Abstract
The goal of this study was to examine factors that contribute to energy balance in female GHR −/− mice. We measured energy intake, energy expenditure (EE), fuel utilization, body mass (Mb) changes and physical activity in 17 month-old female GHR −/− mice and their age-matched wild type littermates. The GHR −/− mice were smaller, consumed more food per unit Mb, had greater EE per unit Mb and had an increase in 24-h EE/Mb that was similar to the increase in their surface-area-to-volume ratio. Locomotor activity (LMA) was reduced in the GHR −/− mice, but the energetic cost associated with their LMA was greater than in wild type controls. Furthermore, Mb and LMA were independent explanatory covariates of most of the variance in EE, and when adjusted for Mb and LMA, the GHR −/− mice had higher EE during both the light and dark phases of the daily cycle. Respiratory quotient was lower in GHR −/− mice during the light phase, which indicated a greater utilization of lipid relative to carbohydrate in these mice. Additionally, GHR −/− mice had higher ratios of caloric intake to EE at several intervals during the dark phase, and this effect was greater and more sustained in the final three hours of the dark phase. Therefore, we conclude that GHR −/− mice are able to overcome the substantial energetic challenges of dwarfism through several mechanisms that promote stable Mb. Relative to wild type mice, the GHR −/− mice consumed more calories per unit Mb, which offset the disproportionate increase in their daily energy expenditure. While GHR −/− mice oxidized a greater proportion of lipid during the light phase in order to meet their energy requirements, they achieved greater energy efficiency and storage during the dark phase through a combination of higher energy consumption and lower LMA.
1. Introduction
Modulation of growth hormone (GH) signaling has been shown to have profound effects on body mass (Mb), energy balance and longevity in rodents (recently reviewed [1]). A useful animal model for examining the role of GH in longevity and health has been the Laron mouse [2], so-named due to its phenotypic recapitulation of, and similarity to, Laron Syndrome individuals [3]. This disorder in humans has several features including: short stature, craniofacial abnormalities, obesity, delayed onset of puberty, hypoglycemia, low serum insulin-like growth factor I (IGF-I) and elevated serum GH levels, and insensitivity or resistance to GH [3–5]. Several independent mutations in the GH receptor (GHR)/binding protein (BP) gene have been found to cause Laron Syndrome [6–10], implicating an insensitivity to the effects of GH in this disorder. Likewise, the Laron mouse was engineered with a targeted disruption of GHR/BP gene [2]. Mice homozygous for this deletion (GHR −/−) demonstrated a dramatic reduction in both linear growth and Mb, greater levels of serum GH, GH insensitivity, and low or non-detectable levels of circulating IGF-I [2]. Other features of the GHR −/− mice include delayed puberty, lower fertility [11, 12], a reduction in both ovarian follicle number and average litter size in females [13, 14], and lower bone mineral content [15].
Remarkably, GHR −/− mice live significantly longer than their wild type littermates [16, 17]. Furthermore, GHR −/− mice have an improved metabolic profile; male GHR −/− mice have lower fasting glucose and insulin levels [17] even when challenged with a high fat diet [18]. Paradoxically, GHR −/− mice have a greater percentage of body fat in comparison to their wild type littermates [18, 19], but higher levels of adiponectin [19]. Significantly, the effect of GHR deletion on fat tissue mass is depot-specific; when calculated as a percentage of Mb, the subcutaneous fat depot is greater in GHR −/− mice, while the epididymal fat pad is unchanged [19]. The stimulation of lipolysis by GH has been well established [20–23]. Therefore, alterations in the partitioning of fat in GHR −/− mice may be due to depot-specific dysregulation that results from the loss of GH signaling [19].
The diminutive body size of GHR −/− mice presents a significant energetic challenge for these animals in terms of Mb and temperature homeostasis. While these mice have a lower core body temperature and lower thyroid function [24], they also have greater heat loss due their higher surface area-to-volume ratio. One compensatory mechanism to offset the loss of energy (as heat) in GHR −/− mice appears to be greater food intake (adjusted for Mb) [18, 19]. Male and female GHR −/− mice fed a chow diet reach their plateau Mb at 28 and 34 weeks, respectively, and maintain their Mb up to 80 weeks [16]. This high degree of stability in the Mb of GHR −/− mice suggests that these animals have a finely tuned homeostatic control of energy balance. However, the control points of energy balance in these long-lived mice have not been rigorously evaluated and have never been evaluated in the female GHR−/− mice [18, 25]. In this study, we examined energy intake, energy expenditure, fuel utilization, changes in Mb and physical activity in 17-month-old female GHR −/− mice and their age-matched wild type littermates.
2. Methods
2.1 Animals
All procedures were approved by the Ohio University Institutional Care and Use Committee and fully complied with federal, state and local policies. Additionally, the Elixir Pharmaceuticals Institutional Animal Care and Use Committee approved all protocols for the experimental use of animals, in accordance with NIH guidelines. At the time of study, the mice that we used were 17-month old female GHR −/− and wild type littermates on a C57BL/6J genetic background (> 99 % congenic), as described previously [2]. Mice were bred from GHR +/− pairs and raised at the animal facility at Ohio University (Athens, OH). Following shipment to Elixir, the mice were housed individually for three weeks in controlled environment rooms with the following conditions; 72°F ambient temperature, ~40% humidity, with 12h phased light/dark cycles at 6am/6pm in ventilated racks (Thoren; Hazelton, PA). Room temperature, humidity, light duration and light intensity were monitored using a HOBO data logging system (Onset Computer Corporation, Pocasset, MA).
2.2 Diet
Animals were weaned at four weeks of age onto a rodent chow diet (ProLab RMH 3000; 14% of kilocalories from fat, 26% from protein, and 60% from carbohydrates). After transfer of the mice to Elixir’s animal facility, they were fed a pelleted chow diet (Lab Diet 5001, Purina; St. Louis, MO). A powdered version of this diet was used for the calorimetry study. The calculated dietary food quotient (FQ), which represents the theoretical 24-h RQ of an animal when it is in a state of energy balance, was determined using the following equation: (0.835 × % protein) + (1.0 × % carbohydrate) + (0.71 × % fat) [26]. Information from the supplier indicated that Lab Diet 5001 consisted of 28.5% energy from protein, 58% energy from carbohydrate, and 13.5% energy from fat, and therefore had a calculated food quotient of 0.914 and an energy equivalent of 3.44 kcal/g. Due to the limitations of using a generalized equation to determine the FQ for relatively undefined chow diets, we also estimated FQ (using another dataset) as the y-intercept of the changes in body mass during calorimetry (x-axis) and the 24-h RQ (y-axis) (FQ = 0.855). The data from the present study suggest that this estimate was closer to the actual FQ than the equation-derived FQ.
2.3 Indirect calorimetry and the measurement of metabolic flexibility
Energy expenditure (EE), food intake, RQ, and x-axis locomotor activity (LMA) were recorded using a 16-chamber indirect calorimetry system (Oxymax™, Columbus Instruments, Columbus, OH). We calibrated the calorimeter’s O2 and CO2 sensors before each experiment using a compressed gas with a highly defined mixture of O2 and CO2. RQ was calculated as the quotient of the rates of CO2 production (VCO2) and O2 consumption (VO2), and energy expenditure (kcal/day) was calculated with the equation (3.815 + (1.232 × RQ)) × VO2, with VO2 defined as liters O2/day [27]. We used relative cumulative frequency (RCF) curve analysis to transform RQ data for ease of visualization [28]. LMA was monitored using a beam-break apparatus aligned with the calorimetry chambers along the x-axis. Measurements were collected from each mouse every 14 minutes for exactly 96 hours. We recorded the Mb of each mouse immediately before and after chamber occupancy. Furthermore, any wasted food was collected, weighed, and accounted for in the final food intake measurements. Daily, light cycle and dark cycle averages were calculated for each parameter.
2.4 Statistics
We used Student’s T-test to assess the effects of genotype on all dependent variables. When assessing the effects of genotype over time, we used repeated measures analysis of variance (RM-ANOVA) to estimate the main effects (genotype and time); the effect of genotype at individual time points was determined using the Bonferroni post hoc test, adjusted for multiple comparisons. The influence of potential covariates on the dependent variables was assessed by analysis of covariance (ANCOVA) using a general linear model. Reported ANCOVA results conformed to the homogeneity of slopes assumption as assessed by Levene’s test, and all data were considered statistically significant at P<0.05. Surface area-to-volume ratios were calculated by using a variant of the Meeh equation [29]; we substituted the Mb of each mouse for the volume of a sphere (1 g = 1 cm3) and solving for radius (r) using the equation volume (V) = 4/3*πr2. Surface area (SA) was determined using the equation SA = 4πr2. Therefore, the unit measure for SA/V was 3/r. Statistical analyses were performed with the software packages SYSTAT (Systat Software, Inc, Chicago, IL) and GraphPad Prism version 5 for Windows (GraphPad Software, San Diego CA). Post hoc power calculations were made in some cases using the program G*Power version 3.0.10 for Windows [30].
3. Results
3.1 Body mass and food intake
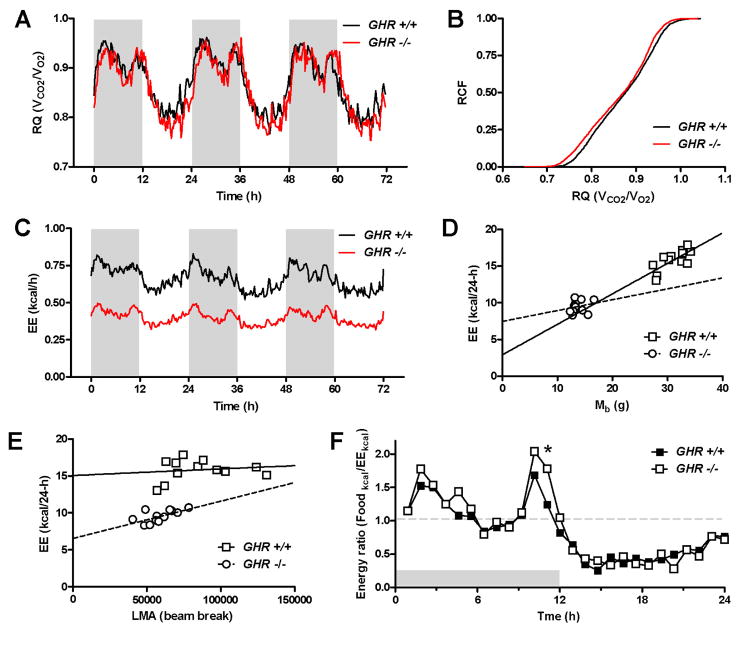
We compared metabolic parameters in female GHR −/− and wild type control mice at 17 months of age, fed a standard chow diet, during fours days of continuous measurements in calorimetry chambers. The results are summarized in Table 1. Importantly, the Mb of the mice remained constant in both groups during the measurement period, indicating that the mice were in a state of energy balance. In terms of Mb, the GHR −/− mice were 56% smaller and had a 31.3% increase in their surface area-to-volume ratio when compared to their wild type controls. The wild type mice had a 24-h EE-to-Mb ratio of 0.51 kcal24h/g. Therefore, if energy expenditure scaled linearly with Mb, the GHR −/− mice would be predicted to have a 24-h EE of 7.01 kcal; however, 24-h EE was in fact 25.8% higher in these mice, similar to the increase in the surface area-to-volume ratio (Table 1). Notably, the 25.8% increase in 24-h EE in the GHR −/− mice was significantly lower than the 31.3% increase in their SA/V ratio (P=0.02), and these two parameters were positively correlated (r=0.627, P=0.022).
Table 1.
Metabolic measurements from 17- month old female wild type and GHR −/− mice.
| Wild type (n=12) |
GHR −/− (n=13) |
||||
|---|---|---|---|---|---|
| Unit | Mean ± SD | Mean ± SD | %Δ | p | |
| Body weightpre | g | 31.17 ± 2.73 | 13.78 ± 1.44 | −56 | <0.0001 |
| Body weightpost | g | 31.01 ± 2.26 | 13.70 ± 1.06 | −56 | <0.0001 |
| Body weightpost-pre | g | −0.16 ± 0.90 | −0.08 ± 0.58 | −51 | 0.396 |
| Surface area/volume | 3/r | 1.54 ± 0.05 | 2.02 ± 0.07 | −31 | <0.0001 |
| Respiratory quotient: | |||||
| 24-h | none | 0.865 ± 0.014 | 0.859 ± 0.015 | −1 | 0.153 |
| 12-h light | none | 0.829 ± 0.017 | 0.816 ± 0.013 | −2 | 0.026 |
| 12-h dark | none | 0.901 ± 0.023 | 0.901 ± 0.022 | 0 | 0.471 |
| Oxygen consumption: | |||||
| 24-h | ml | 3240 ± 294 | 1948 ± 173 | −40 | <0.0001 |
| 12-h light | ml | 1500 ± 144 | 908 ± 80 | −39 | <0.0001 |
| 12-h dark | ml | 1740 ± 153 | 1039 ± 94 | −40 | <0.0001 |
| Energy expenditure: | |||||
| 24-h | kcal | 15.83 ± 1.40 | 9.50 ± 0.82 | −40 | <0.0001 |
| 12-h light | kcal | 7.26 ± 0.69 | 4.38 ± 0.38 | −40 | <0.0001 |
| 12-h dark | kcal | 8.57 ± 0.73 | 5.12 ± 0.45 | −40 | <0.0001 |
| Food intake: | |||||
| 24-h | g | 4.11 ± 0.33 | 2.50 ± 0.23 | −39 | <0.0001 |
| 12-h light | g | 0.46 ± 0.10 | 0.23 ± 0.06 | −50 | <0.0001 |
| 12-h dark | g | 3.65 ± 0.32 | 2.27 ± 0.22 | −38 | <0.0001 |
| Food intake: | |||||
| 24-h | kcal | 14.14 ± 1.15 | 8.62 ± 0.81 | −39 | <0.0001 |
| 12-h light | kcal | 1.60 ± 0.34 | 0.79 ± 0.20 | −50 | <0.0001 |
| 12-h dark | kcal | 12.55 ± 1.09 | 7.82 ± 0.74 | −38 | <0.0001 |
| Locomotor activity: | |||||
| 24-h | beam break | 87108 ± 24786 | 59670 ± 10404 | −31 | 0.001 |
| 12-h light | beam break | 22032 ± 3978 | 17544 ± 3060 | −20 | 0.002 |
| 12-h dark | beam break | 64974 ± 21420 | 42024 ± 7752 | −35 | 0.001 |
Absolute caloric intake in the GHR −/− mice was significantly lower compared to their wild type littermates, but higher in GHR −/− mice after adjusting for body weight (Table 1). Importantly, 24-h caloric intake was roughly equivalent to 24-h EE in both genotypes, consistent with the maintenance of Mb in both genotypes.
3.2 Fuel utilization
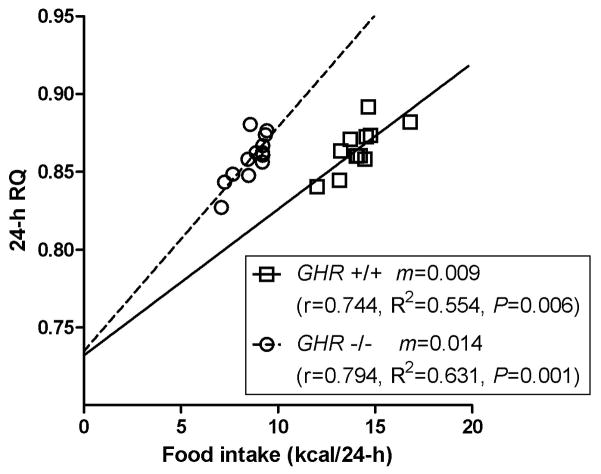
During the 12-h dark period and an entire 24-h cycle, the average respiratory quotient (RQ) did not differ significantly between GHR −/− and wild type mice, indicating similar patterns of fuel oxidation (Table 1, Figure 1A, 1B). However, RQ was significantly lower in GHR −/− mice during the 12-h light period (Table 1), potentially indicating greater oxidation of lipid relative to carbohydrate during the quiescent phase of the daily cycle. (The nonprotein RQ was not determined, and therefore we cannot rule out the possibility that the lower light period RQ in the GHR −/− mice was due to greater protein catabolism [31].) While this RQ shift appeared to be small, it accounted for almost one kilocalorie of energy intake in a 24-h period in the GHR −/− mice (Figure 2). This shift toward lower RQ in the GHR −/− mice is shown clearly in Figure 1b, when the RQ values for each genotype recorded over 24-h were binned and shown as relative cumulative frequencies (RCF). The leftward shift in the RCF curve for the GHR −/− mice indicated a greater ratio of lipid:carbohydrate oxidation (or possibly protein catabolism). Both the GHR −/− and wild type mice had 24-h RQ values that were close to the food quotient of their diet (~0.855), which provided additional evidence that both groups of mice were in a state of energy balance during the experiment.
Figure 1.
Comparison of fuel utilization, energy expenditure and locomotor activity in wild type (n=12) and GHR −/− (n=13) mice. (A) Respiratory quotient (RQ; VCO2/VO2) profiles over a 72-h period. Shaded areas denote the dark phases. (B) Relative cumulative frequency (RCF) analysis of RQ data during the same 72-h period shown in A. (C) Energy expenditure (EE; kcal/h) profiles over a 72-h period. (D) Scatter plot of body mass (Mb; g) and EE (kcal/24-h). (E) Scatter plot of locomotor activity (LMA; beam break) and EE (kcal/24-h). (F) Ratio of food intake (kcal) to EE (kcal) at regular time intervals over a 24-h period, *P<0.05.
Figure 2.
Scatterplot of food intake (kcal/24-h) and 24-h RQ for wild type (n=12) and GHR −/− (n=13) mice. The slope (m), Pearson’s coefficient (r), goodness of fit (R2), and probability (P) are shown for each regression.
3.3 Components of variance in energy expenditure: body mass and activity
Total energy expenditure (EE; kcal/period) was significantly lower in the female GHR −/− mice (F(1,23)=193, P<10−12), with the effect of genotype explaining 89.4% of the experimental variance (Table 1, MFigure 1C) Even though average b loss for each genotype during the 96-h measurement period was minimal, we sought to determine if there was a relationship between 24-h EE and start-to-finish changes in Mb. After adjusting for the covariate ΔMb (Table 1), genotype still predicted differences in 24-h EE (89.1% of total variance; F(1,22)=268, P<10−10), with adjusted least square means of 15.8 and 9.5 kcal/24-h for the wild type and GHR −/− mice, respectively. Importantly, ΔMb made a small but significant contribution to the variance in 24-h EE (3.5% of total variance; F(1,22)=10.6, P=0.004), indicating that animals with greater 24-h EE were more susceptible to Mb loss, regardless of their genotype. While this effect was small, it highlighted the importance of thorough energy accounting for the proper interpretation of our energy balance experiment.
Given the large effect of genotype on Mb, we used ANCOVA to explore the association between energy expenditure and Mb. We analyzed the effect of Mb on EE using ANCOVA instead of simply dividing EE by Mb for two important reasons. First, dividing EE by Mb erroneously assumes that all of the variance in EE is explained completely by Mb. Second, ANCOVA allowed us to determine what percentage of the variance in EE was actually explained by Mb [32]. This analysis demonstrated that the effects of genotype on 24-h EE (0.2% of the variance, F(1,22)=0.1, P=0.776) were explained significantly by the covariate Mb (39.1% of the variance; F(1,22)=14.2, P<0.001) (Figure 1D). The adjusted least square means of 24-h EE for the wild type and GHR −/− mice were 12.5 ± 0.9 and 12.6 ± 1.0 kcal/24-h, respectively. Therefore, the genotypic differences in 24-h EE were only partially explained by Mb; 60.9% of the total variance was unexplained, indicating that factor(s) other than Mb likely contributed to the large genotype-specific differences in EE.
Locomotor activity (LMA) was significantly lower in GHR −/− mice during the 24-h, 12-h light, and 12-h dark periods (Table 1, Figure 1E). Interestingly, when the data were converted to a percentage of total 24-h activity (100%), the GHR −/− mice had a significantly lower percentage of their total LMA in the dark phase (70.4 ± 2.8%) when compared to the wild type mice (73.9 ± 3.7%) (P<0.013). Notably, LMA was correlated significantly with EE in GHR −/− mice during the 24-h period (r=0.623, P=0.023), the 12-h dark phase (r=0.605, P=0.028), and particularly during the 12-h light phase (r=0.721, P<0.001). Furthermore, an analysis of the Mb-adjusted residuals of EE and LMA demonstrated that the relationship between EE and LMA was completely independent of Mb (data not shown) [33]. Interestingly, EE and LMA were not significantly related in the wild type mice (Figure 1E), but the failure to find an association in this group may have been due to a lack of statistical power in the regression. With this concern in mind, the slope of the regression of LMA and EE was greater in the GHR −/− mice indicating that, despite having lower LMA overall, LMA carried a larger proportional energetic cost for the GHR −/− mice relative to wild type mice (Figure 1E).
To understand the relative contributions of Mb and LMA to EE, we performed ANCOVA and found that both Mb and LMA were significant covariates of EE. During the dark cycle, the effect of genotype was minimized (1.8% of the variance; F(1,21)=1.0, P=0.319), and was largely explained by Mb (42% of the variance; F(1,21)=23.7, P<0.001) and LMA12-h dark (19% of the variance; F(1,21)=10.9, P=0.003). Therefore, ~61% of the genotype effect on EE could be explained via the effects of genotype on Mb and LMA. (The 37% residual variance could potentially have been explained by the thermic effect of food, which was not estimated in this study.) The adjusted least square means for the wild type and GHR −/− mice were 6.32 and 7.2 kcal/12-h dark phase, respectively. Similarly, during the light cycle, the effect of genotype was reduced (2.2% of the variance; F(1,21)=1.24, P=0.278), while Mb (41% of the variance; F(1,21)=22.5, P<0.001) and LMA12-h light (19% of the variance; F(1,21)=10.6, P=0.004) were significant covariates. The adjusted least square means for the wild type and GHR −/− mice were 5.33 and 6.16 kcal/12-h light phase, respectively. In each case, no interaction was noted between LMA and Mb. These results demonstrated that Mb and LMA independently explained most of the experimental variance (~61%) in EE, and female GHR −/− mice incurred higher energy costs than the wild type control mice on a per unit activity basis [34].
3.4 Energy balance
In order to assess energy balance in the mice, we constructed an energy ratio plot. This consisted of dividing caloric intake for each mouse by its caloric expenditure at regular time intervals over an average 24-h period. An energy ratio equal to one would indicate energy balance, while ratios greater or less than one would indicate states of energy storage or oxidation, respectively. The overall effect of genotype was not significant (F(1,23)=1.57, P=0.223). GHR −/− and wild type mice had similar ratios of less than one during the light cycle (Figure 1F). However, the dark cycle was punctuated by several periods when the GHR −/− mice had higher ratios than their wild type controls (Figure 1F), and this was significant at one interval near the end of the dark phase (P<0.05). Therefore, relative to the wild type mice, the GHR −/− mice had a greater caloric surplus and were in a more positive energy balance during the dark phase.
Discussion
Many biological traits of the GHR −/− mouse have been well characterized in males, including their smaller Mb [2, 19], longer lifespan compared to normal mice [1, 16, 17], and greater insulin sensitivity [17–19] despite a paradoxical increase in adiposity [19]. GHR −/− mice also have higher energy expenditure per gram Mb, but are able match their caloric intake to their energetic requirements [17, 18, 25], hence maintaining a stable Mb. In this work, we looked carefully at changes in Mb, energy expenditure, energy intake, fuel utilization, and physical activity in order to understand more fully the homeostatic mechanisms underlying Mb regulation in aged female GHR −/− mice.
Perhaps the biggest energetic challenge that the female GHR −/− mice faced in maintaining Mb was the loss of energy as heat. Due to their Mb, GHR −/− mice had a greater surface area-to-volume ratio than their wild type littermates (Table 1). It therefore follows that heat loss (and compensatory EE) should be in proportion to this ratio and should scale allometrically with body mass (∝Mb2/3) [35]. While the value of the scaling exponent for basal metabolic rate has been an ongoing and contentious issue, recent evidence suggests that an exponent of 2/3 is correct [36]. An interesting finding of the current work was that the proportional increase in 24-h EE per unit of Mb in the GHR −/− mice (25.8%) was correlated with, but still less than, the increase in their SA/V ratio (31.3%), indicating that the greater Mb-adjusted 24-h EE in these mice compensated for a large fraction of their size-related heat loss and was probably a key factor in their homeothermic control. Notably, the difference between these two parameters (−5.5%) was significant in the GHR −/− mice, which indicated a small energetic deficiency. This was consistent with previous work that described a slightly lower core body temperature (Tb) in GHR −/− mice and an impaired thyroid axis [24]. While body temperature was not measured in our experimental animals, it is possible that a slightly lower body temperature was a reasonable energetic trade-off for, and played a partial role in, the maintenance of Mb in these mice. (A potential limitation to our mathematical approach for determining the SA/V ratio was that genotypic differences in morphometry and/or composition may have introduced some error in the estimates [37].)
The utilization of energy stored as lipid is crucial for small nocturnal mammals during the light phase, a period of relative quiescence, since energy intake is by definition lower than basal metabolism during this phase. One hypothesis was that light-phase lipid oxidation would be greater in the GHR −/− mice in order to cover the disproportionate heat loss and energetic costs associated with their small Mb. This idea seemed counterintuitive at first, since one of the well-established effects of growth hormone is the stimulation of lipolysis (reviewed in [23]), while loss of growth hormone signaling is associated with the accrual of fat tissue [18, 19]. However, RQ was significantly lower in the GHR −/− mice during their 12-h light phase, which indicated their potential for greater lipid oxidation relative to carbohydrate oxidation (Table 1, Figure 1A & 1B). (One caveat to this conclusion is that since the nonprotein RQs were not determined, genotypic differences in protein catabolism cannot be ruled out as a cause for the difference in light period RQ [31].) Similar results were seen in male GHR −/− mice, indicating that this effect was probably gender-neutral [25]. Therefore, despite the loss of GH signaling, the GHR −/− mice were able to liberate and oxidize stored lipid to a greater degree than the wild type mice. Despite this increase in lipid oxidation, GHR −/− mice were previously shown to have more fat mass (as a percentage of total body mass) than their wild type controls [19]. Furthermore, we postulated that in comparison to the wild type mice, the GHR −/− mice must have restored the fat mass that was lost during the light phase through mechanisms that promoted greater energetic efficiency during the dark phase.
The maintenance of Mb requires that a cycle of energy use is balanced with a cycle of replenishment of energy stores. We explored what factors, after adjusting for the effects of Mb, that might have contributed to greater energy efficiency in the GHR −/− mice. Importantly, the ratio of energy intake to EE (Foodkcal/EEkcal) was greater in GHR −/− mice relative to wild type mice at several (but not all) intervals of the dark phase, and this effect was particularly significant near the end of the dark phase (Figure 1F). The GHR −/− mice achieved this caloric over-matching through two subtle but important mechanisms. First, GHR −/− mice consumed a significantly greater percentage of their total daily caloric intake during the dark phase (2.4%) compared to the wild type mice. Second, the GHR −/− mice had lower LMA during both the light and dark phases, and had a decrease in the percentage of total daily LMA during the dark phase, relative to wild type mice (−4.7%). While this decrease in LMA appeared to be small, its effect on energy conservation was large (Figure 1E), given that the associated energy costs of locomotion are greater disproportionately in smaller mammals [34, 38].
Previous work has shown that the decrease in body temperature in GHR −/− mice was most pronounced during the final four hours of the dark cycle [24]. Perhaps it is not a coincidence that we observed the greatest energy ratio in the GHR −/− mice during this approximate interval (Figure 1F). A reduced thyroid hormone axis in the GHR −/− mice therefore may have resulted in an impairment of meal-stimulated adaptive thermogenesis, or the thermic effect of food. The role that thyroid hormone plays in the thermic effect appears to be species-specific. In humans, thyroid hormone stimulates basal metabolic rate, but does not impact the thermic effect of food [39]. Furthermore, the thermic effect scales linearly with caloric intake and is not different in lean or obese men [40]. However, smaller mammals such as rodents rely more heavily on adaptive thermogenesis from brown fat for heat [41], and this tissue has been shown to increase heat production following a meal, at least in part through a thyroid hormone-dependent mechanism [42, 43]. Interestingly, GHR −/− mice were shown to have greater intrascapular brown adipose tissue mass and uncoupling protein-1 expression [44]. However, despite this, a reduction in the thermogenesis-related component of EE during this interval may have promoted a period of enhanced fat storage immediately before lights-on, thereby offsetting the greater utilization of stored lipid during the light phase in these mice.
In conclusion, we have found that female GHR −/− mice were able to overcome the substantial energetic challenges of being small through several mechanisms that resulted in their ability to maintain body mass. The energetic cost of locomotion was significantly greater in GHR −/− mice in comparison to their wild type controls; however, the GHR −/− mice had lower total locomotor activity, which reduced the total energetic cost of locomotion for these mice. The GHR −/− mice consumed more calories per unit body mass, which offset the disproportionate increase in their daily energy expenditure. Furthermore, while GHR −/− mice oxidized a greater proportion of lipid during the light phase in order to meet their energy requirements; they achieved greater energy efficiency and storage during the dark phase through a combination of increased energy consumption and reduced physical activity.
Acknowledgments
Special thanks to Tim Morrison at the Elixir care and maintenance of the animals and John Speakman for helpful discussion on data interpretation. JJK is supported in part by the State of Ohio’s Eminent Scholars’ Program that includes a gift from Milton and Lawrence Goll and by the following grants: NIH R01 AG019899-06, NIH R15 DK075436-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laron Z, Pertzelan A, Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone--a new inborn error of metabolism? Israel journal of medical sciences. 1966;2:152–155. [PubMed] [Google Scholar]
- 4.Rosenbloom AL, Savage MO, Blum WF, Guevara-Aguirre J, Rosenfeld RG. Clinical and biochemical characteristics of growth hormone receptor deficiency (Laron syndrome) Acta Paediatr Suppl. 1992;383:121–124. [PubMed] [Google Scholar]
- 5.Rosenfeld RG, Rosenbloom AL, Guevara-Aguirre J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocrine reviews. 1994;15:369–390. doi: 10.1210/edrv-15-3-369. [DOI] [PubMed] [Google Scholar]
- 6.Berg MA, Argente J, Chernausek S, Gracia R, Guevara-Aguirre J, Hopp M, Perez-Jurado L, Rosenbloom A, Toledo SP, Francke U. Diverse growth hormone receptor gene mutations in Laron syndrome. American journal of human genetics. 1993;52:998–1005. [PMC free article] [PubMed] [Google Scholar]
- 7.Berg MA, Peoples R, Perez-Jurado L, Guevara-Aguirre J, Rosenbloom AL, Laron Z, Milner RD, Francke U. Receptor mutations and haplotypes in growth hormone receptor deficiency: a global survey and identification of the Ecuadorean E180splice mutation in an oriental Jewish patient. Acta Paediatr Suppl. 1994;399:112–114. doi: 10.1111/j.1651-2227.1994.tb13302.x. [DOI] [PubMed] [Google Scholar]
- 8.Gastier JM, Berg MA, Vesterhus P, Reiter EO, Francke U. Diverse deletions in the growth hormone receptor gene cause growth hormone insensitivity syndrome. Human mutation. 2000;16:323–333. doi: 10.1002/1098-1004(200010)16:4<323::AID-HUMU5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbloom AL, Berg MA, Kasatkina EP, Volkova TN, Skorobogatova VF, Sokolovskaya VN, Francke U. Severe growth hormone insensitivity (Laron syndrome) due to nonsense mutation of the GH receptor in brothers from Russia. J Pediatr Endocrinol Metab. 1995;8:159–165. doi: 10.1515/jpem.1995.8.3.159. [DOI] [PubMed] [Google Scholar]
- 10.Sobrier ML, Dastot F, Duquesnoy P, Kandemir N, Yordam N, Goossens M, Amselem S. Nine novel growth hormone receptor gene mutations in patients with Laron syndrome. The Journal of clinical endocrinology and metabolism. 1997;82:435–437. doi: 10.1210/jcem.82.2.3725. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar V, Bartke A, Coschigano KT, Kopchick JJ. Pituitary and testicular function in growth hormone receptor gene knockout mice. Endocrinology. 1999;140:1082–1088. doi: 10.1210/endo.140.3.6557. [DOI] [PubMed] [Google Scholar]
- 12.Keene DE, Suescun MO, Bostwick MG, Chandrashekar V, Bartke A, Kopchick JJ. Puberty is delayed in male growth hormone receptor gene-disrupted mice. Journal of andrology. 2002;23:661–668. [PubMed] [Google Scholar]
- 13.Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, Chandrashekar V, Coschigano K, Kopchick J. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biology of reproduction. 2002;67:1115–1124. doi: 10.1095/biolreprod67.4.1115. [DOI] [PubMed] [Google Scholar]
- 14.Bachelot A, Monget P, Imbert-Bollore P, Coshigano K, Kopchick JJ, Kelly PA, Binart N. Growth hormone is required for ovarian follicular growth. Endocrinology. 2002;143:4104–4112. doi: 10.1210/en.2002-220087. [DOI] [PubMed] [Google Scholar]
- 15.Sjogren K, Bohlooly YM, Olsson B, Coschigano K, Tornell J, Mohan S, Isaksson OG, Baumann G, Kopchick J, Ohlsson C. Disproportional skeletal growth and markedly decreased bone mineral content in growth hormone receptor −/− mice. Biochemical and biophysical research communications. 2000;267:603–608. doi: 10.1006/bbrc.1999.1986. [DOI] [PubMed] [Google Scholar]
- 16.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 17.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 18.Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- 19.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ng FM, Jiang WJ, Gianello R, Pitt S, Roupas P. Molecular and cellular actions of a structural domain of human growth hormone (AOD9401) on lipid metabolism in Zucker fatty rats. Journal of molecular endocrinology. 2000;25:287–298. doi: 10.1677/jme.0.0250287. [DOI] [PubMed] [Google Scholar]
- 21.Azain MJ, Roberts TJ, Martin RJ, Kasser TR. Comparison of daily versus continuous administration of somatotropin on growth rate, feed intake, and body composition in intact female rats. Journal of animal science. 1995;73:1019–1029. doi: 10.2527/1995.7341019x. [DOI] [PubMed] [Google Scholar]
- 22.Heffernan MA, Thorburn AW, Fam B, Summers R, Conway-Campbell B, Waters MJ, Ng FM. Increase of fat oxidation and weight loss in obese mice caused by chronic treatment with human growth hormone or a modified C-terminal fragment. Int J Obes Relat Metab Disord. 2001;25:1442–1449. doi: 10.1038/sj.ijo.0801740. [DOI] [PubMed] [Google Scholar]
- 23.Moller N, Jorgensen JO. Effects of Growth Hormone on Glucose, Lipid and Protein Metabolism in Human Subjects. Endocrine reviews. 2009 doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 24.Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Experimental biology and medicine (Maywood, NJ) 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- 25.Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. The journals of gerontology. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1988;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- 27.Lusk G. The elements of science and nutrition. 4. Philadelphia: WB Saunders Co; 1928. pp. 64–68. [Google Scholar]
- 28.Riachi M, Himms-Hagen J, Harper ME. Percent relative cumulative frequency analysis in indirect calorimetry: application to studies of transgenic mice. Can J Physiol Pharmacol. 2004;82:1075–1083. doi: 10.1139/y04-117. [DOI] [PubMed] [Google Scholar]
- 29.Diack SL. The determination of the surface area of the white rat. J Nutr III. 1930:289–296. [Google Scholar]
- 30.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 31.Lo HC, Hirvonen MD, Kritsch KR, Keesey RE, Ney DM. Growth hormone or insulin-like growth factor I increases fat oxidation and decreases protein oxidation without altering energy expenditure in parenterally fed rats. Am J Clin Nutr. 1997;65:1384–1390. doi: 10.1093/ajcn/65.5.1384. [DOI] [PubMed] [Google Scholar]
- 32.Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- 33.Speakman JR. Correlations between physiology and lifespan--two widely ignored problems with comparative studies. Aging cell. 2005;4:167–175. doi: 10.1111/j.1474-9726.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Nielsen K. Locomotion: energy cost of swimming, flying, and running. Science. 1972;177:222–228. doi: 10.1126/science.177.4045.222. [DOI] [PubMed] [Google Scholar]
- 35.White CR, Seymour RS. Allometric scaling of mammalian metabolism. The Journal of experimental biology. 2005;208:1611–1619. doi: 10.1242/jeb.01501. [DOI] [PubMed] [Google Scholar]
- 36.White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass2/3. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4046–4049. doi: 10.1073/pnas.0436428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheung MC, Spalding PB, Gutierrez JC, Balkan W, Namias N, Koniaris LG, Zimmers TA. Body surface area prediction in normal, hypermuscular, and obese mice. The Journal of surgical research. 2009;153:326–331. doi: 10.1016/j.jss.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt-Nielsen K. Scaling in biology: the consequences of size. The Journal of experimental zoology. 1975;194:287–307. doi: 10.1002/jez.1401940120. [DOI] [PubMed] [Google Scholar]
- 39.Acheson K, Jequier E, Burger A, Danforth E., Jr Thyroid hormones and thermogenesis: the metabolic cost of food and exercise. Metabolism: clinical and experimental. 1984;33:262–265. doi: 10.1016/0026-0495(84)90048-9. [DOI] [PubMed] [Google Scholar]
- 40.D’Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, Owen LR, Bushman MC, Boden G, Owen OE. Thermic Effect of Food in Lean and Obese Men. J Clin Invest. 1988;81:1781–1789. doi: 10.1172/JCI113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell NJ, Saville ME, Stock MJ. Metabolic responses to fasting and refeeding in lean and genetically obese rats. Am J Physiol. 1983;244:R615–620. doi: 10.1152/ajpregu.1983.244.5.R615. [DOI] [PubMed] [Google Scholar]
- 43.Glick Z, Wu SY, Lupien J, Reggio R, Bray GA, Fisher DA. Meal-induced brown fat thermogenesis and thyroid hormone metabolism in rats. Am J Physiol. 1985;249:E519–524. doi: 10.1152/ajpendo.1985.249.5.E519. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Experimental biology and medicine (Maywood, NJ) 2003;228:207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]