Abstract
Novel antimalarial drugs are urgently needed to treat severe malaria caused by Plasmodium falciparum. Isoprenoid biosynthesis is a promising target pathway, since the biosynthetic route in Plasmodia is biochemically distinct from the mevalonate pathway in humans. The small molecule fosmidomycin is an inhibitor of the enzyme responsible for the first dedicated step in isoprenoid biosynthesis, deoxyxylulose 5-phosphate reductoisomerase (DXR). However, the antimalarial effects of fosmidomycin might not be specific to DXR inhibition and further validation of DXR is warranted. We present the first functional genetic validation of Plasmodium falciparum DXR (PF14_0641). Using a single cross-over strategy, we show that plasmid integration occurs at the DXR locus but only when DXR gene function is preserved, but not when integration disrupts gene function. These data indicate that DXR is required for intraerythrocytic development of Plasmodium falciparum.
Keywords: Malaria, Isoprenoid biosynthesis, drug targets
Novel antiparasitic agents are needed to treat severe malaria caused by Plasmodium falciparum. The isoprenoid biosynthesis pathway of the parasite is a promising drug target. Isoprenoids comprise a large and diverse group of natural products, and play important roles in electron transport, cell signaling, apoptosis, and membrane structure [1,2]. In P. falciparum, the fundamental isoprenoid building blocks (isopentyl pyrophosphate and dimethylallyl pyrophosphate) are generated through eight enzymatic reactions from pyruvate and glyceraldehyde 3-phosphate [3,4]. The first dedicated enzyme of isoprenoid biosynthesis, deoxyxylulose 5-phosphate reductoisomerase (DXR), converts deoxyxylulose 5-phosphate (DXP) to methylerythritol 5-phosphate (MEP), and this metabolic route is therefore alternately referred to as the DXP or MEP pathway. P. falciparum produces the key intermediates of this pathway, and recombinant forms of two of the pathway enzymes have the expected catalytic activities [3,5]. The DXP pathway is also the essential and exclusive metabolic route to isoprenoids in eubacteria, including E. coli [6]. In contrast, the DXP pathway of the parasite biochemically differs from the human isoprenoid biosynthesis pathway, which proceeds through acetyl-CoA with mevalonate as a key intermediate, and this difference adds to its interest as a possible drug target.
The small molecule fosmidomycin, which inhibits recombinant P. falciparum DXR enzyme in vitro, has antimalarial activity both in cell culture and in vivo, and has been well-tolerated in humans [3,7–9]. Modification of the simple structure of fosmidomycin to improve its drug characteristics has been challenging [10–13].
A crucial step in drug discovery is functional validation of potential therapeutic targets. Since fosmidomycin inhibits the DXR enzyme and kills the malaria parasite, de novo isoprenoid biosynthesis has been presumed to be essential in Plasmodia. If the target of fosmidomycin is indeed the parasite DXR enzyme, one would anticipate that fosmidomycin treatment should diminish production of the DXR product, methylerythritol 4-phosphate (MEP). However, two studies have found little alteration in DXP pathway metabolites, and, most notably, levels of the DXR product (MEP) did not change in fosmidomycin-treated trophozoite stage parasites [4,14]. These studies raise the possibility that the antimalarial effects of this compound might not be exerted through DXR inhibition. To test the hypothesis that the DXR gene product is required for intraerythrocytic parasite development, we initiated studies to genetically disrupt DXR, in order to assess the role of this locus in P. falciparum development and survival.
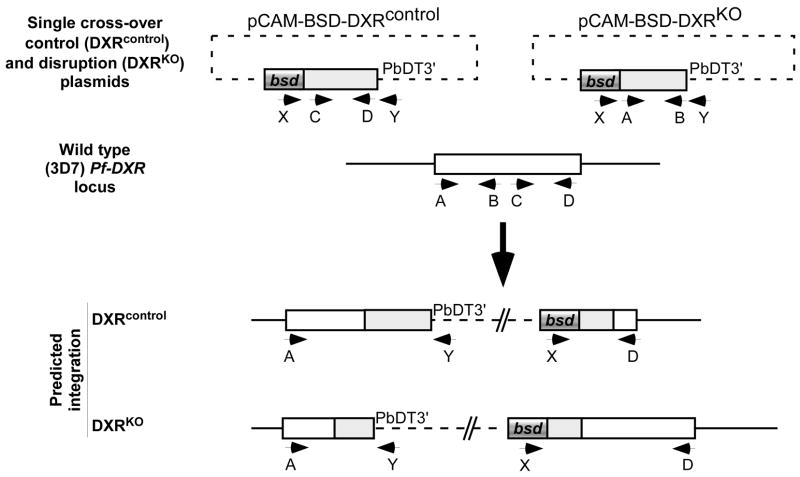
To disrupt the DXR gene locus (PF14_0641), so as to assess its role in survival, we employed a control strategy for gene disruption using single cross-over integrating vectors based on pCAM-BSD, which confers blasticidin resistance (gift of David Fidock, Columbia University) (Figure 1) [15]. Integration of the knockout vector is expected to ablate function, as the recombinant gene product would lack most of the predicted active site region (aa 232–395), based on studies with the E. coli DXR homolog [16]; integration of a separate control vector recapitulates a functional gene with the native Pf-DXR 5′ UTR and the vector-derived 3′ UTR from the P. berghei DHFR gene (Figure 1). Vectors were generated by a PCR-based strategy, with primers designed to amplify bp 31–706 of the DXR genetic locus from 3D7 genomic DNA (A: CATGCCCGGGCTTCATCACAATAAC, B: CATGCTCGAGTTACCCACTATGTTCTGAATCAACAGGT) or bp 747–1463 of the DXR locus (C: CATGCCCGGGCAAAATGTTTACAAGACAA, D: CATGCTCGAGGTTTGTTGTATATATCGGTAG). The purified PCR products were digested with Xma1 and Xho1 (underlined), and cloned into these sites in pCAM-BSD, to generate pCAM-BSD-DXRKO and pCAM-BSD-DXRcontrol, respectively.
Figure 1. Strategy for single cross-over disruption of P falciparum DXR (PF14_0641).
Transfection plasmids contain either an insert spanning positions 31–706 of the 1.5 kb DXR coding sequence (for pCAM-BSD-DXRKO), or an insert spanning positions 248–484 (for pCAM-BSD-DXRcontrol). Single cross-over homologous recombination into the wild-type locus results in a pseudo-diploid configuration. For pCAM-BSD-DXRKO, both copies are truncated: one has a C-terminal truncation resulting in loss of the predicted catalytic domain (232–395), the other copy lacks a promoter and is truncated at the N-terminus. For pCAM-BSD-DXRcontrol, recombination reconstructs a functional copy of the gene as well as a presumably non-functional N-terminal truncation. Locations of PCR primers used for genetic analysis (A,B,C,D, X, and Y) are indicated by labeled arrows.
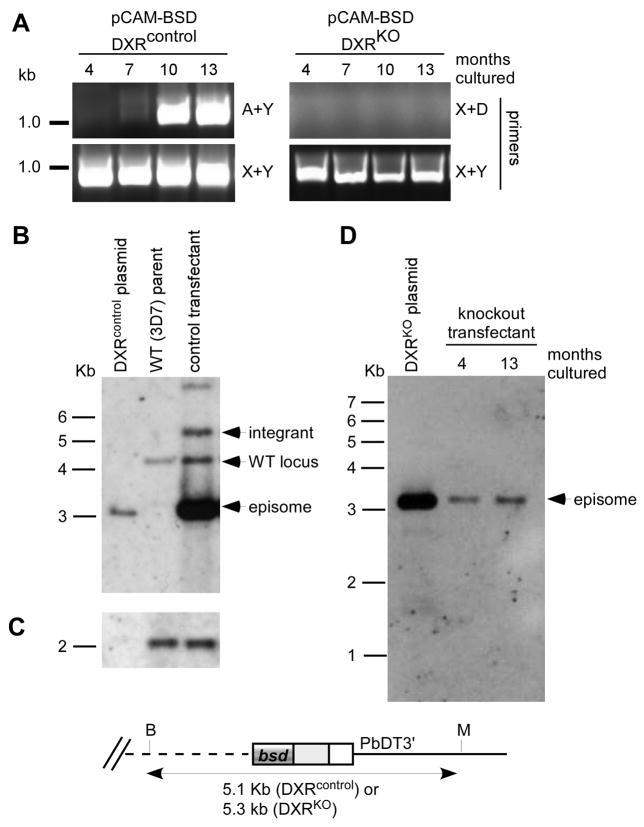
The DXR knockout and control plasmids (100μg each) were electroporated independently into synchronized 3D7 P. falciparum parasites (MR4 strain, courtesy of Pradipsinh Rathod, University of Washington) as described previously [15], and transfectants were selected by growth in the presence of 1 μg/ml blasticidin (Invitrogen). These cell lines were continually cultured in the presence of blasticidin, and screened for spontaneous integration at the genomic DXR locus. Drug-resistant parasites appeared between three to four weeks following transfection. For PCR and Southern blot analysis, genomic DNA was prepared from each strain using the Qiagen DNA mini kit per manufacturer’s instructions. Plasmid-specific primers (X: TAAGAACATATTTATTAAACTGCAG, Y: GAAAAACGAACATTAAGCTGCCATA) were used in various combinations to amplify products from genomic DNA in order to screen blasticidin-resistant transfectant parasites for presence of episomal plasmids (X+Y), integration of the pCAM-BSD-DXRKO plasmid (X+D), and integration of the pCAM-BSD-DXRcontrol plasmid (A+Y) (Figure 2). PCR screening of genomic DNA derived from both cell lines soon after transfection produced amplicons using plasmid-specific primers, but generated no products with integration-specific primers, indicating that the plasmids were episomal, as expected (Figure 2).
Figure 2. Genetic analysis of pCAM-BSD-DXR transfectants.
A. PCR analysis. Genomic DNA isolated from blasticidin-resistant parasites transfected with pCAM-BSD-DXRcontrol (left) or pCAM-BSD-DXRKO (right) was subjected to PCR using the indicated primers. For pCAM-BSD-DXRcontrol, integration events are detected by primers A+Y, while primers X+Y detect the episomal plasmid. For pCAM-BSD-DXRKO, primers X+D would detect integration events, while primers X+Y detect the episomal plasmid.
B. Southern blot analysis of the DXR locus in wild-type 3D7 parental strain, pCAM-BSD-DXRcontrol plasmid, and pCAM-BSD-DXRcontrol blasticidin-resistant transfectant parasites. Genomic and plasmid DNA was digested with BciVI and MfeI, transferred to membrane, and probed with the DXR fragment (C/D) used as insert in the pCAM-BSD-DXRcontrol plasmid.
C. Blot from (B) reprobed for the single copy IspF locus (PFB0420w, bp 9–850).
D. Southern blot analysis of the DXR locus in pCAM-BSD-DXRKO plasmid, and pCAM-BSD-DXRKO blasticidin-resistant transfectant parasites. Genomic and plasmid DNA was digested with BciVI and MfeI, transferred to membrane, and probed with the HindIII-PvuII fragment of pCAM-BSD that contains the blasticidin deaminase gene.
After approximately seven months of continuous culture, recombination-specific amplicons were observed with genomic DNA template from the pCAM-BSD-DXRcontrol transfectant, indicating that this population contained parasites in which the plasmid had integrated into the genomic DXR locus (Figure 2A). In contrast, primers specific to plasmid integration did not amplify template derived from the pCAM-BSD-DXRKO transfectant, even after one year of continuous culture. Plasmid-specific primers X+Y readily amplified DNA from both strains, indicating continued presence of episomes and controlling for quality of the genomic DNA templates. Integrated plasmid concatamers, (which would also amplify with primers X+Y) may explain the more robust amplification seen with the control transfectants. The recombination-specific amplicon (from primers A+Y) generated from the pCAM-BSD-DXRcontrol transfectant was purified and sequenced with both genomic and plasmid-specific primers (A and Y, respectively), to confirm that this appropriately sized PCR product corresponded to the expected DXR locus sequence (data not shown).
Southern blot analyses of genomic DNA from the DXR knockout and control transfectants confirmed the PCR screening results (Figure 2B). For each strain, 2–3 μg of genomic DNA was digested to completion with BciVI and MfeI, separated electrophoretically on a 1.0% agarose gel, and transferred to Nytran Supercharge membrane. Blots were probed with either the same DXR amplicon (C+D) used to generate pCAM-BSD-DXRcontrol, or a HindIII-PvuII fragment from pCAM-BSD (containing the blasticidin deaminase gene). Probes were fluorescein labeled, and hybridization and detection was performed according to the manufacturer’s instructions (Amersham). Southern blotting revealed that the pCAM-BSD-DXRcontrol transfectant parasites contained a mixed population, with bands corresponding to the wild-type DXR genetic locus at 4 kb, the episomal plasmid at 3 kb, and the novel 3′ integration band at 5.1 kb. Cycling of DXRcontrol transfectants off blasticidin-selection pressure for 2 weeks, followed by reapplication of blasticidin, increased the proportion of parasites within the culture that integrated. Reprobing of the blot with a control probe derived from the single copy IspF locus (PFB0420w, bp 9–850) demonstrated similar loading of genomic DNA from the 3D7 parent strain and the DXRcontrol transfectant (Figure 2C).
In contrast, for the pCAM-BSD-DXRKO transfectants, Southern blotting demonstrated only an episomal plasmid at 4 kb and no additional bands, which would have suggested integration at any locus (Figure 2D). No additional bands were revealed even after great overexposure of this blot (data not shown). No bands other than the native locus and episomal plasmid were identified on reprobing of this blot with the DXR amplicon (A+B) used to generate pCAM-BSD-DXRKO (data not shown).
We did not detect integration of plasmid pCAM-BSD-DXRKO either by Southern blot analysis or by PCR screening (which would be more sensitive for detection of site-specific integration in a subset of a mixed culture), despite prolonged intraerythrocytic culture of P. falciparum and multiple cycles off and on drug selective pressure. Two additional independent DXRKO transfectants were each cultured for greater than 6 months without observed integration as well. Genomic DNA from the DXRKO transfectant strain is readily amplified by other primer combinations (Figure 2A, X+Y primer combination), and the recombination-specific primers (X+D) readily amplify from plasmid template pCAM-BSD-DXRcontrol indicating that there is no defect with this primer combination (data not shown). In contrast, we observed site-specific integration of plasmid pCAM-BSD-DXRcontrol at the P. falciparum DXR locus, where integration recapitulates a functional gene. Together, these data demonstrate that the P. falciparum DXR locus is recombinogenic, and strongly suggests that our inability to produce a loss-of-function disruption reflects the fact that the DXR gene is essential for asexual propagation of the parasite. Alternate explanations include a possible dominant-negative effect of recombination of the pCAM-BSD-DXRKO plasmid, since the expected truncated protein product could be toxic to the cell. Additionally, we cannot formally rule out very low levels of integration of the knockout plasmid, below the level of detection by PCR screening.
With an IC50 against P. falciparum in culture between 224 – 1,268 nM, the small molecule fosmidomycin shows moderate activity against P. falciparum [3,9]. Although this paper demonstrates that the presumed target of fosmidomycin, DXR, is required for Plasmodium falciparum growth, further evidence is required to demonstrate conclusively that fosmidomycin acts through DXR inhibition. Additionally, it is not certain that a modified fosmidomycin derivative with improved drug-like qualities can be developed, since fosmidomycin contains charged phosphate, which complicates pharmacokinetic optimization of this compound.
Enzymes of the DXP pathway are attractive for development of broad spectrum antimicrobial agents, since homologues of these proteins are absent in human hosts, and their function is essential in several serious human pathogens [17–19]. We were unable to generate loss-of-function genomic disruption in the likely enzymatic target of fosmidomycin, the DXP pathway enzyme DXR, despite the ability to target recombination to this locus when gene function was preserved. Therefore P. falciparum DXR appears to be amenable to small molecule inhibition and essential for parasite development, supporting further drug development efforts directed against this enzyme.
Acknowledgments
We thank Dr. David Fidock, Columbia University, for the gift of the plasmids and helpful advice on the knockout strategy. We also wish to thank Lynn Barrett, laboratory manager, and Kasey Rivas for technical support, and we thank Drs. Daniel Goldberg, Gregory Storch, and Phillip Tarr, Washington University, for critical reading of this manuscript. Dr. Odom is a Scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine (K12-HD01487), and this work was supported by a Pediatric Infectious Diseases Society Fellowship (Dr. Odom) and NIH K08 AI079010 (Dr. Odom).
Abbreviations
- DXP
deoxyxylulose 5-phosphate
- DXR
deoxyxylulose phosphate reductoisomerase
- MEP
2C-methyl-D-erythritol 5-phosphate
- PCR
polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunter WN. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J Biol Chem. 2007;282:21573–7. doi: 10.1074/jbc.R700005200. [DOI] [PubMed] [Google Scholar]
- 2.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–14. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 3.Jomaa H, Wiesner J, Sanderbrand S, et al. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science. 1999;285:1573–6. doi: 10.1126/science.285.5433.1573. [DOI] [PubMed] [Google Scholar]
- 4.Cassera MB, Gozzo FC, D’Alexandri FL, et al. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J Biol Chem. 2004;279:51749–59. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 5.Rohdich F, Eisenreich W, Wungsintaweekul J, Hecht S, Schuhr CA, Bacher A. Biosynthesis of terpenoids. 2C-Methyl-D-erythritol 2,4-cyclodiphosphate synthase (IspF) from Plasmodium falciparum. Eur J Biochem. 2001;268:3190–7. doi: 10.1046/j.1432-1327.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- 6.Kuzuyama T, Takahashi S, Seto H. Construction and characterization of Escherichia coli disruptants defective in the yaeM gene. Biosci Biotechnol Biochem. 1999;63:776–8. doi: 10.1271/bbb.63.776. [DOI] [PubMed] [Google Scholar]
- 7.Kuemmerle HP, Murakawa T, Soneoka K, Konishi T. Fosmidomycin: a new phosphonic acid antibiotic. Part I: Phase I tolerance studies. Int J Clin Pharmacol Ther Toxicol. 1985;23:515–20. [PubMed] [Google Scholar]
- 8.Missinou MA, Borrmann S, Schindler A, et al. Fosmidomycin for malaria. Lancet. 2002;360:1941–2. doi: 10.1016/S0140-6736(02)11860-5. [DOI] [PubMed] [Google Scholar]
- 9.Tahar R, Basco LK. Molecular epidemiology of malaria in Cameroon. XXV. In vitro activity of fosmidomycin and its derivatives against fresh clinical isolates of Plasmodium falciparum and sequence analysis of 1-deoxy-D-xylulose 5-phosphate reductoisomerase. Am J Trop Med Hyg. 2007;77:214–20. [PubMed] [Google Scholar]
- 10.Haemers T, Wiesner J, Giessmann D, et al. Synthesis of beta- and gamma-oxa isosteres of fosmidomycin and FR900098 as antimalarial candidates. Bioorg Med Chem. 2008;16:3361–71. doi: 10.1016/j.bmc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Kurz T, Behrendt C, Pein M, Kaula U, Bergmann B, Walter RD. gamma-Substituted bis(pivaloyloxymethyl)ester analogues of fosmidomycin and FR900098. Arch Pharm (Weinheim) 2007;340:661–6. doi: 10.1002/ardp.200700107. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner J, Ortmann R, Jomaa H, Schlitzer M. Double ester prodrugs of FR900098 display enhanced in-vitro antimalarial activity. Arch Pharm (Weinheim) 2007;340:667–9. doi: 10.1002/ardp.200700069. [DOI] [PubMed] [Google Scholar]
- 13.Ortmann R, Wiesner J, Silber K, Klebe G, Jomaa H, Schlitzer M. Novel deoxyxylulosephosphate-reductoisomerase inhibitors: fosmidomycin derivatives with spacious acyl residues. Arch Pharm (Weinheim) 2007;340:483–90. doi: 10.1002/ardp.200700149. [DOI] [PubMed] [Google Scholar]
- 14.Cassera MB, Merino EF, Peres VJ, Kimura EA, Wunderlich G, Katzin AM. Effect of fosmidomycin on metabolic and transcript profiles of the methylerythritol phosphate pathway in Plasmodium falciparum. Mem Inst Oswaldo Cruz. 2007;102:377–83. doi: 10.1590/s0074-02762007000300019. [DOI] [PubMed] [Google Scholar]
- 15.Sidhu AB, Valderramos SG, Fidock DA. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol Microbiol. 2005;57:913–26. doi: 10.1111/j.1365-2958.2005.04729.x. [DOI] [PubMed] [Google Scholar]
- 16.Proteau PJ. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase: an overview. Bioorg Chem. 2004;32:483–93. doi: 10.1016/j.bioorg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Brown AC, Parish T. Dxr is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to a lack of uptake. BMC Microbiol. 2008;8:78. doi: 10.1186/1471-2180-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi K, Ehrlich SD, Albertini A, et al. Essential Bacillus subtilis genes. Proc Natl Acad Sci U S A. 2003;100:4678–83. doi: 10.1073/pnas.0730515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerley BJ, Rubin EJ, Novick VL, Amaya K, Judson N, Mekalanos JJ. A genome-scale analysis for identification of genes required for growth or survival of Haemophilus influenzae. Proc Natl Acad Sci U S A. 2002;99:966–71. doi: 10.1073/pnas.012602299. [DOI] [PMC free article] [PubMed] [Google Scholar]