Abstract
In the present study, cellular tropism and relative transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 vectors have been tested for the cornea using primary cultures of human corneal fibroblasts. The AAV6, AAV8 and AAV9 serotypes having AAV2 ITR plasmid encoding for alkaline phosphatase (AP) gene were generated by transfecting HEK293 cell line with pHelper, pARAP4 and pRep/Cap plasmids. Primary cultures of human corneal fibroblasts were exposed to AAV infectious particles at two different doses (1×105 and 2×105 MOI). Cytochemistry and enzyme assays were used to measure delivered transgene expression in samples collected at 4 and 30 hours after AAV infection by counting AP-stained cells or quantifying AP enzyme activity. Cellular toxicity of AAVs was evaluated with TUNEL and trypan blue assays. All three AAV serotypes transduced human corneal fibroblasts. The order of transduction efficiency was AAV2/6>>>AAV2/9>AAV2/8. The transduction efficiency of AAV2/6 was 30-50 fold higher (p <0.001) for the human corneal fibroblasts compared to the AAV2/8 or AAV2/9 at two tested doses. The level of transgene expression at 4 hrs was considerably low compared to 30 hrs suggesting that the transgene delivery did not reach its peak at 4 hrs. Cultures exposed to any of the three AAV serotypes showed more than 97% cellular viability and less than 5 TUNEL positive cells suggesting that tested AAV serotypes do not induce significant cell death and are safe for corneal gene therapy.
Keywords: AAAV2/6, AAV2/8, AAV2/9, Gene transfer, Cornea, Human corneal fibroblasts
1. Introduction
Efficient delivery of therapeutic levels of transgene in the cells/tissues is critical for treating patients with gene therapy. Numerous viral and non-viral vectors have been developed and tested for this purpose. Among recombinant viral vectors, adeno-associated virus (AAV) has emerged as a promising vector for delivering genes efficiently in the ocular cells [15,18,22,27]. AAV has the ability to transduce dividing as well as non-dividing cells and to extend long-term transgene expression. Further, their non-pathogenicity to humans enables them to be an attractive vector for clinical gene therapy. More than 100 serotypes of AAV are known but serotypes 1-9 have been extensively tested for gene therapy [2,25]. The bulk of studies evaluated efficiency of AAV serotypes 2 and 5 for delivering genes in the retina and showed varied degree of transgene delivery into photoreceptors and retinal pigment epithelial cells of rodent, canine and primate retinas in vivo [2,25]. Further, AAV2 has been widely used in preclinical and clinical trials for many retinal diseases including ocular albinism, retinitis pigmentosa and Leber congenital amaurosis [3,16,25]. Contrary to retinal gene therapy, few studies have been performed to evaluate usefulness of AAV vectors for corneal gene therapy [15,19,20]. Our previous studies demonstrated efficacy of AAV2 and AAV5 to deliver genes into the rabbit and rodent stroma in vivo [19,20,23]. The delivered transgene expression appeared 3 days after vector application and continued for up to 10 days or longer. Our subsequent gene transfer studies discovered that delivery of therapeutic genes could be targeted into the stroma by administering the vector in combination with newly-optimized topical and microinjection vector-delivery techniques [20,23]. Other investigators also showed transgene delivery in the rodent corneas with AAV vector [6,11]. Recently, Liu et al compared efficiency of recombinant adenovirus and AAV vectors for delivering genes in the rabbit and human corneal cells (15).
Accumulating evidence suggests that capsid protein is a major determinant of cellular tropism and transduction efficiency of AAV [26]. Thus, many hybrid AAV vectors have been engineered using capsid protein of AAV serotypes 1-9 and genome of AAV serotype 2. Many investigators tested efficiency of hybrid AAV vectors for delivering transgene into ocular and non-ocular tissues using in vitro and in vivo models [2,4,5,13,15,25,29]. Among hybrid AAV vectors, AAV 2/5 has been tested in abundance for delivering genes in the retina [25] and the cornea [20], and has shown considerably enhanced transgene delivery in these ocular tissues compared to the AAV2 [20, 25]. Recently, hybrid vectors AAV2/8 and AAV2/9 have been reported to have up to 5- 100 fold higher efficiency for transgene delivery as compared to AAV2 serotypes in cardiac muscle [4,29], brain [5], skeletal muscle [29], lung [13] and liver [7]. For the ocular tissue, hybrid vectors derived from eight AAV serotypes have been tested for transgene delivery in the retina by several research groups [2,12,25]. Interestingly, AAV2/1, AAV2/4 and AAV2/6 vectors have shown higher transduction efficiency for retinal pigmented epithelium than AAV2/5. However, AAV2/5 had more robust expression in the photoreceptors [25]. On the other hand AAV2/7, AAV2/8 and AAV2/9 showed equal preference for retinal pigmented epithelium and photoreceptors [25] suggesting that hybrid AAV vectors possess unique cellular tropism for different cell types/tissues.
Clinical application of AAV2 vector can be compromised due to the presence of AAV2 neutralizing antibodies in humans. This concern can be minimized by the use of non-human primate derived AAV7 and AAV8 serotypes [8]. Thus, AAV2/7 and AAV2/8 hybrid vectors were developed and found effective for transgene delivery in tissues [12, 25]. However, efficacy of several newly developed hybrid AAV vectors has not been tested for the cornea. In this study we examined and compared the efficiency of hybrid AAV2/6, AAV2/8 and AAV2/9 vectors for delivering genes in the cornea using an in vitro model.
2. Materials and methods
2.1. Human corneal fibroblast culture
Primary cultures of human corneal fibroblasts (HSF) were generated from donor corneas procured from Heartland Eye Bank, St. Louis, Missouri, using previously reported methods [28]. Briefly, the cornea was washed with HSF medium, and the epithelium and endothelium were removed by gentle scraping with a scalpel blade. The corneal stroma was cut into small pieces and incubated in humidified CO2 incubator at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum to obtain HSF.
2.2. AAV vector production
Plasmids for AAV6 rep/cap (pCMV-Cap6, pMT-Rep2) and AAV genomic plasmid (pARAP4) were a generous gift from Dr Dusty Miller, Fred Hutchison Cancer Research Center, Seattle, WA. AAV8 and AAV9 rep/cap plasmids (pAAV2/8; pAAV2/9) were gifted by Dr James M. Wilson, Gene Therapy Program, Division of Medical Genetics, Department of Medicine, University of Pennsylvania, Philadelphia, PA. AAV vector was generated using adenovirus free system following previously published protocol [9]. Briefly, human embryonic kidney (HEK) 293 cells were co-transfected with AAV2-based genomic vector pARAP4, AAV Rep/Cap plasmids and adenovirus helper plasmid (pHelper; Stratagene La Jolla, CA, catalogue # 240071). pARAP4 expresses heat stable placental alkaline phosphatase (AP) under the regulation of Rous sarcoma virus (RSV) promoter/enhancer and simian virus 40 (SV40) polyadenylation sequence. AAV6 was generated using four plasmids, viz. pHelper, pCMV-Cap6, pMT-Rep2 and pARAP4. These plasmids were used at a ratio of 3:3:1:1 respectively. For AAV 8 and AAV9 production, pAAV2/8 and pAAV2/9 encoding for AAV2rep and AAV8 or AAV9cap were used along with pHelper and pARAP4 at a ratio of 3:3:1, respectively. The virus containing cell lysate was harvested at 62 hrs post-transfection. Recombinant viral stocks were purified by two sequential rounds of CsCl gradient ultracentrifugation. Collected viral fractions were pooled and dialyzed through two rounds of HEPES-buffered saline. Viral titer was determined by dot blot analysis using DIG labeled probes (Roche Applied Science, Indianapolis, IN).
2.3. Transduction of human corneal fibroblasts with AAV vectors
Seventy-percent confluent cultures of HSF in 12-well plates were used for the experiments. Three wells for each viral vector transduction were used in every experiment. Cultures were serum-deprived for 2 hours prior to viral transduction (Viral titer 109genomic copies/μl) and were exposed to viral vectors in serum free medium at a multiplicity of infection (MOI) of 105 and 2 × 105. After 6 hours, the medium containing the viral vector was replaced with the fresh serum-containing medium, and cells were incubated for 4 or 30 hours prior to alkaline phosphatase (AP) quantification.
2.4. Cytochemical quantification of alkaline phosphatase
Cultures were washed twice with PBS, fixed in 0.5% glutaraldehyde for 30 min followed by three PBS washes, and 30 min incubation at 65°C in 1mM MgCl2 in PBS to inactivate heat-labile endogenous AP. Cytochemical staining was performed by incubating the cultures with a mixture of BCIP (5-Bromo-4-Chloro-3′-Indolyphosphate p-Toluidine) and NBT (Nitro-Blue Tetrazolium) at 37°C for 2 hrs. The AP-stained cells appeared as dark blue. AP-stained cells were quantified at 100× magnification in six randomly selected, non-overlapping areas of each well.
2.5. Spectrophotometric measurement of alkaline phosphatase in cell lysates
Cell lysates were prepared using RIPA buffer (Tris 50mM, NaCl 150mM, NP40 1%, Na-deoxycholate 0.5% containing 1× Roche protease inhibitor). The protein content for each sample was determined using the Bradford method. AP activity in cell lysates was determined by a spectrophotometric assay using StemTAG Alkaline Phosphatase Activity Assay Kit (Cell Biolabs, Inc., San Diego, CA) following manufacturer’s protocol. Controls included a blank with no cell lysate and cell lysate from uninfected cells. Assay for each sample was run in duplicate and the optical density for AP activity was read at 405 nm. p-nitrophenol was used for plotting the standard curve. The AP activity was expressed as μM of p-nitrophenol generated/μg protein.
2.6. Cellular viability and apoptosis evaluation
To detect the possibility of fibroblast apoptosis due to AAV vector transfection, cells were fixed in paraformaldehyde at room temperature for 5 min and then washed in PBS. A fluorescence-based TUNEL assay was used according to the manufacturer’s instructions using ApopTag apoptosis detection kit (Chemicon international, Temecula, CA, USA). The effect of AAV treatment on cell viability was assessed using trypan blue assay. Briefly, cell suspension was mixed with an equal volume of 0.4% trypan blue solution (Invitrogen, San Diego, CA)) and blue stained and unstained cells were counted using a hemocytometer to calculate the percentage of viable cells.
2.7. Statistical analysis
The results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using two-way analysis of variance (ANOVA) followed by Bonferroni test for comparing data between different AAV vectors. For comparing different doses or time points one way ANOVA followed by Tukey’s multiple comparison test was used.
3. Results
3.1. Characterization of gene delivery by AP cytochemical staining
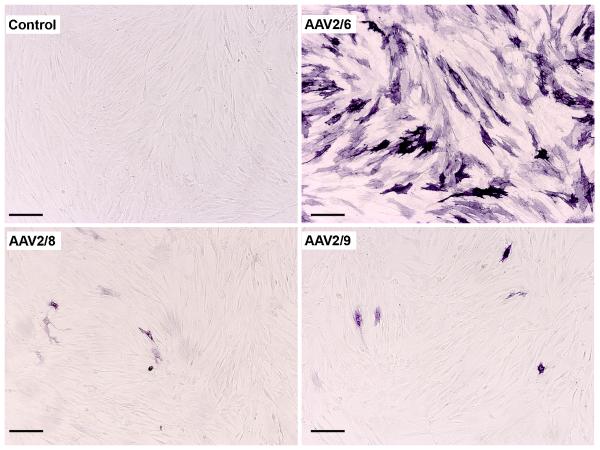
All three tested AAV serotypes successfully transduced human corneal fibroblasts. Fig. 1 demonstrates levels and localization of delivered transgene in human corneal fibroblasts. Thirty hours after AAV treatment, AAV2/6, AAV2/8 or AAV2/9 vector at 1×105 MOI dose showed substantial delivery of AP gene in HSF compared to the control (p<0.001). The AAV2/6 serotype showed highest transduction efficiency followed by AAV2/9 and AAV2/8. The quantification of AP-stained cells in the cultures was performed to determine relative transduction efficiency of the tested AAV serotypes and has been shown in Fig. 2. As evident from the data, all the tested serotypes efficiently transduced HSF and showed statistically significant transgene delivery into human corneal fibroblasts compared to control samples. The AAV2/6 showed highest and AAV2/8 showed lowest transgene delivery. The relative efficiency of the three vectors was AAV2/6, AAV2/9 and AAV2/8. The AAV2/6-treated cultures showed statistically significant 32 fold higher transgene delivery (p<0.001) compared to AAV2/8 and 23 fold higher compared to AAV2/9. Comparative analysis between the AAV 2/8 and AAV2/9 revealed that 1.3 fold higher transgene was delivered by AAV2/9 than AAV2/8 in HSF. Nonetheless, the difference in the levels of transgene delivery between AAV2/8 and 2/9 was not statistically significant probably due to the low levels of transduction with these two AAV serotypes.
Fig. 1.
Transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 for human corneal fibroblasts. Representative images showing alkaline phosphatase (AP) cytochemical staining 30 hours after viral vector application at MOI 1×105. Scale bar denotes 200 μm.
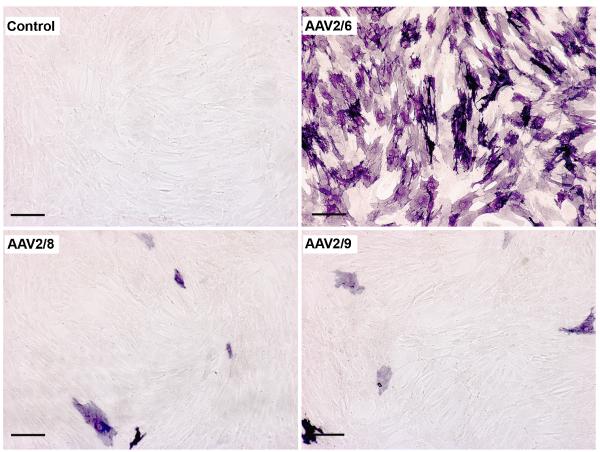
Fig. 2.
Transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 for human corneal fibroblasts. Representative images showing alkaline phosphatase (AP) cytochemical staining 30 hours after viral vector application at MOI 2×105. Scale bar denotes 200 μm.
The transduction efficiency of AAV depends on viral concentration. To test whether virus concentration affects transgene delivery in HSF, we doubled the dose of AAV (2×105 MOI) and compared the transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 for the cornea in vitro. The gene transfer data collected 30 hrs after AAV2/6, AAV2/8 or AAV2/9 serotype infection at the dose of 2×105 MOI confirmed that levels of transgene delivery in the cornea is influenced by the AAV titer. As evident from Figs. 2 and 3, AAV2/6 demonstrated a 2.2 fold increase (p< 0.001) in transduction levels at higher dose (2×105 MOI) compared to the lower dose (1×105 MOI). Similarly, AAV2/8 and AAV2/9 showed a 1.5 and 1.7 fold increase, respectively, in transduction levels at high dose compared to their lower doses. However, this increase was not statistically significant. Furthermore, at high dose transduction efficiency of AAV2/6 was 50 fold more than the same dose of AAV2/8 (p<0.001) or AAV2/9 (p<0.001).
Fig. 3.
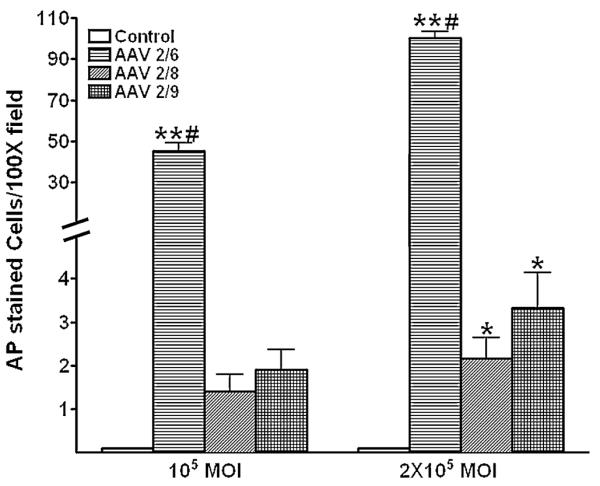
AAV2/6, AAV2/8 and AAV2/9-mediated gene transfer quantification in human corneal fibroblasts. Alkaline phosphatase (AP)-stained cells were counted in 100× magnification 30 hours after viral vector application at MOI 1×105and 2×105.
*p<0.05 vs control; ** p<0.001 vs control or AAV2/8 or AAV2/9 # p<0.001 vs 105 MOI
Various AAV serotypes have shown different onset-timing for delivered-transgene. To insure that the order of transduction efficiency observed for the three tested AAV serotypes for the cornea was not because of the different onset-timing, we measured the AAV2/6, AAV2/8 and AAV2/9 delivered transgene expression in the HSF at an additional randomly selected time point of 4 hours. Qualitative and quantitative analyses of data collected at 4 hrs showed that order of transduction efficiency for the tested AAV serotypes remained the same (AAV2/6<AAV2/9<AAV2/8). However, the total amount of delivered-transgene at 4 hrs was considerably lower than at 30 hrs (data not shown). Another difference observed at the 4 hrs time point was that AAV2/6 showed only 20 fold higher transduction efficiency compared to AAV2/8 or AAV2/9 instead of the 50 fold increase noted at 30 hrs (data not shown). This observation suggests that AAV2/6 mediated transgene delivery did not reach to its peak at 4 hrs.
3.2. Quantification of gene delivery by AP enzyme assay
The gene transduction efficiency of the three selected AAV serotypes was also analyzed by quantifying AP enzyme activity in control and AAV-treated samples. Figure 4 shows levels of enzyme activity measured in cell lysates prepared 30 hrs after exposing HSF cultures at low (1×105 MOI) or high (2×105 MOI) AAV2/6, AAV2/8 or AAV2/9 doses. As evident from Fig. 4, all the three tested serotypes demonstrated significantly more enzyme activity than the control samples (p <0.001 or 0.01). AAV2/6-treated samples showed 2.2 fold more enzyme activity at low dose and 2.8 fold more enzyme activity at high dose compared to their corresponding control groups whereas AAV2/8 and AAV2/9 showed statistically insignificant 0.3 fold increase in AP activity compared to the lower dose (Fig. 4). The comparative analysis of AP activity in cell lysates prepared 30 hrs after high dose (2×105 MOI) treatment of AAV2/6, AAV2/8 or AAV2/9 showed that AAV2/6 serotype was 22 fold (p <0.001) more efficient than the AAV2/9 serotype and 28 fold (p <0.001) more efficient than the AAV2/8 serotype (Fig. 4). On the other hand, samples of AAV2/8 and AAV2/9 serotypes showed insignificant (<0.1-fold) difference in AP enzyme activity.
Fig. 4.
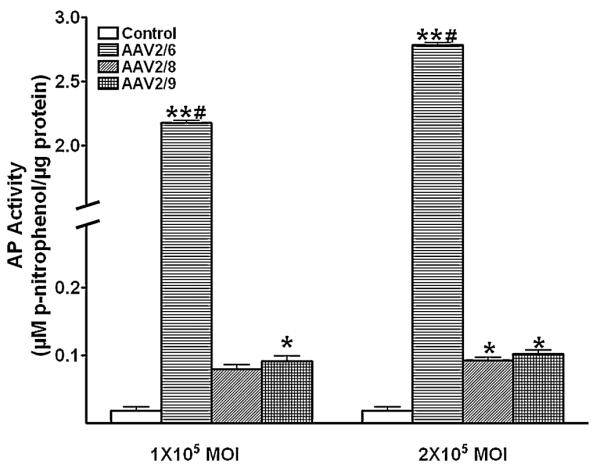
AAV2/6, AAV2/8 and AAV2/9-mediated gene transfer in human corneal fibroblast cell lysates. Alkaline phosphatase (AP) was quantified using enzyme assay 30 hours after viral vector application at MOI 1×105and 2×105.
*p<0.01 vs control; ** p<0.001 vs control or AAV2/8 or AAV2/9 # p<0.001 vs 105 MOI
3.3. Effect of AAV2/6, AAV2/8 and AAV2/9 on cellular viability and death
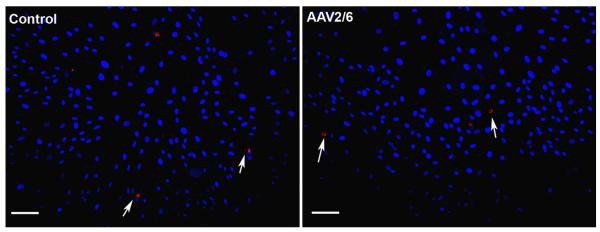
The effect of two tested doses of selected AAV serotypes on HSF viability and death was also investigated. None of the tested doses of the three selected AAV serotypes caused significant loss of cellular viability or induced cell death. Cultures exposed to AAV2/6, AAV2/8 or AAV2/9 showed over 97% viable cells at both the tested AAV doses (1×105 MOI and 2×105 MOI). This viability was comparable to control groups suggesting that selected AAV serotypes do not significantly affect HSF viability. Figure 5 shows results of TUNEL assay which primarily detects apoptosis and to a lesser extent necrosis. Less than 5 TUNEL-positive cells were detected in the control, AAV2/6, AAV2/8 or AAV2/9 treated HSF cultures at two tested doses (1×105 MOI and 2×105 MOI) suggesting that tested AAV serotypes do not induce cell death and are safe for corneal gene therapy. A representative image of TUNEL staining for AAV2/6 treated HSF cultures is shown in Fig. 5.
Fig. 5.
TUNEL assay of human corneal fibroblasts (HSF) treated with AAV2/6. Nuclei are stained blue with DAPI. Few TUNEL-positive cells (Red) were seen in both the control and AAV treated HSF. Scale bar denotes 100 μm. The AAV2/8 or AAV2/9 vcetor treatment to HSF also did not cause significant cell death (Data not shown).
Discussion
AAV serotype 2 has been shown to transport foreign genes efficiently into the cornea, retina and other ocular tissues [2,20,25]. Besides AAV2, many hybrid AAV vectors have been developed and tested for delivering genes in various tissues including the eye [2,15,24]. Hybrid AAV vectors have shown increased transduction efficiency and safety standards compared to AAV2. Nonetheless, these vectors have exhibited unique tropism and showed different transduction efficiency for various tissues/cells. Bish et al [4] found AAV2/9 hybrid vector most efficient for delivering genes in the heart compared to the AAV2/1, AAV2/6, AAV2/7, and AAV2/8. However, AAV2/8 showed up to 200 fold higher transgene delivery in liver compared to AAV2 [7]. On the other hand, AAV2/6 vector was more efficient for delivering genes in the muscle than AAV2/5 or AAV2 vectors [9]. Similar pattern of differences in the transduction efficiency of AAV have been reported for the ocular tissues. The AAV2/5 vectors showed significantly more transgene delivery than AAV2 in corneal stroma, photoreceptors and retinal pigmented epithelium [20,25]. Recently, hybrid AAV2/1, AAV2/6 and AAV2/4 vectors were found more efficient than the AAV2 for retinal pigmented epithelium [25]. The transduction efficiency of AAV2/2 and AAV2/5 serotypes for delivering genes in the mouse cornea has been examined previously [19,20]. However, transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 has not been tested for cornea so far. In this study we report the ability of AAV2/6, AAV2/8 and AAV2/9 vectors to deliver genes in the cornea and demonstrate the relative transduction efficiency of these vectors for delivering therapeutic genes in human corneal fibroblasts. Amongst the three tested serotypes, AAV2/6 showed several folds higher transduction efficiency as compared to AAV2/8 or AAV2/9 for gene delivery in HSF.
Both, in vivo and in vitro models have been used for evaluating transduction efficiency of AAV vectors. The transduction efficiency exhibited by several hybrid AAV vectors for many cultured cells such as lung epithelium, skeletal muscle, gastrointestinal tract cells, etc. was found comparable to the levels of delivered-transgene in vivo [9,14,21] suggesting that in vitro model can provide useful information regarding the efficacy of AAV vectors. Additionally, in vitro models are economical, easily-manageable and allow precise control of experimental conditions for all the groups. Thus, for the initial testing of selected AAV vectors and comparing their transduction efficiencies for the cornea, human corneal fibroblast primary cultures were used. Nonetheless, the findings of the in vitro studies need to be revalidated by the in vivo experiments as multiple studies have reported discrepancy in the amount of transgene delivery in vitro and in vivo [10]. Moreover, the importance of in vivo screening can be realized by the fact that laminin receptor expression (integrin α3β1 and α6β1) has not been found to be the same in adherent and suspension cultures of human corneal fibroblasts [17,24]. The low levels of laminin receptors in cultured corneal fibroblasts may be a contributing factor for low transgene delivery by the AAV2/8 and AAV2/9 vectors as both of these AAV serotype have been reported to use these receptors for entering inside the cell [1]. Besides receptor expression, AAV-mediated gene transfer may also be affected by factors such as lack of endosomal processing, nuclear transport, uncoating, transcription and translation of gene products, etc.
The capsid plays an important role in AAV-mediated gene transfer. It is a primary interface between the host and the vector genome, and is involved in cell binding, internalization and trafficking within the target cell. The capsid influences AAV specificity, transduction efficiency and biodistribution in the tissue [26]. The difference in the transduction efficiency of various AAV vectors has been attributed to the capsid. Subretinal injection of AAV2/4 has preferentially delivered more transgene than AAV2 in the retinal pigment epithelium whereas AAV5 and AAV6 primarily delivered genes in the lung airways cells and AAV9 in alveolar cells [13,14]. On the other hand, AAV6, AAV8 and AAV9 showed robust transgene delivery in muscles in vivo [4,9,29]. Some investigators have identified the involvement of various receptors present on the target cells that might have an effect on cellular tropism and transduction efficiency of AAV. Using lectin competition assays, Wu et al., [30] demonstrated that high transduction efficacy of AAV6 for the skeletal and cardiac tissue compared to AAV8 or AAV9 is due to the involvement of α2,3- or α2,6-linked sialic acid receptor for the cellular entry. The AAV6 uses α2,3- or α2,6-linked sialic acid receptor whereas AAV2/8 and AAV2/9 use laminin receptors. Since the three AAV vectors used in this study carried the same AAV2 ITR plasmid encoding for alkaline phosphatase, it is reasonable to conclude that observed differences in transduction efficiency of AAV2/6 and AAV2/8 or AAV2/9 for human corneal fibroblasts noted in this study are because of different capsid properties.
In conclusion, tested hybrid AAV2/6, AAV2/8 and AAV2/9 vectors efficiently delivered foreign genes in the cornea in vitro. The amount of delivered-transgene in the cornea depends on serotype as AAV2/6 vector showed several fold higher gene transduction for the human corneal fibroblasts compared to AAV2/8 or AAV2/9. This study provides useful information regarding serotype selection for preclinical corneal gene therapy studies.
5. Acknowledgments
This work was supported by the RO1EY17294 (RRM) grant from the National Eye Institute, National Institutes of Health, Bethesda, MD and an unrestricted grant from the Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv. Exp. Med. Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- 3.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 4.Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney L. AAV9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008 doi: 10.1089/hum.2008.123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 6.Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol. Vis. 2007;13:2344–2352. [PubMed] [Google Scholar]
- 7.Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol. Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh A, Yue Y, Duan D. Viral serotype and the transgene sequence influence overlapping adeno-associated viral (AAV) vector-mediated gene transfer in skeletal muscle. J. Gene Med. 2006;8:298–305. doi: 10.1002/jgm.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol. Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J. Biomed. Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 12.Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U S A. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol. Ther. 2008 doi: 10.1038/mt.2008.261. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, Hauswirth WW, Castro MG, Schultz GS, Ljubimov AV. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol. Vis. 2008;14:22087–22096. [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masur SK, Cheung JK, Antohi S. Identification of integrins in cultured corneal fibroblasts and in isolated keratocytes. Invest. Ophthalmol. Vis. Sci. 1993;34:2690–2698. [PubMed] [Google Scholar]
- 18.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog. Retin. Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mohan RR, Schultz GS, Hong JW, Mohan RR, Wilson SE. Gene transfer into rabbit keratocytes using AAV and lipid-mediated plasmid DNA vectors with a lamellar flap for stromal access. Exp. Eye Res. 2003;76:373–383. doi: 10.1016/s0014-4835(02)00275-0. [DOI] [PubMed] [Google Scholar]
- 20.Mohan RR, Schultz GS, Sharma A, Sinha S, Netto MV, Wilson SE. Controlled and site–selective gene delivery into mouse keratocytes by adeno–associated viral, lentiviral and plasmid vectors. Invest. Ophthalmol. Vis. Sci. 2005;46:E-2163. [Google Scholar]
- 21.Polyak S, Mah C, Porvasnik S, Herlihy JD, Campbell-Thompson M, Byrne BJ, Valentine JF. Gene delivery to intestinal epithelial cells in vitro and in vivo with recombinant adeno-associated virus types 1, 2 and 5. Dig. Dis. Sci. 2008;53:1261–1270. doi: 10.1007/s10620-007-9991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saika S, Yamanaka O, Sumioka T, Miyamoto T, Miyazaki K, Okada Y, Kitano A, Shirai K, Tanaka S, Ikeda K. Fibrotic disorders in the eye: targets of gene therapy. Prog. Retin. Eye Res. 2008;27:177–196. doi: 10.1016/j.preteyeres.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Ghosh A, Siddappa C, Mohan RR. Ocular Surface: Gene Therapy. Encyclopedia Eye. 2009 (in press) [Google Scholar]
- 24.Stepp MA. Corneal integrins and their functions. Exp. Eye Res. 2006;83:3–15. doi: 10.1016/j.exer.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359. doi: 10.1016/j.visres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Van Vliet KM, Blouin V, Brument N, Agbandje-McKenna M, Snyder RO. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma I, Weitzman MD. Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 28.Vij N, Sharma A, Thakkar M, Sinha S, Mohan RR. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Mol. Vis. 2008;14:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]