Abstract
Ror1 and Ror2, a small family of tyrosine kinase receptors, have been implicated in multiple aspects of brain development in C. elegans and X. laevis. More recently, we have shown that these receptors modulate the rate of neurite elongation in cultured rat hippocampal neurons. However, no information is available regarding a potential role of these receptors in other developmental milestones in mammalian central neurons. Neither is the identity known of the Ror ligand(s) and/or the signal transduction pathway(s) in which they participate. Here we report that the down regulation of either Ror1 or Ror2 led to a significant decrease in synapse formation in cultured hippocampal neurons. Simultaneous targeting of Ror proteins, however, did not result in an additive phenotype. Our results also indicated that Ror1 and Ror2 physically interact in the mouse brain, suggesting that they might function as heterodimers in central neurons. In addition, these Ror complexes interacted with Wnt-5a mediating its effects on synaptogenesis. Together, these data suggest that Ror proteins play a key role in Wnt-5a-activated signaling pathways leading to synapse formation in the mammalian central nervous system.
Keywords: Ror1, Ror2, synaptogenesis, siRNAs, antisense oligonucleotides
Synapse assembly requires the coordinated activity of multiple extracellular cues and intracellular response machineries linked by cell surface receptors. In the peripheral nervous system, many of the molecular players that regulate synaptogenesis are well characterized (Sanes and Lichtman, 2001). At the level of the muscle cell membrane, environmental signals such as agrin and neuregulin I are transduced by the muscle-specific tyrosine kinase receptor (MuSK) and erbB, respectively, to elicit the formation of neuromuscular junctions (Gautam et al., 1996; Glass et al., 1996; Buonanno and Fischbach, 2001). On the other hand, the molecular mechanisms underlying synapse formation among central neurons are poorly understood. Although recent evidence implicated other members of the superfamily of receptor tyrosine kinases (RTKs) in synapse formation in the central nervous system (CNS), a detailed picture of the mosaic of receptors that are involved in the formation of central synapses is far from complete (Garner et al., 2002; Huang and Reichardt, 2003; Salinas, 2003; Klein, 2004; Umemori et al., 2004).
A recently discovered family of RTKs, the Ror family, has been implicated in brain development based on data obtained in C. elegans and X. laevis (Forrester et al., 1999; Koga et al., 1999; Hikasa et al., 2002; Forrester et al., 2004). These receptors have also been detected in the mammalian CNS. Analysis of their pattern of expression showed that Ror1 and Ror2 levels increased as central neurons developed either in situ or in culture (Oishi et al., 1999; McKay et al., 2001; Paganoni and Ferreira, 2003). This pattern of expression suggested a role for Ror proteins in neurite elongation and synapse formation, two developmental processes essential for the formation of a mature neuronal network. Furthermore, localization studies indicated that Ror1 and Ror2 were highly enriched in dendrites of cultured central neurons (McKay et al., 2001; Paganoni and Ferreira, 2003). This subcellular localization positions them ideally to transduce signals necessary for postsynaptic differentiation leading to synapse formation in the CNS. However, no direct evidence is available regarding the role of Ror1 and Ror2 in the formation of synapses and/or the identity of their ligand(s) in mammalian central neurons.
In this study, we suppressed Ror1 and Ror2 expression by means of RNA interference (RNAi) and antisense oligonucleotides in cultured hippocampal neurons. Our results showed that the down regulation of Ror1 and/or Ror2 led to the formation of fewer synaptic contacts as compared to Ror-expressing controls. Furthermore, we provided evidence suggesting that Ror1 and Ror2 might function as heterodimers in the Wnt-5a signaling pathway in the mammalian brain.
EXPERIMENTAL PROCEDURES
Preparation of hippocampal cultures
Neuronal cultures were prepared from the hippocampi of embryonic day 16 (E16) mouse embryos as previously described (Goslin and Banker, 1991). In brief, embryos were removed and their hippocampi dissected and freed of meninges. The cells were dissociated by trypsinization (0.25% for 15 minutes at 37°C) followed by trituration with a fire-polished Pasteur pipette and plated onto poly-L-lysine-coated coverslips or 60 mm tissue culture dishes in Minimum Essential Medium (MEM) with 10% horse serum (Invitrogen, Carlsbad, CA). Coverslips were then transferred to dishes containing an astroglial monolayer and maintained in MEM containing N2 supplements (Bottenstein and Sato, 1979) plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM). For biochemical experiments, the medium was replaced with glia-conditioned MEM containing N2 supplements (Bottenstein and Sato, 1979) plus ovalbumin (0.1%) and sodium pyruvate (0.1 mM). The Northwestern University Animal Care and Use Committee approved this experimental protocol in accordance with USPHS regulations and applicable federal and local laws.
Small interfering RNA preparation and transfection
Small interfering RNAs (siRNAs) corresponding to Ror1 and Ror2 were designed as described elsewhere (Paganoni and Ferreira, 2005). The following target sequences were used: Ror1 5′-AATCTCCTTCCGGGCAACCAA-3′ and Ror2 5′-AAGATTCGGAGGCAATCGACA-3′, corresponding to nucleotides 312–332 and 107–127 of the Ror1 or Ror2 mouse cDNAs, respectively (Oishi et al., 1999). As controls, scrambled siRNAs (“siRNA SCR”) and siRNAs carrying a two-base pair change (“siRNA MUT”) were used (Ror1-SCR: 5′-AACCTGCGACCTAGCTCCTAA-3′; Ror2-SCR: 5′-AATGTCACAGATAAGCGAGCG-3′; Ror1-MUT: 5′-AATCTCCTTCCGAACAACCAA-3′; Ror2-MUT: 5′-AAGATTCGGAAACAATCGACA-3′).
Transfections were carried out in 60 mm tissue culture dishes 4 days after plating using the TransMessenger transfection reagent (Qiagen, Germantown, MD) as previously described (Paganoni and Ferreira, 2005). Briefly, transfection complexes were prepared by combining siRNA duplexes, Enhancer R and Transmessenger reagent in buffer EC-R (Qiagen). Complexes were then diluted in 2 ml of warm MEM containing N2 supplements (final siRNA concentration: 100 nM). Cultures were incubated in this medium for 3 hours and then returned to the medium in which they were growing before transfection. Analysis was performed 72 hours later.
Antisense treatments
Oligonucleotides were designed to target the 5′ end of the mouse Ror1 and Ror2 cDNAs and were synthesized by Biosource International (Camarillo, CA) as previously described (Paganoni and Ferreira, 2005). The following 18-mer antisense oligonucleotides (AS) were used in this study: for Ror1 5′-CTGTTTCTTGGGCATCAG-3′ and for Ror2 5′-CACAGAGGCACACGGCTC-3′, corresponding to nucleotides +80+97 and +24+41 of the Ror1 and Ror2 mouse cDNAs, respectively (Oishi et al., 1999). Controls were treated with sense (S) oligonucleotides (5′-CTGATGCCCAAGAAACAG-3′ for Ror1 and 5′-GAGCCGTGTGCCTCTGTG-3′ for Ror2). All oligonucleotides contained phosphorothioate groups at the last 3 residues in the 3′ terminal region (Ferreira, 1999).
Sense and antisense oligonucleotides were added directly to the media of hippocampal cultures from day 4 to day 7 after plating every 12 hours at a 50 M (first treatment) or 25 M (following treatments) final concentrations. Cells were harvested for analysis 7 days after plating.
Plasmid transfections
Human embryonic kidney (HEK293) cells were maintained in MEM plus 10% horse serum (Invitrogen) and grown at 37°C in 5% CO2 and 90% humidity. Cultures were fed twice a week and passaged when 80% confluent. Cells were renewed every 10 passages. Expression plasmids encoding mouse Ror1 or Ror2 (pcDNA3-mRor1-Flag, pcDNA3-mRor2-Flag and pcDNA3-mRor2-HA, a generous gift from Dr. Minami, Kobe University, Japan) and expression plasmids encoding HA-tagged mouse Wnt-5a (Upstate, Lake Placid, NY) were transfected into HEK293 cells using the Nucleofector™ apparatus (Amaxa, Gaithersburg, MD). For each reaction, one million HEK cells were resuspended in 100 μl Nucleofector Solution containing 10 μg DNA and electroporated using program Q-01. Cells were plated in MEM plus 10% horse serum and processed 72 hours after plating.
Transfection of HA-tagged Wnt-5a into astrocytes was performed using the Nucleofector™ apparatus (Amaxa) as previously described (Paganoni and Ferreira, 2005). Briefly, confluent astrocyte cultures were trypsinized (0.25% for 15 minutes at 37°C), pelleted down (10 minutes at 1000 rpm) and resuspended in Amaxa glia nucleofector solution, transferred to an electroporation cuvette and “nucleofected” according to the manufacturer’s protocol (program T-20). For each reaction, 4 million astrocytes and 3 μg of cDNA were used. Astrocytes were then plated at a density of 40,000 cells/cm2 and allowed to grow for 48 hours. Transfected and untransfected astrocyte monolayers were then used to set up co-cultures with hippocampal neurons as described above. For some experiments, we collected the media in which untransfected and Wnt-5a-transfected astrocytes had been maintained for 7 days. The media were filtered to remove cell debris before being added directly to untreated hippocampal neurons and to neurons in which Ror1 and/or Ror2 expression has been suppressed using specific antisense oligonucleotides as described above.
Immunocytochemistry
Hippocampal neurons and HEK cells were fixed for 20 minutes with 4% paraformaldehyde in phosphate buffer saline (PBS) containing 0.12 M sucrose. They were then permeabilized in 0.3% Triton X-100 in PBS for 5 minutes and rinsed twice in PBS. The coverslips were preincubated in 10% bovine serum albumin (BSA) in PBS for 1 hour at room temperature and exposed to the primary antibodies (diluted in 1% BSA in PBS) overnight at 4°C. Finally, the cultures were rinsed in PBS and incubated with secondary antibodies for 1 hour at 37°C. The following primary antibodies were used: anti-α-tubulin (clone DM1A, 1:200, Sigma, St Louis, MO), anti-Ror1 and anti-Ror2 (1:20, Paganoni and Ferreira, 2003), monoclonal anti-synaptophysin (clone SY38, 1:50, Millipore Bioscience Research Reagents, Belmont, MA), polyclonal anti-synaptophysin (SYP (H-93), 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), anti-synapsin I (1:100, Zymed, San Francisco, CA), anti-microtubule associated protein 2 (MAP2) (1:100, clone AP-14; Caceres et al., 1984), anti-peptide DYKDDDK (Flag) (1:1000, Millipore Bioscience Research Reagents), anti-HA (1:1000, Upstate Biotechnology, Lake Placid, NY). The following secondary antibodies were used: Alexa Fluor 488 anti-mouse, Alexa Fluor 568 anti-mouse, Alexa Fluor 488 anti-rabbit and Alexa Fluor 568 anti-rabbit IgG (1:200, Invitrogen), anti-rabbit IgG biotin-conjugated (1:50, Millipore Bioscience Research Reagents), Avidin-Rhodamine (1:50, Vector Laboratories, Burlingame, CA) and Avidin-fluorescein (1:50, Roche, Indianapolis, IN).
Synapse count and morphometric analysis
To determine the number of synapses per neuron, hippocampal cultures were double-stained with synaptophysin and tubulin antibodies. The number of synaptophysin immunoreactive spots in randomly selected fields was determined using Metamorph Image Analysis Software (Fryer Company Inc., Huntley, IL). To determine synaptic density, cultures were double-stained with synapsin I and MAP2 antibodies. MAP2 was used as a dendritic marker (Caceres et al., 1984). The number of synapsin I immunoreactive spots and the neuritic length of MAP2 immunoreactive processes from randomly selected fields were determined using Metamorph Image Analysis Software. The number of synapsin I immunoreactive spots in each field was divided by the dendritic length in the same field to obtain synaptic density. Sixty fields from 3 independent experiments were analyzed for each condition. Results were presented as the mean ± S.E.M. Data, obtained blind as to treatment condition, were analyzed using one-way ANOVA followed by Fisher’s LSD post-hoc test.
Protein Electrophoresis and Immunoblotting
Hippocampal neurons and HEK cells were rinsed twice in warmed PBS, scraped into Laemmli buffer, and homogenized in a boiling water bath for 10 minutes. Sodium dodecyl sulfate (SDS)-polyacrylamide gels were run according to Laemmli (1970). Transfer of protein to Immobilon membranes (Millipore Bioscience Research Reagents) and immunodetection were performed according to Towbin et al. (1979) as modified by Ferreira et al. (1989). The following antibodies were used: anti-α-tubulin (clone DM1A, 1:100,000; Sigma); anti-Ror1 (1:500; Paganoni and Ferreira, 2003); anti-Ror2 (1:250; Paganoni and Ferreira, 2003); anti-peptide DYKDDDK (Flag) (1:1000; Millipore Bioscience Research Reagents); anti-HA (1:1000, Upstate Biotechnology); anti-mouse Wnt-5a (1:500; R & D Systems, Minneapolis, MN). Secondary antibodies conjugated to HRP (1:1000; Promega, Madison, WI) followed by enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Chicago, IL) were used for the detection of proteins. In some experiments, blots were stripped in 60 mM Tris, 2% SDS and 0.8% β-mercaptoethanol at 55°C for 30 minutes and then reprobed with a different antibody. Membranes were imaged on ChemiDoc gel documentation system (Bio Rad, Hercules, CA). Bands were analyzed using Quantity One Analysis Software (Bio Rad). Results were expressed as mean ± S.E.M. obtained from 3 independent experiments. Data were analyzed using one-way ANOVA followed by Fisher’s LSD post-hoc test.
Immunoprecipitation
HEK cells and whole mouse embryo brains (E16) were solubilized in ice-cold NP-40 buffer (50 mM Tris, pH 7.5, 150 mM NaCl, NP-40 1%, 50 mM NaF, 1 mM Na3VO4, 250 mM PMSF, protease inhibitor cocktail 1:1000; Sigma). Cell lysates were prepared by centrifugation at 12,000 xg for 30 minutes to remove cell debris and then pre-cleared by rocking for 1 hour at 4°C with Protein A/G PLUS agarose. The supernatants were immunoprecipitated using anti-Flag (Millipore Bioscience Research Reagents), anti-HA (Upstate), anti-Ror1 (Paganoni and Ferreira, 2003) and anti-Ror2 (Paganoni and Ferreira, 2003) antibodies or normal rabbit IgGs (Santa Cruz, Santa Cruz, CA). Samples were incubated with Protein A/G PLUS agarose beads for 3 hours and then centrifuged at 12,000 xg for 1 minute to pull down immunocomplexes. Pellets were washed 4X with NP-40 buffer, eluted with Laemmli buffer and boiled for 10 minutes. Protein electrophoresis and immunoblotting were carried out as described above.
RESULTS
Decreased synapse formation in Ror1 and/or Ror2-depleted hippocampal neurons
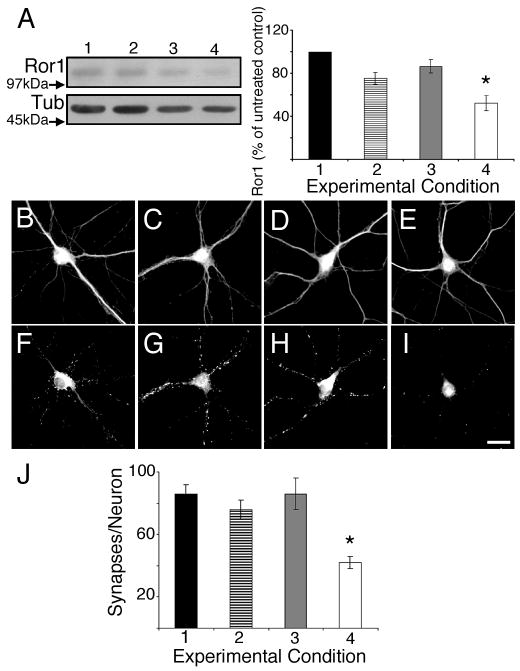
In hippocampal neurons, the expression of Ror proteins increases immediately before, and for the duration, of active synapse formation (Paganoni and Ferreira, 2003). However, no direct evidence of the role of these RTKs in synaptogenesis is available. To gain insights into such a role, we down regulated Ror1 and Ror2 expression in cultured hippocampal neurons taking advantage of siRNAs and antisense oligonucleotides designed to target non-overlapping regions of mouse Ror1 and Ror2 (see Materials and Methods and Paganoni and Ferreira, 2005). These probes’ specificity and effectiveness to suppress the expression of Ror proteins in these neurons have been previously characterized (Paganoni and Ferreira, 2005). In the first set of experiments, we transfected hippocampal cultures with siRNA duplexes 4 days after plating and analyzed them 3 days later. Immunoblot analyses showed that Ror1 and Ror2 targeting by RNAi reduced Ror protein levels by 40–50% as compared to non-treated controls or to cultures that had been transfected with scrambled (SCR) or mutated (MUT) siRNAs (Fig. 1 and Suppl. Fig. 1). To determine whether synaptogenesis was affected by Ror1 or Ror2 down regulation, we immunolabeled sister cultures using synaptophysin as a synaptic marker. In hippocampal neurons that develop in culture, 90% of the synaptophysin immunoreactive dots correspond to presynaptic specializations juxtaposed to postsynaptic densities, as assessed by electron microscopy (Fletcher et al., 1991). Quantitative analysis showed that Ror1 and Ror2 targeting by their corresponding siRNA duplexes resulted in a marked decrease in the number of synaptophysin immunoreactive spots per neuron as compared to non-treated and SCR- or MUT-siRNA transfected cultures (Fig. 1 and Suppl. Fig. 1).
Figure 1. Effect of Ror1 down regulation by RNAi on synapse formation in cultured hippocampal neurons.
(A) Western blot analysis of whole cell extracts prepared from control (lane 1), mutated Ror1 siRNA- (lane 2), scrambled Ror1 siRNA- (lane 3) and Ror1 siRNA- (lane 4) transfected hippocampal cultures. Densitometric analysis of the Ror1 immunoreactive bands is shown on the right. Values represent the mean ± S.E.M for 3 independent experiments normalized using tubulin as an internal control. The numbers correspond to the percent of Ror1 protein present in the siRNA-transfected cultures as compared to the levels detected in the untreated samples (100%). *Differs from untransfected, mutated siRNA- and scrambled siRNA-transfected cultures, P<0.01. (B–I) Seven days in culture control (B, F), mutated Ror1 siRNA- (C, G), scrambled Ror1 siRNA- (D, H) and Ror1 siRNA- (E, I) transfected hippocampal neurons were double stained with tubulin (B–E) and synaptophysin (F–I) antibodies. Note the reduction in the number of synaptophysin immunoreactive dots in the Ror1 siRNA-transfected cultures (I) as compared to the control ones (F, G, H). (J) Quantitative analysis of the number of synapses formed by neurons cultured under the experimental conditions described above. The numbers represent the mean ± S.E.M for 3 independent experiments. * Differs from all other experimental conditions, P< 0.01. Scale bar: 20 μm.
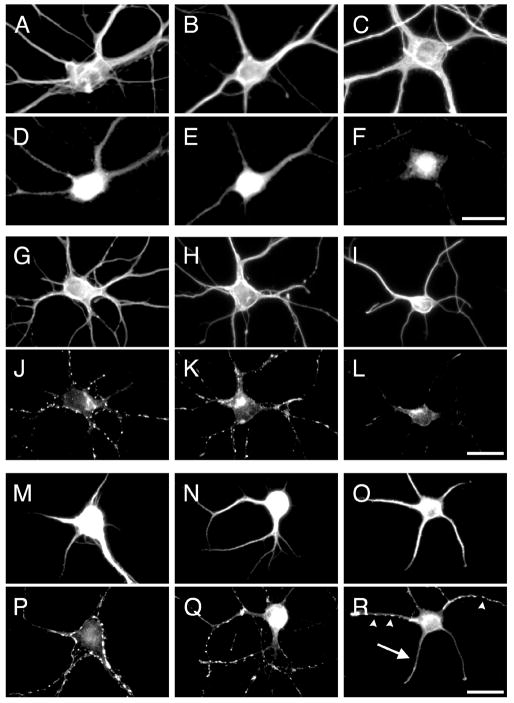
We subsequently tested whether the same phenotype was detected when Ror1 and Ror2 down regulation was achieved using an independent methodology. For these experiments, we used antisense oligonucleotides that significantly reduced Ror-expression in the majority (~70 %) of cultured hippocampal neurons (Paganoni and Ferreira, 2005). Analysis of synaptophysin-labeled control, sense- and antisense-treated hippocampal cultures also revealed a significant decrease in the number of synaptophysin immunoreactive puncta in Ror-depleted neurons (Fig. 2, Suppl. Fig. 2 and Table 1).
Figure 2. Ror1 suppression by antisense oligonucleotide treatment in cultured hippocampal neurons.
(A–F) Seven days in culture control (A, D), Ror1 sense- (B, E) and Ror1 antisense- (C, F) treated hippocampal neurons were double stained with tubulin (A–C) and Ror1 (D–F) antibodies. Ror1 was readily detectable in control (D) and sense-treated (E) neurons. Only faint immunoreactivity was left in the neurons treated with Ror1 antisense oligonucleotides (F). (G–L) Seven days in culture control (G, J), Ror1 sense- (H, K) and Ror1 antisense- (I, L) treated hippocampal neurons were double stained with tubulin (G–I) and synaptophysin (J–L) antibodies. Fewer synaptophysin immunoreactive spots were detected when Ror1 expression was targeted by the addition of Ror1 antisense oligonucleotides as compared to controls (compare L to J and K). (M–R) Seven days in culture control (M, P), Ror1 sense- (N, Q) and Ror1 antisense- (O, R) treated hippocampal neurons were double stained with MAP2 (M–O) and synapsin I (P–R) antibodies. Fewer synapsin I immunoreactive dots (arrowheads) were present in the targeted cultures as compared to controls (R). Note also the diffuse synapsin I immunoreactivity in some of the axons growing on top of MAP2 immunoreactive processes in antisense-treated neurons (arrow in R). Scale bars: 20 μm.
Table 1.
Effects of Ror1 and Ror2 suppression on synapse formation in cultured hippocampal neurons.
| Treatment | Number of synapses/cell1 | Dendritic length2(μm) | Synaptic density3 (synapses/10 μm dendritic length) |
|---|---|---|---|
| None | 64 ± 4 | 269 ± 14 | 1.6 ± 0.1 |
| S-Ror1 | 56 ± 5 | 203 ± 11 | 1.7 ± 0.1 |
| S-Ror2 | 60 ± 6 | 210 ± 13 | 1.8 ± 0.2 |
| S-Ror1 + S-Ror2 | 68 ± 5 | 217 ± 18 | 1.8 ± 0.1 |
| AS-Ror1 | 29 ± 2** | 152 ± 8** | 1.2 ± 0.1** |
| AS-Ror2 | 43 ± 4* | 190 ± 10 | 1.0 ± 0.1** |
| AS-Ror1 + AS-Ror2 | 36 ± 5** | 170 ± 15** | 1.2 ± 0.1** |
At least sixty fields from 3 independent experiments were analyzed for each condition. Results are expressed as mean ± S.E.M.
Differs from controls, P<0.05.
Differs from controls, P<0.01. S: sense oligonucleotides. AS: antisense oligonucleotides.
Cultures were doublestained with synaptophysin and tubulin antibodies. The number of synaptophysin immunoreactive spots in any given field was counted and divided by the number of cells in the same field.
Cultures were doublestained with synapsin I and MAP2 antibodies. The total length of MAP2 immunoreactive processes in any given field was measured (2). The number of synapsin I immunoreactive spots was calculated and divided by the dendritic length (10 μm) in the same field to obtain synaptic density (3).
We determined next to what extent the observed decrease in the number of synaptic contacts was a result of impaired synapse formation or a consequence of altered dendritic elongation. To address this issue, we calculated synaptic density as the mean number of synapses formed per 10 m of dendritic segment. For these experiments, dendritic processes were labeled with MAP2, whereas synapsin I staining was used to detect synapses as previously described (Caceres et al., 1984; Fletcher et al., 1991). Fewer synapsin I immunoreactive spots were detected in Ror-depleted neurons when compared to untreated controls (Fig. 2). It is worth noting that some diffuse synapsin I immunoreactivity was also detected in axons running along dendritic processes of Ror-depleted neurons (Fig. 2). This diffuse synapsin I distribution has been previously described in hippocampal neurons prior to synapse formation (Fletcher et al., 1991). Quantitative analysis showed a significant decrease in synaptic density in both Ror1 and Ror2 antisense-treated cultures when compared to controls (Table 1). These results suggested that the reduction in the number of synapses observed in Ror-depleted cultures was not a reflection of changes in total dendritic length.
Finally, we assessed whether simultaneous targeting of Ror1 and Ror2 resulted in a cumulative phenotype. For these experiments, hippocampal neurons were cultured in the presence of both Ror1 and Ror2 antisense oligonucleotides. Non-treated and sister cultures that had been exposed to both Ror1 and Ror2 sense oligonucleotides were used as controls. Significantly fewer synaptic contacts were detected in Ror1 and Ror2-depleted neurons as compared to sense-treated and untreated controls (Table 1). Synaptic density was also reduced when Ror proteins were knocked down concurrently as compared to Ror-expressing hippocampal neurons. However, no significant differences in synapse formation were detected when Ror1 and Ror2 expression was down regulated separately or simultaneously (Table 1).
Interaction of Ror1 and Ror2 in vitro and in vivo
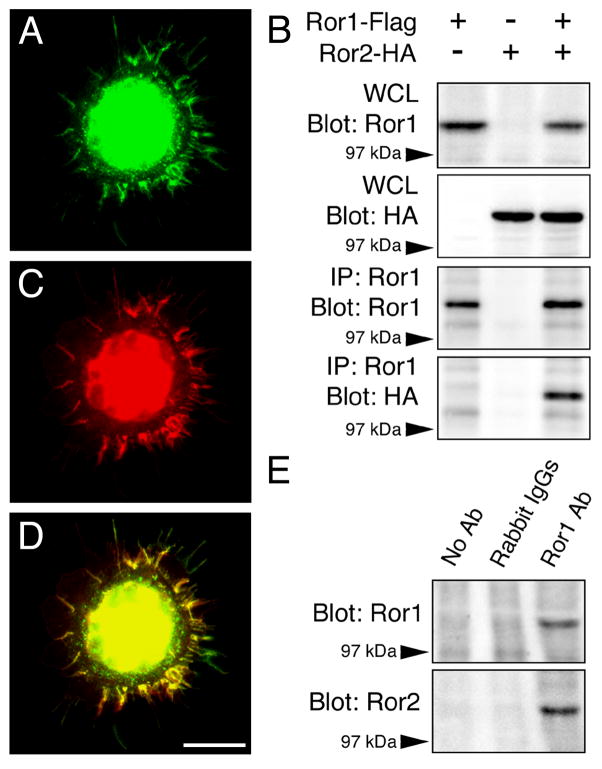
The results described above indicated that the down regulation of Ror1 and Ror2, either individually or combined, resulted in identical phenotypes characterized by the presence of fewer synaptic contacts among cultured hippocampal neurons. These findings suggested that the loss of only one of the two receptors was sufficient to abolish Ror function in these neurons. One likely explanation for such an observation is that Ror1 and Ror2 might function by associating as heterodimers. To directly test this hypothesis, we transfected HEK cells with Ror1 and Ror2 expression plasmids that were tagged with Flag or HA epitopes, respectively. The distribution of Ror1-Flag and Ror2-HA proteins in HEK cells was analyzed by immunocytochemistry 72 hours later. When Ror1-Flag and Ror2-HA were coexpressed in HEK cells, they colocalized in the cell body and in filopodia-like protrusions at the periphery of the cells as determined by staining with anti-Flag and anti-HA antibodies (Fig. 3 A, C, D). Cross-reactivity between anti-Flag and anti-HA antibodies was ruled out by double-staining cultures that had been transfected with only one plasmid. No Flag or HA immunoreactivity was ever detected in non-transfected HEK cells (data not shown).
Figure 3. Ror1 and Ror2 interaction in HEK cells and in the mammalian central nervous system.
(A–D) HEK cells were transfected with Ror1-Flag and Ror2-HA constructs. Immunolabeling with anti-Flag (A) and anti-HA (C) antibodies showed that Ror1 and Ror2 co-localized when co-expressed in HEK cells (A, C, D). (B) Sister cultures were immunoprecipitated with Ror1 antibody. Whole cell lysates (WCL) and immunoprecipitates (IP) were separated by SDS-PAGE and analyzed by Western blotting using HA and Ror1 antibodies. Note that an HA immunoreactive band was detected in the immunoprecipitates prepared from the cultures where Ror1-Flag and Ror2-HA had been co-expressed. (E) Whole brain lysates from E16 mouse embryos were subjected to immunoprecipitation with Ror1 antibody. Controls included samples where the primary antibody was omitted or replaced with normal rabbit IgGs. Samples were analyzed by Western blotting using Ror1 and Ror2 antibodies. When Ror1 was immunoprecipitated from mouse embryonic brains, Ror2 immunoreactive bands were detected in the precipitate. IP: immunoprecipitate; WCL: whole cell lysate. Scale bar: 20 μm.
Next, HEK cells that had been transfected with Ror1-Flag and Ror2-HA, either alone or in combination, were subjected to immunoprecipitation experiments. Western blot analysis showed strong Ror1 and Ror2 immunoreactive bands in the whole cell lysates obtained from transfected cells indicating efficient expression of the corresponding transfected plasmids (Figure 3 B). Ror1 was pulled down from these transfected cultures using an anti-Ror1 antibody (Paganoni and Ferreira, 2003). Analysis of the precipitates revealed a Ror1 immunoreactive band in the Ror1-Flag-transfected samples, demonstrating that Ror1 had been effectively immunoprecipitated. A clear Ror2 band was detected in the samples that had been double transfected with Ror1 and Ror2 constructs. These data indicated that Ror2 co-immunoprecipitated with Ror1. No Ror2 bands were found in the precipitates from cultures that had been transfected with only Ror1-Flag or Ror2-HA. The same results were obtained when co-immunoprecipitation was performed with either anti-Flag or anti-HA antibodies (data not shown).
To assess whether a similar interaction occurred in vivo, we immunoprecipitated Ror proteins from whole brains of mouse embryos. Samples were incubated in the presence of either Ror1 or Ror2 antibodies. Previous studies have shown that these Ror1 and Ror2 antibodies are specific and do not cross react with each other (Paganoni and Ferreira, 2003; Paganoni and Ferreira, 2005). Controls included samples where the primary antibody was omitted or replaced with normal rabbit IgGs. When whole embryonic mouse brains were immunoprecipitated with the Ror1 antibody, a clear Ror1 immunoreactive band was detected by Western blotting (Fig. 3E). These results indicated that Ror1 antibody was able to effectively immunoprecipitate endogenous Ror1. Specificity of the procedure was demonstrated by the fact that no Ror1 immunoreactive bands were ever found in the negative controls. Ror2 antibody, on the other hand, was not able to immunoprecipitate Ror2 under our experimental conditions (data not shown). When control and Ror1-immunoprecipitated samples were probed with Ror2 antibody, a strong immunoreactive band was found in the Ror1-immunoprecipitated samples, but not in the negative controls. These results suggested that Ror1 and Ror2 also associated in mouse embryonic brains (Fig. 3E).
Ror complexes interacted with Wnt-5a in the mouse brain
The results presented above provided evidence suggesting the formation of Ror1-Ror2 complexes in the mammalian CNS. On the other hand, the identity of the ligand(s) that might trigger the activation of these receptors is still elusive. Recent evidence has suggested that Ror2 might mediate the activity of Wnt-5a in HEK and COS7 cells (Hikasa et al., 2002; Oishi et al., 2003; Billiard et al., 2005). Wnt-5a is a member of a family of secreted glycoproteins, Wnt proteins, implicated in different cell processes including the regulation of cell differentiation, migration and patterning formation (Cadigan and Nusse, 1997; Ikeya et al., 1997; Baker et al., 1999; Hall et al., 2000; Lee et al., 2000; Patapoutian and Reichardt, 2000; Peifer and Polakis, 2000; Krylova et al., 2002; Moon et al., 2002; Packard et al., 2002; Castelo-Blanco et al., 2003; Chen and Walsh, 2003; Lyuksyutova et al., 2003; Machon et al., 2003; Rosso et al., 2005). However, it is not clear whether such an interaction might occur in mammalian neurons that develop in situ. To address this issue, we used the Ror1 antibody to immunoprecipitate Ror complexes from embryonic brain. In these samples, a strong Ror1 immunoreactive band was detected, while no band was seen in the negative control in which the primary antibody had been omitted (Fig. 4 B). Ror2 and Wnt-5a immunoreactive bands were also present in the Ror1 antibody-immunoprecipitated lysates, suggesting that Wnt-5a binds Ror1-Ror2 complexes in the mammalian CNS (Fig. 4 B).
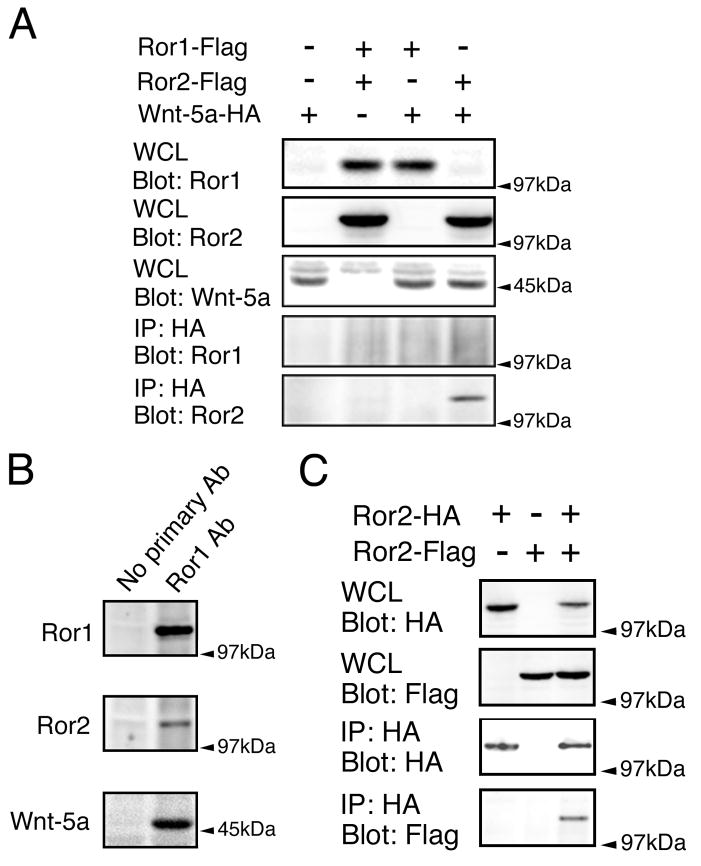
Figure 4. Wnt-5a interaction with Ror proteins in vitro and in the mouse brain.
(A) HEK cells were transfected with mouse Ror1-Flag, Ror2-Flag and Wnt-5a-HA plasmids as indicated. Whole cell lysates (WCL) were analyzed by Western blotting to show expression of the transfected constructs. Lysates were immunoprecipitated with HA antibody and probed for Ror1 and Ror2. No Ror1 band was detected in any of the precipitates (IP). Conversely, a Ror2 immunoreactive band was present in the IP samples prepared from the cultures that had been double transfected with Ror2 and Wnt-5a, but not in the ones prepared from HEK cells that had been transfected with either Ror2 or Wnt-5a alone. (B) Whole brain lysates from E16 mouse embryos were subjected to immunoprecipitation with Ror1 antibodies. In control samples, the primary antibody was omitted. Precipitates were probed for Ror1, Ror2 and Wnt-5a. In the Ror1 immunoprecipitates, Ror2 and Wnt-5a immunoreactive bands were detected. (C) HEK cells were transfected with mouse Ror2 tagged with either Flag or HA epitopes as indicated. Ror2 was pulled down using anti-HA antibodies. Whole cell lysates (WCL) and immunoprecipitates (IP) were immunoblotted with HA and Flag antibodies. Note that Ror2-Flag was detected in the immunoprecipitates when co-expressed with Ror2-HA. IP: immunoprecipitate; WCL: whole cell lysate.
To further analyze Ror-Wnt interactions, we performed immunoprecipitation experiments using HEK cells that had been transfected with expression vectors encoding Ror1-Flag, Ror2-Flag and Wnt-5a-HA, either separately or in combination. The expression of these constructs in transfected cells was confirmed by means of Western blot analysis. Strong Ror1, Ror2, and Wnt-5a bands were detected in the whole cell lysates obtained from transfected cells (Fig. 4 A). Samples were immunoprecipitated with anti-HA antibody to isolate Wnt-5a, and then, immunoblotted with anti-Ror1 and Ror2 antibodies. No Ror1 immunoreactive bands were present in any of the precipitates (Fig. 2 A). On the other hand, a clear Ror2 band was detected when Ror2 and Wnt-5a were coexpressed, suggesting that Ror2, but not Ror1, directly interacted with Wnt-5a in this cellular context (Fig. 4 A).
Ror2 appeared to be sufficient to bind Wnt-5a in vitro. Since most RTKs signal through dimerization, we finally examined whether Ror2 was able to homodimerize in HEK cells. For these experiments, expression plasmids encoding Flag-tagged and HA-tagged mouse Ror2 were expressed in HEK cells, either separately or together. Western blot analysis of whole cell lysates demonstrated that expression of these plasmids was readily detectable in HEK cells. In addition, immunocytochemical analysis showed no cross reactivity between anti-HA and anti-Flag antibodies (Figure 4 C). Cell lysates were subjected to immunoprecipitation experiments with an anti-HA antibody. Immunoprecipitates were then probed with anti-HA and anti-Flag antibodies by Western blotting. HA immunoreactive bands were present in the precipitates prepared from the cultures that had been transfected with Ror2-HA, indicating that Ror2 had been successfully immunoprecipitated by anti-HA antibodies. Furthermore, a Flag immunoreactive band was present when Ror2-HA and Ror2-Flag were coexpressed and immunoprecipitated using the HA antibody. These results suggested that Ror2 homodimerized under these experimental conditions (Fig. 4 C).
Wnt-5a induced synapse formation in Ror-expressing cultured hippocampal neurons
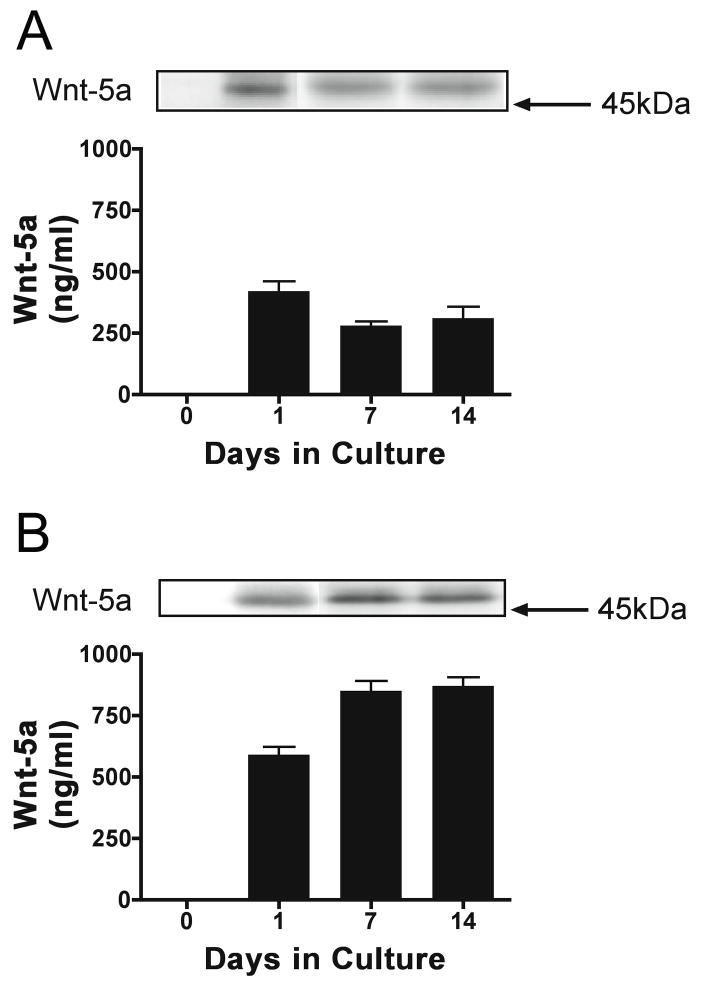
The experiments described above suggested that Ror2-Ror1 heterodimers and/or Ror2 homodimers could be part of the Wnt-5a signaling pathway in central neurons. Although other members of the Wnt family have been implicated in synapse formation, no information is available regarding the biological function Wnt-5a and/or the identity of its receptors in developing central neurons (Lucas and Salinas, 1997; Hall et al., 2000; Ciani and Salinas, 2005; Rosso et al., 2005, Ahamad-Annuar et al., 2006). To obtain insights into a potential role of Wnt-5a in synaptogenesis, we determined first whether this secreted glycoprotein was present in the medium in which hippocampal neurons and astrocytes were co-cultured. Second, we assessed if Wnt-5a was secreted by neurons, glia, or both cell types. For these experiments, hippocampal neurons and astrocytes were cultured independently for up to 14 days. Their media were collected, diluted in Laemmli buffer (1:1), and analyzed by means of Western blot using known concentrations of recombinant Wnt-5a as positive controls. Quantification of immnureactive bands showed that Wnt-5a levels were significantly higher in the medium collected from astrocyte cultures when compared to the one collected from neuronal cultures (Fig. 5). In addition, while the highest Wnt-5a levels in hippocampal neuron-conditioned medium were detected after 1 day in culture, its concentration significantly increased in glia-conditioned medium as astrocytes developed in culture (Fig. 5). These data indicated that astrocytes were the principal source of Wnt-5a in our co-culture system. Therefore, to determine the effects of this Wnt protein on synapse formation, astrocytes were transfected with the HA-tagged Wnt-5a plasmid. The levels of this Wnt protein in the culture medium of transfected astrocytes were significantly higher (~2 fold) than the ones detected in untransfected controls (Fig. 6). Wnt-5a-transfected astrocytes were used to set up co-cultures with hippocampal neurons. Coverslips, containing 1 day in culture hippocampal neurons, were placed facing down on Wnt-5a-transfected and untransfected astrocytes, fixed 6 days later, and stained using synaptophysin as a synaptic marker. Quantitative analysis showed a significant increase in the number of synapses per cell in hippocampal neurons co-cultured with Wnt-5a-overexpressing astrocytes as compared to those co-cultured with non-transfected controls (Fig. 6 and Table 2). Morphometric analysis established that hippocampal neurons cultured in the presence of Wnt-5a transfected astrocytes extended significantly longer dendrites than controls (Table 2). Therefore, we assessed synaptic density under these experimental conditions to determine whether the increased synapse formation observed in the presence of Wnt-5a was only the result of its neuritogenic effects. Quantitative analysis showed that not only the number of synapses but also synaptic density were significantly higher in neurons co-cultured with Wnt-5a-overexpressing astrocytes as compared to those co-cultured with non-transfected controls (Table 2). Similar results were obtained when hippocampal neurons were incubated directly with conditioned media collected from Wnt-5a-transfected astrocytes and untransfected controls (data not shown).
Figure 5. Determination of Wnt-5a levels in hippocampal neuron- and astrocyte- conditioned culture medium.
(A & B) Quantitative analysis of Wnt-5a levels in culture medium obtained from hippocampal neurons (A) and astrocytes (B) cultures maintained up to 14 days. Note that the Wnt-5a concentration was significantly higher in astrocyte-conditioned medium when compared to hippocampal neuron-conditioned one.
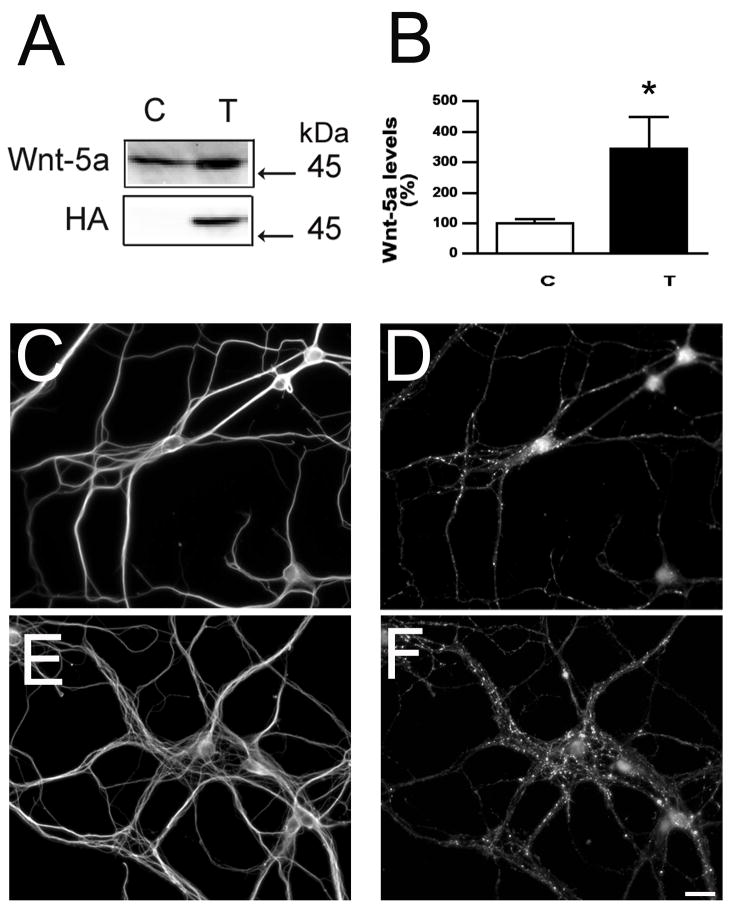
Figure 6. Wnt-5a induced synapse formation in cultured hippocampal neurons.
(A & B) Quantitative analysis of Wnt-5a levels in culture medium obtained from control (C) and Wnt-5a-transfected (T) astrocyte culture maintained for 7 days. Immunoblot membranes were reacted with Wnt-5a and HA antibodies. Values represent the mean ± S.E.M for 3 independent experiments. *Differs from controls, P<0.01. (C–F) Seven days in culture hippocampal neurons maintained in the presence of untransfected (C &D) and Wnt-5a-transfected astrocyte cultures were double stained with tubulin (C & E) and synaptophysin (D & F) antibodies. Note the increase in the number of synaptophysin immunoreactive dots in hippocampal neurons co-cultured with Wnt-5a-transfected astrocytes (F) as compared to the control ones (D). Scale bar: 20 μm.
Table 2.
Effects of Wnt-5a on synapse formation in control and Ror-depleted hippocampal neurons.
| Treatment | Synapses/cell1 | Dendritic length2 (μm) | Synaptic density3 (synapses/10 μm dendritic length) |
|---|---|---|---|
| None | 69 ± 7 | 247 ± 13 | 1.6 + 0.1 |
| Wnt-5a | 105 ± 5** | 324 ± 15** | 2.0 + 0.1* |
| AS-Ror1 | 29 ± 2 | 152 ± 8 | 1.2 + 01 |
| AS-Ror1 + Wnt-5a | 35 ± 7 | 171 ± 8 | 1.3 + 0.2 |
| AS-Ror2 | 43 ± 4 | 190 ± 10 | 1.0 + 0.1 |
| AS-Ror2 + Wnt-5a | 40 ± 9 | 175 ± 11 | 1.2 + 0.1 |
| AS-Ror1 + AS-Ror2 | 41 ± 8 | 170 ± 15 | 1.2 + 0.1 |
| AS-Ror1 + AS-Ror2 + Wnt-5a | 44 ± 5 | 165 + 17 | 1.4 + 0.1 |
At least sixty fields from 3 independent experiments were analyzed for each condition. Results are expressed as mean ± S.E.M. Differs from control cultures grown in the presence of conditioned medium obtained from untransfected astrocytes,
P<0.05 and
P<0.01.
No statistical significant differences were detected in Ror-depleted neurons cultured in the presence of conditioned medium obtained from Wnt-5a transfected astrocytes when compared to those cultured in conditioned medium obtained from untransfected controls in each experimental condition.
Cultures were doublestained with synaptophysin and tubulin antibodies. The number of synaptophysin immunoreactive spots in any given field was counted and divided by the number of cells in the same field.
Cultures were doublestained with synapsin I and MAP2 antibodies. The total length of MAP2 immunoreactive processes in any given field was measured (2). The number of synapsin I immunoreactive spots was calculated and divided by the dendritic length (10 μm) in the same field to obtain synaptic density (3). AS: antisense oligonucleotides.
The results described above indicated that, indeed, Wnt-5a could stimulate synapse formation in cultured hippocampal neurons. We assessed next whether this effect was mediated by Ror receptors. For these experiments, we quantified synaptic contacts in hippocampal neurons in which the expression of Ror1 and/or Ror2 have been suppressed using specific antisense oligonucleotides (see above) and were cultured in the presence of conditioned medium collected from untransfected and Wnt-5a transfected astrocytes. No increase in synapse formation or synaptic density was detected in Ror1-, Ror2-, or both Ror1 and Ror2-depleted neurons cultured in the presence of conditioned medium collected from Wnt-5a-transfected astrocyte cultures when compared to untransfected controls (Fig. 7 and Table 2). Furthermore, no significant differences in synapse formation and/or synaptic density were detected when Ror1 and Ror2 expression was down regulated separately or simultaneously prior to their incubation in the presence of conditioned medium collected from Wnt-5a transfected astrocytes (Fig. 7 and Table 2). Together, these results suggested that Wn-5a could induce synaptogenesis only in Ror expressing hippocampal neurons.
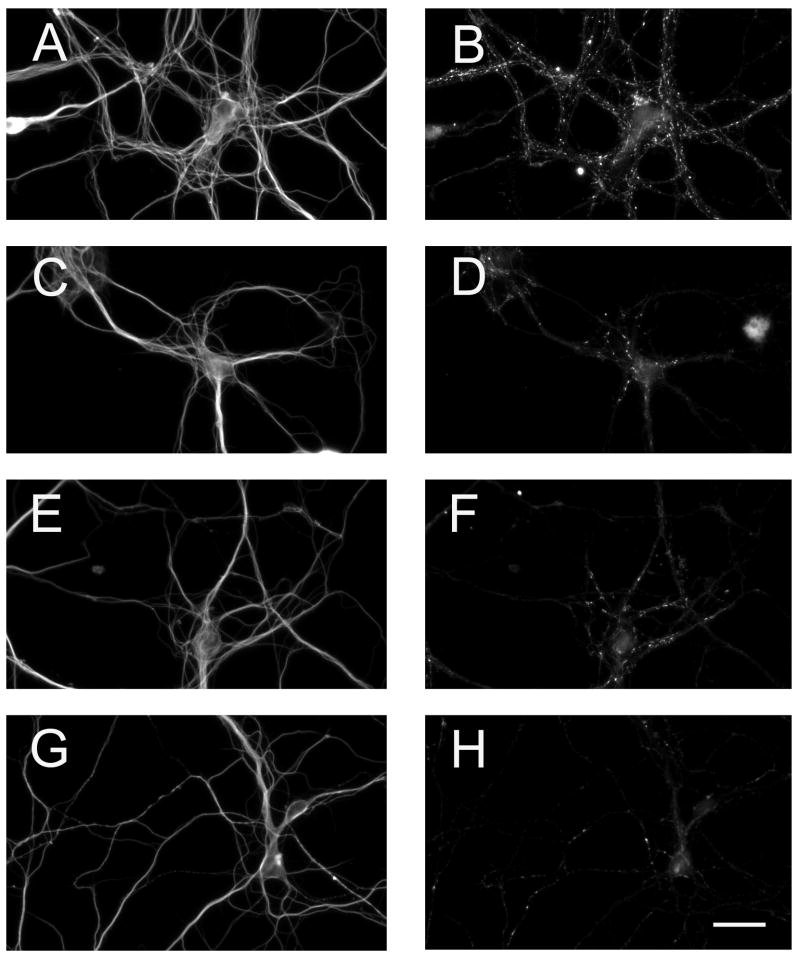
Figure 7. Down regulation of Ror1 and/or Ror2 expression prevented Wnt-5a induced synapse formation in cultured hippocampal neurons.
(A–H) Seven days in culture control (A & B), antisense Ror1- (C & D), antisense Ror2- (E & F), and antisense Ror1/Ror2- (G & H) treated hippocampal neurons maintained in the presence of Wnt-5a-transfected astrocyte cultures were double stained with tubulin (A, C, E, G) and synaptophysin (B, D, F, H) antibodies. Note the decreased in the number of synaptophysin immunoreactive dots in Ror-depleted hippocampal neurons co-cultured with Wnt-5a-transfected astrocytes (D, F, H) as compared to the control ones (B). Scale bar: 20 μm.
DISCUSSION
The results presented herein provide evidence supporting a role for the receptor tyrosine kinases Ror1 and Ror2 in the formation of synapses in hippocampal neurons. Furthermore, they suggest that, through the formation of heterodimers, Ror1 and Ror2 might mediate Wnt-5a signaling in the developing CNS.
The hypothesis that Ror1 and Ror2 might be involved in central synaptogenesis was first raised by the observation that these receptors were highly expressed in the CNS at a developmental time that coincided with the formation of synapses in multiple species (Wilson et al., 1993; Oishi et al., 1997; Forrester et al., 1999; Oishi et al., 1999; Al-Shawi et al., 2001; McKay et al., 2001; Paganoni and Ferreira, 2003). In addition, Ror proteins were localized in the somatodendritic compartment, an area that undergoes extensive remodeling when synapses are being formed (McKay et al., 2001; Paganoni and Ferreira, 2003). Furthermore, a recent study indicated that the Ror family member CAM-1 might be implicated in the formation of peripheral synapses in C. elegans (Francis et al., 2005). However, CAM-1 tyrosine kinase activity may be required for some (i.e. properly orient cell polarity and neurite elongation) but not all of its functions (Forrester et al., 1999; Kim and Forrester, 2003). These observations are now extended by our functional studies showing that Ror1 and/or Ror2-depleted hippocampal neurons formed fewer synapses than control ones. It is worth noting that although the down-regulation of Ror expression was achieved by means of siRNAs or antisense oligonucleotides, the phenotype obtained with each method was identical. These results ruled out non-specific effects of either method.
The suppression of Ror1 and Ror2 expression led to a decrease not only in the number of synapses formed per neuron but also in synaptic density, calculated as the mean number of synapses formed per 10 m of dendritic length. The observed decrease in synaptic density suggested that Ror’s effect on synaptogenesis was independent from changes in dendritic elongation. On the other hand, the effect of Ror molecules on synapse formation might reflect, at least in part, changes in the pattern of axonal extension induced by Ror depletion (Paganoni and Ferreira, 2005). Axons of Ror-depleted neurons elongated fewer branches than controls ones. In contrast, they extended significantly longer axons that Ror-expressing neurons compensating to some extent for the lack of branches (Paganoni and Ferreira, 2005). Therefore, it seems unlikely that the decrease in synapse formation induced by the suppression of Ror expression was only secondary to defects in axonal elongation. Our results showing that Wnt-5a enhanced synaptogenesis only in Ror-expressing neurons provided further support for a role for Ror receptors in the formation of synaptic contacts independently of their neuritogenesis effects in mammalian neurons.
Our results showed a similar decrease in the number of synaptic contacts (both under control conditions and in neurons cultured in the presence of Wnt-5a) when either Ror1 or Ror2 was targeted. These data suggested that the down regulation of either Ror receptor was sufficient to abolish Ror function during synapse formation in hippocampal neurons. These findings are in agreement with a previous study showing that the expression of both Ror1 and Ror2 was required for proper neurite elongation and branching in hippocampal neurons (Paganoni and Ferreira, 2005). Furthermore, the simultaneous down regulation of both Ror1 and Ror2 did not result in an additive phenotype. One likely explanation for such observations is that Ror1 and Ror2 might function in the CNS by associating as heterodimers, a feature that they would share with many other RTKs (Heldin, 1995). Our Ror1 and Ror2 co-immunoprecipitation data suggested that these receptors were indeed able to form heterodimers in both cultured HEK cells and in the mouse brain. Alternatively, Ror1 and Ror2 might mediate distinct signaling pathways that functionally interact at the level of downstream elements. Thus, knock down of either the Ror1 or the Ror2 pathway might disable signaling triggered by activation of the other Ror receptor. These findings suggest that alternative types of interaction might occur between Ror family members, possibly underlying specific interactions between Ror proteins and particular subsets of ligands and/or cellular targets. It is also worth noting that, differently from what we observed, previous studies have shown that Ror proteins might be functionally redundant in some systems. Although the perinatal lethality of Ror mutant mice has prevented a thorough examination of the potential neurological consequences of Ror1 and Ror2 loss in vivo, analysis of Ror2 knockout mice indicated that Ror2 was required for proper skeletal and cardiac development, while Ror1 was not (DeChiara et al., 2000; Takeuchi et al., 2000; Nomi et al., 2001). Together, these results suggest that Ror2 may have a compensatory role for Ror1 loss during the development of the skeletal and cardiovascular systems but not during synapse formation. Alternatively, chronic Ror1 suppression in mutant mice may activate compensatory mechanisms in vivo that are not triggered in hippocampal neurons after acute Ror down regulation.
Ror1 and Ror 2 heterodimers pulled down from mouse brains associated with Wnt-5a, a member of the Wnt family of signaling molecules. These data are consistent with the fact that Ror1 and Ror2 possess a cysteine-rich domain (CRD) similar to the one that is found in Frizzled receptors (Masiakowski and Yancopoulos, 1998; Xu and Nusse, 1998). Frizzled receptors are known to bind to, and mediate the activity of Wnt molecules (Bhanot et al., 1996; Huang and Klein, 2004). Surprisingly, our data showed that isolated Ror2, but not Ror1, physically interacted with Wnt-5a when overexpressed in HEK cells. These results are in agreement with a recent report showing that Ror2 co-immunoprecipitated with some Wnt family members in vitro (Hikasa et al., 2002; Oishi et al., 2003; Billiard et al., 2005). The lack of Ror1-Wnt-5a association might be due to technical reasons (i.e. weak binding that is not detected by co-immunoprecipitation) or to the inability of Ror1 to interact alone or directly with Wnt-5a. Together, these findings suggest that, in the brain, Ror1 and Ror2 might be part of a multimolecular complex. In this complex, Ror2 might directly interact with Wnt-5a, while Ror1 might modulate either this binding or cell signaling, as previously described for other RTK heterodimers (Heldin, 1995; Schlessinger, 2000).
Finally, our results showed that the effects of Wnt-5a on synapse formation were only observed in Ror-expressing neurons. Together, the lack of Wnt-5a effects on neurons depleted of Ror1 and/or Ror2 and its physical interaction with Ror2/Ror1 heterodimers identify Ror as functional Wnt receptors in the Wnt-5a signaling pathway involved in synapse formation. Our co-immunoprecipitation experiments strongly suggested a direct physical interaction between Ror2 and Wnt-5a. However, we cannot rule out that Ror2 might function as coreceptor with any other classical Wnt receptor. A similar mechanism has been described in the case of the mammalian Ryk protein that functions as a coreceptor along with Frizzled for Wnt-3a in dorsal root ganglion neurons (Lu et al., 2004). Ryk also mediated Wnt-5a mediated axon guidance in the corpus callosum and the Wnt-5a repulsion of the corticospinal track (Liu et al., 2005; Keeble et al., 2006).
Regardless of the mechanisms underlying the Wnt-5a-Ror interactions, these results suggest that Ror receptors are novel and key Wnt signaling proteins in hippocampal neurons. Future studies will likely provide further insights regarding the interaction between Ror receptors and Wnt-5a in developing mammalian central neurons.
Supplementary Material
Supplemental Figure 1: Effect of Ror2 down regulation by RNAi on synapse formation in cultured hippocampal neurons.
(A) Western blot analysis of whole cell extracts prepared from control (lane 1), mutated Ror2 siRNA- (lane 2), scrambled Ror2 siRNA- (lane 3) and Ror2 siRNA- (lane 4) transfected hippocampal cultures. Densitometric analysis of the Ror2 immunoreactive bands is shown on the right. Values represent the mean ± S.E.M for 3 independent experiments normalized using tubulin as an internal control. The numbers correspond to the percent of Ror2 protein present in the siRNA-transfected cultures as compared to the levels detected in the untreated samples (100%). *Differs from transfected, mutated siRNA- and scrambled siRNA-transfected cultures: P<0.01. (B–I) Seven days in culture control (B, F), mutated Ror2 siRNA- (C, G), scrambled Ror2 siRNA- (D, H) and Ror2 siRNA- (E, I) transfected hippocampal neurons were double stained with tubulin (B–E) and synaptophysin (F–I) antibodies. Note the reduction in the number of synaptophysin immunoreactive dots in the Ror2 siRNA-transfected cultures (I) as compared to the control ones (F, G, H). (J) Quantitative analysis of the number of synapses formed by neurons cultured under the experimental conditions described above. The numbers represent the mean ± S.E.M for 3 independent experiments. * Differs from all other experimental conditions, P< 0.01. Scale bar: 20 μm.
Supplemental Figure 2: Ror2 suppression by antisense oligonucleotide treatment in cultured hippocampal neurons.
(A–F) Seven days in culture control (A, D), Ror2 sense- (B, E) and Ror2 antisense- (C, F) treated hippocampal neurons were double stained with tubulin (A–C) and Ror2 (D–F) antibodies. Ror2 was readily detectable in control (D) and sense-treated (E) neurons but not in neurons treated with Ror2 antisense oligonucleotides (F). (G–L) Seven days in culture control (G, J), Ror2 sense- (H, K) and Ror2 antisense- (I, L) treated hippocampal neurons were double stained with tubulin (G–I) and synaptophysin (J–L) antibodies. When Ror2 expression was targeted by the addition of Ror2 antisense oligonucleotides, hippocampal neurons formed fewer synapses than controls (compare L to J and K). (M–R) Seven days in culture control (M, P), Ror2 sense- (N, Q) and Ror2 antisense- (O, R) treated hippocampal neurons were double stained with MAP2 (M–O) and synapsin I (P–R) antibodies. Note also the diffuse synapsin I immunoreactivity in some of the axons growing on top of MAP2 immunoreactive processes in antisense-treated neurons (arrow in R). Scale bars: 20 μm.
Acknowledgments
We thank Dr. Minami (Kobe University, Japan) for generously providing the Ror1 and Ror2 expression plasmids. We also thank Dr. Kojima (Northwestern University) for the construction of the pG-SUPER-based silencing Ror constructs. This work was supported by NIH grant NS 46834 to AF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad-Annuar A, Ciani L, Simeonidis L, Herreros J, Ben Fredj N, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and disheveled in presynaptic assembly and neurotransmitter relase. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211:161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, Bodine PV. The Orphan Receptor Tyrosine Kinase Ror2 Modulates Canonical Wnt Signaling in Osteoblastic Cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1977;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Castelo-Blanco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a, and Wnt-5a. Proc Natl Acad Sci USA. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13:599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. Wnts in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Kimble RB, Poueymirou WT, Rojas J, Masiakowski P, Valenzuela DM, Yancopoulos GD. Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24:271–274. doi: 10.1038/73488. [DOI] [PubMed] [Google Scholar]
- Ferreira A. Abnormal synapse formation in agrin-depleted hippocampal neurons. J Cell Sci. 1999;112:4729–4738. doi: 10.1242/jcs.112.24.4729. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Busciglio J, Caceres A. Microtubule formation and neurite growth in cerebellar macroneurons which develop in vitro: evidence for the involvement of the microtubule-associated proteins, MAP-1a, HMW-MAP2 and Tau. Brain Res Dev Brain Res. 1989;49:215–228. doi: 10.1016/0165-3806(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59:83–96. doi: 10.1007/s00018-002-8407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC, Dell M, Perens E, Garriga G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400:881–885. doi: 10.1038/23722. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 Inhibits EGL-20/Wnt Signaling in Cell Migration. Genetics. 2004;168:1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, Norman KR, Maricq AV. The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron. 2005;46:581–594. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Garner CC, Zhai RG, Gundelfinger ED, Ziv NE. Molecular mechanisms of CNS synaptogenesis. Trends Neurosci. 2002;25:243–251. doi: 10.1016/s0166-2236(02)02152-5. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–523. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker GA. Rat hippocampal neurons in low-density culture. In: Banker GA, Goslin K, editors. Culturing nerve cells. The MIT press; Cambridge, MA: 1991. pp. 251–283. [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Heldin CH. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biology. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Keeble TR, Halford MM, Seaman C, Kee N, Macheda M, Anderson RB, Stacker SA, Cooper HM. The Wnt receptor Ryk is required for Wnt-5a-mediated axon guidance on the contralateral side of the courpus callosum. J Neurosci. 2006;26:5840–5848. doi: 10.1523/JNEUROSCI.1175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Forrester WC. Functional analysis of the domains of the C. elegans Ror receptor tyrosine kinase CAM-1. Dev Biol. 2003;264:376–390. doi: 10.1016/j.ydbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Koga M, Take-uchi M, Tameishi T, Ohshima Y. Control of DAF-7 TGF-(alpha) expression and neuronal process development by a receptor tyrosine kinase KIN-8 in Caenorhabditis elegans. Development. 1999;126:5387–5398. doi: 10.1242/dev.126.23.5387. [DOI] [PubMed] [Google Scholar]
- Kojima S, Vignjevic D, Borisy G. Improved silencing vector co-expressing GFP and small hairpin RNA. BioTechniques. 2004;36:74–79. doi: 10.2144/04361ST02. [DOI] [PubMed] [Google Scholar]
- Krylova O, Herreros J, Cleverley KE, Ehler E, Henriquez JP, Hughes SM, Salinas PC. WNT-3, expressed by motoneurons, regulates terminal arborization of neurotrophin-3-responsive spinal sensory neurons. Neuron. 2002;35:1043–1056. doi: 10.1016/s0896-6273(02)00860-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127:457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu C-C, Wang ZB, Lyuksyutova AI, Song X-J, Zou Y. Ryk-mediated Wnt repuslsion regulates posterior-directed growth of corticospinal track. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. Wnt-7a induces axonal modeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;193:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Machon O, van den Bout CJ, Backman M, Kemler R, Krauss S. Role of beta-catenin in the developing cortical and hippocampal neuroepithelium. Neuroscience. 2003;122:129–143. doi: 10.1016/s0306-4522(03)00519-0. [DOI] [PubMed] [Google Scholar]
- Masiakowski P, Yancopoulos GD. The Wnt receptor CRD domain is also found in MuSK and related orphan receptor tyrosine kinases. Curr Biol. 1998;8:R407. doi: 10.1016/s0960-9822(98)70263-5. [DOI] [PubMed] [Google Scholar]
- McKay SE, Hislop J, Scott D, Bulloch AG, Kaczmarek LK, Carew TJ, Sossin WS. Aplysia ror forms clusters on the surface of identified neuroendocrine cells. Mol Cell Neurosci. 2001;17:821–841. doi: 10.1006/mcne.2001.0977. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Nomi M, Oishi I, Kani S, Suzuki H, Matsuda T, Yoda A, Kitamura M, Itoh K, Takeuchi S, Takeda K, et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol Cell Biol. 2001;21:8329–8335. doi: 10.1128/MCB.21.24.8329-8335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Sugiyama S, Liu ZJ, Yamamura H, Nishida Y, Minami Y. A novel Drosophila receptor tyrosine kinase expressed specifically in the nervous system. Unique structural features and implication in developmental signaling. J Biol Chem. 1997;272:11916–11923. doi: 10.1074/jbc.272.18.11916. [DOI] [PubMed] [Google Scholar]
- Oishi I, Takeuchi S, Hashimoto R, Nagabukuro A, Ueda T, Liu ZJ, Hatta T, Akira S, Matsuda Y, Yamamura H, et al. Spatio-temporally regulated expression of receptor tyrosine kinases, mRor1, mRor2, during mouse development: implications in development and function of the nervous system. Genes Cells. 1999;4:41–56. doi: 10.1046/j.1365-2443.1999.00234.x. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Packard M, Koo ES, Gorczyca M, Sharpe J, Cumberledge S, Budnik V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell. 2002;111:319–330. doi: 10.1016/s0092-8674(02)01047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73:429–440. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- Paganoni S, Ferreira A. Neurite extension in central neurons: a novel role for the receptor tyrosine kinases Ror1 and Ror2. J Cell Sci. 2005;118:433–446. doi: 10.1242/jcs.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opinion in Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis-a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Synaptogenesis: Wnt and TGF-beta take centre stage. Curr Biol. 2003;13:R60–62. doi: 10.1016/s0960-9822(02)01429-x. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Takeda K, Oishi I, Nomi M, Ikeya M, Itoh K, Tamura S, Ueda T, Hatta T, Otani H, et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells. 2000;5:71–78. doi: 10.1046/j.1365-2443.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Linhoff MW, Ornitz DM, Sanes JR. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell. 2004;118:257–270. doi: 10.1016/j.cell.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Wilson C, Goberdhan DC, Steller H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc Natl Acad Sci USA. 1993;90:7109–7113. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr Biol. 1998;8:R405–406. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Effect of Ror2 down regulation by RNAi on synapse formation in cultured hippocampal neurons.
(A) Western blot analysis of whole cell extracts prepared from control (lane 1), mutated Ror2 siRNA- (lane 2), scrambled Ror2 siRNA- (lane 3) and Ror2 siRNA- (lane 4) transfected hippocampal cultures. Densitometric analysis of the Ror2 immunoreactive bands is shown on the right. Values represent the mean ± S.E.M for 3 independent experiments normalized using tubulin as an internal control. The numbers correspond to the percent of Ror2 protein present in the siRNA-transfected cultures as compared to the levels detected in the untreated samples (100%). *Differs from transfected, mutated siRNA- and scrambled siRNA-transfected cultures: P<0.01. (B–I) Seven days in culture control (B, F), mutated Ror2 siRNA- (C, G), scrambled Ror2 siRNA- (D, H) and Ror2 siRNA- (E, I) transfected hippocampal neurons were double stained with tubulin (B–E) and synaptophysin (F–I) antibodies. Note the reduction in the number of synaptophysin immunoreactive dots in the Ror2 siRNA-transfected cultures (I) as compared to the control ones (F, G, H). (J) Quantitative analysis of the number of synapses formed by neurons cultured under the experimental conditions described above. The numbers represent the mean ± S.E.M for 3 independent experiments. * Differs from all other experimental conditions, P< 0.01. Scale bar: 20 μm.
Supplemental Figure 2: Ror2 suppression by antisense oligonucleotide treatment in cultured hippocampal neurons.
(A–F) Seven days in culture control (A, D), Ror2 sense- (B, E) and Ror2 antisense- (C, F) treated hippocampal neurons were double stained with tubulin (A–C) and Ror2 (D–F) antibodies. Ror2 was readily detectable in control (D) and sense-treated (E) neurons but not in neurons treated with Ror2 antisense oligonucleotides (F). (G–L) Seven days in culture control (G, J), Ror2 sense- (H, K) and Ror2 antisense- (I, L) treated hippocampal neurons were double stained with tubulin (G–I) and synaptophysin (J–L) antibodies. When Ror2 expression was targeted by the addition of Ror2 antisense oligonucleotides, hippocampal neurons formed fewer synapses than controls (compare L to J and K). (M–R) Seven days in culture control (M, P), Ror2 sense- (N, Q) and Ror2 antisense- (O, R) treated hippocampal neurons were double stained with MAP2 (M–O) and synapsin I (P–R) antibodies. Note also the diffuse synapsin I immunoreactivity in some of the axons growing on top of MAP2 immunoreactive processes in antisense-treated neurons (arrow in R). Scale bars: 20 μm.