Abstract
Background
The NIDA Collaborative Cocaine Treatment Study yielded different efficacies for different psychosocial treatments for cocaine dependence. However, substantial heterogeneity of patient outcomes was evident. Longitudinal data analysis techniques can be helpful in examining differential effects of psychosocial interventions on specific subpopulations of patients.
Methods
Overall drug and cocaine use of 346 patients diagnosed with DSM-IV cocaine dependence and treated with one of four psychosocial interventions were assessed monthly during 6-month treatment. Growth mixture models were used to identify patient subgroups based on typical patterns of change in substance use during treatment and to evaluate differential treatment effects within these subgroups.
Results
Three patient subgroups following different change patterns in cocaine and overall drug use were identified irrespective of the treatment type: (a) those with moderate baseline severity of drug use and very rapid reduction of drug use during treatment, (b) those with moderate baseline severity of drug use and moderate reduction of drug use during treatment, and (c) those with severe levels of baseline drug use with moderate reduction of drug use during treatment. Patient baseline characteristics enabled discrimination between these subgroups. Individual drug counseling was most efficacious among those patients with moderate baseline severity and moderate treatment response. There were no differential treatment effects in the two other patient subgroups.
Conclusions
The population of treatment-seeking cocaine dependent individuals is heterogeneous. Research on patient subgroups with different change patterns revealed its potential to enable classifications of patients that indicate which treatment is most effective for which type of patient.
Keywords: Cocaine Dependence, Psychosocial Treatment, Differential Treatment Effects, Patterns of Change, Growth Mixture Modeling
1. Introduction
Psychosocial interventions form the basis of the treatment of cocaine dependence (Carroll, 2005; Vocci and Montoya, 2009). However, there is an ongoing debate about the optimal type of psychosocial treatment for cocaine dependence and whether different treatment approaches might be best suited for different subgroups of cocaine dependent patients (Carroll et al., 1994a; Maude-Griffin et al., 1998).
So far two main lines of research have been followed in cocaine treatment research: On the one hand, there has been research that primarily focused on outcomes of treatments to establish their efficacy. To compare the efficacy of different treatment approaches, this kind of research usually compared samples of patients treated with different interventions in terms of their mean outcomes. Findings from these studies have been variable, with some suggesting that specialized professional psychosocial treatments are superior to drug counseling approaches (Higgins et al., 1993; Maude-Griffin et al., 1998), other studies indicating no differences between different treatment approaches (Carroll et al., 1998), whereas in the largest study done to date, in the National Institute on Drug Abuse (NIDA) Collaborative Cocaine Treatment Study (CCTS), a combination of individual drug counseling and group drug counseling produced statistically and clinically superior outcomes compared with two types of professional psychotherapy in terms of reducing cocaine use as well as overall drug use (Crits-Christoph et al., 1999).
However, the mean effects of various treatments for a specific disorder in samples of patients usually used to evaluate treatments in randomized controlled trials may mask differential treatment effects on individuals or subgroups within a sample (Cuijpers et al., 2005). Psychotherapy research, e.g., has shown that comparisons of aggregated pre-to-post treatment change between patient samples, relying on the assumption of linear and steady change over the course of treatment, may mask potentially meaningful differences in individual treatment courses (Krause et al., 1998) or different patterns of change that are shared by many individual patients (Lutz et al., in press; Stulz and Lutz, 2007; Stulz et al., 2007), and that this interindividual variation in treatment outcomes may be clinically important (Barkham et al., 1993).
By shifting the focus from mean outcome differences between patient samples to predictors of treatment outcomes, a second line of research was giving more attention to individual differences in treatment outcomes of cocaine dependent patients (e.g., Crits-Christoph et al., 2003; Crits-Christoph et al., 2007; Siqueland et al., 2004; Siqueland et al., 1998). For example, by reanalyzing the data of the NIDA CCTS, Crits-Christoph et al. (2007) identified 4 baseline characteristics (craving, acuity of biomedical problems, belief in the 12-step philosophy, and expectations for improvement) that predicted interindividual differences in sustained abstinence from drug use irrespective of the type of treatment. Also, research on mediators and moderators of treatment outcome increasingly shows that some subpopulations of psychotherapy patients do benefit less than others do from psychosocial treatments (Shadish and Sweeney, 1991).
Overall, these research findings underscore the significance of heterogeneity among psychotherapy patients in general and among cocaine users in particular, and they point to the possible need to develop and use specialized treatments for clinically distinct subgroups of cocaine abusers (Carroll et al., 1994a). Although the two lines of research discussed just before (the one focusing on comparisons of mean outcomes of different treatments and the other focusing on predictors of individual differences in outcomes) usually also looked for predictor × treatment × outcome-interactions, modern techniques for longitudinal data analysis provides an alternative that brings together these two research traditions via the identification of patient subgroups with typical patterns of change during treatment. These Growth Mixture Models (GMMs) permit the identification of distinct groups of individuals who differ in the initial level and the course of a specific behavior (e.g., drug use) through the empirical identification of developmental trajectories (Muthén, 2001). Furthermore, these GMMs also allow examination of whether the effects of different interventions differ for these subpopulations, ascertain which characteristics predict membership to these subpopulations, and establish whether outcomes are different for each of these subpopulations (Muthén, 2001; Muthén et al., 2002). In contrast to cluster analytic approaches, which have also been used to identify typical growth trajectories in outpatient psychotherapy (Barkham et al., 1993) and in substance abuse treatment (Morral et al., 1997; Waldron et al., 2005), GMMs allow simultaneous estimation of subgroup-specific treatment effects, which makes them a promising approach to examine differential treatment effects in patient subgroups. Concerning the examination of moderators of treatment outcomes, there is yet another advantage of GMMs: a fundamental problem inherent in traditional research studies on moderators of treatment outcomes is that these studies are looking for subpopulations who benefit more or benefit less from an intervention, but actually examine only characteristics which may be indicative of these subpopulations (Cuijpers et al., 2005). By contrast, if using the GMM approach, the identification of patient subpopulations within a sample is based on the target behavior itself (e.g., on changes in drug use over time).
These characteristics make GMMs specifically appropriate to examine the following research questions in the NIDA CCTS dataset that go beyond previous reports of average treatment outcomes and predictors of average outcomes: (1) Are there different trajectory classes (i.e., patient subgroups following different patterns of change) of drug and cocaine use during psychosocial treatments for cocaine dependence?; (2) Are there patient baseline characteristics that allow allocation of patients to these trajectory classes?; and (3) Do different psychosocial treatments have differential effects on drug and cocaine use in different trajectory classes (i.e., in different patient subgroups)?
2. Methods
2.1 Design and Procedures
The design and procedures of the NIDA CCTS have already been detailed elsewhere (Crits-Christoph et al., 1997; Crits-Christoph et al., 1999). In brief, the NIDA CCTS was a multi-site randomized clinical trial that compared the efficacy of four psychosocial treatments for cocaine dependence: In two of these treatments, professional psychotherapy, either cognitive therapy (CT; Beck et al., 1993) or supportive-expressive psychodynamic therapy (SE; Luborsky, 1984; Mark and Luborsky, 1992), was added to group drug counseling (GDC; Mercer et al., 1994). A third treatment combined individual drug counseling (IDC; Mercer and Woody, 1992) with GDC, and the fourth consisted of GDC alone. All treatments were planned to include 6 months of active phase treatment and a 3-month booster phase. Individual treatment sessions were held twice per week during the first 12 weeks, weekly during weeks 13 to 24, and monthly during the booster phase. Group drug counseling sessions were held weekly during the active phase treatment and patients in the GDC alone condition met with the group counselor individually once per month during the booster phase.
2.2 Patients
A total of N = 487 outpatients, all of them having a principal diagnosis of cocaine dependence according to the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) and all of them using cocaine during the past 30 days, were randomly assigned to one of the four treatment conditions. Exclusion criteria (e.g., current psychotropic medication) are reported in detail elsewhere (Crits-Christoph et al., 1999). Of these 487 patients who actually began treatment, only those 346 patients who had at least five monthly assessments during the 6-month active phase treatment were included in the current analyses. This is the minimum number of measurement points needed to estimate cubic latent growth curves (see below). By type of treatment, the number of patients excluded was 35 (24.8%) in IDC+GDC, 29 (20.6%) in CT+GDC, 42 (29.8%) in SE+GDC, and 35 (24.8%) in GDC alone (χ2(3, N = 487) = 2.688, n.s.). Of the 346 patients in the final sample, 86 (24.9%) were treated with IDC+GDC, 90 (26.0%) with CT+GDC, 82 (23.7%) with SE+GDC, and 88 (25.4%) with GDC alone. The average number of individual treatment sessions differed significantly between treatment conditions (F(2,255) = 4.12, p < .05), with patients in IDC+GDC attending significantly less sessions (M = 14.72, SD = 10.67) than patients in SE+GDC (M = 18.98, SD = 10.49) but not less than patients in CT+GDC (M = 18.31, SD = 10.09). The mean number of group treatment sessions attended was 10.74 (7.24) in IDC+GDC, 11.42 (7.14) in CT+GDC, 10.37 (6.86) in SE+GDC, and 10.60 (7.30) in GDC alone (F(3,342) = 0.35, n.s.).
The modal patient in our final sample was male (76%), Caucasian (55%; African-American: 43%; other: 2%), lived alone (72%), had children (59%), was employed outside the home (60%), and smocked crack cocaine (82%). The mean age at the beginning of treatment was 34.6 years (SD = 6.3) and the mean duration of cocaine use at baseline was 6.9 years (SD = 4.8). Except for a somewhat higher age (34.6 vs. 32.2 years, p <001) and a marginally lower ASI-Drug Use Composite Score at baseline (0.23 vs. 0.25, p < .05; see below), the patients included in our final sample did not differ significantly from those excluded in all of these characteristics as well as in the number of days using cocaine during the past month. Details on therapies and therapists can be found in previous publications on the NIDA CCTS (Crits-Christoph et al., 1997; Crits-Christoph et al., 1999).
2.3 Instruments and Data Collection
Patients were assessed on a battery of instruments at baseline, monthly during the 6-month active phase treatment, and at quarterly follow-up assessments conducted at month 9, 12, 15, and 18 after randomization. Those instruments analyzed in the our study will be presented.
2.3.1 Addiction Severity Index (ASI; McLellan et al., 1992)
As in the original trial (Crits-Christoph et al., 1999), the Drug Use Composite Score of the interview-based ASI (which assesses the use of alcohol, heroine, opiates, barbiturates, sedatives, cocaine, amphetamines, cannabis, hallucinogens, and inhalants) was the primary outcome measure of this study. Additionally, outcomes on one specific item from the ASI drug use scale, the number of days using cocaine in the past 30 days, were also examined.
To determine measurable change in the ASI–Drug Use Composite Score until treatment termination, we used the reliable change (RC) criterion (Jacobson and Truax, 1991). The RC criterion of an instrument depends on its reliability and equals the minimal amount of change in the score between two repeated assessments that is unlikely (p < .05) to occur if no actual change beyond measurement error has happened. In the present data, the RC criterion for the ASI–Drug Use Composite Score was 0.09 (using rxx = .93 and SD = 0.12; Zanis et al., 1994).
2.3.2 Recovery Attitude And Treatment Evaluator–Clinical Evaluator (RAATE–CE; Mee-Lee, 1988)
The RAATE–CE is a structured interview to measure readiness for substance abuse treatment and aspects of the recovery environment in five subscales: Resistance to Treatment, Resistance to Continuing Care, Acuity of Biomedical Problems, Psychological Acuity, and Environmental/Social Problems. We used the RAATE-CE baseline assessments (ranging from 1 to 4) to predict patterns of change in the ASI scores during active phase treatment.
2.3.3 Addiction Recovery Scale (ARS; Crits-Christoph et al., 2003; Mercer et al., 1993)
This 40-item measure is based on the 12-step approach to recovery. The total score of the ARS ranges from 40 to 200 (each item is rated on a 1–5 scale) and a higher ARS total score indicates greater endorsement of the philosophy and behaviors advocated in 12-step programs. The baseline ARS assessments were also examined as predictors of change patterns in the ASI scores.
2.4 Data Analysis
In a first step of analysis, we identified typical patterns of change in overall drug use and in cocaine use during the 6-month active phase treatment. Patient subgroups with similar growth trajectories in the ASI–Drug Use Composite Score and in the number of days using cocaine during the past 30 days were identified irrespective of the type of treatment using the GMM approach (see below). After evaluating the pre-to-post active phase treatment outcomes in these patient subgroups in terms of effects sizes (Cohen, 1988) and the number of reliably changed patients in the ASI–Drug Use Composite Score and in terms of change in the number of days using cocaine during the past month, we also compared the follow-up assessments in these two outcome dimensions between the patient subgroups by means of analyses of variance (ANOVAs). These analyses of the pre-to-post active phase treatment changes and of the follow-up assessments were performed for those patients with available assessments at the respective measurement points. Subsequently, we explored whether there are associations between the type of treatment and typical patterns of change during the active phase treatment using a χ2-test. To evaluate whether there are other patient baseline characteristics that allow prediction of typical change patterns during active phase treatment, we furthermore examined a series of potential predictors. To do so, the patient baseline characteristics under consideration were implemented into the GMM and they were then evaluated using multinomial logistic regressions. Finally, to evaluate whether there are differences in active phase treatment outcomes within the patient subgroups depending on the type of treatment provided, two types of analysis were performed: On the one hand, we again used the criteria to evaluate pre-to-post active phase treatment change that have been mentioned just before (effects sizes etc) and we compared them between treatments within the patient subgroups. On the other hand, we regressed the most meaningful latent growth parameters (intercept and slope) of the main outcome variable, the ASI–Drug Use Composite Score, on the type of treatment. This was done within each patient subgroup identified with the GMM and it allowed us to determine for each of these subgroups whether the type of treatment was associated with differences in development of overall drug use over time (see below and Muthén et al., 2002).
2.4.1 Growth Mixture Models (GMMs)
Growth Mixture Models allow identification of unobserved groups of individuals with similar growth trajectories over time in one or more outcome variables (Muthén, 2001). The GMM approach is based on conventional latent growth models (LGMs), which analyze longitudinal data by relating an observed outcome variable (e.g., drug use) to time (e.g., months in treatment). In LGMs, individual variation in growth is captured by the fact that growth coefficients (continuous latent variables in a structural equation modeling framework) are random and are therefore allowed to vary across individuals. However, LGM approaches (like, e.g., random coefficient regression models) implicitly assume that all individuals in the sample are drawn from one single population and, hence, that one single growth trajectory can adequately approximate change of the entire population. Thus, LGMs estimate one common mean growth curve and the individual variation around this mean growth curve is captured by individual variation in the continuous latent growth factors. Following the single population assumption, LGMs furthermore also assume that covariates that affect the growth factors influence each individual in the same way.
By implementing a categorical latent class variable into the growth modeling framework, the GMM approach relaxes this single population assumption of LGMs and allows identification of latent classes (subpopulations of individuals) that correspond to different shapes of growth curves. That way, GMMs provide the identification of unobserved subpopulations of individuals that vary around qualitatively different mean growth curves which are shared within homogenous latent classes. Growth mixture models then both estimate the mean growth curve for each latent class and capture individual variation around these growth curves by estimating the growth factor variances for each class (Muthén, 2001). Moreover, GMMs then also allow examination of predictors of these latent classes (through the use of multinomial logistic regressions) and examination of class-specific predictors of individual differences in growth over time (by regressing the growth parameters on potential predictor variables within latent classes) (Muthén et al., 2002).
In this study, GMMs were estimated using the Mplus software (Version 3.11; Muthén and Muthén, 2004). Mplus enables estimation of models with missing values in continuous outcomes using maximum likelihood estimation and an accelerated expectation maximization procedure.
3. Results
As indicated by the lowest value in the Bayesian Information Criterion (BIC; Schwartz, 1978)—a model fit index that balances goodness of fit and parsimony of (mixture) models (Nagin, 1999)—a cubic latent growth model best fitted change data in the ASI–Drug Use Composite Score (intercept-only: 18121.73, linear: 17902.57, loglinear: 17749.67, quadratic: 16927.79, cubic: 15940.14) as well as in the outcome variable counting the number of days with cocaine use during the past 30 days (intercept-only: 25039.91, linear: 23111.49, loglinear: 22384.35, quadratic: 20760.64, cubic: 19042.13). Following these preliminary analyses, the growth models reported below rely on the assumption of a cubic relationship between the amount of treatment (months) and treatment progress (reduction of drug use).
3.1 Patterns of Change and Their Outcomes
In a first step of the analyses, we used unconditional GMMs to identify distinct patient subgroups based on similar development over time in the two outcome dimensions of drug use (ASI–Drug Use Composite Score and number of days using cocaine during the past 30 days). To determine the optimal number of distinct patterns of change, we started with one latent class (i.e., with a conventional LGM), and we then incrementally entered additional latent classes into the GMM until the optimal number of latent classes was found (Muthén, 2001).1 The fit criteria to determine the optimal number of latent classes (patterns of change) in a GMM suggested three latent classes to be most accurate solution: the BIC steadily decreased from one- through three-class solutions (34982.27, 29087.02, 27616.34) and the Vuong-Lo-Mendell-Rubin Likelihood Ratio Test (Lo et al., 2001), which checks whether the implementation of an additional latent class results in a significant improvement of the BIC, also favored a three class solution (1 class vs. 2 classes: p < .001; 2 classes vs. 3 classes: p < .01; 3 classes vs. 4 classes: p = .13). As the entropy—a summary measure of classification accuracy of individuals into the latent classes (Ramaswamy et al., 1993)—was nearly as good for a three-class solution (0.96) as for a two-class solution (0.98), the solution with three latent classes was used for further analyses.
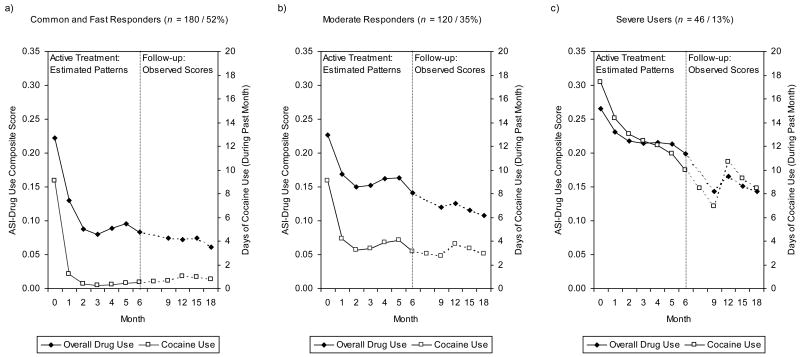
As shown in Figure 1a, a first patient subgroup (n = 180, 52% of the study sample's patients) is characterized by moderate drug use at baseline with very rapid reduction of overall drug use and cocaine use already in an early stage of treatment. The cocaine dependent patients in this largest subgroup were labeled “common and fast responders” since more than half of the cocaine dependent individuals in our study belong to this subgroup. These patients clearly improved during the active phase treatment in the ASI–Drug Use Composite Score (change from baseline to month 6, within-group effect size d = 1.99) and more than two-third of them (69%) were reliably improved on this outcome dimension by the end of the active phase treatment (Table 1). Furthermore, these “common and fast responders” also showed a significant reduction in the number of days using cocaine during the past 30 days (M = -8.75 days, see Table 1). Note that in all patient subgroups there were a few patients who did not have ASI assessments after month 6 (n = 24). The results in Table 1 refer to those patients with completed ASI assessments at treatment termination (month 6).
Figure 1.
Typical Patterns of Change in Overall Drug Use and in Cocaine Use in the Three Patient Subgroups: (a) Common and Fast Responders, (b) Moderate Responders, and (c) Severe Users. The Left Half of Each of the Figures Shows the Estimated Mean Latent Growth Trajectories of the Monthly Assessments During the 6-Month Active Phase Treatment, the Right Half of Each of the Figures Shows the Observed Outcomes at the Follow-Up Assessments 9, 12, 15 and 18 Months After the Randomization. The y-Axes on the Left Refer to Overall Drug Use (ASI–Drug Use Composite Scores), Those on the Right to Cocaine Use (Number of Days Using Cocaine During the Past Month).
Note: Overall drug use was measured by the Addiction Severity Index (ASI)–Drug Use Composite Score, cocaine use by the ASI item counting the number of days using cocaine during the past 30 days.
Table 1.
Pre-to-Post Changes During the 6-Month Active Phase Treatment: Comparisons between Patient Subgroups.
| Outcome Variable / Change Criterion | Patient Subgroup | Test (significant comparisons) |
||
|---|---|---|---|---|
| 1. Common and Fast Responders (n = 169) |
2. Moderate Responders (n = 111) |
3. Severe Users (n = 42) |
||
| ASI–Drug Use Composite Score | ||||
| d | 1.99 | 1.19 | 0.92 |
p < .001a (1.–2.) (1.–3.) |
| No. Reliably Improved (%) | 117 (69 %) | 46 (41 %) | 18 (43 %) | p < .001b |
| Number of Days Using Cocaine During the Past 30 Days | ||||
| Reduction in the Number of Daysc | 8.75 (7.71)*** | 6.05 (7.15)*** | 6.60 (9.51)*** |
p < .05a (1.–2.) |
Note: ASI = Addiction Severity Index; d = pre-to-post effect size (Cohen's d).
Analysis of variance with post-hoc comparisons.
χ2-test.
t-test to compare pre-to-post active phase treatment change within patient subgroups.
p < .001.
Compared to the “common and fast responders”, a second subset of patients (n =120, 35%) was characterized by very similar overall drug use and cocaine use at baseline, but these “moderate responders” then experienced a clearly less rapid improvement in both outcome dimensions (Figure 1b). Thus, by the end of active phase treatment they were significantly less improved than the “common and fast responders” (Table 1).
The third and last patient subgroup (n = 46, 13%) identified in our study includes the “severe users.” These patients were characterized by high overall drug use and cocaine use at baseline with only moderate improvement in both of these outcome dimensions during the active phase treatment (Figure 1c). The magnitude of reduction in drug use in this subset of patients resembled the one in the group of the “moderate responders” (Table 1).
These patterns of change during active phase treatment were shown to be not differential across attrition patterns. Rerunning the GMM for the subsample of those n = 225 patients with no missing values in the two outcome variables yielded very similar change patterns as the ones found in the full study sample.
Figures 1a-c furthermore also show the results of the follow-up assessments of overall drug use and of cocaine use 9, 12, 15, and 18 months after randomization for the three patient subgroups. As can bee seen there, the drug use of the “common and fast responders” remained quite constant over the follow-up period, i.e., they retained their gains in both outcome dimensions during this period. Compared to the two other subgroups, the “common and fast responders” used significantly less drugs and significantly less cocaine in each of the follow-up assessments (Table 2). There were no significant differences between the “moderate responders” and the “severe users” in terms of overall drug use during the follow-up period. However, the “severe users” reported significantly more cocaine use in each of the follow-up assessments (Table 2). Note that these findings on the follow-up assessments again refer to those patients with completed assessments at the respective measurement occasions.
Table 2.
Follow-up Assessments: Comparisons between Patient Subgroups.
| Follow-up Assessment | Outcome Measure | Patient Subgroup | ANOVA F(total) (significant comparisons) |
||
|---|---|---|---|---|---|
| 1. Common and Fast Responders M (SD) (n) |
2. Moderate Responders M (SD) (n) |
3. Severe Users M (SD) (n) |
|||
| 9 month | ASI–Drug Use | 0.074 (0.078) (162) |
0.120 (0.087) (107) |
0.143 (0.084) (42) |
17.460*** (1–2) (1–3) |
| ASI–Cocaine Use | 0.613 (1.362) (163) |
2.704 (4.183) (108) |
6.930 (7.673) (43) |
46.851*** (1–2) (1–3) (2–3) |
|
| 12 month | ASI–Drug Use | 0.073 (0.080) (156) |
0.126 (0.091) (101) |
0.166 (0.101) (40) |
23.607*** (1–2) (1–3) |
| ASI–Cocaine Use | 1.038 (3.120) (159) |
3.748 (5.629) (107) |
10.690 (10.556) (42) |
49.995*** (1–2) (1–3) (2–3) |
|
| 15 month | ASI–Drug Use | 0.075 (0.083) (146) |
0.115 (0.087) (103) |
0.152 (0.098) (38) |
14.643*** (1–2) (1–3) |
| ASI–Cocaine Use | 0.953 (2.798) (150) |
3.390 (5.779) (105) |
9.256 (9.810) (39) |
38.041*** (1–2) (1–3) (2–3) |
|
| 18 month | ASI–Drug Use | 0.062 (0.074) (139) |
0.108 (0.086) (103) |
0.143 (0.099) (41) |
19.138*** (1–2) (1–3) |
| ASI–Cocaine Use | 0.757 (1.776) (140) |
2.951 (3.989) (103) |
8.439 (8.970) (41) |
50.210*** (1–2) (1–3) (2–3) |
|
Note: ASI–Drug Use = Drug Use Composite Score of the Addiction Severity Index (ASI); ASI–Cocaine Use = number of days using cocaine during the past 30 days; ANOVA = analysis of variance.
p < .001.
3.2 Prediction of Patterns of Change
Besides the identification of typical patterns of change by means of unconditional GMMs, we also examined whether the type of treatment and a series of 24 patient baseline characteristics that have already been examined as predictors of sustained abstinence from cocaine in the study of Crits-Christoph et al. (2007) enable prediction of the three typical patterns of change in overall drug use and cocaine use. In addition, current posttraumatic stress disorder and lifetime traumatic events were also examined as predictors of the change patterns (Najavits et al., 2007; Najavits et al., 2003).
As can be derived from Table 3 showing for each of the patient subgroups the number of patients in each of the four psychosocial interventions, treatment modalities were about equally distributed across the three patient subgroups indicating that there was no significant association between the type of treatment and the patterns of change of drug use during active phase treatment (χ2(6, N = 346) = 6.52, n.s.). To determine whether a given patient baseline characteristic enables prediction of the change patterns, preliminary analyses with univariate prediction models for each patient baseline characteristic were run (Stoolmiller et al., 2005). The significant predictors from these univariate analyses (results are not shown here because of space limitations) were then entered into a multivariate prediction model to identify a compact set of predictors for client allocation to the latent classes (patterns of change). In this extended GMM, the predictors of latent class membership probabilities were examined via multinomial logistic regressions. To do so, the “common and fast responders” were designated as the reference subgroup, and each patient baseline characteristic was tested to see whether it discriminated between a given subgroup and the “common and fast responders.” As shown in the upper part of Table 4, the “Environmental/Social Problems” subscale of the RAATE–CE and the ARS total score significantly discriminated between the “moderate responders” and the “common and fast responders”; for example, the significant logistic regression coefficient of 0.63 of the “Environmental/Social Problems” subscale represents the increase in the log odds of being in the “moderate responders” subgroup vs. being in the reference class for a unit increase on the “Environmental/Social Problems” subscale. That is, with each additional point on the “Environmental/Social Problems” subscale at baseline, the probability of a patient belonging to the “moderate responders” subgroup, as compared to the probability of being a member of the “common and fast responders” subgroup, increased by the factor 1.88 (e0.63). Higher scores on the “Environmental/Social Problems” subscale also increased the probability of a patient belonging to the “severe users” (as opposed to the probability belonging to the “common and fast responders”), whereas higher scores on the “Resistance to Treatment” subscale of the RAATE– CE and in the ARS both significantly decreased the probability of belonging to the subgroup of “severe users” (Table 4).
Table 3.
Pre-to-Post Changes During the 6-Month Active Phase Treatment: Comparison between Types of Treatment within Patient Subgroups.
| Subgroup / Outcome Variable / Change Criterion | Type of Treatment | Test | |||
|---|---|---|---|---|---|
| 1. IDC | 2. CT | 3. SE | 4. GDC | ||
| Common and Fast Responders | |||||
| n (%) | 50 (30%) | 41 (24%) | 34 (20%) | 44 (26%) | |
| ASI–Drug Use Composite Score | |||||
| d | 2.34 | 1.86 | 1.89 | 1.80 | n.s.a |
| No. Reliably Improved (%) | 39 (78%) | 25 (61%) | 22 (65%) | 31 (71%) | n.s.b |
| Number of Days Using Cocaine During the Last 30 Days | |||||
| Reduction in the Number of Days | 9.08 (7.93)*** | 8.00 (7.83)*** | 8.97 (7.62)*** | 8.91 (7.65)*** | n.s.a |
| Moderate Responders | |||||
| n (%) | 23 (21%) | 34 (30%) | 30 (27%) | 24 (22%) | |
| ASI–Drug Use Composite Score | |||||
| d | 1.42 | 1.30 | 1.33 | 0.69 | n.s.a |
| No. Reliably Improved (%) | 13 (57%) | 14 (41%) | 13 (43%) | 6 (25%) | n.s.b |
| Number of Days Using Cocaine During the Last 30 Days | |||||
| Reduction in the Number of Days | 8.30 (8.63)*** | 6.15 (7.24)*** | 5.97 (6.59)*** | 3.88 (5.75)*** | n.s.a |
| Severe Users | |||||
| n (%) | 8 (19%) | 10 (24%) | 12 (29%) | 12 (28%) | |
| ASI–Drug Use Composite Score | |||||
| d | 0.69 | 1.19 | 0.63 | 1.33 | n.s.a |
| No. Reliably Improved (%) | 2 (25%) | 5 (50%) | 4 (33%) | 7 (58%) | n.s.b |
| Number of Days Using Cocaine During the Last 30 Days | |||||
| Reduction in the Number of Days | 3.38 (10.18) | 9.70 (8.84) *** | 1.83 (7.60) | 10.92 (9.39) *** | n.s.a |
Note: IDC = individual drug counseling, CT = cognitive therapy, SE = supportive-expressive therapy, GDC = group drug counseling, ASI = Addiction Severity Index, d = effect size (Cohen's d).
Analysis of variance with post-hoc comparisons.
χ2-Test.
t-test to compare pre-to-post active phase treatment change depending on type of treatment within patient subgroups.
p < .001.
Table 4.
Prediction of Patient Subgroups (Patterns of Change) by Patient Baseline Characteristics (Upper Part), and Prediction of Individual Growth in the ASI–Drug Use Composite Score by Type of Treatment Within Patient Subgroups (Lower Part).
| Patient Subgroup | ||||||
|---|---|---|---|---|---|---|
| Common and Fast Responders (n = 180) |
Moderate Responders (n = 120) |
Severe Users (n = 46) |
||||
| Effect | t Statistic | Effect | t Statistic | Effect | t Statistic | |
| Prediction to Patient Subgroup (Pattern of Change) | ||||||
| Resistance to Treatment | —a | —a | 0.277 | 0.635 | -1.662 | -2.595* |
| Environmental/Social a Problems | —a | —a | 0.632 | 2.354* | 1.184 | 3.334* |
| Addiction Recovery Scale | —a | —a | -0.023 | -2.581* | -0.038 | -3.464* |
| Prediction to Intercept (Initial Status)b | ||||||
| IDC | -0.005 | -0.421 | 0.012 | 0.623 | 0.019 | 0.775 |
| CT | -0.001 | -0.105 | -0.003 | -0.198 | -0.008 | -0.513 |
| SE | 0.003 | 0.277 | -0.011 | -0.657 | -0.039 | -2.340* |
| Prediction to Slope (Change)b | ||||||
| IDC | -0.003 | -1.214 | -0.009 | -2.262* | 0.001 | 0.201 |
| CT | -0.001 | -0.165 | -0.004 | -1.225 | -0.002 | -0.257 |
| SE | -0.002 | -0.814 | -0.001 | -0.222 | 0.007 | 1.510 |
Note: IDC = individual drug counseling, CT = cognitive therapy, SE = supportive-expressive therapy.
The “common and fast responders” subgroup was used as reference class for multinomial logistic regressions.
The type of treatment was dummy-coded for these regression analyses with group drug counseling alone being the reference class.
p < .05.
Finally, a supplementary multinomial logistic regression of the latent classes on treatment completion status (completers vs. dropouts) also revealed a significant association between membership in the “severe users” subpopulation and a higher chance of early treatment termination (estimate = -1.96, S.E. = -0.51, p < .001).
3.3 Differential Treatment Effects Within Patient Subgroups: Treatment Progress and Outcomes by Type of Treatment
In a final step of analysis, we again extended our GMM paralleling the approach of Muthen et al. (2002) to evaluate for each of the three patient subgroups whether treatment progress and outcomes differed depending on the type of treatment. In this final GMM, we regressed the intercept and of the slope of the ASI-Drug Use Composite Score dimension on the type of treatment (which was dummy-coded for these analyses) within each of the three patient subgroups, which allowed us to assess the treatment assignment impact on changes in the course within classes (i.e., patient subgroups). When doing these regression analyses, we focused on the ASI–Drug Use Composite Score as this was the primary outcome measure in our study (calculating a GMM to predict change in the number of days using cocaine during the past 30 days would have been computationally very demanding as this count outcome variable requires a numerical integration algorithm for parameter estimation). Predictions of the intercept of the ASI–Drug Use Composite Score then allowed us to estimate whether there had been differences in overall drug use depending on the type of treatment already at intake, whereas the linear slope is the growth parameter best representing change over time. The lower part of Table 4 shows two significant regression coefficients: being treated with SE was associated with lower ASI– Drug Use Composite Scores at intake in the “severe users” subgroup and being treated with IDC predicted a more rapid reduction of the ASI–Drug Use Composite Score during active phase treatment among the “moderate responders.”
Table 3 shows the pre-to-post treatment changes in the ASI–Drug Use Composite Score and in the number of days using cocaine depending on the type of treatment for those patients with a post-assessment after 6 month of active phase treatment. Although not reaching statistical significance, treatment outcomes of IDC again tended to be better than outcomes of the other treatments under consideration in the subgroup of “moderate responders” (Table 3). Corresponding to the findings of the regression analyses, in the other two patient subgroups there were also no statistically significant differences in pre-to-post changes depending on the type of treatment (Table 3).
Finally, in all three patient subgroups there were also no significant differences between the four treatment modalities in the follow-up assessments. However, the “moderate responders” in IDC tended use less drugs compared to the other treatments under consideration also in a longer term perspective (results of these analyses are not shown because of space limitations).
4. Discussion
Whereas the efficacy of psychosocial interventions for the treatment of cocaine dependence has been demonstrated (Carroll, 2005; Dutra et al., 2008; Woody, 2003), there is an ongoing debate whether different psychosocial interventions are equally effective or some are superior to others (Carroll et al., 1998; Crits-Christoph et al., 1999; Higgins et al., 1993; Maude-Griffin et al., 1998). In this study, we examined differential effects of the four psychosocial interventions for cocaine dependence evaluated in the NIDA CCTS (IDC, CT, SE, and GDC) in subgroups of cocaine dependent individuals. Using GMMs, we identified three distinct patient subgroups on the basis of similar initial impairment and change during treatment in the target outcome behaviors of treatments for cocaine dependence–in cocaine use and in overall drug use.
There was a first patient subgroup including more than half (52%) of the patients in our study. Compared to the two other patient subgroups identified in the study, these “common and fast responders” entered treatment with moderate cocaine use and with moderate overall drug use, and they then experienced a very rapid reduction of cocaine and of overall drug use already in the first month of treatment irrespective of the type of psychosocial treatment. These “common and fast responders” also retained their benefits over a one-year period after treatment termination, and there were again no differential treatment effects over this follow-up period indicating that there were no delayed differential effects of professional psychotherapies (CT and SE) as they have been reported in previous studies (Carroll et al., 1994b). These findings suggest that the type of psychosocial treatment does not matter for a large subgroup of patients with cocaine dependence; the common cocaine dependent individual seems to benefit clearly and to the same extent from each of the four psychosocial interventions. Interestingly, this seems to apply even for a minimal intervention such as GDC. If this finding would be replicated in future studies, then other criteria than efficacy (e.g., different costs of treatments) may gain importance when deciding which psychosocial treatment to provide to these patients.
However, when comparing the outcomes of IDC, CT, SE, and GDC alone in terms of sample means, Crits-Christoph et al. (1999) found IDC to be superior to the other three psychosocial interventions in reducing cocaine use as well as overall drug use. According to our findings, this superiority of IDC seems to be mainly attributable to better outcomes of IDC in a subgroup of “moderate responders.” This patient subgroup included about one third (35%) of the patients and was characterized by very similar initial cocaine use and overall drug use as the subgroup of the “common and fast responders”, but the moderate responders then, on average, experienced a clearly less rapid reduction of overall drug and of cocaine use during treatment. Predictor analyses to identify patient characteristics that are indicative for these “moderate responders” revealed that, compared to the more rapid responding “common and fast responders”, the “moderate responders” had more social and environmental problems and reported lower endorsement of the philosophy and behaviors advocated in the 12-step programs at the beginning of treatment. If replicated, these findings suggest that IDC might be a specifically appropriate treatment for moderately severe patients with a psychosocial environment detrimental to recovery (low support of family, friends, or others in the patient's home setting) and with low endorsement of the 12-step philosophy who are prone to only moderate improvement during treatment. (Note that even though these “moderate responders” were characterized by low baseline endorsement of the 12-step philosophy, they seem to benefit most of IDC which relies on the specific stages, tasks and goals based on this philosophy).
Whereas these “moderate responders” cannot be discriminated from the “common and fast responders” in terms of the magnitude of drug use when entering the treatment, there was a third subgroup of patients (about 13% of the patients in our study) who were characterized by high overall drug use and cocaine use. Like the “moderate responders”, these “severe users” also reported more environmental and social problems and lower endorsement of the 12-step philosophy at baseline compared to the “common and fast responders.” Additionally, they also reported less resistance to treatment. Taking into account that the respective subscale of the RAATE-CE includes items asking whether a patient is aware of and accepts having an addiction problem and whether he or she is open to treatment, the severe users seem no longer to be able to deny their addiction problems and their need for treatment as a consequence of their severe drug use. During treatment, they then reduced their cocaine and overall drug use moderately, and it seems that the type of psychosocial intervention does again not matter among these severe users. The magnitude of the reduction of overall drug use and of cocaine use during active phase treatment resembled the one among the moderate responders with moderate improvement. However, the “severe users” then experienced a continued reduction of substance use during the booster phase (i.e., up to the assessment at month 9 after baseline; see Figure 1), though particularly their cocaine use then again increased during the subsequent 3 months. Whereas their overall drug use approached the level of the moderate responders during the year following active phase treatment, they consistently reported higher cocaine use during this phase. These findings suggest that longer treatments might be necessary among severe cocaine users. Taking into account the relatively high drop out rate in this subgroup, clinicians should also be aware to take further measures to aid in the patients retention in this subgroup in order to enhance treatment outcomes among the severe users.
In this study, we demonstrated that several subpopulations can be distinguished among cocaine dependent individuals in terms of initial cocaine and overall drug use and their change during psychosocial interventions. This finding of different treatment courses is clinically appealing and consistent with previous findings from psychotherapy research (Barkham et al., 1993; Lutz et al., in press; Stulz and Lutz, 2007; Stulz et al., 2007). However, our patterns of change differed somewhat from those found by Morral et al. (1997) in another substance use sample. Possible explanations for these differences might be that the 4 response classes identified by Morral et al. (“improving”, “stable-good”, “stable-poor”, and “deteriorating”) were found in a diagnostically different sample (opioid-dependent methadone maintenance patients), based on a different outcome measure (monthly assessed urine specimens) and extracted with a different method (cluster analysis).
Furthermore, although psychotherapy research often failed to find clear outcome differences between different treatment schools (Luborsky et al., 2002; Luborsky et al., 1976; Wampold, 2001), our findings suggest that this is not necessarily true for cocaine dependent patients when using methods that sufficiently account for the heterogeneity among patients. In fact, our findings suggest that different psychosocial treatments for cocaine dependence may have a different impact on the course of substance use in different subgroups of patients. The superiority of IDC to other treatments found in previous analyses of the CCTS data (Crits-Christoph et al., 1999) seems to apply for moderate responders with high social problems and low initial endorsement of 12-step treatment philosophy but not for other patients. In line with findings from previous research (Cuijpers et al., 2005), our results therefore suggest that treatment research should give sufficient consideration to the heterogeneity within samples of patients to provide a thorough evaluation of the efficacy of different interventions.
Finally, our research basing on typical patterns of change in drug use during treatment can also strengthen the research on moderators of treatment effects. If patient baseline characteristics that enable allocation of patients to subgroups are identified, then this allows us to predict for a specific patient already at intake from which type of treatment he or she will benefit most (or whether the type of treatment does most probably not matter for that specific patient). Such predictions of individual treatment courses furthermore might then also be used as benchmark to monitor and evaluate the actual treatment progress of an individual patient and to immediately feed back this information to therapists in the field of patient-focused research (e.g., Haas et al., 2002; Howard et al., 1996; Lutz et al., 2005; Lutz et al., 1999).
However, our study also has some limitations: First, because these analyses were post-hoc exploratory analyses, further research is necessary to examine whether the three patient subgroups that have been identified in our study are typical for patients with cocaine dependence, especially since we draw a subsample from the original CCTS sample that only included those patients with enough assessment to conduct our complex longitudinal analyses. This selection may have affected our findings. Second, it should be kept in mind that the data of the CCTS was generated under controlled conditions which brings some limitations concerning external validity. Therefore, further research should examine whether the typical patterns of drug use are also found in naturalistic settings and in routine care. Third, due to the quite small number of subjects in the severe users subgroup (which is related to a low statistical power), the findings concerning this subgroup should be treated with caution. Fourth, it remains unclear whether our findings also generalize to cocaine patients with more severe psychiatric comorbidity as current psychotropic medication was an exclusion criterion in the CCTS. Fifth, if available, weekly collected urine specimens were used to examine the validity of self-reported drug use as measured by the ASI. Only 15% of the urine tests indicated some drug use during a month when the patient denied use. Despite this good agreement between urine test results and self-reports of cocaine use, whether patients were using cocaine at times when no urine assessments were available is unknown. And finally, future studies should also examine additional predictors as well as change in other (e.g., biomedical) outcome measures.
Despite these limitations, our findings suggest that there is significant heterogeneity among patients with cocaine dependence and that the effects of psychosocial interventions might be different for different subpopulations of patients. Such findings can then also be helpful for patient classifications that indicate which treatment is most effective for which type of patient.
Acknowledgments
The NIDA Collaborative Cocaine Treatment Study was a National Institute on Drug Abuse (NIDA) funded Cooperative Agreement involving four clinical sites, a Coordinating Center, and NIDA staff. The Coordinating Center at the University of Pennsylvania included: Paul Crits-Christoph, Ph.D. (PI), Lynne Siqueland, Ph.D. (Project Coordinator), Karla Moras, Ph.D. (Assessment Unit Director), Jesse Chittams, M.A. and Robert Gallop, M.S. (Director of Data Management), and Larry Muenz, Ph.D. (Statistician). The collaborating scientists at the Treatment Research Branch, Division of Clinical and Research Services at NIDA included Jack Blaine, M.D. and Lisa Simon Onken, Ph.D. The four participating clinical sites were: University of Pennsylvania - Lester Luborsky, Ph.D. (PI), Jacques P. Barber, Ph.D. (CO-PI), Delinda Mercer, Ph.D. (Project Director); Brookside Hospital/Harvard Medical School - Arlene Frank, Ph.D. (PI), Stephen F. Butler, Ph.D. (CO-PI/Innovative Training Systems), Sarah Bishop, M.A (Project Director); McLean/ Mass General Hospital- Harvard University Medical School- Roger D. Weiss, M.D. (PI), David R. Gastfriend, M.D. (CO-PI), Lisa M. Najavits, Ph.D. and Margaret L. Griffin, Ph.D. (Project Directors); University of Pittsburgh/Western Psychiatric Institute and Clinic - Michael E. Thase, M.D. (PI), Dennis Daley, M.S.W. (CO-PI), Ishan M. Salloum, M.D. (CO-PI), and Judy Lis, M.S.N. (Project Director). The Training Unit included Heads of Cognitive Therapy Training Unit: Aaron T. Beck, M.D. (University of Pennsylvania) and Bruce Liese, Ph.D. (University of Kansas Medical Center); Heads of Supportive-Expressive Therapy Training Unit: Lester Luborsky, Ph.D. and David Mark, Ph.D. (University of Pennsylvania); Heads of the Individual Drug Counseling: George Woody, M.D. (Veterans Administration/ University of Pennsylvania Medical School) and Group Drug Counseling Unit: Delinda Mercer (Head), Dennis Daley (Assistant Head; University of Pittsburgh/Western Psychiatric Institute and Clinic), and Gloria Carpenter, M.Ed., (Assistant Head; Treatment Research Unit- University of Pennsylvania. The Monitoring Board included Larry Beutler, Ph.D., Jim Klett, Ph.D., Bruce Rounsaville, M.D., and Tracie Shea, Ph.D. The contributions of John Boren, Ph.D. and Deborah Grossman, M.A., NIDA, the project officers for this cooperative agreement, are also gratefully acknowledged.
Role of Funding Sources: Preparation of this article was supported in part by grant PBBEP11-123652 (Niklaus Stulz) from the Swiss National Science Foundation (SNF). Collection of the original data was supported in part by grant U01-DA07090 and career development awards K05-DA00168, K02-DA00326, U01-DA07663, U01-DA07673, U01-DA07693, and U01-DA07085 from the National Institute on Drug Abuse, Rockville, MD, and Clinical Research Center grant P30-MH-45178 and Career Development Award K02-MH00756 from the National Institute of Mental Health, Rockville, MD.
Footnotes
In this first stage of analyses, we used a special type of GMMs with variances and covariances of the latent growth parameters fixed at zero to determine the number of latent classes. This Latent Class Growth Analysis (LCGA; Nagin, D.S., 1999. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol. Methods 4, 139-157.) can serve as a starting point for conducting GMMs (Jung, T., Wickrama, K.A.S., 2008. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass 2, 302-317.).
Contributors: Niklaus Stulz was responsible for the concept, the data analyses and the final draft of the manuscript. Robert Gallop consulted in data management and analyses. Wolfgang Lutz and Glenda L. Wrenn contributed to the manuscript from the first draft to the final version. Paul Crits-Christoph consulted in data analyses and contributed to the manuscript from the first draft to the final version. All authors contributed significantly to and have approved the final manuscript.
Conflict of interest: All authors declare that they have no conflicting interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition) - DSM IV. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Barkham M, Stiles WB, Shapiro DA. The shape of change in psychotherapy: Longitudinal assessment of personal problems. J Consult Clin Psychol. 1993;61:667–677. doi: 10.1037//0022-006x.61.4.667. [DOI] [PubMed] [Google Scholar]
- Beck AT, Wright FD, Newman CF, Liese BS. Cognitive therapy for substance abuse. Guilford Press; New York, NY: 1993. [PubMed] [Google Scholar]
- Carroll KM. Recent advances in the psychotherapy of addictive disorders. Curr Psychiatry Rep. 2005;7:329–336. doi: 10.1007/s11920-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–728. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, Gawin FH. Psychotherapy and pharmacotherapy for ambulatory cocaine users. Arch Gen Psychiatry. 1994a;51:177–187. doi: 10.1001/archpsyc.1994.03950030013002. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994b;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Crits-Christoph P, Connolly Gibbons MB, Barber JP, Gallop R, Beck AT, Mercer D, Tu X, Thase ME, Weiss RD, Frank A. Mediators of outcome of psychosocial treatments for cocaine dependence. J Consult Clin Psychol. 2003;71:918–925. doi: 10.1037/0022-006X.71.5.918. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Connolly Gibbons MB, Barber JP, Hu B, Hearon B, Worley M, Gallop R. Predictors of sustained abstinence during psychosocial treatments for cocaine dependence. Psychother Res. 2007;17:240–252. [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. The National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Rationale and methods. Arch Gen Psychiatry. 1997;54:721–726. doi: 10.1001/archpsyc.1997.01830200053007. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Shalloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT. Psychosocial treatments for cocaine dependence. National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, van Lier PAC, van Straten A, Donker M. Examining differential effects of psychological treatment of depressive disorder: An application of trajectory analyses. J Affect Disord. 2005;89:137–146. doi: 10.1016/j.jad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Haas E, Hill RD, Lambert MJ, Morell B. Do early responders to psychotherapy maintain treatment gains? J Clin Psychol. 2002;58:1157–1172. doi: 10.1002/jclp.10044. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg FE, Badger GJ. Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Howard KI, Moras K, Brill P, Martinovich Z, Lutz W. The evaluation of psychotherapy. Am Psychol. 1996;52:1059–1064. doi: 10.1037//0003-066x.51.10.1059. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- Krause MS, Howard KI, Lutz W. Exploring individual change. J Consult Clin Psychol. 1998;66:838–845. doi: 10.1037//0022-006x.66.5.838. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Luborsky L. Principles of psychoanalytic psychotherapy: A manual for supportive-expressive treatment. Basic Books; New York, NY: 1984. [Google Scholar]
- Luborsky L, Rosenthal R, Diguer L, Andrusyna TP, Bernman JS, Levitt JT, Seligman DA, Krause ED. The dodo bird verdict is alive and well - mostly. Clin Psychol Sci Pract. 2002;9:2–12. [Google Scholar]
- Luborsky L, Singer B, Luborsky L. Comparative studies of psychotherapies: Is it true that “everybody has won and all must have prizes?”. In: Spitzer R, Klein D, editors. Evaluation of psychotherapies. Johns Hopkins; Baltimore, MD: 1976. [Google Scholar]
- Lutz W, Leach C, Barkham M, Lucock M, Stiles WB, Evans C, Noble R, Iverson S. Predicting change for individual psychotherapy clients based on their nearest neighbors. J Consult Clin Psychol. 2005;73:904–913. doi: 10.1037/0022-006X.73.5.904. [DOI] [PubMed] [Google Scholar]
- Lutz W, Martinovich Z, Howard KI. Patient profiling: An application of random coefficient regression models to depicting the response of a patient to outpatient psychotherapy. J Consult Clin Psychol. 1999;67:571–577. doi: 10.1037//0022-006x.67.4.571. [DOI] [PubMed] [Google Scholar]
- Lutz W, Stulz N, Köck K. Patterns of early change and their relationship to outcome and follow-up among patients with major depressive disorders. J Affect Disord. doi: 10.1016/j.jad.2009.01.019. in press. [DOI] [PubMed] [Google Scholar]
- Mark D, Luborsky L. A manual for the use of supportive-expressive psychotherapy in the treatment of cocaine abuse. Department of Psychiatry. University of Pennsylvania; Philadelphia, PA: 1992. [Google Scholar]
- Maude-Griffin PM, Hohenstein JM, Humfleet GL, Reilly PM, Tusel TJ, Hall SM. Superior efficacy of cognitive-behavioral therapy for urban crack cocaine abusers: Main and matching effects. J Consult Clin Psychol. 1998;66:832–837. doi: 10.1037//0022-006x.66.5.832. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mee-Lee D. An instrument for treatment progress and matching: The Recovery Attitude and Treatment Evaluator (RAATE) J Subst Abuse Treat. 1988;5:183–186. doi: 10.1016/0740-5472(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Mercer D, Carpenter G, Barber JP. Addiction Recovery Scale. University of Pennsylvania; Philadelphia, PA: 1993. [Google Scholar]
- Mercer D, Carpenter G, Daley D, Patterson C, Volpicelli JR. Addiction Recovery Manual Vol 2 Treatment Research Unit. University of Pennsylvania; Philadelphia, PA: 1994. [Google Scholar]
- Mercer D, Woody GE. Addiction counseling. Center for Studies of Addiction. University of Pennsylvania; Philadelphia, PA: 1992. [Google Scholar]
- Morral AW, Iguchi MY, Belding MA, Lamb RJ. Natural classes of treatment response. J Consult Clin Psychol. 1997;65:673–685. doi: 10.1037//0022-006x.65.4.673. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Second-generation structural equation modeling with a combination of categorical and continuous latent variables. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. American Psychological Association; Washington, DC: 2001. pp. 291–332. [Google Scholar]
- Muthén BO, Brown CH, Maysn K, Jo B, Khoo ST, Yang CC, Wang CP, Kellam SG, Carlin JB, Liao J. General growth mixture modeling for randomized preventive interventions. Biostatistics. 2002;3:459–475. doi: 10.1093/biostatistics/3.4.459. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. Muthén & Muthén; Los Angeles, CA: 2004. [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Harned MS, Gallop RJ, Butler SF, Barber JP, Thase ME, Crits-Christoph P. Six-month treatment outcomes of cocaine-dependent patients with and without PTSD in a multiside national trial. J Stud Alcohol Drugs. 2007;68:353–361. doi: 10.15288/jsad.2007.68.353. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Runkel R, Neuner C, Frank AF, Thase ME, Crits-Christoph P, Blaine J. Rates and symptoms of PTSD among cocaine-dependent patients. J Stud Alcohol Drugs. 2003;64:601–606. doi: 10.15288/jsa.2003.64.601. [DOI] [PubMed] [Google Scholar]
- Ramaswamy V, DeSabro W, Reibstein D, Robinson W. An empirical pooling approach for estimating marketing mix elasticities with PIMS data. Marketing Sci. 1993;12:103–124. [Google Scholar]
- Schwartz G. Estimating dimensions of a model. Ann Stat. 1978;6:461–464. [Google Scholar]
- Shadish WRJ, Sweeney RB. Mediators and moderators in meta-analyses: There's a reason we don't let dodo birds tell us which psychotherapies should have prizes. J Consult Clin Psychol. 1991;59:883–893. doi: 10.1037//0022-006x.59.6.883. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Barber JP, Connolly Gibbons MB, Gallop R, Griffin M, Frank A, Thase ME, Luborsky L, Liese B. What aspects of treatment matter to the patient in the treatment of cocaine dependence? J Subst Abuse Treat. 2004;27:169–178. doi: 10.1016/j.jsat.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Frank A, Daley D, Weiss R, Chittams J, Blaine J, Luborsky L. Predictors of dropout from psychosocial treatment of cocaine dependence. Drug Alcohol Depend. 1998;52:1–13. doi: 10.1016/s0376-8716(98)00039-8. [DOI] [PubMed] [Google Scholar]
- Stoolmiller M, Kim HK, Capaldi DM. The course of depressive symptoms in men from early adolescence to young adulthood: Identifying latent trajectories and early predictors. J Abnorm Psychol. 2005;114:331–345. doi: 10.1037/0021-843X.114.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulz N, Lutz W. Multidimensional patterns of change in outpatient psychotherapy: The phase model revisited. J Clin Psychol. 2007;63:817–833. doi: 10.1002/jclp.20397. [DOI] [PubMed] [Google Scholar]
- Stulz N, Lutz W, Leach C, Lucock M, Barkham M. Shapes of early change in psychotherapy under routine outpatient conditions. J Consult Clin Psychol. 2007;75:864–874. doi: 10.1037/0022-006X.75.6.864. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Montoya ID. Psychological treatments for stimulant misuse, comparing and contrasting those for amphetamine dependence and those for cocaine dependence. Curr Opin Psychiatry. 2009;22:263–268. doi: 10.1097/YCO.0b013e32832a3b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron HB, Turner CW, Ozechowski TJ. Profiles of drug use behavior change for adolescents in treatment. Addict Behav. 2005;30:1775–1796. doi: 10.1016/j.addbeh.2005.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampold BE. The great psychotherapy debate: Models, methods, and findings. Earlbaum; Mahwah, NJ: 2001. [Google Scholar]
- Woody GE. Research findings on psychotherapy of addictive disorders. Am J Addict. 2003;12:19–26. [PubMed] [Google Scholar]
- Zanis DA, McLellan AT, Cnaan RA, Randall M. Reliability and validity of the Addiction Severity Index with a homeless sample. J Subst Abuse Treat. 1994;11:541–548. doi: 10.1016/0740-5472(94)90005-1. [DOI] [PubMed] [Google Scholar]