Abstract
Plasmid DNA immunizations induce low levels but a broad spectrum of cellular and humoral immune responses. Here, we investigate the potential of co-stimulation through 4-1BB as an adjuvant for a HIV-1 DNA vaccine in mice. We designed plasmid DNAs expressing either the membrane bound or soluble form of 4-1BBL, and compared with the agonistic anti-4-1BB Ab for their ability to adjuvant the Gag DNA vaccine. Both, anti-4-1BB agonistic Ab as well as 4-1BBL DNA enhanced the Gag-specific cellular immune responses. However, in complete contrast to the agonistic Ab that suppressed humoral immunity to Gag, 4-1BBL DNA adjuvanted vaccines enhanced Gag-specific IgG responses. Importantly, the expression of Gag and 4-1BBL from the same plasmid was critical for the adjuvant activity. Collectively, our data suggest that for a HIV-1 vaccine where both antigen-specific cellular and humoral immunity are desirable, 4-1BBL expressed by a DNA vaccine is a superior adjuvant than anti-4-1BB agonistic Ab.
Keywords: Vaccine adjuvant, DNA vaccines, 4-1BB
1. Introduction
Despite intense multifarious efforts over the past several years, a successful HIV vaccine still remains elusive. Of the many current vaccination strategies, DNA vaccination is relatively safe and can induce a broad spectrum of cellular and humoral immnune responses. Also, problems like pre-existing immunity, as seen with other viral vectors such as Adenovirus type 5 (Ad5), Adeno Associated Virus (AAV) and Modified Vaccinia Ankara (MVA) can be averted with DNA based immunizations. However, DNA alone is a weak immunogen and several strategies are currently under investigation to adjuvant DNA vaccines to increase their potency and clinical utility. One such approach is to modulate and enhance the host immune response by using genetic adjuvants such as cytokines, chemokines and T-cell co-stimulatory molecules as a component of the vaccine.
4-1BB (CD137), a type I transmembrane protein, is a member of the TNFR superfamily (TNFSF9) with several extracellular cysteine-rich domains. 4-1BB expression can be induced on CD4 and CD8 T cells upon activation [1, 2] and is also found on a subset of splenic and bone-marrow derived DCs, mast cells, natural killer (NK) cells and on human monocytes and eosinophills [3]. Surface expression of 4-1BB on activated T cells is transient, reaching the peak at about 48 hrs post activation and declining by 4-5 days [1, 4]. Co-stimulation through 4-1BB delivers pro-survival signals during the peak of effector phase of an immune response that is shown to be important for preventing activation induced cell death of the effector population, thus generating a larger memory pool. Consistent with this, several studies have indicated that 4-1BB and 4-1BB ligand (4-1BBL) interactions are important for inducing robust CTL responses and for the establishment of long-lived memory CTLs [5-8]. All of these favorable effects on the final outcome of an immune response make this co-stimulatory pathway an attractive target for immunotherapy and adjuvanticity.
Because of its crucial role in the generation and sustenance of CD4 and CD8 T cell responses, the 4-1BB/ 4-1BBL co-stimulatory pathway has been exploited both for anti-tumor and anti-viral immunity [9-12]. However, an interesting and important feature of this co-stimulatory pathway is that signaling through 4-1BB can also suppress the immune response. In contrast to its positive regulatory role on T cells and anti-tumor/anti-viral activity, 4-1BB co-stimulation has also been shown to ameliorate or prevent various autoimmune conditions in mice by suppressing immune responses [13-15]. Thus, given its conflicting role on immune regulation, it is important to modulate this pathway appropriately for the purpose of vaccine development where enhancement, and not suppression, of the immune response is desirable.
In this study, we investigated if the form of adjuvant could influence antigen-specific T and B cell responses elicited by a HIV-1 Gag DNA vaccine in mice. We designed plasmid DNAs expressing either membrane bound or soluble 4-1BBL, and compared with the agonistic anti-4-1BB Ab in their ability to adjuvant the DNA vaccine. Our results demonstrate differential adjuvantive effects on the cellular and humoral immunity by the agonistic Ab and ligand DNA. Our results also highlight that by changing the form (agonistic Ab vs. ligand DNA), the same molecular adjuvant can be exploited either to enhance or suppress the humoral arm of adaptive immunity and thus can have varied applications in anti-tumor or anti-viral vaccines or in autoimmune diseases.
2. Material and Methods
2.1. Immunizations
Female BALB/c mice of 6-8 weeks of age were purchased from Charles River Laboratories (Wilmington, Mass.). The DNA immunogen, pGA1/JS8 [16], expresses clade B consensus Gag. The MVA immunogen, MVA/HIV62 expresses HIV-1 clade B Gag-Pol and Env [17]. Both DNA and MVA vaccines were administered intramuscularly. Agonistic anti-4-1BB Ab (clone 3H3) was given intraperitoneally at a dose of 200μg per mouse. Unless otherwise stated, for experiments with agonistic Ab, DNA vaccine was used at a dose of 100μg per mouse. For in vivo depletion of CD4 T cells, mice (n=3) were injected i.p. with 200μg of GK1.5 antibody one day before and after DNA prime along with or without the agonistic anti-4-1BBAb. Gag-tetramer specific CD8 T cells were evaluated in blood for both wild type and CD4 depleted mice at different time points following MVA boost. For experiments with ligand DNA, Gag, m4-1BBL and SPD-4-1BBL DNAs were used at a dose of 20μg per mouse, and the Gag-m4-1BBL DNA was used at a dose of 40μg per mouse. MVA vaccine was used at a dose of 106 pfu. All immunizations were performed in sterile PBS in a final volume of 100μl and 50μl was injected in each of the hind legs. Mice were cared for under guidelines established by the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals” using protocols approved by the Emory University Institutional Animal Care and Use Committee.
2.2. Construction of 4-1BBL DNA plasmids
cDNA encoding murine 4-1BBL was PCR amplified using specific primers. The oligonucleotide primers were designed to include NheI site in the sense primer (TATCGCTAGCATGGACCAGCACACACTTGATG) and AvrII site in the antisense primer (TATCCCTAGGTCATTCCCATGGGTTGTCGGG). The restriction enzyme (RE) -digested PCR fragment was ligated to the pGA1 expression vector at NheI and AvrII sites to create the m4-1BBL plasmid (GenBank Accession#: GQ258348). Both the m4-1BBL and the pGA1/JS8 plasmids were digested with NotI and PvuI and the 4-1BBL gene along with the CMV-IA promoter (insert) was ligated to the pGA1/JS8 expression vector to create the 4-1BBL and Gag co-expressing plasmid (Gag-m4-1BBL plasmid, GenBank Accession#: GQ258349). The insertion and orientation of the 4-1BBL cDNA in the expression vectors were confirmed by RE analysis. The SP-D (lung surfactant protein D) fragment was amplified from pSP-D-CD40L [18] using the following PCR primers: sense GGGGGCTAGCGAATTCCACCAGGAAGC; antisense CTCGGTGCGGCCATCAGGGAACAATGCAGC. The extracellular domain (ECD) of 4-1BBL was PCR amplified using CCCTGATGGCCGCACCGAGCCTCGGC (sense) and TATCCCTAGGTCATTCCCATGGGTTGTCGGG (antisense). The PCR products were gel purified and an overlap PCR was done to create the junction between SP-D on the 5′ end and ECD of 4-1BBL on 3′ end. The amino acid sequence at the junction between SP-D and murine 4-1BBL was KAALFPDG/ RTEPRPAL, where the N-terminal portion is from SP-D (amino acids 1 to 256 of GenBank protein sequence no. NP_033186) and the C-terminal portion is the extracellular sequence of murine 4-1BBL (amino acids 104 to 310 of GenBank protein sequence no. NP_033430.1). The overlap PCR product (SPD-4-1BBL form) was ligated to the pGA1 expression vector between NheI and AvrII sites (GenBank Accession#: GQ258350).
2.3. Protein expression analysis of 4-1BBL
293T cells (human embryonic kidney cell line) were transfected with plasmid DNA using Lipofectamine 2000 following the manufacturer's guidelines (Life Technologies). Intracellullar expression of 4-1BBL and Gag were analyzed by flow cytometry using flurochrome-conjugated mAbs 4-1BBL-FITC (clone 19H3) and KC-57-PE (Beckman Coulter), respectively. To isolate virus-like particles (VLPs), supernatants from plasmid DNA transfected 293T cells were overlayed on a 20% sucrose layer and centrifuged in a Beckman SW41 rotor at 32,000rpm for 2hrs and pellet was resuspended in PBS. VLPs were diluted with sample buffer and were run on 4-15% acrylamide gels (Biorad). Gels were transferred to nitrocellulose membranes and probed sequentially with anti-4-1BBL polyclonal antibody (clone D-20, Santa Cruz Biotechnology), and anti-goat IgG-HRP (Santa Cruz Biotechnology). Western blots were developed using the chemiluminescence reagent (GE Healthcare). For detecting Gag, H12.5C mAb (NIH AIDS reagent resource) was used as the primary antibody and anti-mouse IgG-HRP (Sigma) was used as the secondary antibody. For detecting SPD-4-1BBL in 293T cell culture supernatants, purified anti-mouse 4-1BBL mAb (clone TKS-1) was used as the primary antibody and anti-rat IgG-HRP (Southern Biotech) was used as the secondary antibody. The polyclonal Ab was not used for this purpose, as this Ab was raised against a peptide mapping near the N-terminus of the 4-1BBL protein that had been deleted in the SPD-4-1BBL form. Plasmid DNAs were grown in E.coli DH5α and purified using Endotoxin-free Gigaprep kits (Qiagen).
2.4. In vitro activity assay for 4-1BBL
Total splenocytes (7 × 106 cells/well) were stimulated with soluble anti-CD3 (clone 145.2C11, 1μg/ml) for 72 hrs at 37°C in 6-well plates. After 72hrs, cells were washed and surface expression of 4-1BB was confirmed by flow cytometry. 96 well plates were coated overnight with anti-CD3 (clone 145.2C11, 0.003μg/ml) and mouse IgG (1ug/ml). The 72hr stimulated splenocytes (0.5 ×106 cells/well) were cultured with 50μl of 293T culture supernatants containing either membrane bound 4-1BBL (m4-1BBL) on VLP or SPD-4-1BBL for an additional 48hrs. Cells were pulsed with [3H]thymidine (1 μCi/well) for the last 8 -10 hours before harvesting and proliferation was measured by scintillation counting.
2.5. Tetramer assays
For tetramer analyses, cells were stained using allophycocyanin (APC)-conjugated Gag-tetramer (NIH tetramer core facility), CD4-FITC, CD19-FITC, CD11a-PE and CD8-PerCP in 100μl of complete RPMI at 4°C for 30 min. Cells were washed twice with wash buffer (PBS with 2% FBS) and acquired (approx. 200,000 lymphocytes) on the FACS Calibur (Becton Dickinson) and analyzed using Flowjo software (Tree Star, San Carlos, California). All antibodies were purchased from BD Pharmingen. CD8+ CD11a+ CD4-CD19- and Gag Tetramer+ cells were scored as tetramer positive cells.
2.6. Intracellular Cytokine Staining
Approximately 2×106 splenocytes were stimulated in complete RPMI in a final volume of 200μl. 5 peptide pools specific for HIV-1 Gag HXB2, each containing 25 peptides (15mers overlapping by 11aa) at a final concentration 1μg/ml were used for stimulations. The stimulation medium was supplemented with 1μg/ml of anti-mouse CD28 and anti-mouse CD49d. Cells were cultured for 6 hours (at 37°C) and Golgi stop (10μg/ml, BD Biosciences Pharmingen) was included for the last 4 hours. At the end of stimulation, cells were surface stained with anti-CD3-FITC and anti-CD8-PerCP at 4°C for 20 mins, washed with 2% PBS-FBS, and fixed and permeabilized with Cytofix-Cytoperm solution (Pharmingen). Cells were then washed twice with Perm wash and incubated with anti-mouse IFN-γ-APC and IL-2-PE at 4°C for 30 mins. Following incubation, cells were washed once with Perm wash and once with 2% PBS-FBS and resuspended in PBS with 1% paraformaldehyde (PFA) and analyzed by flow cytometry. All antibodies were purchased from BD Pharmingen.
2.7. Gag-specific ELISA
Sera from immunized mice were assayed by ELISA to measure Gag-specific Ab. Ninety-six well ELISA plates were coated overnight with HIV-1 HXB2-Pr55 (Chiron Corp.) at a concentration of 1μg/ml. Wells were blocked using non-fat dry milk and test sera were assayed at four-fold dilutions in duplicate wells. A standard curve was generated using anti-mouse IgG (2μg/ml, Sigma) followed by a two-fold dilution series of mouse IgG (Sigma) starting from 800ng/ml. Peroxidase conjugated anti-mouse IgG (Southern Biotech) was used to detect bound IgG for both the test sera and the standard curve. ELISA plates were read on a SPECTRAmax PLUS (Molecular Devices, CA). Standard curves were fitted and total Gag-specific IgG was determined using SOFTmax 2.3 software (Molecular Devices, CA).
2.8. Statistical analyses
Statistical analyses were done using Student's t -test or wilcoxon rank-sum test using S-PLUS software. Results are expressed as the means ± SEM. Each experiment was repeated at least twice (n = 3–10).
3. Results
3.1. Agonistic anti-4-1BB Ab adjuvants the Gag-specific CD4 but not CD8 T cell responses following HIV-1 DNA prime
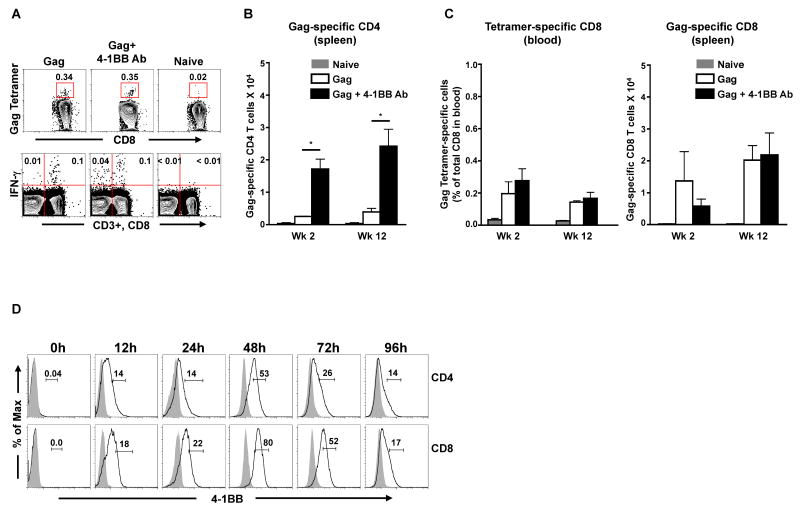
To evaluate the potential of agonistic anti-4-1BB Ab as an adjuvant for a DNA vaccine, we primed mice with 100μg of DNA vaccine expressing HIV-1 Clade B Gag either in the presence or absence of an agonistic anti-4-1BB Ab (Fig. 1). The Gag-specific CD4 and CD8 T cell responses were analyzed by measuring IFN-γ production after ex vivo stimulation of splenocytes with a Gag peptide pool that encompasses the entire Gag protein (Fig. 1A, lower panel). The Gag-specific CD8 responses in blood were also evaluated using a H-2Kd restricted Gag tetramer (Fig. 1A, top panel). Since the agonist Ab treatment resulted in a marginally enlarged spleen during the peak immune response, we are reporting the absolute numbers and not frequencies of the antigen-specific cells in the anti-4-1BB agonist Ab adjuvanted and non-adjuvanted groups. As expected, the DNA prime induced low levels of Gag-specific CD4 as well as CD8 T cell responses (Fig. 1B and 1C). However, the Gag-specific CD4 T cell responses were 6-fold higher in the adjuvant group compared to the non-adjuvant group (Fig. 1B). This enhancement was observed both at the peak (week 2) as well as memory (week 12) time points following prime. In contrast to the CD4 responses, the Gag-specific CD8 responses (evaluated both by tetramer in blood as well as by intracellular IFN-γ staining in spleen) were comparable between the two groups following prime (Fig. 1C). These results demonstrate that co-administration of agonistic anti-4-1BB Ab enhances the antigen-specific CD4 but not CD8 T cell response elicited by a DNA vaccine following prime.
Figure 1. Agonistic anti-4-1BB Ab adjuvants the Gag-specific CD4 but not the CD8 T cell responses following HIV-1 DNA prime.
BALB/c mice (n=3) were primed with HIV-1 Gag DNA vaccine in the presence or absence of agonistic anti-4-1BB Ab. A, Representative FACS plot showing the frequency of Gag-tetramer specific CD8 T cells in blood (top panel) and Gag-specific CD4 and CD8 T cell responses in spleen (lower panel) at 2 weeks following the DNA prime. In the lower panel representative plot, the left quadrants represent CD4 T cells (CD3+, CD8-) and right quadrants represent CD8 T cells (CD3+, CD8+). Numbers indicate Gag-specific IFN-γ+ CD4 or CD8 T cells expressed as a percent of respective total cells. B and C, Summary of Gag-specific CD4 and CD8 T cells respectively following DNA prime. Bars represent the mean absolute number ± SEM per spleen. D, Kinetics of 4-1BB expression on CD4 and CD8 T cells. Total splenocytes were activated in vitro with soluble anti-CD3 for the indicated time periods. Cells were harvested and 4-1BB expression on CD4 (CD3+ CD4+) and CD8 (CD3+ CD8+) T cells were analyzed by flow cytometry. Open histograms correspond to cells stained with anti-4-1BBAb and shaded histograms correspond to cells stained with anti-rat IgG2a isotype control. Percentage of CD4+ 4-1BB+ or CD8+ 4-1BB+ T cells are indicated on the plot. *, p < 0.05.
To further understand whether the observed preferential adjuvanticity of CD4 T cell response in vivo was due to a faster/higher expression of 4-1BB by these cells, we studied the kinetics of 4-1BB expression on CD4 and CD8 T cells following anti-CD3 stimulation in vitro (Fig. 1D). Following stimulation, expression of 4-1BB peaked at 48hrs on both CD4 as well as CD8 T cells, and was marginally higher on CD8 than on CD4 T cells suggesting that the lack of adjuvant effect on CD8 T cells in vivo may not be due to their inability to upregulate the 4-1BB receptor.
3.2. Anti-4-1BB agonistic Ab lowers the DNA dose required for a DNA/MVA HIV vaccine
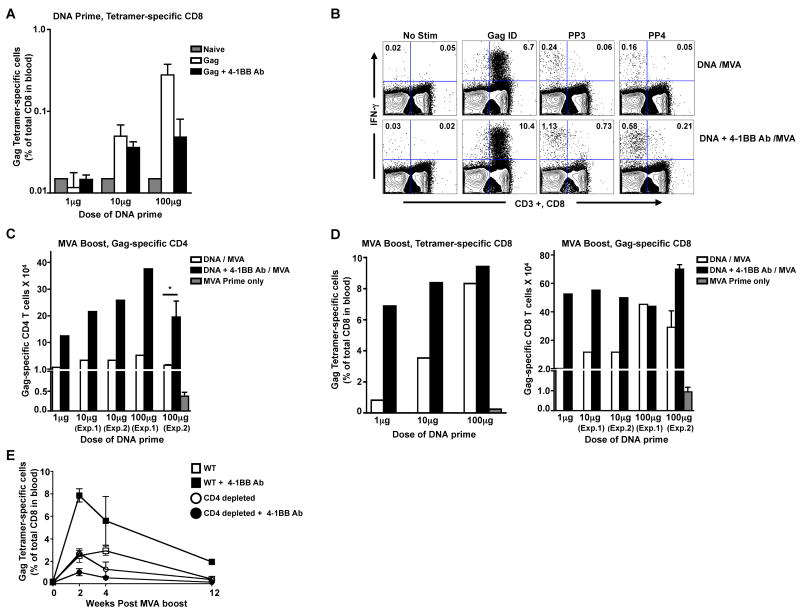
Since the agonistic anti-4-1BB Ab failed to shown any adjuvant effects on Gag-specific CD8 responses following a relatively high dose (100μg) of DNA prime (Fig. 1), we next investigated whether the DNA priming dose had any influence on the adjuvant effects of the agonist Ab. Three doses (1, 10 and 100μg) of Gag DNA were used for priming either in the absence or presence of anti-4-1BB agonistic Ab (Fig. 2). We measured the Gag-specific CD8 T cell response in blood after prime using the H-2Kd restricted Gag tetramer (Fig. 2A). Following DNA prime, low levels of tetramer-specific CD8 T cells were detectable in the 10 and 100μg dose groups with no difference in magnitude between the adjuvanted and non-adjuvanted groups.
Figure 2. Anti-4-1BB agonistic Ab lowers the DNA dose required for a DNA/MVA HIV vaccine.
BALB/c mice (n=5) were primed with three different doses of HIV-1 Gag DNA vaccine in the presence or absence of agonistic anti-4-1BB Ab at week 0 and boosted at week 12. A, Gag-tetramer specific CD8 T cells in blood were analyzed at 2 weeks following the DNA prime. The bars represent the means ± SEM. B, Representative FACS plot showing Gag-specific CD4 and CD8 T cell responses at one week following the MVA boost. Left quadrants represent CD4 T cells (CD3+, CD8-) and right quadrants represent CD8 T cells (CD3+, CD8+). Numbers indicate Gag-specific IFN-γ+ CD4 or CD8 T cells expressed as a percent of respective total cells. Gag ID: Gag immunodominant peptide; PP3: Peptide Pool 3; PP4: Peptide Pool 4. C, Summary of Gag-specific CD4 T cells at 1 week following the MVA boost. Data from two independent experiments (Exp.1 and Exp.2) with the 10μg and 100μg DNA priming doses are shown. Bars represent the mean absolute number per spleen (spleens from each group were pooled for analyses except for Exp.2 with 100μg DNA priming dose). D, Summary of Gag-specific CD8 T cells analyzed at 1 week following the MVA boost. Left panel, Gag-tetramer specific CD8 T cells in blood (pooled data). Right panel, Mean absolute number of IFN-γ producing CD8 T cells per spleen is plotted (spleens from each group were pooled for analyses except for Exp.2 with 100μg DNA priming dose). Data from two independent experiments (Exp.1 and Exp.2) with the 10μg and 100μg of DNA priming dose are shown. E, Adjuvant effect of anti-4-1BB agonistic Ab is CD4 dependent. For in vivo depletion of CD4 T cells, mice (n=3) were injected intraperitoneally with 200μg of GK1.5 antibody one day before and after DNA prime along with or without the agonistic anti-4-1BBAb. Gag-tetramer specific CD8 T cells were evaluated in blood for both WT and CD4 depleted mice at the indicated time points following MVA boost. The results are represented as means ± SEM. *, p < 0.05.
To better assess the differences in immune responses following DNA prime, we boosted these mice with a recombinant MVA expressing HIV-1 clade B Gag, that is known to boost DNA primed responses by more than 10-20 fold[19]. We did not use the DNA to boost because a homologous DNA prime/ DNA boost for 1 and 10 μg priming doses could have yielded very low levels of Gag-specific T cells. The Gag-specific CD4 and CD8 T cells were analyzed by intracellular IFN-γ staining following the boost (Fig. 2B, 2C and 2D). At 1 week following the MVA boost, the Gag-specific IFN-γ producing CD4 T cell responses were about 5-fold higher at all doses tested in the adjuvanted groups compared to the respective non-adjuvanted group (Fig. 2C). However, for Gag-specific CD8 responses, enhancement was observed only at lower doses (1 and 10 μg) and not at the highest dose (100μg) when evaluated both by Gag-tetramer specific as well as by Gag-specific IFNγ producing CD8 responses (Fig. 2D). Interestingly, the frequency of Gag-specific CD8 T cells in the adjuvanted group that received 1μg of DNA was similar to the frequency of Gag-specific CD8 T cells in the non-adjuvanted group that received 100 μg of DNA suggesting that 4-1BB co-stimulation lowered the DNA dose required for priming by 100-fold in CD8 T cells. These results demonstrate that agonistic anti-4-1BB Ab when given during a DNA prime can enhance both antigen-specific CD4 as well as CD8 T cell responses after a MVA boost and suggest that the enhanced CD4 help primed in the adjuvanted group could have contributed for enhanced CD8 T cell response/survival following the boost. Consistent with this hypothesis, transient depletion of CD4 T cells during DNA prime abrogated the adjuvant effects of agonistic anti-4-1BB Ab following the MVA boost (Fig. 2E).
3.3. Construction and in vitro characterization of DNA plasmids expressing different forms of 4-1BBL
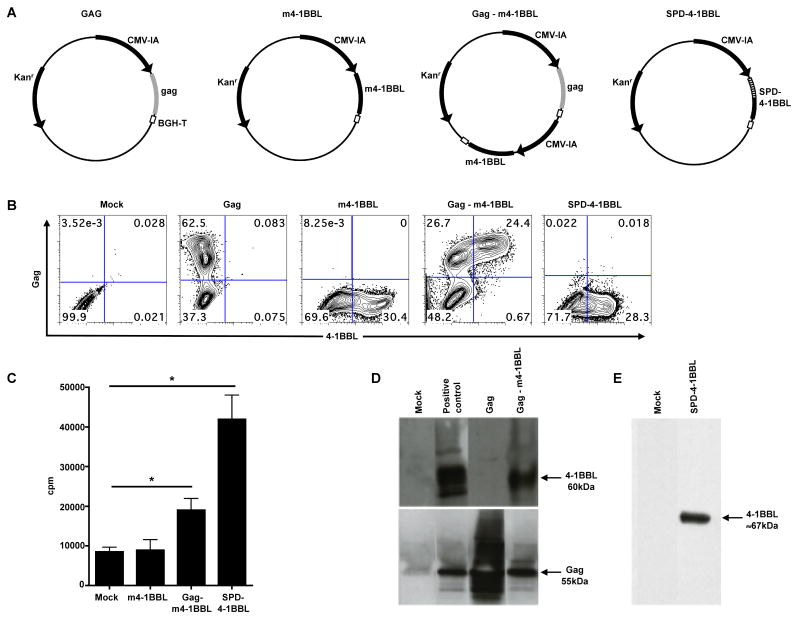
DNA vaccines are more practical than Ab based treatments in terms of affordability for the purpose of mass vaccination. So, we explored the possibility of using 4-1BBL expressed by DNA as an adjuvant for HIV-1 DNA vaccine. In vivo, 4-1BBL exists as a trimer and can be either membrane bound or secreted. Trimerization of the ligand is important for its co-stimulatory activity [20]. We constructed DNA plasmids that express the full-length cell membrane bound 4-1BBL (m4-1BBL) or the secreted multimeric form of 4-1BBL. The m4-1BBL was expressed both in trans (on separate plasmids; m4-1BBL-trans) as well as in cis (on the same plasmid; Gag-m4-1BBL-cis) with respect to Gag expression. For the secreted, multimeric form of the ligand, we fused the extracellular domain of 4-1BBL with the multimerization domain of the lung surfactant protein D (SPD-4-1BBL). This construct is expected to produce a dodecameric form (4 molecules of 4-1BBL trimers covalently linked to each other) of 4-1BBL and will be secreted into the extracellular milieu as has been shown for CD40L previously [18] (Fig.3A).
Figure 3. Construction and in vitro characterization of plasmid DNAs expressing different forms of 4-1BBL.
A, Schematic of the plasmid DNA constructs. The Gag expression vector consists of a HIV-1 Clade B consensus Gag sequence. The m4-1BBL plasmid consists of the membrane-bound (m) 4-1BBL. The Gag-m4-1BBL plasmid consists of the membrane-bound (m) 4-1BBL and the HIV-1 Clade B Gag genes. The SPD-4-1BBL plasmid consists of the extracellular domain of 4-1BBL fused to the multimerization domain of SP-D. CMV-IA: cytomegalovirus immediate-early promoter plus intron A; BGH polyA: bovine growth hormone polyadenylation signal; Kanr: Kanamycin resistance. B, In vitro expression of Gag and 4-1BBL. 293T cells were transfected with the respective plasmid DNA constructs and intracellular expression of 4-1BBL and Gag was analyzed by flow cytometry at 36 hrs post transfection. No DNA was added to the mock well. C, In vitro biological activity of different forms of 4-1BBL. Activated T cells were stimulated with immobilized anti-CD3 (0.003μg/ml) and 50ul of supernatant from 293T cells transfected with respective DNA plasmids. In vitro proliferation was measured at 48 hrs using [3H] incorporation. This experiment was repeated thrice. *, p < 0.05. D, Western blot analysis of 4-1BBL expression in VLPs. VLPs, from the culture supernatant of DNA transfected 293T cells, were purified by ultracentrifugation and western blotting was performed using a polyclonal anti-4-1BBL Ab and H12.5C mAb to detect 4-1BBL and Gag proteins, respectively. Positive Control for 4-1BBL gel: Gag-m4-1BBL cell lysate. Positive control for Gag gel: Gag cell lysate. E, Western blot analysis of 4-1BBL in culture supernatant from 293T cells transfected with SPD-4-1BBL plasmid. For detecting soluble 4-1BBL, purified anti-mouse 4-1BBL mAb was used as the primary antibody.
The intracellular expression of Gag and 4-1BBL was confirmed using antibodies specific to respective proteins (Fig. 3B). As expected, the Gag plasmid expressed only Gag, the m4-1BBL and SPD-4-1BBL plasmids expressed only 4-1BBL, and the Gag-m4-1BBL plasmid expressed both Gag and 4-1BBL. In Gag-m4-1BBL plasmid DNA transfected cultures, about fifty-percent of the Gag-positive cells also co-expressed 4-1BBL. To test for in vitro biological activity of the membrane bound 4-1BBL (m4-1BBL) and SPD-4-1BBL forms, we performed a thymidine proliferation assay that measures the co-stimulatory potential of 4-1BBL following anti-CD3 stimulation (Fig. 3C). We observed a 2.2-fold and 5-fold enhancement in T cell proliferation with the supernatant from 293T cells transfected with Gag-m4-1BBL and SPD-4-1BBL DNAs respectively. As expected, enhancement in proliferation was not observed with supernatant from cells transfected with the m4-1BBL plasmid. The m4-1BBL plasmid expresses only the membrane bound ligand and not the Gag, and thus the adjuvant remains cell-associated. In the Gag-m4-1BBL construct, we co-expressed the membrane bound form of 4-1BBL with HIV-1 Gag that is known to form virus-like particles (VLP) [16] (Fig.3A). This construct is expected to produce VLPs that incorporate multiple trimers of 4-1BBL on their surface and are secreted into the extracellular milieu.
To further confirm the incorporation of 4-1BBL in the Gag VLPs, virus-like particles from cell culture supernatants were purified over a sucrose cushion and tested for expression of 4-1BBL and Gag by Western blot analyses. Both the 4-1BBL and Gag proteins were detected in the VLPs that were generated upon transfection of 293T cells with Gag-m4-1BBL DNA, indicating the co-localization of the antigen and adjuvant (Fig. 3D). As expected, the Gag protein was detected in VLPs made from cells transfected with the Gag plasmid. Also, only the Gag protein was detected in VLPs that were purified from cells co-transfected with DNA plasmids expressing the Gag and SPD-4-1BBL (data not shown). Since the SPD-4-1BBL plasmid expresses the secretory form of the ligand, the Gag VLPs are not expected to incorporate any ligand as it buds off from the cell membrane. However, 4-1BBL protein was detected in 293T cell supernatant that was transfected with the DNA plasmid expressing SPD-4-1BBL (Fig. 3E).
3.4. Membrane bound 4-1BBL expressed in cis with Gag enhances the magnitude of Gag-specific CD8 T cell responses
To test for the in vivo adjuvant potential of DNA vaccines expressing the two forms of 4-1BBL, BALB/c mice were primed and boosted with DNA plasmids expressing either the membrane bound 4-1BBL (m4-1BBL) or soluble SPD-4-1BBL (Fig. 4). Immunizations with the m4-1BBL form compared 4-1BBL expression in cis (Gag and 4-1BBL on the same plasmid) as well as in trans (Gag and 4-1BBL on separate plasmids but co-injected) with respect to co-expression with Gag. Similarly, we also performed immunizations with SPD-4-1BBL form expressed in cis as well as in trans with respect to Gag. However, the expression of SPD-4-1BBL was significantly lower in the cis form compared to the trans form. Thus we are not reporting the immunogenicity data for the cis form in this study. The Gag, m4-1BBL (in trans) and SPD-4-1BBL DNAs were used at a dose of 20μg per mouse, and the Gag-m4-1BBL DNA (in cis) was used at a dose of 40μg per mouse. Since a high Gag DNA priming dose (100μg) failed to adjuvant Gag-specific CD8 responses (as evident from the in vivo data with the agonist antibody), we chose a low Gag DNA dose (20μg) for immunizations along with the ligand DNAs.
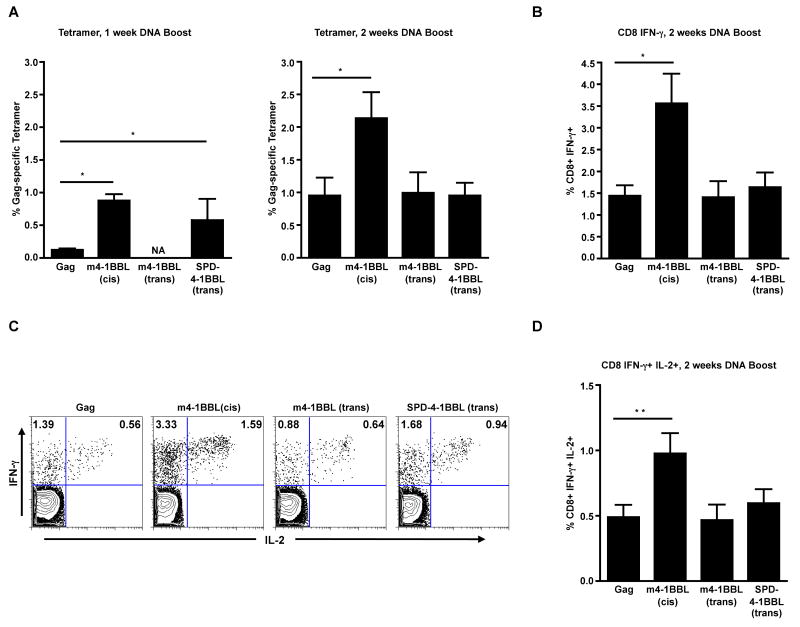
Figure 4. Enhanced magnitude of Gag-specific CD8 T cell responses in the m4-1BBL-cis adjuvanted group following DNA vaccination.
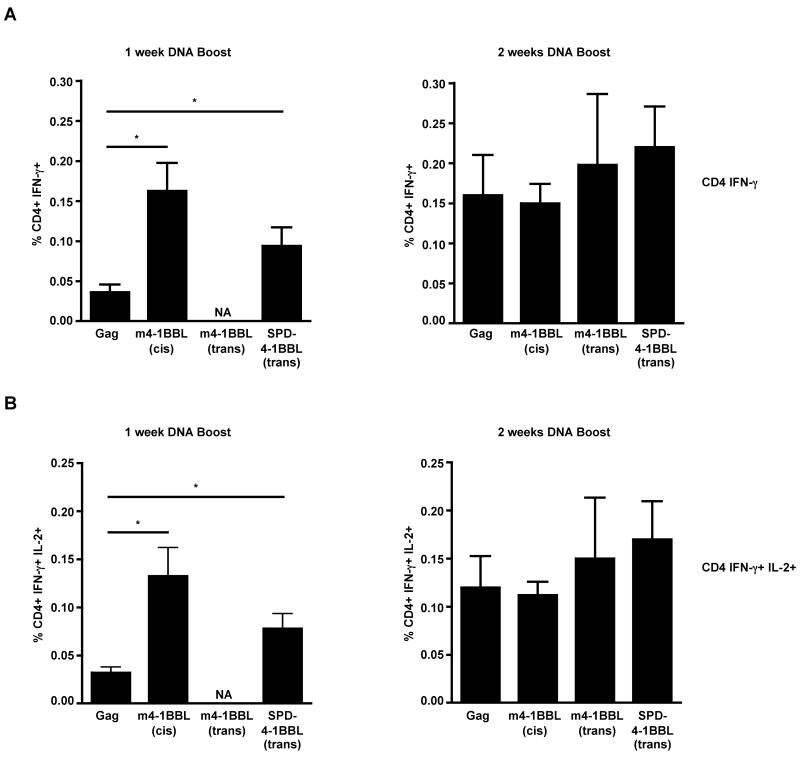
BALB/c mice (n=10) were primed and boosted with indicated DNAs at week 0 and week 4 respectively. A, Gag-tetramer specific CD8 T cells in spleen. B, Gag-specific IFN-γ producing CD8 T cells in spleen as evaluated by intracellular cytokine staining. C, Representative FACS plots showing Gag-specific CD8 T cells that co-produce IFN-γ and IL-2 (cells were gated on CD3 and CD8). Numbers indicate Gag-specific CD8 T cells either secreting IFN-γ or IFN-γ and IL-2 expressed as a percent of total CD8 T cells. D, Summary of the frequency of IFN-γ+ IL-2+ CD8 T cells in spleen. The bars represent mean frequency ± SEM. The Gag group was immunized with 20μg of Gag plasmid and the m4-1BBL-cis group with 40μg of Gag-m4-1BBL plasmid. The m4-1BBL-trans and SPD-4-1BBL-trans groups were immunized with 20μg of the respective plasmids pre-mixed with 20μg of Gag plasmid. *, p <0.05; and **, p <0.001. NA, Not available.
Following DNA prime, we measured the frequency of Gag tetramer-specific CD8 T cells in the blood. These analyses demonstrated marginally higher frequencies of these cells in the m4-1BBL-cis group but not in m4-1BBL-trans and SPD-4-1BBL-trans groups (data not shown). At one week after the DNA boost, Gag tetramer-specific CD8 T cell responses were higher in the m4-1BBL-cis (7-fold) and SPD-4-1BBL-trans (4.6-fold) groups than in the non-adjuvanted controls (Fig. 4A). However, at 2 weeks after boost, only the m4-1BBL-cis group maintained higher (2.2-fold) frequencies of Gag tetramer-specific CD8 T cells. In contrast to the m-4-1BBL-cis group, adjuvancity was not observed in the m4-1BBL-trans or the SPD-4-1BBL-trans group. Consistent with tetramer analyses, the frequency of IFN-γ producing CD8 T cells was 2.5-fold higher in the m4-1BBL-cis group than in the non-adjuvanted controls (Fig. 4B). In addition, the frequency of Gag-specific CD8 T cells that co-produce IFN-γ and IL-2 were also 2-fold greater (Fig. 4C, D) in the m4-1BBL-cis group. As observed with the tetramer analyses, the SPD-4-1BBL-trans and m4-1BBL-trans groups failed to show any augmented cytokine response. These results demonstrate that the membrane bound 4-1BBL (m4-1BBL) expressed in cis but not in trans with Gag can augment antigen-specific CD8 T cell responses elicited by a DNA vaccine. Interestingly, though the SPD-4-1BBL protein showed the highest level of adjuvancity in an in vitro proliferation assay (Fig.3C), yet, it failed to augment any antigen-specific CD8 T cell responses in vivo.
3.5. Rapid expansion of Gag-specific CD4 T cells in the 4-1BBL adjuvanted groups
As seen with the Gag-specific CD8 T cell response, the Gag-specific CD4 T cell responses were also augmented by 4-1BBL mediated co-stimulation. At one week following the DNA boost, the frequency of IFN-γ producing CD4 T cells in the m4-1BBL-cis (4-fold) and the secreted SPD-4-1BBL-trans form (2.3-fold) adjuvanted groups were higher compared to the non-adjuvanted controls (Fig. 5A). Similarly, the frequency of Gag-specific CD4 T cells that co-produced IFN-γ and IL-2 (Fig. 5B) was also 4-fold and 2.4-fold higher in the m4-1BBL-cis and SPD-4-1BBL-trans form adjuvanted groups, respectively. However, by 2 weeks post boost, these responses further expanded in the SPD-4-1BBL-trans form adjuvanted and non-adjuvanted groups but not in the m4-1BBL-cis adjuvanted group. As a result, the frequency of Gag-specific CD4 T cells was similar between the adjuvanted and non-adjuvanted groups at 2 weeks post boost. Also, at 2 weeks post boost, there was no difference in the magnitude of antigen-specific CD4 responses between the non-adjuvanted controls and the m4-1BBL-trans group. These results demonstrate that adjuvanting DNA with the 4-1BBL results in a more rapid expansion of vaccine-specific CD4 T cells rather than an overall increase in the magnitude of this response.
Figure 5. Rapid expansion of Gag-specific CD4 T cells in the m4-1BBL-cis and SPD-4-1BBL adjuvanted groups.
BALB/c mice (n=5) were primed and boosted with indicated DNAs at weeks 0 and 4, respectively. A, Summary of Gag-specific IFN-γ producing CD4 T cells in spleen as evaluated by intracellular cytokine staining. B, Summary of Gag-specific IFN-γ+ IL-2+ CD4 T cells in spleen. The bars represent mean frequency ± SEM. *, p <0.05. NA, Not available.
3.6. Gag-specific IgG response: Suppression with agonistic anti-4-1BB Ab and augmentation with membrane bound 4-1BBL DNA expressed in cis
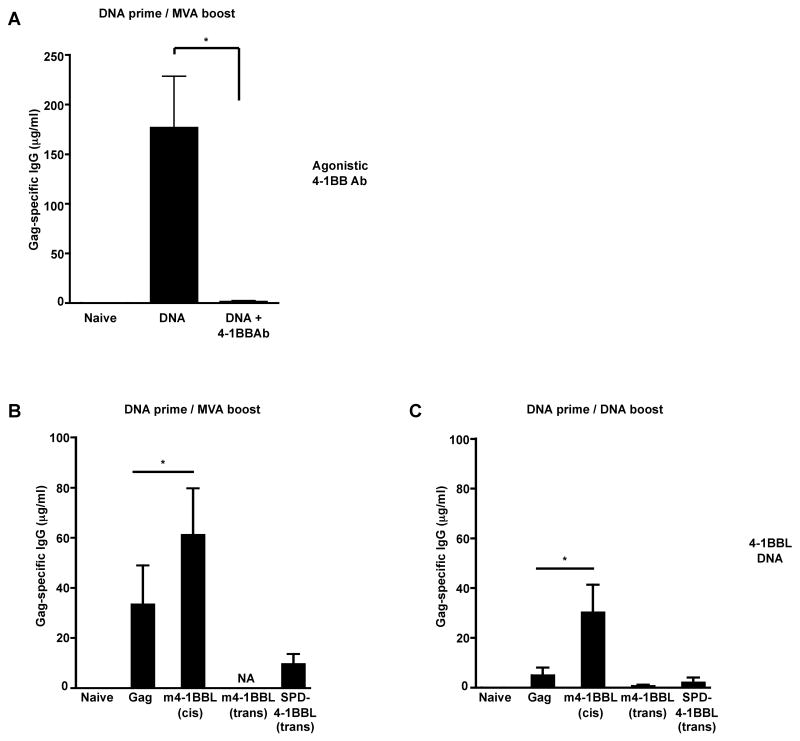
To investigate whether 4-1BB/ 4-1BBL-mediated co-stimulation could also adjuvant the humoral immune response, sera from vaccinated mice were assayed for titer of Gag-specific binding Ab. Mice that were primed with DNA and boosted with MVA generated Gag-specific Ab response with a mean titer of 176 μg/ml of serum at 1 week after the boost (Fig. 6A). However, administration of anti-4-1BB agonistic Ab during the DNA prime resulted in a near complete loss of Gag-specific Ab response. These results demonstrate that co-delivery of anti-4-1BB agonistic Ab during DNA prime enhances the magnitude of cellular immune response but diminishes the humoral immune response. In contrast to the agonistic Ab, co-delivery of 4-1BBL DNA during DNA prime not only preserved the Gag-specific Ab response but also resulted in 1.8-fold enhancement following the MVA boost (Fig. 6B). Similarly, a 6-fold increase in Gag-specific IgG response was also observed when mice were primed and boosted with DNA co-expressing Gag and 4-1BBL (Fig. 6C). Unlike the enhanced humoral immune responses seen in the m4-1BBL-cis adjuvanted group, no augmentation in the Gag-specific Ab responses was observed in the m4-1BBL-trans or SPD-4-1BBL-trans form adjuvanted groups (Fig. 6C). In fact, a trend towards diminished Ab response was observed in the SPD-4-1BBL-trans group compared to the non-adjuvanted control group. These results clearly demonstrate that the anti-4-1BB agonistic Ab and 4-1BBL DNA have opposing effects on the humoral immunity, the former suppressing the response while the latter enhancing the response.
Figure 6. Gag-specific IgG response: Suppression with agonistic anti- 4-1BB Ab, while preservation/augmentation with membrane bound 4-1BBL DNA expressed in cis.
A, BALB/c mice (n=3) were primed with 100μg of HIV-1 Gag DNA vaccine with or without the agonistic anti-4-1BB Ab at week 0 and boosted with MVA-Gag at week 12. Sera were collected at 1 week after the boost and total Gag-specific IgG was determined by ELISA. B, Mice (n=10) were primed with plasmid DNA (20μg) at week 0 and boosted with MVA-Gag at week 4. Gag-specific IgG was determined by ELISA at one week following the boost. C, Mice (n=10) were primed and boosted with plasmid DNA (20μg) at week 0 and at week 4 respectively. Total Gag-specific IgG was evaluated at two weeks after the DNA boost. Sera from unimmunized naïve mice were used as negative control. The bars represent mean ± SEM of Gag-specific IgG titer in serum. *, p= 0.05. NA, Not available.
4. Discussion
The principal goal of this study was to target the 4-1BB/4-1BBL co-stimulatory pathway to adjuvant a HIV DNA vaccine. Several pre-clinical studies have suggested that a strong CTL response is the basis for 4-1BB mediated anti-tumor activity and currently humanized anti-4-1BB monoclonal Abs are also in clinical trials in patients with solid tumors. Given the fact that a strong CTL response is also desirable for an effective anti-HIV immunity, we hypothesized that modulating this co-stimulatory pathway could be a viable option for designing HIV vaccines. In this study, we compared and contrasted the antigen-specific cellular and humoral immune responses raised when 4-1BB co-stimulation is either delivered by an agonistic antibody or by 4-1BBL expressed by a DNA vaccine. Our results demonstrate that while both forms augment the Gag-specific CD8 and CD4 T cell responses, only the ligand form augments the Gag-specific Ab response. In fact, the agonistic Ab suppressed the Gag-specific humoral immune response. These results demonstrate that 4-1BBL rather than anti-4-1BB agonistic Ab is an ideal adjuvant for DNA vaccines for enhancing their immunogenicity.
One of the important findings of our study is that the m4-1BBL-cis group but not m4-1BBL-trans group enhances immunogenicity in vivo demonstrating that for the membrane bound form of the ligand, it is important that both adjuvant and antigen are expressed by the same antigen presenting cell for enhancing vaccine-specific cellular immune responses. In vitro, we observed similar levels of co-expression of Gag and 4-1BBL when 293T cells were either co-transfected with both m4-1BBL and Gag plasmids (4-1BBL in trans) or with the Gag-m4-1BBL plasmid (4-1BBL in cis) (data not shown). Since in vivo transfection efficiency is much lower than in vitro transfection efficiency, one possibility for the reduced in vivo immunogenicity with the trans form could be due to the fact that though the antigen and the adjuvant plasmids were pre-mixed and co-injected in mice, yet in vivo, they were not taken up by the same APC simultaneously. To study this phenomenon, we attempted to detect CD11c positive dendritic cells (DC) co-expressing Gag (intracellular) and 41BBL (surface) in the draining lymph nodes at day 3 following vaccination using antibodies specific to respective proteins. We were successful in detecting 4-1BBL positive cells but not Gag positive cells (data not shown). However, the frequency of 4-1BBL positive DCs were higher in mice that were vaccinated with either of the membrane bound form of 4-1BBL expressing plasmids (in cis or in trans) compared to mice vaccinated with Gag DNA alone suggesting that the failure to enhance the immunogenicity by m4-1BBL plasmid when delivered in trans with respect to Gag was not due to the presence of lower levels of 4-1BBL positive DCs in the draining lymph nodes following vaccination.
Previous studies using other members of the TNF superfamily such as FasL, CD40L and GITRL demonstrated that multimerization is an important aspect for their biological activity [18, 21, 22]. We created the SPD-4-1BBL plasmid to investigate if multimerization had any additive effect on the adjuvant potential of 4-1BBL. In addition, we anticipated that since it is a secreted form of the ligand, co-expression along with Gag antigen (cis form) may not be a requirement. Unexpectedly, though the soluble SPD-4-1BBL form demonstrated higher levels of in vitro co-stimulatory activity in comparison to the m4-1BBL-cis form, yet in vivo, the latter had a greater adjuvant effect. The reason for this disagreement between in vitro and in vivo activity is unclear. However, our data suggest that both for the membrane bound form as well as the multimeric secreted form of the ligand, it may be crucial to co-express the antigen and the adjuvant in cis to achieve any in vivo adjuvancity.
One of the important findings in our study is that 4-1BB mediated co-stimulation, in particular with the agonistic Ab, augments Ag-specific CD4 T cell responses primed by a DNA vaccine and this enhanced CD4 help contributes to the enhanced magnitude of Ag-specific CD8 T cell responses following the boost. In vitro, both CD4 and CD8 T cells can respond to 4-1BB mediated co-stimulation [4, 23, 24]. However, in vivo studies in murine antiviral models have shown that 4-1BB mediated co-stimulation primarily augments CTL responses [11, 25, 26]. Consistently, studies in mice using either the agonistic anti-4-1BB Ab or the 4-1BBL along with poxvirus vectors expressing HIV-1 immunogens also demonstrated an enhancement in antigen-specific CD8 T cell responses but not in CD4 T cell responses [27, 28]. Collectively, these results suggest that the 4-1BB-mediated co-stimulation may be more effective in adjuvanting Ag-specific CD4 T cell responses when administered along with DNA but not with viral vectors. However, in a recent study in macaques that administered three doses of agonistic anti-4-1BB Ab during prime along with a SIV Gag DNA vaccine, enhancement was observed for Gag-specific CD8 and not for CD4 T cell responses [29]. Unlike in our study, where the adjuvant was given at the same time as the DNA prime, the agonistic anti-4-1BB Ab treatments were administered between 12-19 days following prime in the macaque study. We thus suggest that it may be important to deliver the agonistic Ab or 4-1BBL simultaneously with the DNA prime to be able to augment Ag-specific CD4 T cell responses.
Suppressive effects of anti-4-1BB agonistic Ab on antibody responses are well documented and this phenomenon have been therapeutically used in several autoimmune disease models to inhibit immune responses [14, 30]. B cells do not express 4-1BB and effects of 4-1BB co-stimulation on humoral immunity have been shown to be mediated through other cell types. Studies have shown that this suppression of humoral immunity can be mediated by either diminished CD4 helper responses or by CD4-independent mechanisms. The proposed CD4-dependent mechanisms include, anergizing the CD4 T cells during antigen priming [14], induction of suppressor CD8 T cells [15], expansion of CD4+ regulatory T cells [31] and generation of TGFβ secreting effector CD8 T cells [32]. The CD4-independent mechanisms include, diminished dendritic cell networks in B cell follicles [33] and direct killing of B cells by activated monocytes [34], although the latter has only been shown for human B cells. In our study, we augmented the Gag-specific Th1 CD4 T cell responses upon anti-4-1BB agonistic Ab treatment suggesting that the suppression of Gag-specific IgG responses was mediated through some CD4-independent mechanisms.
To further investigate whether the agonistic Ab mediated suppressive effect on IgG responses was due to a block in isotype switching, we evaluated the titer of Gag-specific IgM in serum. Serum IgM levels after a single DNA immunization was low in both the agonistic Ab treated and untreated mice, but were similar between adjuvanted and control groups (data not shown) suggesting that impairment in isotype class switching of Ag-specific B cells could have contributed to the suppressive effect of agonistic Ab on humoral immune response.
In conclusion, our results demonstrate that both anti-4-1BB agonistic Ab as well as 4-1BBL DNA can enhance the antigen-specific cellular immune responses elicited by a DNA vaccine. Our results also demonstrate that administration of agonistic Ab but not the ligand DNA results in suppression of antigen-specific humoral immunity. Collectively, our data suggest that for a HIV-1 vaccine, 4-1BBL expressed by a DNA vaccine is a superior adjuvant than the anti-4-1BB agonistic Ab. These results highlight that by changing the form (agonistic Ab vs. ligand DNA), the same molecular adjuvant can be exploited either to enhance or suppress the humoral arm of adaptive immunity and thus can have varied applications in anti-tumor or anti-viral vaccines or in autoimmune diseases.
Acknowledgments
We thank H.L. Robinson for valuable discussions throughout the study. We thank H. Drake-Perrow for outstanding administrative support. We thank L. Chennareddi for help with statistical analyses and R. Kornbluth for provision of SP-D plasmid. We thank L. Lai for help with in vitro expression studies. We are thankful to the Yerkes Division of Research Resources for the consistent excellence of veterinary care. We thank the NIH AIDS Research and Reference Reagent Program for the provision of peptides. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R01 AI057029 to RRA; R01 AI059290 to RSM; Yerkes National Primate Research Center base grant, P51 RR00165; Emory CFAR grant P30 AI050409.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Seminars in immunology. 1998 Dec;10(6):481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 2.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annual review of immunology. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002 May 1;168(9):4262–7. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 4.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001 Aug 1;167(3):1313–24. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 5.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. The Journal of experimental medicine. 1997 Jul 7;186(1):47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JT, Whitmire JK, Murali-Krishna K, Ahmed R, Altman JD, Mittler RS, et al. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol. 2000 Mar 1;164(5):2320–5. doi: 10.4049/jimmunol.164.5.2320. [DOI] [PubMed] [Google Scholar]
- 7.Wiethe C, Dittmar K, Doan T, Lindenmaier W, Tindle R. Provision of 4-1BB ligand enhances effector and memory CTL responses generated by immunization with dendritic cells expressing a human tumor-associated antigen. J Immunol. 2003 Mar 15;170(6):2912–22. doi: 10.4049/jimmunol.170.6.2912. [DOI] [PubMed] [Google Scholar]
- 8.Myers L, Lee SW, Rossi RJ, Lefrancois L, Kwon BS, Mittler RS, et al. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. International immunology. 2006 Feb;18(2):325–33. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 9.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nature medicine. 1997 Jun;3(6):682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 10.Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002 Aug 15;169(4):1792–800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 11.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nature immunology. 2002 Jun;3(6):536–41. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 12.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2004 Feb 3;101(5):1291–6. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol. 2002 Feb 1;168(3):1457–65. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 14.Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. The Journal of clinical investigation. 2003 May;111(10):1505–18. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nature medicine. 2004 Oct;10(10):1088–94. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 16.Smith JM, Amara RR, Campbell D, Xu Y, Patel M, Sharma S, et al. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res Hum Retroviruses. 2004 Dec;20(12):1335–47. doi: 10.1089/aid.2004.20.1335. [DOI] [PubMed] [Google Scholar]
- 17.Robinson HL, Sharma S, Zhao J, Kannanganat S, Lai L, Chennareddi L, et al. Immunogenicity in macaques of the clinical product for a clade B DNA/MVA HIV vaccine: elicitation of IFN-gamma, IL-2, and TNF-alpha coproducing CD4 and CD8 T cells. AIDS Res Hum Retroviruses. 2007 Dec;23(12):1555–62. doi: 10.1089/aid.2007.0165. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Barzee S, Snarsky V, Kee K, Spina CA, Yu XF, et al. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. Journal of virology. 2006 Feb;80(4):1762–72. doi: 10.1128/JVI.80.4.1762-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Wyatt LS, Amara RR, Moss B, Robinson HL. Studies on in vitro expression and in vivo immunogenicity of a recombinant MVA HIV vaccine. Vaccine. 2006 Apr 12;24(16):3332–9. doi: 10.1016/j.vaccine.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001 Feb 23;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 21.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. European journal of immunology. 2001 Oct;31(10):3094–100. doi: 10.1002/1521-4141(2001010)31:10<3094::aid-immu3094>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, et al. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. The Journal of experimental medicine. 1998 Apr 20;187(8):1205–13. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand. European journal of immunology. 2000 Feb;30(2):392–402. doi: 10.1002/1521-4141(200002)30:2<392::AID-IMMU392>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 24.Cooper D, Bansal-Pakala P, Croft M. 4-1BB (CD137) controls the clonal expansion and survival of CD8 T cells in vivo but does not contribute to the development of cytotoxicity. European journal of immunology. 2002 Feb;32(2):521–9. doi: 10.1002/1521-4141(200202)32:2<521::AID-IMMU521>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999 Nov 1;163(9):4859–68. [PubMed] [Google Scholar]
- 26.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002 Apr 15;168(8):3777–85. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 27.Harrison JM, Bertram EM, Boyle DB, Coupar BE, Ranasinghe C, Ramshaw IA. 4-1BBL coexpression enhances HIV-specific CD8 T cell memory in a poxvirus prime-boost vaccine. Vaccine. 2006 Nov 17;24(4748):6867–74. doi: 10.1016/j.vaccine.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004 Aug;112(4):559–66. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calarota SA, Hokey DA, Dai A, Jure-Kunkel MN, Balimane P, Weiner DB. Augmentation of SIV DNA vaccine-induced cellular immunity by targeting the 4-1BB costimulatory molecule. Vaccine. 2008 Jun 13;26(25):3121–34. doi: 10.1016/j.vaccine.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nature medicine. 2002 Dec;8(12):1405–13. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 31.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol. 2004 Aug 15;173(4):2428–34. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 32.Myers L, Takahashi C, Mittler RS, Rossi RJ, Vella AT. Effector CD8 T cells possess suppressor function after 4-1BB and Toll-like receptor triggering. Proceedings of the National Academy of Sciences of the United States of America. 2003 Apr 29;100(9):5348–53. doi: 10.1073/pnas.0837611100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Blink SE, Chen JH, Fu YX. Regulation of follicular dendritic cell networks by activated T cells: the role of CD137 signaling. J Immunol. 2005 Jul 15;175(2):884–90. doi: 10.4049/jimmunol.175.2.884. [DOI] [PubMed] [Google Scholar]
- 34.Kienzle G, von Kempis J. CD137 (ILA/4-1BB), expressed by primary human monocytes, induces monocyte activation and apoptosis of B lymphocytes. International immunology. 2000 Jan;12(1):73–82. doi: 10.1093/intimm/12.1.73. [DOI] [PubMed] [Google Scholar]