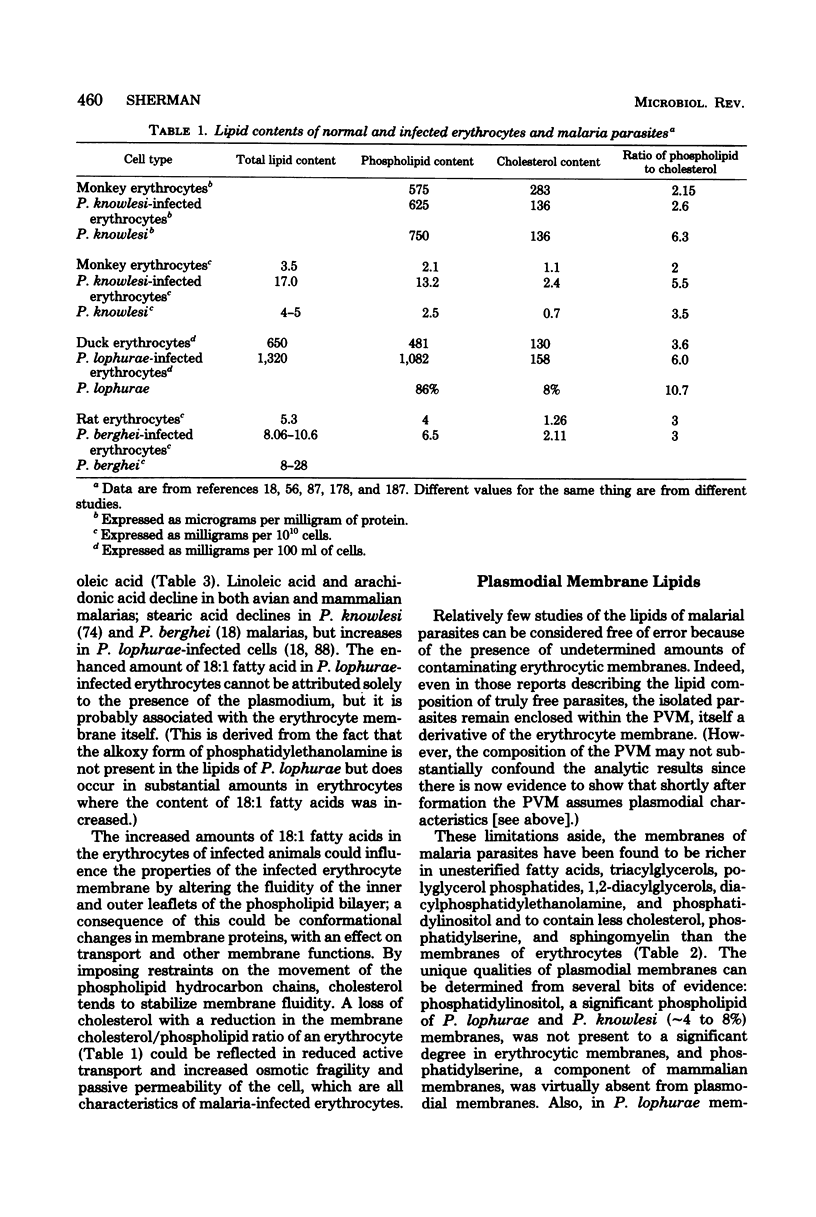
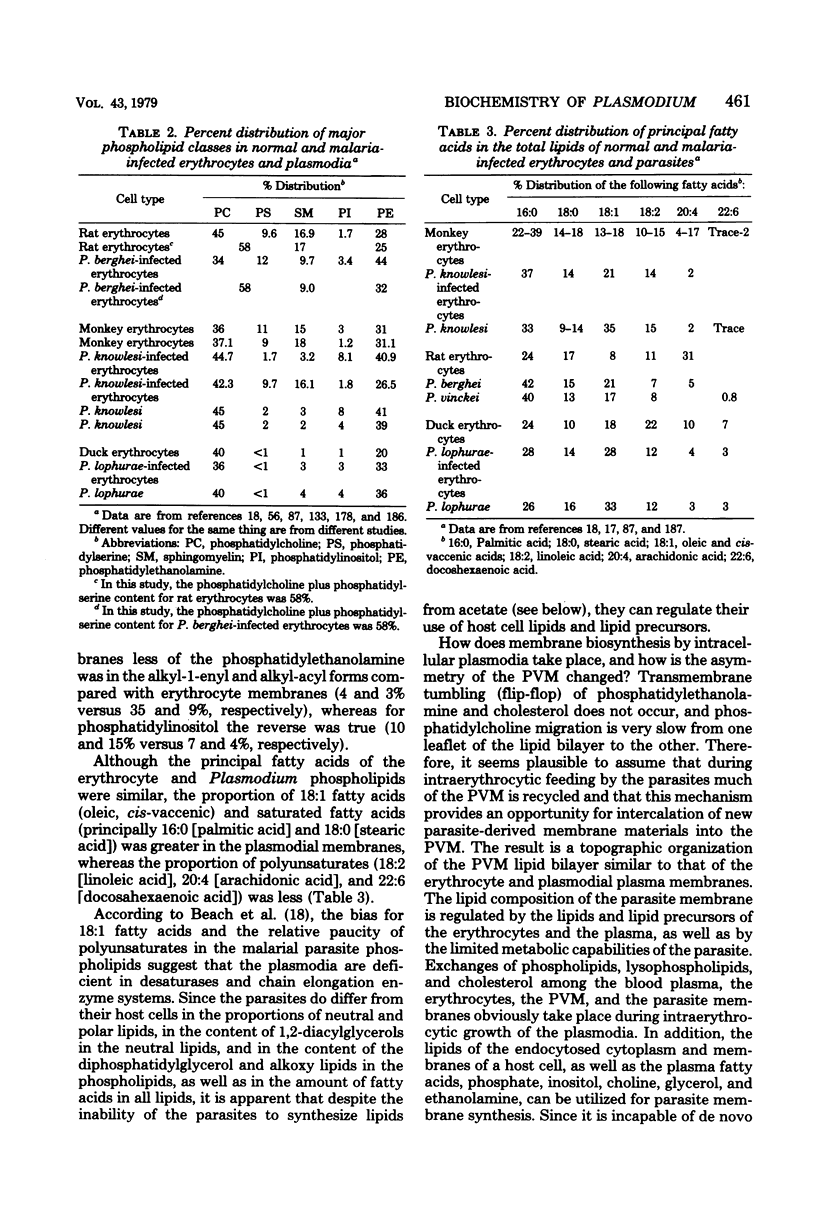
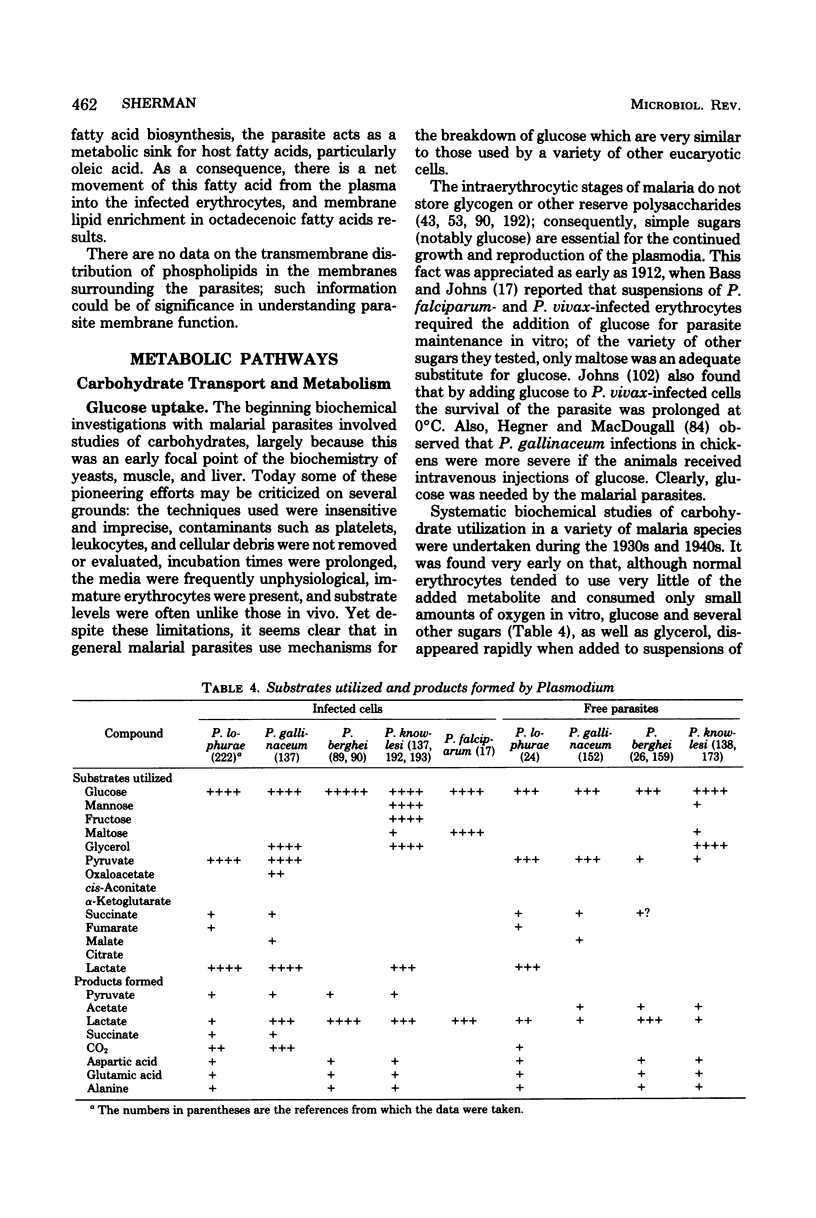
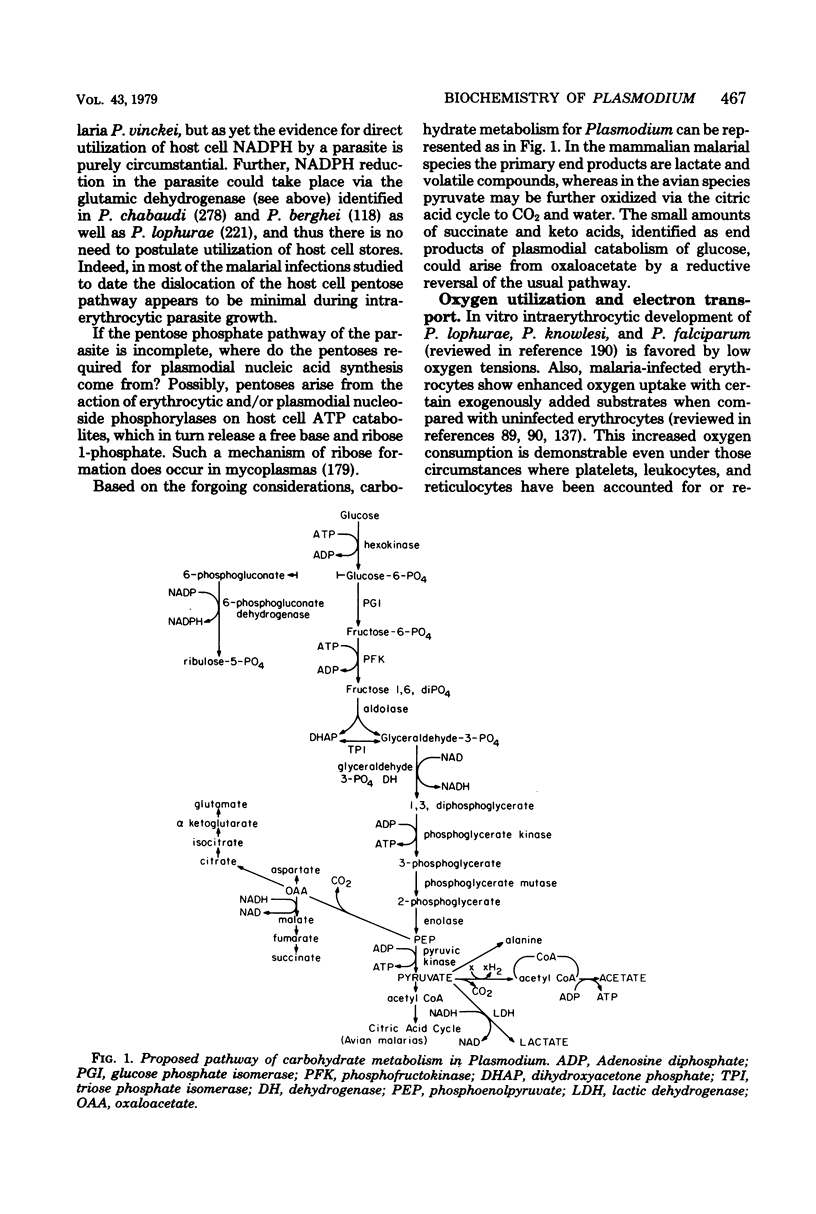
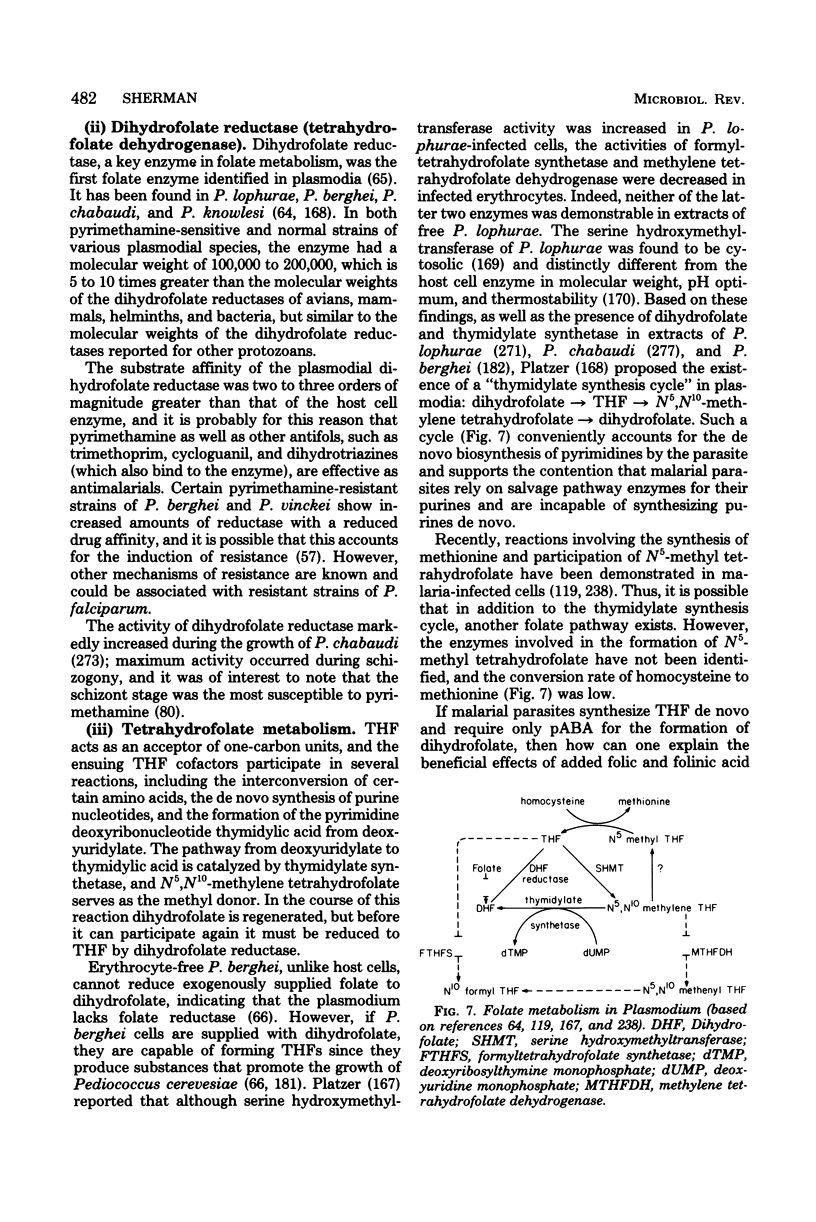
Full text
PDF

































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahr G. F. Quantitative cytochemical study of erythrocytic stages of Plasmodium lophurae Plasmodium berghei. Mil Med. 1966 Sep;131(9 Suppl):1064–1070. [PubMed] [Google Scholar]
- Aikawa M., Cook R. T. Plasmodium: electron microscopy of antigen preparations. Exp Parasitol. 1972 Feb;31(1):67–74. doi: 10.1016/0014-4894(72)90048-3. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Hsieh C. L., Miller L. H. Ultrastructural changes of the erythrocyte membrane in ovale-type malarial parasites. J Parasitol. 1977 Feb;63(1):152–154. [PubMed] [Google Scholar]
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M., Miller L. H., Rabbege J. Caveola--vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P cynomolgi. Unique structures related to Schüffner's dots. Am J Pathol. 1975 May;79(2):285–300. [PMC free article] [PubMed] [Google Scholar]
- Aikawa M. Parasitological review. Plasmodium: the fine structure of malarial parasites. Exp Parasitol. 1971 Oct;30(2):284–320. doi: 10.1016/0014-4894(71)90094-4. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Rabbege J. R., Wellde B. T. Junctional apparatus in erythrocytes infected with malarial parasites. Z Zellforsch Mikrosk Anat. 1972;124(1):72–75. [PubMed] [Google Scholar]
- Aikawa M., Thompson P. E. Localization of acid phosphatase activity in Plasmodium berghei and P. gallinaceum: an electron microscopic observation. J Parasitol. 1971 Jun;57(3):603–610. [PubMed] [Google Scholar]
- Aikawa M. Variations in structure and function during the life cycle of malarial parasites. Bull World Health Organ. 1977;55(2-3):139–156. [PMC free article] [PubMed] [Google Scholar]
- Angus M. G., Fletcher K. A., Maegraith B. G. Studies on the lipids of Plasmodium knowlesi-infected rhesus monkeys (macaca mulatta). IV. Changes in erythrocyte lipids. Ann Trop Med Parasitol. 1971 Dec;65(4):429–439. doi: 10.1080/00034983.1971.11686775. [DOI] [PubMed] [Google Scholar]
- BALL E. G., McKEE R. W. Studies on malarial parasites; chemical and metabolic changes during growth and multiplication in vivo and in vitro. J Biol Chem. 1948 Sep;175(2):547–571. [PubMed] [Google Scholar]
- BOUISSET L., RUFFIE J. Evolution du paludisme à Plasmodium berghei (Vincke et Lips) chez le rat blanc Rattus norvegicus L. var. albus carencé en vitamin A. Ann Parasitol Hum Comp. 1958 Apr-Jun;33(3):209–217. [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C. Oxidation and transamination of glutamate by typhus rickettsiae. J Biol Chem. 1950 Jun;184(2):661–676. [PubMed] [Google Scholar]
- BOWMAN I. B., GRANT P. T., KERMACK W. O. The metabolism of Plasmodium berghei, the malaria parasite of rodents. I. The preparation of the erythrocytic form of P. berghei separated from the host cell. Exp Parasitol. 1960 Apr;9:131–136. doi: 10.1016/0014-4894(60)90021-7. [DOI] [PubMed] [Google Scholar]
- BRYANT C., VOLLER A., SMITH M. J. THE INCORPORATION OF RADIOACTIVITY FROM (14C)GLUCOSE INTO THE SOLUBLE METABOLIC INTERMEDIATES OF MALARIA PARASITES. Am J Trop Med Hyg. 1964 Jul;13:515–519. doi: 10.4269/ajtmh.1964.13.515. [DOI] [PubMed] [Google Scholar]
- BUEDING E., CHARMS B. Cytochrome c, cytochrome oxidase, and succinoxidase activities of helminths. J Biol Chem. 1952 May;196(2):615–627. [PubMed] [Google Scholar]
- Bannister L. H., Butcher G. A., Mitchell G. H. Recent advances in understanding the invasion of erythrocytes by merozoites of Plasmodium knowlesi. Bull World Health Organ. 1977;55(2-3):163–169. [PMC free article] [PubMed] [Google Scholar]
- Bass C. C., Johns F. M. THE CULTIVATION OF MALARIAL PLASMODIA (PLASMODIUM VIVAX AND PLASMODIUM FALCIPARUM) IN VITRO. J Exp Med. 1912 Oct 1;16(4):567–579. doi: 10.1084/jem.16.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach D. H., Sherman I. W., Holz G. G., Jr Lipids of Plasmodium lophurae, and of erythrocytes and plasma of normal and P. lophurae-infected Pekin ducklings. J Parasitol. 1977 Feb;63(1):62–75. [PubMed] [Google Scholar]
- Becker Y. The chlamydia: molecular biology of procaryotic obligate parasites of eucaryocytes. Microbiol Rev. 1978 Jun;42(2):274–306. doi: 10.1128/mr.42.2.274-306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienzle U., Guggenmoos-Holzmann I., Luzzatto L. Malaria and erythrocyte glucose-6-phosphate dehydrogenase variants in West Africa. Am J Trop Med Hyg. 1979 Jul;28(4):619–621. [PubMed] [Google Scholar]
- Bowman I. B., Grant P. T., Kermack W. O., Ogston D. The metabolism of Plasmodium berghei, the malaria parasite of rodents. 2. An effect of mepacrine on the metabolism of glucose by the parasite separated from its host cell. Biochem J. 1961 Mar;78(3):472–478. doi: 10.1042/bj0780472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büngener W. Adenosindeaminase und Nucleosidphosphorylase bei Malariaparasiten. Z Tropenmed Parasitol. 1967 Apr;18(1):48–52. [PubMed] [Google Scholar]
- Büngener W., Nielsen G. Nukleinsäurenstoffwechsel bei experimenteller Malaria. 3. Einbau von Adenin aus dem Adeninukleotidpool der Erythrozyten in die Nukleinsäuren von Malariaparasiten (Plasmodium vinckei) in vivo. Z Tropenmed Parasitol. 1969 Mar;20(1):67–73. [PubMed] [Google Scholar]
- CIUCA M., CIPLEA A. G., BONA C., POZSGI N., ISFAN T., IUGA G. ETUDES CYTOCHIMIQUES SANGUINES DANS L'INFECTION EXP'ERIMENTALE AVEC PLASMODIUM BERGHEI DE LA SOURIS BLANCHE. I. STRUCTURE CYTOCHIMIQUE DU PARASITE, DES GLOBULES ROUGES ET OBSERVATIONS EFFECTU'EES AU MICROSCOPE 'A CONTRASTE DE PHASE. Arch Roum Pathol Exp Microbiol. 1963 Sep;23:503–514. [PubMed] [Google Scholar]
- CLARKE D. H. The use of phosphorus 32 in studies on Plasmodium gallinaceum. I. The development of a method for the quantitative determination of parasite growth and development in vitro. J Exp Med. 1952 Nov;96(5):439–450. doi: 10.1084/jem.96.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE D. H. The use of phosphorus 32 in studies on Plasmodium gallinaceum. II. Studies on conditions affecting parasite growth in intact cells and in lysates. J Exp Med. 1952 Nov;96(5):451–463. doi: 10.1084/jem.96.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK L., GRANT P. T., KERMACK W. O. Proteolytic enzymes of the erythrocytic forms of roden and simian species of malarial plasmodia. Exp Parasitol. 1961 Nov;11:372–379. doi: 10.1016/0014-4894(61)90041-8. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Walliker D. Biochemical markers for strain differentiation in malarial parasites. Bull World Health Organ. 1977;55(2-3):339–345. [PMC free article] [PubMed] [Google Scholar]
- Cenedella R. J., Jarrell J. J., Saxe L. H. Lipid synthesis in vivo from 1-14C-oleic acid and 6-3H-glucose by intraerythrocytic Plasmodium berghei. Mil Med. 1969 Sep;134(10):1045–1055. [PubMed] [Google Scholar]
- Cenedella R. J., Rosen H., Angel C. R., Saxe L. H. Free amino-acid production in vitro by Plasmodium berghei. Am J Trop Med Hyg. 1968 Nov;17(6):800–803. doi: 10.4269/ajtmh.1968.17.800. [DOI] [PubMed] [Google Scholar]
- Chan V. L., Lee P. Y. Host-cell specific proteolytic enzymes in Plasmodium berghei infected erythrocytes. Southeast Asian J Trop Med Public Health. 1974 Sep;5(3):447–449. [PubMed] [Google Scholar]
- Chance M. L., Momen H., Warhurst D. C., Peters W. The chemotherapy of rodent malaria, XXIX DNA relationships within the subgenus Plasmodium (Vinckeia). Ann Trop Med Parasitol. 1978 Feb;72(1):13–22. doi: 10.1080/00034983.1978.11719275. [DOI] [PubMed] [Google Scholar]
- Coggeshall L. T. THE SELECTIVE ACTION OF SULFANILAMIDE ON THE PARASITES OF EXPERIMENTAL MALARIA IN MONKEYS IN VIVO AND IN VITRO. J Exp Med. 1940 Jan 1;71(1):13–20. doi: 10.1084/jem.71.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin K. A., Chou S. C., Siddiqui W. A., Schnell J. V. DNA and RNA syntheses by intraerythrocytic stages of Plasmodium knowlesi. J Protozool. 1973 Nov;20(5):683–688. doi: 10.1111/j.1550-7408.1973.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cook R. T., Aikawa M., Rock R. C., Little W., Sprinz H. The isolation and fractionation of Plasmodium knowlesi. Mil Med. 1969 Sep;134(10):866–883. [PubMed] [Google Scholar]
- Cook R. T., Rock R. C., Aikawa M., Fournier M. J., Jr Ribosomes of the malarial parasite, Plasmodium knowlesi. I. Isolation, activity and sedimentation velocity. Comp Biochem Physiol B. 1971 Aug 15;39(4):897–911. doi: 10.1016/0305-0491(71)90113-1. [DOI] [PubMed] [Google Scholar]
- Cooper S. A unifying model for the G1 period in prokaryotes and eukaryotes. Nature. 1979 Jul 5;280(5717):17–19. doi: 10.1038/280017a0. [DOI] [PubMed] [Google Scholar]
- DASGUPTA B. Polysaccharides in the different stages of the life-cycles of certain sporozoa. Parasitology. 1960 Nov;50:509–514. doi: 10.1017/s0031182000025580. [DOI] [PubMed] [Google Scholar]
- Deans J. A., Dennis E. D., Cohen S. Antigenic analysis of sequential erythrocytic stages of Plasmodium knowlesi. Parasitology. 1978 Dec;77(3):333–344. doi: 10.1017/s0031182000050290. [DOI] [PubMed] [Google Scholar]
- Diggens S. M., Gutteridge W. E., Trigg P. I. Altered dihydrofolate reductase associated with a pyrimethamine-resistant Plasmodium berghei berghei produced in a single step. Nature. 1970 Nov 7;228(5271):579–580. doi: 10.1038/228579a0. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Alterations of red blood cell metabolism in simian malaria: evidence for abnormalities of nonparasitized cells. Mil Med. 1969 Sep;134(10):1100–1105. [PubMed] [Google Scholar]
- Dunn M. J. Alterations of red blood cell sodium transport during malarial infection. J Clin Invest. 1969 Apr;48(4):674–684. doi: 10.1172/JCI106025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- Eckman J. R., Eaton J. W. Dependence of plasmodial glutathione metabolism on the host cell. Nature. 1979 Apr 19;278(5706):754–756. doi: 10.1038/278754a0. [DOI] [PubMed] [Google Scholar]
- Eisen H. Purification of intracellular forms of Plasmodium chabaudi and their interactions with the erythrocyte membrane and with serum albumin. Bull World Health Organ. 1977;55(2-3):333–338. [PMC free article] [PubMed] [Google Scholar]
- FABIANI G., GRELLET P. Etude chez le rat blanc des rapports entre la carence en vitamine A et le paludisme expérimental à Plasmodium berghei. C R Seances Soc Biol Fil. 1952 Mar;146(5-6):441–444. [PubMed] [Google Scholar]
- FLETCHER K. A., MAEGRAITH B. G. Glucose-6-phosphate and 6-phosphogluconate dehydrogenase activities in erythrocytes of monkeys infected with Plasmodium knowlesi. Nature. 1962 Dec 29;196:1316–1318. doi: 10.1038/1961316b0. [DOI] [PubMed] [Google Scholar]
- FRASER D. M., KERMACK W. O. The inhibitory action of some antimalarial drugs and related compounds on the hexokinase of yeast and of Plasmodium berghei. Br J Pharmacol Chemother. 1957 Mar;12(1):16–23. doi: 10.1111/j.1476-5381.1957.tb01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON J. D., GRANT P. T. The sulphur requirements of the erythrocytic from of Plasmodium knowlesi. Biochem J. 1956 Jun;63(2):274–282. doi: 10.1042/bj0630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone R., Burchall J. J., Hitchings G. H. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969 Jan;5(1):49–59. [PubMed] [Google Scholar]
- Ferone R. Folate metabolism in malaria. Bull World Health Organ. 1977;55(2-3):291–298. [PMC free article] [PubMed] [Google Scholar]
- Ferone R., Hitchings G. H. Folate cofactor biosynthesis by Plasmodium berghei. Comparison of folate and dihydrofolate as substrates. J Protozool. 1966 Aug;13(3):504–506. doi: 10.1111/j.1550-7408.1966.tb01951.x. [DOI] [PubMed] [Google Scholar]
- Fletcher A., Maegraith B. The metabolism of the malaria parasite and its host. Adv Parasitol. 1972;10:31–48. doi: 10.1016/s0065-308x(08)60171-2. [DOI] [PubMed] [Google Scholar]
- Fletcher K. A., Canning M. V., Theakston R. D. Electrophoresis of glucose-6-phosphate and 6-phosphogluconate dehydrogenases in erythrocytes from malaria-infected animals. Ann Trop Med Parasitol. 1977 Jun;71(2):125–130. doi: 10.1080/00034983.1977.11687170. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Oxidant damage mediates variant red cell resistance to malaria. Nature. 1979 Jul 19;280(5719):245–247. doi: 10.1038/280245a0. [DOI] [PubMed] [Google Scholar]
- GODFREY D. G. Antiparasitic action of dietary cod liver oil upon Plasmodium berghei and its reversal by vitamin E. Exp Parasitol. 1957 Nov;6(6):555–565. doi: 10.1016/0014-4894(57)90038-3. [DOI] [PubMed] [Google Scholar]
- GROMAN N. B. Dynamic aspects of the nitrogen metabolism of Plasmodium gallinaceum in vivo and in vitro. J Infect Dis. 1951 Mar-Apr;88(2):126–150. doi: 10.1093/infdis/88.2.126. [DOI] [PubMed] [Google Scholar]
- Gutierrez J. Effect of the antimalarial chloroquine on the phospholipid metabolism of avian malaria and heart tissue. Am J Trop Med Hyg. 1966 Nov;15(6):818–822. doi: 10.4269/ajtmh.1966.15.818. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Dave D., Richards W. H. Conversion of dihydroorotate to orotate in parasitic protozoa. Biochim Biophys Acta. 1979 Feb 1;582(3):390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Trigg P. I. Action of pyrimethamine and related drugs against Plasmodium knowlesi in vitr. Parasitology. 1971 Jun;62(3):431–444. doi: 10.1017/s0031182000077581. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Trigg P. I. Incorporation of radioactive precursors into DNA and RNA of Plasmodium knowlesi in vitro. J Protozool. 1970 Feb;17(1):89–96. doi: 10.1111/j.1550-7408.1970.tb05163.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Trigg P. I. Periodicity of nuclear DNA synthesis in the intraerythrocytic cycle of Plasmodium knowlesi. J Protozool. 1972 May;19(2):378–381. doi: 10.1111/j.1550-7408.1972.tb03482.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Trigg P. I., Williamson D. H. Properties of DNA from some malarial parasites. Parasitology. 1971 Apr;62(2):209–219. doi: 10.1017/s0031182000071456. [DOI] [PubMed] [Google Scholar]
- Herman Y. F., Ward R. A., Herman R. H. Stimulation of the utilization of 1-14C-glucose in chicken red blood cells infected with Plasmodium gallinaceum. Am J Trop Med Hyg. 1966 May;15(3):276–280. doi: 10.4269/ajtmh.1966.15.276. [DOI] [PubMed] [Google Scholar]
- Holz G. G., Jr, Beach D. H., Sherman I. W. Octadecenoic fatty acids and their association with hemolysis in malaria. J Protozool. 1977 Nov;24(4):566–574. doi: 10.1111/j.1550-7408.1977.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Holz G. G., Jr Lipids and the malarial parasite. Bull World Health Organ. 1977;55(2-3):237–248. [PMC free article] [PubMed] [Google Scholar]
- Homewood C. A. Carbohydrate metabolism of malarial parasites. Bull World Health Organ. 1977;55(2-3):229–235. [PMC free article] [PubMed] [Google Scholar]
- Homewood C. A., Jewsbury J. M., Chance M. L. The pigment formed during haemoglobin digestion by malarial and schistosomal parasites. Comp Biochem Physiol B. 1972 Nov 15;43(3):517–523. doi: 10.1016/0305-0491(72)90135-6. [DOI] [PubMed] [Google Scholar]
- Homewood C. A., Moore G. A., Wwarhurst D. C., Atkinson E. M. Purification and some properties of malarial pigment. Ann Trop Med Parasitol. 1975 Sep;69(3):283–287. doi: 10.1080/00034983.1975.11687012. [DOI] [PubMed] [Google Scholar]
- Homewood C. A., Neame K. D. Malaria and the permeability of the host erythrocyte. Nature. 1974 Dec 20;252(5485):718–719. doi: 10.1038/252718a0. [DOI] [PubMed] [Google Scholar]
- Howells R. E., Maxwell L. Citric acid cycle activity and chloroquine resistance in rodent malaria parasites: the role of the reticulocyte. Ann Trop Med Parasitol. 1973 Sep;67(3):285–300. doi: 10.1080/00034983.1973.11686889. [DOI] [PubMed] [Google Scholar]
- Howells R. E. Mitochondrial changes during the life cycle of Plasmodium berghei. Ann Trop Med Parasitol. 1970 Jun;64(2):181–187. doi: 10.1080/00034983.1970.11686680. [DOI] [PubMed] [Google Scholar]
- Howells R. E., Peters W., Fullard J. Cytochrome oxidase activity in a normal and some drug-resistant strains of Plasmodium berghei--a cytochemical study. I. Asexual erythrocytic stages. Mil Med. 1969 Sep;134(10):893–915. [PubMed] [Google Scholar]
- Ilan J., Pierce D. R., Miller F. W. Influence of 9-beta-D-arabinofuranosyladenine on total protein synthesis and on differential gene expression of unique proteins in the rodent malarial parasite Plasmodium berghei. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3386–3390. doi: 10.1073/pnas.74.8.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan J., Tokuyasu K. Amino acid activation for protein synthesis in Plasmodium berghei. Mil Med. 1969 Sep;134(10):1026–1031. [PubMed] [Google Scholar]
- Jung A., Jackisch R., Picard-Maureau A., Heischkeil R. DNA-, RNA- und Lipidsynthese sowie die spezifische Aktivität von Glucose-6-Phosphatdehydrogenase und Glucose-6-Phosphatase in den verschiedenen morphologischen Stadien von Plasmodium vinckei. Tropenmed Parasitol. 1975 Mar;26(1):27–34. [PubMed] [Google Scholar]
- Kilejian A. A unique histidine-rich polypeptide from the malaria parasite, Plasmodium lophurae. J Biol Chem. 1974 Jul 25;249(14):4650–4655. [PubMed] [Google Scholar]
- Kilejian A., Abati A., Trager W. Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of "knob-like protrusions" on infected erythrocyte membranes. Exp Parasitol. 1977 Jun;42(1):157–164. doi: 10.1016/0014-4894(77)90073-x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochim Biophys Acta. 1975 May 16;390(3):276–284. doi: 10.1016/0005-2787(75)90348-2. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Does a histidine-rich protein from Plasmodium lophurae have a function in merozoite penetration? J Protozool. 1976 May;23(2):272–277. doi: 10.1111/j.1550-7408.1976.tb03768.x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Histidine-rich protein as a model malaria vaccine. Science. 1978 Sep 8;201(4359):922–924. doi: 10.1126/science.567375. [DOI] [PubMed] [Google Scholar]
- Kilejian A., Jensen J. B. A histidine-rich protein from Plasmodium falciparum and its interaction with membranes. Bull World Health Organ. 1977;55(2-3):191–197. [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Liao T. H., Trager W. On primary structure and biosynthesis of histidine-rich polypeptide from malarial parasite Plasmodium lophurae. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3057–3059. doi: 10.1073/pnas.72.8.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killby V. A., Silverman P. H. Isolated erythrocytic forms of Plasmodium berghei. An electron-microscopical study. Am J Trop Med Hyg. 1969 Nov;18(6):836–859. doi: 10.4269/ajtmh.1969.18.836. [DOI] [PubMed] [Google Scholar]
- Killick-Kendrick R. Parasitic protozoa of the blood of rodents: a revision of Plasmodium berghei. Parasitology. 1974 Oct;69(2):225–237. doi: 10.1017/s0031182000048071. [DOI] [PubMed] [Google Scholar]
- Krooth R. S., Wuu K. D., Ma R. Dihydroorotic acid dehydrogenase: introduction into erythrocyte by the malaria parasite. Science. 1969 May 30;164(3883):1073–1075. doi: 10.1126/science.164.3883.1073. [DOI] [PubMed] [Google Scholar]
- Königk E., Mirtsch S. Plasmodium chabaudi-infection of mice: specific activities of erythrocyte membrane-associated enzymes and patterns of proteins and glycoproteins of erythrocyte membrane preparations. Tropenmed Parasitol. 1977 Mar;28(1):17–22. [PubMed] [Google Scholar]
- Königk E. Salvage syntheses and their relationship to nucleic acid metabolism. Bull World Health Organ. 1977;55(2-3):249–252. [PMC free article] [PubMed] [Google Scholar]
- LEWERT R. M. Changes in nucleic acids and protein in nucleated erythrocytes infected with Plasmodium gallinaceum as shown by ultraviolet absorption measurements. J Infect Dis. 1952 Sep-Oct;91(2):180–183. doi: 10.1093/infdis/91.2.180. [DOI] [PubMed] [Google Scholar]
- Langer B. W., Jr, Phisphumvidhi P., Friedlander Y. Malarial parasite metabolism: the pentose cycle in Plasmodium berghei. Exp Parasitol. 1967 Feb;20(1):68–76. doi: 10.1016/0014-4894(67)90023-9. [DOI] [PubMed] [Google Scholar]
- Langer B. W., Jr, Phisphumvidhi P., Jiampermpoon D. Malarial parasite metabolism: the glutamic acid dehydrogenase of Plasmodium berghei. Exp Parasitol. 1970 Oct;28(2):298–303. doi: 10.1016/0014-4894(70)90100-1. [DOI] [PubMed] [Google Scholar]
- Langer B. W., Jr, Phisphumvidhi P., Jiampermpoon D., Weidhorn R. P. Malarial parasite metabolism: the metabolism of methionine by Plasmodium berghei. Mil Med. 1969 Sep;134(10):1039–1044. [PubMed] [Google Scholar]
- Langreth S. G. Electron microscope cytochemistry of host-parasite membrane interactions in malaria. Bull World Health Organ. 1977;55(2-3):171–178. [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Jensen J. B., Reese R. T., Trager W. Fine structure of human malaria in vitro. J Protozool. 1978 Nov;25(4):443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Nguyen-Dinh P., Trager W. Plasmodium falciparum: merozoite invasion in vitro in the presence of chloroquine. Exp Parasitol. 1978 Dec;46(2):235–238. doi: 10.1016/0014-4894(78)90136-4. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Trager W. Fine structure of the malaria parasite Plasmodium lophurae developing extracellularly in vitro. J Protozool. 1973 Nov;20(5):606–613. doi: 10.1111/j.1550-7408.1973.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Cenedella R. J. Lipid content of Plasmodium berghei-infected rat red blood cells. Exp Parasitol. 1969 Oct;26(2):181–186. doi: 10.1016/0014-4894(69)90110-6. [DOI] [PubMed] [Google Scholar]
- Levy M. R., Chou S. C. Activity and some properties of an acid proteinase from normal and Plasmodium berghei-infected red cells. J Parasitol. 1973 Dec;59(6):1064–1070. [PubMed] [Google Scholar]
- Levy M. R., Chou S. C. Inhibition of macromolecular synthesis in the malarial parasites by inhibitors of proteolytic enzymes. Experientia. 1975 Jan 15;31(1):52–54. doi: 10.1007/BF01924675. [DOI] [PubMed] [Google Scholar]
- Levy M. R., Siddiqui W. A., Chou S. C. Acid protease activity in Plasmodium falciparum and P. knowlesi and ghosts of their respective host red cells. Nature. 1974 Feb 22;247(5442):546–549. doi: 10.1038/247546a0. [DOI] [PubMed] [Google Scholar]
- Lukow I., Schmidt G., Walter R. D., Königk E. Adenosinmonophosphat-Salvage-Synthese bei Plasmodium chabaudi. Z Tropenmed Parasitol. 1973 Dec;24(4):500–504. [PubMed] [Google Scholar]
- MCGHEE R. B. The infection by Plasmodium lophurae of duck erythrocytes in the chicken embryo. J Exp Med. 1953 Jun;97(6):773–782. doi: 10.1084/jem.97.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar M. S., Van Dyke K. Detailed purine salvage metabolism in and outside the free malarial parasite. Exp Parasitol. 1975 Apr;37(2):138–146. doi: 10.1016/0014-4894(75)90064-8. [DOI] [PubMed] [Google Scholar]
- Mason S. J., Miller L. H., Shiroishi T., Dvorak J. A., McGinniss M. H. The Duffy blood group determinants: their role in the susceptibility of human and animal erythrocytes to Plasmodium knowlesi malaria. Br J Haematol. 1977 Jul;36(3):327–335. doi: 10.1111/j.1365-2141.1977.tb00656.x. [DOI] [PubMed] [Google Scholar]
- McClean S., Purdy W. C., Kabat A., Sampugna J., DeZeeuw R. Analysis of the phospholipid composition of Plasmodium knowlesi and rhesus erythrocyte membranes. Anal Chim Acta. 1976 Mar;82(1):175–185. doi: 10.1016/s0003-2670(01)82215-7. [DOI] [PubMed] [Google Scholar]
- McDaniel H. G., Siu P. M. Purification and characterization of phosphoenolpyruvate carboxylase from Plasmodium berghei. J Bacteriol. 1972 Jan;109(1):385–390. doi: 10.1128/jb.109.1.385-390.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGHEE R. B. Factors affecting the susceptibility of erythrocytes to an intracellular parasite. Ann N Y Acad Sci. 1953 Oct 14;56(5):1070–1073. doi: 10.1111/j.1749-6632.1953.tb30289.x. [DOI] [PubMed] [Google Scholar]
- McKee R. W., Ormsbee R. A., Anfinsen C. B., Geiman Q. M., Ball E. G. STUDIES ON MALARIAL PARASITES : VI. THE CHEMISTRY AND METABOLISM OF NORMAL AND PARASITIZED (P. KNOWLESI) MONKEY BLOOD. J Exp Med. 1946 Nov 30;84(6):569–582. doi: 10.1084/jem.84.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D. J., Bannister L. H., Trigg P. I., Butcher G. A. A freeze-fracture study on the parasite-erythrocyte interrelationship in Plasmodium knowlesi infections. Bull World Health Organ. 1977;55(2-3):199–203. [PMC free article] [PubMed] [Google Scholar]
- McLaren D. J., Bannister L. H., Trigg P. I., Butcher G. A. Freeze fracture studies on the interaction between the malaria parasite and the host erythrocyte in Plasmodium knowlesi infections. Parasitology. 1979 Aug;79(1):125–139. doi: 10.1017/s0031182000052021. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Ilan J. The ribosomes of Plasmodium berghei: isolation and ribosomal ribonucleic acid analysis. Parasitology. 1978 Dec;77(3):345–365. doi: 10.1017/s0031182000050307. [DOI] [PubMed] [Google Scholar]
- Miller L. H., Aikawa M., Dvorak J. A. Malaria (Plasmodium knowlesi) merozoites: immunity and the surface coat. J Immunol. 1975 Apr;114(4):1237–1242. [PubMed] [Google Scholar]
- Miller L. H., Dvorak J. A., Shiroishi T., Durocher J. R. Influence of erythrocyte membrane components on malaria merozoite invasion. J Exp Med. 1973 Dec 1;138(6):1597–1601. doi: 10.1084/jem.138.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Haynes J. D., McAuliffe F. M., Shiroishi T., Durocher J. R., McGinniss M. H. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J Exp Med. 1977 Jul 1;146(1):277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H. Hypothesis on the mechanism of erythrocyte invasion by malaria merozoites. Bull World Health Organ. 1977;55(2-3):157–162. [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., McGinniss M. H., Holland P. V., Sigmon P. The Duffy blood group phenotype in American blacks infected with Plasmodium vivax in Vietnam. Am J Trop Med Hyg. 1978 Nov;27(6):1069–1072. doi: 10.4269/ajtmh.1978.27.1069. [DOI] [PubMed] [Google Scholar]
- Momen H. Biochemistry of intraerythrocytic parasites. I. Identification of enzymes of parasite origin by starch-gel electrophoresis. Ann Trop Med Parasitol. 1979 Apr;73(2):109–115. [PubMed] [Google Scholar]
- Momen H. Biochemistry of intraerythrocytic parasites. II. Comparative studies in carbohydrate metabolism. Ann Trop Med Parasitol. 1979 Apr;73(2):117–121. doi: 10.1080/00034983.1979.11687238. [DOI] [PubMed] [Google Scholar]
- Müller M. Biochemistry of protozoan microbodies: peroxisomes, alpha-glycerophosphate oxidase bodies, hydrogenosomes. Annu Rev Microbiol. 1975;29:467–483. doi: 10.1146/annurev.mi.29.100175.002343. [DOI] [PubMed] [Google Scholar]
- NAGARAJAN K. PYRUVATE AND LACTATE LEVELS IN RELATIONSHIP TO THE NICOTINAMIDE--ADENINE DINUCLEOTIDE LEVELS IN MALARIAL PARASITES (PLASMODIUM BERGHEI). Biochim Biophys Acta. 1964 Oct 9;93:176–179. doi: 10.1016/0304-4165(64)90275-2. [DOI] [PubMed] [Google Scholar]
- Nagarajan K. Metabolism of Plasmodium berghei. 3. Carbon dioxide fixation and role of pyruvate and dicarboxylic acids. Exp Parasitol. 1968 Feb;22(1):33–42. doi: 10.1016/0014-4894(68)90076-3. [DOI] [PubMed] [Google Scholar]
- Nagarajan K. Metabolism of Plasmodium berghei. I. Krebs cycle. Exp Parasitol. 1968 Feb;22(1):19–26. doi: 10.1016/0014-4894(68)90074-x. [DOI] [PubMed] [Google Scholar]
- Nagarajan K. Metabolism of Plasmodium berghei. II. 32P incorporation into high-energy phosphates. Exp Parasitol. 1968 Feb;22(1):27–32. doi: 10.1016/0014-4894(68)90075-1. [DOI] [PubMed] [Google Scholar]
- Neame K. D., Homewood C. A. Alterations in the permeability of mouse erythrocytes infected with the malaria parasite, Plasmodium berghei. Int J Parasitol. 1975 Oct;5(5):537–540. doi: 10.1016/0020-7519(75)90046-6. [DOI] [PubMed] [Google Scholar]
- Nowell F. The effect of a milk diet upon Plasmodium berghei, Nuttallia (=Babesia) rodhaini and Trypanosoma brucei infections in mice. Parasitology. 1970 Dec;61(3):425–433. doi: 10.1017/s0031182000041275. [DOI] [PubMed] [Google Scholar]
- OVERMAN R. R., HILL T. S., WONG Y. T. Physiological studies in the human malarial host; blood, plasma, extracellular fluid volumes and ionic balance in therapeutic P. vivax and P. falciparum infections. J Natl Malar Soc. 1949 Mar;8(1):14–31. [PubMed] [Google Scholar]
- Oelshlegel F. J., Jr, Sander B. J., Brewer G. J. Pyruvate kinase in malaria host-parasite interaction. Nature. 1975 May 22;255(5506):345–347. doi: 10.1038/255345a0. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Phisphumvidhi P., Langer B. W., Jr Malarial parasite metabolism: the lactic acid dehydrogenase of Plasmodium berghei. Exp Parasitol. 1969 Feb;24(1):37–41. doi: 10.1016/0014-4894(69)90218-5. [DOI] [PubMed] [Google Scholar]
- Platzer E. G., Campuzano H. C. The serine hydroxymethyltransferase of Plasmodium lophurae. J Protozool. 1976 May;23(2):282–286. doi: 10.1111/j.1550-7408.1976.tb03770.x. [DOI] [PubMed] [Google Scholar]
- Platzer E. G. Dihydrogolate reductase in Plasmodium lophurae and duckling erythrocytes. J Protozool. 1974 May;21(2):400–405. doi: 10.1111/j.1550-7408.1974.tb03678.x. [DOI] [PubMed] [Google Scholar]
- Platzer E. G., Kassis J. A. Pyridoxine kinase in Plasmodium lophurae and duckling erythrocytes. J Protozool. 1978 Nov;25(4):556–559. doi: 10.1111/j.1550-7408.1978.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Platzer E. G. Metabolism of tetrahydrofolate in Plasmodium lophurae and duckling erythrocytes. Trans N Y Acad Sci. 1972 Mar;34(3):200–207. doi: 10.1111/j.2164-0947.1972.tb02675.x. [DOI] [PubMed] [Google Scholar]
- Platzer E. G. Subcellular distribution of serine hydroxymethyltransferase in Plasmodium lophurae. Life Sci. 1977 Apr 15;20(8):1417–1424. doi: 10.1016/0024-3205(77)90370-8. [DOI] [PubMed] [Google Scholar]
- Polet H., Barr C. F. DNA, RNA , and protein synthesis in erythrocytic forms of Plasmodium knowlesi. Am J Trop Med Hyg. 1968 Sep;17(5):672–679. doi: 10.4269/ajtmh.1968.17.672. [DOI] [PubMed] [Google Scholar]
- Polet H., Brown N. D., Angel C. R. Biosynthesis of amino acids from 14C-U glucose, pyruvate, and acetate by erythrocytic forms of P. knowlesi, in vitro. Proc Soc Exp Biol Med. 1969 Sep;131(4):1215–1218. doi: 10.3181/00379727-131-34073. [DOI] [PubMed] [Google Scholar]
- Polet H., Conrad M. E. Malaria: extracellular amino acid requirements for in vitro growth of erythrocytic forms of Plasmodium knowlesi. Proc Soc Exp Biol Med. 1968 Jan;127(1):251–253. doi: 10.3181/00379727-127-32666. [DOI] [PubMed] [Google Scholar]
- Rao K. N., Subrahmanyam D., Prakash S. Plasmodium berghei: lipids of rat red blood cells. Exp Parasitol. 1970 Feb;27(1):22–27. doi: 10.1016/s0014-4894(70)80005-4. [DOI] [PubMed] [Google Scholar]
- Razin S. The mycoplasmas. Microbiol Rev. 1978 Jun;42(2):414–470. doi: 10.1128/mr.42.2.414-470.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. E., Warren L. G., Susskind B., Lo H. S. An energy-conserving pyruvate-to-acetate pathway in Entamoeba histolytica. Pyruvate synthase and a new acetate thiokinase. J Biol Chem. 1977 Jan 25;252(2):726–731. [PubMed] [Google Scholar]
- Reid V. E., Friedkin M. Plasmodium berghei: folic acid levels in mouse erythrocytes. Exp Parasitol. 1973 Jun;33(3):424–428. doi: 10.1016/0014-4894(73)90108-2. [DOI] [PubMed] [Google Scholar]
- Reid V. E., Friedkin M. Thymidylate synthetase in mouse erythrocytes infected with Plasmodium berghei. Mol Pharmacol. 1973 Jan;9(1):74–80. [PubMed] [Google Scholar]
- Rich P. R., Moore A. L. The involvement of the protonmotive ubiquinone cycle in the respiratory chain of higher plants and its relation to the branchpoint of the alternate pathway. FEBS Lett. 1976 Jun 15;65(3):339–344. doi: 10.1016/0014-5793(76)80142-1. [DOI] [PubMed] [Google Scholar]
- Rock R. C. Incorporation of 14 C-labelled fatty acids into lipids of rhesus erythrocytes and Plasmodium knowlesi in vitro. Comp Biochem Physiol B. 1971 Dec 15;40(4):893–906. doi: 10.1016/0305-0491(71)90035-6. [DOI] [PubMed] [Google Scholar]
- Rock R. C. Incorporation of 14 C-labelled non-lipid precursors into lipid of Plasmodium knowlesi in vitro. Comp Biochem Physiol B. 1971 Nov 15;40(3):657–669. doi: 10.1016/0305-0491(71)90141-6. [DOI] [PubMed] [Google Scholar]
- Rock R. C., Standefer J., Little W. Incorporation of 33p-orthophosphate into membrane phospholipids of Plasmodium knowlesi and host erythrocytes of Macaca mullatta. Comp Biochem Physiol B. 1971 Oct;40(2):543–561. doi: 10.1016/0305-0491(71)90239-2. [DOI] [PubMed] [Google Scholar]
- SCHELLENBERG K. A., COATNEY G. R. The influence of antimalarial drugs on nucleic acid synthesis in Plasmodium gallinaceum and Plasmodium berghei. Biochem Pharmacol. 1961 May;6:143–152. doi: 10.1016/0006-2952(61)90158-7. [DOI] [PubMed] [Google Scholar]
- SEAMAN G. R. Inhibition of the succinic dehydrogenase of parasitic protozoans by an arsono and a phosphono analog of succinic acid. Exp Parasitol. 1953 Oct;2(4):366–373. doi: 10.1016/0014-4894(53)90022-8. [DOI] [PubMed] [Google Scholar]
- SHERMAN I. W. Heterogeneity of lactic dehydrogenase in intraerythrocytic parasites. Trans N Y Acad Sci. 1962 Jun;24:944–953. doi: 10.1111/j.2164-0947.1962.tb01454.x. [DOI] [PubMed] [Google Scholar]
- SHERMAN I. W. Molecular heterogeneity of lactic dehydrogenase in avian malaria (Plasmodium lophurae). J Exp Med. 1961 Dec 1;114:1049–1062. doi: 10.1084/jem.114.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGER I., TRAGER W. Coenzyme A changes in liver, spleen and kidney of rats with infections of Plasmodium berghei. Proc Soc Exp Biol Med. 1956 Feb;91(2):315–318. doi: 10.3181/00379727-91-22248. [DOI] [PubMed] [Google Scholar]
- SINGER I. Tissue thiamine changes in rats with experimental trypanosomiasis or malaria. Exp Parasitol. 1961 Nov;11:391–401. doi: 10.1016/0014-4894(61)90043-1. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Ashton S. H., Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979 Jun;47(3):410–418. doi: 10.1016/0014-4894(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Miller J. Cytochrome oxidase activity in platelet-free preparations of Plasmodium knowlesi. J Parasitol. 1969 Aug;55(4):825–829. [PubMed] [Google Scholar]
- Scheibel L. W., Miller J. Glycolytic and cytochrome oxidase activity in Plasmodia. Mil Med. 1969 Sep;134(10):1074–1080. [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F., Lightholder J. Two Plasmodium knowlesi-specific antigens on the surface of schizont-infected Rhesus monkey erythrocytes induce antibody production in immune hosts. J Exp Med. 1979 Jul 1;150(1):86–99. doi: 10.1084/jem.150.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Wallach D. F. Plasmodium knowlesi-induced antigens in membranes of parasitized rhesus monkey erythrocytes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4949–4953. doi: 10.1073/pnas.75.10.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Walter R. D., Königk E. Adenosine kinase from normal mouse erythrocytes and from Plasmodium chabaudi: partial purification and characterization. Tropenmed Parasitol. 1974 Sep;25(3):301–308. [PubMed] [Google Scholar]
- Schnell J. V., Siddiqui W. A., Geiman Q. M. Biosynthesis of coenzymes Q by malarial parasites. 2. Coenzyme Q synthesis in blood cultures of monkeys infected with malarial parasites (Plasmodium falciparum and P. knowlesi). J Med Chem. 1971 Nov;14(11):1026–1029. doi: 10.1021/jm00293a002. [DOI] [PubMed] [Google Scholar]
- Seed T. M., Aikawa M., Sterling C. R. An electron microscope-cytochemical method for differentiating membranes of host red cells and malaria parasites. J Protozool. 1973 Nov;20(5):603–605. doi: 10.1111/j.1550-7408.1973.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Seed T. M., Kreier J. P. Surface properties of extracellular malaria parasites: electrophoretic and lectin-binding characteristics. Infect Immun. 1976 Dec;14(6):1339–1347. doi: 10.1128/iai.14.6.1339-1347.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare P. G., Trigg P. I. Glucose catabolism by the simian malaria parasite Plasmodium knowlesi. Nature. 1973 Feb 23;241(5391):538–540. doi: 10.1038/241538a0. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Amino acid metabolism and protein synthesis in malarial parasites. Bull World Health Organ. 1977;55(2-3):265–276. [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W., Cox R. A., Higginson B., McLaren D. J., Williamson J. The ribosomes of the simian malaria, Plasmodium knowlesi. I. Isolation and characterization. J Protozool. 1975 Nov;22(4):568–572. doi: 10.1111/j.1550-7408.1975.tb05235.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. In vitro studies of factors affecting penetration of duck erythrocytes by avian malaria (Plasmodium lophurae). J Parasitol. 1966 Feb;52(1):17–22. [PubMed] [Google Scholar]
- Sherman I. W., Jones L. A. Plasmodium lophurae: membrane proteins of erythrocyte-free plasmodia and malaria-infected red cells. J Protozool. 1979 Aug;26(3):489–501. doi: 10.1111/j.1550-7408.1979.tb04659.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Jones L. A. Protein synthesis by a cell-free preparation from the bird malaria, Plasmodium lophurae. J Protozool. 1976 May;23(2):277–281. doi: 10.1111/j.1550-7408.1976.tb03769.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Jones L. A. The Plasmodium lophurae (avian malaria) ribosome. J Protozool. 1977 May;24(2):331–334. doi: 10.1111/j.1550-7408.1977.tb00989.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Levels of oxidized and reduced pyridine nucleotides in avian malaria (Plasmodium lophurae). Am J Trop Med Hyg. 1966 Nov;15(6):814–817. doi: 10.4269/ajtmh.1966.15.814. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Malic dehydrogenase heterogeneity in malaria (Plasmodium lophurae and P. berghei). J Protozool. 1966 May;13(2):344–349. doi: 10.1111/j.1550-7408.1966.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Mudd J. B. Malaria infection (Plasmodium iophurae): changes in free amino acids. Science. 1966 Oct 14;154(3746):287–289. doi: 10.1126/science.154.3746.287. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Peterson I., Tanigoshi L., Ting I. P. The glutamate dehydrogenase of Plasmodium lophurae (avian malaria). Exp Parasitol. 1971 Jun;29(3):433–439. doi: 10.1016/0014-4894(71)90052-x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ruble J. A., Ting I. P. Plasmodium lophurae: (U-14C)-glucose catabolism by free Plasmodia and duckling host erythrocytes. Exp Parasitol. 1969 Aug;25(1):181–192. doi: 10.1016/0014-4894(69)90064-2. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Tanigoshi L. Alterations in sodium and potassium in red blood cells and plasma during the malaria infection (Plasmodium lophurae). Comp Biochem Physiol A Comp Physiol. 1971 Oct;40(2):543–546. doi: 10.1016/0300-9629(71)90046-6. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Tanigoshi L. Glucose transport in the malarial (Plasmodium lophurae) infected erythrocyte. J Protozool. 1974 Oct;21(4):603–607. doi: 10.1111/j.1550-7408.1974.tb03711.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Tanigoshi L. Incorporation of 14C-amino acids by malarial plasmodia (Plasmodium iophurae). VI. Changes in the kinetic constants of amino acid transport during infection. Exp Parasitol. 1974 Jun;35(3):369–373. doi: 10.1016/0014-4894(74)90042-3. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. The ribosomes of the simian malaria Plasmodium knowlesi--II. A cell-free protein synthesizing system. Comp Biochem Physiol B. 1976;53(4):447–450. doi: 10.1016/0305-0491(76)90196-6. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ting I. P. Carbon dioxide fixation in malaria (Plasmodium iophurae). Nature. 1966 Dec 17;212(5068):1387–1388. doi: 10.1038/2121387a0. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ting I. P. Carbon dioxide fixation in malaria. II. Plasmodium knowlesi (monkey malaria). Comp Biochem Physiol. 1968 Feb;24(2):639–642. doi: 10.1016/0010-406x(68)91018-9. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ting I. P., Ruble J. A. Characterization of the malaria pigment (hemozoin) from the avian malaria parasite Plasmodium lophurae. J Protozool. 1968 Feb;15(1):158–164. doi: 10.1111/j.1550-7408.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- Sherman I. W., Ting I. P., Tanigoshi L. Plasmodium lophurae: glucose-1-14C nd glucose-6-14C catabolism by free plasmodia and duckling host erythrocytes. Comp Biochem Physiol. 1970 Jun 1;34(3):625–639. doi: 10.1016/0010-406x(70)90289-6. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Transport of amino acids and nucleic acid precursors in malarial parasites. Bull World Health Organ. 1977;55(2-3):211–225. [PMC free article] [PubMed] [Google Scholar]
- Siddiqui W. A., Schnell J. V., Geiman Q. M. Stearic acid as plasma replacement for intracellular in vitro culture of Plasmodium knowlesi. Science. 1967 Jun 23;156(3782):1623–1625. doi: 10.1126/science.156.3782.1623. [DOI] [PubMed] [Google Scholar]
- Siddiqui W. A., Trager W. Folic and folinic acids in relation to the development of Plasmodium lophurae. J Parasitol. 1966 Jun;52(3):556–558. [PubMed] [Google Scholar]
- Siu P. M. Carbon dioxide fixation in plasmodia and the effect of some antimalarial drugs on the enzyme. Comp Biochem Physiol. 1967 Dec;23(3):785–795. doi: 10.1016/0010-406x(67)90341-6. [DOI] [PubMed] [Google Scholar]
- Skelton F. S., Lunan K. D., Folkers K., Schnell J. V., Siddiqui W. A., Geiman Q. M. Biosynthesis of ubiquinones by malarial parasites. I. Isolation of [14C]ubiquinones from cultures of rhesus monkey blood infected with Plasmodium knowlesi. Biochemistry. 1969 Mar;8(3):1284–1287. doi: 10.1021/bi00831a064. [DOI] [PubMed] [Google Scholar]
- Skelton F. S., Rietz P. J., Folkers K. Coenzyme Q. CXXII. Identification of ubiquinone-8 biosynthesized by Plasmodium knowlesi, P. cynomolgi, and P. berghei. J Med Chem. 1970 Jul;13(4):602–606. doi: 10.1021/jm00298a004. [DOI] [PubMed] [Google Scholar]
- Smith C. C., McCormick G. J., Canfield C. J. Plasmodium knowlesi: in vitro biosynthesis of methionine. Exp Parasitol. 1976 Dec;40(3):432–437. doi: 10.1016/0014-4894(76)90111-9. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. E. The effects of cod-liver oil and vitamin E on infections of Plasmodium gallinaceum and Treponema duttoni. Ann Trop Med Parasitol. 1958 Jul;52(2):139–144. doi: 10.1080/00034983.1958.11685854. [DOI] [PubMed] [Google Scholar]
- TRAGER W. Studies on the extracellular cultivation of an intracellular parasite (avian malaria). I. Development of the organisms in erythrocyte extracts, and the favoring effect of adenosinetriphosphate. J Exp Med. 1950 Oct 1;92(4):349–366. doi: 10.1084/jem.92.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAGER W. Studies on the extracellular cultivation of an intracellular parasite (avian malaria). II. The effects of malate and of coenzyme A concentrates. J Exp Med. 1952 Nov;96(5):465–476. doi: 10.1084/jem.96.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Sherman I. W. Plasmodium iophurae: cationized ferritin staining, an electron microscope cytochemical method for differentiating malarial parasite and host cell membranes. Exp Parasitol. 1978 Apr;44(2):145–154. doi: 10.1016/0014-4894(78)90092-9. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Fletcher K. A. A technique for the cytochemical demonstration in the electron microscope of glucose-6-phosphate dehydrogenase activity in erythrocytes of malaria-infected animals. J Microsc. 1973 Apr;97(3):315–320. doi: 10.1111/j.1365-2818.1973.tb03786.x. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Fletcher K. A. An electron cytochemical study of 6-phosphogluconate dehydrogenase activity in infected erythrocytes during malaria. Life Sci. 1973 Aug 16;13(4):405–410. doi: 10.1016/0024-3205(73)90233-6. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Fletcher K. A., Maegraith B. G. The use of electron microscope autoradiography for examining the uptake and degradation of haemoglobin by Plasmodium berghei. Ann Trop Med Parasitol. 1970 Mar;64(1):63–71. doi: 10.1080/00034983.1970.11686664. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Fletcher K. A., Moore G. A. Glucose-6-phosphate and 6-phosphogluconate dehydrogenase activities human erythrocytes infected with Plasmodium falciparum. Ann Trop Med Parasitol. 1976 Mar;70(1):125–127. doi: 10.1080/00034983.1976.11687105. [DOI] [PubMed] [Google Scholar]
- Theakston R. D., Howells R. E., Fletcher K. A., Peters W., Fullard J., Moore G. A. The ultrastructural distribution of cytochrome oxidase activity in Plasmodium Berghei and P. Gallinaceum. Life Sci. 1969 May 15;8(10):521–529. doi: 10.1016/0024-3205(69)90251-3. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K., Ilan J., Ilan J. Biogenesis of ribosomes in Plasmodium berghei. Mil Med. 1969 Sep;134(10):1032–1038. [PubMed] [Google Scholar]
- Tracy S. M., Sherman I. W. Purine uptake and utilization by the avian malaria parasite Plasmodium lophurae. J Protozool. 1972 Aug;19(3):541–549. doi: 10.1111/j.1550-7408.1972.tb03524.x. [DOI] [PubMed] [Google Scholar]
- Trager W. Cofactors and vitamins in the metabolism of malarial parasites. Factors other than folates. Bull World Health Organ. 1977;55(2-3):285–289. [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W. Malaria parasites (Plasmodium lophurae) developing extracellularly in vitro: incorporation of labeled precursors. J Protozool. 1971 Aug;18(3):392–399. doi: 10.1111/j.1550-7408.1971.tb03341.x. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Trigg P. I., Gutteridge W. E. A rational approach to the serial culture of malaria parasites: evidence for a deficiency in RNA synthesis during the first cycle in vitro. Parasitology. 1972 Oct;65(2):265–271. doi: 10.1017/s0031182000045042. [DOI] [PubMed] [Google Scholar]
- Trigg P. I., Hirst S. I., Shakespeare P. G., Tappenden L. Labelling of membrane glycoprotein in erythrocytes infected with Plasmodium knowlesi. Bull World Health Organ. 1977;55(2-3):205–209. [PMC free article] [PubMed] [Google Scholar]
- Trigg P. I., Shakespeare P. G., Burt S. J., Kyd S. I. Ribonucleic acid synthesis in Plasmodium knowlesi maintained both in vivo and in vitro. Parasitology. 1975 Oct;71(2):199–209. doi: 10.1017/s0031182000046655. [DOI] [PubMed] [Google Scholar]
- Trigg P. I. Sterol metabolism of Plasmodium knowlei in vitro. Ann Trop Med Parasitol. 1968 Dec;62(4):481–487. doi: 10.1080/00034983.1968.11686587. [DOI] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J Biol Chem. 1971 May 25;246(10):3111–3119. [PubMed] [Google Scholar]
- WHITFELD P. R. Nucleic acids in erythrocytic stages of a malaria parasite. Nature. 1952 May 3;169(4305):751–752. doi: 10.1038/169751a0. [DOI] [PubMed] [Google Scholar]
- WHITFELD P. R. Studies on the nucleic acids of the malaria parasite, Plasmodium berghei; Vincke & Lips. Aust J Biol Sci. 1953 May;6(2):234–243. [PubMed] [Google Scholar]
- Walsh C. J., Sherman I. W. Isolation, characterization and synthesis of DNA from a malaria parasite. J Protozool. 1968 Aug;15(3):503–508. doi: 10.1111/j.1550-7408.1968.tb02163.x. [DOI] [PubMed] [Google Scholar]
- Walsh C. J., Sherman I. W. Purine and pyrimidine synthesis by the avian malaria parasite, Plasmodium lophurae. J Protozool. 1968 Nov;15(4):763–770. doi: 10.1111/j.1550-7408.1968.tb02209.x. [DOI] [PubMed] [Google Scholar]
- Walter R. D., Königk E. Biosynthesis of folic acid compounds in plasmodia. Purification and properties of the 7,8-dihydropteroate-synthesizing enzyme from Plasmodium chabaudi. Hoppe Seylers Z Physiol Chem. 1974 Apr;355(4):431–437. doi: 10.1515/bchm2.1974.355.1.431. [DOI] [PubMed] [Google Scholar]
- Walter R. D., Königk E. Hypoxanthine-guanine phosphoribosyltransferase and adenine phosphoribosyltransferase from Plasmodium chabaudi, purification and properties. Tropenmed Parasitol. 1974 Jun;25(2):227–235. [PubMed] [Google Scholar]
- Walter R. D., Königk E. Plasmodium chabudi: Die enzymatische Synthese von Dihydropteroat und ihre Hemmung durch Sulfonamide. Z Tropenmed Parasitol. 1971 Sep;22(3):256–259. [PubMed] [Google Scholar]
- Walter R. D., Königk E. Synthese der Desoxythymidylat-Synthetase und der Dihydrofolat-Reduktase bei synchroner Schizogonie von Plasmodium chabaudi. Z Tropenmed Parasitol. 1971 Sep;22(3):250–255. [PubMed] [Google Scholar]
- Walter R. D., Nordmeyer J. P., Königk E. NADP-specific glutamate dehydrogenase from Plasmodium chabaudi. Hoppe Seylers Z Physiol Chem. 1974 May;355(5):495–500. doi: 10.1515/bchm2.1974.355.1.495. [DOI] [PubMed] [Google Scholar]
- Wan Y. P., Porter T. H., Folkers K. Antimalarial quinones for prophylaxis based on a rationale of inhibition of electron transfer in Plasmodium. Proc Natl Acad Sci U S A. 1974 Mar;71(3):952–956. doi: 10.1073/pnas.71.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. J., Saz H. J. Comparative biochemical studies of Litomosoides carinii, Dipetalonema viteae, and Brugia pahangi adults. J Parasitol. 1974 Apr;60(2):316–321. [PubMed] [Google Scholar]
- Warhurst D. C., Williamson J. Ribonucleic acid from Plasmodium knowlesi before and after chloroquine treatment. Chem Biol Interact. 1970 Aug;2(2):89–106. doi: 10.1016/0009-2797(70)90042-6. [DOI] [PubMed] [Google Scholar]
- Weidekamm E., Wallach D. F., Lin P. S., Hendricks J. Erythrocyte membrane alterations due to infection with Plasmodium berghei. Biochim Biophys Acta. 1973 Nov 16;323(4):539–546. doi: 10.1016/0005-2736(73)90162-4. [DOI] [PubMed] [Google Scholar]
- Yamada K. A., Sherman I. W. Plasmodium lophurae: composition and properties of hemozoin, the malarial pigment. Exp Parasitol. 1979 Aug;48(1):61–74. doi: 10.1016/0014-4894(79)90055-9. [DOI] [PubMed] [Google Scholar]
- Yuthavong Y., Wilairat P., Panijpan B., Potiwan C., Beale G. H. Alterations in membrane proteins of mouse erythrocytes infected with different species and strains of malaria parasites. Comp Biochem Physiol B. 1979;63(1):83–85. doi: 10.1016/0305-0491(79)90238-4. [DOI] [PubMed] [Google Scholar]


