Abstract
Dreams are a most remarkable experiment in psychology and neuroscience, conducted every night in every sleeping person. They show that our brain, disconnected from the environment, can generate by itself an entire world of conscious experiences. Content analysis and developmental studies have furthered our understanding of dream phenomenology. In parallel, brain lesion studies, functional imaging, and neurophysiology have advanced our knowledge of the neural basis of dreaming. It is now possible to start integrating these two strands of research in order to address some fundamental questions that dreams pose for cognitive neuroscience: how conscious experiences in sleep relate to underlying brain activity; why the dreamer is largely disconnected from the environment; and whether dreaming is more closely related to mental imagery or to perception.
Contemporary dream research
Although dreams have fascinated us since the dawn of time, their rigorous, scientific study is a recent development[1–4] (Supplementary Fig. 1). In The interpretation of dreams [5] Freud predicted that “Deeper research will one day trace the path further and discover an organic basis for the mental event.” Recent work, which we review in this article, begins to fulfill Freud s prediction.
The study of dreams is a formidable task, because dream consciousness is only accessible via report rather than direct observation (Box 1) and because it is difficult to manipulate dream content experimentally, whether by exposure to stimuli before[6, 7] or during sleep[7, 8]. Therefore, it is difficult to predict the contents of specific dreams[9], and most modern dream research tries to relate neuronal activity retrospectively to dream form rather than dream content, i.e. to focus on properties of all dreams rather than to investigate the neural correlates of a particular dream. Yet, as we shall see, encouraging progress has been made in relating the phenomenology of dreams to underlying brain activity, and to studies of brain damage and development.
BOX 1. Can reports be trusted to accurately convey internal experiences in sleep?
Do dream reports obtained by awakening a sleeping subject accurately convey subjective experiences in sleep? At one extreme, we could be fully conscious throughout sleep but remember dreams well, little, or not at all depending on the brain state when we are awakened. Indeed, we know that dreaming often goes unreported – some people claim they rarely dream, but systematic awakenings in sleep labs have revealed that we greatly underestimate how often and how much we are conscious during sleep. On the other hand, neurological patients who report loss of dreaming are no more likely to have memory disorders than those who report dreaming[22], suggesting that lack of dream reports indeed reflects lack of experience rather than changes in memory alone. Further studies may illuminate this issue since, for example, memory-related regions in the medial temporal lobe are highly active in REM sleep (Fig. 1).
At the other extreme, one could claim that we are unconscious throughout sleep and merely have a tendency to confabulate during the transition into wakefulness. While such a claim is hard to refute conclusively (just as it is hard to prove conclusively that one is not a zombie when awake), it seems highly implausible; when one has just experienced a vivid dream, it seems hard to believe that it was made up in a flash during an awakening. Indeed, (a) the estimated time in dream report correlates well with the time elapsed in REM sleep before awakening[62]; and (b) in REM sleep behavior disorder (where muscle atonia is disrupted), movements seem to match the reported dream[113].
Reports obtained upon awakenings from deep NREM sleep are more difficult to evaluate because of disorientation associated with increased sleep inertia[114]. However, some evidence indicates that indeed dream consciousness can occur in NREM sleep and does not merely reflect recalls of earlier REM sleep dreams[59]: (a) It is sometimes possible to influence dream content by sounds delivered in NREM sleep, and to “tag” NREM reports[59], (b) Some NREM parasomnias (sleep talking, sleep terrors) correspond to reported dream experiences[115], and (c) “Full-fledged” dreams are sometimes reported upon awakening from the first NREM episode, before any REM sleep occurred [59, 66], and even in naps consisting of only NREM sleep[67].
Nevertheless, it is worth keeping in mind that several factors may render dream reports less trustworthy when compared to reports of waking experience, including: (a) a dramatic state change, since we report about a sleep experience when awake; (b) considerable time delay, since dream reports are obtained after the experience, possibly leading to passive forgetting and interference; (c) difficulties in verbally describing experiences that are mainly visual and emotional; and (d) censorship of embarrassing, immoral, sexual and aggressive material.
Phenomenology of dreams and their relation to brain activity
The level and nature of our conscious experience varies dramatically in sleep. During slow wave sleep (SWS) early in the night, consciousness can nearly vanish despite persistent neural activity in the thalamocortical system[10]. Subjects awakened from other phases of sleep, especially but not exclusively during REM sleep, report “typical”, full-fledged dreams - vivid, sensorimotor hallucinatory experiences that follow a narrative structure[3, 11]. The dreamer is highly conscious (she has vivid experiences), is disconnected from the environment (she is asleep), but somehow her brain is creating a story, filling it with actors and scenarios, and generating hallucinatory images. How does the brain accomplish this remarkable feat? And, conversely, what do dreams tell us about the organization and working of the brain?
Since awakenings from REM sleep regularly yield reports of typical dreams, we will first focus on neural activity during REM sleep, to gain insight into brain states that are compatible with dreaming. It should be emphasized at the outset, however, that dreams can occur in other brain states, such as late NREM sleep, as will be discussed below.
Similarities between dreaming and waking
In order to gain insight into the phenomenology and neural basis of dreams, it is useful to consider both similarities and differences between waking consciousness and dreaming consciousness, and to relate these differences to changes in brain activity and organization[11]. Perhaps the most striking feature of conscious experiences in sleep is how altogether similar the inner world of dreams is to the real world of wakefulness. Indeed, at times the dreamer may be uncertain whether he is awake or asleep. Certainly, dreams are not created in a vacuum but closely reflect the organization and functions of our brain.
In most dreams, perceptual modalities and submodalities that dominate in wakefulness are heavily represented. Dreams are highly visual, in full color, rich in shapes, full of movement, and incorporate typical wakefulness categories such as people, faces, places, objects, and animals[3]. Dreams also contain sounds (including speech and conversation), and more rarely tactile percepts, smells and tastes, as well as pleasure and pain[4, 12–14]. Experiences in typical dreams have a clear sensory character (i.e. they are seen, heard, and felt) and are not mere thoughts or abstractions.
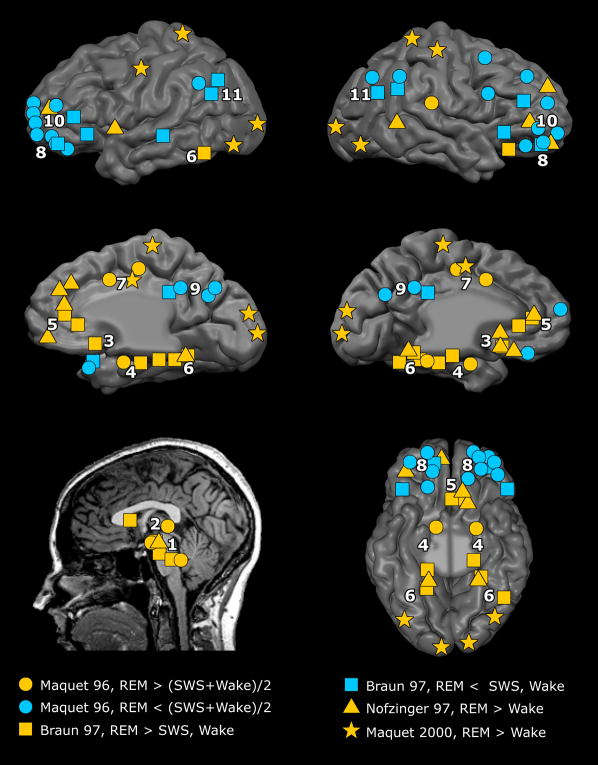
These phenomenological similarities are reflected in neurophysiological similarities between waking and dreaming. For historical and methodological reasons, most electroencephalogram (EEG) and neuroimaging studies have contrasted brain activity during quiet wakefulness with that observed during REM sleep, when subjects are most likely to report dreams[15–20]. At least superficially, the EEG looks remarkably similar in active waking and REM sleep. Positron emission tomography (PET) studies have shown that global brain metabolism is comparable between wakefulness and REM sleep[11, 20]. Such studies have also revealed a strong activation of high-order occipito-temporal visual cortex in REM sleep, consistent with the vivid visual imagery during dreams (Fig. 1)[16, 17, 19].
Figure 1. Functional neuroanatomy of human REM sleep: a meta-analysis of PET results.
Meta-analysis of relative increases and decreases in neuronal activity during REM sleep as seen with PET imaging using H215O measurements of regional cerebral blood flow (rCBF) [15, 16, 19] or [18F]-flurodeoxyglucose measurements of glucose metabolism[17]. Top row: cortical surface, lateral view. Middle row: cortical surface, medial view. Bottom row: subcortical foci (left) and ventral view of cortical surface (right). Analysis is based on published Talairach coordinates of foci whose activity was significant at p<0.001 corrected (Z-score > 3.09). Circles, squares, triangles, and stars denote activity foci as reported by [15] (Maquet 96), [16] (Braun 97), [17] (Nofzinger 97), and [19] (Maquet 2000), respectively. Each symbol marks a region’s center-of-mass regardless of its spatial extent. Yellow symbols denote increased regional activity in the (1) mesopontine tegmentum and midbrain nuclei, (2) thalamus, (3) basal forebrain and diencephalic structures, (4) limbic MTL structures including amygdala and hippocampus, (5) medial prefrontal cortex, (6) occipito-temporal visual cortex, and (7) anterior cingulate cortex. Cyan symbols denote decreased activity in the (8) orbitofrontal cortex, (9) posterior cingulate and precuneus, (10) dorsolateral prefrontal cortex, and (11) inferior parietal cortex.
There is also remarkable consistency between a subject s cognitive and neural organization in dreaming and waking[13, 14]. For instance, children studies demonstrate that dream features show a gradual development that parallels their cognitive development when awake[21] (Box 2). Patients with brain lesions that impair their waking cognition show corresponding deficits in dreams. For example, subjects with impaired face perception also do not dream of faces[22, 23] (Box 3).
BOX 2. The development of dreams in children.
When do children start dreaming, and what kind of dreams do they have? Since children often show signs of emotion in sleep, many assume they dream a great deal. However, a series of studies by David Foulkes showed that children under the age of 7 reported dreaming only 20% of the time when awakened from REM sleep, compared with 80–90% in adults[21].
Preschoolers dreams are often static and plain, such as seeing an animal or thinking about eating. There are no characters that move, no social interactions, very little feeling, and they do not include the dreamer as an active character. There are also no autobiographic, episodic memories, perhaps because children have trouble with conscious episodic recollection in general, as suggested by the phenomenon of infantile amnesia. Preschoolers do not report fear in dreams, and there are few aggressions, misfortunes, and negative emotions. Note that children who have night terrors, in which they awaken early in the night from SWS and display intense fear and agitation, are probably terrorized by disorientation due to incomplete awakening rather than by a dream[116]. Thus, although children of age 2–5 can obviously see and speak of everyday people, objects and events, apparently they cannot dream of them.
Between ages 5 to 7 dream reports become longer, although still infrequent. Dreams may contain sequences of events in which characters move about and interact, but narratives are not well developed. At around age 7, dream reports become longer and more frequent, contain thoughts and feelings, the child s self becomes an actual participant in the dream, and dreams begin to acquire a narrative structure and to reflect autobiographic, episodic memories.
It could be argued that perhaps all children dream, but some do not yet realize that they are dreaming, do not remember their dreams, or cannot report them because of poor verbal skills. Contrary to these intuitive suggestions, dream recall was found to correlate best with abilities of mental imagery rather than language proficiency. Mental imagery in children is assessed by the Block Design Test of the Wechsler intelligence test battery[117]. In this task, children look at models or pictures of red and white patterns, and then recreate those patterns with blocks. Critically, scores on this test are the one parameter that correlates best with dream report in children. Put simply, it is children with the most developed mental imagery and visuo-spatial skills (rather than verbal or memory capabilities) that report the most dreams, suggesting a real difference in dream experience. Visuo-spatial skills are known to depend on the parietal lobes, which are not fully myelinated until age 7. Thus, linking visuo-spatial cognitive development with brain maturation studies[118] is an important field of further research.
The static nature of preschoolers dreams is also in accord with the notion that preoperational children can’t imagine continuous visual transformations[119]. In the “mental rotation” test[120] a subject is asked to determine whether two figures are the same or different. In adults, reaction times (which are used as the score) increase linearly with the degree of rotation, but children do not show this relationship and do not seem to be mentally imagining movement using visuo-spatial imagery. This is consistent with their dream reports lacking movement[21].
Along the same line, people who are blinded after the age of 5–7 seem to have visual imagination and dream with visual imagery throughout life, while blinding at an earlier age leads to absence of visualization in both waking and dreaming[121, 122], though dreaming in blind individuals is a subject of debate[123–125]. Overall, dreaming appears to be a gradual cognitive development that is tightly linked to the development of visual imagination.
The slow development of full-fledged dreams and their intimate relation with imagination cast doubts on whether animals can dream as we do. It is likely that animals, too, can be conscious during sleep. For instance, lesions in parts of the brainstem that control movements cause cats to seemingly act out their dreams[126], very much like humans with REM sleep behavior disorder [113]. However, while a cat may experience images and emotions in sleep, it is less likely that these experiences are tied together by a narrative as is the case in our typical dreams[127]. Altogether, what kind of dreaming consciousness an animal has may reflect the extent to which it is conscious in general, and both waking and dreaming consciousness are best viewed as graded phenomena[80].
BOX 3. Lesion studies of dreaming.
The primary source on neuropsychology of dreaming is a study by Solms[22] who examined 361 neurological patients and asked them in detail about their dreaming. Overall, lesion studies indicate that dreaming depends on specific forebrain regions rather than on the brainstem REM sleep generator[22, 128, 129]. In most cases, global cessation of dreaming follows damage in or near the temporo-parieto-occipital junction (around Brodmann’s Area 40), more often unilaterally than bilaterally[23, 128]. This region supports various cognitive processes that are essential for mental imagery[130]. Accordingly, patients with such damage typically show a parallel decline in waking visuo-spatial abilities[109]. These results strongly suggest that mental imagery is the cognitive ability most related to dreaming (though a link between loss of dreaming and aphasia has also been suggested[131]).
Less frequently, global cessation of dreaming follows bilateral lesions of white matter tracts surrounding the frontal horns of the lateral ventricles, underlying ventromedial prefrontal cortex[22]. Many of these nerve fibers originate or terminate in limbic areas, in line with increased limbic activity in REM sleep as revealed by functional imaging[15, 16, 18]. The ventromedial white matter contains dopaminergic projections to the frontal lobe which were severed in prefrontal leucotomy, once performed on many schizophrenic patients[53]. Most leucotomized patients (70–90%) complained of global cessation of dreaming as well as of lack in initiative, curiosity, and fantasy in waking life[23]. Since dopamine can instigate goal-seeking behavior, these data have been interpreted as supporting the classical psychodynamic view of dreams as fulfillment of unconscious wishes related to egoistic impulses[132].
Apart from global cessation of dreaming, more restricted lesions produce the cessation of visual dreaming [22, 109], or the disruption of particular visual dimensions in dreams. For example, lesions in specific regions that underlie visual perception of color or motion are associated with corresponding deficits in dreaming[23, 109]. In general, it seems that lesions leading to impairments in waking have parallel deficits in dreaming.
Some lesions, especially those in medial prefrontal cortex, the anterior cingulate cortex, and the basal forebrain, are associated with increased frequency and vividness of dreams and their intrusion into waking life[22]. Importantly, many brain-damaged patients report no changes in dreaming, indicating that the neural network supporting dreaming has considerable specificity. For example, lesions of dorsolateral prefrontal cortex, sensorimotor cortex, and V1 do not seem to affect dreaming at all[22]. The fact that patients with V1 lesions report vivid dreaming argues against the notion that reentry to early retinotopic cortex is a necessary condition for visual awareness[133].
Dreams also reflect our interests and personality, just like mental activity during wakefulness. Formal content analysis has revealed that mood, imaginativeness, individuals of interest, and predominant concerns are correlated between our waking and dreaming selves[12–14]. Personal anxieties we experience in wake, such as being inappropriately dressed, being lost, or being late for an examination, can appear in dreams that involve social interactions[24]. Dreams, like our personality in general, are quite stable over time in adulthood[12–14], and share many characteristics across cultures[12–14]. In addition, we feel we are personally participating in many dream events.
Despite these remarkable similarities, what makes dream consciousness so fascinating are the ways in which it differs from our waking experience. Some of these phenomenological differences are accompanied by consistent neurophysiological differences.
Reduced voluntary control and volition
We are generally surprised on awakening from a dream (“it was only a dream”) mainly because we didn’t consciously will that we would dream it. In fact, during dreaming there is a prominent reduction of voluntary control of action and thought. We cannot pursue goals, and have no control over the dream’s content. The fact that we are so surprised, excited and even skeptical about lucid dreaming – possibly a way to control some dreams[25] - illustrates how dreams normally lack voluntary control[9]. Interestingly, recent evidence points to the role of the right inferior parietal cortex (Brodmann’s Area 40) in waking volition[26, 27], an area that is deactivated during REM sleep[15, 16] (Fig. 1).
Reduced self-awareness and altered reflective thought
Our dreaming consciousness consists of a single “track”: we are not contextually aware of where we are (in bed) or of what we are doing (sleeping, dreaming). There is a strong tendency for a distinct narrative of thoughts and images to persist without disruption (“single-mindedness”[28]). Indeed, reports of mental activity in REM sleep are longer than reports obtained from awake subjects[28]. Dreaming is almost always delusional since events and characters are taken for real. Reflective thought is altered in that holding contradictory beliefs is common, and a dreamer easily accepts impossible events such as flying, inconsistent scene switches, sudden transformations and impossible objects[29] such as a pink elephant. There is often uncertainty about space, time, and personal identities[30]. For example, a character may have the name, clothes and hairstyle of a male friend, but have mother’s face. Reduced self-monitoring in dreams may be related to the deactivation of brain regions such as posterior cingulate cortex, inferior parietal cortex, orbitofrontal cortex, and dorsolateral prefrontal cortex[15, 16] (Fig 1). Indeed, deactivation of prefrontal cortex has been shown to accompany reduced self-awareness during highly engaging sensory perception in wakefulness[31]. However, some dreams may have conserved reflective thought processes such as thoughtful puzzlement about impossible events[32], contemplating alternatives in decision-making[32], reflecting during social interactions[32], and “theory of mind”[33], demonstrating that individual dreams can differ from each other substantially.
Emotionality
Some dreams are characterized by a high degree of emotional involvement, including joy, surprise, anger, fear, and anxiety[34–36]. Interestingly, sadness, guilt, and depressed affect are rare[11], possibly due to reduced self-reflection. Some claim that fear and anxiety are enhanced in dreams to a degree rare in waking life[37], in line with Freud’s suggestion that dream narratives originate in perceived threats or conflicts[5]. Whether or not this interpretation has merits, REM sleep is in fact associated with a marked activation of limbic and paralimbic structures such as the amygdala, the anterior cingulate cortex, and the insula[15, 17, 19] (Fig. 1). However, emotions are feeble in other dreams, and are absent altogether in 25–30% of REM sleep reports[34–36], including in situations where emotions would likely be present in waking[34], once again highlighting the variability in dream phenomenology.
Altered mnemonic processes
Memory is drastically altered for the dream and within the dream. Unless the dreamer wakes up, most dreams are forever lost. Upon awakening, memory for the dream often vanishes rapidly unless written down or recorded, even for intense emotional dreams. It is not clear why this is the case since from a neuroimaging perspective, limbic circuits in the medial temporal lobe that are implicated in memory processes, are highly active during REM sleep[15–18] (Fig. 1). Perhaps the hypoactivity of prefrontal cortex, also implicated in mnemonic processes, plays an important role in dream amnesia. Contemporary theories of dreaming (Table 1) offer different accounts of dream amnesia. For example, according to psychodynamic models, dream amnesia is due to processes of active repression[5]. According to Hobson s Activation-Input-Mode [AIM] model, dream amnesia is related to a state-change involving inactivity of monoaminergic systems (“aminergic de-modulation”) and deactivation of dorsolateral prefrontal cortex[11]. The neurocognitive model claims that dreams are usually forgotten because they are internal narratives; unless internal experiences are tied to external cues such as times and places they are bound to be forgotten[13].
Table 1.
Contemporary theories of dreaming
| Psychodynamic (Freud, Solms) | Activation-Input-Modulation [AIM] Model (Hobson) |
Neurocognitive (Foulkes, Domhoff) | |
|---|---|---|---|
| General | Dreams represent fulfillment of unconscious wishes related to egoistic (often infantile sexual) impulses [5]. Latent unconscious content is disguised via censorship creating the bizarre manifest dream content [5]. More recently, the drive for dreaming has been associated with dopaminergic systems and “appetitive interests” [132]. | Our conscious state is determined by three factors: (a) Activation - total and regional brain activity levels, (b) Input - activation generated internally or externally, (c) Modulation - the ratio of aminergic to cholinergic neuromodulators. REM sleep and dreaming are characterized by high levels of activation, internal input, and cholinergic modulation [11]. |
Dreaming is what occurs when the mature brain is adequately activated, disconnected from external stimuli and without self-reflection. Once instigated, dreaming actively draws on memory schemas, general knowledge, and episodic information to produce simulations of the world [13, 14]. |
| Dream amnesia | Since unconscious wishes are noxious to our consciousness, they are actively repressed via censorship processes [5]. Dream amnesia is anything but arbitrary: “our memory reproduces the dream not only incompletely but also untruthfully, in a falsifying manner”[5]. | Dream amnesia largely stems from a state-change. Aminergic de-modulation and deactivation of dorsolateral prefrontal cortex in REM sleep create a brain state which is not favorable for subsequent memory [11]. This also explains why we forget moments of brief awakenings during sleep. | Dream amnesia is primarily related to a cognitive state and lack of context. To remember, we need an external narrative to which internal events can be tied [14, 21]. Dream amnesia cannot be explained by a state- change since dreaming can occur at any state (NREM sleep and wake). |
| Signal propagation in dreaming | “Top-down”: dreams originate from psychic motives which are later instantiated as sensory percepts: “a thought... is objectified in the dream, and represented as a scene”[5]. | “Bottom-up”: dreams originate from activation of sensory cortex by the brainstem (e.g. PGO waves), later to be interpreted and synthesized by mnemonic and high-order modules [11, 47]. | “Top-down”: Dreams originate in abstract knowledge and figurative thought which are processed back into “imaginal copies” of perceptual phenomena [14]. |
| Is REM sleep a good model for dreaming? | No. REM sleep and dreaming can occur one without the other [23, 154], for example in neurological patients. Dream- like experiences are related to forebrain mechanisms rather than to REM sleep generators in brainstem [22, 23]. | Yes. Because REM sleep provides the most favorable brain conditions for dreaming, we can focus on its neurophysiology in our attempt to model the neuronal basis of dreaming [4, 47]. | No. Dream-like experiences can occur also in NREM sleep, sleep onset, and wakefulness [13, 155]. Children studies show that REM sleep may be an important condition for dreaming but not sufficient [13, 21]. |
| Is dreaming largely similar to waking consciousness? | No. The apparent (manifest) aspect of dreams is bizarre and includes nonsensical changes in time and place, as well as incongruities of plot, character, and action [5]. This is because the true (latent) dream content is disguised by the censor [5]. Dreaming may be closely akin to mental illness [5, 156]. | No. Dreaming is altogether comparable to delirium (acute confusional state) that can occur upon alcohol withdrawal [3]; REM sleep shares its physiological substrate with psycho-pathological conditions such as schizophrenia (limbic hyper-activation and frontal hypo-activation) [157, 158]. |
Yes. Dreams are “a remarkably faithful replica of waking life” [159]. Dreams are largely coherent and internally plausible narrative sequences rather than the stereotypical illogical sequences of bizarre images. Content analysis indicate a strong continuity between dream content and waking life[13]. Evidence linking dreams to psychosis is limited[155]: REM sleep deprivation does not alter schizophrenic pathology, aminergic agonists suppress REM sleep with no psychopathological effects. |
| Neurochemistry of dreaming | Dreaming is driven by the ‘wanting’ dopaminergic system: evidence from prefrontal leucotomies & effects of l-DOPA on dreaming [23, 156]. | Primarily a cholinergic role for REM sleep and dreaming [4, 11]. Administration of cholinergic agonists (e.g. pilocarpine) can induce an artificial REM sleep period associated with dream reports [160]. | Dreaming is unlikely to be driven by a specific chemical or brain region. It is most likely related to a complex neurochemical mixture where serotonin, norepinephrine, and histamine are absent while both acetylcholine and dopamine are present [13, 155]. |
| The function of dreaming | According to Freud, dreams preserve sleep in the face of unconscious needs for excitement [5]. More recently (Solms): “the biological function of dreaming remains unknown” [23]. | Dreams may serve a creative function by providing a virtual reality model (protoconsciousness). The brain is preparing itself for integrative functions including learning and secondary consciousness [4]. | Dreams probably have no function, but they do have coherence and meaning, which is often conflated with function[13]: “dreaming is a spandrel of the mind, a by- product of the evolution of sleep and consciousness.” [13] |
| What is the psychological meaning of dreams? | This theory emphasizes dream content: individual dreams carry meaningful information about the dreamer. This theory lacks in power with regards to explaining dreams shared by all people [5] (e.g. flying, teeth falling). | Dreaming is an attempt to best interpret activating signals in a coherent manner, and contents of individual dreams are nearly random. Nevertheless, the process of interpretation may carry some psychological meaning[11]. | This theory emphasizes dream form: dreams are based on stored memory representations and therefore reflect individual ways of abstracting knowledge, but specific dreams are not traceable back to particular episodes in our life[14]. |
| Are dreams directly related to previous experience? | Yes. Dream content is related to daytime experience (“Day’s residue”) that triggers the emergence of related memories. “All the material making up the content of a dream is in some way derived from experience” [5] | No. Dream content is largely unrelated to the preceding day’s experiences[45] and in general does not accurately represent episodic memories which are available during wakefulness[42, 45]. | No. Familiar settings and people are sometimes incorporated into dreams but dreams are not a recollection of everyday life [14]. |
Episodic memory is also impaired within the dream. Indeed, a dream is not like an episode of life being “replayed”. In one example in which subjects had intensively played the computer game Tetris, there was no episodic memory in subsequent dreams that subjects had indeed played Tetris. In fact, dreams of healthy subjects were indistinguishable from those of profoundly amnesic subjects, who could not remember having played Tetris at all. In contrast, both normal and amnesic subjects often reported perceptual fragments, such as falling blocks on a computer screen, at sleep onset[38]. While ‘residues’ from waking experience are incorporated in about 50% of dreams[39–41], they do so in new and unrelated contexts, and verified memories for episodes of recent life are only found in about 1.5% of dreams[42]. Such residual recollections have been interpreted by some to suggest that dreaming may have an active role in forgetting[5, 43]. Finally, many have the impression that the network of associations stored in our memory may become looser than in wake[44, 45], perhaps favoring creativity, divergent thinking, and problem resolution[4, 46].
In summary, dream consciousness is remarkably similar to waking consciousness, though there are several intriguing differences. These include reduced attention and voluntary control, lack in self-awareness, altered reflective thought, occasional hyperemotionality, and impaired memory. Traditionally, dream phenomenology has often been compared to madness or psychosis[3, 11, 47], but in fact the hallucinations, disorientation, and subsequent amnesia of some bizarre dreams may be more akin to the acute confusional state – also known as delirium - which occurs after withdrawal from alcohol and drugs[48]. However, most dreams are less bizarre, perhaps more similar to mind wandering or stimulus independent thoughts[14, 49, 50]. Waking thoughts jump around and drift into bizarre daydreaming, rumination, and worrying far more than stereotypes of rational linear thinking suggest[51]. Importantly, individual dreams are highly variable in their phenomenology, and only some conform to the typical monolithic template that is often portrayed. Thus, just like diverse waking experiences, “Not all dreams are created equal”, and future studies should consider different kinds of dreams and their neural correlates separately.
What mechanisms are responsible for regional differences in brain activity between waking and REM sleep, and thus presumably for some of the cognitive differences between waking and dreaming? Single-unit physiology indicates that generally, cortical activity in REM sleep reaches similar levels as found in active wake (Fig. 2), but variability between brain areas remains poorly explored. Regional differences may likely stem from changes in the activity of neuromodulatory systems (Fig. 2). During REM sleep, acetylcholine is alone in maintaining brain activation, whereas monoaminergic systems are silent, an observation that could explain many features of dreams[11]. For example, consistent with imaging results, cholinergic innervation is stronger in limbic and paralimbic areas than in dorsolateral prefrontal cortex[52], which may explain why limbic regions are highly active in REM sleep while dorsolateral prefrontal cortex is deactivated (Fig. 1). Dopaminergic modulation may also play a role[23], since dreaming is decreased by prefrontal leucotomies that cut dopaminergic fibers[53] and is increased by dopaminergic agonists[23] (Table 1 and Fig. 2).
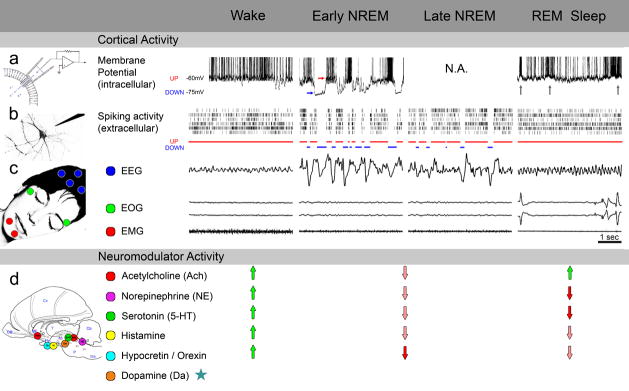
Figure 2. Neurophysiology of wake and sleep states.
A comparison of cortical activity (upper panel) and neuromodulator activity (bottom panel) in wake, early NREM (when sleep pressure is high and dream reports are rare), late NREM (when sleep pressure dissipates, and dream reports are more frequent), and REM sleep (when dreams are most common).
(a) Intracellular studies. The membrane potential of cortical neurons in both wake and REM sleep is depolarized and fluctuates around −63mV and −61mV, respectively [77]. In REM sleep, whenever phasic events such as rapid eye movements and PGO waves occur (gray arrows, events not shown), neurons increase their firing rates to levels that surpass those found in wake [77, 146]. In early NREM sleep, neurons alternate between two distinct states, each lasting tens/hundreds of milliseconds: UP states (red arrow) are associated with depolarization and increased firing, while in DOWN states (blue arrow) the membrane potential is hyperpolarized around −75mV, and neuronal firing fades[78, 147]. Intracellular studies focusing specifically on late NREM sleep are not available (N.A.).
(b) Extracellular studies. Spiking of individual neurons in REM sleep reaches similar levels as in active wake. In both wake and REM sleep, neurons exhibit tonic irregular asynchronous activity [77, 148–151]. Sustained activity in wake and REM sleep can be viewed as a continuous UP state [78] (red bars). In early NREM sleep, UP states are short and synchronous across neuronal populations, and are frequently interrupted by long DOWN states (blue bars). In late NREM sleep, UP states are longer and less synchronized [79].
(3) Polysomnography. Waking is characterized by low-amplitude, high-frequency EEG activity (above 7Hz), occasional saccadic eye movements, and elevated muscle tone. In early NREM sleep, high-amplitude slow waves (below 4Hz) dominate the EEG. Neuronal UP (red) and DOWN (blue) states correspond to positive and negative peaks in the surface EEG, respectively [79]. Eye movements are largely absent and muscle tone is decreased. In late NREM sleep, slow waves are less frequent, while spindles (related to UP states and surface EEG positivity) become more common. Eye movements and muscle tone are largely similar to early NREM sleep [152]. In REM sleep, theta activity (4–7 Hz) prevails, rapid eye movements occur, and muscle tone is dramatically reduced.
(d) Neuromodulator activity. Subcortical cholinergic modulation is highly active in wake and REM sleep (green arrows) and leads to sustained depolarization in cortical neurons and EEG activation [77]. Wake is further maintained by activity of monoamines, histamine, and hypocretin/orexin (green arrows). In sleep, monoaminergic systems including norepinephrine and serotonin reduce their activity (pink arrows), and are silent in REM sleep (red arrows). While dopamine levels do not change dramatically across the sleep-wake cycle (asterisks), phasic events and regional profiles may differ[153].
Data are pooled across different species for illustration purposes. Intracellular cat data adapted with permission from Ref [77]; extracellular and EEG rat data obtained from V. Vyazovskiy (personal communication).
On the whole, relating typical dreams to the neurophysiology of REM sleep has proven to be a useful starting point for revealing the neural basis of dreaming. However, dream consciousness can not be reduced to brain activity in REM sleep. Indeed, some fundamental questions concerning the relationship between the brain and dreaming linger on. We shall discuss three in turn: i) what determines the level of consciousness during sleep; ii) why the dreamer is disconnected from the environment; and iii) whether dreams are more akin to perception or to imagination.
What determines the level of consciousness during sleep?
In principle, studying mental experiences during sleep offers a unique opportunity to explain how changes in brain activity relate to changes in consciousness[3, 54]. In fact, if it were not for sleep, when consciousness fades in and out on a regular basis, it might be hard to imagine that consciousness is not a given, but depends somehow on the way our brain is functioning. Traditionally, studies have focused on differences among reports obtained after awakenings from different sleep stages or at different times of night. When REM sleep was initially distinguished from NREM sleep[55], it was reported that 74–80% of REM sleep awakenings produced vivid dream recall, compared to only 7–9% of awakenings from NREM sleep[56, 57]. It was only natural to conclude that, compared to NREM sleep, the distinct physiology of REM sleep, and especially its fast, low-voltage EEG resembling that of wakefulness, was the reason why we are conscious and dream in REM sleep, and not in NREM sleep[29]. Indeed, for some time, reports of mental activity upon awakenings from NREM sleep were assumed to be recalls of earlier REM sleep dreams, or considered analogous to sleep talking[3], or treated as confabulations made up by subjects confused upon awakening[9] (Box 1). However, when changing the question from “tell me if you had a dream” to “tell me anything that was going through your mind just before you woke up,” reports of conscious experiences in NREM sleep ranged between 23% and 74%[9]. Subsequent studies demonstrated clearly that NREM sleep awakenings yielded reports of mental activity[58, 59].
Specifically, reports from sleep stage N1 are extremely frequent (80–90% of the time), though they are very short[60]. Usually people report vivid hallucinatory experiences, so-called hypnagogic hallucinations. In contrast to typical dreams, hypnagogic hallucinations are often static - like single snapshots[11, 47], and usually do not include a self character[14]. Some activities performed before sleep (e.g. video games) may influence the content of hypnagogic dreams[38, 61]. Awakenings from NREM sleep stages N2 and N3 yield reports about some experienced content 50–70% of the time[59], although there is great variability throughout the night and between subjects. Early in the night, when stage N3 is prevalent and many large slow waves dominate the EEG, awakenings yield few reports[62]. Moreover, these reports are often qualitatively different than typical REM sleep reports, being usually short, thought-like, less vivid, less visual and more conceptual, less motorically animated, under greater volitional control, more plausible, more concerned with current issues, less emotional and less pleasant[9, 11, 63]. Also, the average length of REM sleep reports increases with the duration of the REM sleep episode while this is not true for NREM sleep reports[62]. However, late in the night NREM sleep reports are considerably longer and more hallucinatory. Indeed, 10–30% of all NREM sleep reports are indistinguishable by any criteria from those obtained from REM sleep[64, 65]. Since NREM sleep accounts for 75% of total sleep time, this means that full-fledged NREM sleep dreams actually account for a significant portion of all typical dreams.
Thus, the initial equation of a physiological state (REM sleep) with a mental state (dreaming) was incorrect, or at best, an oversimplification. Moreover, neuropsychological evidence indicates that dreaming and REM sleep can be dissociated: forebrain lesions may abolish dreaming and spare REM sleep, whereas brainstem lesions may nearly eliminate overt features of REM sleep without abolishing dreams[23] (Box 3). But if dream reports can be elicited during any stage of sleep[11, 47, 59, 66, 67], and conversely some awakenings may yield no report, no matter in which sleep stage they were obtained[59], where do we stand today with respect to the relationship between brain activity and consciousness during sleep?
The one thing that seems clear is that we need to move beyond the REM/NREM sleep dichotomy and beyond traditional sleep staging. Though staging is useful, it treats brain activity as uniform in space (only a few electrodes are used) and in time (for 30 sec epochs). Inevitably, subtler features of brain activity, which may well influence the presence, degree, and reportability of consciousness, are missed both in space and in time.
In the spatial domain, increasing evidence suggests that different brain regions may be in different states at the same time. For example, preliminary findings suggest that during sleepwalking, thalamocingulate pathways may be active as in wake, while the rest of the cerebral cortex is in NREM sleep[68]. A related notion of dissociated states is derived from the study of parasomnias, where wake-like behaviors occur during sleep[69]. For instance, the study of REM sleep behavior disorder shows that, contrary to common assumptions, wakefulness, REM sleep and NREM sleep may not be mutually exclusive states[69]. In the current context, it has been suggested that dreaming in NREM sleep is related to ‘covert’ REM processes that occur locally[59]. Thus, refined spatial analysis using fMRI or high-density EEG (hd-EEG) could potentially identify regionally-specific predictors of dreaming, and possibly indicate, in real time, whether dream reports will be obtained.
In the temporal domain, some attempts have been made to relate transient, phasic activities[70] to dreaming. For example, various studies have tried to link dream recall to eye movements[71, 72], PGO waves[73], and EEG power bouts in specific frequency bands[74] but limited success has been achieved, and little has been done for NREM sleep[11, 75, 76]. We now know that slow waves in NREM sleep reflect a slow oscillation of cortical neurons between UP and DOWN states (Fig. 2)[77, 78]. Perhaps long UP states are necessary for dreaming to occur. This is normally the case in REM sleep since slow waves are absent. As for NREM sleep, we would expect that higher occurrence of recalls, and especially of typical dreams in the morning hours, would reflect longer UP periods upon dissipation of sleep pressure (Fig. 2)[79]. In general, focusing on (rather than avoiding) “gray zones” where it is more difficult to predict whether a dream report will be obtained, for example in early REM sleep or late NREM sleep, may be a promising strategy for identifying psychophysiological correlates that go beyond traditional staging.
Finally, theoretical considerations suggest that the level of consciousness may depend on the brain s ability to integrate information[80]. Indeed, during wakefulness external perturbations such as TMS pulses (transcranial magnetic stimulation) cause changing patterns of activation across distant interconnected brain regions[10]. In REM sleep, evoked activity propagates much like it does in wakefulness[81]. By contrast, in deep SWS early in the night, when consciousness is most likely to fade, the response evoked by TMS remains either local (loss of integration), or spreads nonspecifically (loss of information). Apparently, the brain s capacity for information integration is reduced whenever neurons become bistable between UP and DOWN states. Intriguingly, the brain s response to a TMS pulse may offer a more sensitive measure of the inner state than spontaneous EEG. For example, such perturbations can uncover inherent bistability in short stretches of NREM sleep even when the EEG shows a wake-like low-voltage pattern[82].
Why is the dreamer disconnected from the environment?
The most obvious difference between dreaming and waking consciousness is the profound disconnection of the dreamer from his current environment. Such disconnection, of course, is a key feature of sleep: by definition a sleeping person shows no meaningful responses to external stimuli, unless they are strong enough to cause an awakening. This feature is known as “high arousal threshold”, and it persists in REM sleep despite its wake-like low-voltage EEG[83]. Moreover, stimuli not only fail to elicit a behavioral response, but also largely fail to be incorporated in the content of the dream[8, 84–86] (though some stimuli, such as a spray of water, pressure on the limbs, and meaningful words have a slightly higher chance of incorporation[84, 85]). This striking disconnection occurs even when subjects sleep with their eyes taped open and objects are illuminated in front of them[8]. Surely just before awakening, stimuli such as the sound of an alarm clock can enter our dreams, but when sleep is preserved, such relations are by and large surprisingly weak and dream consciousness is remarkably disconnected from the external environment.
The disconnection of the dreamer poses an intriguing paradox, especially if one considers that dreams involve vivid sensory experiences, and that they can occur upon a state of strong cortical activation. Several possibilities come to mind. For example, it has been suggested that during sleep a thalamic “gate” may close and sensory inputs may not reach the cortex effectively[87]. However, evoked responses in primary sensory cortices are largely preserved during REM sleep[88, 89]. Also, olfactory stimuli are not directly incorporated in dreams[90], though they are not routed through the thalamus (their emotional valence, however, may affect dreams). A related notion is that of a cortical “gate” leading to diminished inter-cortical propagation[91], as seems to be the case in the dissociation of primary visual cortex (V1) from high-order visual cortex in REM sleep[18]. It would be interesting to establish whether direct activation of cortical areas can overcome the disconnection from the environment. For example, can TMS over V1 or area MT bypass thalamic or cortical “gates” and produce sensations of phosphenes or movement in dream consciousness?
An intriguing possibility concerns the putative antagonism between externally oriented cortical networks and internally oriented, default-mode networks[92, 93]. Perhaps in dreams intrinsic activity dominates, as it does during stimulus-independent thoughts in wake[50]. This may occur at the expense of the processing of external stimuli, leading to disconnection from the environment. Indeed, both PET and magnetoencephalography (MEG) suggest that medial prefrontal cortex, a part of the default network, is highly active in REM sleep[16, 17, 94] as it is during wakeful rest (Fig. 1). Conversely, other components of the default network, including posterior cingulate and inferior parietal cortices, are deactivated in REM sleep[15, 16], as in highly-engaging waking tasks (Fig. 1). The exact cognitive task associated with the default-mode network is still not well understood[95] and it may be primarily driven by self-related introspective processes rather than general mind wandering[31, 96, 97]. Indeed, since most nodes of this network are deactivated in REM dreaming and mental imagery[98], cognitive states that are oriented internally but away from the self do not seem sufficient to elicit activity in this network.
Another possibility is that dreams may be analogous to altered states of consciousness in which attention is profoundly altered, as may be the case in extreme absorption, hypnosis, neglect[99], and Balint s syndrome, when visual experience may persist for single but unlocalizable objects (simultanagnosia)[100, 101]. The reticular thalamic nucleus has been implicated in redirecting attention across modalities[102, 103] and its activity in sleep may underlie some aspects of disconnection. It would also be interesting to determine whether neuronal correlates of momentary lapses of attention[104] occur regularly while dreaming.
Finally, as we have seen, the neuromodulatory milieu changes drastically in sleep (Fig. 2). Specifically, the levels of norepinephrine, serotonin, histamine, and hypocretin are greatly reduced in REM sleep compared to wake, so the presence of one or more of these neuromodulators may be necessary for external stimuli to be incorporated into our stream of consciousness. This search can be narrowed down by considering cataplexy, which affects people with narcolepsy[105]. Cataplexy is a transient episode of muscle tone loss in which humans report that awareness of external stimuli is preserved, and presumably animals are likewise aware of their environment during cataplectic attacks. Neuromodulatory activity in cataplectic dogs is largely similar to that in REM sleep except that levels of histamine are high, much like during wakefulness[105]. It thus seems that levels of histamine are correlated with our ability to incorporate sensory stimuli into conscious experience. It would be important to establish whether histamine is indeed necessary for such incorporation, and how it may do so. For instance, could it be that in wakefulness histaminergic tone facilitates transmission of feed-forward sensory inputs in cortical layer 4, at the expense of backward signal propagation?
Are dreams more like perception or imagination?
Whether dreams are generated in a “bottom-up” or a “top-down” manner is a question that has been asked since at least Aristotle[106]. To put the question in a modern context, do dreams start from activity in low-level sensory areas, which is then interpreted and synthesized by higher-order areas, as is presumably the case in waking perception? Or do they begin as wishes, abstract thoughts, and memories deep in the brain, which are then enriched with perceptual and sensory aspects, as in imagination? Of course, it is possible that such a dichotomy is misguided, and dreams may be best conceptualized as global attractors that emerge simultaneously over many brain areas. However, as we shall see, the available data do indeed suggest that there may be a privileged direction of dream generation.
In the 19th century, sensory experience was often regarded as the source of dreams, which were considered to be an attempt of the mind to interpret somatic nerve-stimuli (Supplementary Fig. 1). A similar notion was later adopted by Henri Beaunis, and recently championed by Allan Hobson (Table 1)[4, 11, 47]. According to his AIM model, internally generated signals originating in the brainstem during REM sleep, such as PGO waves, excite visual cortex and are later processed and synthesized by higher-order areas. High levels of acetylcholine in the absence of aminergic neuromodulation may enhance feed-forward transmission and suppress back-propagation[3, 107]. By contrast, Freud and some of his followers asserted that dreams originate from psychic motives that are later instantiated as sensory percepts, much like mental imagery[5].
Deciding between these alternative views will most likely require difficult experiments in which the direction of signal flow during dreaming sleep is evaluated and compared to that during waking perception and imagery[108] (Box 4). However, various lines of evidence already suggest that dreaming may be more closely related to imagination than to perception. From lesion studies (Box 3) we know that dreaming requires an intact temporo-parieto-occipital junction[22, 23] and lesions in this region also affect mental imagery in wakefulness[109]. Cognitive studies indicate that the skill that maximally correlates with dream recall in adults is visuo-spatial imagery[110]. In children, dream recall develops hand in hand with visuo-spatial imagery (Box 2). In epileptic patients, direct electrical stimulation in high-order regions such as the medial temporal lobe, rather than in visual cortex, can elicit “dream-like” experiences[111], although such patients are simultaneously aware of their surroundings. Other evidence comes from lucid dreamers[25] who report that it is impossible to focus on fine-grain details of visual objects, as is the case in mental imagery[112]. Perhaps top-down connections lack the anatomical specificity to support detailed representations. The rare occurrences of smells or pain in dreams may also be related to our difficulty in imagining them vividly when awake. However, one important difference between dreaming and mental imagery is that while imagining we are aware that the images are internally generated (preserved reflective thought).
Box 4. Future directions.
1. Signal propagation in dreams
During wakefulness, sensory responses precede responses in higher-order areas by more than 100ms[134, 135]. Does neural activity during dreaming sleep show a similar feed-forward progression as in perception? Or does neural activity propagate backwards, from higher to lower areas, as it is thought to do during imagery? This issue, which is crucial to our understanding of dream generation, could be resolved by examining unit and field potential recordings from the same neuronal populations in wake and REM (or late NREM) sleep in both animals and humans[135]. One can also apply directional measures of signal propagation (e.g. Granger causality) to hd-EEG data, and check whether the main direction of signal flow inverts between wake and sleep. Finally, one could use TMS with concurrent hd-EEG during both wake and REM sleep, and examine whether there may be a preferential direction of the brain s response to perturbations depending on behavioral state[10].
2. Functional networks underlying dreaming
So far, most regional studies of brain activity during sleep have employed PET. While PET allows for quantification of cerebral blood flow and comparison across vigilance states, functional MRI (fMRI) offers superior spatial and temporal resolutions. Event-related fMRI has been already used to map brain activity associated with phasic events such as slow waves[136] and eye movements[137, 138]. Studies of functional and effective connectivity[139] may be especially well suited to map the functional networks underlying dreaming. Notably, perceptual awareness is associated with specific functional connectivity patterns within sensory modalities[140], between modalities[141], and with a striking segregation between sensory systems and the default-mode/intrinsic system[31, 93, 104]. Are such connectivity patterns also a hallmark of activity in the dreaming brain? What regional brain activity underlies dreaming in NREM sleep? How do functional networks of mental imagery and dreaming compare in the same subjects? Finally, hd-EEG may be particularly suited for sleep imaging since it (a) allows for relatively undisturbed sleep, (b) upon source modeling can provide a spatial resolution roughly comparable to PET, (c) offers high temporal resolution suitable for evaluating signal propagation, and (d) can be combined with TMS during sleep.
3. Initial steps towards studying dream content
Progress in signal decoding may ultimately enable us to investigate the neural correlates not only of dream form – what is common to all dreams – but also of dream content – what is specific to a particular dream. This can be done, for instance, by using classification techniques applied to fMRI or hd-EEG data[142]. At least initially, it may be worthwhile to consider some coarse properties of individual dreams, such as the frequency of occurrence of faces or places in a dream report, the amount of movement, or the dominant affective valence. In principle, it should be possible to predict not only the likelihood of a report upon awakening, but also the likelihood of specific features based on preceding brain activity. An important step in this direction would be to identify the contents of internally generated mental imagery using the same approach[143]. Furthermore, some patients with epilepsy or post-traumatic stress disorder who experience recurring dream contents[144, 145] may provide a unique opportunity to relate specific dream content to its neural basis.
If the flow of brain activity during dreaming were shown to be largely backwards, as one would expect in imagery, rather than forwards, as in perception, many of the seemingly bizarre properties of dreams, such as blended characters and scene switches, would be easier to explain, as they are standard features of our imagination. Such a top-down mode may disrupt the encoding of new memories, and thus underlie dream amnesia. In addition, top-down mental imagery could obstruct the processing of incoming stimuli and disconnect us from the environment. If this view is correct, waking consciousness is more like watching the news in real time, while dreaming is more like watching a movie created by an imaginative director[81]. As in some B-movies, the director is not particularly choosey and any actor, dress, means of transportation, or object that is readily available will do. Albert Einstein said that “imagination points to all we might yet discover and create”, and indeed, dreaming may turn out to be the purest form of our imagination.
Concluding remarks
In summary, dream consciousness is remarkably similar to waking consciousness, though there are several intriguing differences in volition, self-awareness and reflection, affect, and memory, and there is great variability between individual dreams. The neurophysiology of REM sleep, and in particular recent insights into its regional activity patterns, offers a useful starting point for relating dream phenomenology to underlying brain activity. However, the initial equation of REM sleep with dreaming has been shown to be inaccurate. Thus, it is time we moved beyond sleep stages when trying to link dream consciousness to neuronal events, and focused on more subtle features of brain activity in space and time. Our profound disconnection from the external environment when dreaming poses a central unsolved paradox, the answer to which may be instrumental for understanding dreams. Converging evidence from multiple fields of study, including phenomenology, development, neuropsychology, functional imaging, and neurophysiology, support the notion that dreaming may be closely related to imagination, where brain activity presumably flows in a “top-down” manner. Viewing dreams as a powerful form of imagination can help explain many of their unique features, such as sudden transitions, uncertainty about people and places, poor subsequent recall, disconnection from the environment, and offers testable predictions for future studies.
Supplementary Material
Acknowledgments
We apologize to those whose work was not cited because of space constraints. We thank Michal Harel, Lior Fisch, and Vlad Vyazovskiy for help with figures; Chiara Cirelli, Rafi Malach, Simone Sarasso, Brady Riedner, and Fabio Ferrarelli for helpful discussions and comments; our anonymous reviewers for valuable suggestions. Y.N. is supported by an EMBO long term fellowship and the Brainpower for Israel Fund. G.T is supported by an NIH Director’s Award DP1 OD000579 and NIH Conte Center Award P20 MH077967.
Glossary
- Slow waves
Oscillations of cortical origin that have frequencies below 4Hz
- Spindles
Waxing and waning oscillations of thalamic origin that have frequencies in the sigma band (12–15Hz)
- Ponto-Geniculo-Occipital (PGO) waves
Phasic field potentials occurring immediately before and during REM sleep (originally discovered in cats). They propagate from the pontine brainstem (p) via the lateral geniculate nucleus of the thalamus (g) to the occipital visual cortex (o)
- Rapid Eye Movement (REM) sleep
Sleep occurring mostly late at night, with low amplitude EEG as in wake, presence of theta activity (4–7Hz), reduced muscle tone, and involuntary saccadic rapid eye movements
- Non Rapid Eye Movement (NREM) sleep
Sleep comprising stages N1, N2 and N3 (slow wave sleep), characterized by slow waves and spindles in the EEG
- Stage N1 (NREM1)
Sleep where the EEG is intermediate between wake and deep sleep, with presence of theta activity (4–7Hz), occasional vertex sharp EEG waves, and slow eye movements
- Stage N2 (NREM2)
Sleep occurring throughout the night, where the EEG may contain spindles and occasional slow waves
- Stage N3 (NREM3)/Slow Wave Sleep (SWS)
Sleep occurring mostly early at night, with many large slow waves in the EEG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arkin AM, et al. The mind in sleep: Psychology and psychophysiology. Lawrence Erlbaum Associates; 1978. [Google Scholar]
- 2.Ellman SJ, Antrobus JS. The mind in sleep: Psychology and psychophysiology. Willey; 1991. [Google Scholar]
- 3.Hobson JA. The Dreaming Brain. Basic Books; 1988. [Google Scholar]
- 4.Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 5.Freud S. The modern library. 1900. The Interpretation of Dreams. [Google Scholar]
- 6.Foulkes D, Rechtschaffen A. Presleep Determinants of Dream Content: Effect of Two Films. Percept Mot Skills. 1964;19:983–1005. doi: 10.2466/pms.1964.19.3.983. [DOI] [PubMed] [Google Scholar]
- 7.Arkin A, Antrobus JS. The effects of external stimuli applied prior to and during sleep on sleep experience. In: Arkin A, et al., editors. The Mind in Sleep. Wiley; 1991. [Google Scholar]
- 8.Rechtschaffen A, Foulkes D. Effect of Visual Stimuli on Dream Content. Percept Mot Skills. 1965;20(SUPPL):1149–1160. doi: 10.2466/pms.1965.20.3c.1149. [DOI] [PubMed] [Google Scholar]
- 9.Rechtschaffen A. The Psychophysiology of Mental Activity During Sleep. In: McGuigan FJ, Schoonover RA, editors. The Psychophysiology of Thinking: Studies of Covert Processes. Academic Press; 1973. p. 196. [Google Scholar]
- 10.Massimini M, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 11.Hobson JA, et al. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23:793–842. doi: 10.1017/s0140525x00003976. discussion 904–1121. [DOI] [PubMed] [Google Scholar]
- 12.Hall C, Van de Castle R. The content analysis of dreams. Appleton-Century-Crofts; 1966. [Google Scholar]
- 13.Domhoff GW. The Scientific Study of Dreams: Neural Networks, Cognitive Development, and Content Analysis. American Psychological Association; 2003. [Google Scholar]
- 14.Foulkes D. Dreaming: A Cognitive-Psychological Analysis. Lawrence Erlbaum Associates; 1985. [Google Scholar]
- 15.Maquet P, et al. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- 16.Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120 (Pt 7):1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 17.Nofzinger EA, et al. Forebrain activation in REM sleep: an FDG PET study. Brain Res. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]
- 18.Braun AR, et al. Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science. 1998;279:91–95. doi: 10.1126/science.279.5347.91. [DOI] [PubMed] [Google Scholar]
- 19.Maquet P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 20.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res. 2000;9:207–231. doi: 10.1046/j.1365-2869.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 21.Foulkes D. Children’s Dreaming and the Development of Consciousness. Harvard University Press; 1999. [Google Scholar]
- 22.Solms M. The neuropsychology of dreaming: a Clinico-Anatomical Study. Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 23.Solms M. Dreaming and REM sleep are controlled by different brain mechanisms. Behav Brain Sci. 2000;23:843–850. doi: 10.1017/s0140525x00003988. discussion 904–1121. [DOI] [PubMed] [Google Scholar]
- 24.Domhoff GW. Finding meaning in dreams: A quantitative approach. Plenum; 1996. [Google Scholar]
- 25.LaBerge S. Lucid dreaming: Evidence and methodology. Behavioral and Brain Sciences. 2000;23:962. [Google Scholar]
- 26.Goldberg I, et al. Neuronal correlates of “free will” are associated with regional specialization in the human intrinsic/default network. Conscious Cogn. 2008;17:587–601. doi: 10.1016/j.concog.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Desmurget M, et al. Movement intention after parietal cortex stimulation in humans. Science. 2009;324:811–813. doi: 10.1126/science.1169896. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A. The single-mindedness and isolation of dreams. Sleep. 1978;1:97–109. doi: 10.1093/sleep/1.1.97. [DOI] [PubMed] [Google Scholar]
- 29.Hobson JA, et al. The neuropsychology of REM sleep dreaming. Neuroreport. 1998;9:R1–14. doi: 10.1097/00001756-199802160-00033. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz S, Maquet P. Sleep imaging and the neuro-psychological assessment of dreams. Trends Cogn Sci. 2002;6:23–30. doi: 10.1016/s1364-6613(00)01818-0. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg II, et al. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Wolman RN, Kozmova M. Last night I had the strangest dream: Varieties of rational thought processes in dream reports. Conscious Cogn. 2007;16:838–849. doi: 10.1016/j.concog.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Kahn D, Hobson A. Theory of Mind in Dreaming: Awareness of Feelings and Thoughts of Others in Dreams. Dreaming. 2005;15:48–57. [Google Scholar]
- 34.Foulkes D, et al. Appropriateness of dream feelings to dreamed situations. Cognition & Emotion. 1988;2:29–39. [Google Scholar]
- 35.Strauch I, et al. In search of dreams: results of experimental dream research. State University of New York Press; 1996. [Google Scholar]
- 36.Fosse R, et al. The mind in REM sleep: reports of emotional experience. Sleep. 2001;24:947–955. [PubMed] [Google Scholar]
- 37.Nielsen TA, et al. Emotions in dream and waking event reports. Dreaming. 1991;1:287–300. [Google Scholar]
- 38.Stickgold R, et al. Replaying the game: hypnagogic images in normals and amnesics. Science. 2000;290:350–353. doi: 10.1126/science.290.5490.350. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann E. The day residue: Time distribution of waking events. Psychobiology. 1968;5:222. [Google Scholar]
- 40.Harlow J, Roll S. Frequency of day residue in dreams of young adults. Percept Mot Skills. 1992;74:832–834. doi: 10.2466/pms.1992.74.3.832. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen T, Powell R. The day-residue and dream-lag effect. Dreaming. 1992;2:67–77. [Google Scholar]
- 42.Fosse MJ, et al. Dreaming and episodic memory: a functional dissociation? J Cogn Neurosci. 2003;15:1–9. doi: 10.1162/089892903321107774. [DOI] [PubMed] [Google Scholar]
- 43.Crick F, Mitchinson G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 44.Fiss H, et al. Waking fantasies following interruption of two types of sleep. Arch Gen Psychiatry. 1966;14:543–551. doi: 10.1001/archpsyc.1966.01730110095015. [DOI] [PubMed] [Google Scholar]
- 45.Stickgold R, et al. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 46.Wagner U, et al. Sleep inspires insight. Nature. 2004;427:352–355. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 47.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 48.Hobson JA. Dreaming as delirium: a mental status analysis of our nightly madness. Seminars in Neurology. 1997;17:121–128. doi: 10.1055/s-2008-1040921. [DOI] [PubMed] [Google Scholar]
- 49.Singer JL. Daydreaming: An introduction to the experimental study of inner experience. Crown Publishing Group/Random House; 1966. [Google Scholar]
- 50.Mason MF, et al. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klinger E. Daydreaming and fantasizing: Thought flow and motivation. In: Markman K, et al., editors. Handbook of Imagination and Mental Stimulation. Psychology Press; 2008. [Google Scholar]
- 52.Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- 53.Panksepp J. Mood changes. In: Vinken P, et al., editors. Handbook of Clinical Neurology 45. Elsevier; 1985. pp. 271–285. [Google Scholar]
- 54.Rees G, et al. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 55.Aserinsky E, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- 56.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–690. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 57.Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957;53:339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- 58.Foulkes WD. Dream reports from different stages of sleep. J Abnorm Soc Psychol. 1962;65:14–25. doi: 10.1037/h0040431. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23:851–866. doi: 10.1017/s0140525x0000399x. discussion 904–1121. [DOI] [PubMed] [Google Scholar]
- 60.Foulkes D. The Psychology of Sleep. Charles Scribner’s Sons; 1966. [Google Scholar]
- 61.Wamsley EJ, et al. Cognitive replay of visuomotor learning at sleep onset: temporal dynamics and relationship to task performance. doi: 10.1093/sleep/33.1.59. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stickgold R, et al. Brain-mind states: I. Longitudinal field study of sleep/wake factors influencing mentation report length. Sleep. 2001;24:171–179. doi: 10.1093/sleep/24.2.171. [DOI] [PubMed] [Google Scholar]
- 63.Fosse R, et al. Brain-mind states: reciprocal variation in thoughts and hallucinations. Psychol Sci. 2001;12:30–36. doi: 10.1111/1467-9280.00306. [DOI] [PubMed] [Google Scholar]
- 64.Monroe LJ, et al. Discriminability of Rem and Nrem Reports. J Pers Soc Psychol. 1965;12:456–460. doi: 10.1037/h0022218. [DOI] [PubMed] [Google Scholar]
- 65.Antrobus J, et al. Dreaming in the late morning: summation of REM and diurnal cortical activation. Conscious Cogn. 1995;4:275–299. doi: 10.1006/ccog.1995.1039. [DOI] [PubMed] [Google Scholar]
- 66.Cavallero C, et al. Slow wave sleep dreaming. Sleep. 1992;15:562–566. doi: 10.1093/sleep/15.6.562. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki H, et al. Dreaming during non-rapid eye movement sleep in the absence of prior rapid eye movement sleep. Sleep. 2004;27:1486–1490. doi: 10.1093/sleep/27.8.1486. [DOI] [PubMed] [Google Scholar]
- 68.Bassetti C, et al. SPECT during sleepwalking. Lancet. 2000;356:484–485. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 69.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 70.Moruzzi G. Active Processes in the Brain Stem during Sleep. Harvey Lect. 1963;58:233–297. [PubMed] [Google Scholar]
- 71.Roffwarg HP, et al. Dream imagery: relationship to rapid eye movements of sleep. Arch Gen Psychiatry. 1962;7:235–258. doi: 10.1001/archpsyc.1962.01720040001001. [DOI] [PubMed] [Google Scholar]
- 72.Moskowitz E, Berger RJ. Rapid eye movements and dream imagery: are they related? Nature. 1969;224:613–614. doi: 10.1038/224613a0. [DOI] [PubMed] [Google Scholar]
- 73.Pivik RT. Tonic States and Phasic Events in Relation to Sleep Mentation. Wiley; 1991. [Google Scholar]
- 74.Esposito MJ, et al. Reduced Alpha power associated with the recall of mentation from Stage 2 and Stage REM sleep. Psychophysiology. 2004;41:288–297. doi: 10.1111/j.1469-8986.00143.x. [DOI] [PubMed] [Google Scholar]
- 75.Antrobus J. How does the dreaming brain explain the dreaming mind? Behav Brain Sci. 2000;23:904–907. [Google Scholar]
- 76.Pivik RT. Psychophysiology of dreams. In: Kryger M, et al., editors. Principles and practices of sleep medicine. 3. Saunders; 2000. pp. 491–501. [Google Scholar]
- 77.Steriade M, et al. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- 78.Destexhe A, et al. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 81.Tononi G. Sleep and dreaming. In: Laureys S, Tononi G, editors. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Elsevier; 2009. pp. 89–107. [Google Scholar]
- 82.Massimini M, et al. Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci U S A. 2007;104:8496–8501. doi: 10.1073/pnas.0702495104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rechtschaffen A, et al. Auditory awakening thresholds in REM and NREM sleep stages. Percept Mot Skills. 1966;22:927–942. doi: 10.2466/pms.1966.22.3.927. [DOI] [PubMed] [Google Scholar]
- 84.Dement W, Wolpert EA. The relation of eye movements, body motility, and external stimuli to dream content. J Exp Psychol. 1958;55:543–553. doi: 10.1037/h0040031. [DOI] [PubMed] [Google Scholar]
- 85.Berger RJ. Experimental Modification of Dream Content by Meaningful Verbal Stimuli. Br J Psychiatry. 1963;109:722–740. doi: 10.1192/bjp.109.463.722. [DOI] [PubMed] [Google Scholar]
- 86.Koulack D. Effects of somatosensory stimulation on dream content. Arch Gen Psychiatry. 1969;20:718–725. doi: 10.1001/archpsyc.1969.01740180102010. [DOI] [PubMed] [Google Scholar]
- 87.Steriade M. Neuronal Substrates of Sleep and Epilepsy. Cambridge University Press; 2003. [Google Scholar]
- 88.Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–14480. doi: 10.1523/JNEUROSCI.3086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colrain IM, Campbell KB. The use of evoked potentials in sleep research. Sleep Med Rev. 2007;11:277–293. doi: 10.1016/j.smrv.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schredl M, et al. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. J Sleep Res. 2009 doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 91.Esser SK, et al. Breakdown of effective connectivity during slow wave sleep: investigating the mechanism underlying a cortical gate using large-scale modeling. J Neurophysiol. 2009 doi: 10.1152/jn.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Golland Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- 94.Ioannides AA, et al. MEG identifies dorsal medial brain activations during sleep. Neuroimage. 2009;44:455–468. doi: 10.1016/j.neuroimage.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 95.Buckner RL, et al. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 96.Gusnard DA, et al. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Amedi A, et al. Negative BOLD differentiates visual imagery and perception. Neuron. 2005;48:859–872. doi: 10.1016/j.neuron.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 99.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robertson L, et al. The Interaction of Spatial and Object Pathways: Evidence from Balint’s Syndrome. J Cogn Neurosci. 1997;9:295–371. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- 101.Pollen DA. Fundamental requirements for primary visual perception. Cereb Cortex. 2008;18:1991–1998. doi: 10.1093/cercor/bhm226. [DOI] [PubMed] [Google Scholar]
- 102.Zikopoulos B, Barbas H. Circuits for multisensory integration and attentional modulation through the prefrontal cortex and the thalamic reticular nucleus in primates. Rev Neurosci. 2007;18:417–438. doi: 10.1515/revneuro.2007.18.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guillery RW, et al. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- 104.Weissman DH, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 105.John J, et al. Cataplexy-active neurons in the hypothalamus: implications for the role of histamine in sleep and waking behavior. Neuron. 2004;42:619–634. doi: 10.1016/s0896-6273(04)00247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aristotle (350 B.C.) On Dreams (“De Insomniis”).
- 107.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 108.Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 109.Kerr NH, Foulkes D. Right hemispheric mediation of dream visualization: a case study. Cortex. 1981;17:603–609. doi: 10.1016/s0010-9452(81)80066-4. [DOI] [PubMed] [Google Scholar]
- 110.Butler S, Watson R. Individual differences in memory for dreams: The role of cognitive skills. Percept Mot Skills. 1985;53:841–864. [Google Scholar]
- 111.Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Little Brown; 1954. [Google Scholar]
- 112.Finke RA, Kurtzman HS. Mapping the visual field in mental imagery. J Exp Psychol Gen. 1981;110:501–517. doi: 10.1037//0096-3445.110.4.501. [DOI] [PubMed] [Google Scholar]
- 113.Mahowald M, Schenck C. REM sleep parasomnias. In: Kryger M, et al., editors. Principles and practices of sleep medicine. W. B. Saunders; 2000. pp. 724–741. [Google Scholar]
- 114.Chugh DK, et al. Neurobehavioral consequences of arousals. Sleep. 1996;19:S198–201. doi: 10.1093/sleep/19.suppl_10.s198. [DOI] [PubMed] [Google Scholar]
- 115.Arkin AM. Sleep talking: Psychology and Psychophysiology. Lawrence Erlbaum Associates; 1981. [Google Scholar]
- 116.Broughton RJ. Sleep disorders: disorders of arousal? Enuresis, somnambulism, and nightmares occur in confusional states of arousal, not in “dreaming sleep”. Science. 1968;159:1070–1078. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- 117.Wechsler D. Manual for the Wechsler preschool and primary scale of intelligence. Psychological Corporation; 1967. [Google Scholar]
- 118.Casey BJ, et al. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 119.Piaget J, Barbel I. Mental Imagery in the Child. Basic Books; 1966. [Google Scholar]
- 120.Shephard RN, Cooper LA. Mental images and their transformations. MIT Press; 1982. [Google Scholar]
- 121.Hollins M. Styles of mental imagery in blind adults. Neuropsychologia. 1985;23:561–566. doi: 10.1016/0028-3932(85)90009-0. [DOI] [PubMed] [Google Scholar]
- 122.Buchel C, et al. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain. 1998;121 (Pt 3):409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- 123.Lopes da Silva FH. Visual dreams in the congenitally blind? Trends Cogn Sci. 2003;7:328–330. doi: 10.1016/s1364-6613(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 124.Hurovitz CS, et al. The dreams of blind men and women: a replication and extension of previous findings. Dreaming. 1999;9:183–193. [Google Scholar]
- 125.Holzinger B. The dreams of the blind: considerations of the congenitally and adventitiously blind. J Sleep Res. 2000;9:83. [Google Scholar]
- 126.Sastre JP, Jouvet M. Oneiric behavior in cats. Physiol Behav. 1979;22:979–989. doi: 10.1016/0031-9384(79)90344-5. [DOI] [PubMed] [Google Scholar]
- 127.Foulkes D. Cognitive processes during sleep: Evolutionary aspects. In: Mayes A, editor. Sleep mechanisms and functions in humans and animals: An evolutionary perspective. Van Nostrand Reinhold; 1983. pp. 313–337. [Google Scholar]
- 128.Murri L, et al. Dream recall after sleep interruption in brain-injured patients. Sleep. 1985;8:356–362. doi: 10.1093/sleep/8.4.356. [DOI] [PubMed] [Google Scholar]
- 129.Bischof M, Bassetti CL. Total dream loss: a distinct neuropsychological dysfunction after bilateral PCA stroke. Ann Neurol. 2004;56:583–586. doi: 10.1002/ana.20246. [DOI] [PubMed] [Google Scholar]
- 130.Kosslyn S. Image and Brain. MIT press; 1994. [Google Scholar]
- 131.Epstein AW, Simmons NN. Aphasia with reported loss of dreaming. Am J Psychiatry. 1983;140:108–109. doi: 10.1176/ajp.140.1.108. [DOI] [PubMed] [Google Scholar]
- 132.Solms M. Freud returns. Sci Am. 2004;290:82–88. doi: 10.1038/scientificamerican0504-82. [DOI] [PubMed] [Google Scholar]
- 133.Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 134.Naya Y, et al. Backward spreading of memory-retrieval signal in the primate temporal cortex. Science. 2001;291:661–664. doi: 10.1126/science.291.5504.661. [DOI] [PubMed] [Google Scholar]
- 135.Mormann F, et al. Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. J Neurosci. 2008;28:8865–8872. doi: 10.1523/JNEUROSCI.1640-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dang-Vu TT, et al. Spontaneous neural activity during human slow wave sleep. Proc Natl Acad Sci U S A. 2008;105:15160–15165. doi: 10.1073/pnas.0801819105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peigneux P, et al. Generation of rapid eye movements during paradoxical sleep in humans. Neuroimage. 2001;14:701–708. doi: 10.1006/nimg.2001.0874. [DOI] [PubMed] [Google Scholar]
- 138.Hong CC, et al. fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum Brain Mapp. 2009;30:1705–1722. doi: 10.1002/hbm.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Friston KJ. Modalities, modes, and models in functional neuroimaging. Science. 2009;326:399–403. doi: 10.1126/science.1174521. [DOI] [PubMed] [Google Scholar]
- 140.Nir Y, et al. Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation. Neuroimage. 2006;30:1313–1324. doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 141.von Kriegstein K, et al. Interaction of face and voice areas during speaker recognition. J Cogn Neurosci. 2005;17:367–376. doi: 10.1162/0898929053279577. [DOI] [PubMed] [Google Scholar]
- 142.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 143.Lee JH, et al. Automated classification of fMRI data employing trial-based imagery tasks. Med Image Anal. 2009;13:392–404. doi: 10.1016/j.media.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 145.Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11:295–310. doi: 10.1016/j.smrv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 146.Yamamoto M, Nakahama H. Stochastic properties of spontaneous unit discharges in somatosensory cortex and mesencephalic reticular formation during sleep-waking states. J Neurophysiol. 1983;49:1182–1198. doi: 10.1152/jn.1983.49.5.1182. [DOI] [PubMed] [Google Scholar]
- 147.Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Evarts EV. Temporal Patterns of Discharge of Pyramidal Tract Neurons during Sleep and Waking in the Monkey. J Neurophysiol. 1964;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- 149.Noda H, Adey WR. Firing of neuron pairs in cat association cortex during sleep and wakefulness. J Neurophysiol. 1970;33:672–684. doi: 10.1152/jn.1970.33.5.672. [DOI] [PubMed] [Google Scholar]
- 150.Hobson JA, McCarley RW. Cortical unit activity in sleep and waking. Electroencephalogr Clin Neurophysiol. 1971;30:97–112. doi: 10.1016/0013-4694(71)90271-9. [DOI] [PubMed] [Google Scholar]
- 151.Destexhe A, et al. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Werth E, et al. Selective REM sleep deprivation during daytime. II. Muscle atonia in non-REM sleep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R527–532. doi: 10.1152/ajpregu.00466.2001. [DOI] [PubMed] [Google Scholar]
- 153.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–133. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 154.Vogel GW. An alternative view of the neurobiology of dreaming. Am J Psychiatry. 1978;135:1531–1535. doi: 10.1176/ajp.135.12.1531. [DOI] [PubMed] [Google Scholar]
- 155.Domhoff GW. Refocusing the neurocognitive approach to dreams: a critique of the Hobson versus Solms debate. Dreaming. 2005;15:3–20. [Google Scholar]
- 156.Solms M, Turnbull O. The brain and the inner world. Other press; 2002. [Google Scholar]
- 157.Doricchi F, Violani C. In: The Neuropsychology of Sleep and Dreaming. Antrobus JS, Bertini M, editors. Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 158.Hobson JA. The Dream Drug Store. MIT Press; 2001. [Google Scholar]
- 159.Snyder F. The phenomenology of dreaming. In: Madow L, Snow L, editors. The psychodynamic implications of the physiological studies on dreams. Thomas; 1970. pp. 124–151. [Google Scholar]
- 160.Gillin J, Christian, et al. Cholinergic Mechanisms in REM Sleep. In: Wauquier A, et al., editors. Sleep: Neurotransmitters and Neuromodulators. Raven Press; 1985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.