Abstract
Episodes of ventricular fibrillation (VF) and myocardial dysfunction commonly occur after cardiac resuscitation compromising the return of stable circulation. We investigated in a pig model of VF whether limiting Na+-induced cytosolic Ca2+ overload using the sarcolemmal sodium-hydrogen exchanger isoform-1 (NHE-1) inhibitor cariporide promotes resuscitation with stable circulation.
Methods
VF was electrically induced in 20 male pigs and left untreated for 6 minutes after which CPR was initiated and continued for 8 minutes before attempting defibrillation. Pigs were randomized to receive 3-mg/kg cariporide (n = 10) or 0.9%-NaCl (n = 10) before chest compression.
Results
Seven of 10 pigs in each group were successfully resuscitated and survived 2 hours. Cariporide ameliorated post-resuscitation ventricular ectopic activity such that fewer singlets (5 ± 5 vs 26 ± 21; p < 0.05) and fewer bigemini (1 ± 3 vs 33 ± 25; p < 0.05) were observed during the initial 5 minutes post-resuscitation. Additionally, cariporide-treated pigs did not require additional post-resuscitation shocks for ventricular tachycardia or recurrent VF (0.0 ± 0.0 vs 5.3 ± 7.8 shocks; p = 0.073). During the initial 60 minutes cariporide-treated pigs had higher), cardiac index (6.1 ± 0.7 vs 4.4 ± 1.1, L/min/m2; p < 0.01), left ventricular stroke work index (45 ± 9 vs 36 ± 10 gm·m/beat/m2; p < 0.05), and numerically higher mean aortic pressure (104 ± 11 vs 91 ± 12 mmHg; p = 0.054).
Conclusion
Cariporide administered at the start of chest compression may help restore electrically and mechanically stable circulation after resuscitation from cardiac arrest.
INTRODUCTION
Ventricular arrhythmias commonly occur after successful resuscitation from cardiac arrest with a reported incidence of ventricular fibrillation (VF) as high as 79%.1 Some studies have reported an inverse relationship between the number of VF episodes and survival.1 These episodes were noted to occur within a time window of 23 to 115 seconds with a median of 45 seconds after the return of spontaneous circulation.1 The prevailing concept is that post-resuscitation ventricular arrhythmias, including episodes of VF are to a large extent a manifestation of cytosolic Ca2+ accumulation in cardiomyocytes.2–4 Such Ca2+ accumulation is a central manifestation of ischemia and reperfusion injury and reflects in part increased sarcolemmal Na+ entry followed by cytosolic Ca2+ overload consequent to reverse mode operation of the sarcolemmal Na+-Ca2+ exchanger.5,6 One mechanism of sarcolemmal Na+ entry and cytosolic Na+ accumulation during ischemia and reperfusion is activation of the sarcolemmal sodium-hydrogen exchanger isoform-1 (NHE-1) with concomitant inactivation of the Na+-K+ ATPase activity.7,8
Along with reperfusion arrhythmias, the myocardium during the post-resuscitation period also suffers varying degrees of global dysfunction that can compromise hemodynamic function.9,10 These electrical and mechanical abnormalities occur early in the post-resuscitation phase, coinciding with the prehospital phase and may account for the nearly 40% deaths that have been reported before hospital admission in initially resuscitated victims.11 Thus, treatments that could provide initial electrical and mechanical stability could have potential beneficial survival effects on victims of out-of-hospital cardiac arrest. In this study we examined the effects of NHE-1 inhibition using cariporide on post-resuscitation ventricular arrhythmias and myocardial dysfunction using a pig model of VF and closed-chest resuscitation.
METHODS
The studies were approved by our Institutional Animal Care and Utilization Committee and conducted according to institutional guidelines.
Animal preparation
Male domestic pigs (29 to 39 kg, Oak Hill Genetics) were sedated with intramuscular ketamine (30 mg/kg) and anesthetized with intravenous sodium pentobarbital administering 30 mg/kg for induction and 8 mg/kg for maintenance every 30 minutes. Ventilation was provided through an orotracheal tube using a volume-controlled ventilator (Bear 1000, Bear Medical Systems, Inc) set to deliver a tidal volume of 10 mL/kg, peak flow of 40 L/min, and FiO2 of 0.4. The respiratory rate was adjusted to maintain an end-tidal PCO2 (PETCO2) between 35 and 45 mm Hg. Rectal temperature was maintained between 36.5 and 37.5 °C using a servo-controlled water-circulated blanket. A lead-II ECG was recorded through skin electrodes and two self-adhesive conductive gel pads were positioned on the chest for electrical defibrillation. Through the right cephalic vein, a 5F pacing electrode was advanced into the right ventricle until an injury current was observed and used for induction of VF. A 7-F angiographic catheter was advanced through the right femoral artery into the descending thoracic aorta for pressure measurements and blood sampling. A 7-F thermodilution balloon-tipped catheter was advanced through the left cephalic vein into the pulmonary artery for measuring cardiac output (Edwards Critical Care Explorer™, Baxter Healthcare Corporation), right atrial pressure, pulmonary artery pressure, and blood sampling. A 7F high-fidelity Micro-Tip pressure-transducer pigtail-catheter (SPC-474A, Millar Instruments) was advanced through the right carotid artery into the left ventricle for pressure measurements. Proper catheter placement was confirmed under fluoroscopy.
Measurements
Cardiac output was measured in triplicate by thermodilution after bolus injection of 10 ml ice-cold 0.9% NaCl solution into the right atrium, and the values normalized to body surface area (BSA) using the Kelly equation (BSA [m2] = 0.073 * weight2/3 [kg]). Left ventricular stroke work index was calculated as the difference between systolic and diastolic left ventricular pressure multiplied by the stroke volume index and expressed as gm·m/beat/m2 by applying the conversion factor 0.0136. The coronary perfusion pressure corresponded to the aortic minus right atrial pressure at the end of diastole during spontaneous circulation and at the end of chest relaxation during chest compression.
To characterize the effect on electrical stability during the initial five minutes post-resuscitation, the numbers of recurrent episodes of VF and the corresponding number of electrical shocks and cumulative energy delivered were analyzed. Additionally, ventricular ectopic activity during the same interval was assessed by tallying the incidence of singlets, bigemini, salvos (three consecutive ventricular complexes), and ventricular tachycardia (VT) (≥4 consecutive ventricular complexes).
For NHE-1 inhibition, cariporide (4-isopropyl-methylsulphonylbenzoyl-guanidine methanesulphonate, Aventis Pharma Deutschland GmbH, Frankfurt, Germany) was prepared in 0.9% NaCl by the investigators on the day of the experiment who were not blinded to the treatment assignment.
Experimental protocol
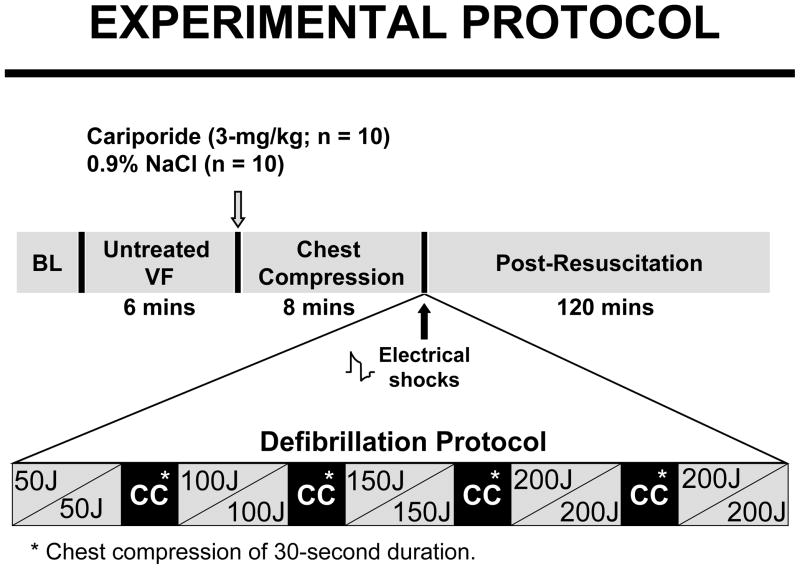
VF was induced by an alternating current (1 to 5 mA) delivered to the right ventricular endocardium and mechanical ventilation discontinued. Ten pigs in each group were randomized to receive a bolus of either 3-mg/kg cariporide or 0.9% NaCl into the right atrium at 5 minutes and 45 seconds of untreated VF. Chest compression and ventilation with 100% oxygen were initiated 15 seconds later using a pneumatically driven chest compressor/ventilator (Thumper® Model 1007, Michigan Instruments) programmed to deliver 80 compressions/min and one breath every six compressions. The force of compression was gradually increased attempting to generate within three minutes a coronary perfusion pressure above the minimal resuscitability threshold of 10 mmHg in pigs. Defibrillation was attempted after 8 minutes of chest compression using a biphasic waveform defibrillator (Smart Biphasic Heartstream XL M4735A, Agilent Technologies) and an escalating energy protocol of 50, 100, 150, and 200 Joules. This escalating energy protocol – starting at levels not expected to successfully terminate VF – was used to determine whether cariporide could lower the threshold for electrical defibrillation given previous studies in rats in which cariporide prompted spontaneous defibrillation during chest compression.13 Up to two shocks were delivered within each energy level. If VF persisted or an organized rhythm with a mean aortic pressure ≤25 mmHg ensued, chest compression was resumed for 30 seconds. The compression-defibrillation sequence was repeated for a maximum of four additional cycles, increasing the energy of individual shocks to the next level in instances of refractory VF (Figure 1). Spontaneous circulation was defined as an organized cardiac activity with a mean aortic pressure ≥ 60 mmHg for ≥5 minutes. Recurrent VF prompted 100-Joule shocks without additional chest compressions. Resuscitated pigs were monitored for 120 minutes and then euthanized by intravenous pentobarbital (150 mg/kg).
Figure 1.
Experimental protocol. Cariporide or 0.9% NaCl was given immediately before the start of chest compression. Resuscitated pigs were monitored for 120 minutes.
Statistical analysis
To examine whether cariporide ameliorated myocardial abnormalities at different intervals during the post-resuscitation period continuous variables between groups were analyzed by one-way ANOVA (SigmaStat 3.0.1, SPSS Inc.). Categorical variables were analyzed using Fisher exact test. Equivalent non-parametric tests were substituted when tests for normality or equal variance failed. The data are presented as mean ± SD unless otherwise stated. A two-tail p value < 0.05 was considered significant.
RESULTS
No differences in hemodynamic variables were observed between groups at baseline. From the 2nd to the 8th minute of closed-chest resuscitation the averaged coronary perfusion pressure was 16 ± 4 mmHg in pigs treated with cariporide and 15 ± 3 mmHg in control pigs. During the same interval the PETCO2 was higher in the cariporide group (30 ± 6 vs 27 ± 6 mmHg) but the difference was not statistically significant.
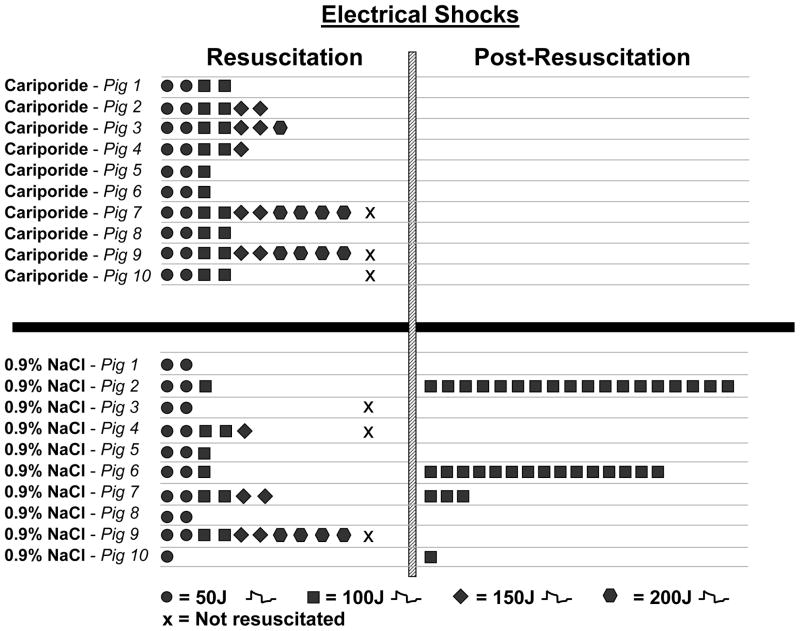
Seven of 10 pigs in each group were successfully resuscitated. Failure to resuscitate was associated in the cariporide group with pulseless electrical activity in two pigs and with refractory VF in one pig, and in the control group with pulseless electrical activity in one pig and with refractory VF in two pigs. A numerically higher number of electrical shocks (4.6 ± 1.5 vs 2.9 ± 1.6 shocks, p = 0.06) and higher cumulative energy (407 ± 224 vs 207 ± 184 Joules, p < 0.05, Figure 2) was required to terminate VF in successfully resuscitated pigs treated with cariporide. The energy level of the shock that terminated VF was numerically higher in pigs that received cariporide (129 ± 39 vs 86 ± 38 Joules, p = 0.06, Figure 2). During the initial 5 minutes after return of spontaneous circulation, intense ventricular ectopic activity was observed in control pigs contrasting with electrical stability in cariporide treated pigs (Table). Post-resuscitation episodes of VF occurred only in control pigs (4/7 vs 0/7 pigs, p = 0.07) which required the delivery of additional electrical shocks (5.3 ± 7.8 vs 0.0 ± 0.0 shocks, p = 0.073) and therefore additional cumulative energy (529 ± 778 vs 0.0 ± 0.0 Joules, p = 0.073) to reestablish spontaneous circulation (Figure 2).
Figure 2.
Shown are the electrical shocks required to initially terminate VF (resuscitation) and to subsequently reverse episodes of refibrillation (post-resuscitation). Pigs marked by “x” during resuscitation failed to regain spontaneous circulation.
Table.
Ventricular Ectopic Activity during the Initial 5 minutes Post-resuscitation.
| Singlets (n) | Bigemini (n) | Salvos (n) | Episodes of VT (n) | Episodes of VF (n) | |
|---|---|---|---|---|---|
| Cariporide (n = 7) | 5 ± 5† | 4 ± 3† | 4 ± 8 | 0 ± 0 | 0 ± 0 |
| 0.9% NaCl (n = 7) | 26 ± 21 | 4 ± 25 | 17 ± 24 | 1 ± 1 | 4 ± 6 |
VT = Ventricular tachycardia. VF = Ventricular fibrillation. Mean ± SD.
p < 0.05 vs 09% NaCl using one-way ANOVA.
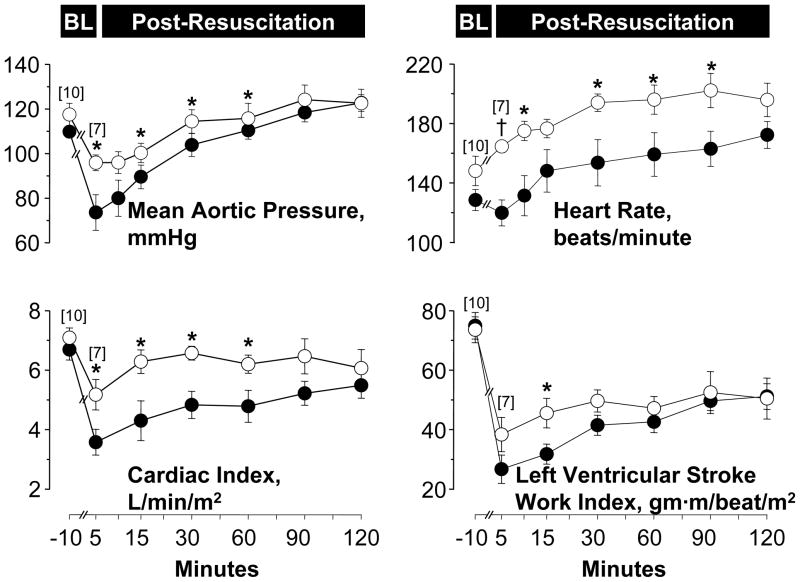
Post-resuscitation, pigs experienced reversible hemodynamic and myocardial dysfunction that lasted approximately 120 minutes but was less prominent in cariporide treated pigs (Figure 3). Averaged over the initial 60 minutes post-resuscitation observation interval cariporide-treated pigs had higher cardiac index (6.1 ± 0.7 vs 4.4 ± 1.1, L/min/m2, p < 0.01), left ventricular stroke work index (45 ± 9 vs 36 ± 10 gm·m/beat/m2, p < 0.05), and numerically higher mean aortic pressure (104 ± 11 vs 91 ± 12 mmHg, p = 0.054). All resuscitated pigs survived 120 minutes.
Figure 3.
Baseline and post-resuscitation left ventricular and hemodynamic function in pigs randomized to receive cariporide (open symbols) or 0.9% NaCl (closed symbols). Numbers in brackets indicate sample size. Values are mean ± SEM. *p < 0.05; †p < 0.001 vs 0.9% NaCl analyzed by one-way ANOVA.
DISCUSSION
Cariporide in this pig model of electrically induced VF and closed-chest resuscitation attenuated post-resuscitation myocardial dysfunction enabling greater post-resuscitation hemodynamic stability. Additionally, there was a trend favoring less post-resuscitation episodes of VF in cariporide-treated pigs.
Post-resuscitation ventricular arrhythmias
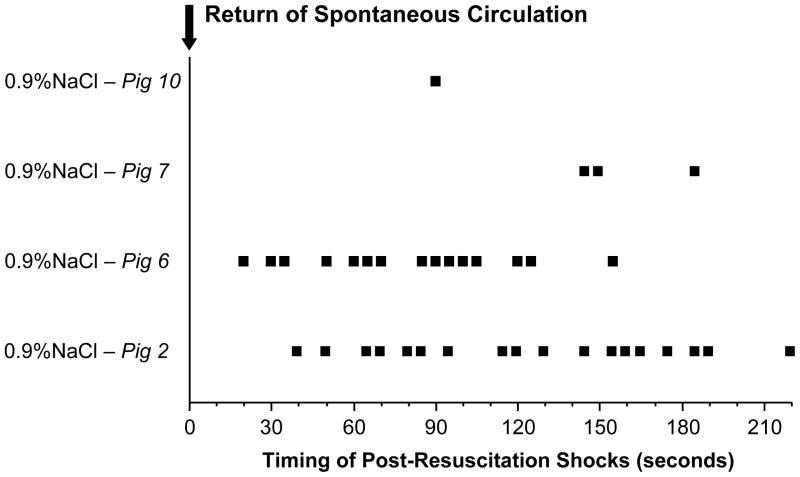
The time course and severity of the ventricular arrhythmias demonstrated in the present study in our pig model was remarkably similar to what has been reported in victims of out-of-hospital cardiac arrest. In our study, VF spontaneously occurred after return of spontaneous circulation in 4 of 7 control pigs (57%) within a time window of 20 to 220 seconds (median, 103 seconds) and with an average of ≈ 5 episodes per pig (Figure 4). As mentioned earlier, VF in victims of out-of-hospital cardiac arrest has been reported to occur within a time window of 23 to 115 seconds (median, 45 seconds) in up to 79% of resuscitated patients and with an average of ≈ 2 episodes per patient.1
Figure 4.
Timing of each electrical shock delivered after the return of spontaneous circulation to 0.9% NaCl-treated pigs during an episode of refibrillation.
Mechanistically, they reflect abnormalities linked to reperfusion of ischemic myocardium in which Na+-induced cytosolic Ca2+ overload plays a prominent role. Electrophysiological, there are repolarization abnormalities, including shortening of the action potential duration.14 In a previous study,15 shortening of the action potential duration was evident immediately upon return of spontaneous circulation with gradual resolution within approximately 15 minutes. This time window coincides with the restoration of electrical stability. In the present study, cariporide suppressed episodes of VF during the post-resuscitation intervention and attenuated ventricular ectopic activity (Table). We16 and others17 have reported previously that cariporide – for reasons not well understood – attenuates shortening of the action potential duration upon reperfusion. Thus the beneficial effects of cariporide on ventricular ectopic activity coincide in time with its effect on action potential duration.
Post-resuscitation myocardial dysfunction
Resuscitation from cardiac arrest is commonly associated with varying severity of reversible left ventricular systolic and diastolic dysfunction.11,18 Preventing or reversing post-resuscitation myocardial abnormalities is challenging because the underlying pathogenic mechanisms are poorly understood. Our group has reported that Na+-induced Ca2+ overload is an important mechanisms of injury that contributes to post-resuscitation myocardial dysfunction.15 In the present study, pigs that had received cariporide during chest compression had less myocardial dysfunction during the early post-resuscitation interval. This finding is consistent with previous report by our group in a rat model of VF and chest compression,13,19 in a pig model of VF and chest compression in which epinephrine was used during chest compression,20 and in an open-chest pig model of VF and resuscitation by use of extracorporeal circulation.21 Accordingly, limiting Na+-induced Ca2+ overload by use of cariporide could provide more competent myocardial function during the early post-resuscitation interval.
Limitations of the study
We acknowledge that results pertaining to post-resuscitation ventricular arrhythmias attained borderline statistical significance (i.e., p values between 0.060 and 0.073) which we attribute to the small sample size (i.e., n=7) rather than the lack of drug effectiveness. In previous studies by our group we reported significant attenuation of post-resuscitation ventricular arrhythmias including prevention of recurrent episodes of VF with cariporide and other NHE-1 inhibitors in both pig and rat models of cardiac arrest and resuscitation.13,15,19 Caution should be exercised when extrapolating these findings to clinical settings. The current experiments were performed in anesthetized pigs using agents that can exert independent myocardial protective effects.22 Moreover, the model differed from the typical adult victim in that we used immature healthy pigs without underlying coronary artery disease. The extent to which underlying coronary artery disease and acute coronary occlusion may moderate the effects of the experimental interventions investigated is currently unknown. We have also observed differences between the beneficial effects associated with cariporide and newer highly selective NHE-1 inhibitors in our animal models of cardiac arrest and resuscitation. These differences may be attributed to non-selective effects of cariporide that may offer additional cardioprotection when used in animal models of electrically induced VF and closed-chest resuscitation.
An issue of potential clinical relevance is the effects of cariporide on defibrillation threshold. Because previous studies in rats suggested that cariporide could lower the defibrillation threshold,13 we applied an escalating energy protocol starting at levels not expected to terminate VF. We found that the energy level of the shock that terminated VF was numerically higher in pigs treated with cariporide (129 ± 39 vs 86 ± 38 Joules, p = 0.06) promoting numerically more electrical shocks to be delivered (4.6 ± 1.5 vs 2.9 ± 1.6 shocks, p = 0.06). The additional electrical shocks delivered to cariporide treated pigs had no measurable electrical or mechanical consequences and replicated similar observations reported in another series of experiments using the combination of cariporide and epinephrine.20 In contrast, no such effect was observed in another series using an open-chest pig model in which electrical shocks were delivered through paddles directly applied to the heart.21 We have previously discussed the relationship between cariporide and defibrillation energy23 proposing that the effect may be the result of cariporide maintaining a larger left ventricular cavity size during closed-chest resuscitation.15,23 The larger left ventricular cavity size would “shunt” current away from the myocardium mass requiring increases in such current to terminate VF.24 However, the magnitude of the effect appears not large enough to be clinically relevant for resuscitation.
Clinical implications
Promoting return of cardiac activity with electrical and mechanical stability early after resuscitation from out-of hospital cardiac arrest could potentially impact survival by reducing the nearly 40% early deaths reported to occur between return of spontaneous circulation and hospital admission. Thus, cariporide or equivalent pharmacological interventions could promote hemodynamic stability for safe transport of these victims to the hospital.
Conclusions
Cariporide in this study promoted early post-resuscitation electrical and mechanical cardiac stability. These favorable effects may allow safer transfer of initially resuscitated victims of cardiac arrest to a receiving hospital.
Acknowledgments
Work supported by National Institutes of Health grant R01 HL71728-01 entitled “Myocardial Protection by NHE-1 Inhibition,” VA Merit Review Grant entitled “Myocardial Protection during VF. The authors wish to acknowledge the logistic support of Biomedical Engineering; Pharmacy Services, and the Supply, Processing, and Distribution Center at the North Chicago VA Medical Center.
Footnotes
CONFLICT OF INTEREST STATEMENT
Authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation. 2003;59:181–8. doi: 10.1016/s0300-9572(03)00208-9. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Clusin WT. Cellular mechanism for ischemic ventricular arrhythmias. Annu Rev Med. 1990;41:231–8. doi: 10.1146/annurev.me.41.020190.001311. [DOI] [PubMed] [Google Scholar]
- 3.Shivkumar K, Deutsch NA, Lamp ST, Khuu K, Goldhaber JI, Weiss JN. Mechanism of hypoxic K loss in rabbit ventricle. J Clin Invest. 1997;100:1782–8. doi: 10.1172/JCI119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, Stowe DF. Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- 5.Kusuoka H, Chacko VP, Marban E. Myocardial energetics during ventricular fibrillation investigated by magnetization transfer nuclear magnetic resonance spectroscopy. Circ Res. 1992;71:1111–22. doi: 10.1161/01.res.71.5.1111. [DOI] [PubMed] [Google Scholar]
- 6.Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res. 1999;84:1401–6. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 7.Gazmuri RJ, Ayoub IM, Kolarova JD, Karmazyn M. Myocardial protection during ventricular fibrillation by inhibition of the sodium-hydrogen exchanger isoform-1. Crit Care Med. 2002;30:S166–S171. doi: 10.1097/00003246-200204001-00010. [DOI] [PubMed] [Google Scholar]
- 8.Karmazyn M, Sawyer M, Fliegel L. The na(+)/h(+) exchanger: a target for cardiac therapeutic intervention. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:323–35. doi: 10.2174/1568006054553417. [DOI] [PubMed] [Google Scholar]
- 9.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med. 1996;24:992–1000. doi: 10.1097/00003246-199606000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J Am Coll Cardiol. 1996;28:232–40. doi: 10.1016/0735-1097(96)00130-1. [DOI] [PubMed] [Google Scholar]
- 11.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40:2110–6. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 12.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22:656–65. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 13.Gazmuri RJ, Ayoub IM, Hoffner E, Kolarova JD. Successful ventricular defibrillation by the selective sodium-hydrogen exchanger isoform-1 inhibitor cariporide. Circulation. 2001;104:234–9. doi: 10.1161/01.cir.104.2.234. [DOI] [PubMed] [Google Scholar]
- 14.Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- 15.Ayoub IM, Kolarova JD, Yi Z, Trevedi A, Deshmukh H, Lubell DL, Franz MR, Maldonado FA, Gazmuri RJ. Sodium-hydrogen exchange inhibition during ventricular fibrillation: Beneficial effects on ischemic contracture, action potential duration, reperfusion arrhythmias, myocardial function, and resuscitability. Circulation. 2003;107:1804–9. doi: 10.1161/01.CIR.0000058704.45646.0D. [DOI] [PubMed] [Google Scholar]
- 16.Ayoub IM, Kolarova JD, Sehgal MA, Sanders R, Sheth O, Gazmuri RJ. Sodium-hydrogen exchange inhibition minimizes adverse effects of epinephrine during cardiac resuscitation. Circulation. 2003;108:IV–420. [Google Scholar]
- 17.Wirth KJ, Maier T, Busch AE. NHE1-inhibitor cariporide prevents the transient reperfusion-induced shortening of the monophasic action potential after coronary ischemia in pigs. Basic Res Cardiol. 2001;96:192–7. doi: 10.1007/s003950170070. [DOI] [PubMed] [Google Scholar]
- 18.Xu T, Tang W, Ristagno G, Wang H, Sun S, Weil MH. Postresuscitation myocardial diastolic dysfunction following prolonged ventricular fibrillation and cardiopulmonary resuscitation. Crit Care Med. 2008;36:188–92. doi: 10.1097/01.CCM.0000295595.72955.7C. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Radhakrishnan J, Ayoub IM, Kolarova JD, Taglieri DM, Gazmuri RJ. Limiting sarcolemmal Na+ entry during resuscitation from VF prevents excess mitochondrial Ca2+ accumulation and attenuates myocardial injury. J Appl Physiol. 2007;103:55–65. doi: 10.1152/japplphysiol.01167.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ayoub IM, Kolarova J, Kantola RL, Sanders R, Gazmuri RJ. Cariporide minimizes adverse myocardial effects of epinephrine during resuscitation from ventricular fibrillation. Crit Care Med. 2005;33:2599–605. doi: 10.1097/01.ccm.0000186773.88576.83. [DOI] [PubMed] [Google Scholar]
- 21.Ayoub IM, Kolarova J, Kantola R, Radhakrishnan J, Gazmuri RJ. Zoniporide preserves left ventricular compliance during ventricular fibrillation and minimizes post-resuscitation myocardial dysfunction through benefits on energy metabolism. Crit Care Med. 2007;35:2329–36. doi: 10.1097/01.ccm.0000280569.87413.74. [DOI] [PubMed] [Google Scholar]
- 22.Kato R, Foex P. Myocardial protection by anesthetic agents against ischemia-reperfusion injury: an update for anesthesiologists. Can J Anaesth. 2002;49:777–91. doi: 10.1007/BF03017409. [DOI] [PubMed] [Google Scholar]
- 23.Gazmuri RJ, Ayoub IM. The case for sodium-hydrogen exchanger isoform-1 inhibition during cardiac resuscitation remains strong. Crit Care Med. 2006;34:1580–2. doi: 10.1097/01.CCM.0000216687.86553.EC. [DOI] [PubMed] [Google Scholar]
- 24.Idriss SF, Anstadt MP, Anstadt GL, Ideker RE. The effect of cardiac compression on defibrillation efficacy and the upper limit of vulnerability. J Cardiovasc Electrophysiol. 1995;6:368–78. doi: 10.1111/j.1540-8167.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]