Abstract
Rural populations living in the northern Ecuadorian Amazon (NEA) experience the highest health burden of any region in the country. Two independent studies of colonist and indigenous groups living in the NEA are used to compare their morbidity and mortality experiences. Colonist data are from a probability sample of land plots in 1999, while indigenous data are from a representative sample of the five largest ethnicities (Quichua, Shuar, Huaorani, Cofan, Secoya) collected in 2001. Poisson regression was used to compare morbidity. Results indicate clear differences in health between populations. Indigenous groups had 30% higher probability of mortality and 63% higher incidence rate of all-cause morbidity compared to colonists. Vector-borne, chronic, gastrointestinal, and diseases of unknown origin were particularly high among indigenous groups. Factors associated with morbidity varied: morbidity rates were similar for the two youngest age groups (0–4 and 5–9), but indigenous people aged 15–39 and 40+ had almost double the morbidity compared to colonists; larger households, later months of data collection and less pollution were associated with less morbidity in both groups; better infrastructure access (electricity and roads) was generally associated with lower morbidity in both groups; and associations of land use were different by group with more cultivation of perennials and fewer annuals associated with less morbidity for colonists, but more for indigenous groups. These results demonstrate the health disparities that exist among indigenous and non-indigenous populations even when living in the same geographic region. Land use itself exemplifies the cultural and contextual differences that are evident in health, since land use decisions are related to broader demographic and economic factors that influence overall ecological and human health. Ongoing population-environment and/or environment-health research needs to recognize the broader factors involved when studying relationships between population health, development and deforestation.
Keywords: Colonists, Indigenous, Morbidity and Mortality, Human-environment dynamics, Ecuador Amazon, land use
INTRODUCTION
There is a growing global concern regarding the fate of the Amazon forest and its impact on climate change, infectious disease transmission, and irrevocable loss of natural resources. The fate of the forests is tied to colonist and indigenous populations that inhabit the Amazon Basin and who have been identified as primary agents of land use change in the tropics (Carr & Bilsborrow, 2001; Gray, Bilsborrow, Bremner, & Lu, 2008; Rudel & Roper, 1996). People living in the Amazon are among the most vulnerable in Latin America and are often constrained in their decisions pertaining to agricultural land use, labor opportunities (particularly for women), fertility regulation, migration, and health care due to factors that include limited access to infrastructure and technical assistance, severe poverty, low education, and environmental conditions (e.g., Angelsen & Kaimowitz, 1999; Carr, Pan, & Bilsborrow, 2006; Coomes, Grimard, & Burt, 2000; Murphy, 2001; Pichón, 1997). At a macro-level, these constraints are often framed within a vicious cycle of poverty, rising population (i.e., high fertility), ill health, and environmental degradation (Carr, 2004; Dasgupta, 1995). At the micro level, consensus on factors associated with household decisions has yet to be attained, and although health outcomes have been directly tied to environmental degradation (Patz, Daszak, Tabor, Aguirre, Pearl, Epstein et al., 2004; Sawyer, 1993; Yasuoka & Levins, 2007), land use and land cover change research has largely ignored health.
Data on the health status of rural populations in developing countries are usually obtained from Demographic and Health Surveys (DHS) or studies focused on individual health outcomes. In the Amazon, DHS data highlight regional or country-level health outcomes, such as malnutrition, reproductive health, and infant mortality (Ali, Cleland, & Shah, 2003; Cleland, Bicego, & Fegan, 1992; Larrea & Freire, 2002; MACRO, 1997; Valdivia, 2004). However, DHS data have been criticized for often not being representative of rural areas and possibly exhibiting reporting bias (Manesh, Sheldon, Pickett, & Carr-Hill, 2008). A number of focused epidemiological studies have been conducted in the Amazon that provide invaluable information pertaining to health burden, such as gastrointestinal illnesses and malnutrition, elevated blood-mercury levels, tuberculosis, and vector-borne and zoonotic diseases (e.g., Akhavan, Musgrove, Abrantes, & Gusmão, 1999; Basta, Coimbra, Escobar, Santos, Alves, & Fonseca, 2006; Carvalho-Costa, Gonçalves, Lassance, Silva Neto, Salmazo, & Bóia, 2007; Passos, Da Silva, Lemire, Fillion, Guimarães, Lucotte et al., 2008). An unfortunate shortcoming of these studies is the (usually) inherent focus on a few key health outcomes due to study design and objectives that exclude the wider array of ailments that affect inhabitants and potentially overstating the impact of a particular disease for a region.
Regardless of whether one is concerned (or not) with potential biases in regional data or focused studies, one easily surmises that health quality is not equally distributed. In Amazon Basin countries, publically available data easily display these disparities at a regional level and across some large municipalities. However, the extent to which regional disparity exists in the Amazon itself is not well defined. Thus, the primary objective for this study is to evaluate disparities in health between two distinct populations living in the northeastern Ecuadorian Amazon (NEA): migrant colonists and indigenous people. We address disparities associated with (1) overall morbidity and mortality and (2) factors associated with morbidity. Data are from two independent studies of anthropogenic land use change that obtained information from colonists living in the Northern Ecuadorian Amazon (NEA) in 1999 and from five indigenous groups in 2001. Surveys administered focused on factors associated with land use decisions, with modules exclusively designed to capture morbidity and mortality of each person living in a household. Established demographic methods of mortality estimation are applied and regression methods are used to compare factors associated with morbidity. The findings from this study highlight important health disparities in tropical regions.
ECUADOR’S AMAZON
The three geographic regions of Ecuador include: the Costa (~5.6 million population) along the Pacific Ocean; the Sierra (~6.3 million) along the central highlands of Ecuador that include the Andes Mountains and Quito, Ecuador’s capital; and the Oriente (~0.6 million) comprised of six provinces in the Amazon Basin and home to a number of Ecuador’s indigenous tribes (i.e. Quichua, Siona, Secoya, Huaorani, Cofán, Shuar, Saparo, Achuar). The Oriente population has increased 10-fold since 1960 (when it was mostly indigenous) to an estimated 2001 census population of about 500,000 with approximately 30% indigenous (INEC, 2001b). In-migration has been rapid since 1967 when oil was discovered near the Colombian border, leading to extensive road construction, laying of oil pipelines, and regional development. Over 90% of these migrants are from outside the Amazon, mostly from rural areas of the Sierra (Bilsborrow, Barbieri, & Pan, 2004; Pichón, 1997; Rudel & Richards, 1990). The convergence of new land settlement and regional development resulted in Ecuador experiencing the highest deforestation rate among all Amazon Basin countries since 1980 (FAO, 2001, 2005, 2007), leading to 282 endemic plant species being critically endangered and almost 50 endemic species already extinct (Pitman, Jørgensen, Williams, León-Yánez, & Valencia, 2002).
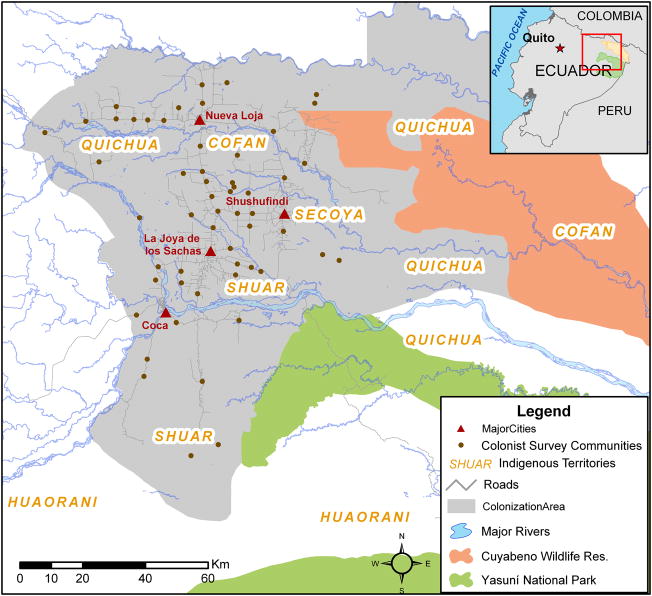
Our study region, the NEA, covers an area greater than 700,000 hectares (ha), including much of the provinces of Sucumbios and Orellana and parts of Napo and Pastaza. The study region is expansive due to the very low population density among both colonist and indigenous settlements throughout the region (Figure 1); however, the area extent is also a key strength as most research on health and/or environment in tropical regions are based on convenience samples of limited geographic size. Colonist settlements are considerably different from indigenous. Colonists occupy government-defined plots (fincas, typically 40–60 ha) that are arranged in lines parallel to roads in settlement “sectors” (i.e., line 1 is on the road, line 2 is ~2 KM from the road, line 3 ~4km, etc.). Colonist farms are relatively new, with most fincas first established during the early 1980s. Almost all colonist families live on their land and have variable access to infrastructure depending on the finca line, sector’s proximity to a town, and road quality. In contrast, most indigenous settlements are arranged in household clusters (communities) with farm land located in surrounding areas and most family members born and raised in the Oriente. Over half of the indigenous communities in the NEA are located in the same general area as colonist settlements, with groups such as the Shuar claiming land in a similar fashion as colonists. These differences led to the design of two separate studies to examine factors associated with land use change (viz. deforestation) among colonist and indigenous groups, respectively.
Figure 1.
Colonist (left) and indigenous (right) study regions in the northern Ecuadorian Amazon. The bolded box outlines the dimensions of the colonist survey locations within the scope of the indigenous survey locations.
Indigenous Populations in the Northern Ecuadorian Amazon
The indigenous population of the NEA comprises just under one-third of the region’s population (INEC, 2001b). In the NEA, the Quichua are the most numerous (~21,000 people), followed by the Shuar (~1,700 people), the Cofán (~500), and the Huaorani and Secoya (each ~300). Other ethnicities in the NEA numbered fewer than 100 in the 2001 Ecuadorian census. Previous studies have discussed several key differences among ethnic groups ranging from land use, fertility and market orientation (Bremner, Bilsborrow, Feldacker, & Holt, 2009; Bremner & Lu, 2006; Gray, Bilsborrow, Bremner et al., 2008). The Quichua are widely dispersed with equally diverse economic activities that range from small-scale subsistence agriculture to cash-cropping, hunting, and timber extraction. The Shuar are the largest group in the southern Ecuadorian Amazon, but have several communities in the NEA as a result of migration. Thus, many Shuar communities have claimed land along roads in a similar way to colonists and are thus more market-oriented than other ethnicities. The Cofán are limited to just seven communities in the NEA and southern Colombia. Activities of the Cofán differ among those communities that have chosen to isolate themselves in the Cuyabeno Wildlife Refuge and those in close proximity to colonists (Dureno and Duveno) who, as expected, are more market-oriented. The Huaorani were traditionally semi-nomadic with a violent reputation; however, missionaries in the 1950s influenced the establishment of permanent settlements. In general, the Huaorani rely on subsistence agriculture, but also depend on wage labor income from oil companies. Finally, the Secoya have been greatly affected by colonization and the border wars between Peru and Ecuador, which caused them to move west, eventually restricting them to just five communities in the NEA. Due to these disruptions, the Secoya have received considerable aid for cattle ranching and market-oriented agriculture.
Although characteristics of indigenous groups vary, as a whole they are considerably different from colonists. Indigenous households are 20% larger in size; have less access to electricity (27% compared to 42% of colonist households having electricity); have more limited road access (42% of indigenous vs. 90% of colonists households have road access); and land ownership is generally communal with indigenous people clearing an average of 1.5–5 ha depending on their market orientation, while colonists aspire for title ownership and clear over 50% of their plots (~13 ha) for agricultural use. Indigenous groups are also linked politically through CONAIE, a unified national federation to support indigenous rights. These clear demographic, economic and cultural differences between colonists and indigenous people, as well as the statistical constraints due to small group-level sample sizes, led to the decision to pool indigenous groups to make comparisons with colonists.
HEALTH IN ECUADOR
Since the 1960s, Ecuador as a whole has experienced substantial improvements in health. Between 1960 and 2000, life expectancy increased 37%, infant mortality decreased 75% and under-5 mortality dropped 82%. In addition, vaccination coverage has improved, for example, measles immunization increased from 24% in 1980 to 84% in 2000 and 95% in 2005 (United Nations Statistics Division (UNSD), 2008; WHO & UNICEF, 2004).
Although health has improved, Ecuador faces significant challenges in addressing disparities across regions and population groups. Most of the health improvements have occurred in the Costa and Sierra regions, particularly in the major cities of Guayaquil and Quito. Persons living in the Oriente have significantly higher risks for disease (Table 1) and approximately thirty percent of reported mortality has no known cause due to the lack of health resources compared to just 10–15% in the Costa and Sierra (INEC, 2001a). Although vector-borne and zoonotic diseases are slightly more common in coastal provinces, several diseases are endemic in the NEA such as leishmaniasis (Calvopina, Armijos, & Hashiguchi, 2004), Chagas disease (Aguilar V, Abad-Franch, Racines V, & Paucar, 1999; Grijalva, Escalante, Paredes, Costales, Padilla, Rowland et al., 2003), and malaria, which was considered eliminated in the 1970s but reemerged partly due to lack of coordination in the design and implementation of effective control strategies (San Sebastian, Jativa, & Goicolea, 2000). As with other regions of Amazonia, chronic malnutrition, stunting and parasitic infection is endemic, particularly among indigenous children (Buitrón, Hurtig, & San Sebastián, 2004; Orr, Dufour, & Patton, 2001; Quizhpe, San Sebastián, Hurtig, & Llamas, 2003; San Sebastián & Santi, 1999), which increases their risks for other diseases.
Table 1.
Morbidity Rates per 1000 population by region of Ecuador, 2000
| DISEASE CATEGORIES | REGIONS OF ECUADOR | ORIENTE PROVINCES | |||
|---|---|---|---|---|---|
| SIERRA | COSTA | ORIENTE | Study Region (Sucumbios, Napo, Orellana) | Other Provinces | |
| ACUTE RESPIRATORY INFECTIONS | 50.6 | 56.1 | 95.2 | 87.3 | 102.9 |
| GASTROINTESTINAL ILLNESSES (e.g., diarrhea, salmonella, cholera, etc.) | 17.1 | 23.1 | 34.2 | 31.4 | 36.9 |
| VECTOR-BORNE DISEASE (e.g., malaria, dengue, yellow fever) | 2.4 | 15.8 | 15 | 26.7 | 3.7 |
| CHRONIC INFECTIONS (e.g., TB, AIDS, leprosy, etc.) | 0.5 | 0.7 | 1.6 | 2.3 | 0.9 |
| CHRONIC NON-TRANSMISSIBLE INFECTIONS (e.g., diabetes) | 0.7 | 0.9 | 0.8 | 0.8 | 0.7 |
| ACCIDENTS & INJURIES (e.g., poisoning, violence, snakebite, etc.) | 4.1 | 2.5 | 5.4 | 7.2 | 3.6 |
| MENTAL HEALTH (e.g., suicide, depression, alcoholism) | 1 | 0.3 | 1.5 | 1.0 | 2.0 |
| VACCINE-PREVENTABLE (e.g., hepatitis, whooping cough, tetanus, diphtheria, etc.) | 0.1 | 0.1 | 0.3 | 0.3 | 0.3 |
| ZOONOTIC (e.g., cysticercosis, rabies, etc.) | 2.1 | 3.0 | 2.4 | 2.7 | 2.2 |
Reference: INEC 2001 Situación de la salud en el Ecuador: Indicadores básicos por región y provincia. “Principales enfermedades de notificación obligatoria. Numero de casos reportados por región y provincia” Quito.
Environmental exposures are increasingly implicated as disease agents. Petroleum contamination has been linked to skin and respiratory irritations, elevated cancer mortality, and spontaneous abortions (Hurtig & San Sebastian, 2002a, 2004; Hurtig & San Sebastian, 2002b; San Sebastian, Armstrong, Córdoba, & Stephens, 2001; San Sebastian, Armstrong, & Stephens, 2002). While some of these findings have been refuted due to their methodology in estimating the population at risk (Arana & Arellano, 2007), the debate regarding the health effects of petroleum contamination continues, motivated by the ongoing litigation of the government of Ecuador against Chevron-Texaco, to seek remediation for environmental degradation and pollution caused by oil exploration and extraction activities since 1967.
METHODS
Data Collection
The study undertaken was approved by the ethics committee at the University of North Carolina at Chapel Hill. As noted earlier, data were obtained from two separate projects studying land use among colonist and indigenous inhabitants of the NEA. Survey data were comprehensive and included modules on migration, reproductive health, mortality, and morbidity (surveys are available online at www.cpc.unc.edu/projects/ecuador/). Sampling methods applied resulted in a representative sample for each group. An abbreviated description follows with more detailed methods described in previous studies (Bilsborrow, Barbieri, & Pan, 2004; Gray, Bilsborrow, Bremner et al., 2008).
Data pertaining to colonists are from a probability sample of land plots that had been surveyed by the Ecuadorian government land agency (IERAC) and allocated to migrant colonist families. In 1990, IERAC made available a listing of all settlement sectors with the number and size of the land plots allocated (referred to as fincas) from which a 2-stage PPS (probability proportional to size) selection of 62 settlement sectors and 459 fincas was carried out, representing a 5.9% sample of all fincas existing in the study region. All households located on fincas were interviewed in 1990 and again in 1999. In 1990, 418 farm households on 398 fincas provided data (34 fincas were uninhabited and 27 did not have a proper respondent); however, between 1990 and 1999, 40% of the fincas were subdivided with several households containing more than one family unit. Thus, 1999 colonist data were collected from 767 farm households on 392 fincas (one sector near the Colombian border was not revisited due to safety concerns) living together in 652 households representing a total population of 3802.
Data pertaining to indigenous people were collected in two phases: an ethnographic study in 8 communities followed by household surveys in an additional 28 communities. The 8 communities that provided both ethnographic and household survey data were selected based on willingness to participate, ethnicity and geographic diversity. The 28 additional communities and their households were selected using controlled sampling (Goodman & Kish, 1950) to ensure adequate representation of both large and smaller ethnic groups in the study region, ensuring heterogeneity of location (close to roads and colonists towns or not), province, and population size. Indigenous health data were obtained from 479 households with the following distribution by ethnicity:
| Ethnic Group | # Communities | # Households | Population |
| Quichua | 14 | 221 | 1454 |
| Shuar | 10 | 99 | 637 |
| Huaorani | 7 | 78 | 519 |
| Cofan | 3 | 50 | 281 |
| Secoya | 2 | 31 | 149 |
The survey instruments used in both the colonist and indigenous household data collection were nearly identical, with a focus on factors thought to influence land use. In both studies, questionnaires were administered separately to male and female household heads (the male household head was usually the person mainly responsible for generating income). If more than one family occupied a household, the female head was defined as the spouse of the head who originally occupied the land. Surveys were comprehensive, covering a large number of topical areas. Household survey data and variables used in this study are described below.
Variable Definitions
Household demographic composition includes age, year moved to the NEA, education, and total number of live births and surviving children (for women over 12). Birth histories were used for mortality estimation and the household roster was used to compute morbidity rates (person-months at risk and age-specific rates). We expect household size to be positively associated with morbidity, while years’ living in the Amazon, a proxy for experience in minimizing health risks, and higher education of the female household head to be inversely related to morbidity.
Household quality variables defined to capture healthy behaviors and the extent of regional infrastructure. Water use practices (boiling, obtaining water from a well or spring, or other source) and electricity access are used to capture household quality.
Health status, quality and perception variables include: number of household members ill during the three months prior to the survey; whether a person reported ill received care (physician, nurse or other caregiver); perceived environmental pollution (water, air, soil) and the perceived source of contamination; where household members usually obtain health care (hospital, health center, private clinic, traditional healer or other); date, location (home, hospital, private clinic) and type of attendant (physician, nurse, midwife, relative, no one) at last birth; vaccines the last child born received (BCG, DPT, Polio, measles, other); perceived causes of morbidity (i.e., climate, insects, parasites, lack of potable water, poor hygiene, malnutrition, contaminant exposure, inadequate health care, physically demanding work); and whether poor health is the primary cause for desired migration. Colonists and indigenous people reported one of eleven types of illnesses for each person (viz., cold/flu, diarrhea, parasites, malaria, skin disease, respiratory disease, snakebite, accident/injury, cancer, heart, witchcraft, and other) that were assigned to nine categories (Tables 1 and 2). Ailments reported as “other” were examined by a physician to recognize typical illnesses of the region and assigned to categories. Morbidity rates are defined as the number of illnesses reported in the household divided by person months (3 x household size, since we rely on 3-month morbidity recall). Environmental pollution is expected to be positively related to morbidity. Other variables defined here are used to define cluster-level variables (below) and to evaluate the importance of perceived health status.
Table 2.
Ratesa and 95% confidence intervals of reported illnesses for Colonists (1999) and Indigenous (2001) in the northeastern Ecuadorian Amazon
| DISEASE CATEGORIES | COLONISTS (pop: 3802) | INDIGENOUS (pop: 3040) |
|---|---|---|
| RESPIRATORY INFECTIONS (e.g., Respiratory disease, Cold/Flu, headaches, sore throat, cough, fever) | 25.8 (22.9–28..9) | 27.4 (24.1–31.0) |
| GASTROINTESTINAL ILLNESSES (e.g., diarrheal disease, parasitic infection, dysentery, stomach pain) | 7.6 (6.1–9.4) | 12.8 (10.6–15.4) |
| VECTOR-BORNE DISEASE (e.g., malaria, dengue, yellow fever) | 7.2 (5.7–8.9) | 28.5 (25.1–32.2) |
| CHRONIC INFECTIONS (e.g., conjunctivitis, skin disease, TB, genital infection, herpes) | 2.9 (2.0–4.1) | 7.9 (6.2–9.9) |
| CHRONIC NON-TRANSMISSIBLE INFECTIONS (e.g., diabetes, cancer, heart disease, anemia, epilepsy, prostate) | 3.0 (2.1–4.2) | 1.4 (0.8–2.4) |
| ACCIDENTS & INJURIES (e.g., chemical poisoning, snakebite, hernia, concussion, hemorrhage) | 2.6 (1.8–3.8) | 1.6 (1.1–2.7) |
| REPRODUCTIVE HEALTH (e.g., miscarriage, pre-/post-natal problems) | 0.3 (0.1–0.8) | 0.5 (0.2–1.3) |
| VACCINE-PREVENTABLE (e.g., chickenpox, mumps, measles, hepatitis) | 1.8 (1.1–2.8) | 1.2 (0.6–2.2) |
| UNKNOWN/OTHER ILLNESSES (e.g., Witchcraft, Bone/Muscle pain, other/don’t know) | 4.5 (3.3–5.9) | 8.9 (7.1–11.0) |
Rates are computed as the number of morbidity events divided by (3 x population size) x 1000 for interpretation as events per 1000 person-months
Land use variables include: total agricultural land and land use type (pasture, perennial, and annual crops). The relationship between land use and morbidity is largely unknown – one may expect total land in agriculture to be protective since it can serve as a proxy for wealth and food security, thus lowering health risk. However, research has shown that land use transitions in tropical agricultural frontiers (i.e., farm establishment characterized by initial subsistence farming to eventual market-oriented agriculture consisting of pasture and perennial crops) are a general path toward greater wealth, and thus longer establishment (presence of pasture and perennials) would be expected to be associated with lower risk. This is consistent with the Frontier Malaria Hypothesis (Sawyer & Sawyer, 1992) in which early settlement is associated with greater risk for malaria. However, since indigenous settlements generally do not follow this transition, we would expect more land use for agriculture (regardless of type) to be associated with lower health risk since it is related to greater food security and less reliance on petroleum-based labor.
Accessibility was obtained from both the male and female household head as well as from GPS data recorded during data collection. Three variables describe a household’s accessibility to health facilities: type of health facility usually used for care (hospital, clinic, traditional healer or other), distance of a health facility to the household, and vehicle access.
Cluster variables capture access to and utilization of health facilities and services. Among colonists, clusters are defined as settlement sectors, while indigenous households are naturally clustered in communities. We define five cluster-level measures, all hypothesized to be inversely related to morbidity: percentage of births in the past five years attended by a trained physician, nurse or midwife; percentage of births in the past five years in a hospital or health center; percentage of children born in the past six years who are at least one year old that were vaccinated (measles plus any other vaccine); percentage of persons reported ill who were seen by a physician or nurse; and percentage of households that usually go to a hospital, health center or private clinic for care.
Temporal Alignment of Data
On average, data being compared for this study were collected 16 months apart (1999 colonists vs. 2001 indigenous). In 1999, Ecuador defaulted on their public debt following 15 years of negative GDP growth resulting in de facto dollarization. In January 2000, President Mahuad was forced to step aside and Vice President Gustavo Noboa took control. Noboa expanded petroleum development and helped stabilize the economy, which led to an increase in per capita social spending from $55 to $77 between 1999 and 2001 and decreased poverty from 51% to 38% between 2000 and 2006 (Weisbrot, Sandoval, & Cadena, 2006). Although economic improvements have not been equally distributed, if we assume that better socio-economic status is associated with better health, then if colonists were interviewed in 2001, we would expect them to report fewer health problems due to generally improved economic conditions (conversely, if indigenous surveys were administered in 1999 we would expect more health ailments reported). This temporal mismatch should be kept in mind when comparing results.
Data Analysis
To compare mortality trends between indigenous and colonist groups, we use Trussel’s modification of the Brass techniques to estimate mortality (Trussel, 1975; United Nations, 1983), which is a widely used technique to estimate mortality from DHS data and for Global Burden of Disease estimates. This technique estimates the probability of dying based on children ever born, children surviving and total number of women classified by five-year age groups. The probability is computed as:
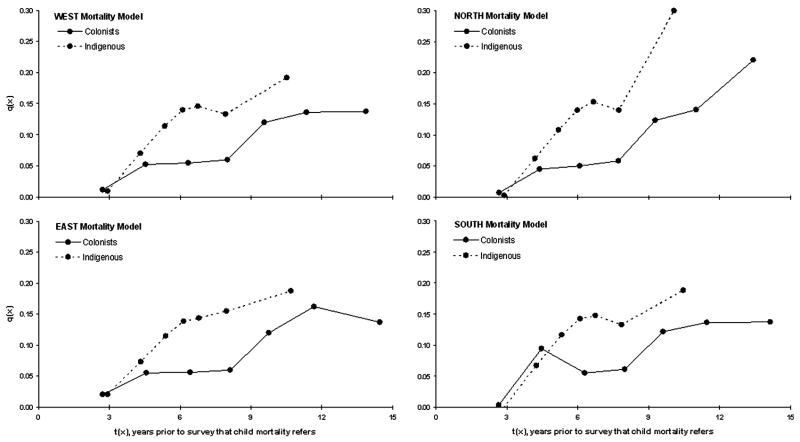
where q(x) is the probability of dying between birth and age x (x=1, 2, 3, 5, 10, 15, and 20), D(i) is the proportion dead among children ever born to women by five-year age groups, and k(i) is a multiplier to adjust for non-mortality factors associated with D(i) (i refers to five-year age groups, 15–19, 20–24, etc.). The reference period, t(x), for each q(x) is also computed, which is the number of years prior to the survey that each q(x) value refers. Multipliers are defined by the mortality pattern characteristic of the region under interest. We use four standard mortality patterns derived by Ansley Coale and Paul Demney for the United Nations (1983) to define our multipliers. These patterns are classified into four groups: (1) East model: a U-shaped pattern that assumes higher mortality among young children and the elderly, and lower mortality among older children and adults; (2) North model: assumes low infant mortality, high child mortality, and high mortality for ages over 50; (3) South model: assumes relatively high mortality under age 5, lower mortality for 40–60 year olds and higher mortality over age 65; and (4) West model: a general mortality pattern that was derived using residual tables and thus is recommended as the first choice to represent mortality in countries that lack evidence for a particular model. All four patterns are reported. Mortality is compared by computing the percent differences for areas under each mortality pattern curve (AUC) between estimated colonist and indigenous q(x) values using the trapezoidal rule to interpolate between ages (Yeh, 2002). The overall percent difference across the four mortality patterns is used to estimate the difference in mortality.
Morbidity estimates are based on three-month recall of illnesses reported by household members (Table 2). Illnesses reported include respiratory (cold/flu, cough, sore throat), gastrointestinal (diarrhea, dysentery, parasitic infection), vector-borne disease (malaria, dengue, yellow fever), skin infection, snakebites, accidents, cancer, heart conditions, and an open ended response for type of illness, which includes unknown causes and witchcraft (Table 2). Rates of illnesses per 1000 are reported and 95% confidence intervals are estimated assuming that the number of ailments reported follows a Poisson distribution.
Poisson regression is used to identify differential risks associated with household morbidity. Unadjusted risk ratios are reported for each variable and adjusted risk ratios are computed for a subset of variables selected in a two-stage selection process: first, variables in the unadjusted model that had Wald chi-square p-values under 0.35 were considered for the final model; second, a forward stepwise procedure was implemented in which variables from step one were ranked by importance and added sequentially. They remained if their Wald p-value was under 0.35. We recognize that Akaike’s Information Criterion (AIC) is likely a better statistic for inclusion, but models were fit using pseudo-likelihood, which does not permit accurate comparisons using IC statistics. To compare results, the same minimum set of predictors was included in the final colonist and indigenous models by including non-significant variables previously excluded. Random effects are also estimated to control for correlated observations within households and sectors.
RESULTS
The rate at which the probability of mortality increased with age varied for colonists and indigenous people across all four Brass models (Figure 2). Overall, indigenous groups had a 30% higher average mortality curve than colonists. The average probability of dying (q(x), across all four models) is estimated as 1.1%, 6.2%, 5.4%, 6.0%, and 12.2% among colonists to ages 1, 2, 3, 5, and 10, respectively, but among indigenous people, q(x) increased more rapidly across the same age groups: 0.8%, 6.8%, 11.4%, 14.0%, and 14.8%. This pattern, which suggests lower average mortality for indigenous children to age 1, but more than double the mortality probability to ages 3 and 5, indicate significant differentials in child health.
Figure 2.
Brass mortality model estimates for colonists (1999) and indigenous (2001) populations living in the northern Ecuadorian Amazon
All-cause morbidity rates for indigenous people were 90 per 1000 (95% CI: 84.5–96.4) compared to 56 per 1000 (95% CI: 51.5–60.0) among colonists. This difference corresponds to an incidence rate ratio of 1.62 (95% CI: 1.49–1.77) or a 62% higher incidence of disease among indigenous people. Cause-specific morbidity (Table 2) among indigenous people is particularly high compared to colonists for vector-borne diseases (4 times higher), chronic infections (2.5 times higher), diseases of unknown origin (2 times higher), and gastrointestinal illnesses (1.7 times higher). The high rate of unknown illness alludes to the lack of health services and likely less health care knowledge in general. In fact, indigenous groups reported 33 cases of witchcraft compared to just 2 among colonists. Only chronic non-transmissible infections, accidents/injuries, and vaccine-preventable diseases were reported more often by colonists, although rates were low and differences not significant. It is poignant that respiratory infections are among the top two causes of morbidity for both colonists and indigenous people, demonstrating their importance regardless of culture, context and spatial location.
Morbidity Risk Models
Unadjusted model results are generally consistent in the direction of effects for all variables across population groups (Table 3), with a few exceptions: cultivation of more perennials and pasture, “sometimes” boiling water, and road access are associated with less morbidity among colonists, but more among indigenous people. None of these effects are significant in both groups. The association for boiling water is notable as “always” boiling water is associated with higher morbidity. It is possible this association is spurious; however, boiling water for purification/decontamination also requires practices regarding consumption (i.e., using a separate cup to pour and consume water, not dipping hands into the water after boiling, etc.), which may not be practiced by either group.
Table 3.
Unadjusted Morbidity Risk Ratios and 95% CIs household and community characteristics, and health care accessibility among Colonists (1999) and Indigenous (2001) living in the NEA
| COLONISTS | INDIGENOUS | ||
|---|---|---|---|
| HOUSEHOLD CHARACTERISTICS | |||
| Age group | 0 – 4 vs. 40+ | 0.93 (0.75–1.15)† | 0.45 (0.37–0.56)† |
| 5 – 14 vs. 40+ | 0.62 (0.51–0.77)† | 0.40 (0.33–0.49)† | |
| 15 – 39 vs. 40+ | 0.53 (0.43–0.65)† | 0.62 (0.52–0.74)† | |
| Time | Month of data collection | 0.99 (0.93–1.05) | 0.93 (0.87–0.98)† |
| Population & Land Use | Household Population | 0.89 (0.86–0.92)† | 0.95 (0.91–0.98)† |
| Total Agricultural Land Use (ha/10) | 0.93 (0.89–1.04) | 0.95 (0.87–1.03)† | |
| Agricultural Land: HH Pop | 1.05 (1.01–1.09)† | 1.16 (1.03–1.31)† | |
| Annuals (Ha) | 0.95 (0.88–1.02)† | 0.90 (0.84–0.96)† | |
| Perennials (Ha) | 0.99 (0.96–1.03) | 1.01 (0.98–1.04) | |
| Pasture (Ha) | 0.99 (0.98–1.01)† | 1.01 (0.98–1.04) | |
| Education, Female head | Complete vs. Incomplete Primary/None | 1.18 (0.85–1.65)† | 1.09 (0.94–1.26)† |
| >Primary vs. Incomplete Primary/None | 1.04 (0.85–1.28)† | 1.17 (0.88–1.54)† | |
| Drinking Water | Sometimes vs. Never Boil | 0.96 (0.78–1.17) | 1.10 (0.96–1.26)† |
| Always vs. Never Boil | 1.00 (0.79–1.27) | 1.09 (0.93–1.27)† | |
| Collect from a well/spring vs. Other | 0.93 (0.76–1.13) | 0.96 (0.77–1.20) | |
| Sewage disposal | Forest or field vs. Other | 1.10 (0.89–1.36) | 1.14 (0.98–1.33)† |
| Pollution | Any vs. None | 1.29 (1.07–1.56)† | 1.12 (0.92–1.37)† |
| SOURCE: Air vs. (none or other) | 1.36 (1.15–1.60)† | 1.12 (0.89–1.40)† | |
| SOURCE: River vs. (none or other) | 1.29 (1.07–1.56)† | 1.08 (0.89–1.31) | |
| SOURCE: Soil vs. (none or other) | 1.19 (0.96–1.49)† | 1.19 (0.96–1.48) | |
| Years living in Amazon | 0.99 (0.98–1.00)† | 0.95 (0.83–1.07) | |
| Electricity | Electricity in HH vs. None | 0.82 (0.64–1.04)† | 0.97 (0.72–1.31) |
| HEALTH CARE ACCESS VARIABLES | |||
| Distance to Hospital/Health Center | 1.00 (0.99–1.01) | 1.00 (0.99–1.00) | |
| Vehicle access | All year vs None | 0.80 (0.66–0.98)† | 1.01 (0.79–1.28) |
| Occasionally vs. None | 0.97 (0.69–1.38)† | ||
| Where HH gets health care | Hospital vs. Traditional Healer/Other | 0.91 (0.63–1.32) | 0.93 (0.75–1.15) |
| Health Cl. vs. Traditional Healer/Other | 1.00 (0.72–1.38) | 1.03 (0.78–1.38) | |
| Private Cl. vs. Traditional Healer/Other | 1.15 (0.78–1.72) | 1.07 (0.81–1.42) | |
| COMMUNITY VARIABLES | |||
| Trained Birth Attendant (%) | Physician or Nurse | 1.00 (0.99–1.01) | 1.00 (0.98–1.02) |
| Physician, Nurse or Midwife | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) | |
| Births at a Health Ctr/Hospital (%) | 1.01 (0.99–1.00)† | 1.00 (0.98–1.02) | |
| Vaccination of 1–5 year olds | % Received any vaccine | 1.00 (0.99–1.01) | 1.00 (0.98–1.01) |
| % Received Measles | 1.00 (0.99–1.01) | 0.99 (0.99–1.00)† | |
| % Ill seen by a trained health practitioner | 1.00 (0.99–1.00)† | 1.00 (0.99–1.00) | |
| Where HHs go to get health care (%) | Hospital | 0.99 (0.66–1.48) | 1.00 (0.99–1.01) |
| Health Center | 0.98 (0.65–1.49) | 1.00 (0.99–1.00) | |
| Private Clinic | 1.59 (0.78–3.27)† | 1.01 (1.00–1.01)† | |
Tested for inclusion to the multivariate (adjusted) morbidity risk ratio model
Table 4 shows the multivariate model results with shaded boxes indicating variables that were originally excluded, but re-entered to make comparisons (re-entry of these variables did not affect significance or magnitude of the final model variables). Factors that exhibit similarities in effect and significance are age, month of data collection, and household size. Although consistent, the effect of age indicates different morbidity patterns. Colonist morbidity follows a U-shaped pattern in which the predicted probability of morbidity decreases significantly for each sequential age group (i.e., 0.08 [95% confidence interval: 0.066–0.096] for 0–4 year olds, 0.05 [0.045–0.064] for 5–14 year olds, and 0.04 [0.038–0.052] for 15–39 year olds) then increases to age group 40+ to the same level as 0–4 year olds (0.08 [0.069–0.097]). Indigenous people have a relatively flat predicted morbidity from ages 0–4 to 5–14 (0.07 [0.056–0.085] and 0.06 [0.053–0.078], respectively), which significantly increases to age group 15–39 (0.09 [0.079–0.111]) and to age group 40+ (0.15 [0.123–0.183]). Later months of data collection were associated with less morbidity, likely due to later months (February–June) having higher average precipitation, which has been associated with higher risk for respiratory and vector-borne disease. Larger household population size was unexpectedly associated with lower morbidity. This may result either from reporting biases (i.e., morbidity may be more accurately reported in smaller households), improved health practices for larger families due to experience, or household risk diversification as larger households are more able to diversify risks (assuming household members are not children).
Table 4.
Adjusted Morbidity Risk Ratios and 95% Confidence Intervals for Colonists (1999) and Indigenous (2001).
| COLONISTS 1999a | INDIGENOUS 2001b | ||
|---|---|---|---|
| HOUSEHOLD CHARACTERISTICS | |||
| AGE GROUP (ref group: ages 40+) | 0 – 4 | 0.97 (0.76–1.25) | 0.46*** (0.36–0.58) |
| 5 – 14 | 0.65** (0.52–0.83) | 0.43*** (0.34–0.53) | |
| 15 – 39 | 0.54*** (0.43–0.67) | 0.62*** (0.51–0.76) | |
| TIME | Month of data collection | 0.94* (0.89–1.00) | 0.92** (0.87–0.98) |
| POPULATION & LAND USE | Household Population | 0.89*** (0.86–0.92) | 0.96** (0.93–0.98) |
| Perennials (Ha) | 0.99 (0.97–1.02) | 1.02 (0.99–1.06) | |
| Annuals (Ha) | 1.02 (0.95–1.08) | 0.90* (0.83–0.97) | |
| POLLUTION (ref group: None or Other) | Air Pollution | 1.32* (1.05–1.65) | 1.13 (0.93–1.37) |
| River Pollution | 1.26* (1.01–1.57) | 1.07 (0.90–1.26) | |
| ELECTRICITY | Electricity in HH vs. None | 0.85† (0.71–1.03) | 0.94 (0.76–1.17) |
| HEALTH CARE ACCESS VARIABLES | |||
| VEHICLE ACCESS | All year vs (Occasional or None) | 0.83† (0.68–1.01) | 1.10 (0.86–1.41) |
| SECTOR/COMMUNITY VARIABLES | |||
| % OBTAIN HEALTH CARE HERE | Private Clinic | 1.46 (0.80–2.64) | 1.01† (1.00–1.02) |
p<0.0001;
p<0.01;
p<0.05;
p<0.10
Intercept values for each model are:
−1.7348; and
−1.1102.
Variables exhibiting consistency in directions of effects, but not in significance include pollution, electricity and community-level effects. Air and river pollution are associated with a 32% and 24% increase in morbidity among colonists, respectively, compared to 13% and 7% among indigenous people, respectively. Presence of air pollution was reported equally in both groups (21%), but 30% of colonists households reported river pollution compared to 47% of indigenous households, and 6% of colonists reported soil pollution compared to 20% of indigenous households. The absence of a significant effect for indigenous is likely due to the over-sensitivity of indigenous households to pollution presence, which is related to the litigation brought by the Ecuadorian government against Chevron-Texaco. The lawsuit is widely supported by indigenous groups and it is possible that indigenous households are over-reporting pollution on their property.
Access to electricity is protective, but only borderline significant for colonists (p=0.09) and was not included in the final indigenous model. Electricity access has implications of access to both infrastructure and information: only 30% of colonists located further than 15km from a large town have electricity compared to almost 55% less than 15km; and most households who have electricity have a radio or television, which improves their ability to obtain information. At the community level, a positive effect with morbidity was also found for both groups when more people obtain health care at a private clinic, but the effect was of borderline significance for indigenous people (p=0.06) and not significant in the final colonist model. Although the direction of effect is unexpected, private clinics include those that are operated by traditional health workers (i.e., not just operated by physicians or nurses). In addition, access to a private clinic may increase the likelihood of reporting an illness in general.
The two remaining model variables – land use and road access – have opposite effects on colonist and indigenous morbidity, but neither is significant in both groups or included in the final selected models. Annual crops are significantly associated with less morbidity for indigenous people, but (non-significantly) more morbidity for colonists. Alternatively, perennial crops are associated with more morbidity among indigenous people, and less among colonists, although neither effect is significant. The opposite effects of land use are important, implying differences in environmentally-induced risks, consistent with the Frontier Malaria Hypothesis. This will be addressed in more detail in the Discussion Section. Road access is associated with a 17% reduction in morbidity among colonists (p=0.06), but a non-significant increase among indigenous people. The relationship among indigenous groups is likely due to road access not being equivalent to vehicle transport – i.e., most colonist communities have organized vehicle transport, but not indigenous settlements. Indigenous people use a mix of walking, horses, water and vehicles for travel.
Perceived Health Quality
44% of colonists perceived their quality of health as worse than prior to living in the NEA, compared to 47% among indigenous people (who compared their health to ten years prior). Perceived worse health was associated, as expected, with 51% more illnesses reported by colonists, but only 20% more by indigenous. The primary causes for perceived poorer health among colonists were harsher climate and presence of insects and parasites, while indigenous people blamed contamination by petroleum companies and presence of insects and parasites. In fact, 83% of colonists and 75% of indigenous people who reported environmental pollution blamed petroleum companies. In addition, although desire to migrate and reported morbidity were not directly related, 30% of colonists and 20% of indigenous respondents who believed their children should migrate from the NEA indicated poor health and lack of services as reasons for leaving.
DISCUSSION
This study confirms significant health disparities between colonist and indigenous populations living in the NEA. Differences in economic and health outcomes between indigenous and non-indigenous populations have been documented for Latin America countries in general by World Bank and the IMF (Fischer, 2001; Hall & Patrinos, 2006), but this study demonstrates that health quality and outcomes are distinct even at small scales when the two groups are living in the same geographic region. We show that indigenous mortality probabilities are 30% higher than colonists, with the largest deviation occurring between ages 2 and 5. Indigenous people report 62% more morbidity due to all causes as well as 2–4 times higher rates of vector-borne disease, chronic infections, gastrointestinal illnesses and diseases of unknown origin. The top three causes for morbidity among both colonist and indigenous people are the same (respiratory, gastrointestinal and vector-borne diseases), although not in ranking. The high rate of unknown illnesses among indigenous people indicates both a lack of access to or use of health services and less knowledge of health in general. Although data are not aligned temporally, the economic improvements that Ecuador experienced likely indicates that the disparities noted are even more severe than those we estimate here – i.e., it is likely that health among colonists has improved slightly from 1999 to 2001. In addition, based on regional and national data from the World Bank and IMF, it is likely these disparities have existed for decades. Given our sample size constraints, we were unable to distinguish disparities among indigenous ethnicies; however, exploratory analyses (not shown) indicate that the Cofan have more than double the rate of respiratory illness than other ethnicities, the Shuar have more than double the rate of vector-borne disease than other ethnicities, and all ethnicities have approximately the same rate of gastrointestinal illness. These differences in health among the various indigenous ethnic groups are possibly a result of spatial differences of access, lack of health services, and living in areas that have greater disease vector abundance.
When considering factors associated with morbidity, similarities and differences demonstrate the variation in culture, spatial context and policy in the NEA. Colonist and indigenous morbidity by age have slightly different patterns. Estimates of health risk do not differ significantly for the two youngest age groups, but the risk of illness among 15–39 year olds and persons older than 40 are almost double among indigenous people compared to colonists. This is an important contrast to mortality, as the younger age groups have the largest differential in mortality. A possible explanation relates to differences in rates of immunization and vaccine-preventable disease. Survey data indicate that indigenous children have lower rates of immunization and vaccine-preventable disease than colonists, even lower than colonists interviewed in 1990. For example, among women reporting a birth in the five years prior to the survey, 72% and 78% of colonist children 2 months to 5 years of age received DPT in 1990 and 1999, respectively, compared to 59% of indigenous children; 64% and 69% of colonist children (1990 and 1999, respectively) received polio vaccine compared to 43% indigenous; and 72% and 73% of colonist children aged 1–5 years received measles vaccine compared to 54% of indigenous children. Thus, the combined lack of immunizations among indigenous and the possible under-reporting of vaccine-preventable disease due to lack of knowledge and/or access to health care may result in higher death rates in younger age groups among indigenous children. This would be an important future area of research.
Another important finding is that of household population. In urban and peri-urban settings, greater household population density is usually associated with higher health risks in developing countries; however, in this rural setting, larger households are associated with lower risks for both colonists and indigenous. As noted earlier, there are several potential explanations for this finding; however, assuming data are accurately reported, diversification of risk likely explains lower morbidity. Research on land use change in tropical regions has shown that households that diversify economic and agricultural risk are better positioned to accumulate wealth and transition from subsistence to market-based agriculture (e.g., Carr, Suter, & Barbieri, 2005; Schelhas, 1996).
The effects of land use (perennials and annuals) are in opposite directions for each land use type. Clear differences in culture are manifested in this relationship: Colonists manage a defined area (finca), on which they typically hold title and can subdivide to children, other relatives, or incoming migrants. Land uses vary and include annuals, perennials, pasture, and fallow, with the goal of most colonist farmers being a transition from subsistence to market production. This transition is a function of several factors that influence several demographic decisions, particularly migration to rural (new property) or urban areas. Among indigenous people, land is communal and highly egalitarian in each community – farm plots are allocated more or less equally with little agricultural clearing to maintain the vast majority of forest for hunting and gathering (e.g., every household in a Quichua community El Pilche on the Napo river has 100 ha available to manage, but clear fewer than 5 hectares). These cultural differences manifest into health risks through environmental modification as explained by the Frontier Malaria Hypothesis, although not necessarily exclusive to malaria. For example, recently established household farms clear forest for annual crops and create culverts, irrigation pools, and other habitat that are ideal for malaria and other disease-causing vectors. Individuals living in these newly established households tend to have higher exposures to disease risks due to extended hours needed to work on the farm with usually less access to health care and information regarding protection from disease. In contrast, established farms with annual crops (i.e., mostly indigenous farms), have lived on the land for a longer period of time and are likely to have gained more control over their surrounding environmental conditions, such as areas of standing water where anophelines (malaria vectors) breed. The transition to perennial crops and pasture follows these latter characteristics, but also corresponds to less time required by household members to work the land and thus less time exposed to potential disease-causing agents.
In light of population-environment research in the tropics that focuses on demographic and economic factors associated with land use change and deforestation, the most important question is whether and how human health influences land cover. While this may be more appropriately addressed using longitudinal data, it is important to consider both the perception and actual state of health. Our data show that among those who wish their children to migrate from the Oriente, one-third of colonists and one-fifth of indigenous cite poor health quality as the primary reason for this belief. Thus, if health outcomes are associated with migration decisions, to what extent are health outcomes related to deforestation and associated climate change? The answer is not so simple, but is likely related to (1) how strong the relationship is between migration and health and (2) when people migrate due to health reasons, where do they go. Evidence from the Brazilian and Peruvian Amazon indicates that settlement regions of high health risk (viz., malaria) tend to be areas of unstable settlement (Castro, Monte-Mor, Sawyer, & Singer, 2006; Sawyer, 1993; Vittor, Gilman, Tielsch, Glass, Shields, Lozano et al., 2006; Vittor, Pan, Gilman, Tielsch, Glass, Shields et al., 2009), indicating that the absence of health services in rural settlement regions can have adverse impacts on the environment. Evidence regarding migration destinations on the other hand is not as well documented due to the focus on rural-to-urban rather than rural-to-rural migration. In our study site, 70% of colonists who migrate out of a household move to another rural destination in the Oriente, with females being more likely than males to migrate to an urban area (Barbieri & Carr, 2005). This process of rural-to-rural migration is a primary driver of tropical deforestation as rural migrants push the boundaries of deforestation further into the forest frontier. In order to better quantify the impact of health on land use processes, research needs to be conducted to identify rural destinations among rural migrants who move due to health reasons.
Interpretation of our results must be in light of several potential limitations. First, the overall goal of the survey instrument was to study factors associated with land use change; therefore, data related to health, with the exception of mortality and fertility, are based on non-standardized recall periods. Three-month morbidity recall is likely too long a time span for accurately recalling health events, particularly ones as common as gastrointestinal and respiratory illnesses. Surveys such as the Living Standards Measurement (LSMS) and Demographic and Health Surveys (DHS) typically use two- or four-week morbidity recall. Second, all morbidity, mortality and vaccination data are reported by female heads of household without record verification. In fact, it was not possible in several cases since people often did not obtain any medical care. Third, it is possible that larger households have larger reporting errors since it may be difficult for the female head to recall all morbidity events for each person in the home if several persons are away (i.e., she may not be able to confirm illnesses). Fourth, we treat colonist data as independent and do not take full advantage of the longitudinal nature of the data, though that would obviate comparisons with the indigenous populations. Nevertheless, a model focusing on changes among colonists would have advantages over the cross-sectional models used here. A final potential limitation relates to perceived causes of health problems. Research by San Sebastian and colleagues has implicated petroleum extraction in a wide array of ailments, and has led to advocating popular epidemiology, in which local organizations assess health problems and their causes (San Sebastián & Hurtig, 2005). But while oil companies may be responsible for elevated morbidity and mortality, the engagement of local organizations in the conduct of scientific investigations may bias results toward the desired hypothesis. For example, political and economic indigenous activism toward oil production may have resulted in the indigenous implicating petroleum as the cause of health problems 2.5 times more than colonists. Increased awareness is important for people to choose how to live a healthy life, but in order to properly test the influence of oil production on health, one must conduct more biologically-based studies that combine environmental (soils, air, water, etc.) and human (blood, urine, etc.) sampling to properly link petroleum pollution to health.
This work illustrates the health disparities that exist between the indigenous and colonist populations in the northern Ecuadorian Amazon. While this work links differences in health to culture and context, we must also recognize that the health of the region’s residents is likely related to the health of the ecosystem as a whole. Land use and land cover changes (notably widespread deforestation) are known to affect the health of ecosystems, through changes in climate as well as nutrient/water cycling and loss of biodiversity, so it is important to examine whether these changes also affect human health. Given that land use and land cover changes may affect the epidemiological character of a region by changing the proximity or interactions between vectors and hosts, future research should aim to link land cover changes to reports of illness at various spatial scales.
Acknowledgments
This research is made possible through grants from the US National Aeronautics and Space Administration (NASA) (NCC5-295), the Mellon, Summit, and Compton Foundations, PROFORS (Proyecto Forestal, in Lago Agrio, Ecuador, funded by GTZ, the German foreign aid program), and the National Institute for Child Health and Human Development (HD07168 and 1R03HD054939). Financial support for the 1990 data collection was provided by the US National Science Foundation, the World Wildlife Federation, and the Carolina Population Center (CPC), with logistical support from several Ecuadorian government agencies, including CONADE (the former National Planning Agency), the Ministry of Agriculture, and IERAC (Ecuadorian Institute for Agrarian Reform and Colonization). We would like to thank all the families who participated in our surveys.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
William Kuang-Yao Pan, The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD UNITED STATES.
Christine Erlien, Duke University.
Richard E Bilsborrow, University of North Carolina at Chapel Hill.
References
- Aguilar VHM, Abad-Franch F, Racines VJ, Paucar A. Epidemiology of Chagas Disease in Ecuador. A Brief Review. Mem Inst Oswaldo Cruz. 1999;94(Suppl I):387–393. doi: 10.1590/s0074-02761999000700076. [DOI] [PubMed] [Google Scholar]
- Akhavan D, Musgrove P, Abrantes A, Gusmão RdA. Cost-effective malaria control in Brazil: Cost-effectiveness of a Malaria Control Program in the Amazon Basin of Brazil, 1988–1996. Social Science & Medicine. 1999;49(10):1385–1399. doi: 10.1016/s0277-9536(99)00214-2. [DOI] [PubMed] [Google Scholar]
- Ali MM, Cleland J, Shah IH. Trends in reproductive behavior among young single women in Colombia and Peru: 1985–1999. Demography. 2003;40(4):659–673. doi: 10.1353/dem.2003.0031. [DOI] [PubMed] [Google Scholar]
- Angelsen A, Kaimowitz D. Rethinking the causes of deforestation: Lessons from economic models. The World Bank Research Observer. 1999;14(1):73–98. doi: 10.1093/wbro/14.1.73. [DOI] [PubMed] [Google Scholar]
- Arana A, Arellano F. Cancer incidence near oilfields in the Amazon basin of Ecuador revisited. Journal of Occupational and Environmental Medicine. 2007;64(7):490. [PMC free article] [PubMed] [Google Scholar]
- Barbieri A, Carr DL. Gender-specific out-migration, deforestation, and urbanization in the Ecuadorian Amazon. Global and Planetary Change. 2005;47(2–4):99–110. doi: 10.1016/j.gloplacha.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta PC, Coimbra CE, Jr, Escobar AL, Santos RV, Alves LC, Fonseca Lde S. Survey for tuberculosis in an indigenous population of Amazonia: the Surui of Rondonia, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(6):579–585. doi: 10.1016/j.trstmh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Bilsborrow RE, Barbieri A, Pan WK. Changes in population and land use over time in the Ecuadorian Amazon. Acta Amazonica. 2004;34(4):635–647. [Google Scholar]
- Bremner J, Lu F. Common property among Indigenous peoples of the Ecuadorian Amazon. Conservation and Society. 2006;4(4):499–521. [Google Scholar]
- Bremner J, Bilsborrow RE, Feldacker C, Holt FL. Fertility beyond the frontier: indigenous women, fertility, and reproductive practices in the Ecuadorian Amazon. Population and Environment 2009 [Google Scholar]
- Buitrón D, Hurtig A, San Sebastián M. Nutritional status of Naporuna children under five in the Amazon region of Ecuador. Revista Panamericana Salud Publica. 2004;15(3):151–159. doi: 10.1590/s1020-49892004000300003. [DOI] [PubMed] [Google Scholar]
- Calvopina M, Armijos R, Hashiguchi Y. Epidemiology of leishmaniasis in Ecuador: current status of knowledge – a review. Memórias do Instituto Oswaldo Cruz. 2004;99(7):663–672. doi: 10.1590/s0074-02762004000700001. Epub. [DOI] [PubMed] [Google Scholar]
- Carr DL, Bilsborrow RE. Population and land use/cover change: A regional comparison between Central America and South America. Journal of Geography Education. 2001;43:7–16. [Google Scholar]
- Carr DL. Proximate population factors and deforestation in tropical agricultural frontiers. Population and Environment. 2004;25(6):585–612. doi: 10.1023/B:POEN.0000039066.05666.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DL, Suter L, Barbieri AF. Population dynamics and tropical deforestation: State of the debate and conceptual challenges. Population and Environment. 2005;27(1):89–113. doi: 10.1007/s11111-005-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DL, Pan WK, Bilsborrow RE. Declining fertility on the frontier: The Ecuadorian Amazon. Population and Environment. 2006;28:17–39. doi: 10.1007/s11111-007-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Costa F, Gonçalves A, Lassance S, Silva Neto L, Salmazo C, Bóia M. Giardia lamblia and other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev Inst Med Trop Sao Paulo. 2007;49(3):147–153. doi: 10.1590/s0036-46652007000300003. [DOI] [PubMed] [Google Scholar]
- Castro MCd, Monte-Mor RL, Sawyer DO, Singer BH. Malaria risk on the Amazon frontier. Proceedings of the National Academy of Sciences. 2006;103(7):2452–2457. doi: 10.1073/pnas.0510576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland J, Bicego G, Fegan G. Socioeconomic inequalities in childhood mortality: the 1970s to the 1980s. Health Transition Review. 1992;2(1):1–18. [PubMed] [Google Scholar]
- Coomes OT, Grimard F, Burt GJ. Tropical forests and shifting cultivation: secondary forest fallow dynamics among traditional farmers of the Peruvian Amazon. Ecological Economics. 2000;32(1):109–124. [Google Scholar]
- Dasgupta P. Population, poverty and the local environment. Scientific American. 1995 February;:40–45. [Google Scholar]
- FAO. UNFaA Organization. New York: Department of Forestry, United Nations Secretariat; 2001. State of the World’s Forests. [Google Scholar]
- FAO. FaAOotU Nations. Rome: Department of Forestry, United Nations Secretariat; 2005. State of the World’s Forests. [Google Scholar]
- FAO. FaAOotU Nations. Rome: Department of Forestry, United Nations Secretariat; 2007. State of the World’s Forests. [Google Scholar]
- Fischer S. Ecuador and the IMF. In: Alesina A, Barro RJ, editors. Currency Unions. Hoover Institution Press; 2001. p. 86. [Google Scholar]
- Goodman R, Kish L. Controlled selection -- A technique in probability sampling. Journal of the American Statistical Association. 1950;45:350–372. [Google Scholar]
- Gray C, Bilsborrow RE, Bremner J, Lu F. Indigenous land use in the Ecuadorian Amazon: A cross-cultural and multilevel analysis. Human Ecology. 2008;36(1):97–109. [Google Scholar]
- Grijalva M, Escalante L, Paredes R, Costales J, Padilla A, Rowland E, Aguilar H, Racines J. Seroprevalence and risk factors for Trypanosoma cruzi infection in the Amazon region of Ecuador. American Journal of Tropical Medicine and Hygiene. 2003;69(4):380–385. [PubMed] [Google Scholar]
- Hall G, Patrinos HA. Indigenous peoples, poverty, and human development in Latin America. Houndmills, Basingstoke, Hampshire; New York: Palgrave Macmillan; 2006. p. xix.p. 308. [Google Scholar]
- Hurtig AK, San Sebastian M. Geographical differences in cancer incidence in the Amazon basin of Ecuador in relation to residence near oil fields. International Journal of Epidemiology. 2002a;31:1021–1027. doi: 10.1093/ije/31.5.1021. [DOI] [PubMed] [Google Scholar]
- Hurtig AK, San Sebastian M. Incidence of childhood leukemia and oil exploration in the Amazon basin of Ecuador. International Journal of Occupational and Environmental Health. 2004;10:245–250. doi: 10.1179/oeh.2004.10.3.245. [DOI] [PubMed] [Google Scholar]
- Hurtig AK, San Sebastian M. Gynecologic and breast malignancies in the Amazon basin of Ecuador, 1985–1998. International Journal of Gynecology and Obstetrics. 2002b;76:199–201. doi: 10.1016/s0020-7292(01)00546-x. [DOI] [PubMed] [Google Scholar]
- INEC. Situacion de la salud en el Ecuador: Indicadores basicos por region y provincia. Quito: Instituto Nacional de Estadistica y Censos (INEC); 2001a. Poblacion y principales causas de muerte por provincia de residencia habitiual de la person fallecida y sexo. Tasa de mortalidad por 10000 habitantes. [Google Scholar]
- INEC. VI censo de poblacion y V censo de vivienda, 2001: Resultados Definitivos. Quito, Ecuador: INEC (Instituto Nacional de Estadistica y Censos); 2001b. [Google Scholar]
- Larrea C, Freire W. Social inequality and child malnutrition in four Andean countries. Revista Panamericana de Salud Pública. 2002;11(5–6):356–364. doi: 10.1590/s1020-49892002000500010. [DOI] [PubMed] [Google Scholar]
- MACRO. Improvements in child survival documented in DHS study. Newsletter (Macro Systems. Institute for Resource Development. Demographic and Health Surveys) 1997;8(2):8. [PubMed] [Google Scholar]
- Manesh AO, Sheldon TA, Pickett KE, Carr-Hill R. Accuracy of child morbidity data in demographic and health surveys. International Journal of Epidemiology. 2008;37(1):194–200. doi: 10.1093/ije/dym202. [DOI] [PubMed] [Google Scholar]
- Murphy L. Colonist farm income, off-farm work, cattle, and differentiation in Ecuador’s northern Amazon. Human Organization. 2001;60(1) [Google Scholar]
- Orr C, Dufour D, Patton J. A comparison of anthropometric indices of nutritional status in Tukanoan and Achuar Amerindians. American Journal of Human Biology. 2001;13(3):301–309. doi: 10.1002/ajhb.1053. [DOI] [PubMed] [Google Scholar]
- Passos CJS, Da Silva DS, Lemire M, Fillion M, Guimarães JRD, Lucotte M, Mergler D. Daily mercury intake in fish-eating populations in the Brazilian Amazon. Journal of Exposure Science and Environmental Epidemiology. 2008;18(1):76–87. doi: 10.1038/sj.jes.7500599. [DOI] [PubMed] [Google Scholar]
- Patz JA, Daszak P, Tabor GM, Aguirre AA, Pearl M, Epstein J, Wolfe ND, Kilpatrick AM, Foufopolous J, Molyneux D, Bradley D. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environmental Health Persectives. 2004;112(10):1092–1098. doi: 10.1289/ehp.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichón FJ. Settler households and land use patterns in the Amazon frontier: Farm-level evidence from Ecuador. World Development. 1997;25(1):67–91. [Google Scholar]
- Pitman NCA, Jørgensen PM, Williams RSR, León-Yánez S, Valencia R. Extinction-rate estimates for a modern neotropical flora. Conservation Biology. 2002;16(5):1427–1431. [Google Scholar]
- Quizhpe E, San Sebastián M, Hurtig AK, Llamas A. Prevalence of anaemia in schoolchildren in the Amazon area of Ecuador. Revista Panamericana de Salud Pública. 2003;13(6):355–361. doi: 10.1590/s1020-49892003000500003. [DOI] [PubMed] [Google Scholar]
- Rudel T, Roper J. Regional patterns and historical trends in tropical deforestation, 1976–1990. Ambio. 1996;25(3):160–166. [Google Scholar]
- Rudel TK, Richards S. Urbanization, roads, and rural population change in the Ecuadorian Andes. Studies in Comparative International Development. 1990;25(3):73–89. doi: 10.1007/BF02687180. [DOI] [PubMed] [Google Scholar]
- San Sebastian M, Jativa R, Goicolea I. Epidemiology of malaria in the Amazon basin of Ecuador. Revista Panamericana de Salud Pública. 2000;7(1):24–28. doi: 10.1590/s1020-49892000000100004. [DOI] [PubMed] [Google Scholar]
- San Sebastian M, Armstrong B, Córdoba J, Stephens C. Exposures and cancer incidence near oil fields in the Amazon basin of Ecuador. Journal of Occupational and Environmental Medicine. 2001;58(5):517–522. doi: 10.1136/oem.58.8.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Sebastian M, Armstrong B, Stephens C. Outcomes of pregnancy among women living in the proximity of oil fields in the mazon basin of Ecuador. International Journal of Occupational and Environmental Health. 2002;8(4):312–319. doi: 10.1179/107735202800338650. [DOI] [PubMed] [Google Scholar]
- San Sebastián M, Santi S. The health status of rural school children in the Amazon basin of Ecuador. Journal of Tropical Pediatrics. 1999;45(6):379–382. doi: 10.1093/tropej/45.6.379. [DOI] [PubMed] [Google Scholar]
- San Sebastián M, Hurtig AK. Oil development and health in the Amazon basin of Ecuador: the popular epidemiology process. Social Science & Medicine. 2005;60(4):799–807. doi: 10.1016/j.socscimed.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Sawyer D. Economic and social consequences of malaria in new colonization projects in Brazil. Social Science and Medicine. 1993;37(9):1131–1136. doi: 10.1016/0277-9536(93)90252-y. [DOI] [PubMed] [Google Scholar]
- Sawyer DR, Sawyer DO. The Malaria Transition and the Role of Social Science Research. In: Chen LL, Kleinman A, Ware NC, editors. Advancing Health in Developing Countries. New York: Auburn House; 1992. pp. 105–127. [Google Scholar]
- Schelhas J. Land use choice and change: Intensification and diversification in the lowland tropics of Costa Rica. Human Organization. 1996;55(3):298–306. [Google Scholar]
- Trussel JT. A re-estimation of the multiplying factors for the Brass technique for determining childhood survivorship rates. Population Studies. 1975;29(1):97–107. doi: 10.1080/00324728.1975.10410187. [DOI] [PubMed] [Google Scholar]
- United Nations. Manual X: Indirect Techniques for Demographic Estimation. New York: United Nations; 1983. [Google Scholar]
- United Nations Statistics Division (UNSD) United Nations Common Database (UNCDB) New York: UN; 2008. [Google Scholar]
- Valdivia M. Poverty, health infrastructure and the nutrition of Peruvian children. Economics and Human Biology. 2004;2(3):489–510. doi: 10.1016/j.ehb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Vittor AY, Gilman R, Tielsch J, Glass GE, Shields TM, Lozano WS, Pinedo-Cancino V, Patz JA. The effect of deforestation on the human biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene. 2006;74(1):3–11. [PubMed] [Google Scholar]
- Vittor AY, Pan WK, Gilman RH, Tielsch J, Glass G, Shields T, Sanchez-Lozano W, Pinedo-Cancino V, Patz J. Linking deforestation to malaria in the Amazon: Characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. American Journal of Tropical Medicine and Hygiene. 2009;81(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Weisbrot M, Sandoval L, Cadena B. Ecuador’s Presidential Election: Background on Economic Issues. Washington DC: Center for Economic and Policy Research (CEPR); 2006. p. 16. [Google Scholar]
- WHO, & UNICEF. Review of National Immunization Coverage, 1980–2003: Ecuador. Geneva, Switzerland: World Health Organization, Department of Immunization Vaccines and Biologicals; 2004. [Google Scholar]
- Yasuoka J, Levins R. Impact of deforestation and agricultural development on Anopheline ecology and malaria epidemiology. American Journal of Tropical Medicine and Hygiene. 2007;76(3):450–460. [PubMed] [Google Scholar]
- Yeh S-T. SAS Users Group International (SUGI) Vol. 27. Orlando, FL: 2002. Using trapezoidal rule for the area under a curve calculation. [Google Scholar]