Abstract
No vaccine exists for the prevention of infection with the ubiquitous gastric pathogen Helicobacter pylori, and drug therapy for the infection is complicated by poor patient compliance, the high cost of treatment, and ineffectiveness against drug resistant strains. A new medical advancement is required to reduce the incidence of peptic ulcer disease and stomach cancer, two conditions caused by infection with H. pylori. Clinical trials have been performed with a formalin-inactivated Helicobacter pylori Whole Cell (HWC) vaccine, given orally in combination with the mucosal adjuvant mLT(R192G), a mutant of E. coli heat-labile toxin. Following the initial dose of this vaccine, some subjects experienced gastrointestinal side effects. To reduce side effects and potentially further increase the amount of adjuvant that can safely be administered with the HWC vaccine, experiments were performed with a form of LT that carried two mutations in the A subunit, a substitution of G for R at position 192, and A for L at position 211. The double-mutant LT (dmLT) adjuvant stimulated immune responses as effectively as the single mutant LT in mice. Additionally, following a challenge infection, the dmLT-adjuvanted vaccine was as effective as single mutant LT in reducing gastric urease levels (diagnostic for H. pylori infection), and H. pylori colonization in the stomach as assessed by quantitative analysis of stomach homogenates. A lyophilized formulation of HWC was developed to improve stability and to potentially reduce reliance on cold chain maintenance. It was observed that a dmLT-adjuvanted lyophilized vaccine was equally as protective in the mouse model as the liquid formulation as assessed by gastric urease analysis and analysis of stomach homogenates for viable H. pylori. No readily detectable effect of tonicity or moisture content was observed for the lyophilized vaccine within the formulation limits evaluated. In an accelerated stability study performed at 37°C the lyophilized vaccine remained equally as protective as vaccine stored at 2–8°C. The formulation selected for clinical development consisted of 2.5×1010 formalin-inactivated cells per ml in 6.5% trehalose, 0.5% mannitol, and 10 mM citrate buffer at pH 6.8.
Keywords: Helicobacter pylori, vaccine, adjuvant
1. Introduction
Helicobacter pylori is a spiral-shaped Gram-negative rod that infects the stomach of as much as 50% of the population in the industrialized world, and the prevalence of infection is markedly higher in developing countries [1–3]. These microaerophilic bacteria are vigorous producers of the enzyme urease, which allows for efficient colonization of the hostile acidic environment found in mammalian stomachs. Many of the bacteria are free-swimming in the stomach mucus layer, while others attach to the underlying gastric mucosal epithelial cells [4]. Despite the induction of an immune response targeted to the pathogen, H. pylori can persist for life on the mucosal surface of the stomach unless treatment is given [5].
H. pylori has been strongly implicated in the etiology of chronic active gastritis, a condition where colonization causes an inflammation of the gastric mucosa. This disease is usually life-long in the absence of treatment and is often accompanied by mucosal atrophy and metaplasia [6]. Although most of those infected experience no symptoms, approximately 10% will develop peptic ulcer disease [7;8]. H. pylori infection is also a significant risk factor for stomach cancer and low-grade, B-cell lymphomas of the gastric mucosa-associated lymphoid tissue (MALT) [9–11]. In addition, H. pylori has been linked to the development of hypochlorhydria, atrophic gastritis, and intestinal metaplasia/dysplasia, which are conditions that predispose to gastric cancer [12–14]. Importantly, H. pylori was listed as a class I carcinogen by the World Health Organization in 1994 [15], because of its association with cancers of the stomach.
Not long after the discovery that Helicobacter pylori infection was associated with the development of gastric ulcers and more serious gastrointestinal diseases, investigators sought strategies to treat the infection, through the use of antimicrobials and vaccines. Antimicrobial “triple therapy” (e.g. amoxicillin, clarithromycin and a proton pump inhibitor such as omeprazole) remains the standard of care for H. pylori infection with complications, but research on vaccines continues, owing in part to the fact that strains resistant to antibiotics commonly used for treatment have emerged [16;17]. Both subunit and whole cell vaccines were shown in animal models not only to be able to prevent infection or reduce its intensity in a prophylactic setting, but could also act therapeutically in established infections to reduce colonization levels, sometimes to the extent that they were below the level of detection. Whole cell preparations and lysates were the first type of vaccine shown to be prophylactically [18;19] and therapeutically [20] protective using mouse-adapted H. pylori strains. Further studies demonstrated that urease or urease subunits were also prophylactically [18;19;21;22] and therapeutically [23] protective in the H. pylori mouse model. Numerous other H. pylori antigens have been discovered which have been shown to provide protection in animal models [5].
Orally delivered whole cell vaccines offer an attractive approach for the prevention and/or treatment of H. pylori infection in that they cause few side effects, are completely avirulent, and are generally easy to produce. An additional advantage of whole cell vaccines is that they offer advantages that individual antigens do not, in that they present to the immune system both known, and unknown protective antigens. It was expected based upon pre-clinical data that a mucosal adjuvant would be required to maximize immunogenicity and the potential for protection with the whole cell vaccine. This was because immunization with the Helicobacter vaccines alone resulted in poor immunogenicity and no protection from infection, while adjuvanted vaccines were immunogenic and protective [18;19;24]. The adjuvant initially chosen for clinical evaluation of (HWC vaccine was a mutant form of E. coli heat-labile toxin (mLT) known to have reduced toxicity compared to wild-type LT, but which retained adjuvant activity. This mutant differed from wild-type LT in that it contained a glycine at position 192 rather than an arginine in the enzymatically active A1 subunit of the holotoxin which reduced its sensitivity to protease activation [25]. This molecule has a number of properties that suggested it would be less toxic than wild-type LT in humans, including 100–1,000-fold less activity compared to native LT in the mouse Y-1 adrenyl cell assay [25;26], insensitivity to proteolytic activation, and absence of in vitro NAD:agmatine ADP ribosyltransferase activity [25;27].
Although a small proportion (14.6%) of subjects who received mLT with or without HWC experienced diarrhea at any time during the trial [28], it was apparent that the potency of mLT to induce diarrhea was significantly reduced compared to that of wild type LT [29;30]. Similar to that which was observed in the urease trials in which wild-type LT was used, diarrhea was most often observed following the initial vaccine dose [28]. In a second Phase I trial in which non-infected subjects received HWC vaccine and either 10 or 25 μg of mLT, similar results were observed (unpublished observations).
Because mLT(R192G) when co-administered with the HWC vaccine still retained some enterotoxicity, and because it was of interest to evaluate doses of adjuvant greater than 25 μg, a form of mLT was created that had an additional mutation at position 211, where a leucine was replaced with alanine [27]. This position was chosen for modification because it was identified as a potential pepsin cleavage site. Additionally, position 211 in the A1 subunit was of interest as a target for a second mutation because a single mutant mLT(L211A) protein had been shown to have reduced enterotoxicity in the patent mouse model [27]. This reduction in enterotoxicity observed with mLT(L211A) protein was roughly equivalent to that seen with single mutant mLT(R192G) [27].
The double mutant molecule was evaluated in assays to assess its potential for toxicity, including assays to measure resistance to trypsin and pepsin proteolysis, and activity in the patent mouse enterotoxicity assay [31]. The single mutant mLT(L211A) remained sensitive to trypsin, while the dmLT was insensitive [27]. That the dmLT was resistant to trypsin digestion was expected, since the single mutant mLT(R192G) itself imparts this resistance. When the dmLT molecule was evaluated, it was found to have essentially no detectable enterotoxicity in the patent mouse enterotoxicity model [31], where the gut to carcass ratio did not differ from that seen in untreated control mice [27]. For this reason, the dmLT adjuvant was considered for evaluation with the HWC vaccine. In addition to modifying the adjuvant for the vaccine, it was also of interest to develop a lyophilized formulation for the HWC vaccine to provide for optimal stability.
2. Materials and Methods
Manufacturing of killed whole cell H. pylori vaccine (HWC)
The method used to manufacture HWC vaccine was as described [28;32;33] with some modifications. Vaccine for the present studies was produced at 30L scale, and cGMP vaccine was produced at 400L scale. For the preparation of final containers, 4 ml of killed cell suspension was transferred to 5 ml sterile glass vials, which were capped with sterile siliconized rubber tops and sealed. Final vials, containing approximately 2.5 × 1010 cells, were released based upon a battery of tests including visual inspection (color, appearance and uniformity), sterility, pH, residual formaldehyde, bacterial concentration, general safety, and potency as assessed by immunogenicity.
Lyophilization of HWC vaccine
A freeze-dried formulation of HWC vaccine was developed with the ultimate goal of producing a vaccine that has long-term physico-chemical stability, rapid reconstitutability, and maintenance of bioactivity throughout the intended shelf-life. Preliminary experiments were carried out to subject various placebo formulations to an initial working freeze-drying cycle. Using a standard freezing process, cell-minus formulations containing different concentrations of trehalose, mannitol, sodium acetate and sodium citrate at a pH of 6.5 were subjected to automated freeze-drying cycles. Moisture analysis (via Karl Fisher assay) after up to four hours of secondary drying resulted in samples with residual moisture of approximately 2.0%, which was within the preferred target of ≤ 3%. The lyophilized cakes appeared acceptable in appearance and upon reconstitution formed a clear solution within one minute.
Lyophilization was performed using a Lyostar II lyophilizer (FTS Systems, Stone Ridge, NY). To prepare vials for lyophilization, 1.05 ml of the cellular suspension (2.5×1010 cells per vial) was filled in a 5 ml tubing vial. The lyophilization process consisted of the standard three steps: freezing, primary drying and secondary drying. Freezing was undertaken at −40°C for 2h at 150 mT. Primary drying was carried out by successive cycles at −40°C, −30°C, −25°C, 0°C and finally 25°C with pressure control set at 30 mT. Secondary drying was carried out at 10°C for 7h, with a final hold pressure at 50 mT, after which the vials were brought to a temperature of 4°C.
Tonicity adjustments were made by varying the trehalose concentration from 5.5–8.0%, and vaccines with osmolarity of 245, 280, and 325 mOsm were compared in immunization and challenge experiments. Levels of mannitol, sodium citrate, and the HWC cell concentration (2.5×1010 per vial) in the formulations were kept constant. Moisture content was varied in final vials by extending the duration of secondary drying.
E. coli heat-labile toxin single mutant
To generate the first generation single mutant LT adjuvant (mLT) used in previous clinical trials [28;34], the A subunit of E. coli heat-labile toxin was modified by substituting arginine with glycine at amino acid position 192 [25]. The substitution at a disulfide-forming region makes the protein less toxic by targeting a trypsin-sensitive site. The resulting protein was designated as mLT(R192G). mLT(R192G) was expressed from E. coli expression vector pLC326 which contains a kanamycin resistance gene as a selective marker. The vector was transformed into E. coli JM83 and a master cell bank (MCB) was generated for JM83(pLC326) at the Walter Reed Army Institute of Research (WRAIR).
Cloning of double mutant heat-labile toxin adjuvant (dmLT)
To further reduce the potential for enterotoxicity of the adjuvant, a double mutant LT (dmLT) was designed [27]. This double mutant contains a leucine to alanine substitution at amino acid 211 (L211A) in addition to the R192G mutation. E. coli JM83 host cells were grown overnight in 50 ml of LB medium at 37°C, and were made chemically competent to be used as host cells for the plasmid containing the dmLT sequence. Plasmid pLC326 was isolated from the JM83(pLC326) MCB and was used as a template to make the double mutant construct. The second mutation was introduced using overlap extension PCR. The four primers used were: 1) forward primer (dmLT-F1) corresponding to −180 to −141 bp from the initiation codon of pLC326 coding region (including the signal sequence), 2) the reverse primer (dmLT-R1) corresponding to positions 263 to 292 from the stop codon, 3) LT-L211A-F, containing the second mutation (GCA for the L211A mutation) in the forward direction and 4) dmLT-R1, containing the L211A mutation (TGC) in the reverse direction. Using the four primers and the pLC326 template, overlap extension PCR was performed. The first round of PCR generated two fragments, each containing the desired L211A mutation. The two fragments were combined together by performing the second round of PCR. The resulting PCR product contained the full-length coding region with the desired L211A mutation.
The 1260 bp PCR fragment was digested with BamHI and XmaI. The single mutation plasmid pLC326 was also digested with the same enzymes and the backbone without the coding region was isolated. The PCR fragment and the pLC326 backbone were then ligated together to generate the double mutant plasmid. The ligation product was used to transform NovaBlue (Novagen) competent cells. Once the transformants were verified by selection on LB plates containing 50 μg/ml kanamycin (Kan 50 plates), a stock strain was made and the plasmid was isolated by miniprep. The plasmid (designated pEIS101) contained both the R192G (from the pLC326 plasmid) and the L211A (from the overlap extension PCR reaction) mutation. After verifying that pEIS101 indeed contained both mutations by DNA sequencing, pEIS101 was used to transform JM83 competent cells. Three colonies were chosen to be used for generation of a research cell bank. As a final verification of plasmid sequence, frozen cultures from the research cell bank were inoculated to five 5 ml of LB media containing 50 μg/ml kanamycin and incubated overnight at 37°C with shaking. Plasmids were isolated from each culture and pooled together. Plasmids were then digested with BamHI and the size of the plasmid (5.4 kb) was verified by agarose gel electrophoresis. The plasmid was subsequently fully sequenced and the result verified that pEIS101 contained both the R192G and L211A mutations.
Manufacturing of mutant LT adjuvants
Since dmLT and mLT(R192G) differ by only one amino acid and are produced using the same E. coli expression vector, the process for manufacturing dmLT adjuvant did not substantially differ from that used to produce mLT(R192G), used in previous HWC trials [28]. Three L of medium from inoculated shake flasks was used to seed a 400 L fermenter. Cells were harvested and the cell paste was collected and stored at −80°C prior to purification. Bulk lots of dmLT protein were prepared by lysis of the bacteria with a microfluidizer, clarification of the lysate by centrifugation, and purification of the solubilized dmLT protein by affinity column chromatography, followed by concentration using ultrafiltration, size exclusion chromatography and a step concentration using ultrafiltration. Release testing of the final containers included final container visual inspection (color, appearance, uniformity), sterility, endotoxin content by LAL (gel-clot), pH, protein content by A280, moisture content, osmolarity, HPLC-SEC, general safety, purity by SDS-PAGE (trypsin & non-trypsin treated), cyclic AMP induction activity, potency by adjuvant activity for tetanus toxoid, and in vivo enterotoxicity as assessed by the patent mouse assay [31]. The resulting product contained >99% double mutant holotoxin, <1.2 endotoxin units/mL, and was not sensitive to trypsin activation.
Animals and animal facility
Six to eight-week old female Helicobacter-free C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). All animals were shipped directly to a contract animal facility (Biocon Inc., Rockville MD) from the Charles River Frederick, MD facility. Biocon is a controlled access, UDSA-registered and American Association for Accreditation of Laboratory Animal Care (AAALAC) accredited facility. Animals were housed in USDA approved, micro-isolation cages with food and water provided ad libitum. The Helicobacter infected animals are maintained in a separate room with air-lock entry and an internal workspace so that animals need not be removed from the room for routine examinations or non-invasive procedures. All animal protocols were approved by Biocon’s Institutional Animal Care and Use Committee (IACUC).
Oral vaccination procedure
Mice were immunized with HWC vaccines via oral gavage using a three dose schedule. An initial dose was given, followed by booster doses on days 14 and 28. A quantity of vaccine was aseptically removed with a 1cc syringe and mixed with sterile PBS without Mg+ or Ca+ and adjuvant to give a final concentration of 2×109 cells/ml, with a 0.5 ml instilled volume yielding 1×109 cells/dose. Experimental concentrations of mLT adjuvants for oral use in clinical trials have ranged from 5 to 25 μg per dose, and it has been determined experimentally that a 25 μg dose of mLT is reproducibly effective in C57BL/6 mice. This dose of adjuvant is the same as that which was used in a Phase 1 trial with the HWC vaccine [28], and is it within the dose range of the CT/LT family of adjuvants that is typically used to evaluate Helicobacter vaccines in animals [19;35–37].
Animals were physically restrained so that a 22 gauge ball-tipped feeding needle, attached to a 1cc tuberculin syringe, could be passed into the stomach. A volume of 0.5 ml of ACS grade sterile 5% sodium bicarbonate solution (5 g Sigma product S-6014, dissolved in 100 ml deionized water) was first instilled in order to neutralize gastric acidity. The needle was then withdrawn and the animals observed for ill effects. After 10 minutes the needle was reinserted and 0.25 ml of solution containing 1×109 HWC, and 25 g mLT(R192G) or dmLT adjuvant was instilled. After immunization the animals were observed for several minutes for any indication of distress.
Challenge strain
H. pylori Sydney Strain 1 (SS1) [38], subculture 5, was originally supplied to Emergent in 1997 by Dr. Adrian Lee of the University of New South Wales. Working stocks were created by re-passaging the donated strain in mice. The supplied strain was passed once, re-isolated, and then stored frozen at −80°C.
Oral challenge procedure
The Sydney strain stocks used for experimental infections were from material that had been expanded in vitro fewer than five times since isolation from an infected mouse. To generate a challenge culture, a shake flask containing BHI broth plus 4% fetal calf serum (FCS) was inoculated, and allowed to incubate for 25–48 hours at 37°C, in an atmosphere of 10% CO2 + 5% O2, shaking at 125 rpm. This culture had to yield a pure population of highly motile urease positive bacteria having the expected morphology to be used for infection. Approximately two weeks following the final immunization, animals were infected with 1 × 108 live Helicobacter pylori strain SS1. The animals were restrained and the feeding needle passed into the stomach. A 0.25 ml volume of the mixed suspension was instilled over approximately 30 seconds to deliver the challenge to each animal. Animals were again watched for approximately 10 minutes for adverse signs then taken to the isolation housing room. This procedure was performed three times in a 5-day period, with 24h between dosing.
Analysis of stomach colonization
Two weeks following the infection, mice were sacrificed and stomachs were removed for quantitative assessment of colonization. Animals were sacrificed by CO2 inhalation and the fur was saturated with 70% ethanol and skin incision is made with sterile surgical scissors. The stomachs and duodenum were removed and transferred to labeled sterile Petri plates containing 5–10 ml of sterile PBS, and were then transferred to a biosafety cabinet where the stomachs were opened by midline incision and the contents gently cleaned using sterile gauze. The antrum was visualized and aseptically dissected away from the rest of the stomach, which was discarded. The antral section was then diced, using sterilized single-edge razor blades, and the pieces placed in a pre-weighed 5 ml tube containing brain-heart infusion broth (BHI) media. Tubes containing antral sections were re-weighed to 0.001 gram accuracy and placed in a biosafety cabinet. The sections were then mechanically macerated using sterile plastic tissue homogenizers and serial 1:10, 1:100, and 1:1000 dilutions of the homogenates were made in BHI media. From each dilution tube, a 100 μl aliquot was placed on a sterile BHI agar plate and a full plate spread was performed. The media on which homogenates were plated contained BHI agar (Difco), 4% fetal bovine serum, bacitracin, nalidixic acid, amphoteracin B, and Campylobacter selective supplement (Oxoid, Lenexa, KS). Plates were placed in anaerobic jars containing BBL CampyPak Plus® microaerophilic envelopes (Becton Dickinson, Franklin Lakes, NJ; product # 271045) and incubated at 37°C for 6–7 days. Growth control plates were included in each jar, inoculated with a freshly grown preparation of the SS1 challenge strain (“positive control”). The limit of detection in this assay was approximately 500 H. pylori per g of stomach tissue. Colonies on plates were confirmed to be Helicobacter by use of the urease reaction, oxidase reaction, and wet-mount morphology. Colony urease activity was demonstrated by picking a colony with a sterile loop and swirling the material in 200 μl of urease indicator reagent (see below) and assessing color development.
Rapid urease test
An aliquot of the antral tissue homogenate, prepared as described above, was used for the detection of urease activity [39]. Shortly after homogenates were prepared, a 100 μl volume of the original stomach homogenate was added aseptically to a sterile Eppendorf tube containing 100 μl of freshly prepared urease indicator reagent [39]. The tubes were vortexed and incubated at room temperature. After four hours, the tubes were centrifuged at RCF 6000 for 5 minutes and 100 μl of the supernatant transferred to a 96-well plate to be read spectrophotometrically at 550 nm. Tissue homogenates from uninfected mice served as negative controls in this assay and a positive control (“positive control”) containing a known concentration of cultured H. pylori, was also used. Results were recorded using the spectrophotometric data to express activity in OD/gram of antral tissue.
ELISA to measure serum anti-HWC responses
Sera from immunized mice were analyzed for the presence of IgG antibodies to the HWC vaccine using an enzyme-linked immunosorbent assay (ELISA). MaxiSorp 96-well plates (Nunc product #442408) were coated overnight at 4°C with a 1:400 (20 μg/ml) suspension of HWC. After four washes in an automated plate washer the plates are blocked with 0.5% BSA/0.5% casein then re-washed with PBS containing Tween-20. Two, three or four-fold serial dilutions were performed and the plates sealed with Parafilm. Plates were incubated overnight (16–18 hours) at 4°C and then washed. Peroxidase-linked goat anti-mouse IgG (KPL #074-1306) or goat anti-mouse IgA (KPL #ZA086) was then added to plates at a dilution of 1:20,000. The plates were then incubated at room temperature for 1.5 hours. ELISA plates were again washed and a single-step TMB substrate (KPL #52-00-03) was added to all wells. The reaction was stopped after 15 minutes with a commercial stop solution (KPL #50-85-05) and optical density was read at 450nm.
Statistical analysis
The Mann-Whitney Test was used to determine statistical significance for antibody titers, and bacterial colony counts from stomach homogenates.
3. Results
Adjuvant properties of dmLT for HWC vaccine
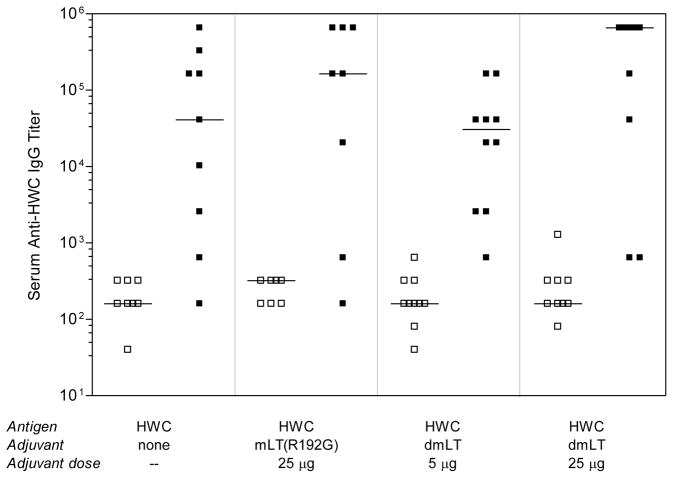
The dmLT adjuvant was used to immunize mice orally using the liquid HWC vaccine. In this study a negative control group received HWC alone with no adjuvant, and the positive control group received HWC plus 25 μg/dose mLT(R192G). We have previously found in at least 10 experiments using this model that protection afforded by vaccination with unadjuvanted HWC does not substantially differ from that afforded by vaccination with diluent alone (data not shown), and thus we did not include the later group in this study. Groups of 10 mice were immunized three times via oral gavage with 1 × 109 inactivated whole cells and adjuvant, and sera were collected on day 35 post-immunization (one week after the final dose was administered). As shown in Figure 1, unadjuvanted HWC was immunogenic for most mice. Co-administration of either mLT(R192G) or dmLT adjuvant with HWC did not significantly increase anti-HWC serum IgG responses compared to those seen in mice immunized with unadjuvanted HWC.
Figure 1.
Serum IgG response to HWC following immunization with unadjuvanted HWC, or HWC plus either mLT(R192G) or dmLT. Groups of 8–10 adult Helicobacter-free C57BL/6 mice were immunized orally on days 0, 14 and 28 with the 109 HWC cells and adjuvant as indicated, and sera were collected on day 35 post-immunization. Sera were evaluated for reactivity in an ELISA using plates coated with HWC cells. Open squares (□) represent pre-immune sera whereas closed squares (■) represent post-immune sera. Horizontal lines through data sets denote the geometric mean for each group.
Protection against H. pylori infection using HWC + dmLT
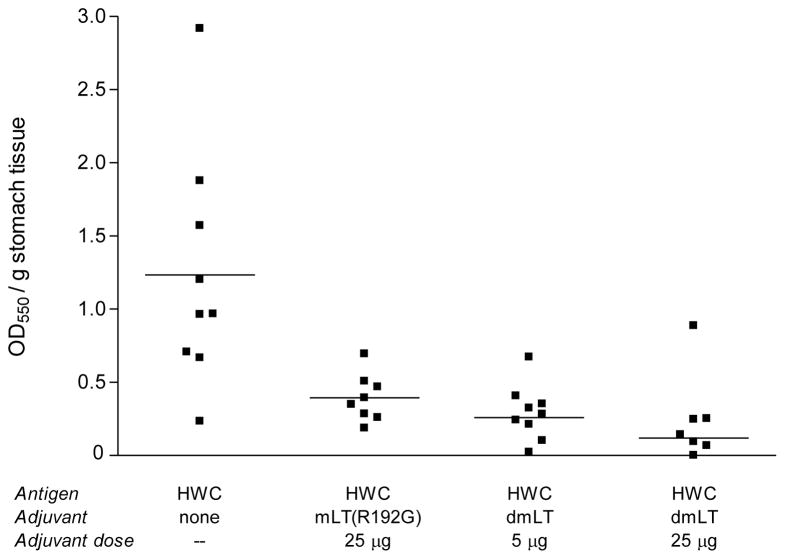
Studies were performed to assess the effect of the dmLT adjuvant on the ability of the liquid HWC vaccine to provide protection against an oral challenge infection with H. pylori. Animals were immunized as described in the legend for Figure 1, and were challenged three times in the third week after the final immunization. All animals were sacrificed two weeks after the challenge infection and mouse stomach tissues were subjected to microbiological analysis. Two different assays were performed on stomach homogenates to assess resistance to H. pylori infection, the urease assay, and the quantitative colony forming unit assay. As shown in Figure 2, significantly reduced urease activity (P <0.05) was found in stomach homogenates prepared from mice that had been vaccinated with HWC plus either mLT(R192G) or dmLT, compared to that seen in animals administered unadjuvanted HWC. Importantly, when mLT(R192G) was directly compared to dmLT on a mass basis, the dmLT was equally capable of reducing urease activity. In fact, optical densities of the samples tested from the dmLT (25 μg) group were significantly lower (P <0.01) than those from the mLT(R192G) (25 μg) group, suggesting the possibility that the dmLT is even more potent than mLT(R192G) on a mass basis.
Figure 2.
Post-infection urease activity in stomachs of mice prophylactically immunized with HWC plus single (R192G) or double (dmLT) mutant LT adjuvant, and challenged with H. pylori SS1. Groups of 8–10 animals were immunized orally on days 0, 14 and 28 with the 109 HWC cells and adjuvant as indicated, and were subsequently challenged orally three times in the third week post-immunization with 108 H. pylori. Urease activity in stomach homogenates was assessed two weeks after the final challenge infection.
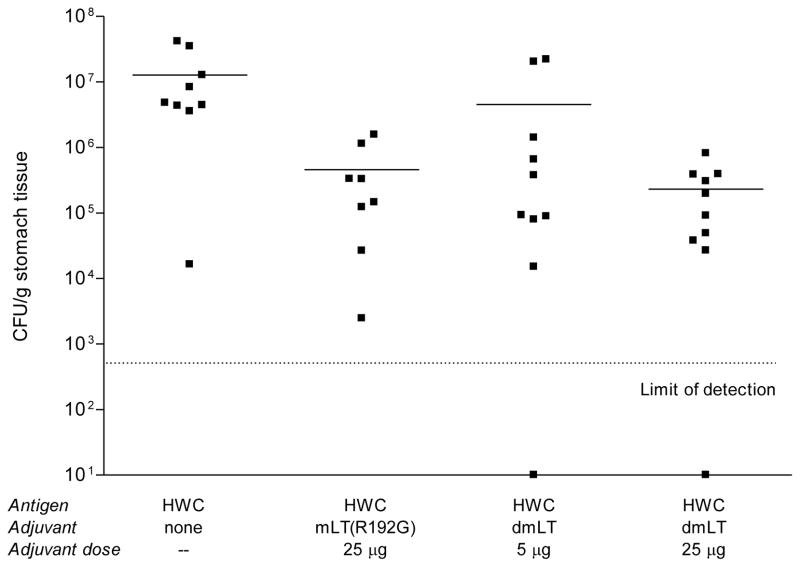
A similar pattern of results was found when the numbers of H. pylori that were present in the stomach homogenates post-challenge were quantified (Figure 3). A statistically significant reduction in bacterial load in the stomachs of mice was achieved using HWC plus 25 μg/dose mLT(R192G) (P <0.01), and the dmLT adjuvant was equally as protective at the 25 μg/dose level (P <0.01). Immunization of mice with HWC and 5 μg/dose dmLT reduced mean colony counts obtained post-challenge to the extent that the reduction approached statistical significance (P =0.056). Importantly, we also observed following the challenge infection that two mice immunized with HWC plus dmLT (one from Group 3 and one from Group 4) had undetectable levels of infection in their stomachs. These data, along with the data from the urease assay demonstrate that for the HWC vaccine, the dmLT adjuvant is at least as potent as the mLT(R192G) adjuvant, despite the fact that the dmLT was significantly less toxic [27].
Figure 3.
H. pylori colony counts in stomachs of mice prophylactically immunized with HWC plus single (R192G) or double (dmLT) mutant LT adjuvant, following challenged with H. pylori. Mice were immunized and challenged as described in the legend of Figure 2. H. pylori colony counts in stomach homogenates were quantified as described in Materials and Methods. Values shown for animals that had undetectable colony counts were arbitrarily shown on the figure as 101. The limit of detection in this assay was approximately 500 H. pylori per g of stomach homogenate.
Evaluation of lyophilized formulations of HWC vaccine
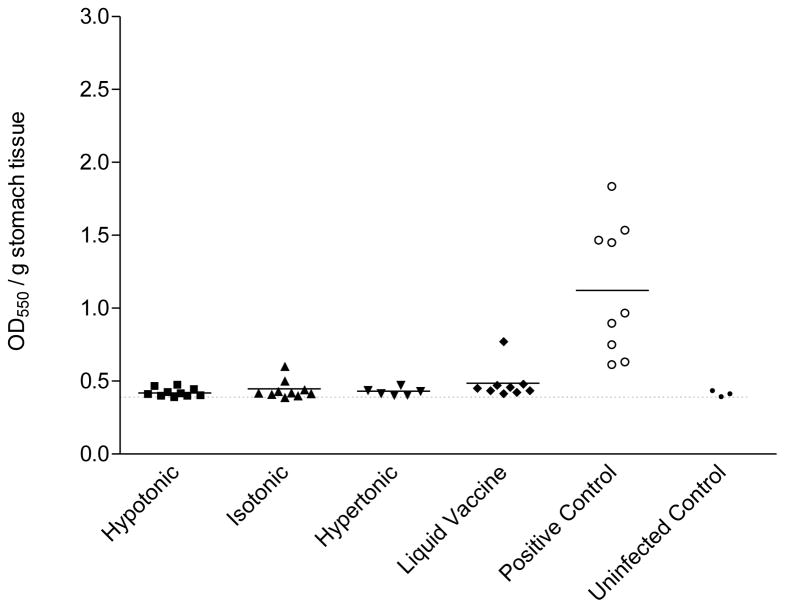
To assess the protective efficacy of the lyophilized HWC vaccine, mice were immunized on days 0, 14 and 28, with either 108 or 109 cells and two weeks after the final immunization, were challenged with an oral dose of 1 × 108 H. pylori. All animals were sacrificed 18 days after H. pylori challenge and individual mouse stomachs were isolated and subjected to microbiological analysis. As shown in Figure 4, stomach homogenates prepared from mice vaccinated with 109 HWC cells generally lacked detectable urease activity, which was significantly reduced compared to the mice which received PBS (P <0.002). Equivalent optical densities were measured from stomach homogenates of mice administered lyophilized HWC vaccines and liquid HWC vaccine and were not different than those measured from naïve mice (non-immunized, non-infected). Additionally, no differences were seen among vaccines prepared with different tonicities. Similar results were seen in mice vaccinated with 108 cells per dose (data not shown).
Figure 4.
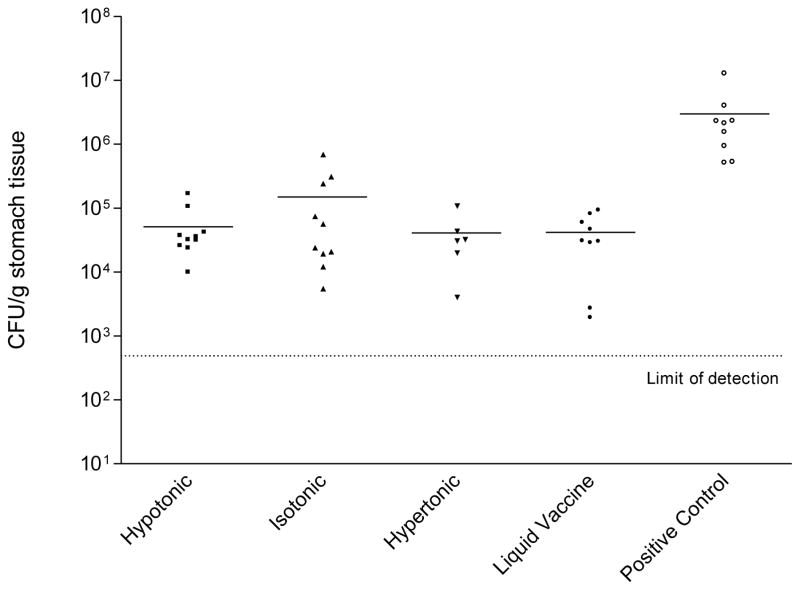
Urease activity detected in stomachs of mice orally vaccinated with 1 × 109 HWC cells plus 25 μg of dmLT adjuvant. Vaccines were prepared as hypertonic, isotonic, or hypertonic solutions prior to lyophilization, and were compared to liquid (never lyophilized) vaccine. The number of animals per group was as follows: hypertonic (10), isotonic (10), hypertonic (6), liquid vaccine (9), positive control (9), uninfected control (3). The baseline optical density in the assay (shown as horizontal dashed line) was established using stomach homogenates from three non-immunized, uninfected C57BL/6 mice. The “positive control” were cultures of freshly grown H. pylori cells, included in the assay to ensure that the urease reagent was reactive to viable H. pylori cells.
When the numbers of H. pylori present in the stomach homogenates after challenge were quantified, a statistically significant reduction in the number of H. pylori colonies was seen in mice immunized with each of the HWC vaccines compared to mice administered PBS (P <0.05). The three lyophilized HWC vaccine lots were equally effective in protecting from H. pylori challenge at both vaccine doses tested, i.e. 109 (Figure 5) and 108 cells (data not shown), and importantly, the efficacy of each of the lyophilized HWC vaccines was equivalent to that of the liquid HWC vaccine control. It was concluded from these studies that the different buffering conditions used in the lyophilization process resulted in vaccines that were equally protective, and that protection was not compromised as a result of lyophilization.
Figure 5.
H. pylori colony counts detected in stomachs of mice orally vaccinated with 1 × 109 HWC cells plus 25 μg of dmLT adjuvant. Vaccines were prepared as hypertonic, isotonic, or hypertonic solutions prior to lyophilization, and were compared to liquid vaccine. The number of animals per group was as described in the legend of Figure 4. Adult Helicobacter-free C57BL/6 mice were immunized orally on days 0, 14 and 28 with the 109 HWC cells, and were challenged as described in Materials and Methods. Animals were sacrificed two weeks after challenge for analysis of H. pylori colonization in the stomach. The “positive control” were cultures of freshly grown H. pylori cells, and were included in the assay to ensure that proper growth conditions in the microaerophilic chambers were present.
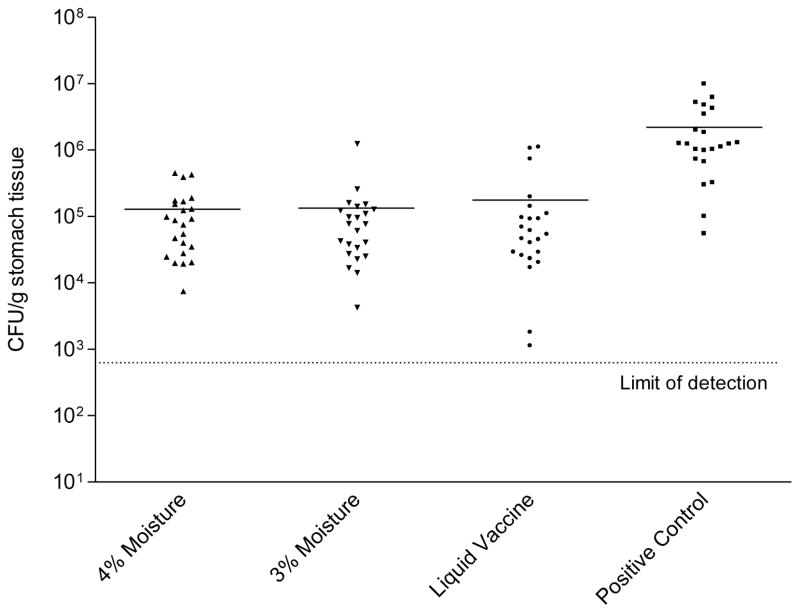
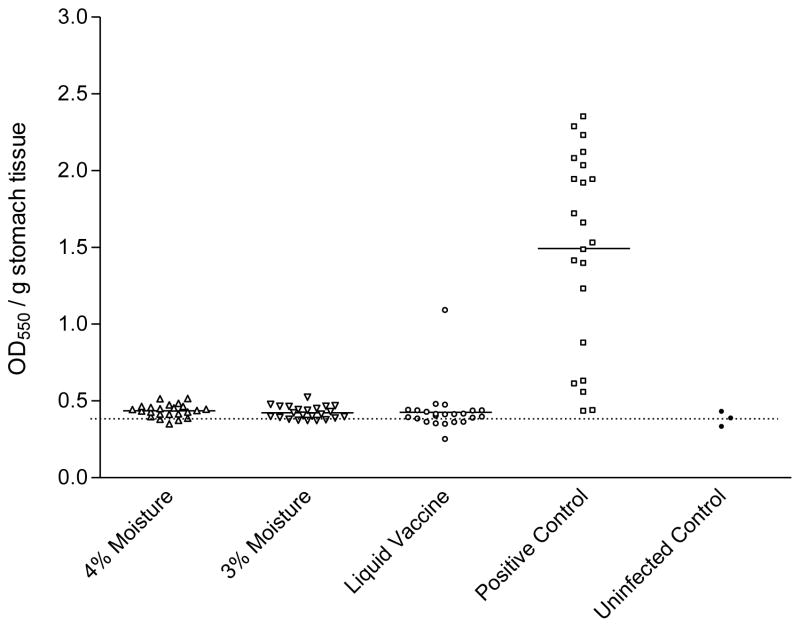
The protective efficacy of lyophilized HWC vaccines varying in moisture content was then evaluated. The lots of lyophilized HWC vaccine evaluated contained either 3% or 4% water, (pH 6.6–7.2; cell count 2.5×1010/ml) and both were lyophilized from an isotonic buffered suspension (290–312 mOsm). Lyophilized HWC vaccines were resuspended with 1 ml of sterile water and mice were orally immunized three times with 1×109 cells from either vaccine preparation. The dmLT adjuvant was added to each vaccine at a concentration of 25 μg per dose. Inoculation of the vaccines was on days 0, 14 and 28, and mice were challenged two weeks after the final vaccination. As shown in Figure 6 the two lyophilized HWC vaccines containing either 3% or 4% water were equally effective in significantly reducing the numbers of H. pylori colonies enumerated from the stomach homogenates from challenged mice. Statistical analysis revealed no significant differences observed in the mean CFU/gram stomach tissue weight when comparing the two lots. As observed in previous studies, there was also no difference in the protective efficacy of lyophilized versus liquid vaccines, and all vaccines afforded significant production versus animals immunized with PBS (P <0.001). Similar results were seen when stomach homogenates were analyzed for urease activity (Figure 7). No readily detectable effect of tonicity or moisture content was observed within the formulation limits evaluated.
Figure 6.
H. pylori colony counts detected in stomachs of mice orally vaccinated with 1 × 109 HWC cells plus 25 μg of dmLT adjuvant. Vaccines tested contained either 3% or 4% moisture following lyophilization, and were compared to liquid (never lyophilized) vaccine. Groups of 23 animals were immunized with the indicated vaccines as described in the legend of Figure 1, were challenged, and analyzed for H. pylori colonization two weeks after challenge. The “positive control” were cultures of freshly grown H. pylori cells, included in the assay to ensure that proper growth conditions in the microaerophilic chambers were present. Horizontal lines through data sets denote the geometric mean for each group.
Figure 7.
Urease activity detected in stomachs of groups of 23 mice orally vaccinated with 1 × 109 HWC cells plus 25 μg of dmLT adjuvant. Vaccines tested contained either 3% or 4% moisture following lyophilization, and were compared to liquid (never lyophilized) vaccine. Urease activity in stomach homogenates was assessed two weeks following challenge. The baseline optical density in the assay (shown as horizontal dashed line) was established using stomach homogenates from three non-immunized, uninfected C57BL/6 mice
To assess vaccine stability, a one month accelerated stability study showed that lyophilized vaccines stored at 4°C or 37°C did not lose biological activity, when vaccines were assessed by their ability to protect against H. pylori infection in the mouse model. Both gastric colony counts and urease activity in stomachs were evaluated in this study (data not shown). Based upon the results of efficacy studies using the mouse model, the selected formulation for the lyophilized HWC vaccine consisted of 2.5×1010 formalin-inactivated cells per ml in 6.5% trehalose (bulking agent), 0.5% mannitol (caking agent) and 10 mM citrate buffer, pH 6.8.
4. Discussion
While H. pylori infection can generally be treated with a regimen of two antimicrobials and a proton pump inhibitor [40], a rationale for an H. pylori vaccine still exists. The primary factors driving the need for an H. pylori vaccine are the emergence of drug-resistant, treatment-refractory strains [16], and the expense of drug therapy, which makes treatment difficult for most people in the developing world, where H. pylori is a more predominant public health problem.
A great variety of H. pylori vaccines have been developed and tested in animal models, but few have been tested clinically. The majority of the clinical work has focused on raising immune responses to urease, the enzyme that the bacterium makes in order to survive in the highly acidic gastric environment [41]. Such vaccines include recombinant urease delivered orally with or without wild-type LT as an adjuvant [29;30;42], recombinant urease delivered rectally [43], and recombinant Salmonella expressing urease delivered orally [44–47]. Additionally, a subunit vaccine composed of the antigens CagA, VacA and NAP adsorbed to aluminum adjuvant for injection has been tested in humans [44]. To date no subunit or vectored vaccine has had an obvious advantage over whole cell vaccines administered orally with an LT-based adjuvant with respect to immunogenicity or safety, nor have they shown any significant degree of efficacy in therapeutic use.
The mLT(R192G) adjuvant was first clinically tested in the mid-1990s in studies sponsored by the U.S. Army in collaboration with the U.S. Naval Medical Research Institute, as part of a Campylobacter jejuni vaccine development program in which Antex Biologics (now Emergent BioSolutions) was a sponsor [49;unpublished data]. The adjuvant was first tested alone in healthy adults in a placebo-controlled Phase 1 trial in which mLT(R192G) was administered orally to subjects in doses of 5, 25, 50 or 100 μg. Of the 27 subjects that that received doses up to 50 μg, no subjects developed diarrhea. No difference in the incidence of side effects were seen at these dose levels compared to placebo. At the 100 μg dose, approximately 17% of subjects (2/12) met the protocol definition for severe diarrhea, although the diarrhea was self-resolving and the affected subjects did not require intravenous fluid. When compared to clinical safety data generated by the same group in trials evaluating the tolerability of orally-administered wild-type LT (unpublished results), native LT was found to be 4–10 fold more enterotoxic than mLT(R192G). For studies on the killed whole cell Campylobacter vaccine (CWC), and later for the HWC vaccine, a fixed dose of 25 μg of mLT(R192G) was used in initial clinical trials because this dose of LT had been shown to significantly improve the immunogenicity of the CWC vaccine in mice and non-human primates [50;51], and because this dose was 4-fold below that at which dose-limiting toxicity was observed in healthy adults.
Two clinical trials have been performed using the HWC vaccine adjuvanted with single mutant mLT(R192G). This vaccine has been shown to be protective in both prophylactic and therapeutic models of H. pylori infection. On the basis of positive pre-clinical data using HWC and mLT(R192G), an initial Phase 1a clinical trial was performed in uninfected and infected asymptomatic subjects using a mLT(R192G) dose of 25 μg. A follow-up Phase 1b trial was performed using either 10 μg or 25 μg of mLT(R192G) to further define the optimal vaccine formulation and regimen, and to evaluate the incidence of side effects. In both trials the vaccine was found to be well tolerated and generated both mucosal and systemic immune responses in some vaccinated individuals [28;34] and unpublished results]. In the first trial, the predominant side effects seen in subjects were diarrhea and other gastrointestinal-related complaints [28]. Mild to moderate, self-limiting diarrhea was most commonly observed after the first vaccine dose. These gastrointestinal side effects occurred in subjects that received adjuvant in combination with HWC vaccine and in subjects that received mLT(R192G) alone (1 of 3 subjects).
Subsequent steps taken to modify this vaccine included the replacement of the mLT(R192G) adjuvant with the double-mutant LT(R192G, L211A) adjuvant, and the development of a lyophilized formulation of HWC. The modification to the adjuvant was undertaken because of the observation that the dmLT adjuvant was less reactive in the mouse model of intestinal inflammation and fluid secretion than mLT(R192G) but nevertheless had equivalent adjuvant properties compared to mLT(R192G) [27]. This could potentially allow for higher doses of adjuvant to be used clinically, in order to improve vaccine immunogenicity. Lyophilization was desired because of the potential need for this vaccine in areas of the world where maintaining a cold chain would be difficult or expensive.
We found that an acceptable formulation and lyophilization process could be developed for the killed whole cell H. pylori vaccine, and that the lyophilized vaccine was as protective as liquid, never lyophilized HWC vaccine in the mouse model. The final formulation selected was also found to be stable, in that lyophilized vaccine stored at 37°C had demonstrable stability for at least one month. Ongoing stability studies are evaluating long-term stability at 2–8°C for both the HWC vaccine and lyophilized dmLT.
On the basis of the preclinical formulation and challenge studies performed on the lyophilized HWC vaccine, cGMP lots of both HWC vaccine and dmLT adjuvant were produced at the 300L scale at the WRAIR Pilot Bioproduction Facility. These materials passed all lot release tests and thus could be used for future clinical evaluation.
Acknowledgments
This work was funded under NIH grant 5U01AI060624. The authors wish to thank the staff at the Walter Reed Army Institute of Research Pilot Bioproduction Facility, and Dr. Robert Hopkins for reviewing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Graham DY, Malaty HM, Evans DG, Evans DJ, Klein PD, Adam E. Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology. 1991;100(6):1495–501. doi: 10.1016/0016-5085(91)90644-z. [DOI] [PubMed] [Google Scholar]
- 2.Goodman KJ, Correa P. The transmission of Helicobacter pyori. A critical review of the evidence. International Journal of Epidemiology. 1995;24:875–87. doi: 10.1093/ije/24.5.875. [DOI] [PubMed] [Google Scholar]
- 3.Torres J, Perz-Perez G, Goodman KJ, et al. A comprehensive review of the natural history of Helicobacter pylori infection in children. Archives of Medical Research. 2000;31:431–69. doi: 10.1016/s0188-4409(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 4.Lee A. The microbiology and epidemiology of Helicobacter pylori infection. Scandinavian Journal of Gastroenterology. 1994;29:2–6. [PubMed] [Google Scholar]
- 5.Svennerholm AM, Lundgren A. Progress in vaccine development against Helicobacter pylori. FEMS Immunol Med Microbiol. 2007;50(2):146–56. doi: 10.1111/j.1574-695X.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 6.Sipponon P, Hyvarinen H, Seppala K, Blaser MJ. Pathogenesis of the transformation from gastritis to malignancy. Alimentary Pharmacology & Therapeutics. 1998;12(Suppl 1):61–71. doi: 10.1111/j.1365-2036.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 7.Rauws E, Tytgat G. Cure of duodenal ulcer disease associated with eradication of Helicobacter pylori. Lancet. 1990;335(1233):1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 8.Graham DY, Lew GM, Klein P, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric and duodenal ulcer. A randomized controlled study. Ann Intern Med. 1992;116(9):705–8. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 9.Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–5. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132–6. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- 11.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and risk for gastric cancer. N Engl J Med. 1991;325(16):1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 12.Correa P, Fox J, Fontham E, et al. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer. 1990;66:2569–74. doi: 10.1002/1097-0142(19901215)66:12<2569::aid-cncr2820661220>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Garnier J, Mohar A, Parsonnet J, Halperin D. The association of Helicobacter pylori with gastric cancer and preneoplastic gastric lesions in Chiapas, Mexico. Cancer. 1993;71:297–301. doi: 10.1002/1097-0142(19930115)71:2<297::aid-cncr2820710205>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Lambert J, Lin SK. Prevalence/disease correlates of H. pylori. In: Hunt R, Tygat G, editors. Helicobacter pylori: Basic Mechanisms to Clinical Cure. New York, NY: Springer-Verlag; 1994. [Google Scholar]
- 15.World Health Organization. Schistosomes, Liver Flukes and Helicobacter pylori: Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon: World Health Organization; 1994. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Helicobacter pylori; pp. 177–240. [Google Scholar]
- 16.Koletzko S, Richy F, Bontems P, et al. Prospective multicentre study on antibiotic resistance to Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711–6. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham DY. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–7. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–8. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti M, Rossi M, Giannelli V, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–7. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 20.Ghiara P, Rossi M, Marchetti M, et al. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ermak TH, Giannasca PJ, Nichols R, et al. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–88. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleanthous H, Myers GA, Georgakopoulos KM, et al. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–86. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy B, Hessler C, Fourage S, Rokbi B, Millet MJ. Comparison between targeted and untargeted systemic immunizations with adjuvanted urease to cure Helicobacter pylori infection in mice. Vaccine. 1999;17:1130–5. doi: 10.1016/s0264-410x(98)00332-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit-whole-cell vaccine. Infect Immun. 1994;62(8):3594–7. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995 May;63(5):1617–23. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sack D, Sack RB. Test for enteropathogenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975;11:334–6. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clements JD. The Administrators of Tulane Educational Fund, assignee. Double mutant enterotoxin for use as an adjuvant. 6,033,673. LA, USA patent. 2000 Mar 7; inventor.
- 28.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69(6):3581–90. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michetti P, Kreiss C, Kotloff KL, et al. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology. 1999;116(4):804–12. doi: 10.1016/s0016-5085(99)70063-6. [DOI] [PubMed] [Google Scholar]
- 30.Banerjee S, Medina-Fatimi A, Nichols R, et al. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut. 2002;51(5):634–40. doi: 10.1136/gut.51.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason HS, Haq TA, Clements JD, Arntzen CJ. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16(13):1336–43. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 32.Pace JL, Walker RI, Frey SM, inventors; Vaccines comprising enhanced antigenic Helicobacter spp. 5,897,475. 1999 Apr 27.
- 33.Pace JL, Walker RI, Frey SM, inventors; Methods for producing enhanced antigenic Helicobacter spp. 6,051,416. 2000 Apr 18.
- 34.Losonsky GA, Kotloff KL, Walker RI. B cell responses in gastric antrum and duodenum following oral inactivated Helicobacter pylori whole cell (HWC) vaccine and LTR192G in H. pylori seronegative individuals. Vaccine. 2003;21(5–6):562–5. doi: 10.1016/s0264-410x(02)00259-1. [DOI] [PubMed] [Google Scholar]
- 35.Lee CK. Vaccination against Helicobacter pylori in non-human primate models and humans. Scand J Immunol. 2001;53(5):437–42. doi: 10.1046/j.1365-3083.2001.00911.x. [DOI] [PubMed] [Google Scholar]
- 36.Raghavan S, Hjulström M, Holmgren J, Svennerholm AM. Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines. Infect Immun. 2002;70(11):6383–8. doi: 10.1128/IAI.70.11.6383-6388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutton P, O’Rourke J, Wilson J, Dixon MF, Lee A. Immunisation against Helicobacter felis infection protects against the development of gastric MALT Lymphoma. Vaccine. 2004;22(20):2541–6. doi: 10.1016/j.vaccine.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997 Apr;112(4):1386–97. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 39.Nedrud JG, Blanchard TG. Helicobacter animal models. Current Protocols in Immunology. 2000 doi: 10.1002/0471142735.im1908s36. [DOI] [PubMed] [Google Scholar]
- 40.Wong BC, Lam SK, Lai KC, et al. Triple therapy for Helicobacter pylori eradication is more effective than long-term maintenance antisecretory treatment in the prevention of recurrence of duodenal ulcer: a prospective long-term follow-up study. Alimentary Pharmacology & Therapeutics. 1999;13:303–9. doi: 10.1046/j.1365-2036.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 41.Eaton KA, Brooks CL, Morgan DR, Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991;59(7):2470–5. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreiss C, Buclin T, Cosma M, Corthesy-Theulaz I, Michetti P. Safety of oral immunisation with recombinant urease in patients with Helicobacter pylori infection. Lancet. 1996 Jun 8;347(9015):1630–1. doi: 10.1016/s0140-6736(96)91119-8. [DOI] [PubMed] [Google Scholar]
- 43.Sougioultzis S, Lee CK, Alsahli M, et al. Safety and efficacy of E. coli enterotoxin adjuvant for urease-based rectal immunization against Helicobacter pylori. Vaccine. 2002;21(3–4):194–201. doi: 10.1016/s0264-410x(02)00467-x. [DOI] [PubMed] [Google Scholar]
- 44.Angelakopoulos H, Hohmann EL. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect Immun. 2000;68(4):2135–41. doi: 10.1128/iai.68.4.2135-2141.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL. Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine. 1999;18(5–6):449–59. doi: 10.1016/s0264-410x(99)00246-7. [DOI] [PubMed] [Google Scholar]
- 46.Bumann D, Metzger WG, Mansouri E, et al. Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2001;20(5–6):845–52. doi: 10.1016/s0264-410x(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 47.Metzger WG, Mansouri E, Kronawitter M, et al. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine. 2004;22(17–18):2273–7. doi: 10.1016/j.vaccine.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Malfertheiner P, Schultze V, Rosenkranz B, et al. Safety and Immunogenicity of an Intramuscular Helicobacter pylori Vaccine in Noninfected Volunteers: A Phase I Study. Gastroenterology. 2008;135:787–795. doi: 10.1053/j.gastro.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 49.Scott DA. Vaccines against Campylobacter jejuni. J Infect Dis. 1997;176(Suppl 2):S183–S188. doi: 10.1086/513791. [DOI] [PubMed] [Google Scholar]
- 50.Baqar S, Applebee LA, Bourgeois AL. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun. 1995;63(9):3731–5. doi: 10.1128/iai.63.9.3731-3735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baqar S, Bourgeois AL, Schultheiss PL, et al. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13(1):22–8. doi: 10.1016/0264-410x(95)80006-y. [DOI] [PubMed] [Google Scholar]