Abstract
Oxidative stress and aggregation of the presynaptic protein α-synuclein (α-Syn) are implied in the pathogenesis of Parkinson disease and several other neurodegenerative diseases. Various posttranslational modifications, such as oxidation, nitration and truncation, have significant effects on the kinetics of α-Syn fibrillation in vitro. α-Syn is a typical natively unfolded protein, which possesses some residual structure. The existence of long-range intra-molecular interactions between the C-terminal tail (residues 120–140) and the central part of α-Syn (residues 30–100) was recently established (Bertoncini et al. (2005) Proc Natl Acad Sci U S A 102, 1430–1435). Since α-Syn has four methionines, two of which (Met 1 and 5) are at the N-terminus and the other two (Met 116, 127) are in the hydrophobic cluster at the C-terminus of protein, the perturbation of these residues via their oxidation represents a good model for studying the effect of long-range interaction on α-Syn fibril formation. In this paper we show that Met 1, 116, and 127 are more protected from the oxidation than Met 5 likely due to the residual structure in the natively unfolded α-Syn. In addition to the hydrophobic interactions between the C-terminal hydrophobic cluster and hydrophobic central region of α-Syn, there are some long-range electrostatic interactions in this protein. Both of these interactions likely serve as auto-inhibitors of α-Syn fibrillation. Methionine oxidation affects both electrostatic and hydrophobic long-range interactions in α-Syn. Finally, oxidation of methionines by H2O2 greatly inhibited α-Syn fibrillation in vitro, leading to the formation of relatively stable oligomers, which are not toxic to dopaminergic and GABAergic neurons.
1. Introduction
α-Synuclein (α-Syn), a 140-residue protein, is the major fibrillar component of Lewy bodies (LBs) [1] in Parkinson’s Disease (PD) and several other neurodegenerative diseases. Purified α-Syn lacks ordered tertiary and secondary structure [2–5]; i.e., belongs to the family of intrinsically disordered (or natively unfolded) proteins (reviewed in [6–11]). In vitro, natively unfolded α-Syn easily forms amyloid-like fibrils, which are rich in cross-β sheet and have morphology similar to filamentous deposits isolated from LBs [12, 13]. The core region of α-Syn fibrils comprises the central part of α-Syn (residues 30–100) that contains non-amyloid component (NAC) [14–16]. Mature α-Syn amyloid fibrils and/or intermediates (oligomers or proto-fibrils) transiently populated during fibrillation have been suggested to be toxic to neuron cells[17 –22].
Despite being termed natively unfolded, α-Syn is not a completely structure-less random coil. Small angle X-ray scattering analysis showed that the gyration radius of native α-Syn is ~40Å, which is much larger than a value of folded globular protein of similar size (15Å), but is noticeably smaller than the radius of gyration of a fully unfolded random coil with a similar molecular mass (52Å) [3]. Molecular dynamics simulations also showed that native state of α-Syn is composed of an ensemble of structures that, on average, more compact than a random coil [23]. Recently, NMR studies revealed the existence of long-range intra-molecular interactions between the C-terminal tail (residues 120–140) and the central part of α-Syn (residues 30–100) [15]. These long-range interactions were proposed to serve as an auto-inhibitor of the α-Syn fibrillation since the release of them effectively promoted the native α-Syn aggregation. The C-terminus of α-Syn (residues 120–140) is very acidic and negatively charged (− 8 net charge: 8 negative charges, no positive charges), whereas the central region (residues 30–100) is slightly positively charged (+3 net charge: 9 postive and 6 negative charges). The electrostatic attraction between these two regions might, at least in part, be responsible for the long-range interactions in α-Syn. This hypothesis is supported by accelerated fibrillation of α-Syn induced by polyamine that binds and neutralize the negative charges of C-terminus [15, 24]. Furthermore, a hydrophobic cluster was found at the C-terminus of of α-Syn. This cluster was shown to be stabilized by the long-range interactions too [15].
The familial mutations [25–29] and various posttranslational modifications of α-Syn, such as oxidation, nitration and truncation, have significant effects on the kinetics of its fibrillation [30–33], suggesting that the fibrillation of α-Syn can be modulated via altering native structure of this protein. Among all the posttranslational modifications found in α-Syn, its methionine oxidation is of particular interest since higher oxidative stress in dopaminergic neurons has been proposed as one of the potential causes of PD and because of the fact that all four methionines of α-Syn are highly accessible for oxidation. It was shown that methionine oxidation inhibited α-Syn fibrillation and led to the formation of soluble oligomers [33, 34]. However, it remains unclear how methionine oxidation modulates the α-Syn fibrillation and whether the methionine oxidation-induced oligomers are beneficial or toxic.
Interestingly, none of the four methionines (Met 1, 5, 116, and 127) are located in the core region (residues 30–100) that is primarily responsible for α-Syn fibrillation; two of them (Met 1 and 5) are at the N-terminus and the other two (Met 116, 127) are in the hydrophobic cluster at the C-terminus of protein. This provides a good model to study the effect of long-range interaction on α-Syn fibrillation. In this paper we show that: (i) three methionines (Met 1, 116, and 127) are more resistant to oxidation than Met 5, suggesting that Met 1, 116, and 127 are somehow protected by the residual structure of the natively unfolded α-Syn; (ii) methionine oxidation greatly inhibits α-Syn fibrillation in vitro, being accompanied by the formation of soluble oligomers; (iii) the resulting methionine oxidation-induced stable oligomers are not toxic to dopaminergic neurons; (iv) methionine oxidation affects both the electrostatic and hydrophobic long-range interactions of α-Syn which are assumed to serve as an auto-inhibitor of α-Syn fibrillation.
2. Materials and Methods
2.1. Materials
Recombinant wild type (WT) human α-Syn and its two mutants (Y125W; Y125W/133F/136F) were expressed in Escherichia coli and purified similarly as described previously [35]. All chemicals were analytical grade and purchased from Fisher chemicals or EM science.
2.2. Methionine oxidation of α-Syn and its mutants
α-Syn and its mutants were completely oxidized with 300 mM H2O2 in 20mM Tris buffer (pH 7.2) for 15 min at room temperature. Molar equivalent of sodium thiosulfate was added to stop oxidation. Samples were then dialysed against the 10mM Tris buffer (pH 7.2) to remove sodium thiosulfate. Products of methionine oxidation, but not trytophan, oxidized to sulfoxides were confirmed by ESI mass spectrometry. In the case of kinetic studies of methionine oxidation of wild type α-Syn, lower concentration of H2O2 (30 mM) was used to slow down the reaction in order to observe the difference in oxidation rates of four methionines. Oxidation was stopped by 100mM Na2S2O3 and the resulting solutions were digested by tosyl lysine chloromethyl ketone (TLCK) treated chymotrypsin for 24 or 48 hours at room temperature (molar ration of chymotrypsin to α-Syn1:200).
2.3. Fibril formation and ThT assay
Fibril formation was monitored by ThT (thioflavine T) fluorescence using a Fluoroskan Ascent CF plate reader (Labsystems, Inc.). Protein solutions with 70 μM α-Syn and 20 μM ThT were incubated in 100 mM NaCl, 30 mM Tris buffer (pH 7.2) on a 96-well plate, each well containing a 1/8 inch diameter Teflon sphere bead. The plate was incubated at 37°C with shaking at 600 rpm, 2 mm diameter. The fluorescence was measured at 30 min intervals with excitation at 450 nm and emission at 485 nm. Data from six wells were averaged and plotted as a function of time, then fitted to a sigmoidal curve using Sigmaplot software. Lag times were calculated based on the equation described previously [3]. The kinetics of fibrillation are very sensitive to small changes in the rate of agitation. In this paper, wild type α-Syn forms fibrils with a lag phase of ~12 hours when shaking on plate reader.
2.4. Size exclusion chromatography (SEC) HPLC measurements
500 μL of intact or Met-oxidized wild type α-Syn (70μM) was incubated in 100 mM NaCl, 30 mM Tris buffer (pH 7.2) with constant stirring with a mini stir-bar. In this case, intact wild type α-Syn forms fibrils with a lag phase of 20 hours. At different time points, 15 μL of solutions were taken out during incubation and centrifuged for 20 min at 14000 rpm to remove any insoluble materials before running SEC chromatography. HPLC was performed on a TSK-GEL G2000SWXL size-exclusion column (7.8 mm ID × 30 cm) at a flow rate of 0.5 mL/min using a Waters 2695 separations module with a Waters 996 Photodiode Array (PDA) detector, and data were collected and analyzed by Millennium software. Absorbance of the eluant was monitored over the wavelength range from 220 to 450 nm with bandwidth of 1.2 nm. For FPLC (AKTA instrument), spectra were monitored at 280 nm.
2.5. Far-UV circular dichroism (CD) measurements
Far-UV CD spectra were collected with an Aviv 60DS spectrometer at 1-nm intervals, 1.5 nm bandwidth and averaging time of 1.0 second. Measurements were performed in a 0.02cm cell at room temperature over the range 190–250 nm. Five scans were applied continuously and the data were averaged. Protein concentrations used for CD measurements are around 35μM.
2.6. Light scattering measurements
7μM α-Syn in 20mM phosphate buffer (pH 7) was adjusted to the desired pH with hydrochloric acid at room temperature right before static light scattering measurements. Static light scattering measurements were done using a FluoroMax-3 Spectrofluorimeter (Jobin Yvon Horiba) with excitation and emission at 295 nm.
2.7. Mass spectrometry
Samples for MS analysis were desalted with C8 reverse phase column and eluted out with 4 acetonitrile: 1 H2O (pH adjusted to 2.0 by trifluoroacetic acid) solution. The desalted solution was directly loaded onto Mass spectrometer. Mass spectra were obtained using a MicroMass Quattro II electrospray mass spectrometer operating in positive ion mode. Injection was carried out using a syringe pump (Harvard Apparatus, Holliston, MA) at a flow-rate of 20μl/min. The source temperature was set to 80°C and the capillary voltage was 3.25 kV. Protein molecular weight was determined from mass-to-charge ratios by MassLynx software.
2.8. Electron Microscopy Measurements
Samples for electron microscopy (EM) were deposited on the carbon-coated pioloform 300 mesh copper grids and negatively stained with 1% (w/v) aqueous uranyl acetate. EM images were obtained on a JEOL JEM-100B transmission electron microscope operated at 80 kV.
2.9. Atomic Force Microscopy
Atomic force microscopy (AFM) images were collected with a PicoScan LE system (Molecular Imaging, Phoenix, AZ) equipped with Acoustic AC mode (Tapping mode) for ex-situ experiment. Triangular cantilevers with 160 kHz resonance frequency and 2 N/m spring constant, a V-shaped cantilever NSC16/AIBS (MikroMasch), were used in Tapping mode imaging. The imaging was carried out at a scan rate of 1 line/s with 512 data points per line, at a drive current of 10 ± 4 Å.
Aliquots of 10 μL of sample containing 100 mM NaCl were placed on a freshly cleaved mica substrate. After incubation at least 60 min, the substrate was rinsed with water several times to remove salt and loosely bound protein and dried with high-purity Nitrogen.
Heights ranging from 0.1 to 100 nm were estimated by section analysis, and lateral sizes were calibrated with standard calibration grid. At least four regions of the mica surface were examined to verify that similar structures existed through the sample. No filter treatment was used to modify the images. SPIP 4.0 (Image Metrology) was used to analyze the height, area and volume distribution of the α-Syn aggregations.
2.10. Small angle X-ray scattering (SAXS) Measurements
SAXS measurements were performed on Beam line 4-2 at the Stanford Synchrotron Radiation Laboratory (SSRL). The SAXS instrument was configured with a Mo:CB4 multilayer monochromator, an 18 mm beamstop, and a 218 cm sample-to-detector distance. The fresh prepared samples were centrifuged at 10,000 rpm for 15 minutes before being illuminated to the beam. A PTFE flow-cell with 1.3 mm path length was used to minimize radiation damage. Data were collected with the accumulating time of 10 minutes at 25°C. Buffer was subtracted and radii of gyration were calculated using the Guinier approximation: ln (I(Q)) =ln(I(0))−Rg2Q2/3, where Q is the scattering vector (Q= (4πsinθ)/λ), 2θ is the scattering angle and λ is the wavelength of x-ray. Kratky plot was got by plotting I(S)*S2 against S where S equals 2sinθ/λ and I(S) is the averaged scattering intensity.
2.11. Primary rat ventral mesencephalic cultures
Cultures were prepared from the ventral mesencephalon of Sprague-Dawley rats of 14-days gestation. Experimental protocols were in accordance with the NIH guidelines for animal use and were approved by the Institutional Animal Care and Use Committee. Dissected tissues were incubated in calcium-free Hank’s Balanced Salt Solution (HBSS) containing trypsin (0.1%) and DNase (0.5 mg/ml) for 6 min. The trypsinization process was terminated by addition of HBSS containing soybean trypsin inhibitor (0.1 mg/ml) and DNase (0.5 mg/ml). Cells were dissociated by gentle trituration using a fire-polished Pasteur pipette and were then centrifuged at 100g for 10 min. The supernatant was discarded and the cells were resuspended in DMEM:F12 medium supplemented with 10% Fetal Bovine Serum (FBS). Cultures were plated in 96-well plates coated with poly-D-lysine (100mg/ml) at a density of 100,000 cells/well in DMEM:F12 containing 10% FBS, penicillin and streptomycin. Cells were exposed to α-Syn oligomers starting on day 5 (d5) or day 6 (d6) in culture for 24 to 48 hrs. α-Syn species were diluted in DMEM/F12 to 11X the needed concentration. 10 μl of this concentrated stock was added to 100 μl of existing feeding media in wells to obtain the final desired concentration.
2.12. [3H] dopamine and [3H] GABA uptake
For assay of dopamine uptake, cultures were rinsed with DMEM:F12 containing 1mM ascorbic acid and incubated for 30 min at 37 C with the same buffer containing 1μCi/ml [3H]dopamine. Non-specific binding was determined in cultures treated with the dopamine uptake inhibitor mazindol (10μM). For GABA uptake, cultures were preincubated with DMEM:F12 containing 500μM β-alanine and 10uM amino oxyacetic acid to block high affinity glial uptake and enzymatic degradation of GABA, respectively. 1μCi/ml [3H]GABA in medium was added to cells and incubated for 30 min at 37 C. After rinsing with phosphate-buffered saline (PBS, pH 7.4), the radioactivity was extracted with Optiphase SuperMix Cocktail and measured using a Liquid Scintillation Counter.
3. RESULTS
3.1. Methionine oxidation of α-Syn and its mutants
Wild type α-Syn (WT α-Syn) has four methionine residues (Met 1, 5, 116 and 127), four tyrosine residues (Tyr 39, 125, 133 and 136) and has no tryptophan and cysteine residues. To probe the effects of the C-terminal hydrophobic cluster on the α-Syn structure and aggregation propensity, a single Tyr125-Trp mutant (Y125W) and a triple Ty125r-Trp/Tyr133-Phe/Tyr136-Phe mutant (Y125W/133F/136F) were analyzed under variety of conditions. Tryptophan is less sensitive to H2O2 than methionine. Under conditions we used in this study, all four methionines of α-Syn were readily oxidized to methionine sulfoxides by 30 mM (for partial oxidation) or 300 mM (for full oxidation) H2O2 whereas tryptophan in α-Syn mutants remained intact. Oxidation of four methionines to methionine sulfoxides resulted in an increase of mass of α-Syn by 64 (four oxygen atoms were added on α-Syn). This was confirmed by mass spectroscopy (data not shown).
3.2. The oxidation rates of four methionine residues are different supporting the existence of a hydrophobic cluster at the α-Syn C-terminus
The C-terminus of α-Syn is highly disordered in many known conformations of α-Syn (natively unfolded monomer, fibril and complex with lipids). However, based on the relative resistance to the extensive proteolytic degradation, the unexpected rigidity was reported for this part of α-Syn in the SDS micelle-bound form, likely due to the calcium binding to the negatively charged C-terminal region [36]. In fact, it has been shown that binding of calcium to α-Syn occurs with an IC50 of ~300 μM Ca2+ [37]. The interaction of α-Syn with metal ions induces shielding of the negatively charged residues and affects several properties of this protein, such as its solubility [38], its aggregation kinetics and fibril formation [38, 39], and the morphology of the fibrils [40]. In particular, the charge shielding effect induced by calcium binding promotes the aggregation of α-syn, in analogy to the removal of the C-terminal tail from the protein [30, 41].
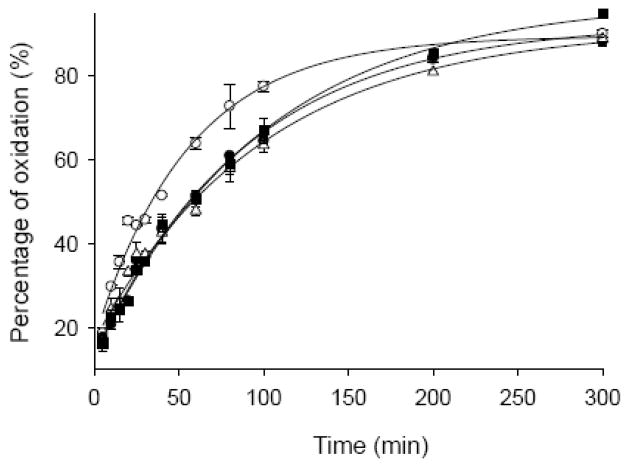
Recently, a hydrophobic cluster involving residues 115–119 (Met116, Val118) and 125–129 (Tyr125, Met127) was identified in the C-terminal region of α-Syn [15]. We hypothesized that the C-terminal methionines Met 116 and 127 should be protected by this hydrophobic cluster and thus they should be more resistant to H2O2 oxidation. To check this hypothesis, α-Syn (70 μM) was oxidized by 30 mM H2O2. At different time points, aliquots of reaction mixture were taken out and excess Na2S2O3 was added to stop the oxidation process. The resulting α-Syn samples were digested by chymotrypsin. This treatment allowed the independent analysis of four Met residues since they fall into four different fragments after digestion (Table 1). The ratios of oxidized to non-oxidized fragment in a given α-Syn sample obtained after the exposure to H2O2 for a definite amount of time reflect the susceptibility of the corresponding Met residue to oxidation. Results of this analysis are illustrated by Fig. 1. Our data clearly showed that Met 5 was more susceptible to H2O2 oxidation in comparison with three other Met residues (Fig. 1). This supported the presence of a hydrophobic cluster at the C-terminus. Interestingly, our data also indicated that the N-terminal Met 1 was partially protected too, probably by some long-range interactions within the α-Syn molecule.
Table 1.
Masses of unoxidized and oxidized α-Syn fragments after chymotrypsin digestion
| Fragments of α-Syn after digestion by chymotrypsin | Calculated | Expected unoxidized m/z | Expected oxidized m/z | Observed unoxidized m/z | Observed oxidized m/z |
|---|---|---|---|---|---|
| MDVF (1–4) | 510.6 | 510.6 | 510.6+16 = 526.6 | 511.1 ± 0.1 | 527.2 ± 0.1 |
| MKGL (5–8) | 447.6 | 447.6 | 447.6+16=463.6 | 448.1 ± 0.1 | 4643 ± 0.1 |
| VKKDQLGKNEEGAPQEGILED MPVDP DNEAY (95–125) | 3429.7 | 3429.7/3 = 1143.2 | (3429.7+16)/3 = 1148.6 | 1143.8 ± 0.2 | 1149.5 ± 0.2 |
| EMPSEEGYQDYEPEA (126–140) | 1773.8 | 1773.8/2 = 886.9 | (1773.8+16)/2 = 894.9 | 887.4 ± 0.2 | 895.5 ± 0.2 |
Fig. 1.
Time course oxidation of individual methionines (Met 1 ●; Met 5 ○; Met 116 ■; Met l27 △) of α-Syn (70 μM) by H2O2 (30 μM), the percentage of oxidation on individual methionine was calculated as oxidized/(oxidized + unoxidized) based on the MS data.
3.3. Methionine oxidized α-Syn is more extended and in contrary to the non-oxidized protein forms stable oligomers
Earlier, FTIR and far-UV spectra revealed that the Met-oxidized α-Syn is more disordered than the intact protein [33]. Here we showed that in comparison with the intact protein, the peak corresponding to the Met-oxidized α-Syn was slightly shifted toward left on the SEC HPLC profile, indicating the more extended conformation (Fig. 2). Although Met-oxidized α-Syn did not form fibrils after 60 hours of incubation [33], the protein effectively aggregated into oligomers as shown by EM (Fig. 3A). Furthermore, the appearance of a significant SEC HPLC peak corresponding to the oligomeric species suggested that these Met-oxidized α-Syn oligomers were stable whereas the oligomers formed by the intact α-Syn were unstable (Fig. 2). The presence of oligomers in the incubated samples of intact α-Syn was proven by AFM analysis [42]. However, these oligomers can not be observed by SEC HPLC as they are unstable and dissociate during the chromatographic process due to the significant dilution of a protein solution on a SEC HPLC column.
Fig. 2.
Time course of changes in hydrodynamic properties of intact (A) or oxidized (B) α-Syn (70 μM) incubated at pH 7.4, 37°C with mini-stir-bar agitation as monitored by size exclusion HPLC. Absorbance was monitored at 280 nm. In these experiments, 15 μL of incubated solutions were injected on a TSK-GEL G2000SWXLsize -exclusion column at a flow rate of 0.5 mL/min.
Fig. 3.
EM image (A), an AFM image (B), Guinier (C) and Kratky plot (D) from small angle X-ray scattering analysis of methionine oxidized α-Syn after incubation at 37°C, pH 7.2 with agitation for 60 hours. The scale bars in the EM AFM images are 200 nm. In Guinier plot, Rg was calculated from the slope, which equals −Rg2/3. Two slopes, corresponding to the biggest and smallest oligomers, were indicated in plot. In Kratky plot, the pronounced peak at low angles suggests that the Met-oxidized oligomers are compact and have relatively tightly packed globular core.
Since α-Syn is a natively unfolded protein, whose hydrodynamic dimensions dramatically exceed those of the globular protein of similar size (RS of 31.8 Å instead of 19.1 Å expected for a globular protein with the molecular mass of 14,460 Da), it migrates on the SEC HPLC column as a protein with high apparent molecular mass (~60 kDa). Therefore, the calibration of SEC column using a set of globular proteins with known molecular masses would not provide any adequate information on the molecular masses of various species formed during the α-Syn aggregation. However, in our experiments SEC was used only to show that the soluble oligomers transiently populated during the fibrillation of non-modified α-Syn were not stable since they completely dissociated during the SEC experiments. On the other hand, the oxidation of methionines in α-Syn noticeably increases the stability of these oligomers.
Small angle X-ray scattering (SAXS) is a very useful method for the investigation of conformation, shape and dimensions of biopolymers in solution. Analysis of the scattering curves using the Guinier approximation provides the radius of gyration, Rg. Scattering data in the form of the Kratky plots provides information about the globularity (packing density) and conformation of a protein [43]. SAXS analysis of the Met-oxidized α-Syn showed the presence of relatively compact oligomers, with Rg ranging from 8 to 11.6 nm, formed during the incubation of Met-oxidized α-Syn (Fig. 3C). The Rg was calculated from the slope of the Guinier plot, which is proportional to −Rg2/3. The non-linearity of the Guinier plot reflected the non-homogeneity of the studied samples which likely contained several oligomers with different hydrodynamic properties. The non-linear Guinier plot, being fit by several successive linear dependencies, can provide information on the largest and smallest members of this heterogeneous pool of species. This analysis of the Guinier plot gave the highest Rg of 11.6 nm (from a line with highest slope at the beginning of the Guinier plot) and the lowest Rg of 8 nm (from the line with the lowest slope at the end of the plot) (Fig. 3B). It is important to emphasize that all the oligomers possessed hydrodynamic dimensions noticeably larger than those of a “natively unfolded” α-Syn monomer, which had Rg around 4 nm[3].
The compactness and relatively tight packing of Met-oxidized α-Syn oligomers was further confirmed by the analysis of SAXS data in the form of the Kratky plot. For a native globular protein this plot has a characteristic maximum, whereas unfolded and partially folded polypeptides have significantly different-shaped Kratky plots. The analysis of the X-ray scattering data in the form of the Kratky plot shows that the intact α-Syn does not have a well-developed globular structure in the aqueous solution [3, 44]. However, the Kratky plot profile for the Met-oxidized protein shows very distinctive features. In particular, a characteristic maximum at low angles is observed, which is indicative of globular structure in the oligomer(s) (Fig. 3D). Analogous behavior has been earlier reported for the associated forms of partially folded intermediates of staphylococcal nuclease [45, 46], for the TMAO-induced oligomers of α-Syn [47] and for the γ-synuclein oligomers [44]. Therefore, SAXS data taken together suggested that the Met-oxidized α-Syn represented a heterogeneous mixture of relatively compact oligomers with some globular structure. It is important to emphasize here that the mentioned SAXS data are in a great agreement with the results of SEC and EM analyses. In fact, Fig. 2B shows that Met-oxidized α-Syn incubated for 60 hours (a time at which SAXS data were taken) eluted from as SEC HPLC column as a peak corresponding to the oligomeric species. Fig. 3A shows that Met-oxidized α-Syn did not form fibrils after 60 hours of incubation effectively forming small oligomers. This fact is further confirmed by Fig. 3B, which represents an AFM image of Met-oxidized α-Syn sample incubated for 60 hours and clearly shows the lack of amyloid fibrils in the solution.
3.4. The intra-molecular long-range charge-charge interactions dominate the auto-inhibition of α-Syn fibrillation and methionine oxidation strengthens these auto-inhibitory effects
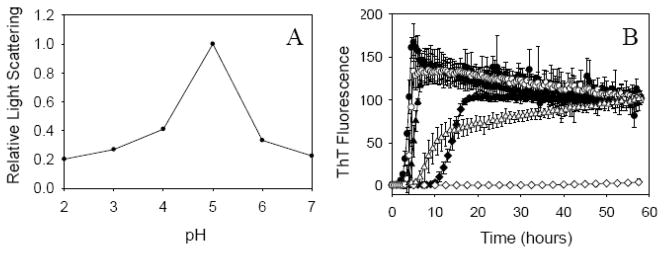
Protein fibrillation consists of two major steps: self-association of protein molecules followed by the specific inter-molecular interactions that favor formation of fibrils. It is known that static light scattering from protein solutions reflects the formation of large particles, i.e., this is an association-dependent parameter. In contrast to ThT, the increase in static light scattering reflects the formation of all large particles, such as large soluble oligomers, amorphous aggregates and amyloid-like fibrils. In order to compare the aggregation state of fresh, non-incubated, α-Syn under the variety of conditions, pH dependence of static light scattering was analyzed. Since the major goal of these experiments was to evaluate how does the intrinsic propensity of α-Syn to oligomerize and aggregate change at different pH, relatively low protein concentrations were used. As expected, fresh, non-incubated α-Syn possessed the highest propensity to aggregate around pH 5.0 (close to its pI) where the protein net charge was close to zero and therefore the intermolecular electrostatic repulsion was minimal. This is illustrated by Fig. 4A which clearly shows that the fresh protein is more prone to aggregate at pH 5.0 than at the neutral or acidic pH. Assuming that self-association is crucial for fibrillation, the fastest rate of α-Syn fibril formation was expected at its pI 4.67. However, the analysis of α-Syn fibrillation at different pH (pH 3.0, 5.0, and 7.2) revealed that although protein was able to form amyloid-like fibrils at all pH studied the fastest fibrillation rate was achieved at pH 3, rather than at pH 5.0, where α-Syn has the highest intrinsic propensity to oligomerize/aggregate (Fig. 4B) (see also [3]). Since the negatively charged C-terminus of α-Syn (residue 102–140) has a pI of 3, this observation suggested that the neutralization of C-terminal charges represented the rate-limiting step in the α-Syn fibrillation. Such mechanism likely complements the acidic pH-induced formation of partially folded intermediate which is know to dramatically accelerate the process of α-Syn fibrillation [3]. This hypothesis is supported by the fact that the truncated α-Syn (1–108) with removed C-terminus fibrillates much faster than the intact full-length α-Syn [30]. The faster fibrillation was also reported for the calcium-loaded form of the full-length α-Syn, where calcium binding promotes the aggregation of α-syn via the charge shielding effects [39, 40].
Fig. 4.
(A) Static light scattering of intact wild type α-synuclein (7 μM) at different pH (λex = 295 nm; λem = 295 nm); (B) ThT assay of fibrillation of 70 μM intact and oxidized wild type α-Syn at different pH (3, 5 and 7.2). Symbols are as follows: intact α-synuclein at pH3 (●), pH5 (▲) and pH7.2 (◆); oxidized α-synuclein pH3 (○), pH5 (△) and pH7.2 (◇). ThT fluorescence intensities were normalized. The decrease after a max of ThT signal at pH 3 might reflect ThT fluorescence quenching due to the association of fibrils.
In other words, there are several mechanisms by which the C-terminus can slow down the α-Syn fibrillation at neutral pH. These mechanisms include: (i) the simple inhibition of the α-Syn association due to the inter-tail charge-charge repulsion; (ii) the existence of intra-molecular long-range hydrophobic interactions between the C-terminus (residues 120–140) and the central region (residues 30–100) of α-Syn [15]; (iii) the existence of the long-range intra-molecule electrostatic interactions between the negatively charged C-terminal region (residues 120–140, which has a net charge of −8, containing 8 negative charges and no positive charges) and the positively charged central region (residues 30–100), which has a net charge of +3 containing 9 positive and 6 negative charges, thus preventing α-Syn from changing its structure from natively unfolded state to the partially folded intermediate, which is required for fibril formation.
Our analysis revealed that although the methionine oxidation inhibited α-Syn fibrillation, these inhibitory effects were released at low pH which completely restored the ability of the oxidized α-Syn to fibrillate (Fig. 4B). In fact, Fig. 4B shows that although no fibrils were formed at neutral pH, the acidification of solution changed this situation dramatically. At pH 5.0, Met-oxidized α-Syn was able to form fibrils (although slower than the intact protein). At pH3.0, the fibrillation kinetics of the intact and oxidized WT α-Syn were rather similar since both forms possessed rather similar lag0times and the elongation rates (see Fig. 4B). These observations suggested that the methionine oxidation might inhibit fibrillation through strengthening the auto-inhibitory effects of the negative charges at C-terminus of α-Syn which contributes to the increased stability of the Met-oxidized α-Syn oligomers.
3.5. Hydrophobic cluster at C-terminus modulates the α-Syn fibrillation
Earlier, residual dipolar coupling (RDC) analysis of the NMR data revealed that there is a hydrophobic cluster involving residues 115–119 and 125–129 at the C-terminus of α-Syn (note: Met 116 and 127 are located in this cluster) [15]. This cluster was stabilized by the long-range interaction since an α-Syn C-terminal peptide (105–136) showed lower RDCs compared to the same region in the full-length protein [15]. This suggested that hydrophobic attraction between C-terminus and central region of monomeric α-Syn can contribute to their long-range interactions.
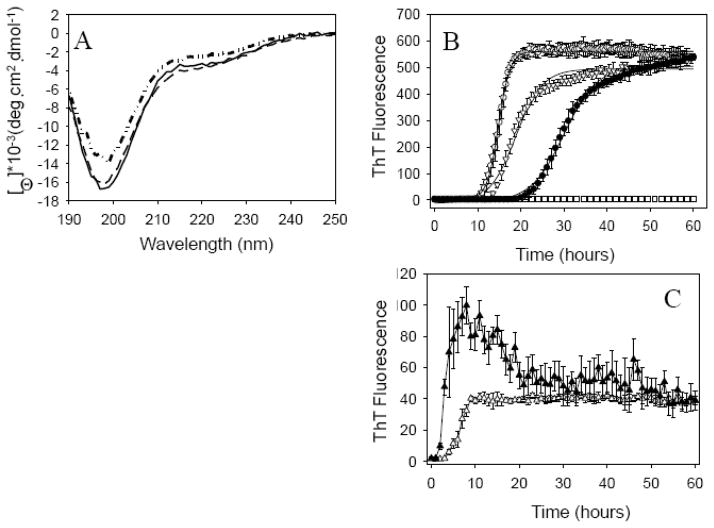
To check this hypothesis, two mutants, Y125W and Y125W/133F/136F, were designed to increase the hydrophobicity of the C-terminal hydrophobic cluster of α-Syn. Since α-Syn has four tyrosines and no tryptophan residues, a tyrosine to tryptophan mutation at position 125 was designed to create an intrinsic fluorescence probe and allow additional characterization of the aggregation process. Fig. 5A shows that at neutral pH, all three proteins possessed almost indistinguishable far-UV CD spectra, which were typical of an essentially unfolded polypeptide chain. In fact, these spectra showed characteristic minima in the vicinity of 196 nm and the absence of bands in the 210–230 nm region.
Fig. 5.
(A) Far-UV circular dichroism spectra of intact wild type α-Syn (solid), Y125W (dash) and Y125W/133F/136F (dash dot); (B) ThT assay of fibrillation of 70 μM wild type α-Syn (○) and its mutants: Y125W (∇) and Y125W/133F/136F (●) at pH 7.2. ThT fluorescence signals were normalized. Solid lines represent fit sigmoidal curves. All Met-oxidized proteins (□) do not form fibrils after incubation over 60 hours. (C) ThT assay of fibrillation of 70 μM intact (▲) and oxidized (△) Y125W/133F/136F at pH 3.0.
Next, fibrillation of WT α-Syn and its mutants were monitored by ThT assay at pH 7.2 (Fig. 5B). Y125W mutation had some inhibitory effect on α-Syn fibrillation, being characterized by a little longer lag-time and a much smaller elongation rate. The Y125W/133F/136F triple mutation significantly inhibited fibrillation of α-Syn showing much longer lag-time and much smaller elongation rate(Table 2). This suggested that the Tyr 125 to Trp mutation might intensify the long-range intra-molecular interactions through the enhanced hydrophobic attraction, thereby strengthening the auto-inhibitory effects of C-terminus on the α-Syn fibrillation. Additional mutations of Tyr 133 and Tyr 136 to Phe in the Y125W/133F/136F mutant further stabilized this hydrophobic cluster, substantially reinforcing the inhibitory effects of the long-range hydrophobic interactions on the fibrillation of α-Syn.
Table 2.
Kinetic Parameters for the Fibrillation of wild type α-Syn and its mutants
| Proteins | Lag-time (hrs) | Elongation Rate (1/h) |
|---|---|---|
| Wild Type | 12.2 ± 0.4 | 0.74 ± 0.01 |
| Y125W | 12.8 ± 1.0 | 0.33 ± 0.02 |
| Y125W/Y133F/Y136F | 22.4 ± 2.0 | 0.26 ± 0.03 |
Similar to WT α-Syn, methionine oxidation on these two α-Syn mutants completely inhibited their fibrillation whereas low pH restored their ability to fibrillate. However, at pH 3 where all the negative C-terminal charges are neutralized, the oxidized Y125W/133F/136F mutant formed fibrils significantly slower than the intact mutant (Fig. 5C). This suggested that Met-oxidation not only strengthened the auto-inhibitory effects of C-terminal negative charges on fibrillation, but also increased the inhibitory effects of the C-terminal hydrophobic cluster.
3.6. Methionine oxidation leads to a decrease in the Trp fluorescence anistropy of α-Syn mutants
Y125W/133F/136F had higher Trp fluorescence anisotropy (0.058, λex = 295 nm and λem = 355 nm) than the Y125W mutant (0.052) (Table 3). The small changes in the Trp fluorescence anisotropy might reflect the changes in the local environments of Trp since all α-Syn mutants retain “natively unfolded” structure (as shown by far-UV CD) and their molecular weights were close. Together with the fact that the Y125 residue is in the hydrophobic cluster at the C-terminus of α-Syn (residues 115–119 and 125–129), the higher Trp fluorescence anisotropy of the Y125W/133F/136F mutant might be attributed to the increased stability of this hydrophobic cluster since the increased stability of this cluster can result in the increased restrictions of the Trp 125 mobility leading to the higher anisotropy.
Table 3.
Tip Anistropy of α-Syn Mutants
| α-Syn Mutants | Trp Anistropy |
|---|---|
| Y125W/133/F/136F | 0.058 ± 0.002 |
| Ox-Y125W/133/F/136F | 0.050 ± 0.002 |
| Y125W | 0.052 ± 0.002 |
| Ox-Y125W | 0.048 ± 0.002 |
For both α-Syn mutants, Met-oxidation resulted in small decreases in the Trp fluorescence anisotropy (Table 3). Oxidation of Met residues, at least oxidation of the two Mets at the C-terminus, might destabilize the hydrophobic cluster at C-terminus (see above) making it more extended, thereby giving the Trp residue at this cluster more freedom to move or rotate (Trp anisotropy decreases). However, this change somehow allows stronger inter-molecular interactions promoting α-Syn oligomerization.
Y125W/133F/136F mutant was also designed to probe the intra-molecular distance between Y39 and W125 residues by fluorescence resonant energy transfer (FRET) due to the overlap between the Tyr emission and the Trp excitation. The 39th (donor) and 125th (acceptor) residues were selected because they were involved in the long-range interaction between the central part (residues 30–100) and C-terminus (residues 120–140) of α-Syn [15]. Shorter distance between them should be reflected on more fluorescence resonance energy transfer between Tyr 39 and Trp 125 residue. FRET efficiency between Tyr and Trp can be measured via analysis of the Trp fluorescence excitation intensity [48, 49]. The Trp excitation spectra of the unmodified and oxidized Y125W/133F/136F mutant were almost the same suggesting that there was no significant distance change between the 39th and the 125th residues induced by the Met oxidation (data not shown). This possibly means that Y39 is not involved in the structural change induced by Met-oxidation or the change is too small to be detected by FRET.
3.7. Methionine oxidized α-Syn oligomers are not toxic
The oligomers formed by methionine oxidation of wild-type α-Syn were tested for cytotoxicity using a primary culture of ventromedial cells from rat brain. In these mixed cultures containing dopaminergic as well as non-dopaminergic (e.g. GABAergic) neurons, loss of neuronal integrity was assessed as a decrease in neurotransmitter (dopamine or GABA) uptake [50]. Monomeric α-Syn showed minimal toxicity in these assays, whereas α-Syn fibrils (unpublished data) and 4-Hydroxy-2-nonenal (HNE) stabilized α-Syn oligomers [51] showed the time- and dose-dependent toxicity. In fact, this earlier study revealed that when cells were incubated for 48 h in the presence of 1 μM HNE-modified oligomers, no significant loss of neuronal integrity was observed. However, at a higher concentration (5 μM), the HNE-stabilized α-Syn oligomers displayed marked neurotoxicity and caused a 95 and 85% loss of dopaminergic and GABAergic neurons, respectively [51]. Contrarily to these earlier observations with the HNE-stabilized α-Syn oligomers, the Met-oxidized α-Syn oligomers showed no toxicity at 5 μM and a relatively little toxicity at 10 μM. Importantly, the mentioned toxicity of 10 μM Met-oxidized α-Syn was not specific for dopaminergic cells based on the lack of the statistically significant difference between the dopamine and GABA uptake (Fig. 6). This suggested that the methionine oxidation was able to shift α-Syn away from the fibrillation pathway toward the formation of the relatively non-harmful oligomers. Therefore, methionine oxidation can be beneficial leading to the measurable decrease in the toxicity of α-Syn aggregates.
Fig. 6.
Neurotransmitter uptake as a surrogate of cell viability for dopaminergic and GABAergic cells exposed for 48hrs to 5μM and 10μM Met-oxidized oligomeric α-Syn. Loss of neuronal integrity was measured as a decrease in dopamine (black bars) or GABA (white bars) uptake. The data represent the means±S.E. of measurements in six separate wells. Note that there is no statistically significant selective toxicity toward dopaminergic neurons exerted by these oligomers.
4. Discussion
α-Syn has been known as a natively unfolded protein. Its detailed native conformations were not well understood. Recently, NMR data showed that long-range intra-molecular interactions existed in native α-Syn [15]. These long-range interactions were between the central region and part of the C-terminus of α-Syn. A hydrophobic cluster at C-terminus, involving residues 115–119 and 125–129, was also found by NMR and this hydrophobic cluster was shown to be involved in the long-range intra-molecular interactions. Increase in the hydrophobicity of this cluster induced by Tyr to Trp and/or by Tyr to Phe mutations partially inhibited fibrillation of α-Syn at neutral pH. Surprisingly, although the methionine oxidation was expected to decrease the hydrophobicity of this cluster (since Met 116 and 127 are located there) and therefore promote fibrillation, no fibrils were found when oxidized mutants were incubated at neutral pH. We propose that methionine oxidation gives more freedom of this cluster to move or rotate, attracting more hydrophobic residues in long-range intra-molecular interaction within an α-Syn molecule or promoting more effective interactions between the α-Syn molecules (likely through the domain swapping mechanism, where the C-terminal hydrophobic cluster of one molecule interacts with the hydrophobic central region of another molecule).
Our analysis also revealed an interesting and unexpected role of the negative charges at the C-terminus. In fact, highly negatively charged C-terminus seems to be able to inhibit fibrillation of α-Syn not only through inter-molecular charge-charge repulsion, but also through the charge attraction (both intra- and inter-molecular). Therefore, two main forces might contribute to the long-range intra-molecular interactions in α-Syn: (1) the hydrophobic interaction between the hydrophobic cluster of C-terminus and the hydrophobic NAC part of α-Syn; (2) electrostatic attraction between negatively charged residues at the C-terminus and positively charged residues at the central region of α-Syn. Both these interaction types might be important in maintaining the native structure of α-Syn, preventing α-Syn changing from natively unfolded to intermediate conformation, which is required for fibrillation.
In this simplified model, the role of C-terminus was analyzed whereas the impact of N-terminus was neglected. Actually, N-terminus turned out to be important for modulating fibrillation of α-Syn too, since the N-terminus truncation accelerated whereas the N-terminus extension inhibited the α-Syn fibrillation [52]. It is important to remember that the N-terminus of α-Syn also contains two methionines, whose oxidation contributes to the inhibition of fibrillation [34]. Therefore, better understanding the peculiarities of the N-terminus conformation is required for revealing the native structure of α-Syn.
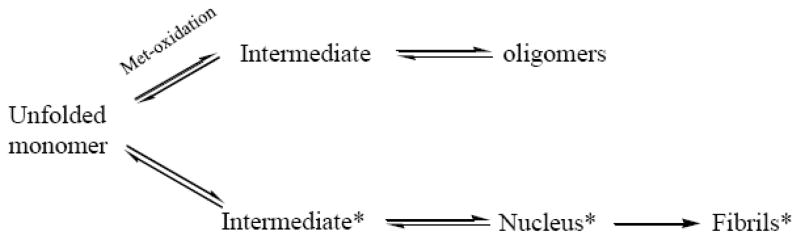
The inhibition of fibrillation did not block the α-Syn oligomerization. Based on the results of SAXS analysis, incubation of Met-oxidized α-Syn led to the appearance of large globular (at least partially) oligomers. These Met-oxidized oligomers are stable in SEC HPLC experiments since the peak corresponding to oligomeric species was observed in the SEC HPLC profiles. On the other hand, the transient oligomers formed at the incubation of intact α-Syn were not HPLC stable since only the chromatographic peak corresponding to monomeric intact α-Syn was observed by SEC-HPLC (Fig. 2). Importantly, these Met-oxidized α-Syn oligomers were non-toxic to the dopaminergic and GABAergic neurons (Fig. 6), whereas α-Syn fibrils (unpublished data) and 4-Hydroxy-2-nonenal stabilized oligomers [51] showed significant toxicity. We propose that there are at least two aggregation pathways for α-Syn: one leads to the fibril formation, during which some neurotoxic species can be produced; the other pathway results in the formation of oligomers that appear to be non-toxic. Normally, the fibrillation pathway dominates since fibrils are much more stable. However, when fibrillation is inhibited (e.g. by methionine oxidation or by some other means), non-toxic oligomerization pathway will dominate (Fig. 7).
Fig. 7.
Two aggregation pathways of α-Syn (* indicates potential toxic species). Methionine oxidation brings α-Syn into a non-toxic oligomerization pathway.
We propose that the long-range intra-molecular interactions in α-Syn are selected for several reasons: firstly, they maintain natively unfolded structure of α-Syn, thereby ensuring the normal function of this protein; secondly, they prevent formation of toxic species, such as oligomers on the fibrillation pathway and protofibrils, by inhibiting fibrillation of α-Syn; thirdly, these long-range interactions being not very strong, ensure the efficient α-Syn fibrillation under some extreme conditions, which can help to remove toxic oligomers and proto-fibrils.
Acknowledgments
This research was supported in part by grants R01 NS39985 (A.L.F.), R01 LM007688-01A1 (V.N.U.) and GM071714-01A2 (V.N.U.) from the National Institutes of Health, and from the Program of the Russian Academy of Sciences «Molecular and Cellular Biology» (V.N.U.). We gratefully acknowledge the support of the IUPUI Signature Centers Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 3.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 4.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 5.Bussell R, Jr, Eliezer D. Residual structure and dynamics in Parkinson’s disease-associated mutants of alpha-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 6.Uversky VN, Fink AL. Biophysical properties of human alpha-synuclein and its role in Parkinson’s disease. In: Pandalai SG, editor. Recent Research Developments in Proteins. Transworld Research Network; Kerala, India: 2002. pp. 153–186. [Google Scholar]
- 7.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 8.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 9.Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 10.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Uversky VN, Eliezer D. Biophysics of Parkinson’s Disease: Structure and Aggregation of alpha-Synuclein. Curr Protein Pept Sci. 2009 doi: 10.2174/138920309789351921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 13.Iwai A, Yoshimoto M, Masliah E, Saitoh T. Non-A beta component of Alzheimer’s disease amyloid (NAC) is amyloidogenic. Biochemistry. 1995;34:10139–10145. doi: 10.1021/bi00032a006. [DOI] [PubMed] [Google Scholar]
- 14.Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277:19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- 15.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Der-Sarkissian A, Jao CC, Chen J, Langen R. Structural organization of alpha-synuclein fibrils studied by site-directed spin labeling. J Biol Chem. 2003;278:37530–37535. doi: 10.1074/jbc.M305266200. [DOI] [PubMed] [Google Scholar]
- 17.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 18.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Schaack J, Zawada WM, Freed CR. Overexpression of human alpha-synuclein causes dopamine neuron death in primary human mesencephalic culture. Brain Res. 2002;926:42–50. doi: 10.1016/s0006-8993(01)03292-9. [DOI] [PubMed] [Google Scholar]
- 20.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 21.van der Putten H, Wiederhold KH, Probst A, Barbieri S, Mistl C, Danner S, Kauffmann S, Hofele K, Spooren WP, Ruegg MA, Lin S, Caroni P, Sommer B, Tolnay M, Bilbe G. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20:6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 23.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez CO, Hoyer W, Zweckstetter M, Jares-Erijman EA, Subramaniam V, Griesinger C, Jovin TM. NMR of alpha-synuclein-polyamine complexes elucidates the mechanism and kinetics of induced aggregation. EMBO J. 2004;23:2039–2046. doi: 10.1038/sj.emboj.7600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann N Y Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 26.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha -synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Uversky VN, Fink AL. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 30.Murray IV, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, Trojanowski JQ, Lee VM. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–8540. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- 31.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 32.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 33.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517:239–244. doi: 10.1016/s0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 34.Hokenson MJ, Uversky VN, Goers J, Yamin G, Munishkina LA, Fink AL. Role of individual methionines in the fibrillation of methionine-oxidized alpha-synuclein. Biochemistry. 2004;43:4621–4633. doi: 10.1021/bi049979h. [DOI] [PubMed] [Google Scholar]
- 35.Kaylor J, Bodner N, Edridge S, Yamin G, Hong DP, Fink AL. Characterization of oligomeric intermediates in alpha-synuclein fibrillation: FRET studies of Y125W/Y133F/Y136F alpha-synuclein. J Mol Biol. 2005;353:357–372. doi: 10.1016/j.jmb.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 36.de Laureto PP, Tosatto L, Frare E, Marin O, Uversky VN, Fontana A. Conformational Properties of the SDS-Bound State of alpha-Synuclein Probed by Limited Proteolysis: Unexpected Rigidity of the Acidic C-Terminal Tail. Biochemistry. 2006;45:11523–11531. doi: 10.1021/bi052614s. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen MS, Vorum H, Lindersson E, Jensen PH. Ca2+ binding to alpha-synuclein regulates ligand binding and oligomerization. J Biol Chem. 2001;276:22680–22684. doi: 10.1074/jbc.M101181200. [DOI] [PubMed] [Google Scholar]
- 38.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer W, Cherny D, Subramaniam V, Jovin TM. Impact of the acidic C-terminal region comprising amino acids 109–140 on alpha-synuclein aggregation in vitro. Biochemistry. 2004;43:16233–16242. doi: 10.1021/bi048453u. [DOI] [PubMed] [Google Scholar]
- 40.Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crowther RA, Jakes R, Spillantini MG, Goedert M. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 1998;436:309–312. doi: 10.1016/s0014-5793(98)01146-6. [DOI] [PubMed] [Google Scholar]
- 42.Zhou W, Gallagher A, Hong DP, Long C, Fink AL, Uversky VN. At low concentrations, 3,4-dihydroxyphenylacetic acid (DOPAC) binds non-covalently to alpha-synuclein and prevents its fibrillation. J Mol Biol. 2009;388:597–610. doi: 10.1016/j.jmb.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glatter O, Kratky O. Small-angle X-ray scattering. Academic Press; London, New York: 1982. [Google Scholar]
- 44.Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta-and gamma -synucleins. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 45.Uversky VN, Karnoup AS, Khurana R, Segel DJ, Doniach S, Fink AL. Association of partially-folded intermediates of staphylococcal nuclease induces structure and stability. Protein Sci. 1999;8:161–173. doi: 10.1110/ps.8.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uversky VN, Karnoup AS, Segel DJ, Seshadri S, Doniach S, Fink AL. Anion-induced folding of Staphylococcal nuclease: characterization of multiple equilibrium partially folded intermediates. J Mol Biol. 1998;278:879–894. doi: 10.1006/jmbi.1998.1741. [DOI] [PubMed] [Google Scholar]
- 47.Uversky VN, Li J, Fink AL. Trimethylamine-N-oxide-induced folding of alpha-synuclein. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 48.Eisenhawer M, Cattarinussi S, Kuhn A, Vogel H. Fluorescence resonance energy transfer shows a close helix-helix distance in the transmembrane M13 procoat protein. Biochemistry. 2001;40:12321–12328. doi: 10.1021/bi0107694. [DOI] [PubMed] [Google Scholar]
- 49.Eisinger J. Intramolecular energy transfer in adrenocorticotropin. Biochemistry. 1969;8:3902–3908. doi: 10.1021/bi00838a004. [DOI] [PubMed] [Google Scholar]
- 50.Driscoll BF, Law MJ, Crane AM. Cell damage associated with changing the medium of mesencephalic cultures in serum-free medium is mediated via N-methyl-D-aspartate receptors. J Neurochem. 1991;56:1201–1206. doi: 10.1111/j.1471-4159.1991.tb11411.x. [DOI] [PubMed] [Google Scholar]
- 51.Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4 -hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282:5862–5870. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- 52.Kessler JC, Rochet JC, Lansbury PT., Jr The N-terminal repeat domain of alpha-synuclein inhibits beta-sheet and amyloid fibril formation. Biochemistry. 2003;42:672–678. doi: 10.1021/bi020429y. [DOI] [PubMed] [Google Scholar]