains.
Conclusions
The present paper conceptualized attentional biases in terms of three complimentary aspects: the attentional components, mediating mechanisms, and stages of information processing. Research has identified elements within each domain and suggested specific interrelations between the domains. Though future research is still needed to clarify inconsistencies and ambiguities, there has been clear progress in identifying the mechanisms of attentional biases. Future research in this area will hopefully further elucidate the specific processes underlying attentional bias and clarify how this research informs a larger understanding of the processes of anxiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algom D, Chajut E, Lev S. A rational look at the emotional Stroop paradigm: a generic slowdown, not a Stroop effect. Journal of Experimental Psychology: General. 2004;133:323–338. doi: 10.1037/0096-3445.133.3.323. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M. Attention training in individuals with generalized social phobia: a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117:860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Elias J, Klumpp H, Przeworski A. Attentional bias to threat in social phobia: Facilitated processing of threat or difficulty disengaging attention from threat? Behaviour Research and Therapy. 2003;41:1325–1335. doi: 10.1016/s0005-7967(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Amir N, Najmi S, Morrison AS. Attenuation of attention bias in obsessive-compulsive disorder. Behaviour Research and Therapy. doi: 10.1016/j.brat.2008.10.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK. Affective influences on the attentional dynamics supporting awareness. Journal of Experimental Psychology: General. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia: Bleuler's ‘fragmented phrene’ as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg, van IJzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders: the nature and treatment of anxiety and panic. Guilford Press; New York: 2002. [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130:553–573. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Clark DA. An information processing model of anxiety: Automatic and strategic processes. Behaviour Research and Therapy. 1997;35:49–58. doi: 10.1016/s0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: General emotionality or disorder specificity? Journal of Anxiety Disorders. 2001;15:147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Gibb BE, McGeary JE, Miller IW. Serotonin transporter genetic variation and biased attention for emotional word stimuli among psychiatric inpatients. Journal of Abnormal Psychology. 2007;116:208–212. doi: 10.1037/0021-843X.116.1.208. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar N, White J. Selective processing of negative information: effects of clinical anxiety, concurrent depression, and awareness. Journal of Abnormal Psychology. 1995;104:532–536. doi: 10.1037//0021-843x.104.3.532. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: manipulation of stimulus duration. Cognition & Emotion. 1998;12:737–753. [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. The British Journal of Clinical Psychology. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing threatening information in posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Hickling EJ. Automatic and strategic processing of threat stimuli: a comparison between PTSD, panic disorder, and non-anxiety controls. Cognitive Therapy and Research. 2002;26:97–115. [Google Scholar]
- Byrne A, Eysenck MW. Trait anxiety, anxious mood, and threat detection. Cognition and Emotion. 1995;9:549–562. [Google Scholar]
- Calvo MG, Avero P. Time course of attentional bias to emotional scenes in anxiety: gaze direction and duration. Cognition and Emotion. 2005;19:433–451. doi: 10.1080/02699930441000157. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Reinke KS. Masked fearful faces modulate the orienting of covert spatial attention. Emotion. 2008;8:522–529. doi: 10.1037/a0012653. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47:1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neuroscience and Biobehavioral Reviews. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: a critical review. Cognitive Therapy and Research. 2009;33:221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Olatunji BO. Components of attentional biases in contamination fear: evidence for difficulty in disengagement. Behaviour Research and Therapy. doi: 10.1016/j.brat.2009.09.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Taghavi R, Neshat-Doost H, Moradi A, Canterbury R, Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. Journal of Clinical Child and Adolescent Psychology. 2003;32:10–21. doi: 10.1207/S15374424JCCP3201_02. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development and Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Annals of the New York Academy of Sciences. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning JP, Hajack G. See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46:28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Ricon T, Bar-Haim Y. Plasticity in attention: implications for stress response in children. Behaviour Research and Therapy. 2008;46:450–461. doi: 10.1016/j.brat.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Evans J. St. B. T. Dual-processing accounts of reasoning, judgment, and social cognition. Annual Review of Psychology. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Feldner MT, Zvolensky MJ, Eifert GH, Spira AP. Emotional avoidance: an experimental test of individual differences and response suppression using biological challenge. Behaviour Research and Therapy. 2003;41:403–411. doi: 10.1016/s0005-7967(02)00020-7. [DOI] [PubMed] [Google Scholar]
- Foa EG, Ilai D, McCarthy PR, Shoyer BS, Murdock T. Information processing in obsessive-compulsive disorder. Cognitive Therapy and Research. 1993;17:173–189. [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Attentional bias for threat: evidence for delayed disengagement from emotional faces. Cognition and Emotion. 2002;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Hollon SD. What can specificity designs say about causality in psychopathology research? Psychological Bulletin. 1991;110:129–136. doi: 10.1037/0033-2909.110.1.129. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and maintenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115:760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Foa EB, Amir N. Attentional biases for facial expressions in social phobia: the face-in-the-crowd paradigm. Cognition and Emotion. 1999;13:305–318. [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;386-398 doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Handbook of Emotion Regulation. Guilford Press; New York, NY: 2007. [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: timing is everything. Current Directions in Psychological Science. 2001;10:214–219. [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Review of General Psychology. 1998a;2:271–299. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998b;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana b. S., Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Bryant RA, Rapee RM. Preconscious processing of threat in posttraumatic stress disorder. Cognitive Therapy and Research. 1996;20:613–623. [Google Scholar]
- Hazen RA, Vasey MW, Schmidt NB. Attentional retraining: a randomized clinical trial for pathological worry. Journal of Psychiatric Research. doi: 10.1016/j.jpsychires.2008.07.004. in press. [DOI] [PubMed] [Google Scholar]
- Horenstein M, Segui J. Chronometrics of attentional processes in anxiety disorders. Psychopathology. 1997;30:25–35. doi: 10.1159/000285025. [DOI] [PubMed] [Google Scholar]
- Johnson DR. Goal-directed attentional deployment to emotional faces and individual differences in emotion regulation. Journal of Research in Personality. 2009;43:8–13. [Google Scholar]
- Juth P, Lundqvist D, Karlsson A, Öhman A. Looking for foes and friends: perceptual and emotional factors when finding a face in the crowd. Emotion. 2005;5:379–395. doi: 10.1037/1528-3542.5.4.379. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kindt M, Bierman D, Brosschot JF. Stroop versus Stroop: comparison of a card format and a single-trial format of the standard color-word Stroop task and emotional Stroop task. Personality and Individual Differences. 1996;21:653–661. [Google Scholar]
- Kindt M, Van Den Hout M. Selective attention and anxiety: a perspective on developmental issues and the causal status. Journal of Psychopathology and Behavioral Assessment. 2001;23:193–202. [Google Scholar]
- Koole SL. The psychology of emotion regulation: an integrative review. Cognition and Emotion. 2009;23:4–41. [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, De Houwer J. Does imminent threat capture and hold attention? Emotion. 2004b;4:312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, De Houwer J. Signals for threat modulate attentional capture and holding: fear-conditioning and extinction during the exogenous cueing task. Cognition and Emotion. 2005;19:771–780. [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, De Houwer J. Attention to threat in anxiety-prone individuals: mechanisms underlying attentional bias. Cognitive Therapy and Research. 2006b;30:635–643. [Google Scholar]
- Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behaviour Research and Therapy. 2004a;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Vershuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour Research and Therapy. 2006a;44:1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Verschuere B, Crombez G, Van Damme S. Time-course of attention for threatening pictures in high and low trait anxiety. Behaviour Research and Therapy. 2005;43:1087–1098. doi: 10.1016/j.brat.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lavy EH, Van den Hout M, Arntz A. Attentional bias and spider phobia: conceptual and clinical issues. Behaviour Research and Therapy. 1993;31:17–24. doi: 10.1016/0005-7967(93)90038-v. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. Simon & Schuster; New York: 1996. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li S, Tan J, Qian M, Liu X. Continual training of attentional bias in social anxiety. Behaviour Research and Therapy. 2008;46:905–912. doi: 10.1016/j.brat.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Lipp OV. Human fear learning: contemporary procedures and measurement. In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and Learning: from basic processes to clinical implications. American Psychological Association; Washington, DC: 2006. pp. 37–52. [Google Scholar]
- Lipp OV, Waters AM. When danger lurks in the background: attentional capture by animal fear-relevant distracters is specific and selectively enhanced by animal fear. Emotion. 2007;7:192–200. doi: 10.1037/1528-3542.7.1.192. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW. Negative affectivity, effortful control, and attention to threat-relevant stimuli. Journal of Abnormal Child Psychology. 2009;37:387–399. doi: 10.1007/s10802-008-9284-y. [DOI] [PubMed] [Google Scholar]
- Macleod C, Campbell L, Rutherford E, Wilson E. The causal status of anxiety-linked attentional and interpretive bias. In: Yiend J, editor. Cognition, emotion, and psychopathology: Theoretical, empirical and clinical directions. Cambridge University Press; New York, NY: 2004. pp. 172–189. [Google Scholar]
- MacLeod C, Hagan R. Individual differences in the selective processing of threatening information, and emotional responses to a stressful life event. Behaviour Research and Therapy. 1992;30:151–161. doi: 10.1016/0005-7967(92)90138-7. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in the emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E. Information-processing approaches: assessing the selective functioning of attention, interpretation, and retrieval. In: Heimberg RG, Turk CL, editors. Generalized Anxiety Disorder: Advances in Research and Practice. Guilford Press; New York, NY: 2004. pp. 109–142. [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Lin H. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111:107–123. [PubMed] [Google Scholar]
- Mathews A, Mackintosh B, Fulcher EP. Cognitive biases in anxiety and attention to threat. Trends in Cognitive Sciences. 1997;1:340–345. doi: 10.1016/S1364-6613(97)01092-9. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cognition and Emotion. 2002;16:331–354. [Google Scholar]
- Mathews A, Mogg K, Kentish J, Eysenck M. Effect of psychological treatment on cognitive bias in generalized anxiety disorder. Behaviour Research and Therapy. 1995;33:293–303. doi: 10.1016/0005-7967(94)e0022-b. [DOI] [PubMed] [Google Scholar]
- Matthews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- Matthews G, Wells A. Attention, automaticity, and affective disorder. Behavior Modification. 2000;24:69–93. doi: 10.1177/0145445500241004. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Automaticity and the anxiety disorders. Behaviour Research and Therapy. 1995;33:747–754. doi: 10.1016/0005-7967(95)00015-p. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Cognitive bias in the anxiety disorders. In: Hope DA, editor. Nebraska Symposium on Motivation, 1995: perspectives on anxiety, panic, and fear. University of Nebraska Press; Lincoln, NE: 1996. pp. 211–250. [PubMed] [Google Scholar]
- McNally RJ, Kaspi SP, Bradley CR, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. Journal of Abnormal Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miltner WHR, Krieschel S, Hecht H, Trippe R, Weiss T. Eye movements and behavioral responses to threatening and nonthreatening stimuli during visual search in phobic and nonphobic subjects. Emotion. 2004;4:323–339. doi: 10.1037/1528-3542.4.4.323. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behaviour Research and Therapy. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Bono JD, Painter M. Time course of attentional bias for threat information in non-clinical anxiety. Behaviour Research and Therapy. 1997;35:297303. doi: 10.1016/s0005-7967(96)00109-x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Dixon C, Fisher HT, McWilliams A. Trait anxiety, defensiveness and selective processing of threat: an investigation using two measures of attentional bias. Personality and Individual Differences. 2000;28:1063–1077. [Google Scholar]
- Mogg K, Bradley BP, Hallowell N. Attentional bias to threat: Roles of trait anxiety, stressful events, and awareness. The Quarterly Journal of Experimental Psychology A: Human Experimental Psychology. 1994;47:841–864. doi: 10.1080/14640749408401099. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon C. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18:689–700. [Google Scholar]
- Mogg K, Bradley BP, Millar N, White J. A follow-up study of cognitive bias in generalized anxiety disorder. Behaviour Research and Therapy. 1995;33:927–935. doi: 10.1016/0005-7967(95)00031-r. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Mogg K, Holmes A, Garner M, Bradley BP. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behaviour Research and Therapy. 2008;46:656–667. doi: 10.1016/j.brat.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, Woldehawariat G, Montgomery LA, Zarahn E, McClure EB, et al. Experience-dependent plasticity for attention to threat: behavioral and neurophysiological evidence in humans. Biological Psychiatry. 2004;56:607–610. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Zoellner L, Mollenholt N. Are expressive suppression and cognitive re-appraisal associated with stress-related symptoms? Behaviour Research and Therapy. 2008;46:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors A, De Houwer J. Automaticity: a theoretical and conceptual analysis. Psychological Bulletin. 2006;132:297–326. doi: 10.1037/0033-2909.132.2.297. [DOI] [PubMed] [Google Scholar]
- Moritz S, Fischer B, Hottenrott B, Kellner M, Fricke S, Randjbar S, et al. Words may not be enough! No increased emotional Stroop effect in obsessive-compulsive disorder. Behaviour Research and Therapy. 2008;46:1101–1104. doi: 10.1016/j.brat.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Moritz S, von Muhlenen A. Investigation of an attentional bias for fear-related materials in obsessive-compulsive checkers. Depression and Anxiety. 2008;25:225–229. doi: 10.1002/da.20294. [DOI] [PubMed] [Google Scholar]
- Morris J,S, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nay WT, Thorpe GL, Roberson-Nay R, Hecker JE, Sigmon ST. Attentional bias to threat and emotional response to biological challenge. Journal of Anxiety Disorders. 2004;18:609–627. doi: 10.1016/j.janxdis.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohman A. Preferential preattentive processing of threat in anxiety: preparedness and attentional biases. In: Rapee RM, editor. Current Controversies in the Anxiety Disorders. Guilford Press; New York: 1996. [Google Scholar]
- Öhman A. Fear and anxiety: evolutionary, cognitive, and clinical perspectives. In: Lewis M, Haviland-Jones JM, editors. Handbook of emotions. 2nd edition Guilford Press; New York, NY: 2000. pp. 573–593. [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Öhman A, Wiens S. The concept of an evolved fear module and cognitive theories of anxiety. Feelings and emotions: the Amsterdam symposium. 2004:58–80. [Google Scholar]
- Osinksy R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N, et al. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- Peers PV, Lawrence AD. Attentional control of emotional distraction in rapid serial visual presentation. Emotion. 2009;9:140–145. doi: 10.1037/a0014507. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLP polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pflugshaupt T, Mosimann UP, von Wartburg R, Schmitt W, Nyffeler T, Muri RM. Hypervigilance-avoidance pattern in spider phobia. Journal of Anxiety Disorders. 2005;19:105–116. doi: 10.1016/j.janxdis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MR, Rothbart MK. Developing mechanisms of self-regulation. Development and Psychopathology. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. Methods for dealing with reaction time outliers. Psychological Bulletin. 1993;114:510–532. doi: 10.1037/0033-2909.114.3.510. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rinck M, Becker ES, Kellermann J, Roth WT. Selective attention in anxiety: distraction and enhancement in visual search. Depression and Anxiety. 2003;18:18–28. doi: 10.1002/da.10105. [DOI] [PubMed] [Google Scholar]
- Rinck M, Reinecke A, Ellwart T, Heuer K, Becker ES. Speeded detection and increased distraction in fear of spiders: Evidence from eye movements. Journal of Abnormal Psychology. 2005;114:235–248. doi: 10.1037/0021-843X.114.2.235. [DOI] [PubMed] [Google Scholar]
- Rohner J-C. The time-course of visual threat processing: high trait anxious individuals eventually avert their gaze from angry faces. Cognition and Emotion. 2002;16:837–844. [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behavioral and Cognitive Neuroscience Reviews. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Salemink E, van den Hout MA, Kindt M. Selective attention and threat: quick orienting versus slow disengagement and two versions of the dot probe task. Behaviour Research and Therapy. 2007;45:607–615. doi: 10.1016/j.brat.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19:595–605. [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Divergent cognitive costs for online forms of reappraisal and distraction. Emotion. 2008;8:870–874. doi: 10.1037/a0013711. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Siegrist M. Test-retest reliability of different versions of the Stroop test. The Journal of Psychology. 1997;131:299–306. [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biological Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. (Reprinted in 1992 in the Journal of Experimental Psychology: General, 121, 15-23. [Google Scholar]
- Tata PR, Leibowitz JA, Prunty MJ, Cameron M, et al. Attentional bias in obsessional compulsive disorder. Behaviour Research and Therapy. 1996;34:53–60. doi: 10.1016/0005-7967(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Urry HI, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme S, Crombez G, Hermans D, Koster EHW, Eccleston C. The role of extinction and reinstatement in attentional bias to threat: A conditioning approach. Behaviour Research and Therapy. 2006;44:1555–1563. doi: 10.1016/j.brat.2005.11.008. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- van den Hout M, Tenney N, Huygens K, de Jong P. Preconscious processing of bias in specific phobia. Behaviour Research and Therapy. 1997;35:29–34. doi: 10.1016/s0005-7967(96)00080-0. [DOI] [PubMed] [Google Scholar]
- van den Hout M, Tenney N, Huygens K, Merckelbach H, Kindt M. Responding to subliminal threat cues is related to trait anxiety and emotional vulnerability: a successful replication of MacLeod and Hagan (1992). Behaviour Research and Therapy. 1995;33:451–454. doi: 10.1016/0005-7967(94)00062-o. [DOI] [PubMed] [Google Scholar]
- Van Dillen LF, Koole SL. How automatic is ’automatic vigilance”? The role of working memory in attentional interference of negative information. Cognition and Emotion. in press. [Google Scholar]
- Van Dillen LF, Koole SL. Clearing the mind: a working memory model of distraction from negative mood. Emotion. 2007;7:715–723. doi: 10.1037/1528-3542.7.4.715. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, van den Hout M, Putman P, de Haan E, Stam H. Selective attention to unmasked and masked threatening words: relationships to trait anger and anxiety. Personality and Individual Differences. 2001;30:711–720. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Watts FN, McKenna FP, Sharrock R, Trezise L. Colour naming of phobia-related words. British Journal of Psychology. 1986;77:97–108. doi: 10.1111/j.2044-8295.1986.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Weierich MR, Treat TA, Hollingworth A. Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion. 2008;22:985–1018. [Google Scholar]
- Wells A, Matthews G. Attention and emotion: a clinical perspective. Lawrence Erlbaum Associates; Hillsdale, NJ: 1994. [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. Wiley; Chichester: 1988. [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A. Cognitive psychology and emotional disorders. 2nd ed. Wiley; Chistester: 1997. [Google Scholar]
- Wilson E, Macleod M. Contrasting two accounts of anxiety-linked attentional bias: selective attention to varying levels of stimulus threat intensity. Journal of Abnormal Psychology. 2003;112:212–218. doi: 10.1037/0021-843x.112.2.212. [DOI] [PubMed] [Google Scholar]
- Yiend J, Mathews A. Anxiety and attention to threatening pictures. The Quarterly Journal of Experimental Psychology. 2001;54A:665–681. doi: 10.1080/713755991. [DOI] [PubMed] [Google Scholar]