SUMMARY
Relaxin is a polypeptide hormone that triggers multiple signaling pathways through its receptor RXFP1. Many of relaxin’s functions, including vascular and antifibrotic effects, are similar to those induced by activation of PPARγ. In this study, we tested the hypothesis that relaxin signaling through RXFP1 would activate PPARγ activity. In cells overexpressing RXFP1 (HEK-RXFP1), relaxin increased transcriptional activity through a PPAR response element (PPRE) in a concentration-dependent manner. In cells lacking RXFP1, relaxin had no effect. Relaxin increased both the baseline activity and the response to the PPARγ agonists rosiglitazone and 15d-PGJ2, but not to agonists of PPARα or PPARδ. In HEK-RXFP1 cells infected with adenovirus expressing PPARγ, relaxin increased transcriptional activity through PPRE, and this effect was blocked with an adenovirus expressing a dominant-negative PPARγ. Knockdown of PPARγ using siRNA resulted in a decrease in the response to both relaxin and rosiglitazone. Both relaxin and rosiglitazone increased expression of the PPARγ target genes CD36 and LXRα in HEK-RXFP1 and in THP-1 cells naturally expressing RXFP1. Relaxin did not increase PPARγ mRNA or protein levels. Treatment of cells with GW9662, an inhibitor of PPARγ ligand binding, effectively blocked rosiglitazone-induced PPARγ activation, but had no effect on relaxin activation of PPARγ. These results suggest that relaxin activates PPARγ activity, and increases the overall response in the presence PPARγ agonists. This activation is dependent on the presence of RXFP1. Furthermore, relaxin activates PPARγ via a ligand-independent mechanism. These studies represent the first report that relaxin can activate the transcriptional activity of PPARγ.
Keywords: Relaxin, Relaxin Family Peptide Receptor 1 (RXFP1), Peroxisome proliferator-activated receptor gamma (PPARγ)
INTRODUCTION
Relaxin is a polypeptide hormone of the insulin superfamily, which also includes the relaxin-like peptides relaxin-3 and insulin-like peptide-3 (InsL3) (Sherwood, 2004). Despite considerable structural similarity to insulin, relaxin does not bind to the insulin receptors and has no insulin-like glucocoregulatory effects. The identification of a relaxin receptor proved elusive, which hampered progress in relaxin research for many years, until Hsu et al. identified two relaxin receptors in 2002. Somewhat surprisingly, unlike the receptors for insulin and insulin-like growth factor I, which signal through tyrosine kinase receptors, the leucine-rich G protein-coupled receptors 7 and 8 (LGR7 and LGR8) were identified as relaxin receptors (Hsu et al., 2002). These receptors are now known as relaxin family peptide receptor (RXFP) 1 and 2, respectively (Bathgate et al., 2006). Although relaxin will bind to and activate both receptors in vitro, the evidence thus far suggests that only RXFP1 is activated by relaxin in vivo, and therefore RXFP1 is considered to be the cognate relaxin receptor, while RXFP2 is the InsL3 receptor.
Activation of RXFP1 stimulates adenylyl cyclase and increases cAMP through a complex, biphasic mechanism (Halls et al., 2006, Nguyen et al., 2003). Initially, RXFP1 couples to Gαs, resulting in rapid increase in cAMP (Halls et al., 2006). This is followed by coupling to Gαo and a transient decrease in cAMP production, then finally a delayed and sustained increase in cAMP mediated through coupling to Gαi and activation of phosphoinositide 3’-kinase and protein kinase Cζ through Gβ/γ subunits (Halls et al., 2006, Nguyen et al., 2003). In addition to stimulation of cAMP, a number of other pathways can also be triggered by relaxin signaling. Relaxin effects in a number of tissues are mediated by stimulation of nitric oxide synthase and increased nitric oxide production (Baccari and Bani, 2008). In some cells, relaxin can activate the extracellular signal-related kinase 1 and 2 (ERK1/2) pathway, in a process that may involve the nuclear factor NF-κB (Dschietzig et al., 2003, Ho et al., 2007, Zhang et al., 2002, Mookerjee et al., 2009). Additional studies have suggested that relaxin can activate a tyrosine kinase pathway in some cells (Anand-Ivell et al., 2007, Bartsch et al., 2001, Palejwala et al., 2001). There is also a report that relaxin can activate the glucocorticoid receptor through a mechanism that may be independent of RXFP1 (Dschietzig et al., 2005). Therefore, the accumulating evidence suggests that relaxin signals through highly diverse and complex mechanisms.
The traditional functions attributed to relaxin are related to pregnancy, where its functions include inhibition of uterine contraction, and inducement of cervical growth and softening (Sherwood, 2004). However, recent studies have revealed that relaxin has many nonreproductive functions. It has been long known that relaxin has antifibrotic effects related to its ability to regulate the expression and degradation of extracellular matrix components. In fibroblasts and myofibroblasts, relaxin inhibits transforming growth factor beta (TGF-β)-induced collagen production and promotes matrix degradation, and decreases fibrillar collagen in experimental models of dermal, pulmonary, renal, and hepatic fibrosis (Samuel, 2005). This property was strongly supported by studies of the relaxin-null mouse. These mice developed age-related fibrosis in a number of tissues including the lung, skin, heart, and kidney (Samuel et al., 2005), suggesting a role for relaxin in the treatment of fibrosis, and in the prevention of the development of age-related fibrosis. In addition, relaxin exhibits properties related to the control of cardiovascular functions. Relaxin promotes vasodilation and angiogenesis, protects against ischemic damage, and displays anti-inflammatory properties, largely through nitric oxide-dependent pathways (Bani, 2008, Baccari and Bani, 2008). Therefore, in addition to its reproductive role, relaxin is an important modulator of a number of physiological processes outside of pregnancy. Many of these nonreproductive functions of relaxin are similar to those regulated by peroxisome proliferator-activated receptor gamma (PPARγ).
The peroxisome proliferators-activated receptors (PPARs) are nuclear receptors that heterodimerize with the retinoid X receptors (RXR) to induce transcription of target genes (Evans et al., 2004). Three major PPARs have been identified to date. PPARα, the earliest identified form, is involved in peroxisome proliferation and lipid utilization. Less is known about PPARδ (also known as PPARβ), but its main functions appear to be in fatty acid utilization. The third form, PPARγ is a major regulator of adipogenesis (Tontonoz and Spiegelman, 2008). Agonists of PPARγ, such as the antidiabetic thiazolidinedione drugs, promote lipid storage in adipose tissue, decrease serum lipid levels, and regulate adipokine secretion from the adipose tissue, and increase insulin responsiveness in the liver and muscle (Tontonoz and Spiegelman, 2008). In addition, PPARγ agonists have antifibrotic properties in the skin, heart, kidney, lung and liver (Calkin et al., 2006, Genovese et al., 2005, Iglarz et al., 2003, Wu et al., 2009, Galli et al., 2002), and induce anti-inflammatory, cardiovascular, and antifibrotic effects (Tontonoz and Spiegelman, 2008, Varga and Nagy, 2008).
Because relaxin and PPARγ share a number of biological effects, it is possible that they possess common signaling pathways. Relaxin stimulates the production of cAMP and nitric oxide, both regulators of PPARγ activity (Lazennec et al., 2000, Ptasinska et al., 2007, Watanabe et al., 2003). Therefore, it is possible that activation of PPARγ may be one mechanism for the antifibrotic effects of relaxin. We hypothesized that relaxin activation of RXFP1 would result in increased PPARγ activity. To explore this possibility, we sought to determine the effect of relaxin on the levels and activity of PPARγ in cells expressing RXFP1.
MATERIALS AND METHODS
Materials
Highly purified porcine relaxin (Sherwood and O'Byrne, 1974) was provided by O. David Sherwood (University of Illinois Urbana-Champaign). The RXFP1 expression plasmid (Hsu et al., 2000) was provided by Aaron Hsueh (Stanford University). The PPAR luciferase reporter plasmid ACO-PPRE (Jiang et al., 1998) was provided by Brian Seed (Harvard University). Adenoviruses expressing wild-type and mutant PPARγ and β-galactosidase (Park et al., 2003) were provided by J. Larry Jameson (Northwestern University). Human relaxin-3 (H3 relaxin) and insulin-like peptide 3 (InsL3) were from Phoenix Pharmaceuticals. Rosiglitazone, GW0742, GW9662, and 15-Δ12,14-deoxy prostaglandin J2 (15d-PGJ2) were from Cayman Chemical. WY-14,643 was from Sigma Chemical.
Production of cell lines stably expressing RXFP1
To generate cells stably expressing RXFP1, a plasmid encoding LGR7/RXFP1 containing a FLAG epitope (Hsu et al., 2000) was transfected into HEK-293T cells (American Type Culture Collection) using Fugene-6 according to the manufacturer’s instructions. Cells stably expressing the FLAG-RXFP1 were selected using zeocin (Invitrogen) at 400µg/mL, and individual clones were isolated and screened for RXFP1 expression using an in-cell Western blot procedure. Cells grown on poly-L-lysine coated 96-well plates were fixed in 4% paraformaldehyde for 20 minutes, the washed and solubilized in PBS containing 0.1% Triton X-100 for 5 minutes for a total of 5 washes. After blocking in Odyssey block (Li-Cor) for 2 hours, wells were incubated with either M2 anti-FLAG antibody (Sigma) or an antibody to a control protein (rabbit antiinsulin- degrading enzyme, Millipore) at a 1:1000 dilution in Odyssey block overnight at 4°C. After washing 5 times with PBS containing 0.1% Tween-20 for 5 minutes each, the cells were incubated in the dark with IRDye-680 labeled goat anti-rabbit or IRDye-800 labeled goat anti-mouse secondary antibodies for 1 hour at room temperature. After 5 washes with PBS containing 0.1% Tween-20 and one final PBS wash, fluorescence was measured in an Odyssey infrared fluorescence scanner (Li-Cor). The clones with the highest FLAG content relative to the control protein were selected and expanded. The cells were then screened for relaxin responsivity (cAMP production), and a final clone (HEK-RXFP1) was selected and used for all studies.
Reporter and cAMP assays
PPAR activation was monitored using a dual-luciferase reporter assay. Cells were transfected with a plasmid (ACO-PPRE) containing three copies of the acyl CoA oxidase PPRE element upstream of the firefly luciferase gene (Jiang et al., 1998), and a plasmid containing the renilla luciferase gene under the control of the thymidine kinase promoter (pRL-TK, Promega) to control for transfection efficiency and cell number. After 24 hours, cells were treated and incubated for 24 hours, and then firefly and renilla luciferase activities were measured using the Dual-Glo assay (Promega). For cAMP determinations, cells were treated for 30 minutes at room temperature, and cAMP content was determined using the cAMP-Glo assay (Promega) following the manufacturer’s instructions.
Overexpression and knockdown of PPAR
To increase expression of PPARγ, HEK-RXFP1 cells were infected with a total of 45 ifu of replication-incompetent adenovirus containing the genes for β-galactosidase (β-Gal), wild-type PPARγ, or dominant-negative Leu466Ala-PPARγ (Park et al., 2003). After 24 hours, cells were transfected with ACO-PPRE and pRL-TK plasmids, treated with relaxin or rosiglitazone for 24 hours, then subject to the PPRE luciferase reporter assay as described above. For knockdown of PPARγ, siRNA and reporter plasmids were applied to HEK-RXFP1 cells using Nucleofection Kit V (Amaxa) as directed by the manufacturer. Each nucleofection reaction contained 2µg siRNA (either PPARγ siRNA or nontargeting control SMARTpool siRNA, Dharmacon), 1µg ACO-PPRE and 0.1µg pRL-TK. After nucleofection, cells were seeded onto poly-L-lysine coated 96-well or 24-well plates. After 24 hours, cells in 96-well plates were treated with relaxin or rosiglitazone for 24 hours, then subject to the dual-luciferase assay as described above. Cells in 24-well plates were lysed and knockdown of PPARγ was verified using anti-PPARγ (Santa Cruz) and anti-GAPDH (Millipore) primary antibodies, followed by IR-Dye labeled secondary antibodies (Li-Cor). Fluorescence was detected using Odyssey infrared fluorescence scanner (Li-Cor).
Gene expression assays
Cells were treated for 16 hours, then total cellular RNA was extracted using the Purelink kit (Invitrogen). The RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and then subject to TaqMan real-time PCR. The gene expression assays (Applied Biosystems) used were PPARγ (Hs00234592_m1), CD36 (Hs00169627_m1), LXRα (Hs00172885_m1), and 18S rRNA (Hs99999901_s1). Gene expression was normalized to the level of 18S rRNA within each sample using the relative CT method.
Statistical Analysis
Curve-fitting and statistical analysis was performed using Prism 5 software (GraphPad). Differences were analyzed using one-way or two-way analysis of variance as appropriate, with Bonferroni’s post-test. Data are expressed as mean ± S.E.M. of at least three independent determinations except as indicated in the figure legends.
RESULTS
Relaxin increases PPAR transcriptional activity in RXFP1-expressing cells
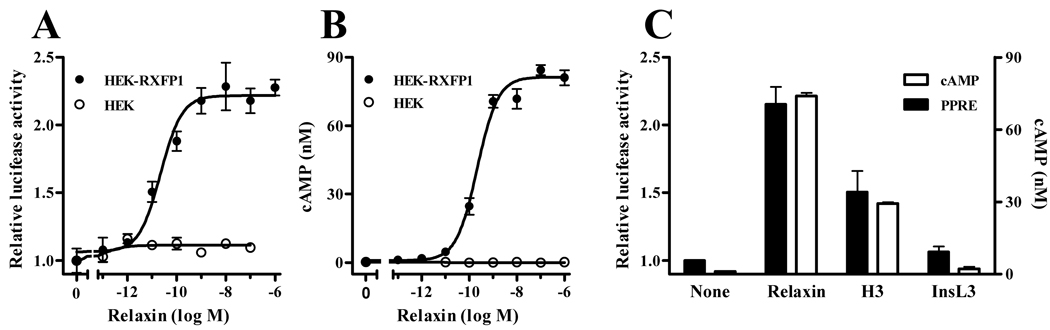
Activation of PPARs induces binding to peroxisome proliferator response elements (PPRE) in target gene promoters, resulting in gene transcription. To determine the effect of relaxin on PPAR activity, a reporter plasmid containing three tandem copies of the PPRE from acyl-CoA oxidase upstream of the firefly luciferase gene was used to monitor activation of PPAR. Relaxin treatment of HEK-RXFP1 cells resulted in a concentration-dependent increase in PPAR activity (Figure 1A). The apparent EC50 was 22.39pM, with a maximal increase in PPRE transcriptional activity of approximately two-fold. There was no response in the parental HEK cells (not expressing RXFP1). Consistent with activation of RXFP1, relaxin treatment increased cAMP production in HEK-RXFP1 cells, but not cells lacking RXFP1 (Figure 1B). The calculated pEC50 value for cAMP stimulation was 214.7pM. The effect was specific to RXFP1 ligands, as both relaxin and the alternative RXFP1 agonist H3 relaxin (human relaxin-3) induced PPRE transcriptional activity, but the relaxin-like peptide InsL3, which is the physiological ligand for RXFP2, and has very low affinity for RXFP1, had no significant effect (Figure 1C).
Fig. 1.
Relaxin activates PPAR activity through RXFP1. Cells expressing RXFP1 (HEK-RXFP1) or without RXFP1 (HEK) were treated with relaxin or related peptides. A. Cells transfected with ACO-PPRE and pRL-TK reporter plasmids were treated with the indicated concentrations of relaxin for 24 hours, then subject to firefly and renilla luciferase assays. The data are expressed as the ACO-PPRE luciferase activity relative to that in untreated cells, mean ± S.E.M. (N=3). B. HEK-RXFP1 or HEK cells were treated with the indicated concentrations of relaxin for 30 minutes, then subject to cAMP quantitation. Data are expressed as the actual cAMP concentration (nM) mean ± S.E.M of triplicate wells. C. Cells were treated with 100nM relaxin, H3-relaxin or InsL3 for 24 hours or 30 minutes for PPRE-luciferase assay (N=3) or cAMP determination (triplicate wells), respectively.
Relaxin activates PPARγ
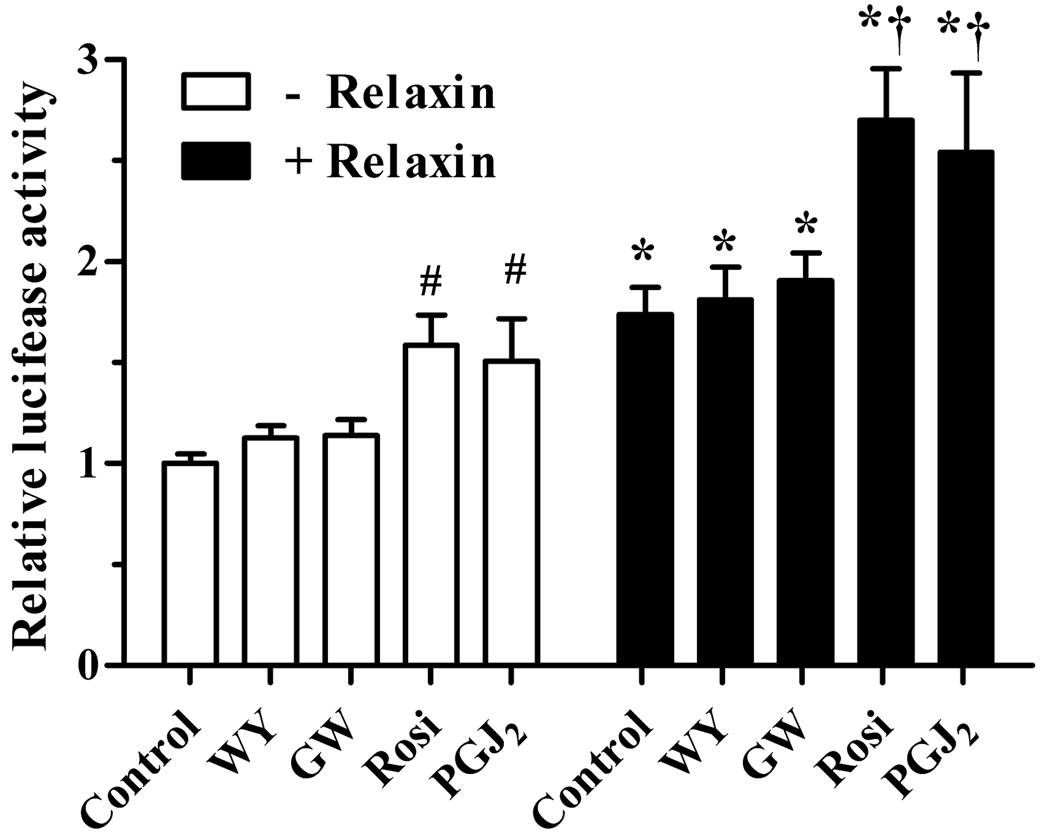
The PPRE reporter plasmid used above is not specific, and can be activated by PPARα, PPARδ, or PPARγ. To explore the potential role of each of the PPARs in the cell model, HEK-RXFP1 cells were treated with PPAR subtype-specific agonists, with or without relaxin. Agonists for either PPARα or PPARδ (WY-14,643 and GW0742, respectively) slightly increased the activity of the PPRE reporter (Figure 2). The combination of either WY-14,643 or GW0742 with relaxin resulted in PPRE transcriptional activity that did not differ from relaxin alone. In contrast, agonists of PPARγ (rosiglitazone and 15d-PGJ2) induced a much greater increase in activation of the PPRE reporter. Furthermore, the combination of relaxin and PPARγ agonists resulted in an induction of PPRE that was significantly greater than either treatment alone. The low responses of PPARα and PPARδ to their agonists either alone or in combination with relaxin make it unlikely that they are the target of the relaxin effects on the PPRE reporter, while the greater response to PPARγ agonists makes it more likely that PPARγ is the target of relaxin signaling.
Fig. 2.
Relaxin increases the overall response to PPARγ agonists. HEK-RXFP1 cells were treated for 24 hours with or without 1nM relaxin in the presence or absence of specific PPAR agonists, then subject to the PPRE luciferase assay. The agonists used were WY-14,643 (WY, 2µM) for PPARα; GW-0742 (GW, 10nM) for PPARδ; and rosiglitazone (Rosi, 1µM) or 15-deoxy-Δ12,14-PGJ2 (PGJ2, 1µM) for PPARγ. The data are expressed as the ACO-PPRE luciferase activity relative to that in untreated cells, mean ± S.E.M (N=5). *p<.05 compared to corresponding agonist in the absence of relaxin; #p<.05 compared to no treatment; †p<.05 compared to either relaxin or corresponding agonist alone.
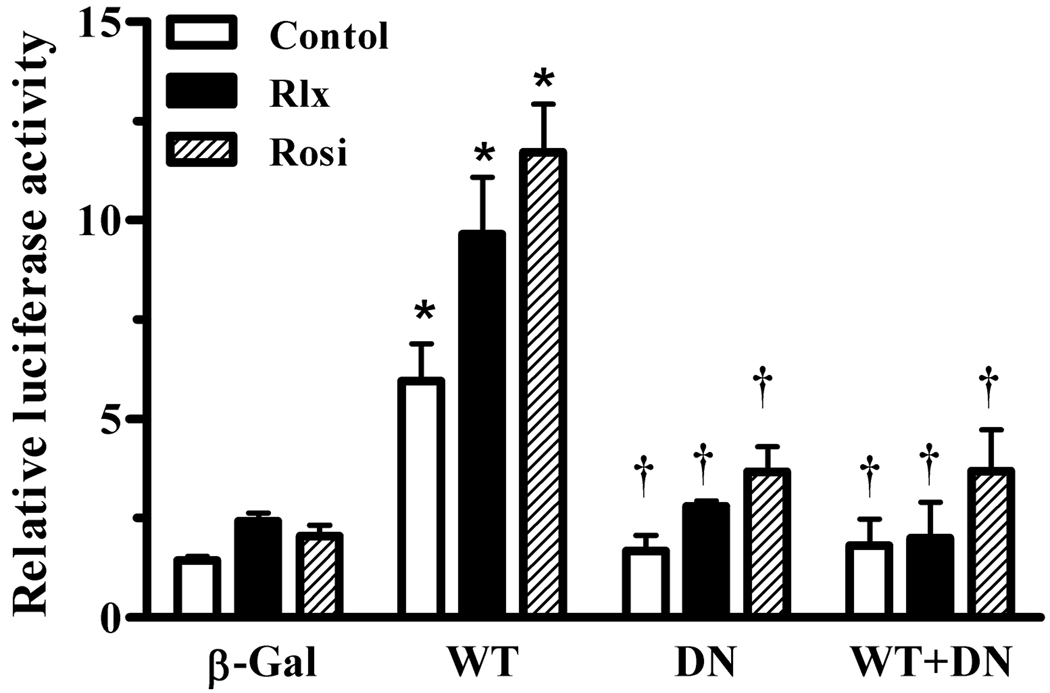
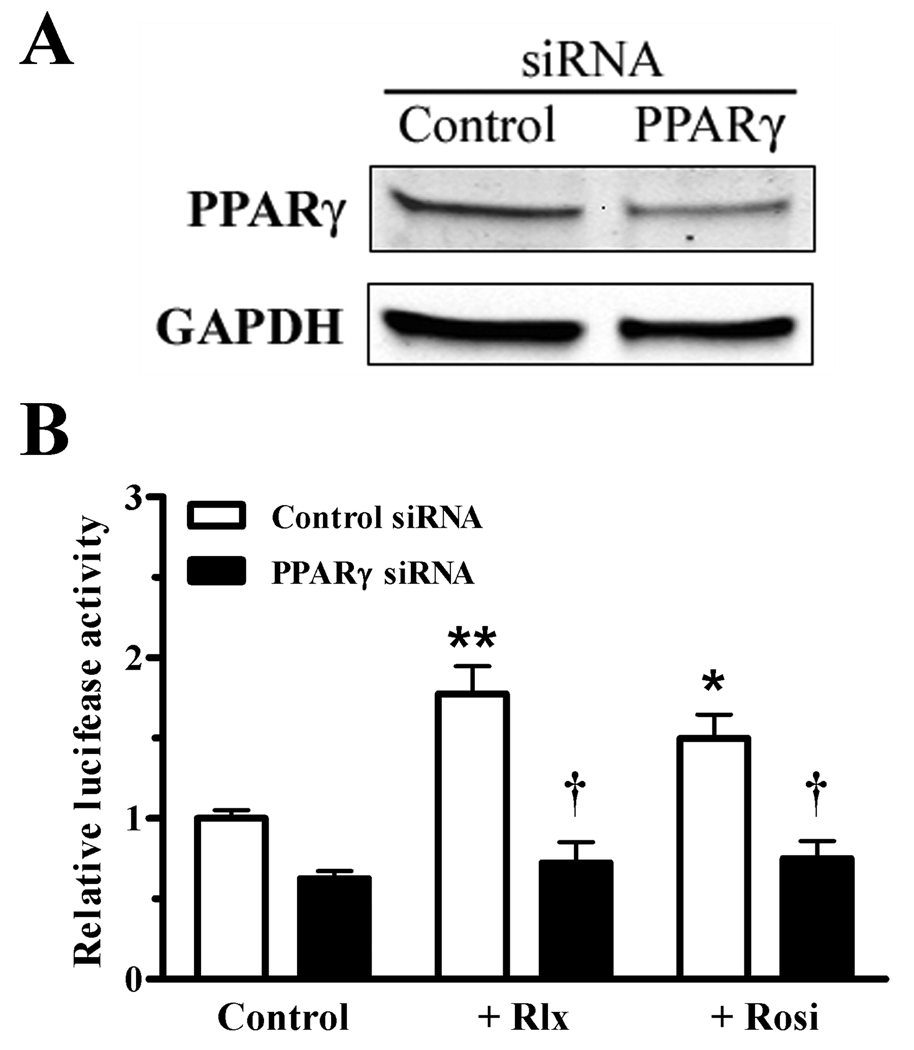
To confirm that the target of relaxin signaling was PPARγ, cells were infected with adenoviruses expressing either wild-type (wtPPARγ) or dominant-negative PPARγ (dnPPARγ). The dnPPARγ possesses DNA binding activity, but has reduced transcriptional activity due to preferential binding to corepressors (Park et al., 2003), and thus can inhibit the wtPPARγ. Infection with wtPPARγ caused an increase in the basal and rosiglitazone-induced PPRE transcriptional activation (Figure 3). Relaxin also increased the activation of wtPPARγ, as well as the residual activity in the presence of the dnPPARγ. However, when cells were coinfected with both wtPPARγ and dnPPARγ, the response to both relaxin and rosiglitazone was greatly suppressed. To more directly identify PPARγ as the target of relaxin signaling, siRNA was used to reduce the expression of PPARγ. Introduction of PPARγ siRNA, but not control siRNA, reduced the level of PPARγ protein in HEK-RXFP1 cells (Figure 4A). Furthermore, in the presence of PPARγ siRNA, transcription through PPRE in response to relaxin or rosiglitazone was reduced, whereas control siRNA had no effect (Figure 4B).
Fig. 3.
Effect of relaxin on wild-type and dominant-negative PPARγ. HEK-RXFP1 cells were infected with adenoviruses expressing β-galactosidase (β-Gal), wild-type PPARγ (WT), dominant-negative PPARγ (DN) or WT:DN at a 1:2.5 ratio. Cells were then treated with relaxin (1 nM), rosiglitazone (1 µM), or vehicle for 24 hours, then subject to the PPRE luciferase assay. The data are expressed as the ACO-PPRE luciferase activity relative to that in uninfected untreated cells. Data are mean ± S.E.M., N=3. *p<.001 compared to β-Gal. †p<.01 compared to WT.
Fig. 4.
Knockdown of_PPARγ reduces the response to relaxin. PPARγ-specific or nontargeting control siRNA were introduced into HEK-RXFP1 cells by nucleofection. A. After 24 hours, a portion of the cells were lysed and subject to Western blot analysis to determine the levels of PPARγ or GAPDH. B. The remaining cells were treated with relaxin (1 nM), rosiglitazone (1 µM), or vehicle for 24 hours, then subject to the PPRE luciferase assay. The data are expressed as the ACO-PPRE luciferase activity relative to untreated cells, mean ± S.E.M of six wells per condition. The experiment was repeated three times, and a representative result is shown. *p<.05, **p<.001 compared with untreated control; †p<.001 comparted to nontargeting siRNA under the same treatment.
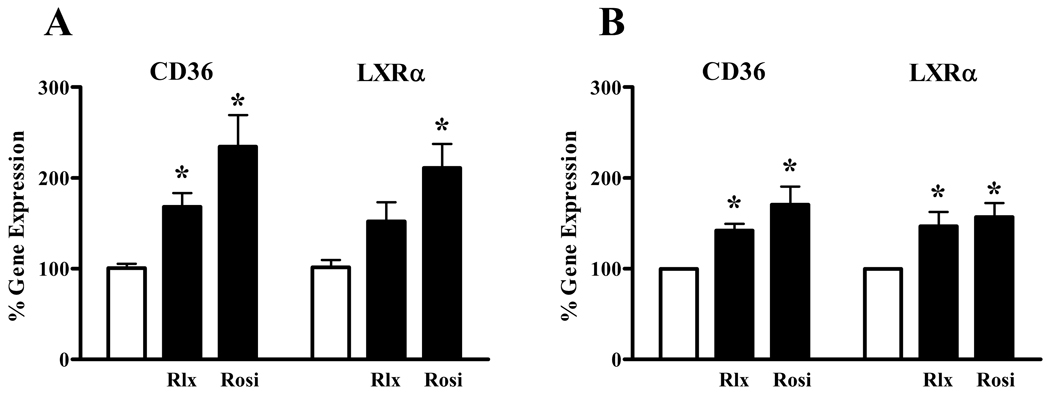
To confirm that relaxin activation of PPARγ resulted in activation of gene transcription, the ability of relaxin to induce the expression of PPARγ target genes was determined in HEK-RXFP1 cells. Both relaxin and rosiglitazone induced significant increases in the expression levels of CD36 (Figure 5A). The expression level of LXRα was significantly increased by rosiglitazone, and was also increased by relaxin, although not to the point of statistical significance. To assess the effect of relaxin on cells naturally expressing RXFP1, a similar experiment was performed in THP-1 cells, a monocyte/macrophage cell line responsive to relaxin. Treatment of THP-1 cells with relaxin significantly increased the expression of both CD36 and LXRα (Figure 5B), similar to the response induced by rosiglitazone.
Fig. 5.
Relaxin increases the expression of PPARγ target genes. Cells were treated with 1nM relaxin (Rlx) or 1µM rosiglitazone (Rosi) for 16 hours. Total RNA was extracted, and the mRNA for CD36 and LXRα were quantified by real-time RT-PCR, and normalized to the level of 18S rRNA using the relative CT method. The cell lines tested were A. HEK-RXFP1 cells, and B. THP-1 monocytes. Data are expressed as the percent gene expression compared with untreated cells, mean ± S.E.M., N=3. *p<.05 compared with untreated cells.
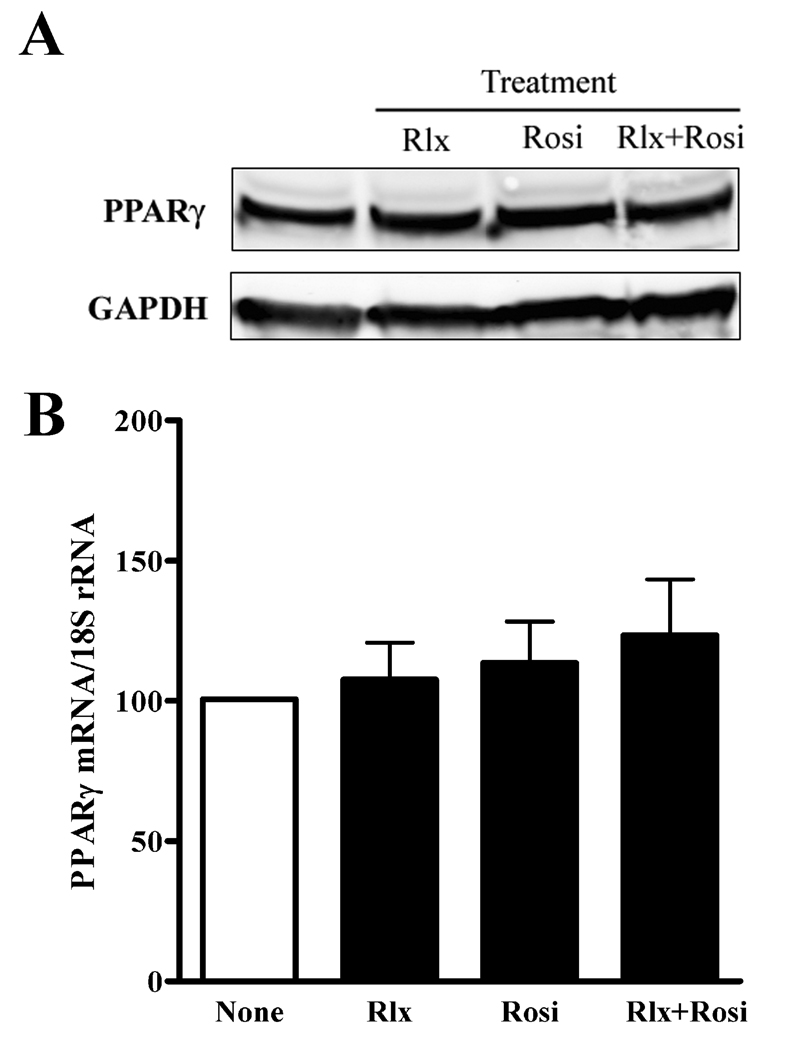
Relaxin does not increase PPARγ mRNA or protein levels
The relaxin effects on PPARγ activity did not require the addition of exogenous PPARγ agonists, raising the possibility that relaxin increased the expression or stability of PPARγ. However, using real-time RT-PCR, it was found that relaxin, rosiglitazone, or their combination did not significantly increase PPARγ mRNA in HEK-RXFP1 cells (Figure 6B). Likewise, no change was detectable in the protein levels of PPARγ (Figure 6A). Therefore, the increase in PPRE transcriptional activity in response to relaxin in HEK-RXFP1 was not due to increased expression or stability of PPARγ.
Fig. 6.
Relaxin does not increase PPARγ mRNA or protein. A. HEK-RXFP1 cells were treated with 1nM relaxin (Rlx), 1µM rosiglitazone (Rosi), or both for 24 hours. Total protein was extracted and analyzed by Western blotting for PPARγ or GAPDH as indicated. Blot shown is representative of at 4 independent experiments. B. HEK-RXFP1 cells were treated with 1nM relaxin (Rlx) or 1µM rosiglitazone (Rosi), or both for 16 hours. Total RNA was extracted, and the mRNA for PPARγ was quantified by real-time RT-PCR, and normalized to the level of 18S rRNA using the relative CT method. Data shown are mean ± S.E.M, N=3.
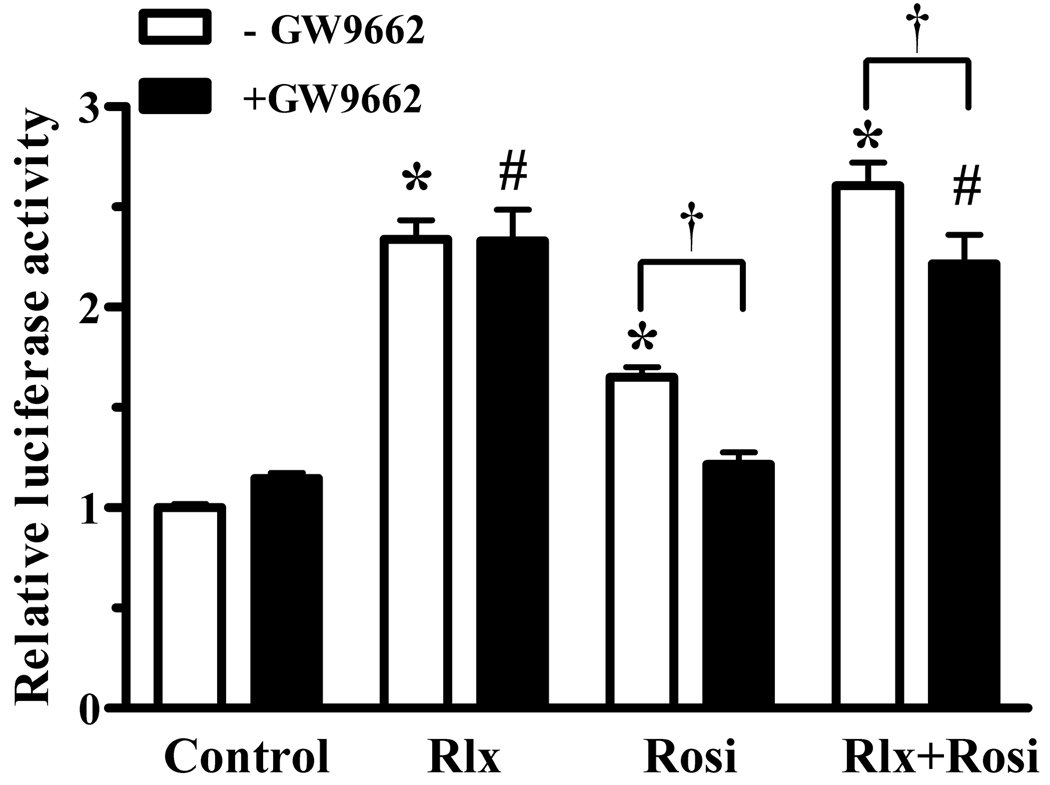
Relaxin activation of PPARγ is ligand-independent
Relaxin activation of PPARγ did not require exogenous PPARγ ligands, and did not increase PPARγ levels. Therefore, it is possible that relaxin induces production of an endogenous PPARγ ligand, or causes ligand-independent activation of PPARγ. To address these possibilities, a covalent modifier of PPARγ that prevents ligand binding (GW9662) was used to block ligand-dependent activation of PPARγ transcriptional activity. As expected, PPARγ activation by rosiglitazone was blocked by GW9662 (Figure 7). In contrast, GW9662 had no effect on relaxin signaling, and only partially blocked the combined effect of relaxin and rosiglitazone, reducing the response to the level achieved by relaxin alone. Therefore, this data suggests that relaxin does not increase the level of an endogenous PPARγ ligand in these cells, but may instead increase PPARγ activity through a ligand-independent mechanism.
Fig. 7.
Relaxin activation of PPARγ is not ligand-dependent. HEK-RXFP1 cells were treated for 24 hours with 1nM relaxin (Rlx), 1µM rosiglitazone (Rosi), or both, in the presence and absence of the inhibitor of PPARγ ligand binding (GW9662, 100nM) or vehicle control (DMSO) for 24 hours, then subject to the PPRE-luciferase assay. The data are expressed as the ACO-PPRE luciferase activity relative to that of untreated cells, mean ± S.E.M, N=4. *p<.001 compared to untreated controls; #p<.01 compared to GW9662 alone; †p<.05.
DISCUSSION
We have shown that relaxin activates transcriptional activity through PPRE in cells expressing RXFP1. Despite the low expression levels of PPARs in these cells, relaxin consistently increased activity through the PPRE reporter vector, with an efficacy equal to or greater than the PPARγ agonists. The relaxin effect was reduced in the presence of dominant-negative PPARγ, or by siRNA silencing. Finally, relaxin treatment resulted in increased expression of the PPARγ target genes CD36 and LXRα, both in cells overexpressing RXFP1 and cells naturally expressing the receptor. Therefore, these are the first experiments demonstrating activation of PPARγ transcriptional activity by relaxin.
The primary relaxin receptor is RXFP1, but potential alternative receptors have been identified. Experiments in vitro have shown that relaxin can also activate RXFP2, although the relevance of this interaction has not been demonstrated in vivo, and InsL3 is the cognate ligand for RXFP2 (Bogatcheva et al., 2003, Hsu et al., 2002). In some cells, relaxin can activate the glucocorticoid receptor, apparently in an RXFP1-independent manner (Dschietzig et al., 2005). In our studies, PPARγ was activated in HEK- 293T cells expressing RXFP1, but not in the untransfected cells that do not express RXFP1 or RXFP2. The alternative RXFP1 ligand relaxin-3 also activated PPARγ, but InsL3, which has extremely low affinity for RXFP1, had no effect. Therefore, the activation of PPARγ required the presence of RXFP1, and was not the result of action through alternative relaxin receptors or glucocorticoid receptor activation.
The combination of relaxin and rosiglitazone caused a greater increase in PPAR activity than either agent alone, suggesting that they were acting through different mechanisms. Importantly, this relaxin effect did not require the addition of exogenous PPARγ ligands. Therefore it was possible that relaxin was increasing the expression of PPARγ or increasing the stability of PPARγ protein. However, when PPARγ was overexpressed, the relaxin effect was maintained. There was no change in PPARγ gene expression in response to relaxin, and no increase in the levels of PPARγ protein. Another possible mechanism for relaxin activation of PPARγ activity could be increased production of an endogenous PPARγ ligand. Our data suggests that this is not the responsible mechanism. Treatment with GW9662, a covalent modifier of PPARγ that prevents ligand-dependent activation, effectively blocked rosiglitazone-induced PPARγ. Conversely, the effect of relaxin was not blocked by GW9662. If relaxin increased the production of an endogenous inhibitor, then GW9662 should have blocked relaxin activation of PPARγ. Therefore, other mechanisms may be responsible.
As stated above, the ability of relaxin to activate PPARγ did not require the addition of exogenous PPARγ agonists. Furthermore, unlike the results using the PPRE reporter vector, the expression of the PPARγ target genes CD36 and LXRα was greater in response to rosiglitazone than to relaxin, providing further evidence that relaxin and rosiglitazone act on PPARγ through different mechanisms. Together with the insensitivity to GW9662, the data suggests that relaxin may be acting to stimulate PPARγ through a ligand-independent mechanism, an area of increasing interest (Xu and Li, 2008). In addition to its heterodimerization partner RXRα, PPARγ associates with a number of transcriptional coactivators and corepressors, which can regulate its activity and specificity both in the absence and presence of PPARγ ligands (Feige and Auwerx, 2007, Lonard and O'Malley, 2007, Puigserver and Spiegelman, 2003). For example, PPARγ association with the coactivators PPARγ coactivator 1α (PGC1α) or p300 results in increased PPARγ ligand-independent transcriptional activity, and altered target gene specificity (Gelman et al., 1999, Puigserver et al., 1998). In addition, the activity of PPARγ, or its binding to coactivators and corepressors can be regulated by phosphorylation (Burns and Vanden Heuvel, 2007). Further study is needed to determine if relaxin activation of PPARγ transcriptional activity involves one of these mechanisms.
Through RXFP1, relaxin activates the cAMP pathway (Halls et al., 2007, Sherwood, 2004). We have found that the EC50 for relaxin-activated PPARγ transcriptional activity was approximately ten-fold lower than its ability to elevate cAMP levels. This finding raises the possibility that relaxin activates PPARγ through additional signaling pathways. Indeed, relaxin has been shown to act through nitric oxide, tyrosine kinase, and mitogen activated protein kinase pathways (Anand-Ivell et al., 2007, Baccari and Bani, 2008, Dschietzig et al., 2003, Mookerjee et al., 2009). Importantly, these same pathways have all been implicated in the regulation of PPARγ activity (Burns and Vanden Heuvel, 2007, Lazennec et al., 2000, Ptasinska et al., 2007). Indeed, there is considerable overlap in the properties of relaxin and PPARγ in the response of fibroblasts to fibrotic stimuli. For example, both relaxin and PPARγ agonists reduced the response to the profibrotic cytokine TGFβ in skin (Unemori and Amento, 1990, Ghosh et al., 2004), lung (Burgess et al., 2005, Unemori et al., 1996), and renal fibroblasts (Masterson et al., 2004, Zafiriou et al., 2005), and both agents have been effective in treatment of fibrosis in vivo (Samuel, 2005, Bennett, 2009, Michalik, 2006). Interestingly, in recent study provided evidence that in renal fibroblasts, relaxin acts to decrease the TGFβ-induced fibrotic phenotype through multiple mechanisms including cAMP, nitric oxide, and mitogen-activated protein kinase (Mookerjee et al., 2009), providing further support for cross-talk between relaxin and PPARγ signaling.
In summary, we have provided the first evidence that relaxin signaling through RXFP1 activates the transcriptional activity of PPARγ. These findings provide a possible mechanism for the antifibrotic effects of relaxin. Because RXFP1 expression appears to be lacking in glucoregulatory insulin-sensitive cells (Hsu et al., 2003, Kamat et al., 2004), relaxin treatment may be an approach to the treatment of fibrosis by activation of PPARγ, but without the glucoregulatory and adipogenic effects of thiazolidinediones and other PPARγ agonists.
ACKNOWLEDGMENTS
This work was supported by funding through NIAAA (AA015509), The Department of Veterans Affairs Biomedical Laboratory Research & Development Program, and the Bly Memorial Research Fund (RGB). We wish to thank Dr. O. David Sherwood (University of Illinois Urbana-Champaign) for providing the porcine relaxin, Dr. Aaron Hsueh for providing the RXFP1 expression plasmid, Dr. Brian Seed (Harvard University) for providing the PPRE reporter, and Dr. J. Larry Jameson (Northwestern University) for providing the adenoviruses expressing wild-type and mutant PPARγ and β-galactosidase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anand-Ivell R, Heng K, Bartsch O, Ivell R. Relaxin signalling in THP-1 cells uses a novel phosphotyrosine-dependent pathway. Molecular and Cellular Endocrinology. 2007;272:1–13. doi: 10.1016/j.mce.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Baccari MC, Bani D. Relaxin and nitric oxide signalling. Curr Protein Pept Sci. 2008;9:638–645. doi: 10.2174/138920308786733921. [DOI] [PubMed] [Google Scholar]
- Bani D. Relaxin as a natural agent for vascular health. Vasc Health Risk Manag. 2008;4:515–524. doi: 10.2147/vhrm.s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod. 2001;7:799–809. doi: 10.1093/molehr/7.9.799. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Ivell R, Sanborn BM, Sherwood OD, Summers RJ. International Union of Pharmacology LVII: Recommendations for the Nomenclature of Receptors for Relaxin Family Peptides. Pharmacol Rev. 2006;58:7–31. doi: 10.1124/pr.58.1.9. [DOI] [PubMed] [Google Scholar]
- Bennett RG. Relaxin and its role in the development and treatment of fibrosis. Transl Res. 2009;154:1–6. doi: 10.1016/j.trsl.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Truong A, Feng S, Engel W, Adham IM, Agoulnik AI. GREAT/LGR8 is the only receptor for insulin-like 3 peptide. Mol Endocrinol. 2003;17:2639–2646. doi: 10.1210/me.2003-0096. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPAR{gamma} agonists inhibit TGF-{beta} induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771:952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin AC, Giunti S, Jandeleit-Dahm KA, Allen TJ, Cooper ME, Thomas MC. PPAR-{alpha} and -{gamma} agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol. Dial. Transplant. 2006;21:2399–2405. doi: 10.1093/ndt/gfl212. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Greinwald M, Baumann G, Stangl K. The pregnancy hormone relaxin binds to and activates the human glucocorticoid receptor. Ann N Y Acad Sci. 2005;1041:256–271. doi: 10.1196/annals.1282.039. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res. 2003;92:32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, Ridolfi F, Trozzi L, Surrenti C, Casini A. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- Gelman L, Zhou G, Fajas L, Raspe E, Fruchart J-C, Auwerx J. p300 Interacts with the N- and C-terminal Part of PPARgamma 2 in a Ligand-independent and -dependent Manner, Respectively. J. Biol. Chem. 1999;274:7681–7688. doi: 10.1074/jbc.274.12.7681. [DOI] [PubMed] [Google Scholar]
- Genovese T, Cuzzocrea S, Di Paola R, Mazzon E, Mastruzzo C, Catalano P, Sortino M, Crimi N, Caputi AP, Thiemermann C, Vancheri C. Effect of rosiglitazone and 15-deoxy-{Delta}12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25:225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Bhattacharyya S, Lakos G, Chen S, Mori Y, Varga J. Disruption of transforming growth factor beta signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor gamma. Arthritis & Rheumatism. 2004;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- Halls ML, Bathgate RA, Summers RJ. Relaxin family peptide receptors RXFP1 and RXFP2 modulate cAMP signaling by distinct mechanisms. Mol Pharmacol. 2006;70:214–226. doi: 10.1124/mol.105.021691. [DOI] [PubMed] [Google Scholar]
- Halls ML, Van Der Westhuizen ET, Bathgate RAD, Summers RJ. Relaxin family peptide receptors - former orphans reunite with their parent ligands to activate multiple signalling pathways. British Journal of Pharmacology. 2007;150:677–691. doi: 10.1038/sj.bjp.0707140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T-Y, Yan W, Bagnell CA. Relaxin-induced matrix metalloproteinase-9 expression is associated with activation of the NF-{kappa}B pathway in human THP-1 cells. J Leukoc Biol. 2007;81:1303–1310. doi: 10.1189/jlb.0906556. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, Van Der Spek PJ, Van Duin M, Hsueh AJ. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14:1257–1271. doi: 10.1210/mend.14.8.0510. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Bathgate RA, Sherwood OD, Hsueh AJ. Relaxin signaling in reproductive tissues. Mol Cell Endocrinol. 2003;202:165–170. doi: 10.1016/s0303-7207(03)00078-9. [DOI] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJW. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–674. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- Iglarz M, Touyz RM, Viel EC, Paradis P, Amiri F, Diep QN, Schiffrin EL. Peroxisome Proliferator-Activated Receptor-{alpha} and Receptor-{gamma} Activators Prevent Cardiac Fibrosis in Mineralocorticoid-Dependent Hypertension. Hypertension. 2003;42:737–743. doi: 10.1161/01.HYP.0000083511.91817.B1. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPARγ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kamat AA, Feng S, Bogatcheva NV, Truong A, Bishop CE, Agoulnik AI. Genetic targeting of relaxin and insulin-like factor 3 receptors in mice. Endocrinology. 2004;145:4712–4720. doi: 10.1210/en.2004-0515. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Canaple L, Saugy D, Wahli W. Activation of Peroxisome Proliferator-Activated Receptors (PPARs) by Their Ligands and Protein Kinase A Activators. Mol Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, O'malley BW. Nuclear Receptor Coregulators: Judges, Juries, and Executioners of Cellular Regulation. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Masterson R, Hewitson TD, Kelynack K, Martic M, Parry L, Bathgate R, Darby I, Becker G. Relaxin down-regulates renal fibroblast function and promotes matrix remodelling in vitro. Nephrol Dial Transplant. 2004;19:544–552. doi: 10.1093/ndt/gfg598. [DOI] [PubMed] [Google Scholar]
- Michalik L. Involvement of PPAR nuclear receptors in tissue injury and wound repair. The Journal of Clinical Investigation. 2006;116:598–606. doi: 10.1172/JCI27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookerjee I, Hewitson TD, Halls ML, Summers RJ, Mathai ML, Bathgate RA, Tregear GW, Samuel CS. Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2. FASEB J. 2009;23:1219–1229. doi: 10.1096/fj.08-120857. [DOI] [PubMed] [Google Scholar]
- Nguyen BT, Yang L, Sanborn BM, Dessauer CW. Phosphoinositide 3-kinase activity is required for biphasic stimulation of cyclic adenosine 3',5'-monophosphate by relaxin. Mol Endocrinol. 2003;17:1075–1084. doi: 10.1210/me.2002-0284. [DOI] [PubMed] [Google Scholar]
- Palejwala S, Stein DE, Weiss G, Monia BP, Tortoriello D, Goldsmith LT. Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology. 2001;142:3405–3413. doi: 10.1210/endo.142.8.8295. [DOI] [PubMed] [Google Scholar]
- Park Y, Freedman BD, Lee EJ, Park S, Jameson JL. A dominant negative PPARgamma mutant shows altered cofactor recruitment and inhibits adipogenesis in 3T3-L1 cells. Diabetologia. 2003;46:365–377. doi: 10.1007/s00125-003-1037-4. [DOI] [PubMed] [Google Scholar]
- Ptasinska A, Wang S, Zhang J, Wesley RA, Danner RL. Nitric oxide activation of peroxisome proliferator-activated receptor gamma through a p38 MAPK signaling pathway. FASEB J. 2007;21:950–961. doi: 10.1096/fj.06-6822com. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome Proliferator-Activated Receptor-{gamma} Coactivator 1{alpha} (PGC-1{alpha}): Transcriptional Coactivator and Metabolic Regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Samuel CS. Relaxin: antifibrotic properties and effects in models of disease. Clin Med Res. 2005;3:241–249. doi: 10.3121/cmr.3.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Zhao C, Bathgate RA, Du XJ, Summers RJ, Amento EP, Walker LL, Mcburnie M, Zhao L, Tregear GW. The relaxin gene-knockout mouse: a model of progressive fibrosis. Ann N Y Acad Sci. 2005;1041:173–181. doi: 10.1196/annals.1282.025. [DOI] [PubMed] [Google Scholar]
- Sherwood CD, O'byrne EM. Purification and characterization of porcine relaxin. Arch Biochem Biophys. 1974;160:185–196. doi: 10.1016/s0003-9861(74)80025-1. [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin's Physiological Roles and Other Diverse Actions. Endocr Rev. 2004;25:205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265:10681–10685. [PubMed] [Google Scholar]
- Unemori EN, Pickford LB, Salles AL, Piercy CE, Grove BH, Erikson ME, Amento EP. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98:2739–2745. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga T, Nagy L. Nuclear receptors, transcription factors linking lipid metabolism and immunity: the case of peroxisome proliferator-activated receptor gamma. Eur J Clin Invest. 2008;38:695–707. doi: 10.1111/j.1365-2362.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Inukai K, Katagiri H, Awata T, Oka Y, Katayama S. Regulation of PPAR[gamma] transcriptional activity in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications. 2003;300:429–436. doi: 10.1016/s0006-291x(02)02860-7. [DOI] [PubMed] [Google Scholar]
- Wu M, Melichian DS, Chang E, Warner-Blankenship M, Ghosh AK, Varga J. Rosiglitazone Abrogates Bleomycin-Induced Scleroderma and Blocks Profibrotic Responses Through Peroxisome Proliferator-Activated Receptor-{gamma} Am J Pathol. 2009;174:519–533. doi: 10.2353/ajpath.2009.080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HE, Li Y. Ligand-Dependent and -Independent Regulation of PPAR{gamma} and Orphan Nuclear Receptors. Sci. Signal. 2008;1:pe52. doi: 10.1126/scisignal.148pe52. [DOI] [PubMed] [Google Scholar]
- Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone Inhibits Cell Growth and Reduces Matrix Production in Human Kidney Fibroblasts. J Am Soc Nephrol. 2005;16:638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu SH, Erikson M, Lewis M, Unemori E. Relaxin activates the MAP kinase pathway in human endometrial stromal cells. J Cell Biochem. 2002;85:536–544. doi: 10.1002/jcb.10150. [DOI] [PubMed] [Google Scholar]