Abstract
Cigarette craving is an important contributor to cigarette smoking, and clinical approaches that focus on regulation of craving are effective in reducing rates of relapse. However, a laboratory model that targets the use of cognitive strategies to regulate craving is lacking. To develop such a model, twenty heavy cigarette smokers (>12/day), twenty-two tobacco “chippers” (<6/day), and twenty non-smoking controls completed this outpatient study, during which they were presented with photographs of cigarettes and foods that have been previously shown to induce craving. During each trial, participants were instructed to think of the stimulus in one of two ways: by focusing either on the short-term consequences associated with consuming the item (e.g., it will taste good) or on the long-term consequences associated with regular consumption (e.g., I may get lung cancer). Participants reported significantly reduced food cravings when focusing on the long-term consequences associated with eating. For cigarette-smoking participants, cigarette craving was significantly reduced when focusing on the long-term consequences associated with smoking. This latter finding confirms clinical data and extends it by highlighting the importance of cognition in the modulation of craving. Future studies using this laboratory model could test the efficacy of different cognitive strategies and develop targeted interventions for smoking cessation based on the regulation of craving.
Keywords: Cigarette smokers, tobacco chippers, craving, cognitive strategies, emotion regulation
1. Introduction
In the United States, there are more than 430,000 smoking-attributable deaths each year (Center for Disease Control and Prevention, 2007), which has earned cigarette smoking the distinction of being the leading preventable cause of disease and death in the United States. Despite this fact, 60 million Americans smoke cigarettes, and nearly 40 million of them are daily smokers (Substance Abuse and Mental Health Services Administration, 2008). Indeed, while considerable efforts have been devoted to the development of smoking cessation treatments, the modal outcome for these interventions is smoking relapse (Fiore et al., 2000; Piasecki, 2006).
Craving has long been considered an important contributory factor in cigarette smoking. Data from clinical research have shown that the degree of craving for cigarettes increases prior to relapse (Allen et al., 2008; Shiffman et al., 1996). Moreover, craving has been found to predict relapse in several prospective studies (Catley et al., 2000; Killen and Fortmann, 1997; O’Connell et al., 2004; Shiffman et al., 1997). One potent trigger for craving is exposure to smoking cues, such as photos depicting others smoking (Carter and Tiffany, 1999; Conklin et al., 2000; McBride et al., 2006; Mucha et al., 1999). Such cue exposure elicits both classically- and operantly-conditioned craving responses (Kober et al., in press), renders smokers particularly vulnerable to cigarette use (Tiffany, 1990), and increases the likelihood of relapse in the context of smoking cessation (Bliss et al., 1989; Shiffman et al., 1996). Further, data from experimental paradigms directly links increased cue-exposure to increased smoking behavior (Payne et al., 1991).
Although craving is not the only factor that leads to drug use (e.g., Tiffany and Carter, 1998), these data indicate that craving is associated with cigarette smoking, and further suggest that cigarette smoking and relapse rates could be decreased by regulation of craving. This has not been studied experimentally, but this idea is consistent with data showing that cognitive-behavioral and relapse prevention approaches that include the use of cognitive strategies for regulation of craving are effective at reducing craving across various substance use disorders (Carroll, 1996; McCrady and Ziedonis, 2001). In cigarette smokers, cognitive coping strategies have been shown to reduce craving as well as reduce instances of relapse during smoking cessation (Bliss et al., 1989; Bliss et al., 1999; O’Connell et al., 2007; Shiffman et al., 1996).
The above suggest that the effective use of cognitive strategies can reduce both craving and smoking behavior in cigarette smokers. Yet, a few important questions remain unresolved. First, the modulatory effect of cognitive strategies on craving has not been studied in a laboratory model under controlled conditions. Although a few laboratory studies have demonstrated that craving predicts preferences for cigarettes over monetary rewards (Bisaga et al., 2007) or the willingness to work for cigarettes (Willner et al., 1995), none have directly examined the modulatory effects of specific cognitive strategies on craving. Second, the effects of different cognitive strategies on craving have not been directly compared. Cognitive-behavioral approaches to smoking cessation often include a component targeting regulation of craving (McDonald et al., 2003; Piasecki and Baker, 2001; Shiffman, 1993), but they are not designed to determine which specific strategies are most effective in reducing craving. Addressing these two questions should contribute to our understanding of why specific cognitive strategies are effective for curbing smoking behavior and could provide the basis for developing targeted interventions for smoking cessation.
We addressed these issues in a novel paradigm that combines elements of studies of cue-induced craving and emotion regulation. Cigarette smoking and non-smoking participants were first trained to use two cognitive strategies, adapted from studies showing that affective responses can be modulated by consciously controlling how one cognitively appraises the meaning of affect-eliciting stimuli (Mischel et al., 1989; Ochsner and Gross, 2005). Participants then completed a series of trials where these strategies were used to enhance or reduce their craving for cigarettes, using cues that have been previously shown to induce craving in cigarette smokers (Mucha et al., 1999). Photographs of high-calorie foods also were used as control stimuli to determine whether smokers differed specifically in their craving for cigarettes. Craving was operationally defined as ratings of subjective desire for food or cigarettes (on a 5-point scale) made at the end of each trial. Following previous studies of cue-induced craving, we predicted that craving for cigarettes but not for food would increase linearly with self-reported smoking. Following studies of emotion regulation, we predicted that craving for both food and cigarettes would be increased or decreased by the use of cognitive strategies.
2. Methods
2.1 Participants
Sixty-two participants (24 female) completed a single session outpatient study. Their age ranged from 18 to 44 years (mean age = 25.11, SD = 6.57). All gave informed consent in accordance with the Columbia University Institutional Review Board.
Participants were divided into three groups based on self-reported cigarette use: Heavy Smokers (Smokers; N=20) smoked at least 12 cigarettes a day, 7 days a week (Mean cigarettes per week = 110.6; Range 84–175; SD = 32.90); Tobacco Chippers (Chippers; N=22) smoked up to 5 cigarettes a day, at least 4 days a week (Mean cigarettes per week = 20.33; Range 8–35; SD = 8.8). This group of long term, yet very light smokers has been characterized as non-nicotine-dependent and as distinct from heavy cigarette smokers (Shiffman et al., 1994a; Shiffman et al., 1994b). Further, Sayette and colleagues (Sayette et al., 2001) have shown that this group is reactive to smoking cues, but reports lower cigarette craving compared to heavy smokers. Finally, Non Smokers (N=20) never smoked cigarettes regularly. The groups differed significantly in the average amount of cigarettes smoked per week (ps<.001) but did not differ significantly in any demographic characteristics.
2.2 Task
This study was a 3 (Groups: heavy smokers, chippers, and non smokers) X 2 (Cues: Cigarettes vs Food) X 2 (Strategies: NOW vs LATER) within-subjects design with group as a between-subjects factor. Each trial began with a 2-second instructional cue (NOW or LATER) followed by a 6 second presentation of a stimulus (either a picture of food or cigarettes). Instruction cues directed participants to think about either the immediate consequence of consuming the pictured substance (NOW cue) or the long-term consequences of repeatedly consuming the substance (LATER cue). These strategies were developed based on prior work showing that, in general, cognitive appraisals modulate experiential, neural, and physiological components of affective responses (Ochsner and Gross, 2005), and specifically, that one can delay consumption of appetitive stimuli by thinking about them in abstract terms rather than focusing concretely on the experience of consuming them (Mischel et al., 1989). The former strategy is one that is often used in cognitive-behavioral treatment for substance abuse. Importantly, to minimize experimental demand the instructions did not include explicit information about the experimental hypothesis for each condition. Following a brief delay, participants next indicated how much they wanted to consume the food or smoke the cigarette at that moment using a 1 (not at all) to 5 (very much) rating scale that appeared on the computer monitor for up to three seconds or until the participants made a response. Exposure to study stimuli and the order of the instructional cues were counterbalanced across participants. A total of 100 trials lasting approximately 17 seconds each were completed. Upon completion of the experiment participants were paid $6 and debriefed.
2.3 Strategy Training
Prior to beginning the task participants underwent a structured training session. During this session, participants received strategy instructions (as detailed above), learned the cue-strategy associations, and then viewed eight sample trials. Sample trials provided participants experience with using both cognitive strategies while looking directly at food and cigarette photographs. Note that the photographs used during the training session were not used during the experimental session. The experiment began when the training session was complete and participants indicated to the experimenter that the directions and procedures were understood.
2.4 Stimuli
To elicit craving responses pictures of cigarettes and fatty foods were collected from three sources: prior research that has used similar photographic cues (Mucha et al., 1999; Simmons et al., 2005; Sobik et al., 2005), pictures from the International Affective Picture System (Lang et al., 1993), and images downloaded from public online sources. The final stimulus set consisted of 100 pictures: 50 of each stimulus type. All images were rated equally desirable to smokers in a separate pilot study.
2.5 Data analysis
Data were subjected to a 2 (Cue: Food vs. Cigarette cues) X 2 (Strategy: NOW vs. LATER) X 3 (Group: Smokers vs. Chippers vs. Non Smokers) mixed ANOVA, with Cues and Strategy as within participants factors, Group as a between participants factor. An alpha level of p<.05 was used to indicate statistical significance. Pairwise comparisons between conditions were performed using t-tests to further clarify the nature of the observed effects.
3. Results
3.1. Craving Across Groups
We observed a significant main effect of Group on craving (F(2,59) = 18.32, p < .001), as well as a significant main effect of Cue type (F(1,59) = 40.30, p < .001). These main effects were qualified by a Group X Cue interaction, (F(2, 59) = 4.08, p < .05), indicating that although the three experimental groups did not differ in their overall craving for food, they did differ in their reported craving for cigarettes (Fig. 1A). Both smokers and chippers reported greater craving for cigarettes than non-smokers (t(38) = 8.67, p< .001; t(40) = 7.21, p < .001, respectively). Further, as expected based on prior work (e.g., Sayette et al., 2001; Shiffman et al., 1994; Shiffman et al., 1995), smokers reported greater cigarette craving compared to chippers (t(40) = 1.86, p = .035, one-tailed). Because we predicted that craving for cigarettes would be linearly related to level of smoking (similarly to a “dose response function”) we subjected the data to a linear contrast on cigarettes cues, and found that this pattern was significant (F(2,59) = 38.01, p < .001). Further, a regression analysis showed that the number of cigarettes smoked per week was a significant predictor of reported craving in both cigarette smoking groups (See Fig. 1B; b = .005, t(40) = 2.22, p < .05). Amount smoked per week explained a significant portion of the variance in reported craving (R2 = .11, F(1, 41) = 4.94, p<.05).
Figure 1.
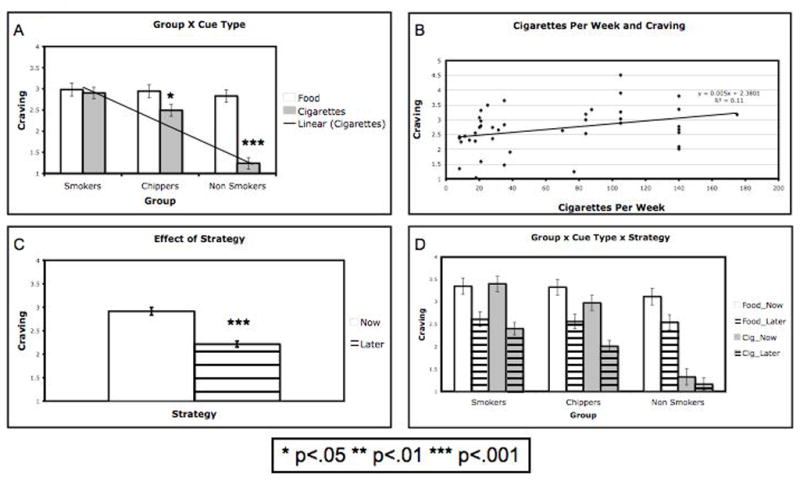
(A) Mean craving reported by smokers, chippers, and non-smokers, on trials in which images of food or cigarette were presented. The three experimental groups did not differ in their overall craving for food. A significant linear trend was found for cigarettes across groups. (B) Number of cigarettes smoked per week plotted against average cigarette craving reported (for smokers and chippers only). Regression analysis showed that the number of cigarettes smoked per week was a significant predictor of reported craving, and explained a significant portion of the variance in reported craving. (C) Mean craving reported in NOW compared to LATER trials across groups and stimuli; Overall, participants reported greater desire to consume food and cigarettes when engaging in the NOW strategy compared to the LATER strategy. (D) Mean craving reported by smokers, chippers, and non-smokers across all trial types. These data represent a significant three-way Group X Cue X Strategy interaction. As shown, the difference in craving for food reported in the NOW vs. LATER was similar across groups, while the difference in craving for cigarettes in NOW vs. LATER conditions varied across groups. Asterisks (*) indicate significant differences. * p<.05, ** p<.01, *** p<.001.
3.2. Effect of Cognitive Strategies
A significant main effect of Strategy was observed, indicating that all participants reported greater desire to consume food and cigarettes when engaging in the NOW strategy compared to the LATER strategy (F(1,59) = 72.00, p < .001; Fig. 1C). This main effect was not moderated by the type of stimulus (Strategy X Cue interaction: F(1,59) = .06, p = .81). Consistent with predictions, however, it was moderated by Group (Strategy X Group interaction: F(2,59) = 4.08, p < .05) and further qualified by a three-way Group X Cue X Strategy interaction (F(2, 59) = 8.31, p=.001; Fig. 1D). This 3-way interaction reflects two patterns in the data: a) the difference in craving for food reported in the NOW vs. LATER was similar across groups, while b) the difference in craving for cigarettes in NOW vs. LATER conditions varied across groups, such that non-smokers reported significantly smaller NOW vs. LATER differences compared to both smokers and chippers (t(38) = 4.81, p< .001; t(40) = 4.02, p < .001, respectively). Importantly, the difference in reported craving for cigarettes in the NOW vs. LATER conditions was equivalent for smokers and chippers (t(40) = .123, p=.9).
4. Discussion
These findings show that smokers, chippers, and non-smokers differ in their reported craving for cigarettes but do not differ in their craving for control food stimuli. The data replicate previous results that chippers report lower cue-induced cigarette craving than smokers (Sayette et al., 2001) and that non-smokers report no significant craving for cigarettes, lending support to the validity of the self-report measure of craving. The data extend previous findings regarding craving in smokers and chippers by finding that the number of cigarettes smoked per week predicted the level of reported craving, illustrating another association between craving and smoking behavior. Finally, the data suggest that smokers do not differ from non-smokers in the intensity of their desire in general, focusing attention on their ability to regulate their desire for cigarettes.
Importantly, the data suggest that the intensity of craving is modulated by cognitive strategies in a laboratory model. Subjective reports of craving for cigarettes were lower when cigarette-smoking participants considered the long-term consequences associated with smoking, a strategy that is often taught as a component of smoking cessation treatments. These data suggest that smokers can use cognitive strategies to reduce their craving for cigarettes, which confirms clinical findings that have demonstrated the efficacy of cognitive strategies in reducing craving and preventing relapse during smoking cessation (Bliss et al., 1989; Bliss et al., 1999; O’Connell et al., 2007; Shiffman et al., 1996).
Although our data suggest a modulatory role of cognition in craving, it does not directly link craving to smoking behavior. Ongoing work in our laboratory will measure the effects of cognitive strategies on both craving and smoking behavior. The present study was an initial first step in creating a laboratory model for studying the effects on cognitive strategies on both craving and smoking. As such, we relied on self-reports of craving, which may be subject to experimental demand. Demand seems an unlikely explanation for the present findings, however, given a) prior work showing that cognitive strategies like those used here modulate autonomic and neural measures of affective responding in addition to self-reports (for reviews, see Ochsner and Gross, 2005, 2008), and b) that our instructions minimized explicit reference to expected behavioral outcomes. In addition, it is possible that the observed effects are due to variations in smoking expectancy between conditions. However, this alternative explanation is less likely because opportunity to smoke was equal across conditions in this within-subject experiment.
In the future, an experimental model like the one described here could be useful in two ways. First, it could be used to measure the relative efficacy of different cognitive strategies in reducing craving and cigarette smoking, thereby aiding in the development of targeted smoking cessation programs. Second, it could be used to investigate the neural mechanisms that underlie the regulation of craving to determine whether smokers successfully recruit neural systems required for the effective regulation of craving. This latter aim is especially important in light of recent suggestions that substance-dependent individuals show impaired control over drug taking that is related to disruption in prefrontal circuits associated with regulation (Volkow et al., 2003).
Acknowledgments
Role of funding source: Funding for this research was provided by NIDA grant DA22541. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Yer Xiong, Landon Fuhrman, and Katherine Remy who assisted with the recruitment and running of study participants. We also thank Peter Mende-Siedlecki who helped with running of study participants, handling of data, and with proofreading of the manuscript.
Footnotes
Contributors: Ms. Kober, Drs. Kross, Mischel, Hart and Ochsner designed the study. Ms. Kober and Dr. Kross wrote the protocol. Ms. Kober managed the literature searches and summaries of previous related work, undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest: The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tobacco Res. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Padilla M, Garawi F, Sullivan MA, Haney M. Effects of alternative reinforcer and craving on the choice to smoke cigarettes in the laboratory. Human Psychopharmacology - Clinical and Experimental. 2007;22:41–47. doi: 10.1002/hup.816. [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. Journal of Consulting and Clinical Psychology. 1989;57:443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- Bliss RE, Garvey AJ, Ward KD. Resisting temptations to smoke: Results from within-subjects analyses. Psychology of Addictive Behaviors. 1999;13:143–151. [Google Scholar]
- Carroll KM. Relapse Prevention as a Psychosocial Treatment: A Review of Controlled Clinical Trials. Experimental and Clinical Psychopharmacology. 1996;4:46–54. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Catley D, O’Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychology of Addictive Behaviors. 2000;14:73–76. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Cigarette Smoking Among Adults - United States, 2006. MMWR Morbidity and Mortality Weekly Report. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST, Vrana SR. The impact of imagining completed versus interrupted smoking on cigarette craving. Experimental Clinical Psychopharmacology. 2000;8:68–74. doi: 10.1037//1064-1297.8.1.68. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaén CR, Kottke TE, Lando HA. Treating tobacco use and dependence: clinical practice guideline. US Department of Health and Human Services; 2000. [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Kober H, Turza AC, Hart CL. Risk Factors for Substance Use, Abuse, and Dependence: Learning. In: Kranzler HR, Korsmeyer P, editors. Encyclopedia of Drugs, Alcohol, and Addictive Behavior. Macmillan Reference; USA: in press. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McCrady BS, Ziedonis D. American Psychiatric Association practice guideline for substance use disorders. Behavior Therapy. 2001;32:309–336. [Google Scholar]
- McDonald P, Colwell B, Backinger CL, Husten C, Maule CO. Better Practices for Youth Tobacco Cessation: Evidence of Review Panel. American Journal of Health Behaviour. 2003;27:144–158. doi: 10.5993/ajhb.27.1.s2.5. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Pauli P. Modulation of craving by cues having differential overlap with pharmacological effect: evidence for cue approach in smokers and social drinkers. Psychopharmacology. 1999;147:306–313. doi: 10.1007/s002130051172. [DOI] [PubMed] [Google Scholar]
- O’Connell K, Schwartz J, Gerkovich M, Bott M, Shiffman S. Playful and rebellious states vs. negative affect in explaining the occurrence of temptations and lapses during smoking cessation. Nicotine Tobacco Res. 2004;6:661–674. doi: 10.1080/14622200410001734049. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation. Health Psychol. 2007;26:77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Currents Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: Effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Baker TB. Any further progress in smoking cessation treatment? Nicotine Tobacco Res. 2001;3:311–323. doi: 10.1080/14622200110050484. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Smoking cessation treatment: Any progress. Journal of Consulting and Clinical Psychology. 1993;61:718–722. doi: 10.1037//0022-006x.61.5.718. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: predicting smoking lapse from daily urge. J Abnorm Psychol. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kassel JD, Paty J, Gnys M, Zettler-Segal M. Smoking typology profiles of chippers and regular smokers. J Subst Abuse. 1994a;6:21–35. doi: 10.1016/s0899-3289(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine Withdrawal in Chippers and Regular Smokers: Subjective and Cognitive Effects. Health Psychology. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994b;2:126–142. [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of Appetizing Foods Activate Gustatory Cortices for Taste and Reward. Cereb Cortex. 2005;15:1602–1608. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Sobik L, Hutchison K, Craighead L. Cue-elicited craving for food: a fresh approach to the study of binge eating. Appetite. 2005;44:253–261. doi: 10.1016/j.appet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Series H-34, DHHS Publication No SMA 08–4343. Office of Applied Studies; Rockville, MD: 2008. Results from the 2007 National Survey on Drug Use and Health (NSDUH): National Findings. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharm. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Hardman S, Eaton G. Subjective and behavioural evaluation of cigarette cravings. Psychopharmacology. 1995;118:171–177. doi: 10.1007/BF02245836. [DOI] [PubMed] [Google Scholar]


