Abstract
A sensitive and precise LC-ESI-MS/MS method for determination of nutlin-3a in murine plasma using ketoconazole as an internal standard was developed and validated. Plasma nutlin-3a samples were prepared by either a simple protein precipitation (PP) for the high concentration range (10 – 20,000 ng/mL) or by liquid-liquid extraction (LLE) for the low concentration range (0.25 – 300 ng/mL). Nutlin-3a and ketoconazole were separated on a modified C18 analytical column (4µ, 75x2 mm) with an isocratic mobile phase (acetonitrile/5mM HCOONH4 = 70/30, v/v). The retention times of nutlin-3a and ketoconazole were 1.14 and 1.45 minutes. Detection was achieved by a tandem MS system, monitoring m/z 582/99 and m/z 532/82 for nutlin-3a and ketoconazole, respectively. The PP method was linear in a range of 10 – 20,000 ng/mL (R2 ≥0.993) and the LLE method was linear in a range of 0.25 – 300 ng/mL (R2 ≥0.992). The mean recoveries for PP and LLE were 24% and 78%, respectively. Within-day and between-day precisions were ≤ 4.5% for PP and were ≤ 4.9% for LLE. Within-day and between-day accuracies (% error) ranged from 4.8 to −7.9 for PP, and from −0.2 to −8.4 for LLE. The two extraction methods produced equivalent results, allowing use of both within the same study. This method has been applied to the measurement of nutlin-3a concentrations in murine plasma samples obtained from a preclinical pharmacokinetic study.
Keywords: nutlin-3a, liquid-liquid extraction (LLE), protein precipitation (PP), liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS)
1. Introduction
The tumor suppressor p53 is impaired in a wide range of human cancers [1,2]. Although p53 activity may be compromised by genetic deletions or mutations, many cancers retain the wild-type p53 gene. In these cases, p53 activity may be suppressed by other regulatory proteins. Murine double minute 2 (MDM2, or HDM2 for the human ortholog) is a central protein in the p53 regulatory pathway that provides negative feedback to p53 activity [3]. p53 activates MDM2 gene expression and the MDM2 protein directly binds to p53, inhibiting its transactivation [4]. MDM2 levels are increased in some human cancers due to gene amplification, increased transcription or translation, or protein stabilization [5,6]. High MDM2 expression levels correlate with a worse clinical prognosis and poor response to therapy [7]. Inhibition of the MDM2-p53 interaction is therefore proposed as a novel strategy for treatment of cancers that do not have p53 alterations [7,8].
Nutlins are a class of small molecules that target the p53-binding pocket of MDM2, thereby allowing p53 activation and tumor suppression [9,10]. Nutlin-3a, an enantiomer of the racemic nutlin-3 mixture, potently disrupts the MDM2-p53 interaction with an IC50 value of 90 nM [9]. Treatment of tumor cells with nutlin-3a induced p-53-dependent cell cycle arrest and cell death, whereas in normal cells nutlin-3a led to cell cycle arrest without cell death [9,11,12]. In addition, nutlin-3a has shown activity against several types of pediatric cancer cell lines, including leukemias [13], neuroblastoma [14], and retinoblastoma [15].
The next step in the development of nutlin-3a as a therapeutic molecule is to describe its pharmacokinetics in preclinical models. However, no analytical method to measure nutlin-3a has yet been reported. Thus, we developed and validated a sensitive and precise liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) method to measure nutlin-3a in murine plasma.
2. Experimental
2.1. Chemicals
cis-nutlin-3a (2-Piperazinone, 4-[[(4S,5R)-4,5-bis(4-chlorophenyl)-4,5-dihydro-2-[4-methoxy-2-(1-methylethoxy)phenyl]-1H-imidazol-1-yl]carbonyl]-) (98% purity, Lot No: 08252008) was supplied by the Department of Chemical Biology and Therapeutics at St Jude Children's Research Hospital (Memphis, TN, USA). Ketoconazole was purchased from Sigma (St. Louis, MO, USA; >98% purity, K-1003, lot 121H0524). HPLC grade acetonitrile was obtained from Burdick & Jackson (Muskegon, MI, USA), formic acid (HCOOH; 95% purity) was purchased from Sigma, and ammonium formate (HCOONH4; 99% purity) was purchased from Fluka BioChemika (Buchs, Switzerland). Blank murine plasma was obtained from Hilltop Lab Animals, Inc. (Scottdale, PA, USA). All water was distilled, deionized, and further purified via a Millipore Milli-Q UV plus and Ultra-Pure Water System (Tokyo, Japan). Other chemicals were purchased from standard sources and were of the highest quality available.
2.2. Apparatus and conditions
2.2.1. Chromatographic conditions
The HPLC system consisted of a Shimadzu (Kyoto, Japan) system controller (SCL-10AVP), pump (LC-20AD), autoinjector (SIL-20ACHT), and online degasser (DGU-14A). The chromatography of the analytes was performed at 25°C using a Phenomenex Synergi Hydro-RP analytical column (75×2.0 mm, 4µ; Torrance, CA, USA) preceded by a Phenomenex C18 guard column (4×2.0 mm). Analytes were eluted with an isocratic mobile phase (acetonitrile/5mM HCOONH4 = 70/30, v/v, pH 6.2) at a flow rate of 0.30 mL/min for 2.5 min.
2.2.2. Mass spectrometry
Detection was performed on an API-3000 LC-MS/MS System (Toronto, Canada) equipped with a Turbo IonSpray® source (TIS; thermally and pneumatically assisted electrospray). The optimized conditions were as follows: ion spray source temperature at 550°C, nebulizer gas at 12, curtain gas at 15, turbo gas flow at 6.0 L/min, ionspray voltage at 5000 V, and collision-activated dissociation at 7.0 units; declustering potential at 80 V, focusing potential at 345 V, entrance potential at 13 V, collision energy at 70 V, and collision exit potential at 5 V. The mass spectrometer was interfaced to a computer workstation running Analyst software (Version 1.4.1 Applied Biosystems, Foster City, CA) for data acquisition and processing. Data acquisition was performed at unit (Q1) and low (Q3) resolution in positive multiple-reaction monitoring (MRM) mode, monitoring the transition of the nutlin-3a protonated [M+H]+ ion m/z 582 to product ion m/z 99, and of the internal standard ketoconazole [M]+ ion m/z 532 to the product ion m/z 82.
2.3. Sample preparation
2.3.1. Stock solutions
Stock solutions were prepared by dissolving nutlin-3a or ketoconazole (the internal standard) into methanol to yield a concentration of 1.0 mg/mL. Stock solutions were stored at −80 °C (less than 5% change from nominal concentration over 6 months). The working stock solutions (nutlin-3a at concentrations of 5, 10, 40, 100, 200, 2000, and 10000 ng/mL for the low range, and 0.2, 1, 5, 50, 100, and 200 µg/mL for the high range, with ketoconazole at 500 ng/mL and 10 µg/mL, respectively) were prepared by diluting the stock solution with 80% methanol/water (v/v).
2.3.2. Calibration curve and quality controls
Two calibration curves were made to quantitate low and high levels of nutlin-3a in murine plasma. Calibrators were made by adding working solutions of nutlin-3a and ketoconazole to blank murine plasma for final concentrations of nutlin-3a of 10, 50, 200, 500, 2000, 8000, and 20000 ng/mL with 800 ng/mL ketoconazole as the internal standard (high concentration range) or 0.25, 0.5, 2, 5, 10 100, and 300 ng/mL with 20 ng/mL of ketoconazole (low concentration range). Separate quality control samples were prepared using the same methodology at nutlin-3a concentrations of 16, 5000, and 16000 ng/mL for the high range, and 0.4, 8.0, and 200 ng/mL for the low range.
2.3.3. Murine plasma sample preparation
One of two extraction procedures was performed, depending on the expected concentration range of the murine plasma samples. Protein precipitation (PP) was performed for samples in the high concentration range and liquid-liquid extraction (LLE) was performed for samples in the low concentration range. For the protein precipitation, a total of 50 µL of murine plasma sample was spiked with 4.0 µL of 10 µg/mL ketoconazole in a 1.5mL centrifuge tube containing 150 µL of 100% acetonitrile and vortexed for 10 s, and then was centrifuged at 13,000 rpm for 5 min at 4°C. 100 µL of the supernatant was transferred to an autoinjector vial and 10 µL was injected onto the LC-MS/MS system. For the liquid-liquid extraction, 100 µL of murine plasma sample was spiked with 4.0 µL of 0.5 µg/mL ketoconazole in a 2.0 mL screw-top tube with 1.5 mL of t-butyl methyl ether and vortexed for 45 s. Samples were then centrifuged at 13,000 rpm for 5 min at 4°C, and the top organic layer (~1.3 mL) was transferred to a new tube and was dried over a stream of nitrogen. The dried sample was reconstituted with 50 µL of 60% acetonitrile in 5 mM HCOONH4. Each tube was vortexed for 30 s, the solution was transferred to an autoinjector vial, and 35 µL was injected onto the LC-MS/MS system.
2.4. Assay validation
2.4.1 Linearity and lower limit of quantitation
Two calibration curves of the high and low ranges were analyzed during the validation process. The linear regression of the ratio of nutlin-3a/ketoconazole peak areas was weighted by 1/x2. The coefficient of determination (R2) was used to evaluate the linearity of each calibration curve. The lower limit of quantitation (LLOQ) was defined as the lowest concentration in each calibration curve, which had a precision and accuracy within 20% and a signal/noise (S/N) ratio greater than 5.
2.4.2 Accuracy, precision, and recovery
The method developed for the measurement of nutlin-3a in murine plasma was validated over five days by analysis of plasma quality control samples, and the within-day and between-day precision and accuracy for the method was determined. Recovery was assessed by preparing low, middle, and high concentration LLE quality control samples in triplicate alongside neat samples of the same concentrations in 60% acetonitrile water. Both were extracted and analyzed to assess percent recovery.
2.4.3 Selectivity, carryover, and ion suppression
Selectivity of the assay was assessed by monitoring all selected ion transitions of nutlin-3a and ketoconazole after injection of blank murine plasma from three different sources. Carryover was assessed by injecting nutlin-3a at 20 ng/ml and monitoring selected ion transitions through four blank plasma samples. Ion suppression was evaluated by post-column infusion of the analyte [16]. A solution of neat nutlin-3a (20 ng/mL) and ketoconazole (8 ng/ml) was infused, post-analytical column, through a Valco zero dead volume tee using a Harvard Apparatus syringe pump 11 (Harvard Apparatus, Holliston, MA, USA) at a constant flow rate of 5.0 µL/min into the LC effluent (300 µL/min) prior to entering the mass spectrometer. Mobile phase solution, commercially available pooled blank murine plasma (Hilltop Lab Animals, Inc.), and blank murine plasma from 4 different C57/BL6 mice were then injected and all selected ion transitions were monitored.
2.4.4 Stability
The stability of nutlin-3a in murine plasma was assessed at low (16 ng/mL) and high (16,000 ng/ml) concentrations in duplicate at room temperature (25°C) and at 4°C. Blank murine plasma was spiked with the indicated concentrations of nutlin-3a and left unextracted at room temperature or 4°C for 0, 4, 6, and 24 h. Samples were extracted after ketoconazole was added in at each scheduled time, and then nutlin-3a was quantitated. Long term stability of nutlin-3a in murine plasma at −80°C was assessed. Unextracted spiked murine plasma (16 ng/mL and 16,000 ng/mL) was frozen at −80°C for 0, 7, 14, 21, 30, 40, 60, and 90 days. Samples were thawed, immediately extracted after adding ketoconazole, and quantitated. Freeze-thaw stability was assessed by freezing unextracted spiked murine plasma (16 ng/mL and 16,000 ng/mL) at −80°C overnight, thawing at 25°C, and extracting an aliquot before refreezing, for a total of 3 freeze-thaw cycles. Samples were analyzed in duplicate.
2.4.6 Comparison of protein precipitation and liquid-liquid extraction
Six plasma samples of various concentrations were extracted using PP, and also diluted 100-fold and extracted with LLE. The extraction methods were considered compatible if the mean concentration using LLE was within 10% of the mean concentration using PP for all samples and the %CV was ≤10%. Furthermore, linear regression was performed on data from duplicate calibrator curves made with PP and LLE. The two methods were considered to be compatible if the slope of the line from LLE was within the 95% confidence interval of the slope using PP.
2.5. Application of method to pre-clinical pharmacokinetic samples
Nutlin-3a (100 mg/kg) was orally administered to male C57/BL6 mice. At 0.5, 1, 2, 4, 8, 12, and 24 h, blood samples (1 mL) were collected in tubes containing EDTA (Ricca Chemical Company, Arlington, TX) and immediately centrifuged at 10,000 rpm for 3 min to separate the plasma. The plasma was removed and stored at −80°C until analysis. Samples were extracted and quantitated using the described method. A one-compartment pharmacokinetic model was fit to the plasma concentration-time data using non-linear regression.
3. Results and discussion
3.1. Chromatography
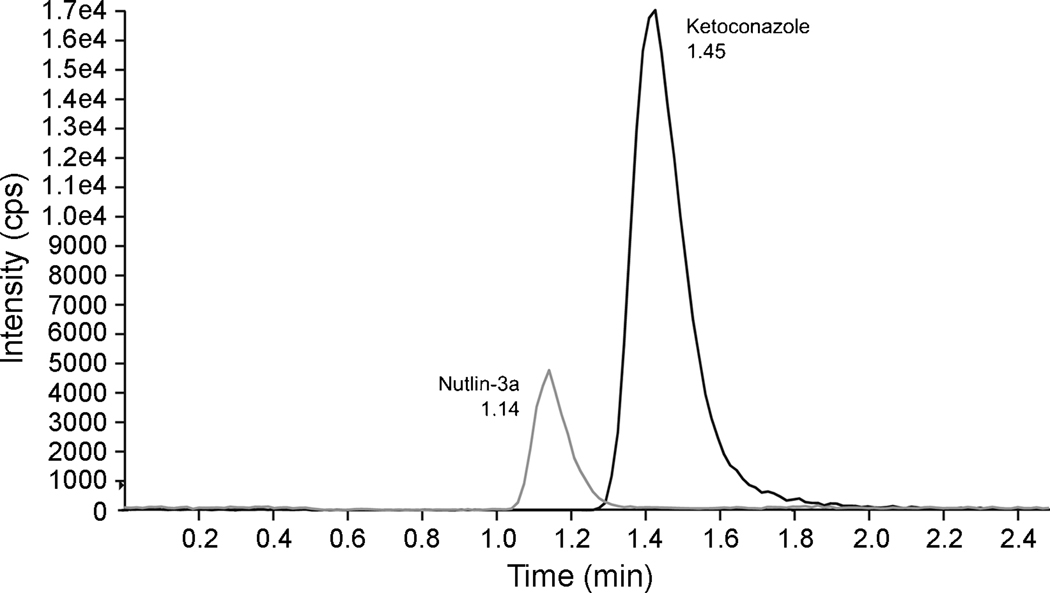
A Synergi 4µ Hydro-RP (75×2 mm, 80A) column was chosen for separation of nutlin-3a and ketoconazole because it allowed a short retention time with at least 95% separation of the peaks. The HPLC conditions were optimized to have the best peak shape and maximal response in terms of counts per second (cps) while achieving good separation of nutlin-3a and ketoconazole within 3 minutes. The pH and percent acetonitrileof the mobile phase were found to affect the separation of the two compounds and also the stability of ketoconazole [17,18]. At pH 3, ketoconazole was rapidly degraded, so the pH was adjusted to near neutral at 6.2. Raising the pH increased the retention time of ketoconazole, but did not significantly affect the retention time of nutlin-3a. The best separation was achieved with 70% acetonitrile. The column temperature was set at 25°C with a flow rate at 0.30 mL/min, which resulted in the best signal response and sufficient separation of both compounds within 2.5 min. The retention times of nutlin-3a and ketoconazole were 1.14 min and 1.45 min, respectively (Fig. 1).
Figure 1.
HPLC chromatogram of murine plasma spiked with nutlin-3a (50 ng/mL) and ketoconazole (800 ng/mL) after protein precipitation.
3.2 Mass spectrometry
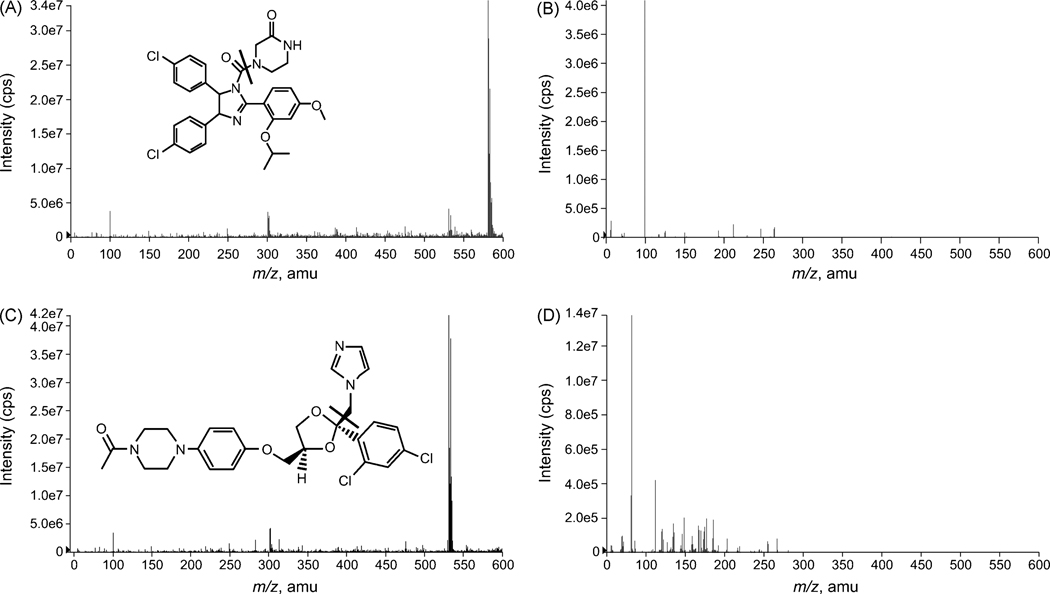
Detection was achieved by an API-3000 LC/MS/MS system at unit resolution for both MS1 and MS2 in positive multiple reaction monitoring (MRM) mode. All parameters related to MS detection were optimized to achieve the best signal response of each selected ion, particularly the nutlin-3a ions. The full-scan MS1 mass spectra, obtained by infusion of nutlin-3a in mobile phase, showed the protonated molecule [M+H]+ for nutlin-3a at m/z 582 (Fig. 2A). The [M+H]+ ion was used as the precursor for MRM detection and the product ion full-scan MS2 spectra showed one major fragment ion at m/z 99 (Fig. 2B). As previously reported [17], the full-scan MS1 spectra of ketoconazole showed the protonated [M]+ ion of ketoconazole at m/z 531 (Fig. 2C). The product ion full-scan MS2 spectra showed one major fragment ion at m/z 82, which was used for detection (Fig. 2D).
Figure 2.
Positive-ion full-scan MS1 spectra for A) nutlin-3a, and C) ketoconazole, and fragment ion MS2 spectra of B) the precursor [M+H]+ ion of nutlin-3a, and D) the precursor [M]+ ion of ketoconazole.
3.3. Precision, accuracy and recovery
To assess within-day and between-day precision and accuracy, we evaluated validation parameters for nutlin-3a (Table 1). Variability was reported as relative standard deviation (%RSD) and accuracy was expressed as percentage error (%Error) in Table 1. Within-day and between-day precision for PP was ≤4.5% and accuracies ranged from 92.1 to 104.8%. For LLE, precisions were ≤4.9% and accuracies ranged from 91.6 to 99.8%. The average recovery of plasma nutlin-3a following PP was 23.5% and following LLE was 77.6%. Percent recoveries were similar in low, medium, and high concentration samples.
Table 1.
Validation parameters of nutlin-3a in murine plasma
| Nutlin-3a | Within-day (n=10) | Between-day (n=5) | |||
|---|---|---|---|---|---|
| (ng/mL) | %RSD | %Error | %RSD | %Error | |
| Protein precipitation | 16 | 4.5 | 4.8 | 2.3 | 0.8 |
| 16,000 | 3.7 | −5.9 | 1.8 | −7.9 | |
| Liquid-liquid extraction | 0.4 | 4.9 | −0.2 | 4.9 | −1.2 |
| 200 | 1.4 | −7.9 | 1.2 | −8.4 | |
3.4 Linearity and lower limit of quantitation
The nutlin-3a plasma calibration curves were linear from 10 to 20,000 ng/mL for the PP (Y = 0.0033 [s.e. 0.0012] + X * 1.830 [s.e. 0.036]) and from 0.25 to 300 ng/mL for the LLE (Y = 0.0143 [s.e. 0.0013] + X * 1.820 [s.e. 0.045]), with correlation coefficients (R2) greater than 0.996 for both extraction methods. The LLOQ for nutlin-3a after PP was 10 ng/mL (S/N = 11, %CV = 3.6, n = 5) and the LLOQ for nutlin-3a after LLE was 0.25 ng/mL (S/N = 9, %CV = 3.1, n = 5), both of which were used as the lowest calibrator in the curve.
3.5. Selectivity, ion suppression, and carryover
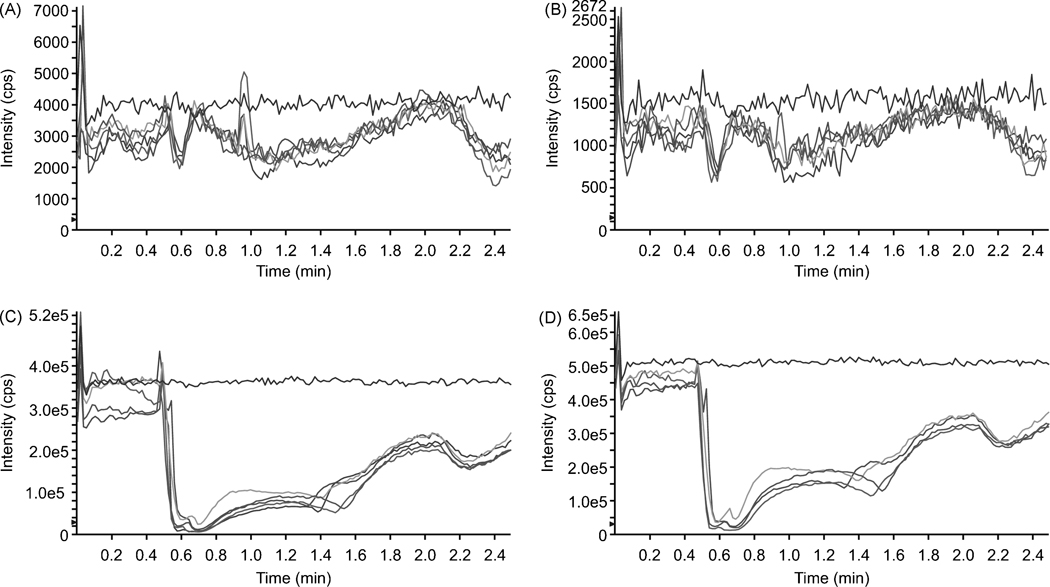
Selectivity of the assay was assessed by monitoring all selected ion transitions using blank murine plasma from four different sources. No co-eluting peak at or around the retention time of either nutlin-3a or ketoconazole were observed. No carryover was seen through four sequential blank plasma samples after injection of nutlin-3a/ketoconazole at 20/0.8 µg/mL. The ion suppression study showed that following LLE, murine plasma caused ion suppression of both nutlin-3a (Fig. 3A) and ketoconazole (Fig. 3B) at their retention times. After PP, there was also significant ion suppression of nutlin-3a (Fig. 3C) and ketoconazole (Fig. 3D) at their retention times. However, the amount of ion suppression was consistent in all plasma sources, indicating that it does not significantly contribute to variability in the response of nutlin-3a in mouse plasma after either PP or LLE preparation.
Figure 3.
Ion suppression of nutlin-3a (A, C) and ketoconazole (B, D). A neat mixture of nutlin-3a (2000 ng/mL for PP and 20 ng/mL for LLE) and ketoconazole (800 ng/mL for PP and 8 ng/mL for LLE) was infused, post-column at a rate of 5.0 µL/min into the LC effluent while mobile phase, commercially-available pooled murine plasma, and murine plasma from 4 different mice were injected into the column after LLE (A,B) or PP (C, D). The top line in each panel is from the injection of mobile phase.
3.6. Stability
At 25°C, spiked murine plasma samples were stable for up to 6 h (<6% change in concentration), but were degraded by 24 h (Table 2a). Similarly, samples at 4°C were stable for 6 h (<1.5% change in concentration), but were degraded by >10% after 24 h (Table 2a). Nutlin-3a plasma samples were stable (<9% change) for at least 90 days at −80°C (Table 2b) and through three freeze-thaw cycles (<3% change; Table 2c).
Table 2.
| Table 2a. Stability of nutlin-3a in murine plasma at 25°C and 4°C. | ||||
|---|---|---|---|---|
| Nutlin-3a | % difference from control |
|||
| (ng/mL) | 4 h | 6 h | 24 h | |
| 25°C | 16 | 1.75 | −5.85 | −23.2 |
| 16,000 | −2.06 | −4.47 | −11.7 | |
| 4°C | 16 | −1.49 | −0.6 | −22.9 |
| 16,000 | −10.31 | −10.65 | −1.4 | |
| Table 2b. Stability of nutlin-3a in murine plasma at −80°C. | ||||||
|---|---|---|---|---|---|---|
| Nutlin-3a | % difference from control |
|||||
| (ng/mL) | 7 day | 14 day | 21 day | 30 day | 40 day | 90 day |
| 16 | 0.62 | 1.23 | −0.6 | 6.83 | −0.93 | 4.32 |
| 16,000 | 0.35 | 0.35 | 0.3 | 7.96 | 8.97 | 1.03 |
| Table 2c. Stability of nutlin-3a in murine plasma through 3 freeze-thaw cycles. | |||
|---|---|---|---|
| Nutlin-3a | % difference from control |
||
| (ng/mL) | 1st cycle | 2nd cycle | 3rd cycle |
| 16 | 0.61 | −2.43 | −1.2 |
| 16,000 | −0.75 | 2.61 | 1.1 |
3.7. Comparison of extraction methods
The mean difference of the six samples extracted with PP and LLE was −2.8% with a %CV of 8.3%. The slope of calibrator curve made with LLE was 1.82, which was within the 95% confidence interval of the slope of the calibrator curve made with PP (1.75 to 1.91), indicating that low-concentration samples quantitated after LLE extraction may be directly compared to high-concentration samples quantitated after PP.
3.8. Application of method to pre-clinical pharmacokinetic samples
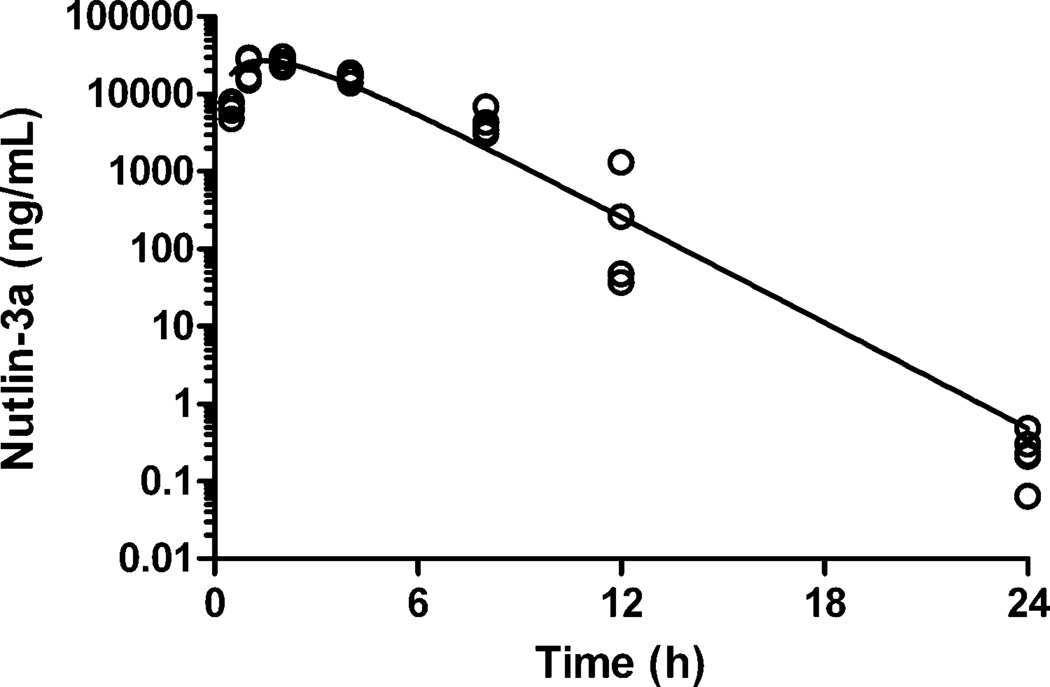
Plasma samples from mice receiving oral nutlin-3a (100 mg/kg) were analyzed to demonstrate the applicability of the method. A representative plasma concentration-time profile for nutlin-3a after oral administration is depicted in Figure 4. Early time points (up to 12 h) were extracted using PP and the 24 h time point was extracted using LLE.
Figure 4.
Nutlin-3a plasma concentration-time profile of mice administered oral nutlin-3a (100 mg/kg). Individual plasma nutlin-3a concentrations from 4 mice per time-point are plotted along with the solid line representing the best-fit curve from the pharmacokinetic analysis.
4. Conclusion
We report here a rapid and sensitive LC-MS/MS method for quantitation of nutlin-3a in murine plasma samples. This analytical method was developed for pre-clinical pharmacokinetic studies of nutlin-3a, and it shows sufficient precision and accuracy for application in these studies. The rapid separation within 2.5 min allows for a short assay time. The two different extraction methods allow a wide range of plasma nutlin-3a concentrations to be quantitated. The two extraction methods show equivalent results, allowing both to be used within the same study. We have successfully applied this LC-MS/MS method by measuring nutlin-3a concentrations in murine plasma for a pharmacokinetic study of oral nutlin-3a.
Acknowledgements
This research was supported by NIH Awards PO1 CA 23099, Cancer Center Support (CORE) Grant CA 21765, and ALSAC. We also thank Julie Groff for her assistance with the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, Hovig E, Smith-Sorensen B, Montesano R, Harris CC. Nucleic Acids Res. 1994;22:3551. [PMC free article] [PubMed] [Google Scholar]
- 2.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. Oncogene. 2007;26:2157. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 3.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. Cell. 1992;69:1237. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DA, Wu L, Levine AJ. Cell Mol Life Sci. 1999;55:96. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bond GL, Hu W, Levine AJ. Curr Cancer Drug Targets. 2005;5:3. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 6.Giglio S, Mancini F, Gentiletti F, Sparaco G, Felicioni L, Barassi F, Martella C, Prodosmo A, Iacovelli S, Buttitta F, Farsetti A, Soddu S, Marchetti A, Sacchi A, Pontecorvi A, Moretti F. Cancer Res. 2005;65:9687. doi: 10.1158/0008-5472.CAN-05-0450. [DOI] [PubMed] [Google Scholar]
- 7.Shangary S, Wang S. Annu Rev Pharmacol Toxicol. 2009;49:223. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurie NA, Shih CS, Dyer MA. Curr Cancer Drug Targets. 2007;7:689. doi: 10.2174/156800907782418266. [DOI] [PubMed] [Google Scholar]
- 9.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. Science. 2004;303:844. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 10.Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 11.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Proc Natl Acad Sci U S A. 2006;103:1888. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Cancer Cell. 2006;10:501. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Gu L, Zhu N, Findley HW, Zhou M. Leukemia. 2008;22:730. doi: 10.1038/leu.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, Shohet JM. Mol Cancer Ther. 2006;5:2358. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]
- 15.Elison JR, Cobrinik D, Claros N, Abramson DH, Lee TC. Arch Ophthalmol. 2006;124:1269. doi: 10.1001/archopht.124.9.1269. [DOI] [PubMed] [Google Scholar]
- 16.Annesley TM. Clinical Chemistry. 2003;49:1041. doi: 10.1373/49.7.1041. [DOI] [PubMed] [Google Scholar]
- 17.Chen YL, Felder L, Jiang X, Naidong W. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:67. doi: 10.1016/s1570-0232(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 18.Skiba M, Skiba-Lahiani M, Marchais H, Duclos R, Arnaud P. Int J Pharm. 2000;198:1. doi: 10.1016/s0378-5173(99)00279-3. [DOI] [PubMed] [Google Scholar]