Abstract
Supportive social relationships, including a positive patient-practitioner relationship, have been associated with positive health outcomes. Using the data from a randomized controlled trial (RCT) undertaken in the Boston area of the United States, this study sought to identify baseline factors predictive of patients' response to an experimentally applied supportive patient-practitioner relationship. To sort through the hundreds of potential attributes affecting the patient-practitioner relationship, we applied a false discovery rate method borrowed from the field of genomics and bioinformatics. To our knowledge such a method has not previously been applied to generate hypotheses from clinical trial data. In a previous RCT, our team investigated the effect of the patient-practitioner relationship on symptom improvement in patients with irritable bowel syndrome (IBS). Data were collected on a sample of 289 individuals with IBS using a three-week, single blind, three arm, randomized controlled design. We found that a supportive patient-practitioner relationship significantly improved symptomatology and quality of life. A complex, multi-level measurement package was used to prospectively measure change and identify factors associated with improvement. Using a local false discovery rate procedure, we examined the association of 452 baseline subject variables with sensitivity to treatment. Out of 452 variables, only two baseline factors, reclusiveness, and previous trial experience increased sensitivity to the supportive patient-practitioner relationship. A third variable, additional opportunity during the study for subjects to discuss their illness through experiential interview, was associated with improved outcomes among subjects who did not receive the supportive patient-practitioner relationship. The few variables associated with differential benefit suggest that a patient-centered supportive patient-practitioner relationship may be beneficial for most patients. This may be especially important for reclusive individuals. Within the context of our study, additional study attention in the form of repeated experiential interviews compensated for a lack of positive patient-practitioner support. A supportive patient-practitioner relationship may also help overcome low provider expectations for subjects with previous trial experience. These results converge with the results of the parent trial, implicating the importance of the social world in healing.
Keywords: USA, Patient-practitioner relationship, social factors, randomized controlled trial, false discovery rate analysis
Introduction
Patients who are satisfied with their care are more likely to be self-confident, motivated, practice healthy behaviors, and follow medical advice (Greenfield, Kaplan, Ware, Yano, & Frank, 1988). Satisfied patients have greater confidence in their practitioner thereby maximizing nonspecific healing mechanisms (Roter & Hall, 1992). In contrast, patients who are dissatisfied with care are less likely to make return visits, more likely to switch practitioners, and are likely to have low trust in their practitioner, which undermines nonspecific contextual effects of the medical encounter (Roter & Hall, 1992). From the point of view of health services delivery, poor patient-practitioner communication can jeopardize patients' health and well-being, interfere with practitioners' therapeutic efforts, and waste health resources (DiMatteo & DiNocola, 1982). Conversely, good patient-practitioner communication can improve physiological health status. For example, better patient-practitioner communication has been associated with improvements in blood pressure in patients with hypertension (Orth, Stiles, Scherwitz, Hennrikus, & Vallabone, 1987), in blood sugar levels in diabetics (Kaplan, Greenfield, & Ware, 1989), and in recovery from surgery as measured by decreased use of pain medications and shortened hospital stays (Mumford, Schlesinger, & Glass, 1982).
The patient-practitioner relationship may influence patients' health status by serving as a primary bond and offering social support (Kaplan, Greenfield, & Ware, 1989; Roter & Hall, 1992). High perceived or observed social support has been linked to improved health outcomes in both human and animal studies. Low levels of social support are associated with altered immune function in observational and experimental studies of both humans and animals (e.g. Cohen, 2004; Kiecolt-Glaser, Fisher, Ogrocki, Stout, Speicher, 1987; Pressman, Cohen, Miller, Barkin, Rabin, & Treanor, 2005; Thomas, Goodwin, & Goodwin, 1985; Uchino, Cacciopo, Kiecolt-Glaser, 1996). The reciprocal is also true: adequate social support may buffer or mediate responses to stress and allow for the maintenance of a healthy immune system. For example, chronic stress has been reported to alter immune function and cytokine production (Wright, Cohen, Wand, & Gold, 2004) and to reduce the immune system's response to anti-inflammatory signals (Miller, Cohen, & Ritchey, 2002). The chronic stressor of low socioeconomic status in childhood is inversely related to immune function independent of higher socioeconomic status later in life (Cohen, Doyle, Turner, Alper & Skoner, 2004). Generally, people with better quality relationships feel valued and are healthier (Uchino, Cacciopo, & Kiecolt-Glaser, 1996; Berkman & Kawachi, 2000).
Positive relationships can be found in the therapeutic context as well as an individual's greater social context. Most randomized controlled trials (RCTs) are designed with the idea that the placebo effect includes effects of therapeutic context (Kaptchuk, 1998). Thus if the effect of a specific pharmacologic agent or treatment is under study, the placebo arm controls for the social context of the medical interaction. In a previous study, we were able to demonstrate that a supportive patient-practitioner relationship can offer significant clinical benefit to patients with irritable bowel syndrome (IBS) (Kaptchuk et al., 2008). Participants improved across a range of measures of IBS symptomatology and quality of life in parallel with the amount of support offered in the supportive patient-practitioner relationship.
Irritable bowel syndrome was chosen as the target illness for the original study because we were interested in the effects of psychosocial variables and their mechanisms of action in healing. IBS belongs to a class of illnesses (such as chronic pain and depression) in which psychosocial interactions have been observed to play a critical role in symptom expression. Also, IBS is one of the ten top reasons for consultation with a primary care physician; nearly one third of all consultations by gastroenterologists are for IBS (Mitchell & Drossman, 1987). The annual estimated direct cost of IBS treatment in the United States, including physician visits, diagnostic testing, and outpatient care, is between $1.7 billion and $10 billion dollars; this is excluding prescription and over the counter drug costs (Martin, Barron, & Zacker, 2001; Sandler et al., 2002). Still, current treatments are inadequate for many patients (Drossman, Whitehead, & Camilleri, 1997). IBS also appears to have a complex etiology, with perhaps multiple subtypes and multiple causes. To capture this variation and generate hypotheses as to the mechanisms of IBS symptomatology and healing, the parent trial included multiple measures beyond the main outcome; for example, measures of psychological, social, and physiological change. Taken together, these multiple measures allowed the study to capture change at multiple levels and for different subtypes of IBS in order to better understand the causes and mechanisms of this disease.
In this study we look for characteristics that modify the potential positive effects of a supportive relationship given in a therapeutic context. In particular, we look for patient characteristics that predict who is most or least likely to benefit from a supportive patient-practitioner relationship. This secondary data analysis project uses a false discovery rate (FDR) (Benjamini & Hochberg, 1995) procedure to identify characteristics of IBS patients that differentiate their response to the three different healing contexts employed in the parent IBS trial. The FDR procedure is widely used in fields with high-dimensional data, e.g., gene-wide association studies where thousands, perhaps 100,000's, of genes are tested for association with a condition of interest. The large number of statistical tests performed in such cases would lead to high rates of false rejections of the null hypotheses without appropriate adjustment. The FDR procedure is a powerful method for drawing appropriate inference in the face of this multiple comparison problem. It allows an investigator to limit the expected proportion of outcomes declared significant that are in fact spurious or false. This paper identifies the predisposing factors that are most sensitive to a supportive patient-practitioner interaction and uses the FDR procedure to limit our selection to those with sufficiently strong support to infer that their likelihood of representing real associations is high.
Methods
The data for this study come from 289 individuals in our previous three-week, three-arm RCT testing the impact of the patient-practitioner relationship on a sample of IBS patients drawn from the Boston area of the United States (Kaptchuk et al., 2008). The trial was approved by applicable ethics boards and participants gave written informed consent. We hypothesized that non-specific contextual factors, or what are commonly called placebo effects, could be experimentally separated into three progressively applied components which defined the three arms of the trial: (1) assessment and observation (operationalized as a wait-list condition), (2) a therapeutic ritual (operationalized as a placebo treatment with a “limited” or minimal patient-practitioner interaction), and (3) an “augmented” or supportive patient practitioner relationship (operationalized as a supportive patient-practitioner interaction added to a placebo treatment). Both the “limited” and “augmented” patient-practitioner relationships were scripted. The placebo was a validated, non-invasive sham acupuncture device. In the limited condition, each subject was told that the practitioner had read his/her intake and that interactions needed to be kept to a minimum because of the scientific nature of the treatment. In contrast, the augmented patient practitioner interaction included a review of general health, emotional concerns, lifestyle questions, and an exploration of the meaning of the disease for the subject. The augmented patient practitioner interaction included specific context enhancements: natural expressions of empathy, attentive listening, thoughtful silence, and natural expressions of positive expectations and the appropriateness of the intervention. In all three treatment arms, no direct medical advice was given and practitioners were instructed not to use any form of psychotherapy found to be effective in IBS (such as cognitive behavioral therapy). Fidelity to treatment was accomplished through the use of extensive training of the practitioners. Independent fidelity assessments based on videotaped treatment sessions conducted throughout the trial yielded excellent agreement between raters judging practitioners on predetermined measures of adherence to treatment protocol (kappa = 0.91) (Kaptchuk et al., 2008; Conboy et al., 2006). A subsample of 27 individuals (9 from each treatment arm) was also simultaneously randomized to receive a qualitative interview exploring the subjects' experience with the illness, trial participation, and their social world. Details of the research design (Conboy et al., 2006), main quantitative results (Kaptchuk et al., 2008), and of the qualitative study are described elsewhere (Kaptchuk et al., 2009).
We sought patient characteristics predictive of differential benefit from a supportive patient-practitioner relationship from among 452 baseline variables. The variables included single item questions as well as constructed scales and included measures and items evaluating: (1) psychological status assessed by the Beck anxiety inventory (Kabacoff, Segal, Hersen, & Van Hasselt, 1997; Piotrowski, 1999) and the Carroll depression scale (Carroll, Feinberg, Smouse, Rawson, & Greden, 1981), (2) social factors including demographics and Social Network Index (Cohen, Doyle, Skoner, Rabin, & Gwaltney, 1997; Cohen & Hoberman, 1983; Cohen, Kamarck, & Mermelstein, 1983; Cohen, Mermelstein, Kamarck, & Hoberman, 1985; Cohen & Williamson, 1988), (3) IBS symptomatology including measures of Adequate Relief (Camilleri, Mayer, Drossman, Heath, Dukes, McSorley, Kong, Mangel, & Northcut, 1999; Camilleri et al., 2000) i,ii and Global Symptom Improvement (Lembo et al., 2001; Gordon et al., 2003), and (4) inflammatory cytokines as blood markers for elevated stress and inflammation. Missing data were addressed in scale construction by dropping a subject's scale response if more than 20% of items were missing; if 20% or fewer items were missing, the scale was constructed by imputing the individual's average on the scale for missing items.
We selected four outcome measures for this analysis (Table 1). Each was measured at baseline and three weeks later. These measurements were chosen as unique descriptors of IBS symptomatology. The IBSSS (Francis, Morris & Whorwell, 1997) assesses effects of IBS on quality of life, IBS severity, stool frequency, and satisfaction with bowel habit. The IBSSS is constructed as a 500-point scale, offering fine-grained assessment. The IBS QOL (Drossman et al., 2007) assesses how the illness affects the subject's life and relationships. The McGill Pain scale (Melzack, 1975) is widely used in pain research and allows for the greatest potential to generalize from our results. The Bristol Stool Form scale (Lewis & Heaton, 1997) is well-recognized clinically and may avoid some reporting bias as patients record the shape of the stool using pictorial cues rather than reporting subjective levels of discomfort.
Table 1.
Descriptions of the four outcome measures. The four treatment outcome measurements used and a brief description are offered.
| Scale | Description |
|---|---|
| Irritable Bowel Symptom Severity Scale (IBSSS) (Francis et al., 1997) | Measures the sum of the participant's evaluation on a 100-point scale in each of five IBS areas: pain severity, pain frequency, severity of abdominal distension, dissatisfaction with bowel habits, and interference with quality of life. All 5 areas are equally rated yielding a theoretical range of 0-500. Higher scores indicate greater severity of symptoms. |
| Irritable Bowel Syndrome Quality Of Life (IBS-QOL) (Drossman et al., 2007; Drossman & Lembo, 2002) | This 35-item scale is designed to assess the impact of IBS on 8 dimensions of health status including: dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, and sexual relationships. It shows high internal consistency, reproducibility, good convergent and discriminant validity. Higher scores indicate a higher quality of life. |
| McGill Pain Scale (Melzack, 1975) | This well-validated, reliable 15-item measure evaluates the quality of subjective IBS pain experience using 3 major classes of word descriptors--sensory, affective and evaluative. Higher scores indicate greater pain. |
| Bristol Stool Form Scale (Lewis & Heaton, 1997) | This seven-level scale asks the subject to rate the form of the stool using pictorial cues. The form of the stool indicates transit time from severe constipation to severe diarrhea. We transformed this 7-point scale to a 4-point scale with 0 indicating normal stool at the midpoint between constipation and diarrhea and higher scores indicating greater deviation from normal stool, either toward constipation or diarrhea. |
Statistical Analysis
The relationships among the three experimentally imposed patient-practitioner interactions, 452 baseline characteristics, and four outcomes were estimated by analysis of covariance (ANCOVA), with a separate model fit for each baseline by outcome combination. In each model, a given outcome measured at 3 weeks was predicted by the same outcome measured at baseline, a given baseline characteristic, treatment group as a categorical variable, and the treatment × baseline characteristic interaction. The interaction between treatment group and baseline characteristic provided an estimate of the degree to which differential effects of a supportive patient-practitioner interaction depended on an individual's baseline characteristic. To focus on dependencies between patient-practitioner interactions and baseline characteristics that reflected a dose-effect of practitioner support, our primary measure of the treatment × baseline characteristic interaction was the Wald score from a one degree of freedom contrast estimating the linear dependence between treatment group and baseline characteristic. Specifically, we tested for a linear dose-response across treatment groups (wait-list < limited < augmented with unit intervals between treatments) in the dependence of any outcome on a given baseline characteristic using a contrast c = {-1, 0, 1} applied to the wait-list × baseline, limited × baseline, and augmented × baseline coefficients, with coefficients estimated using reference cell coding. The contrast was large when participants with high scores for a given baseline characteristic accrued greater benefit from the most supportive patient-practitioner context relative to the wait-list context and participants with low baseline scores accrued less benefit from the supportive context or even greater benefit when randomized to the wait-list group. Contrasts were also large, although negative, when high baseline scores were associated with less benefit from supportive contexts relative to participants with low baseline scores for a given characteristic assuming that higher scores on the outcome measure indicate better health.
With over 450 baseline characteristics evaluated for differential association with each of four outcomes, use of nominal comparison-wise p-values would lead to recognition of a large number of false associations. If none of our measured characteristics were truly associated with differential response to supportive patient-practitioner environment but we evaluated each comparison using a conventional threshold of p < 0.05, then we would expect 0.05 * 452 ≈ 23 characteristics falsely declared significant for each outcome. Applying a Bonferroni adjustment to control for multiple comparisons would require p < 0.05 / 452 ≈ 0.0001 to declare significance if we viewed analyses of each outcome separately. This would provide strong protection against family-wise false positives, holding the probability of any false positives across all characteristics tested to 5%, but by only recognizing associations that meet the p < 0.0001 threshold, power for detecting true associations would be very low. To increase our probability of detecting true associations, we used a local false discovery rate (FDR) procedure (Gordon et al., 2003) to control the expected false positive rate, a less stringent criterion than controlling the family-wise error rate.
False discovery rate techniques solve the multiple comparison problem by calculating a probability that a given test is a false discovery and accepting as potentially real discoveries only those with an acceptably low probability of being false. This follows the approach of traditional hypothesis testing in which low probabilities of observed data given the null hypothesis are a basis for accepting the alternative. The FDR technique controls multiple comparison error rates by calculating the expected probability of false discovery across all comparisons. The FDR technique is widely used in bioinformatic analyses, e.g., genome-wide association studies where many thousands of potential associations between genetic variation, generally single-nucleotide polymorphisms, and presence of a clinical condition are tested. FDR procedures have rarely been used in clinical trials because a large number of predictors is needed for reliable estimation. Yet, given a large enough data set, FDR procedures provide an objective tool with good power for exploratory analysis and identification of associations that are worthy of further investigation. We employed the procedure in this study to generate hypotheses for future research.
The false discovery proportion (FDP), the proportion of “discoveries” that do not reflect true associations, yielded by the FDR technique when assuming a standard normal distribution for the distribution of test results matches the nominal false discovery rate when the tests evaluated are independent, but the FDP is higher than the nominal level when test results are positively correlated (and lower than the nominal level when results are negatively correlated). Similar bias can arise if we omitted important covariates from the model that affect the linear interaction term. We employed a local FDR technique implemented in the locfdr package (version 1.1-6, Efron 2006) of R. An empirical null distribution was used, estimated by maximum likelihood from the observed distribution of statistics. This procedure corrects for bias due to correlated outcomes and model misspecification (Efron, 2007). This was beneficial given the presence of strong associations among many of the characteristics we investigated and the possibility of unmodeled confounders. Specifically, the approach assumes that the great majority of predictors are not associated with differential benefit from patient-practitioner interactions (null predictors), with only a few “interesting”, non-null predictors mixed in. Using this assumption, the FDR technique (Efron, 2007) estimates the full distribution of null predictors based on the center of the Wald score distribution and then calculates a local false discovery rate for a given predictor as the posterior probability of being a false discovery using an empirical Bayes rule (Efron, 2004). Specifically, the local FDR for a test with a given Wald score zi is the probability of zi given that it is from a null predictor divided by the probability of zi among all the data. This yields a conservative FDR estimate based on an implicit prior probability of being a null predictor of 100%. We used an FDR threshold of 0.20 to identify “true” predictors, i.e., those with less than a 20% probability of being a false discovery.
Given the sample size of the current study and the observed variation and covariation among our estimates of interactions between participant characteristics and treatment, an FDR threshold of 0.20 would be expected to identify only 21% of the true predictors of differential response to patient-physician environments. To achieve an 80% probability of identifying true predictors of differential response as “interesting” based on an FDR threshold of 0.20, we would need to increase the sample size 3.54 times, from 289 participants to 1022 (Efron, 2007). Despite this low power, the exploratory nature of the analysis supports the use of this technique. This approach is more powerful than traditional alternatives for controlling family-wise error rates. Moreover, our protection against false discoveries is not diminished by low power.
Results
The 289 IBS patients enrolled in the study were predominantly white, well-educated, and female. Most participants had experienced their IBS symptoms for more than a year. The sample did not exhibit higher than expected levels of psychological distress and symptom levels were moderate. Other specifics of patient demographics and study design are reported elsewhere (Kaptchuk et al., 2008).
Only one of the four outcomes investigated, the IBS Symptom Severity Scale (IBSSS), yielded three baseline predictors with adequate support at a false discovery rate of 0.20 (Table 2). Among the other three outcomes, no predictor had an estimated probability of being a false discovery less than 20%—all baseline characteristics are interpreted as null predictors, unassociated with differential sensitivity to patient-practitioner environment.
Table 2.
Baseline characteristics with probability of false discovery less than 20%. Four outcomes of symptom improvement (described in Table 1) were considered and 452 variables were tested as predictors for each. Three interesting predictors were found for the prediction of the IBSS outcome only and are listed below.
| Interesting predictors for IBSSS | Interpretation | Wald score |
|---|---|---|
| Single item from the Carroll depression scale (Caroll, Feinberg, Smouse, Rawson, & Greden, 1981)related to reclusiveness. | Participants who disagreed with the statement, “I still like to go out and meet people”, were more sensitive to the most supportive treatment. | 2.864 |
| Inclusion in the qualitative substudy group. | Participants in the qualitative group were less sensitive to the most supportive treatment. | 3.128 |
| Experience as a clinical research subject | Participants who have ever participated in any clinical research trial for health problems other than IBS were more sensitive to the most supportive treatment. | -3.148 |
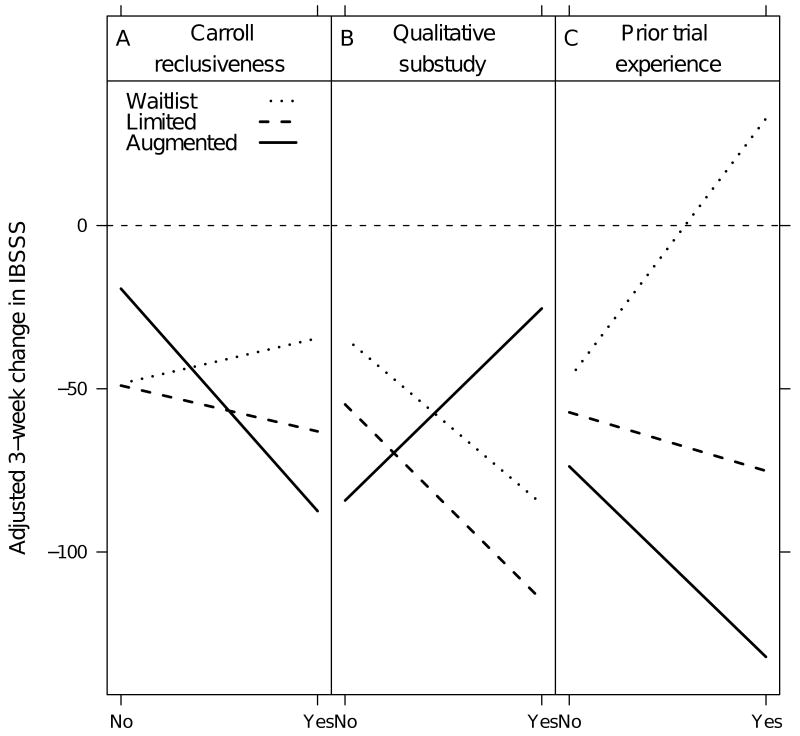
Figure 1 illustrates the interactions between the three predictors discovered and response to each of the three treatment groups. A larger decrease in IBSSS scores indicates that the group in question achieved greater reductions in IBS symptoms over 3 weeks of treatment. For example, Figure 1a addresses differential response to the three treatments based on participants' reclusiveness as measured by a single item from the Carroll Depression scale. Participants whose depression manifests as reclusiveness (denoted as yes) were more sensitive to the most supportive treatment than those that did not report this type of reclusiveness (as indicated by the sharp decrease of the “augmented” or most supportive group). Figure 1b demonstrates that those in the qualitative interview group and who were in waitlist or limited interaction protocols (denoted as yes) experienced more improvement than those not in the qualitative (denoted as no). In contrast, those qualitative interview participants who were in the augmented protocol, received less benefit from the augmented context as indicated by increased slope. Figure 1c indicates that previous trial experience (denoted as yes) was associated with increased sensitivity to the supportive patient-practitioner relationship (Figure 1c).
Figure 1.
Predictors of change in IBSSS scores for three groups, where Yes denotes item endorsement and No denotes lack of endorsement.
Discussion
Our results suggest that only a few factors are likely correlated with differential change in IBSSS scores across treatments; most of the baseline characteristics were not related more strongly than expected by chance. While this could be an issue of power, it is also possible that the supportive patient-practitioner relationship is beneficial for participants presenting with a variety of baseline characteristics; that is, a supportive patient-centered patient-practitioner relationship is universally beneficial in this sample. Further, the three variables found to be of interest in this analysis follow the results we found in the parent trial and underscore the importance of social factors in healing.
It appears that a certain amount of support from either the individual's greater social world or from the study environment is related to likelihood of healing. A lack of support is related to worse outcomes. For example, the found variable from the Carroll Depression scale suggest that patients whose depression manifests as reclusiveness were more sensitive to the most supportive treatment than those that did not report this type of reclusiveness. These more reclusive individuals appear to benefit from the highly supportive patient-practitioner interaction, and this support may make up for a lack of interaction in their greater social world.
This same idea of a lack of support may underlie what was found in the second item concerning participation in the qualitative substudy. The additional qualitative interview may make up for a lack of therapeutic interaction for those in the waitlist group. Participation in the qualitative substudy can be considered an additional dose of these same supportive factors found in the most supportive patient-practitioner interaction. During the interviews, subjects were encouraged to talk about their social relationships and consider how IBS may be related to social factors. The interviewer asked subjects about their social world and if IBS impacted their relationships and ability to perform daily activities. Second, for the duration of the study, these qualitative participants had an additional therapeutic relationship in the form of a partnership with an empathic interviewer. Evidence of this bond was clear at debriefing when subjects were asked how they felt about the interview process. All of the subjects were positive about the interview process and most of the subjects spoke of the therapeutic nature of the qualitative interview (manuscript with this data under preparation). This was the case even though the interviews were not designed to be directly therapeutic. Interviewers merely showed interest, asked questions, and listened. It was surprising then that individuals who received the most supportive patient-practitioner relationship and the qualitative interview showed lower levels of improvement than those without the most supportive interaction. These two types of theoretically supportive relationships do not appear to be additive in their benefit in this sample. This finding could merely be a statistical artifact or, just as likely, because the sample size of the qualitative group was so small, inclusion in this group may be a proxy for another variable. Further analysis will explore these possibilities.
The finding that previous clinical trial experience increases sensitivity to the supportive patient-practitioner relationship could be interpreted in a number of ways. Perhaps seasoned subjects had experienced negative relationships during their previous trial experience. The augmented interventions may have appeared to them particularly therapeutic given their previous experience. Alternatively, wait list participants who had previous trial experience may have expected more from the trial. That previous trial experience may increase sensitivity to treatment raises concerns for interpretation of the results of clinical trials more generally. The short duration and scientific goals of a clinical trial do not typically allow for the establishment of a rich patient-provider relationship. It appears that this rich relationship can be very powerful. The lack of this rich relationship in experimental trials may be one reason why clinical and experimental results often differ (Kaptchuk, 2001). This finding fits with a growing literature on the powerful influence of patient experiences in clinical trials and their understandings of these experiences (Murtagh, Thomson, May, Rapley, Heaven, Graham, Kaner, Stobbart, & Eccles, 2007; Heaven, Murtagh, Rapley, May, Graham, Kaner, & Thomson, 2006).
Moreover, although our method necessarily results in tentative, hypothesis generating findings we have found support for the need to better understand the influence of patient-provider interactions in healing, both toward the goal of harnessing the powerful effects of this relationship and to better understand the limits to the generalizability of results gathered using experimental designs. More research into the mechanisms of how empathic social relationships aid healing may reveal how individuals' understandings of their social world can be modified to produce positive clinical outcomes. Such social treatments may prove highly beneficial, as our parent study found. Furthermore, as this study demonstrates, this supportive, emergent, therapeutic environment appears to benefit universally across the IBS sample, supporting further study of such an intervention in other diseases, health states, and with known active medications.
Acknowledgments
This analysis and research was supported by Samueli Institute for Information Biology. The research was also supported by Grant Number 1K24 AT004095 from the National Center for Complementary and Alternative Medicine (NCCAM), Grant Number 1R01 AT01414-01 from NCCAM and the National Institutes of Digestive, Diabwetes and Kidney Disease (NIDDK), Grant Number 1R21 AT002860-01 from NCCAM and the Office of Behavioral and Social Science Research (OBSSR).
Footnotes
Camilleri, M., Mayer, E. A., Drossman, D. A., Heath, A., Dukes, G. E., McSorley, D., Kong, S., Mangel, A. W., & Northcutt, A. R. (1999). Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Alimentary Pharmacology & Therapeutics, 13, 1149-59.
Camilleri, M., Northcutt, A. R., Kong, S., Dukes, G. E., McSorley, D., & Mangel, A. W. (2000). Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet, 355, 1035-1040.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lisa Ann Conboy, Harvard Medical School, Boston, MA UNITED STATES [Proxy], lisa_conboy@hms.harvard.edu.
Eric Macklin, Department of Biostatistics, Massachusetts General Hospital, Boston, MA.
John Kelley, Department of Psychiatry, Massachusetts General Hospital, Boston, MA.
Efi Kokkotou, 4Department of Gastroenterology, Beth Israel Hospital, Boston, MA.
Anthony Lembo, 4Department of Gastroenterology, Beth Israel Hospital, Boston, MA.
Ted Kaptchuk, 1Osher Research Center, Harvard Medical School, Boston, MA.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. (B).Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Berkman L, Kawachi I. Social Epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- Camilleri M, Mayer EA, Drossman DA, Heath A, Dukes GE, McSorley D, Kong S, Mangel AW, Northcutt AR. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Alimentary Pharmacology & Therapeutics. 1999;13:1149–59. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–1040. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Smouse PE, Rawson SG, Greden JF. The Carroll rating scale for depression. I. Development, reliability and validation. The British Journal of Psychiatry. 1981;138:194–200. doi: 10.1192/bjp.138.3.194. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood Socioeconomic Status and Host Resistance to Infectious Illness in adulthood. Psychosomatic Medicine. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hoberman H. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research, and application. The Hague, Holland: Martinus Nijhoff; 1985. [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the U.S. In: Spacapam S, Oskamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Conboy L, Wasserman RH, Legedza AT, Park M, Rivers A, Morey EB, Nam BH, Jacobson EE, Lembo AJ, Kaptchuk TJ, Kerr CE. Investigating Placebo Effects in Irritable Bowel Syndrome: A Novel Research Design. Journal of Controlled Clinical Trials. 2006;27:123–134. doi: 10.1016/j.cct.2005.11.012. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, DiNocola DD. Achieving Patient Compliance. New York: Pergamon Press; 1982. [Google Scholar]
- Drossman DA, Lembo AJ. Contemporary Diagnosis and Management of Irritable Bowel Syndrome. Newtown, PA: 2002. [Google Scholar]
- Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 1997;112:2120–2137. doi: 10.1053/gast.1997.v112.agast972120. [DOI] [PubMed] [Google Scholar]
- Drossman D, Morris CB, Hu Y, Toner DB, Diamant N, Whitehead WE, Dalton CB, Leserman J, Patrick DL, Bangdiwala SI. Characterization of Health Related Quality of Life (HRQOL) for Patients with Functional Bowel Disorder (FBD) and its response to treatment. American Journal of Gastroenterology. 2007;102:1–12. doi: 10.1111/j.1572-0241.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- Efron B. Large-Scale Simultaneous Hypothesis Testing: The Choice of a Null Hypothesis. Journal of the American Statistical Association. 2004;99:96–104. [Google Scholar]
- Efron B. Size, power and false discovery rates. Annals of Statistics. 2007;35:1351–1377. [Google Scholar]
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary & Pharmacological Therapy. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- Gordon S, Ameen V, Bagby B, Shahan B, Jhingran P, Carter E. Validation of irritable bowel syndrome Global Improvement Scale: an integrated symptom end point for assessing treatment efficacy. Digestive Diseases & Sciences. 2003;48:1317–1323. doi: 10.1023/a:1024159226274. [DOI] [PubMed] [Google Scholar]
- Greenfield S, Kaplan SH, Ware JE, Jr, Yano EM, Frank HJL. Patient's participation in medical care: Effects on blood sugar control and quality of life in diabetics. Journal of General Internal Medicine. 1988;3:448–457. doi: 10.1007/BF02595921. [DOI] [PubMed] [Google Scholar]
- Heaven BR, Murtagh MJ, Rapley T, May CR, Graham RH, Kaner EF, Thomson RG. Patients or research subjects? A qualitative study of participation in a randomized controlled trial of a complex intervention. Patient Education and Counseling. 2006;62(2):260–270. doi: 10.1016/j.pec.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Kabacoff RI, Sega DL, Hersen M, Van Hasselt VB. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. Journal of Anxiety Disorders. 1997;11(1):33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- Kaplan SH, Greenfield S, Ware JE., Jr Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Medical Care. 1989;27:S110–127. doi: 10.1097/00005650-198903001-00010. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Lembo AJ, Csordas TJ, Jacobson EE. Maybe I made up the whole thing: Placebos and patients' experiences in a randomized controlled trial. Culture Medicine & Psychiatry. 2009;33:382–411. doi: 10.1007/s11013-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ. The double-blind randomized controlled trial: gold standard or golden calf. Journal of Clinical Epidemiology. 2001;54:541–49. doi: 10.1016/s0895-4356(00)00347-4. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ. Powerful placebo: the dark side of the randomized controlled trial. Lancet. 1998;351:1722–1725. doi: 10.1016/S0140-6736(97)10111-8. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kelley JM, Conboy LA, Kerr CE, Jacobson EE, Kirsch I, Schnyer RN, Nguyen L, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AL. Components of placebo effect: randomized controlled trial in patients with irritable bowel syndrome. BMJ. 2008;336:999–1003. doi: 10.1136/bmj.39524.439618.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE. Marital quality, marital disruption and immune function. Psychosomatic Medicine. 1987;49:13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Lembo AL, Wright RA, Bagby B, Decker C, Gordon S, Jhingran P. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. American Journal of Gastroenterology. 2001;96:2662–2670. doi: 10.1111/j.1572-0241.2001.04128.x. [DOI] [PubMed] [Google Scholar]
- Lewis S, Heaton K. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology. 1997;32(9):920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- Martin R, Barron JJ, Zacker C. Irritable bowel syndrome: toward a cost effective management approach. American Journal of Managed Care. 2001;7(Suppl 8):S268–S275. [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey KA. Chronic Psychological Stress and the Regulation of Pro-Inflammatory Cytokines: A glucocorticoid-Resistance Model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Mitchell CM, Drossman DA. Survey of the AGA membership relating to patients with functional gastrointestinal disorders. Gastroenterology. 1987;92:1282–1284. doi: 10.1016/s0016-5085(87)91099-7. [DOI] [PubMed] [Google Scholar]
- Mumford E, Schlesinger HJ, Glass GV. The effects of psychological intervention on recovery from surgery and heart attacks: An analysis of the literature. American Journal of Public Health. 1982;72:141–151. doi: 10.2105/ajph.72.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh MJ, Thomson RG, May CR, Rapley T, Heaven BR, Graham RG, Kaner EF, Stobbart L, Eccles MP. Qualitative methods in a randomised controlled trial: the role of an integrated qualitative process evaluation in providing evidence to discontinue the intervention in one arm of a trial of a decision support tool. Quality and Safety in Health care. 2007;16(3):224–229. doi: 10.1136/qshc.2006.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth JE, Stiles WB, Scherwitz L, Hennrikus D, Vallabone C. Patient exposition and provider explanation in routine interviews and hypertensive patients' blood pressure control. Health Psychology. 1987;6(1):29–42. doi: 10.1037//0278-6133.6.1.29. [DOI] [PubMed] [Google Scholar]
- Piotrowski C. The status of the Beck Anxiety Inventory in contemporary research. Psychological Reports. 1999;85(1):261–262. doi: 10.2466/pr0.1999.85.1.261. [DOI] [PubMed] [Google Scholar]
- Pressman S, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor J. Loneliness, social network size and immune response to influenza vaccination in college freshmen. Health Psychology. 2005;24(3):297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Roter DL, Hall JA. Doctors talking with patients: Patients talking with doctors. Auburn House, CT: 1992. [Google Scholar]
- Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- Thomas PD, Goodwin JM, Goodwin JS. Effect of social support on stress-related changes in cholesterol levels, uric acid level and immune function in an elderly sample. American Journal of Psychiatry. 1985;142:735–7. doi: 10.1176/ajp.142.6.735. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacciopo JT, Kiecolt-Glaser JK. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Wright RJ, Cohen S, Wand M, Gold DR. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy & Clinical Immunology. 2004;113:1051–1057. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]