Abstract
Plasticity at corticostriatal synapses is thought to underlie both normal and aberrant forms of reinforcement-driven learning. Studies in brain slices have found bidirectional, spike-timing dependent plasticity in striatum; however it is not known whether similar rules govern corticostriatal plasticity in awake behaving animals. To assess whether behavioral state is a key regulator of plasticity in this pathway, we examined the effects of 5Hz cortical stimulation trains on evoked striatal field potentials, in either anesthetized or awake, unrestrained rats. Consistent with prior studies we observed long-term potentiation in intact, barbiturate-anesthetized animals. However, when an identical stimulation pattern was applied to the same animals while awake, long-term depression was observed instead. Our results demonstrate that the rules governing corticostriatal plasticity depend critically on behavioral state, and suggest that the dynamic context of cortical-basal ganglia loops must be considered while investigating synaptic mechanisms underlying reinforcement learning and neurological disorders.
Keywords: striatum, plasticity, long-term potentiation, anesthesia, barbiturate, learning
Synaptic plasticity in cortical-basal ganglia circuits is a likely core mechanism by which animals learn context-dependent action sequences leading to rewards (e.g. Houk and Wise, 1995, Schultz 1998). Dysregulation of plasticity may be critically involved in drug addiction (Berke and Hyman, 2000), dyskinesias (Graybiel et al., 2000) and Parkinson’s Disease (Shen et al., 2008). Understanding the rules governing striatal plasticity is thus essential for determining how the basal ganglia contribute to both normal and pathological behaviors.
Most information flow into the basal ganglia occurs at glutamatergic synapses onto striatal medium spiny neurons (MSNs), whose spines provide a major locus of synaptic change (e.g. Robinson & Kolb 2004). Corticostriatal plasticity has been extensively investigated in brain slices, and both long-term potentiation (LTP) and long-term depression (LTD) have been observed depending on experimental conditions (for review see DiFillipo et al. 2009). Recent studies that varied the precise timing between synaptic input and post synaptic spiking revealed bidirectional spike-timing-dependent plasticity at synapses onto MSNs (STDP; Fino et al., 2005; Pawlak and Kerr, 2008; Shen et al., 2008), but different groups have obtained very different STDP functions, again possibly due to distinct experimental conditions. Studies in intact animals have also found both LTD and LTP in striatum, and a relationship to dopamine modulation (e.g. Charpier and Deniau, 1997; Charpier et al., 1999; Reynolds and Wickens, 2000; Reynolds et al., 2001; Goto & Grace 2005). However these studies were performed in anesthetized animals, and striatal physiology is very different in awake states (e.g. West 1998, Mahon et al. 2006).
We therefore directly compared corticostriatal plasticity under awake versus anesthetized conditions, using rats with chronically implanted electrodes. To facilitate investigation in freely moving animals, we made use of a cortical stimulation protocol previously shown to produce LTP in barbiturate-anesthetized animals without requiring current injection into the post-synaptic cell (Charpier et al., 1999).
Experimental Procedures
Electrophysiology
All procedures were approved by the University of Michigan’s University Committee on Use and Care of Animals. We used 15 adult male Long-Evans rats (350–550g). Thirteen rats were implanted with a chronic microdrive assembly containing 6 independently moveable tetrodes, each consisting of four strands of 12.5μm nichrome wire (Kanthal Palm Coast, FL, USA). Target coordinates in striatum were AP +1.0mm (from bregma), ML 2.0–4.0mm, DV 3.5–5.5mm (from brain surface). Two rats were implanted with a 4×8 array of microwires (Tucker Davis Technology, Alachua, FL, USA). Array target coordinates were AP +0.5–2.5mm, ML 2.75–4.5mm, DV 3.5–5.0mm (see Supplemental Figure 2). Each animal was also implanted with a bipolar stimulating electrode (either SNEX 200 obtained from David Kopf Instruments, Tujunga, CA or Plastics1 item # 303/3, Roanoke, VA) into contralateral motor cortex (AP +3.2mm, ML +3.3mm DV 1.6mm). Electrophysiologically-guided electrode positioning began immediately following surgery, and continued during post-surgical recovery (minimum 10 days).
Animals were housed on a 12:12 (light:dark) hour cycle and tested during the light phase. Each conditioning stimulation session was conducted with the rat placed on a familiar, elevated octagonal platform (16″ diameter, walls 5.5″ high). For awake sessions, animals were free to move during the course of the experiment, but tended to rest quietly. For experiments under anesthesia, animals were given an initial dose of sodium pentobarbital (66mg/kg, IP), with supplements (22mg/kg) if an animal twitched in response to hind paw pinch. Awake and anesthetized experiments were conducted on the same day, separated by 1 hour if the animal was tested first under the awake condition or a minimum of 4 hours if the animal was first tested under anesthesia. Electrodes were not moved between testing sessions.
Electrical stimulation used single, 0.1ms, biphasic square wave pulses (1mA), from an analog stimulus isolator (A-M Systems, Sequim, WA). The experimental sequence consisted of 30 minutes of baseline measurements with stimulation at 0.1Hz (180 pulses), “conditioning stimulation” at 5Hz (1000 pulses), and 60 minutes of post conditioning pulses at 0.1Hz (360 pulses). Single test pulses did not result in any overt movement or vocalization. Occasionally, 5Hz stimulation would result in repetitive neck movements that ceased immediately with stimulation offset. Electrophysiological signals were wide-band filtered (1–9000Hz) and sampled at 25–31.25kHz. Recording, visualization and stimulation were controlled and synchronized using LabVIEW software (National Instruments, Austin TX). After completion of experiments on each rat, current (20μA, 10s) was passed through each recording electrode to create marker lesions. Recording sites were not included in analyses if located outside striatum, or if the noise pattern suggested a non-functional contact.
Data Analysis
Evoked field potentials were averaged in successive sets of 10 stimulation events. For each such average, the highest amplitude positive peak within 22ms of stimulation was detected (“P1”). Electrodes were excluded if there was no P1 peak (within 22ms). Next, the most negative peak that preceded P1 (excluding 0–2ms to avoid the stimulation artifact) was detected (“N1”). The N1-P1 voltage difference was our estimate of synaptic strength. Just one single wire of each tetrode was used for field potential analyses.
For each electrode estimates of synaptic strength were compared between baseline (30min epoch just prior to conditioning stimulation) and post-conditioning (30min epoch, beginning 30min after conditioning stimulation). The null hypothesis of no change in synaptic strength was rejected if the means were significantly different (t-test, alpha=0.01). If, in a given animal, no electrodes had a significant change in synaptic strength then we reported the result as no change (nc). Otherwise we reported LTP if the mean normalized post conditioning synaptic strength was above 100% and LTD if below 100%. In no cases did an individual animal show a significant increase in synaptic strength on one tetrode or electrode but a significant decrease on another.
Results
Anesthesia reverses the direction of corticostriatal plasticity
We assessed the effects of 5Hz cortical stimulation on corticostriatal synaptic strength in fifteen animals, tested while awake and unrestrained, under barbiturate anesthesia, or both (Figures 1,2; Table 1). The shape of the evoked potential was similar in both states, although we occasionally observed lower amplitude and variance in baseline measurements of synaptic strength when under anesthesia (Supplementary Figure 1.)
Figure 1.
Field potential measurement of evoked monosynaptic strength. (A) Electrical stimulation was applied in orofacial motor cortex, and evoked potentials were recorded from multiple electrodes in the contralateral striatum. (B) Illustration of stimulation protocol. (C) Monosynaptic strength was estimated as the voltage difference between the first negative (N1) and positive (P1) peaks. Trace is an average from 10 consecutive stimulation events. Stimulation artifact is at 0 ms. (D, E) Sample measurements of series averaged evoked field potentials from a single electrode recorded under both the awake state (D) and the barbiturate anesthetized state (E). Dashed lines indicate epochs used to calculate synaptic strength at baseline and following conditioning.
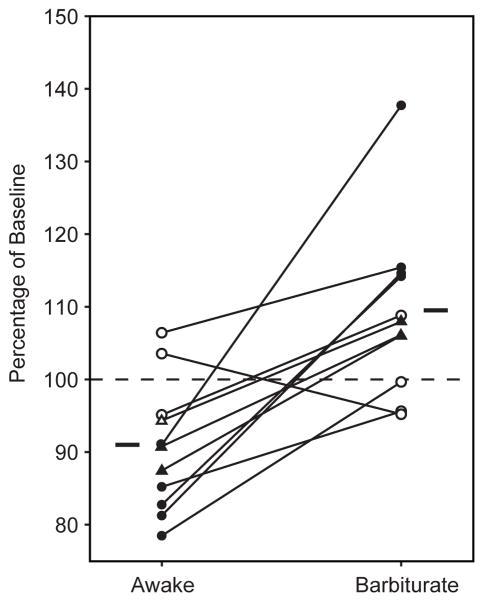
Figure 2.
Direction of plasticity is dependent upon brain state. Eleven animals received 5 Hz stimulation under two brain states, awake/unrestrained and barbiturate anesthesia. Closed markers indicate significant changes in each state, and horizontal lines indicate state means. A two tailed paired samples t-test reveals a significant effect of brain state on plasticity outcome (p=0.002). Seven animals were tested in the awake state first (circles) followed by barbiturate anesthesia. In 3 animals the order of testing was reversed (triangles).
Table 1.
Summary of plasticity results for all experiments.
| Awake State | Barbiturate Anesthesia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ID# | note | n | Min. | Max. | Mean | n | Min. | Max. | Mean | ||
| 92 | 4/4 | 76.8 | 87.3 | 84.2 | LTD | ||||||
| 93 | 0/3 | 97.8 | 103.2 | 100.1 | nc | ||||||
| 100 | 4/4 | 77.2 | 87.3 | 81.3 | LTD | 4/4 | 110.2 | 119.9 | 114.6 | LTP | |
| 107 | 0/4 | 105.2 | 107.2 | 106.4 | nc | 4/4 | 112.3 | 121.1 | 115.4 | LTP | |
| 108 | 0/1 | - | - | 95.1 | nc | 0/1 | - | - | 108.8 | nc | |
| 109 | 3/3 | 83.7 | 87.6 | 85.2 | LTD | 0/3 | 94.0 | 98.2 | 95.6 | nc | |
| 112 | a | 3/3 | 72.2 | 81.9 | 78.5 | LTD | 0/3 | 96.7 | 101.5 | 99.6 | nc |
| 113 | a | 0/1 | - | - | 103.6 | nc | 0/1 | - | - | 95.2 | nc |
| 115 | a | 1/3 | 86.6 | 93.9 | 91.0 | LTD | 3/3 | 123.9 | 155.0 | 137.7 | LTP |
| 122 | a | 4/4 | 79.2 | 86.1 | 82.7 | LTD | 3/4 | 105.6 | 119.1 | 114.2 | LTP |
| 132 | a,b | 1/2 | 85.9 | 95.7 | 90.8 | LTD | 1/2 | 99.6 | 112.7 | 106.1 | LTP |
| 133 | a,b | 3/3 | 84.3 | 90.4 | 87.4 | LTD | 2/3 | 101.6 | 108.6 | 106.1 | LTP |
| 136 | a,b | 0/1 | - | - | 94.4 | nc | 1/1 | - | - | 108.0 | LTP |
| 138 | a,c | 6/14 | 74.6 | 92.0 | 83.8 | LTD | |||||
| 139 | a,c | 0/23 | 88.0 | 111.0 | 95.7 | nc | |||||
ID# indicates each animal, and n indicates the fraction of striatal electrodes where a significant change in synaptic strength was observed. The minimum, maximum and mean synaptic strength, following tetanus, is reported as a percentage of baseline for each animal with two or more tetrodes or electrodes, otherwise a single result is presented under mean change. Notes on experimental protocol:
These animals had a stimulating electrode with a slightly larger tip separation (0.6 mm).
These animals were tested in the barbiturate anesthetized state first.
These animals were implanted with a microwire array.
Of eleven animals that received the conditioning stimulation under barbiturate anesthesia, seven showed LTP and four showed no change; in no case did we observe LTD. These observations are consistent with prior findings of corticostriatal LTP in barbiturate-anesthetized rats (Charpier and Deniau, 1997; Charpier et al. 1999), even though experimental conditions were not identical (e.g. they used intracellular recording and ipsilateral stimulation). Results were very different under the awake condition, with the majority of animals (9/15) showing LTD instead; in no case did we observe LTP. The result of the experiment did not depend on the order of treatment, since the three animals which the anesthesia session was performed first displayed the same pattern of results as animals who received stimulation under the awake condition first (Figure 2).
Direction of plasticity does not strongly reflect intrastriatal location
Reports in brain slices have found evidence that synaptic plasticity may operate differently in distinct striatal subregions (Partridge et al., 2000; Smith et al., 2001). We therefore examined the contribution of intra-striatal location to our results (Figure 3). Although some possible weak trends were apparent, location was not a major factor – our overall finding of LTD in the awake state, LTP under anesthesia held across a wide range of antero-posterior, medio-lateral, and dorso-ventral coordinates.
Figure 3.
No clear relationship between intrastriatal location and plasticity direction. For each striatal tetrode tested under both awake (A–C) and anesthetized (D–F) conditions, post-conditioning synaptic strength is plotted against mediolateral (A, D), dorsoventral (B, E) and anteroposterior (C, F) position. The overall direction of change does not vary with position, although in the anesthetized state (only) there was a weakly significant (p = 0.042) linear correlation between synaptic change and mediolateral position, if we do not correct for multiple comparisons. This appeared to be largely due to a single outlier point.
Evoked corticostriatal field potential originates from within the striatum
Although evoked potentials are often used to assess synaptic strength they are an indirect measure, and the origin of field potentials measured in striatum is not fully clear (Berke 2005). To gain increased confidence in our measure we examined the spatiotemporal pattern of evoked potentials in two animals implanted with a three-dimensional, fixed-geometry array of 32 individual recording electrodes (Supplementary Figure 2). The largest amplitude P1 response was measured from electrodes located deep within the striatum. This is consistent with our evoked potential measure corresponding to striatal physiological events (Ryan et al. 1986), rather than (for example) volume conduction from the overlaying cerebral cortex.
Discussion
This study provides the first description, to our knowledge, of corticostriatal plasticity in intact, unrestrained animals. We found that such plasticity was strikingly dependent on the state of the animal. We confirmed observations of LTP under barbiturate anesthesia (Charpier et al., 1999), but found LTD in the same animals when awake. This finding has broad implications for the study of neural plasticity, and in particular for investigations into relationships between corticostriatal synapses and reinforcement-based learning.
Evoked potentials have been widely used to study synaptic plasticity both in other brain regions and in other areas of striatum (e.g. Goto and Grace 2005). We cannot be certain which intrastriatal processes are contributing to the observed evoked potential in our experiments, although synaptic and postsynaptic currents, close to the recording electrode, are believed to be the dominant factor (Mitzdorf, 1985; Berke, 2005; Katzner et al., 2009). Strong evidence that our evoked potential measure mirrors excitatory post-synaptic potentials (EPSPs) comes from combined intracellular and extracellular recordings in striatum (Ryan et al. 1986). They observed cortically-evoked striatal potentials with a very similar or identical time course to ours, and found that the extracellular P1 peak corresponded to intracellular EPSPs. The short latency of this peak makes it very likely to reflect monosynaptically evoked EPSPs, though we cannot entirely rule out the possibility that fast polysynaptic events in cortex are involved.
The reversal of synaptic plasticity with barbiturate anesthesia may reflect either the actions of this drug locally within the striatum, and/or drug-induced changes in the overall patterns of synaptic input to this structure. We consider the former possibility first. Barbiturate is a sedative-hypnotic drug that tends to prolong the opening of GABAA channels (D’Hulst et al. 2009) and it is worth noting that related manipulations have previously been shown to have a profound effect on striatal plasticity. For example, Yin et al (2007) observed in brain slices that another sedative-hypnotic, ethanol, can reverse the direction of striatal plasticity in dorsomedial striatum (although this reversal was from LTP to LTD). Alterations in striatal GABAA transmission may also be involved in the very different STDP functions described for striatum in vitro. Two groups (Pawlak and Kerr, 2008; Shen et al., 2008) reported a Hebbian plasticity function in the presence of GABAA inhibitors, while a third study (Fino et al., 2005) omitted this treatment and reported an anti-Hebbian plasticity function.
Under barbiturate anesthesia cortical activity patterns are quite different to the awake state, and can drive spontaneous ~5Hz membrane potential oscillations in striatal MSNs (Charpier et al. 1999). Successful induction of corticostriatal LTP with artificial 5Hz stimulation correlates with the entrainment of large-scale cortical spindle oscillations (Charpier et al. 1999). This would result in more broadly coherent inputs to striatum, and a higher probability of postsynaptic spiking in synchrony with the directly stimulated inputs. In addition, cortical stimulation results in feed-forward inhibitory input from fast spiking interneurons (FSIs), via GABAA receptors, onto MSNs. FSIs show marked paired pulse depression at ~100–200ms intervals (e.g. Mallet et al. 2005), so coordinated 5Hz stimulation may result in enhanced postsynaptic MSN activity via disinhibition. Further work is needed to explore the contributions of interneurons to striatal synaptic plasticity, both as coordinators of MSN timing and as a site of plasticity themselves (Fino et al. 2008).
Overall, our results support the general idea that the background patterns of cortical activity are a critical determinant of the direction of synaptic change (Mahon et al. 2004). In contrast to prior in vivo results they are more consistent with LTD as a predominant form of plasticity in the awake, behaving striatum. However, our experiments have significant limitations: we also used a highly artificial form of cortical stimulation in our experiments, and cannot directly control the timing of postsynaptic spiking. Despite this, there are interesting parallels between the striatum and other structures involved in procedural learning that employ an anti-Hebbian plasticity algorithm, including the dorsal cochlear nucleus (Tzounopoulos and Kraus 2009) and cerebellum. Anti-Hebbian rules can support the formation of a “negative image” of one’s own actions, that enable their sensory consequences to be distinguished from other events (Bell et al., 2008). This may also be of great importance in striatum, which receives efference copy of corticospinal outputs as part of an overall algorithm in which unexpected outcomes drive reinforcement learning (Redgrave and Gurney, 2006).
Supplementary Material
Supplementary Figure 1: Magnitude and variability of evoked potentials as a function of behavioral state. Crosses show mean and inter-quartile range for baseline (grey) and post-conditioning (black) measurements for all individual tetrodes tested in both awake (A) and anesthetized (B) states. Included tetrodes are grouped together by animal (grey shading). An asterisk indicates a significant difference between baseline and postconditioning means.
Supplementary Figure 2: Evoked corticostriatal field potential originates from within the striatum. (A) 32-site electrode array configuration, showing the two wedge-shaped groups with deepest electrodes positioned laterally. (B) Evoked field potential measurements from a representative series average are presented in grid electrode array order (in register with C, D, E). Vertical black line at 0 ms is the stimulation artifact. Voltage data depicted in B was used to generate contour color maps of voltage at 5, 10 and 15 ms following stimulation (C, D, E). Interpolated contour maps are shown for each group of 16 coplanar electrodes (black circles, smallest are deepest). Time points were selected to illustrate that the P1 peak appears earliest and with highest amplitude in deep, striatally-located electrodes (F).
Acknowledgments
We thank members of the Berke Laboratory for critical discussions. This work was funded by the University of Michigan, and the National Institute on Drug Abuse (T32 DA007281; R01 DA014318).
List of Abbreviations
- LTP
long-term potentiation
- LTD
long-term depression
- STDP
spike-timing-dependent plasticity
- GABA
gamma-aminobutyric acid
- FSI
fast-spiking interneuron
- EPSP
excitatory post-synaptic potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci. 2008;31:1–24. doi: 10.1146/annurev.neuro.30.051606.094225. [DOI] [PubMed] [Google Scholar]
- Berke JD. The basal ganglia VIII. New York: Springer; 2005. Participation of striatal neurons in large-scale oscillatory networks. [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Charpier S, Deniau JM. In vivo activity-dependent plasticity at cortico-striatal connections: evidence for physiological long-term potentiation. Proc Natl Acad Sci U S A. 1997;94:7036–7040. doi: 10.1073/pnas.94.13.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpier S, Mahon S, Deniau JM. In vivo induction of striatal long-term potentiation by low-frequency stimulation of the cerebral cortex. Neuroscience. 1999;91:1209–1222. doi: 10.1016/s0306-4522(98)00719-2. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Atack JR, Kooy RF. The complexity of the GABAA receptor shapes unique pharmacological profiles. Drug Discov Today 2009. 2009 Sep;14(17–18):866–75. doi: 10.1016/j.drudis.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, Tozzi A, Parnetti L, Calabresi P. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory. Behav Brain Res. 2009;199:108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Deniau JM, Venance Cell-specific spike-timing-dependent plasticity in GABAergic and cholinergic interneurons in corticostriatal rat brain slices. J Physiol. 2008;586(1):265–82. doi: 10.1113/jphysiol.2007.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. 2000;23:S71–77. doi: 10.1016/s1471-1931(00)00027-6. [DOI] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon S, Deniau JM, Charpier S. Corticostriatal plasticity: life after the depression. Trends Neurosci. 2004;27:460–467. doi: 10.1016/j.tins.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Le Moine C, Charpier S, Gonon F. Feedforward inhibition of projection neurons by fast-spiking GABA interneurons in the rat striatum in vivo. J Neurosci. 2005;25:3857–3869. doi: 10.1523/JNEUROSCI.5027-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:37–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JN. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99:199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Tepper JM, Young SJ, Groves PM. Frontal cortex stimulation evoked neostriatal potentials in rats: intracellular and extracellular analysis. Brain Res Bull. 1986;17:751–758. doi: 10.1016/0361-9230(86)90086-9. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Musleh W, Akopian G, Buckwalter G, Walsh JP. Regional differences in the expression of corticostriatal synaptic plasticity. Neuroscience. 2001;106:95–101. doi: 10.1016/s0306-4522(01)00260-3. [DOI] [PubMed] [Google Scholar]
- West MO. Anesthetics eliminate somatosensory-evoked discharges of neurons in the somatotopically organized sensorimotor striatum of the rat. J Neurosci. 1998;18:9055–9068. doi: 10.1523/JNEUROSCI.18-21-09055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Park BS, Adermark L, Lovinger DM. Ethanol reverses the direction of long-term synaptic plasticity in the dorsomedial striatum. European Journal of Neuroscience. 2007;25:3226–3232. doi: 10.1111/j.1460-9568.2007.05606.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Magnitude and variability of evoked potentials as a function of behavioral state. Crosses show mean and inter-quartile range for baseline (grey) and post-conditioning (black) measurements for all individual tetrodes tested in both awake (A) and anesthetized (B) states. Included tetrodes are grouped together by animal (grey shading). An asterisk indicates a significant difference between baseline and postconditioning means.
Supplementary Figure 2: Evoked corticostriatal field potential originates from within the striatum. (A) 32-site electrode array configuration, showing the two wedge-shaped groups with deepest electrodes positioned laterally. (B) Evoked field potential measurements from a representative series average are presented in grid electrode array order (in register with C, D, E). Vertical black line at 0 ms is the stimulation artifact. Voltage data depicted in B was used to generate contour color maps of voltage at 5, 10 and 15 ms following stimulation (C, D, E). Interpolated contour maps are shown for each group of 16 coplanar electrodes (black circles, smallest are deepest). Time points were selected to illustrate that the P1 peak appears earliest and with highest amplitude in deep, striatally-located electrodes (F).