Abstract
We sought to determine whether there are structural and metabolic changes in the brains of older adults with cognitive complaints yet who do not meet MCI criteria (i.e., preMCI). We compared the volumes of regional lobar gray matter (GM) and medial temporal lobe structures, including the hippocampus, entorhinal cortex (ERC), fusiform and parahippocampal gyri, and metabolite ratios from the posterior cingulate in individuals who had a Clinical Demetia Rating (CDR) of 0.5, but who did not meet MCI criteria (preMCI, N = 17), patients with mild cognitive impairment (MCI, N = 13), and cognitively normal controls (N = 18). Controls had more ERC, fusiform, and frontal gray matter volume than preMCI and MCI subjects and greater parahippocampal volume and more posterior cingulate N-acetylaspartate (NAA)/myoinosotil (mI) than MCI. There were no significant differences between MCI and preMCI subjects on any of these measures. These findings suggest there are neurodegenerative changes in the brains of older adults who have cognitive complaints severe enough to qualify for CDR = 0.5 yet show no deficits on formal neuropsychological testing. The results further support the hypothesis that detection of individuals with very mild forms of Alzheimer's disease (AD) may be facilitated by use of the CDR, which emphasizes changes in cognition over time within individuals rather than comparison with group norms.
Keywords: Aging, MCI, AD, Structural MRI, MRS
1. Introduction
The ability to detect evidence of prodromal Alzheimer's disease (AD) is important for clinical management and care planning. The recent development of therapies that may potentially slow disease progression in AD has further highlighted the significance of identifying prodromal AD at the earliest stage possible. Numerous studies have used magnetic resonance imaging (MRI) to investigate the in vivo pathology of mild cognitive impairment (MCI), considered by many to be a transitional phase between normal aging and AD (Petersen et al., 1999). The clinical criteria for MCI require impairment in one or more cognitive domains, as demonstrated by neuropsychological testing, yet not severe enough for a diagnosis of dementia (Petersen et al., 1999). Although there are many older adults who have cognitive complaints but who do not meet MCI criteria (Saykin et al., 2006; Storandt et al., 2006), few imaging studies have examined stages of prodormal AD earlier than MCI (i.e., preMCI). One exception is a recent study by Saykin et al. (2006) in which the authors reported structural MRI changes in the brains of older adults who, despite having cognitive complaints, performed normally relative to peers of same age and education on memory tests. Based on these findings, Saykin and colleagues suggested that cognitive complaints in older adults, even when unaccompanied by deficits on formal testing, may indicate underlying neurodegenerative changes. This is an important finding because there is evidence to suggest that even though these older individuals do not meet MCI criteria, they may still be at increased risk for future cognitive decline and conversion to AD (Storandt et al., 2006).
Saykin and colleagues used multiple inventories to quantify cognitive complaints older adults, including the memory self-rating questionnaire (Squire et al., 1979), self and informant versions of the neurobehavioral function and activities of daily living scale (Saykin, 1992; Saykin et al., 1991), self and informant versions of the informant questionnaire on cognitive decline in the elderly (Jorm and Jacomb, 1989), the four cognitive items from the Geriatric Depression Scale (GDS) (Yesavage et al., 1982), 10 cognitive items from a telephone-based screening for MCI (Rabin et al., 2004), and 23 items from the Memory Assessment Questionnaire, adapted in part from the Functional Activities Questionnaire (Pfeffer et al., 1982). Because it is not practical to administer this many questionnaires and inventories in a clinical setting, we sought to quantify cognitive complaints in older adults using just the Clinical Dementia Rating (CDR) (Morris, 1993). The CDR was developed at the Memory and Aging Project at Washington University School of Medicine in 1979 for the evaluation and staging of dementia severity. It involves an informant-based interview to ascertain decline in the previously established cognitive and functional abilities of the individual, a clinical examination of the individual, and the assessor's synthesis of all observations from the interview and examination to determine the presence of dementia, and when present, its severity. A CDR score of 0 denotes no cognitive impairment while a CDR of 0.5 suggests that an individual is “neither demented nor clearly healthy” (Hughes et al., 1982).
The goal of this study was to determine whether structural and spectroscopic magnetic resonance imaging can detect changes in brains of older adults who have a CDR of 0.5 but who show no deficits on formal neuropsychological testing and therefore do not meet MCI criteria. We compared these preMCI subjects to patients with MCI and cognitively normal controls.
Hippocampal atrophy is a well-described feature of AD (Hsu et al., 2001; Kantarci and Jack, 2003; Zakzanis et al., 2003), risk for developing AD (den Heijer et al., 2006; Mungas et al., 2005), and to some extent, in normal aging (de Leon et al., 1997; Hedden and Gabrieli, 2005; Jack et al., 2000; Petersen et al., 2000). Therefore, the first objective of this study was to compare hippocampal volume in preMCI, MCI, and cognitively normal controls. Convit et al. (2000) reported that including the volume of the fusiform gyrus along with hippocampal volume significantly improved the ability to separate patients with MCI from those with AD. Therefore, the second objective of this study was to compare fusiform volume in preMCI, MCI, and control subjects.
Previous histopathological findings indicate that the neurofibrillary tangles of AD first appear in the anterior parahippocampal region, which encompasses the ectorhinal (Brodmann area 36), perirhinal (Brodmann 35), and entorhinal (Brodmann 28) areas of the medial temporal lobe (MTL) (Braak and Braak, 1991; Morris and Price, 2001). Consistent with these findings, several imaging studies have noted atrophy in the parahippocampal gyrus (Visser et al., 1999; Wolf et al., 2003) and entorhinal cortex (ERC) (de Toledo-Morrell et al., 2000; Xu et al., 2000; Dickerson et al., 2001; Du et al., 2001) in MCI patients compared to normal elderly. ERC atrophy has also been reported to accurately predict future conversion of MCI to AD (de Toledo-Morrell et al., 2000; Killiany et al., 2000; Dickerson et al., 2001; de Leon et al., 2001). Therefore, the third objective of this study was to compare the volumes of the ERC and parahippocampal gyrus in preMCI, MCI, and control subjects.
The results from in vivo neuroimaging studies suggest that normal aging, MCI, and AD are all associated with gray matter (GM) loss. Compared to normal controls, several research groups have reported GM atrophy in the temporal lobe (Baron et al., 2001; Kidron et al., 1997), posterior cingulate (Baron et al., 2001; Busatto et al., 2003; Scahill et al., 2002), and precuneus (Baron et al., 2001; Busatto et al., 2003; Frisoni et al., 2002) in AD and GM atrophy in the MTL (Karas et al., 2004; Pennanen et al., 2005), insula, and thalamus (Karas et al., 2004) in MCI. Global brain atrophy has also been shown to predict the imminent progression from MCI to AD (Jack et al., 2004). In normal aging, cross-sectional (Bartzokis et al., 2001; Jernigan et al., 2001; Raz et al., 2004) and longitudinal (Pfefferbaum et al., 1998; Resnick et al., 2003; Scahill et al., 2003; Raz et al., 2005) studies have reported GM atrophy, most prominently in the prefrontal cortex. Therefore, the fourth objective of this study was to compare patterns of GM atrophy in the four lobes of the brain (i.e., frontal, parietal, temporal, and occipital) in preMCI, MCI, and control subjects.
Singl-voxel 1H MR spectroscopy from the posterior cingulate region has been shown to be sensitve to the biochemical changes in the brains of patients with AD and MCI (Kantarci et al., 2000; Catani et al., 2001). Kantarci et al. (2000) found higher myoinosotil (mI)/creatine (Cr) ratios in patients with MCI and AD relative to controls. Furthermore, N-acetylaspartate (NAA)/mI ratio has been shown to be an efficient predictor of memory and cognitive function in MCI and AD (Kantarci et al., 2002). Therefore, the final objective of this study was to examine metabolite differences in the posterior cingulate region of preMCI, MCI, and control subjects.
2. Methods
2.1. Study participants
Forty-eight subjects participated in the study. Participants were referred to the study by memory clinics in the San Francisco Bay Area, including the Memory Disorders Clinic at the San Francisco Veterans Affairs Medical Center, the Memory and Aging Center at the University of California, San Francisco, and the Memory Disorders Clinic at the California Pacific Medical Center or recruited by flyers and advertisements in local newspapers. All participants gave written informed consent, approved by review boards of the University of California San Francisco and the San Francisco VA Medical Center. In addition to structural and spectroscopic imaging, all participants received medical and neurological examinations and neuropsychological testing.
Participants were required to have an informant who knew them well and could answer questions about their cognition and general health. Exclusion criteria included any medical, psychiatric, or neurologic condition (other than MCI) that could significantly affect brain structure or cognition, history of head trauma with loss of consciousness lasting more than 5 min, and factors contraindicating MRI. None of the participants used anti-seizure medication, anxiolytics, antidepressant, or cholinesterase inhibitors. Participants with hypertension controlled by medication (5 cases) were included in the study. Hypertensive participants took standard hypertension treatment (e.g., beta-blockers, calcium channel blockers, ACE-inhibitors and potassium-sparing diuretics). Other medication used by study participants included antacid/antiulcer agent (e.g., omeprazole, 3 cases), anticoagulant (e.g., warfarin, baby aspirin, 4 cases), anti-gout medication (e.g., allopurinol, 2 cases), cholesterol lowering agent (e.g., atorvastatin, 2 cases), hormone replacement therapy (2 cases), prostate enlargement treatment (e.g., terazosin, 2 cases), analgesics (e.g., acetaminophen and indomethacin, 2 cases), and various vitamin, multivitamin, and nutritional supplements (e.g., glucosamine, chondroitin sulfate, fish oil, folic acid, magnesium, methylsulfonylmethane, 8 cases).
2.2. Neuropsychological tests
Participants were assessed with the California Verbal Learning Test (CVLT II) (Delis et al., 2000) and the Delis–Kaplan Executive Function System (D-KEFS) (Delis et al., 2001). Item response theory (IRT) analytic methods (Baker, 1985; Hambleton and Swaminathan, 1985; Hambleton et al., 1991) were used to create psychometrically matched scales of verbal episodic memory and an executive function. The development and characteristics of these scales have been previously described by Mungas et al. (2003). Briefly, item scores from a comprehensive neuropsychological test battery in a sample of 400 elderly persons who varied in cognitive status from cognitively healthy to mildly demented served as the basis for scale development. Within the IRT framework, scales are matched when they demonstrate equivalent reliability (test information values) over a similar range of measured ability. In terms of classical test theory, this translates to a reliability coefficient of 0.93 for each scale. It also means that the scales have linear measurement properties (i.e., an absence of floor or ceiling effects) over this broad range of cognitive function. The scales also are near-normally distributed, which presents advantages for statistical analyses. Each scale was transformed to have a mean of 100 and a standard deviation of 15. Donor items for the memory composite score included CVLT immediate recall trials 1–5, short delay free- and cued-recall, and total recognition discriminability scores. Donor scales for the executive function composite score included the FAS verbal fluency test, Stroop and Trail making tests from the D-KEFS battery.
2.3. Criteria for group assignment
None of the study participant met the clinical diagnostic criteria for dementia. Thirteen participants were diagnosed with MCI by clinicians from the referring memory clinics by standard criteria (Petersen et al., 2001). Seventeen participants were classified as “preMCI” for purposes of this study. These participants had CDR of 0.5 at time of entry into the study but were not impaired on formal neuropsychological testing (i.e., these subjects had memory and executive function composite scores that were above the mean of 100). For the most part, clinicians from the referring memory clinics had given these participants research diagnoses of “meets no criteria”. Eighteen cognitively normal controls with a CDR of 0 and memory and executive function composite scores that were above the mean of 100 participated in the study.
The CDRs were administered by clinical nurses from the referring memory clinic. On some occasions, the CDRs of cognitively normal control subjects not referred to the study by memory clinics were administered by a clinical assessor other than a nurse. All staff who administered the CDR completed the Brief Training and Reliability Protocol (BTRP) offered by the Washington University Alzheimer's Disease Research Center. The BTRP includes an introduction to the CDR by Dr. John Morris, three videotaped patient interviews for training purposes, and six videotaped interviews for reliability certification. Successful completion of the six reliability tapes is achieved with agreement with a “gold standard” on at least five out of the six tapes. Prior research has shown that physicians and non-physician health professionals demonstrate good reliability in administering the CDR after appropriate training (Rockwood et al., 2000; Schafer et al., 2004).
2.4. Magnetic resonance imaging
All MR imaging were performed on a clinical 1.5 tesla MR scanner (Vision, Siemens Medical Systems, Iselin, NJ). Although imaging typically occurred on the same day as the neuropsychological assessment, due to scheduling conflicts, a few participants were scanned within 2–3 weeks of the neuropsychological assessment. Coronal T1-weighted images (TR/TI/TE = 9/300/4 ms, 1 mm × 1 mm in-plane resolution, 1.5 mm slabs), axial proton density (PD) and T2-weighted images (TR/T1/TE = 5000/20/85, 1 mm × 1.25 mm in-plane resolution, 3 mm slabs) were obtained for volumetry purposes.
2.4.1. Tissue segmentation and lobar definition
Each participant's MRI was transformed with multi-grid free form volume deformation (Studholme et al., 2004) into an age-appropriate atlas on which the four lobes of the brain had been marked (see Fig. 1D). In order to obtain the GM volume for each lobe, the lobar markings were combined the with tissue segmentation, performed with expectation-maximization segmentation (EMS) (Van Leemput et al., 1999, 2001, 2003) according to procedures previously described (Chao et al., 2007) (see Fig. 1). Due to systematic errors partially introduced by the left/right asymmetry of the warping template, we combine volume data from both hemispheres in the analyses.
Fig. 1.

Examples of (A) T-1 weighted image, (B) gray (in green), and (C) white (in red) matter segmentation, and (D) lobar markings.
2.4.2. Hippocampal measurement
Hippocampal volumes were measured using a semiautomated high dimensional brain-warping algorithm (Medtronic Surgical Navigation Technologies, Louisville, CO) (Hsu et al., 2002). Raters blinded to clinical information of the participants visually inspected results of the high dimensional brain-warping. In some instances, hippocampal masks that including non-hippocampal tissue were manually edited. Reliability of the hippocampal measurements has previously been determined by same-day test–retest MRI acquisitions on nine subjects, yielding an intraclass coefficient of 0.94 (Du et al., 2004).
2.4.3. Freesurfer parcellation
We used Freesurfer (Fischl and Dale, 2000) to examine the volumes of the three a priori MTL regions of interest—the parahippocampal and fusiform gyri and the ERC. All the Freesurfer results were visually inspected and manual for corrections to the segmentations were made when necessary. Approximately 75% of the data had to be manually edited for reasons such as dura inclusions and gyral cut offs due to poor white/gray contrasts in the MR images.
2.4.4. Single voxel 1H MRS
Single voxel 1H MRS spectra from a 20 mm3 region of interest in the posterior cingulate cortex was acquired using single voxel STEAM MRS (TR/TM/TE = 1800/10/25 ms, 128 averages), including CHESS water suppression achieved with optimized Shinnar LeRoux pulse design (Matson, 1994). Prior to each STEAM MRS acquisition, EPI images were acquired to allow alignment of MRS with MRI data for atrophy correction, assuming no head movement between MRS and EPI scans.
2.5. Statistical analyses
Data were analyzed by SPSS packate for Windows (15.0). Group effects for demographic, clinical, and the MRI variables were analyzed using the general linear model. Following correction for multiple comparisons (Wilks' Lambda), multivariate analyses of variance and planned comparisons were performed. Group differences in regional GM, hippocampal, parahippocampal, fusiform, and ERC volumes were tested using multivariate analysis of covariance (MANCOVA) with planned comparisons after adjusting for age and total intracranial volume (TIV). A Shapiro-Wilks test of normality revealed that all these variables approached a normal distribution when age was included as an additive term in the model. Because we did not have any a priori hypotheses about laterality, we combine volume data from both hemispheres in the analyses to reduce the number of comparisons.
3. Results
Table 1 summarizes the demographic, clinical, and cognitive composite scores in three study groups. A χ2-test of independence revealed no difference in the male-to-female ratios among the three study groups. There were also no group difference in years of formal education; however, MCI patients were significantly older than control and preMCI subjects. As expected, MCI patients had higher CDR sum of boxes scores and lower mean MMSE, memory and executive composite scores and than controls and preMCI subjects (see Table 1).
Table 1.
Description of the three study groups
| Characteristic | Statisticsa | Control | preMCI | MCI |
|---|---|---|---|---|
| N | 18 | 17 | 13 | |
| Male:female ratio | χ2 = 1.5 | 6:12 | 6:11 | 7:6 |
| Age, year, range | 61–79 | 59–89 | 66–91 | |
| Age, year, mean (S.D.)b | F = 11.8*** | 67.5 (5.0) | 68.6 (7.9) | 78.8 (7.4) |
| Education, year, range | 12–21 | 12–20 | 9–22 | |
| Education, year, mean (S.D.) | F = 0.61 | 16.4 (2.3) | 17.4 (2.2) | 17.2 (3.2) |
| CDR total, mean (S.D.) | 0.0 (0.0) | 0.5 (0.0) | 0.5 (0.0) | |
| CDR sum boxes, mean (S.D.)b | F = 16.5*** | 0.1 (0.2) | 0.6 (0.3) | 1.6 (1.0) |
| MMSE, mean (S.D.)c | F = 12.7*** | 29.6 (0.6) | 29.1 (1.3) | 27.0 (2.2) |
| Memory composite, mean (S.D.)c | F = 45.4*** | 118.6 (13.0) | 121.0 (13.3) | 79.2 (11.5) |
| Executive function composite, mean (S.D.)c | F = 17.5*** | 114.4 (10.1) | 115.8 (12.4) | 89.8 (16.8) |
Degrees of freedom for χ2-test of independence = 2, degrees of freedom for MANOVA = 2.47.
Tukey's HSD post hoc test: MCI > CN (p < 0.0001); MCI > preMCI (p < 0.003).
Tukey's HSD post hoc test: MCI < CN (p < 0.0001); MCI < preMCI (p < 0.001).
The first objective of this study was to compare hippocampal volumes in preMCI, MCI, and control subjects. Analyses revealed no significant group effect for hippocampal volume.
The second objective of this study was to compare fusiform volume in preMCI, MCI, and control subjects. We were unable to obtain Freesurfer parcellation for five control subjects, six preMCI subjects, and two MCI subjects due to issues with scan quality. Analyses of the remaining data revealed a significant main group effect for fusiform (F2,34 = 7.60, p = 0.002) volume (see Table 2). Planned contrasts revealed that controls had more fusiform volume than MCI and preMCI subjects.
Table 2.
Mean volumes and metabolite ratios for three study groups
| Variable | Controls | PreMCI | MCI | F | p |
|---|---|---|---|---|---|
| Volume (cm3) | |||||
| Intracranial | 1275.1 (139.6) | 1228.1 (154.4) | 1225.0 (102.9) | 0.71 | 0.50 |
| Hippocampusa | |||||
| Right | 2.33 (0.22) | 2.30 (0.34) | 2.10 (0.25) | 0.97 | 0.39 |
| Left | 2.31 (0.20) | 2.27 (0.36) | 2.03 (0.34) | 1.37 | 0.26 |
| Frontal GMa | 217.23 (24.94) | 201.80 (27.44) | 195.24 (16.25) | 5.91 | 0.01 |
| Parietal GMa | 105.40 (13.51) | 99.91 (12.85) | 95.66 (8.56) | 0.78 | 0.46 |
| Temporal GMa | 140.53 (16.31) | 134.85 (17.34) | 125.00 (11.51) | 2.77 | 0.07 |
| Occipital GMa | 53.48 (6.43) | 49.60 (10.09) | 48.47 (5.95) | 0.62 | 0.54 |
| Entorhinal Cortexb | 3.49 (0.33) | 2.99 (0.53) | 2.50 (0.49) | 7.62 | 0.002 |
| Parahippocampal gyrusb | 4.66 (0.68) | 4.21 (0.67) | 3.62 (0.44) | 6.58 | 0.04 |
| Fusiform gyrusb | 22.74 (2.76) | 18.40 (1.95) | 17.94 (3.03) | 7.60 | 0.002 |
| Metabolite | |||||
| NAA/Crc | 1.34 (0.09) | 1.33 (0.12) | 1.23 (0.10) | 1.02 | 0.37 |
| mI/Crc | 0.86 (0.04) | 0.86 (0.10) | 0.94 (0.08) | 2.91 | 0.07 |
| NAA/mIc | 1.57 (0.14) | 1.55 (0.18) | 1.32 (0.13) | 5.26 | 0.01 |
Note: standard deviations in parentheses.
Data from 18 controls, 17 preMCI, 13 MCI, degrees of freedom for F-statistic = 2,47.
Data from 13 controls, 11 preMCI, 11 MCI, degrees of freedom for F-statistic = 2,34.
Data from 9 controls, 9 preMCI, 13 MCI, degrees of freedom for F-statistic = 2,30.
The third objective of this study was to compare the volumes of the ERC and parahippocampal gyrus in preMCI, MCI, and control subjects. Analyses revealed significant main group effects for both ERC (F2,34 = 7.62, p = 0.002) and parahippocampal (F2,34 = 3.58, p = 0.04) volume (see Table 2). Planned contrasts revealed that controls had more ERC volume than MCI and preMCI and more parahippocampal volume than MCI.
The fourth objective of this study was to compare the patterns of GM atrophy in the four lobes of the brain in preMCI, MCI, and controls. Analyses revealed a significant main group effect for frontal GM volume (F2,47 = 5.91, p = 0.005). Planned contrasts showed that controls had more frontal GM volume than MCI (p = 0.016) and preMCI (p = 0.003).
The final objective of the study was to examine metabolite ratios in the posterior cingulate in preMCI, MCI, and controls. Single-voxel 1H MRS data was available for 9 CN, 9 preMCI and 13 MCI subjects. Analysis revealed a signifi-cant main group effect for NAA/mI (F2,30 = 5.26, p = 0.012). Planned contrasts showed that controls had higher levels of NAA/mI (1.57 ± 0.14) in the posterior cingulate region than MCI (1.32 ± 0.13, p = 0.005) and marginally more NAA/mI than preMCI subjects (1.55 ± 0.18, p = 0.06).
Because there were significant group differences in posterior cingulate NAA/mI, we used the Freesurfer parcellation in a post hoc analysis to examine whether there was a similar group difference in posterior cingulate volume. As shown in Fig. 2, the region where we placed the voxel for proton spectroscopic imaging was part of a region classified by Freesurfer as the isthmus of the cingulate. Post hoc analysis revealed a significant main group effect for volume (F2,34 = 3.45, p = 0.45). Planned contrasts showed that while MCI had less volume (p = 0.013) in this region of the brain than controls, there were no significant difference between MCI and preMCI subjects.
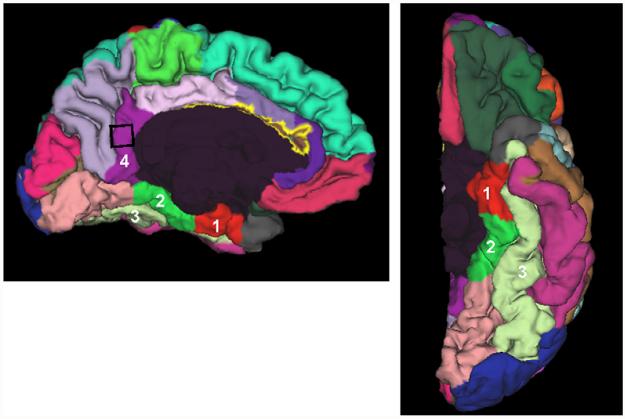
Fig. 2.
Examples Freesurfer parcellation of the (1) entorhinal cortex, (2) parahippocampal and (3) fusiform gyri, and (4) the isthmus of the cingulate gyrus. The black square denotes the approximate location of the single MRS voxel.
4. Discussion
The present study sought to characterize the pattern of MRI/MRS changes in older adults who had a CDR score of 0.5, but who showed no deficits on formal neuropsychological testing, and therefore did not fulfill MCI criteria. The results indicate that these CDR 0.5 individuals (i.e., preMCI subjects) had abnormalities in the ERC, parahippocampal and fusiform gyri, frontal GM, isthmus of the cingulate, and posterior cingulate NAA/mI levels similar to those found in individuals diagnosed with MCI and who are considered by some to have early forms of AD (Morris et al., 2001). These results support the hypothesis that detection of individuals with very mild forms of AD may be facilitated by use of the CDR, which emphasizes changes in cognition over time within individuals rather than comparison with group norms (Storandt et al., 2006).
Compared to controls, preMCI subjects had reduced ERC, fusiform, and frontal GM volume. This finding should dispel the notion that preMCI subjects were mistakenly assigned to this group because of unreliable informants. Interestingly, there were no significant verbal episodic memory, executive function, or MMSE differences between preMCI and control subjects despite the significant structural MRI differences. We assessed other cognitive domains (i.e., visual and semantic memory) and also did not find significant differences between preMCI and control subjects. There are several explanations for this. First, it is possible that changes in frontal GM, ERC, and fusiform gyri occur prior to alternations in cognitive function. Alternatively, it is possible that many of the preMCI subjects were high functioning individuals who were able to compensate for their frontal GM, ERC, and fusiform atrophy, possibly due to incipient AD, by recruiting other brain regions (i.e., cognitive/brain reserve hypothesis) (Schmand et al., 1997; Stern, 2002). Another possible, although not mutually exclusive, explanation is that imaging has greater statistical power to detect changes than neuropsychological testing because of higher test–retest reliability.
We found frontal GM atrophy in MCI subjects relative to controls, consistent with previous reports by our group and others of inferior frontal atrophy in patients with MCI (Bell-McGinty et al., 2005; Duarte et al., 2006). We also found significant frontal GM atrophy in preMCI subjects relative to controls. Cross-sectional (Bartzokis et al., 2001; Jernigan et al., 2001; Raz et al., 2004) and longitudinal (Pfefferbaum et al., 1998; Resnick et al., 2003; Scahill et al., 2003; Raz et al., 2005) studies have noted prominent pre-frontal GM volume reductions in normal aging. Although the MCI group was older than the other two study groups, there was no significant age difference between controls and preMCI. Therefore, it is unlikely that aging alone can explain the frontal GM atrophy that we found in the preMCI group. It is noteworthy that Saykin et al. also found frontal GM density differences in their voxel-based morphometry study on older adults with cognitive complaints (CC) relative to controls. Although Saykin et al. did not utilize the CDR, their CC subjects were similar to the preMCI subjects in the present study in that they performed normally relative to peers of the same age and education on cognitive tests but had cognitive difficulties based on informant questionnaires and/or self-reports. Although MTL structures have been the focus of most previous volumetric studies, several studies have shown that structural lesions (e.g., neurofibrillary tangles, neuritic plaques) are more widely distributed in MCI, and include neocortical areas (Kordower et al., 2001; Price and Morris, 1999). Furthermore, amyloid deposits have been identified in vivo in the frontal lobes of patients with mild AD (Klunk et al., 2004). Our results, together with those of Saykin et al., suggest diffuse neurodegenerative changes also occur in individuals who express cognitive complaints but who do not yet meet MCI criteria.
Consistent with previous studies, we found ERC, parahippocampal, and fusiform atrophy, and decreased posterior cingulate NAA/mI in MCI patients relative to controls (Visser et al., 1999; Kantarci et al., 2000; Xu et al., 2000; de Toledo-Morrell et al., 2000; Dickerson et al., 2001; Catani et al., 2001; Wolf et al., 2003; Chetelat et al., 2005). However, the lack of a significant main group effect for hippocampal volume was somewhat surprising given that hippocampal atrophy has been well documented in MCI (Convit et al., 1997; Jack et al., 1999; Du et al., 2001). Some authors have found that ERC measurements show better classification between controls than MCI than hippocampal volume (Pennanen et al., 2004). de Toledo-Morrell and colleagues reported that baseline ERC volume was a better predictor than hippocampal volume of conversion to AD among a group of non-demented subjects who had cognitive complaints (de Toledo-Morrell et al., 2000; Dickerson et al., 2001). It is interesting to note that not everyone in that group had demonstrable cognitive impairment, similar to the preMCI subjects in the present study and the CC subjects studied by Saykin and colleagues. Saykin et al. (2006) also did not find significant hippocampal atrophy in their CC subjects relative to controls. Together, these results suggest that volumetric measurements of the entire hippocampus may not be sensitive enough to detect abnormalities in the small and complexly shaped structures of the MTL (Ashburner et al., 2003). For example, we have some preliminary evidence that suggest that, early in the disease process, abnormalities in surface of the hippocampus corresponding to the subiculum and CA1 subfield may be more sensitive indicators of future cognitive decline than volumetric measurements of the whole hippocampus.
This study had several limitations: (1) The cross-sectional nature of the study did not allow us to directly quantify longitudinal change within an individual. Instead, we had to rely on the CDR to assess longitudinal decline via informant-based questionnaires and self-reports. Because previous studies have shown that individuals with cognitive complaints and a CDR of 0.5 had higher incidences of converting to dementia (Albert et al., 2001), we are in the process of following these preMCI subjects longitudinally to determine the relative rates of conversion to dementia and to determine the trajectory of cognitive decline, the progression of structural and metabolic brain changes, and the diagnostic outcomes. For example, it is unclear whether these preMCI subjects will be more likely to eventually meet non-amnestic (i.e., dysexecutive) MCI criteria given their reduced frontal GM volume. Following the subjects longitudinally will also allow us to determine whether the present abnormalities seen on MRI are indeed related to AD pathology. It is possible that some of the preMCI subjects may eventually convert to other types of dementia given their frontal GM atrophy and early functional impairment (i.e., frontotemporal lobar degeneration, FTLD). (2) The sample sizes were small. (3) Due to the lengthy scan time and some data quality issues, we did not have imaging data for all subjects in all modalities. This prevented us from comparing regional lobar volume differences with volumes of the MTL structures of interest derived from FreeSurfer and single-voxel spectroscopy data. (4) We did not formally test the consistency of the CDR ratings by acquiring two CDRs on one subject at the same visit. However, all staff who administered the CDR completed the Brief Training and Reliability Protocol offered by the Washington University Alzheimer's Disease Research Center and prior research has shown that physicians and non-physician health professionals demonstrate good reliability in administering the CDR after appropriate training (Rockwood et al., 2000; Schafer et al., 2004). (5) Our results may have limited generalizability because most study participants had high levels of education and estimated baseline intellect. These limitations notwithstanding, our results suggest that historical/informant-based functional decline, even in the absence of overt cognitive impairment on formal neuropsychological testing, warrant close monitoring over time. Furthermore ERC, fusiform, and frontal lobe GM, atrophy may useful indices for detecting early transition from normal to abnormal aging, and possibly to dementia in older individuals who express cognitive complaints.
Acknowledgements
National Institute on Aging (Grant AG10897), Jennifer Hlavin, Kari Haws, Prediction of Cognitive Decline volunteers, and the San Francisco Veterans Affairs Medical Center.
Footnotes
Conflict of interest
None.
References
- Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J. Int. Neuropsychol. Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Csernansky JG, Davatzikos C, Fox NC, Frisoni GB, Thompson P. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003;2:79–88. doi: 10.1016/s1474-4422(03)00304-1. [DOI] [PubMed] [Google Scholar]
- Baker FB. The Basics of Item Response Theory. Heinemann; Portsmouth, NH: 1985. [Google Scholar]
- Baron JC, Chételat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal volume in men: a magnetic resonance imaging study. Arch. Gen. Psychiatry. 2001;60:393–398. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch. Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Garrido GE, Almeida OP, Castro CC, Camargo CH, Cid CG, Buchpiguel CA, Furuie S, Bottino CM. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer's disease. Neurobiol. Aging. 2003;24:221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12:2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- Chao LL, Schuff N, Clevenger EM, Mueller SG, Rosen HJ, Gorno-Tempini ML, Kramer JH, Miller BL, Weiner MW. Patterns of white matter atrophy in frontotemporal lobar degeneration. Arch. Neurol. 2007;64:1–7. doi: 10.1001/archneur.64.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease. Neurobiol. Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Convit A, de Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, George AE. Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol. Aging. 1997;18:131–138. doi: 10.1016/s0197-4580(97)00001-8. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-d-glucose/poitron-emission tomography (FDG/PET) Proc. Natl. Acad. Sci. U.S.A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, George AE, Golomb J, Tarshish C, Convit A, Kluger A, De Santi S, McRae T, Ferris SH, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller DC, Wisniewski HM. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol. Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann. NY Acad. Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. 2001 doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Adult version. Manual, Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test-2nd edition. [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch. Gen. Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol. Aging. 2001;22:747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Kramer JH, Ganzer S, Zhu XP, Jagust WJ, Miller BL, Reed BR, Mungas D, Yaffe K, Chui HC, Weiner MW. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62:422–427. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Hayasaka S, Du A, Schuff N, Jahng GH, Kramer J, Miller B, Weiner M. Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 2006;406:60–65. doi: 10.1016/j.neulet.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J. Neurol. Neurosurg. Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton RK, Swaminathan H. Item Response Theory Principles and Applications. Kluwer Nijhoff Publishing; Dordrecht: 1985. [Google Scholar]
- Hambleton RK, Swaminathan H, Rogers HA. Fundamentals of Item Response Theory. Sage Publications Inc.; London: 1991. [Google Scholar]
- Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Curr. Opin. Neurol. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Du AT, Schuff N, Weiner MW. Magnetic resonance imaging and magnetic resonance spectroscopy in dementias. J. Geriatr. Psychiatry Neurol. 2001;14:145–166. doi: 10.1177/089198870101400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, Weiner MW. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J. Magn. Reson. Imaging. 2002;16:305–310. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danizer WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br. J. Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CRJ, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–490. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fenema-Notestine C, Gamst AC, Stout J, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Jacomb PA. An informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates reliability, validity and some norms. Psychol. Med. 1989;18:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR., Jr. Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin. N. Am. 2003;13:197–209. doi: 10.1016/s1052-5149(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Smith GE, Ivnik RJ, Petersen RC, Boeve BF, Knopman DS, Tangalos EG, Jack CR., Jr. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer's disease. J. Int. Neuropsychol. Soc. 2002;8:934–942. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SARB, Visser PJ, van Schijndel RA, Fox NC, Barkhof F. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kidron D, Black SE, Stanchev P, Buck B, Szalai J, Parker J, Szekely C, Bronskill MJ. Quantitative MR volumetry in Alzheimer's disease. Topographic markers and the effects of sex and education. Neurology. 1997;49:1504–1512. doi: 10.1212/wnl.49.6.1504. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann. Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann. Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Matson GB. An integrated program for amplitude-modulated RF pulse generation and re-mapping with shaped gradients. Magn. Reson. Imaging. 1994;12:1205–1225. doi: 10.1016/0730-725x(94)90086-7. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J. Mol. Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner WW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Häanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol. Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Häanninen T, Kivipelto M, Könönen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H. A voxel based morphometry study on mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Jack CR, Jr., Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Hurrah CHJ, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch. Gen. Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann. Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Saykin AJ, Wishart HA, Copenhaver BR, Flashman LA, Santulli RB. Telephone-based screening for MCI and cognitive complaints: preliminary validation by comprehensive assessment; Paper presented at the International Neuropsychological Society Meeting; 2004. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol. Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, WIlliamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J. Neurosci. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Strang D, MacKnight C, Downer R, Morris JC. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J. Am. Geriatr. Soc. 2000;48:558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x. [DOI] [PubMed] [Google Scholar]
- Saykin AJ. Neurobehavioral Function and Activities of Daily Living Rating Scale (NBFADL-63 item version) Darthmouth Medical School; Hanover: 1992. [Google Scholar]
- Saykin AJ, Janssen R, Sprehn G, Spira T, Kaplan J, O'Connor B. Longitudinal evaluation of neuropsychological function in homosexual men with HIV-1 infection; 18 month follow-up. J. Neuropsychiatry Clin. Neurosci. 1991;3:286–298. doi: 10.1176/jnp.3.3.286. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, Fox NC. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003;60:989–994. doi: 10.1001/archneur.60.7.989. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid-registered serial MRI. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer KA, Tractenberg RE, Sano M, Mackell JA, Thomas RG, Gamst A, Thal LJ, Morris JC, Study A.s.D.C. Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis. Assoc. Disord. 2004;18:219–222. [PMC free article] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia A test of the brain reserve hypothesis. Psychol. Med. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: assessment with a new self-rating instrument. Biol. Psychiatry. 1979;14:791–801. [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs. revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Studholme C, Cardenas V, Song I, Ezekiel F, Maudsley A, Weiner M. Accurate template-based correction of brain MRI intensity disorder with application to dementia and aging. IEEE Trans. Med. Imaging. 2004;23:99–110. doi: 10.1109/TMI.2003.820029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Colchester A, Suetens P. Automated segmentation of multiple sclerosis lesions by model outlier detection. IEEE Trans. Med. Imaging. 2001;20:677–688. doi: 10.1109/42.938237. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. Automated model-based tissue classification of MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:897–908. doi: 10.1109/42.811270. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Maes F, Vandermeulen D, Suetens P. A unifying framework for partial volume segmentation of brain MR images. IEEE Trans. Med. Imaging. 2003;22:105–109. doi: 10.1109/TMI.2002.806587. [DOI] [PubMed] [Google Scholar]
- Visser P, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J. Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol. Scand. Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jack CR, Jr., O'Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000;54:1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of Geriatric Depression Rating Scale: a preliminary report. J. Psychiatr. Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Graham SJ, Campbell Z. A meta-analysis of structural and functional brain imaging in dementia of the Alzheimer's type: a neuroimaging profile. Neuropsychol. Rev. 2003;13:1–18. doi: 10.1023/a:1022318921994. [DOI] [PubMed] [Google Scholar]