Abstract
TNF-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) cytokine superfamily which regulates a number of cellular responses, including inflammation and proliferation. TWEAK is primarily secreted by phagocytic cells and its receptor, fibroblast growth factor-inducible 14 (Fn14), is expressed on non-lymphoid cells, including epithelial, endothelial and mesenchymal cells. The TWEAK/Fn14 pathway is highly conserved from an evolutionary standpoint, and has been shown to play a role in tissue regeneration and inflammation in the liver, kidney, lung and skeletal muscle. We hypothesized that TWEAK/Fn14 might have a physiological role in regulating infection-induced inflammation in the lower female genital tract. To test this hypothesis, we examined expression of the receptor Fn14 in relevant cells and tissue. Receptor function was tested by treating cells with recombinant TWEAK, with and without other known pro-inflammatory stimuli. Flow cytometric analysis of vaginal and cervical epithelial cells revealed that Fn14 was highly expressed at the cell surface. We also detected both Fn14 and TWEAK in whole cervical tissue by RT-PCR. Treatment of vaginal and cervical epithelial cells with recombinant TWEAK led to a weak induction of the chemokine IL-8. However, TWEAK potentiated the effects of IL-1ß, the TLR2 ligand Pam3CysSK4, and live Neisseria gonorrhoeae in a synergistic manner. These data reveal a novel pathway for regulation of microbial-induced inflammation in the female reproductive tract and suggest that interference with the TWEAK/Fn14 pathway might be an approach to abrogate excessive infection-induced inflammation caused by sexually transmitted pathogens.
Keywords: Innate Immunity, Epithelial Cells, Cytokine Receptors
1. Introduction
TNF-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) family of cytokines. This multifunctional protein was first described as a weak inducer of apoptosis; however, it has now been shown to regulate a variety of cellular functions, including cell survival, cell growth, angiogenesis, and inflammation [reviewed in (Burkly et al., 2007)]. The TWEAK receptor Fn14 is related to the TNF receptor (TNFR) superfamily, and contains a single cysteine rich domain in its extracellular region and a TNFR-associated factor (TRAF) binding motif in its intracellular domain (Wiley et al., 2001). TWEAK is made primarily by lymphoid cells as a type II transmembrane protein that is cleaved to produce the biologically active cytokine (Chicheportiche et al., 1997). In contrast, Fn14 is expressed by nonlymphoid cells, such as epithelial, endothelial and mesenchymal cells, and its engagement with TWEAK leads to the activation of the transcription factor NF-κB and MAP kinase pathways (Meighan-Mantha et al., 1999). The TWEAK/Fn14 pathway is believed to be evolutionarily ancient, and both proteins have been identified in species as divergent as zebra fish and mammals; some evidence suggests that TWEAK/Fn14 may even have preceded evolution of vertebrates [reviewed in (Burkly et al., 2007)]. While the regulation of TWEAK is not fully understood, Fn14 appears to be expressed at low levels in a variety of cell types, and its expression is readily up regulated by growth factors and has been shown to be induced within injured tissue.
A number of in vitro and in vivo studies suggest that the TWEAK/Fn14 interaction may play a role regulating inflammation within tissues, such as the kidney, lung, liver, and muscle (Campbell et al., 2006; Girgenrath et al., 2006; Jakubowski et al., 2005; Sanz et al., 2008; Xu et al., 2004). For example, Fn14 is expressed in the kidney (Campbell et al., 2006) and systemic administration of TWEAK to mice induced renal tubular inflammation (Sanz et al., 2008). In the liver, transgenic over expression of TWEAK has been shown to induce progenitor cell inflammation in the mouse and in human tissue, increased expression of Fn14 in some forms of chronic liver disease suggest that it may contribute to liver damage (Jakubowski et al., 2005). While these studies suggest TWEAK has a proinflammatory role, others suggest the opposite. Maecker and colleagues reported the first TWEAK knockout mouse, and found that mice had enlarged spleens, overabundant natural killer cells, and were hypersensitive to LPS injection (Maecker et al., 2005). Because TWEAK suppressed interferon (IFN)-γ and interleukin (IL)-12, they hypothesized that TWEAK functioned to suppress the innate immune response and inhibit Th1 immunity. A similar anti-inflammatory role for TWEAK was recently reported in implantation and embryo survival, where it is thought to counter the high TNF environment of the pre- and periimplantation uterus (Mas et al., 2008).
We were interested in determining if the TWEAK/Fn14 pathway was active within the lower female genital tract and how it might function in regulating inflammation. Using flow cytometry we found that the TWEAK receptor Fn14 was indeed expressed on the surface of both vaginal and cervical epithelial cells. Both TWEAK and Fn14 could also be detected in cervical tissue by RT-PCR. The surface expressed Fn14 was functional, and incubation of these cells with recombinant TWEAK led to the up regulation of the chemokine IL-8 and activation of the transcription factor NF-κB. While TWEAK alone was a weak stimulus in cells, it potentiated the effects of treatment with interleukin (IL)-1ß, the Toll-like receptor (TLR)-2 synthetic ligand Pam3CysSK4, and the genital pathogen Neisseria gonorrhoeae in a synergistic manner. This suggests a novel receptor cross-talk between the TLR/IL-1R and Fn14 pathways that can amplify inflammatory signals in this compartment.
We conclude that TWEAK fuels the induction of chemokines and other proinflammatory signals by mucosal epithelial cells via the receptor Fn14, amplifying the innate immune response, and hypothesize that this pathway may play a role in the pathogenesis of genital tract infections and other inflammatory states.
2. Materials and methods
2.1. Cell lines
The human papillomavirus 16/E6E7 immortalized endocervical (End1/E6E7), ectocervical (Ect1/E6E7), and vaginal (Vk2/E6E7) epithelial cell lines were a gift from Dr. Raina Fichorova (Brigham and Women's Hospital, Boston, MA) and are described elsewhere (Fichorova et al., 1997; Fichorova et al., 2002). HeLa cells were obtained from the American Type Culture Collection. HEK293 cells stably expressing Flag-tagged human TLR2 were a gift from Evelyn Kurt-Jones and are described elsewhere (Kurt-Jones et al., 2002). All cells lines used were free of mycoplasma by PCR (Ossewaarde et al., 1996).
2.2. Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Cervical tissue from two anonymous donors, aged 48 and 53 years, was obtained from the National Disease Research Interchange (Philadelphia, PA). For extraction of RNA, 100 µg of tissue was excised and pulverized in 1 ml of RLT lysis buffer (Qiagen; Valencia, CA). The tissue lysate was collected and RNA isolated using the RNeasy kit from Qiagen. From the purified RNA, cDNA was reverse transcribed using SuperScript First Strand kit from Invitrogen (Carlsbad, CA), with oligo dT as a primer. The cDNA template was then subjected to 35 cycles of a three step PCR program to detect Fn14 and TWEAK products. PCR was carried out with an Applied Biosystems 2720 Thermal Cycler using Taq polymerase (Stratagene; LaJolla, CA) and an annealing temperature of 60°C. Primers for amplifying TWEAK, Fn14, and GAPDH were designed utilizing Primer3 software (frodo.wi.mit.edu), with and were as follows (5′ to 3′): TWEAK, TGAAGTTCATCCACGACCTG and AGCCTTCCCCTCATCAAAGT; Fn14, GACCGCACAGCGACTTCT and GTCTCCTCTATGGGGGTGGT; GAPDH, GAGTCAACGGATTTGGTCGT and GACAAGCTTCCCGTTCTCAG.
2.3. Antibody staining and flow cytometry
Heparinized venous blood for normal, healthy volunteers was obtained under a human subjects protocol approved by Boston University Medical Center Institutional Review Board. B cells and T cells were identified during flow cytometric analysis based on their forward and side scatter patterns, and immunostaining with phycoerythrin (PE)-Cy5-conjugated monoclonal antibody against CD19 or CD3 antibody to detect B cells and T cells, respectively (BD-Bioscience). Purified, PE-conjugated monoclonal antibody against human Fn14 (clone ITEM-4) and TWEAK (clone CARL-1), along with their isotype controls, were purchased from BioLegend (San Diego, CA). Antibody to CD54 (also known as ICAM-1) and its isotype control were purchased from BD Biosciences (San Jose, CA). Cells were incubated on ice with antibody at a final concentration of 8 μg/ml in phosphate buffered saline containing 1% fetal bovine serum for 15 minutes. Labelled cells were analyzed by flow cytometry using a fluorescence activated cell sorter (FACScan) microfluorimeter (BD Biosciences). A total of 10,000 events were analyzed for each condition.
2.4. Assay for chemokine expression
Cells were plated in 96-well tissue culture dishes at a density of 2×104 cells per well using growth medium one day prior to stimulation. Cells were subsequently treated with recombinant TWEAK (Fitzgerald Industries; Concord, MA) at a concentration of 1 μg/ml, with or without other stimuli: human IL-1ß from Biosource/Invitrogen (Camarillo, CA); synthetic lipopeptide Pam3Cys-SK4 from EMC Microcollections (Tuebingen, Germany); and Neisseria gonorrhoeae strain F62 (Schneider et al., 1982). Supernatant was harvested 24 hours later and assayed for human IL-8 production using a DuoSet ELISA kit from R&D Systems (Minneapolis, MN). All data points were assayed in triplicate, and reported as the mean ± the standard error of the mean (SEM). Data was analysed using an unpaired t-test and significance was concluded when p<0.05. All ELISA assays were repeated at least twice.
2.5. Luciferase reporter assays
The NF-κB luciferase reporter plasmid, consisting of five NF-κB sites upstream of luciferase and the IL-8 promoter luciferase reporter plasmid, were gifts from from Dr. Kate Fitzgerald (Univ. Massachusetts Medical School) (Fitzgerald et al., 2001). HeLa cells were plated in 48-well tissue culture plates at a density of 50,000 cells per well. The next day, cells were transfected using 1 μl Genejuice transfection reagent (Novagen/EMD, Madison, WI), plus 75 ng of the luciferase reporter plasmid, and again allowed to recover overnight. The following day, cells were treated with recombinant human TWEAK, TNFα, or left untreated. After 18 hours, the cells were assayed for luciferase activity according to the manufacturer's protocol (Promega; Madison, WI) using a Synergy HT microplate reader (BioTek; Winooski, VT). All data points were assayed in triplicate, and the data are reported as the mean ± the standard error of the mean (SEM). Data was analysed using an unpaired t-test and significance was concluded when p<0.05. All transfection experiments were repeated at least twice.
3. RESULTS
3.1. Vaginal and cervical epithelial cells express Fn14 at the surface
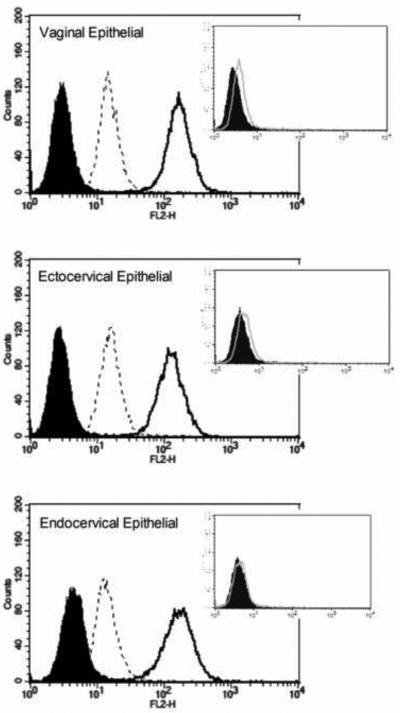
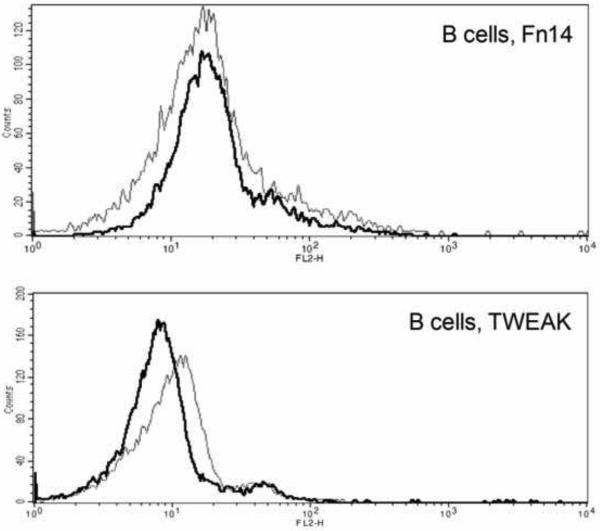
We have established an in vitro model to study epithelial-microbial interaction in the lower female reproductive tract utilizing immortalized epithelial cells derived from human vagina, ectocervix and endocervix. In order to determine if Fn14 is expressed on the surface of epithelial cells from the lower female genital tract, we stained cells using a commercially available monoclonal antibody, and analyzed them by flow cytometry. We found all three cell lines demonstrated a significant shift in fluorescence when stained with the specific antibody compared to the isotype control (Fig. 1A). HeLa cells similarly expressed Fn14 at the surface. In contrast to Fn14, which was highly expressed at the cell surface, we found little or no staining of the cell surface with TWEAK antibody (Fig. 1A, inset). In contrast to the human cervical epithelial cells, we found that human B cells fail to express Fn14 or TWEAK at the cell surface (Fig. 1B), confirming previously published data from an independent laboratory (Desplat-Jego et al., 2009).
Figure 1. Vaginal and cervical epithelial cells express Fn14 at the cell surface.
(A) Shown above are FACS histograms for immortalized vaginal (top histogram), ectocervical (middle histogram), and endocervical (lower histogram) epithelial cell lines. Cells were stained with antibody to Fn14 (solid line) or mouse IgG2bκ as an isotype control (shaded histogram). The surface expression of Fn14 following a 24 hour incubation with 500 ng TWEAK per ml is shown in the dashed line. The vertical axis represents the relative cell number, while the horizontal axis represents the intensity of fluorescence staining for the antibody. In the inset for each histogram, is the surface expression of TWEAK vs. IgG3κ isotype control antibody for each of the three epithelial cell lines. Similar results were found for HeLa cells (data not shown). These histograms are representative of at least three separate experiments. (B) Human B cells, identified by their forward and side scatter properties as well as surface CD19 expression, were stained for surface expression of either Fn14 (top histogram) or TWEAK (lower histogram). Staining with the specific antibody is shown in the thick black line, while the appropriate isotype control is represented by the thin gray line. Similar results were found for T cells (data not shown) and confirmed in two independent blood donors.
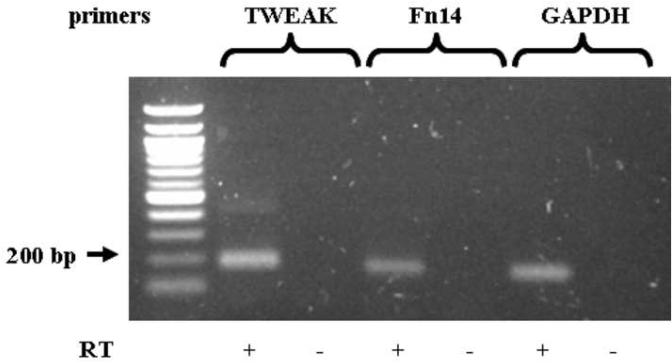
In order to confirm that FACS data and demonstrate the expression of Fn14 in the lower female genital tract in vivo, we also screened human cervical tissue from two different anonymous donors by RT-PCR. Fn14 mRNA could be detected in whole cervical tissue (Fig. 2). We also found evidence of strong TWEAK mRNA expression in whole cervical tissue, which could reflect the presence of phagocytic cells in the tissue, such as resident macrophages and dendritic cells, which are reported to be common sources of the cytokine in tissues.
Figure 2. TWEAK and Fn14 are expressed in whole cervical tissue.
RNA was prepared from the cervical tissue and subjected to RT-PCR. The resulting cDNA was then assayed by PCR to determine the expression of TWEAK and Fn14 as described in the text. GAPDH served as the endogenous control and reverse transcriptase (RT) was excluded in samples testing for chromosomal DNA contamination. The identity of the PCR product was confirmed by DNA sequencing. Similar results were obtained from the two independent tissue donors.
3.2. Incubation with TWEAK down regulates Fn14 surface expression
The expression of Fn14 is known to be highly inducible by various growth factors [reviewed in (Burkly et al., 2007)], however there is little data on the effect inflammation might have on the regulation of expression. In order to address this, we incubated cells with TWEAK, IL-1ß, or live Neisseria gonorrhoeae, and stained for surface expression of Fn14 before and after stimulation. While neither the cytokine IL-1ß nor incubation with live N. gonorrhoeae altered Fn14 surface expression, we did find that Fn14 expression was down regulated in response to treatment with the ligand, TWEAK (Fig. 1A). Similar to Fn14, we found no change in the surface expression of TWEAK in response to incubation with IL-1ß (data not shown).
3.3. TWEAK activates NF-κB and IL-8 pathways in genital tract epithelial cells
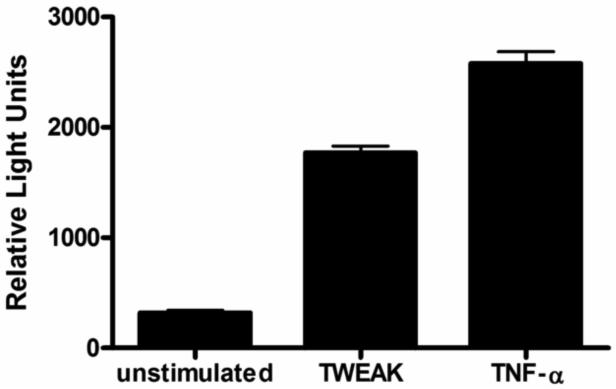
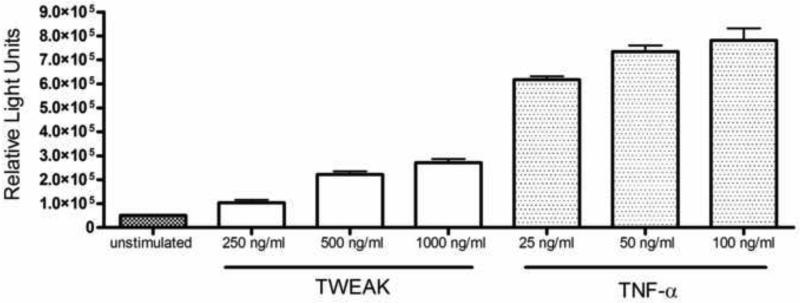
TWEAK signals through the receptor Fn14, which contains TRAF binding sequences. Engagement of the receptor results in the activation of the transcription factor NF-κB which is a central mediator of inflammatory signals, including the release of several pro-inflammatory cytokines and chemokines [reviewed in (Burkly et al., 2007; Winkles, 2008)]. In order to determine if Fn14 activation in genital tract epithelium could activate NF-κB-dependent pathways, we treated HeLa cells transiently transfected with a strong NF-κB luciferase reporter construct with TWEAK or the related cytokine, TNFα, as a control. Both TWEAK and TNFα potently induced NF-κB activity, as measured by increased luciferase activity over untreated cells (Fig. 3A). In order to compare the potency of the two cytokines further, we treated cells with increasing concentrations of each cytokine, and measured the induction of IL-8 using an IL-8 luciferase reporter assay. The two cytokines showed vastly different potencies in their ability to induce IL-8 activity (Fig. 3B). Even a 40-fold higher dose of TWEAK induced only half the IL-8 as TNFα. These data demonstrate that TWEAK can activate NF-κB and other downstream signaling events, but that it is much less potent than the related cytokine, TNFα.
Figure 3. TWEAK stimulation results in activation of the transcription factor NF-κB and IL-8 in HeLa cells.
Hela cells transiently transfected with an NF-κB or IL-8 luciferase reporter plasmid were stimulated with TWEAK or TNFα, as described in the text. Luciferase activity in each cell lysate was measured in relative light units (RLU) with the error bars representing the SEM from single measurements taken from three separate treatments. (A) Cells were treated with 500 ng TWEAK per ml or 50 ng TNFα per ml. Significance is as follows: p<0.0001, stimulated (TWEAK or TNFα) vs. unstimulated. These results are representative of three independent experiments. (B) Cells were treated with increasing concentrations of either TWEAK or TNFα as indicated. Data are representative of two independent experiments.
*p<0.0001 for highest dose of TWEAK (1000 ng/ml) vs. the lowest dose of TNFα (25 ng/ml).
3.4. TWEAK stimulates IL-8 release from vaginal and cervical epithelial cells, and potentiates the effects of IL-1ß and TLR ligands
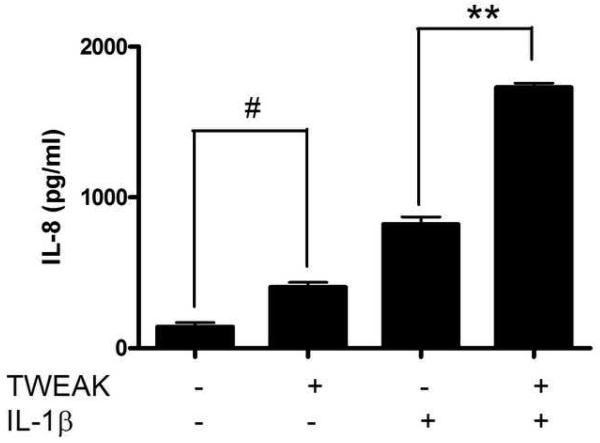
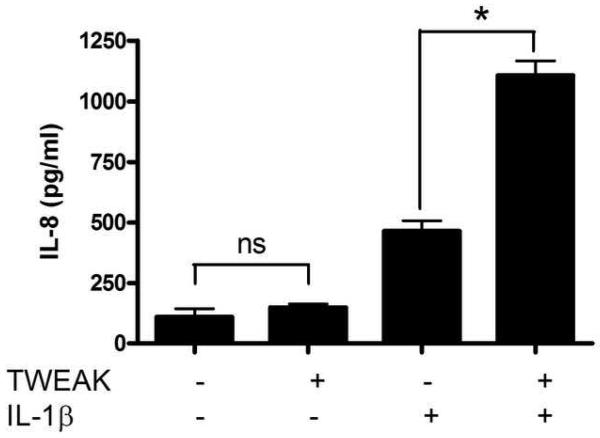
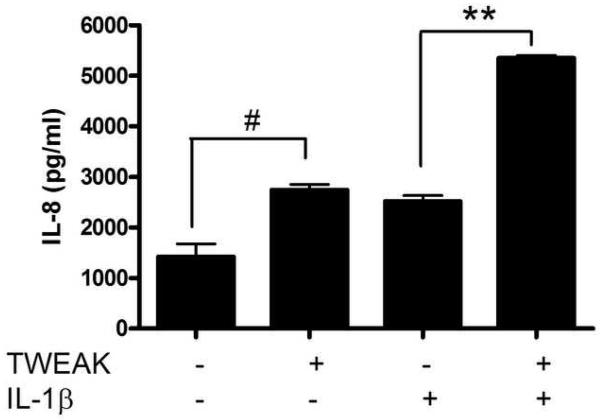
To further examine the proinflammatory pathways induced by TWEAK in lower genital tract epithelial cells, we treated vaginal, ectocervical, and endocervical epithelial cells with TWEAK, and compared the ability of this cytokine to induce IL-8 release with that of the proinflammatory cytokine, IL-1ß. In contrast to the TWEAK receptor Fn14, which signals via TRAF binding sites, the IL-1 receptor activates a MyD88-dependent pathway via its Toll/IL-1R (TIR) domain [reviewed in (Martin and Wesche, 2002)]. When cells were treated with TWEAK alone, it was only a weak inducer of IL-8 secretion compared to IL-1ß (Fig. 4A-C). However, we found that when cells were treated simultaneously with TWEAK and IL-1ß, the effect was enhanced in a synergistic manner, as defined by an effect that is greater than the additive effect of each treatment alone.
Figure 4. TWEAK induces the secretion of IL-8 by vaginal and cervical epithelial cells and potentiates the effect of IL-1ß.
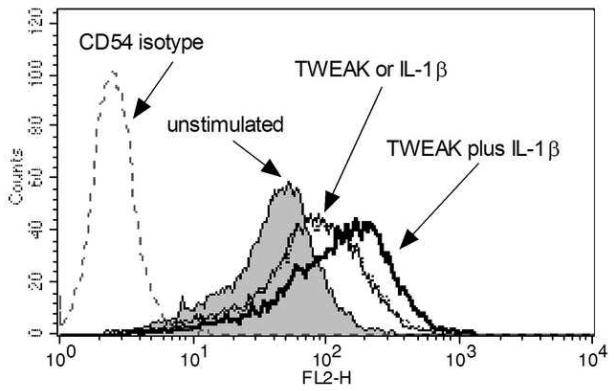
Immortalized vaginal (A), ectocervical (B), and endocervical (C) epithelial cells were stimulated with 1 μg TWEAK per ml, 5 ng IL-1ß per ml, or both, for 24 hours. Culture supernatants were harvested and assayed for IL-8 by ELISA. The values are derived from IL-8 standard curves and represent the mean ± the SEM from triplicate wells, and are representative of at least three independent experiments. (D) Immortalized vaginal epithelial cells were stimulated with 1 μg TWEAK per ml, 5 ng IL-1ß per ml, or both, for 24 hours. Cells were harvested, stained for surface expression of CD54, and analyzed by FACS. The vertical axis represents the relative cell number, while the horizontal axis represents the intensity of anti-CD54 staining. Basal expression of CD54 is shown in the gray shaded histogram. Treatment with TWEAK alone is shown in the thin line while treatment with IL-1ß alone is in the dashed lines. Treatment with TWEAK plus IL-1ß is shown in the thick line. Surface staining with the isotype control antibody for unstimulated cells is shown in the gray dashed line, and stimulation of the cells had no effect on isotype staining (data not shown). Similar results were found with the ectocervical epithelial cells (data not shown). These data are representative of two independent experiments.
* p<0.001; ** p<0.0001 for IL-1ß alone vs. TWEAK + IL-1ß.
# p<0.01 for unstimulated vs. TWEAK alone. ns, not significant .
In addition to secreting IL-8, these cell lines have been previously shown to upregulate the constitutive expression of the surface adhesion molecule CD54 (ICAM-1) in response to proinflammatory signals (Fichorova et al., 2001). In order to determine if TWEAK could similarly amplify CD54 upregulation, we treated cells overnight with TWEAK, in the presence or absence of IL-1ß, and stained them for surface CD54 expression. Vaginal epithelial cells expressed CD54 at baseline, and its expression was equally upregulated upon treatment with either TWEAK or IL-1ß (Fig. 4D). When the two treatments was combined the effect was amplified, as demonstrated by a further shift in the surface expression to the right.
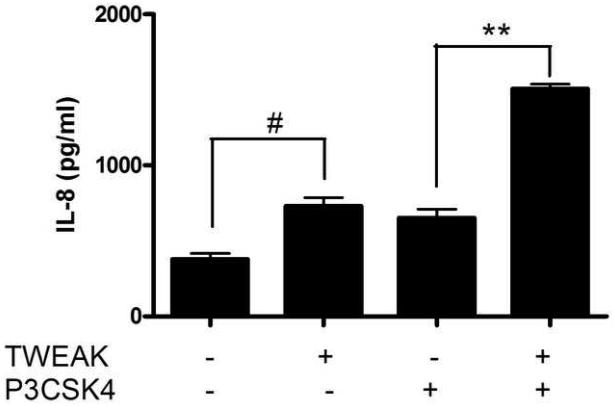
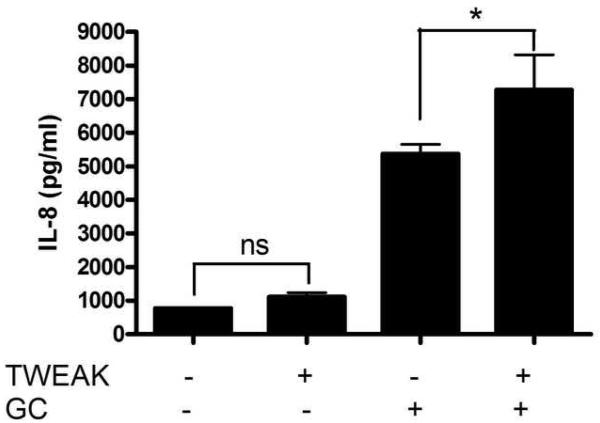
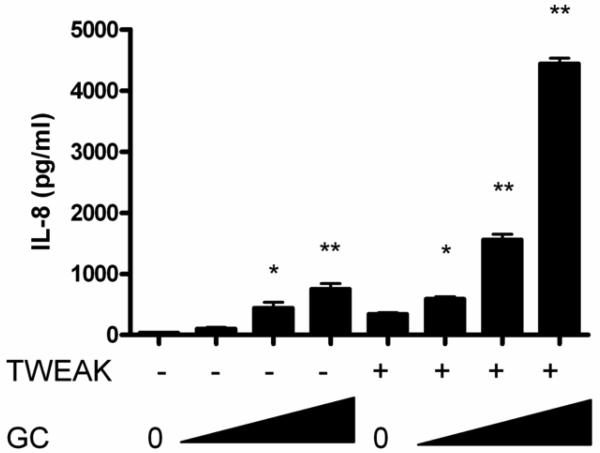
We previously reported that cervical and vaginal epithelial cells express a subset of TLRs (Andersen et al., 2006; Fichorova et al., 2002). Specifically, they fail to express the endotoxin receptor complex of TLR4 and MD2, and appear to respond to Gram-negative bacteria such as the genital tract pathogen N. gonorrhoeae via TLR2 (Fichorova et al., 2002). To further investigate the interaction of TWEAK/Fn14 activation with the innate immune receptor TLR2, we next stimulated endocervical epithelial cells with the synthetic lipopeptide and TLR2 ligand, Pam3CysSK4 (Fig. 5A) and N. gonorrhoeae (Fig. 5B) in the presence or absence of TWEAK, and assayed cell supernatants for secretion of IL-8. Similar to what we observed with IL-1ß, both the synthetic TLR2 ligand and the live bacteria stimulated the release of IL-8, with a synergistic effect when co-incubated with TWEAK.
Figure 5. TWEAK potentiates the effects of Pam3CysSK4 and Neisseria gonorrhoeae on endocervical epithelial cell activation.
Immortalized endocervical cells were stimulated with 1 μg TWEAK per ml, 50 ng Pam3CysSK4 (P3CSK4) per ml, or both, for 24 hours (A). Cells were also treated with 1 μg TWEAK per ml, Neisseria gonorrhoeae strain F62 at an MOI of 10 (GC), or both (B). Culture supernatants were harvested and assayed for IL-8 by ELISA. The values are derived from IL-8 standard curves and represent the mean ± the SEM from triplicate wells. The data are representative of two independent experiments.
* p=0.1; ** p<0.002 for P3CSK4 or GC alone vs. TWEAK + P3CSK4 or GC.
# p<0.01 for unstimulated vs. TWEAK alone. ns, not significant.
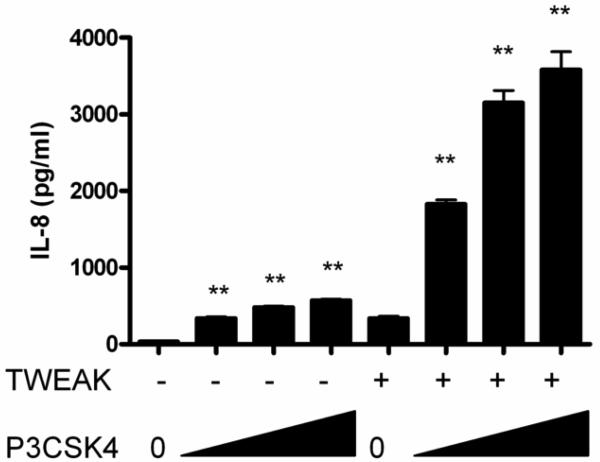
In order to determine if the effect of TWEAK stimulation on TLR and IL-1R activation was unique to the cervical and vaginal epithelial cells only, or if it could be generalized to other cell types, we examined HEK293 cells stably transfected with TLR2. We and others have shown that these cell do not express endogenous TLR2, but when transfected with TLR2 they become highly sensitive to activation with a variety of TLR2 ligands (Fisette et al., 2003; O'Connell et al., 2006). We first examined HEK293/TLR2 cells for expression of endogenous Fn14, and found that, like the other cell lines tested, they expressed Fn14 at the cell surface (data not shown). We then stimulated cells with IL-1ß, Pam3CysSK4 or N. gonorrhoeae in the presence or absence of TWEAK, and assayed cell supernatants for secretion of IL-8. Similar to what we observed with the vaginal and cervical epithelial cells, we found that TWEAK potentiated the IL-8 inducing capacity of the other ligands in a synergistic manner (Fig. 6). This suggests a novel receptor cross-talk between the TLR/IL-1R and Fn14 that is applicable to a variety of cell types.
Figure 6. TWEAK potentiates the effects of Pam3CysSK4 and Neisseria gonorrhoeae on HEK293/TLR2 cell activation.
HEK293/TLR2 cells were stimulated with increasing concentrations of Pam3CysSK4 (5, 50, 500 ng/ml; P3CSK4) or N. gonorrhoeae (multiplicity of infection = 0.1, 1, 10 CFU; GC), in the presence or absence of 1 μg TWEAK per ml. After 24 hours, culture supernatants were harvested and assayed for IL-8 by ELISA. The values are derived from IL-8 standard curves and represent the mean ± the SEM from triplicate wells. Significance is noted as follows for P3CSK4 or GC alone vs. TWEAK + P3CSK4 or GC: *, p<0.001; **, p<0.0001. For unstimulated vs. TWEAK alone, p<0.001. These results are representative of two independent experiments.
In summary, these data demonstrate that while activation of Fn14 alone may have only a minor effect on inflammatory signals, the activation of Fn14 in the context of Toll/IL-1 receptor pathway activation leads to significantly enhanced cell activation.
4. Discussion
TWEAK and Fn14 appear to be ubiquitously expressed proteins in mammalian hosts. Surveys in mice, for example, have demonstrated the expression of TWEAK in heart, brain, placenta, lung, liver, skeletal muscle, kidney, pancreas, and a number of cell lines; in fact, it was reported to be absent only in fetal liver (Chicheportiche et al., 1997). Likewise, the receptor Fn14 can be found in brain, heart, kidney, lung, ovary, skeletal muscle, skin, and testes, while it is undetected in the liver or spleen (Meighan-Mantha et al., 1999). While a variety of cell types have previously been shown to express Fn 14 and respond to TWEAK, our report is the first to examine its expression and function at the genital mucosal epithelial surface. In this study, we demonstrate that both the cytokine TWEAK, and its receptor Fn14, are present in normal cervical tissue. While vaginal and cervical epithelial cells appear to constitutively express the TWEAK receptor Fn14 at their surface, with little or no detectable surface TWEAK, we hypothesize that in vivo, professional immune cells provide a source for the ligand. Thus, taken in the context of previously published data, it would be reasonable to conclude that this cytokine-receptor pair are to be found in nearly all tissues and organs.
Similar to what has been reported for cells derived from other epithelial surfaces, stimulation of these cells with TWEAK resulted in the up regulation of pro-inflammatory pathways, namely activation of the transcription factor NF-κB, and secretion of the chemokine IL-8. Compared to the related cytokine TNFα, TWEAK was significantly less potent in the induction of IL-8. Even a 40-fold higher dose of TWEAK induced only half the IL-8 seen with TNFα. While TWEAK alone was only a weak agonist, it clearly potentiated the activity of other proinflammatory stimuli, specifically IL-1ß, purified TLR2 ligands, and live bacteria in a synergistic manner. Of note, although TWEAK was originally described as a weak inducer of apoptosis, we were unable to demonstrate any effect on cell death or cell proliferation in response to TWEAK using this in vitro system (data not shown).
Interestingly, synergy has also been reported between IL-1ß and TNFα for a variety of read-outs (Haddad et al., 1996; Martensson et al., 2004; Wankowicz et al., 1988). The basis of the synergy between Fn14 and TLR/IL-1R remains unclear, but it does not appear to be a result of receptor upregulation since we were unable to detect any change in surface expression of Fn14 in response to these stimuli. However, interactions between surface receptors remain possible, and the formation of a multimeric receptor complex between the TLR/IL-1R family and Fn14 that might stabilize or enhance signaling events can not be ruled out. Alternatively, cross-talk between proteins in the intracellular signaling pathways of the TLR/IL-1R and the TNFR families is also possible. In any event, these data suggest that activation of Fn14 by TWEAK, in the setting of other proinflammatory signals, may serve to amplify the innate immune response seen in the genital tract during infections and other inflammatory conditions.
Based on our results, we hypothesize that TWEAK helps regulate the proinflammatory response of the lower female genital tract to infectious agents and other inflammatory stimuli in this compartment. However, given its reported effects on cell proliferation and cell survival, it may also play a role in responses to cyclic hormonal changes, pregnancy, and other physiologic conditions. Regulation of Fn14 expression in general remains unclear and, in our genital tract epithelial model, we were unable to discern any obvious change in surface protein, at least in response to inflammatory stimuli. Furthermore, hormonal signals are unlikely to be required for endogenous expression of the receptor since our cell lines are not maintained in the presence of estrogen or progesterone. However, regulation of receptor expression by hormonal changes is still a possibility, and additional human studies to examine its expression in vivo under various physiologic and diseased states will be helpful.
The precise role of TWEAK in modulating inflammatory responses in the genital tract remains unclear. Certainly, regulation of inflammation in a dynamic tissue such as the genital mucosal surface is important in maintaining homeostasis. For example, it is known that the mucosal compartment is exposed to a number of inflammatory stimuli, such as hormones, seminal plasma, and a variety of colonizing microorganisms. On top of this, they must maintain the ability to respond to pathogenic microorganisms and other dangers that might be encountered at that site. A mechanism by which inflammatory signals originating from the epithelia could be amplified through a positive feedback loop might help differentiate between friend and foe. Future studies to determine which cell types in the genital tract express TWEAK and under what conditions will be helpful in defining the role of this cytokine in both physiologic and disease states. While the presence of the receptor in epithelial cells is suggestive that it is biologically relevant, our data are limited by the in vitro nature of our model. While we were mainly interested in examining human-specific responses, the availability of murine-specific reagents, including both Fn14 and TWEAK deficient mice, may enable future studies to examine the role of the TWEAK/Fn14 pathway in response to specific genital tract infections using available mouse models.
Acknowledgements
The authors wish to thank Miss Samrawit Mekasha for technical assistance, the BU Medical Campus Flow Cytometry Core Facility for the use of their core instruments, and the National Disease Research Interchange for providing frozen cervical tissue samples. This work was supported by grants from the National Institutes of Health AI064749 and AI46613 to RRI, and T32 AI052070 to ESH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen JM, Al-Khairy D, Ingalls RR. Innate immunity at the mucosal surface: role of toll-like receptor 3 and toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol Reprod. 2006;74:824–831. doi: 10.1095/biolreprod.105.048629. [DOI] [PubMed] [Google Scholar]
- Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C. Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol. 2006;176:1889–1898. doi: 10.4049/jimmunol.176.3.1889. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Feuillet L, Creidy R, Malikova I, Rance R, Khrestchatisky M, Hahm K, Burkly LC, Pelletier J, Boucraut J. TWEAK is expressed at the cell surface of monocytes during multiple sclerosis. J Leukoc Biol. 2009;85:132–135. doi: 10.1189/jlb.0608347. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA. Distinct Proinflammatory Host Responses to Neisseria gonorrhoeae Infection in Immortalized Human Cervical and Vaginal Epithelial Cells. Infection and Immunity. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–2432. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- Fisette PL, Ram S, Andersen JM, Guo W, Ingalls RR. The Lip lipoprotein from Neisseria gonorrhoeae stimulates cytokine release and NF-kB activation in epithelial cells in a TLR2-dependent manner. J Biol Chem. 2003;278:46252–46260. doi: 10.1074/jbc.M306587200. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O'Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. TWEAK, via its receptor Fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. Embo J. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad EB, Rousell J, Lindsay MA, Barnes PJ. Synergy between tumor necrosis factor alpha and interleukin 1beta in inducing transcriptional down-regulation of muscarinic M2 receptor gene expression. Involvement of protein kinase A and ceramide pathways. J Biol Chem. 1996;271:32586–32592. doi: 10.1074/jbc.271.51.32586. [DOI] [PubMed] [Google Scholar]
- Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B, Bissell DM, Burkly LC. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115:2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Mandell L, Whitney C, Padgett A, Gosselin K, Newburger PE, Finberg RW. Role of toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR2-mediated interleukin 8 responses in neutrophils. Blood. 2002;100:1860–1868. [PubMed] [Google Scholar]
- Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, Hurst S, Danilenko D, Li J, Filvaroff E, Yang B, Daniel D, Ashkenazi A. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Martensson K, Chrysis D, Savendahl L. Interleukin-1beta and TNFalpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004;19:1805–1812. doi: 10.1359/JBMR.040805. [DOI] [PubMed] [Google Scholar]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- Mas AE, Petitbarat M, Dubanchet S, Fay S, Ledee N, Chaouat G. Immune regulation at the interface during early steps of murine implantation: involvement of two new cytokines of the IL-12 family (IL-23 and IL-27) and of TWEAK. Am J Reprod Immunol. 2008;59:323–338. doi: 10.1111/j.1600-0897.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA. The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem. 1999;274:33166–33176. doi: 10.1074/jbc.274.46.33166. [DOI] [PubMed] [Google Scholar]
- O'Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem. 2006;281:1652–1659. doi: 10.1074/jbc.M510182200. [DOI] [PubMed] [Google Scholar]
- Ossewaarde JM, de Vries A, Bestebroer T, Angulo AF. Application of a Mycoplasma group-specific PCR for monitoring decontamination of Mycoplasma-infected Chlamydia sp. strains. Appl Environ Microbiol. 1996;62:328–331. doi: 10.1128/aem.62.2.328-331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A. The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol. 2008;19:695–703. doi: 10.1681/ASN.2007050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Griffiss JM, Williams GD, Pier GB. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982;128:13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z, Megyeri P, Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988;43:349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC. A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity. 2001;15:837–846. doi: 10.1016/s1074-7613(01)00232-1. [DOI] [PubMed] [Google Scholar]
- Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–425. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Okamoto A, Ichikawa J, Ando T, Tasaka K, Masuyama K, Ogawa H, Yagita H, Okumura K, Nakao A. TWEAK/Fn14 interaction stimulates human bronchial epithelial cells to produce IL-8 and GM-CSF. Biochem Biophys Res Commun. 2004;318:422–427. doi: 10.1016/j.bbrc.2004.04.036. [DOI] [PubMed] [Google Scholar]