Abstract
B cell responses after immunization with a drifted H5 influenza/A/Vietnam/1203/04 vaccine were characterized in the peripheral blood of human subjects primed with experimental recombinant H5 influenza A/Hong Kong/156/97 vaccine. Antibody secreting cells were assayed by ELISPOT against a panel of recombinant hemagglutinin and control proteins. Increased frequencies of H5 HA specific antibody secreting and memory B cells could be observed within 7 days of re-vaccination. Furthermore, these responses were cross-reactive to both H5 HA variants, but not H3 or avian H6 HA strains. These observations suggest prior vaccination against H5 influenza HA induces cellular immune responses that cross-react among drifted variants, without precluding a response to new, or existing HA strains.
Keywords: Influenza, B cell, hemagglutinin
Introduction
Pandemics occur when an influenza virus emerges to which humans are immunologically naïve, and has the properties needed to easily transmit from person to person [1]. In spite of the emergence of the 2009 H1N1 influenza, the threat of a pandemic caused by a well-adapted virus related to the H5 avian influenza remains a significant threat to the world’s population. H5 influenza viruses continue to expand in circulation within the wild bird populations, and cases of bird-to-human transmission periodically occur, though a virus that efficiently transmits among people has not yet appeared. These viruses continue to evolve [2], undergoing antigenic drift, and several major surveillance efforts are underway to monitor the changes that occur in the viruses. The 2009 H1N1 virus shares several characteristics with avian influenzas [3], and has been anecdotally detected in birds [4], raising the possibility that it will re-assort with H5N1 influenza viruses, though the outcome of such an event is unpredictable.
One of the challenges of developing vaccines against influenza is the need to produce a vaccine that is antigenically well matched to the strain that emerges as a pandemic. At this time, the H5 influenza viruses that have infected humans fall into several distinct clades and subclades. [5]. The first highly-pathogenic H5 influenza A to cross into humans, influenza A/Hong Kong/156/97, occurred in 1997 in Hong Kong, and falls into clade 0 [5]. Since that time, two other clades have developed characterized by the H5 influenza A/Vietnam/1203/04 clade 1 virus, and the influenza A/Indonesia/05/2005-like clade 2.1 strains [5, 6]. Experimental vaccines against H5 influenza have been developed and tested for at least three clades of the virus. As for seasonal influenza, it is difficult to predict which clade of H5 is the most likely one to emerge, making it difficult to choose the formulation of an H5 vaccine. This is obviously not an ideal pre-pandemic strategy. Although there are substantial efforts under way to improve vaccine manufacturing capacity, and develop technology to shorten the time it takes to choose and develop a vaccine candidate, there nevertheless will be a significant delay in being able to produce enough well-matched vaccine to immunize the US, European, Asian and developing nations.
One potential strategy to improve H5 influenza vaccine efficacy would be to begin pre-immunizing the population with the current H5 vaccines to produce partial immunity against the virus. Few studies have been conducted to determine whether immunization with a vaccine based on one clade of H5 influenza can affect subsequent responses to vaccination with different clades or subclades. One concern is that immunization with the “wrong” variant of influenza will result in a misdirected immune response when exposed to the emergent virus, such that antibodies specific to the vaccine will be produced at the expense of those against the antigenically distant virus. This perception has arisen from classic experiments that showed priming by infection with one type of influenza skewed a subsequent response to a second infection, favoring the production of antibodies to the first virus, a phenomenon termed “Original Antigenic Sin” (OAS) [7, 8]. More recent experiments suggest this does not apply to human subjects after seasonal influenza subunit vaccination [9], and immunization with experimental H5 vaccines in animal models show broad cross protection to multiple influenza subtypes [10–13].
The cellular contribution of previous immunity to the success of vaccination in humans is not well understood. Especially in the case of influenza virus, although cross-reactive T cells that respond to conserved regions of the viral proteins are known to exist in humans [14], their role in vaccine-induced immune responses is only beginning to be characterized [15–21]. The presence of B cell mediated immunity to influenza is generally measured indirectly via serum antibody titers determined using hemagglutination inhibition (HAI) and microneutralization (MN) assays. These assays measure the presence of antibodies that function to inhibit attachment of the virus to cells, or the ability to infect cells, respectively, and are well correlated with clinical protection from influenza illness [22, 23]. ELISA based assays, on the other hand, are not well correlated with protection, possibly confounded by their ability to measure antibodies that recognize non-neutralizing epitopes. Cellular assays of B cell responses to influenza are possible, and like the ELISA, are likely to detect both neutralizing and non-neutralizing, virus-specific B cells. Nevertheless, determining the cellular characteristics of the immune responses to influenza vaccines could shed light on the mechanisms that result in long-lived protective immunity and durable memory.
In the present study, we analyzed the peripheral blood of the subjects receiving re-vaccination with a second strain (and clade) of experimental H5 influenza A vaccine for the presence of memory B cells (MBC) specific for hemagglutinin proteins. Assays were performed on cryopreserved PBMC using a method designed to stimulate MBC [24]. B cell Enzyme Linked ImmunoSpot (ELISPOT) assays were used to detect HA specific and total IgG-secreting B cells in the cultures. The data show that a substantial and rapidly responding population of B cells specific and cross-reactive to H5 antigens could be detected in a majority of the subjects after vaccination. This suggests that primary vaccination with non-replicating influenza subunit vaccines against avian influenza results in the establishment of a long-lived population of MBC, even when assays for functional antibody fail to demonstrate a response in some subjects [25]. These observations raise questions as to whether pre-pandemic vaccination could enhance subsequent immune responses to reformulated vaccines or exposure to the related viruses.
Methods
Study Design
The study was conducted as an open label, phase I evaluation of a single dose of an investigational, inactivated, subunit influenza A /Vientam/1203/2004 H5 (Clade 1) vaccine. The subjects for the study were individuals who had participated in a previous study evaluating baculovirus-expressed recombinant H5 hemagglutinin of the A/Hong Kong/156/97 (Clade 0) vaccine [25]. In that study, conducted in late 1997 and early 1998, subjects were exposed to two doses of recombinant H5 vaccine at 25 µg, 45 µg, or 90 µg, or 90 µg followed by 10 µg, at intervals of 21 days, 28 days, or 42 days. There were a total of 117 recipients of H5 vaccine in 12 groups in this study who were eligible for the current study. Subjects who had received the H5 vaccine at any dose or schedule were recruited using an existing database of previous subjects. To participate, subjects had to be in good health, not pregnant, and not receiving immunosuppressive medications.
All subjects received a single 90µg dose of egg-derived, inactivated subvirion H5N1 vaccine, as previously described [26]. Sera for assessment of neutralizing and hemagglutination-inhibition (HAI) responses were obtained prior to vaccination and on days 28 and 56 following vaccination. Peripheral blood was collected prior to vaccination, and at 7, 28, and 56 days after. Subjects were followed for local and systemic reactions to the vaccine for 7 days and adverse events for 56 days after vaccination using the same assessment tools used in our previous evaluation of the subunit A/Vietnam/1203/04 vaccine [26].
Vaccine
The seed virus for the production of the H5N1 vaccine was generated from the human isolate influenza A/Vietnam/1203/2004 (H5N1) virus using a plasmid rescue system and produced in eggs as previously described [27]. The vaccine was produced as a partially purified HA and NA preparation and formulated at a concentration of 90 µg/mL as determined by single radial immunodiffusion (SRID) using sheep antisera to bromelain-cleaved native H5 HA in the agar. Vaccine was administered as a single dose of 1 mL by deep intramuscular injection into the deltoid of the non-dominant arm.
Sample collection and processing
Blood was collected at days 0, 7, 28, and 56 into CPT tubes (Becton-Dickinson, Franklin Lakes, NJ) and the lymphocytes separated per the manufacturer’s protocol. PBMC were cryopreserved using a modified Immune Transplantation Network protocol. Briefly, isolated PBMC were resuspended in 90% human AB serum (Gemini Bio-Products, Woodland, Ca) 10% high-grade DMSO (Sigma, St. Louis, MO) and frozen to −80° C at a density of 107 per ml using an isopropanol-filled, controlled-rate freezing device. After 24–48h at −80°C, vials were transferred into liquid nitrogen for long-term storage. Prior to immune assay, cells were rapidly thawed, then slowly diluted by adding cold RPMI + 10% human AB serum drop wise. Viability and recovery (>80%) were assessed by counting cells in a hemacytometer in the presence of trypan blue dye.
Memory B cell restimulation
MBC among the PBMC were restimulated following an established protocol [24]. Briefly, PBMC were suspended in RPMI 1640 medium supplemented with 10% human AB serum. Cells were plated at 5 × 105 per well in 24 well plates. A polyclonal mitogens cocktail was added to final concentrations of pokeweed mitogen (PWM) 1/100,000 (Kind gift of Dr. S Crotty, La Jolla, CA), pansorbin (Staphylococcus aureus Cowen strain I) 1/10,000 (Calbiochem, San Diego, CA), phospothioated CpG 6µg/ml (Integrated DNA Technologies, Coralville, IA). Cells were cultured for 6 days in 5% CO2 at 37°C, 95% humidity, then collected and washed in medium before assay.
Antigens
Baculovirus-derived, purified recombinant influenza hemagglutinins (HA) corresponding to H1 influenza A/New Caledonia/20/99 and H3/Wyoming/3/03 were purchased from Protein Sciences Corp. (Meriden, CT). Recombinant baculovirus HA for H5/HK/156/97, H5/VN/1203/04, H3/Wisconsin/67/05, and control H6/Teal/HK/W312/97 were provided by the NIH Biodefense and Emerging Infections Resource (BEIR) repository. Keyhole limpet hemocyanin (KLH) was purchased from Calbiochem (San Diego, CA).
B cell IgG ELISPOT
Anti human immunoglobulin G (Biosource/Invitrogen AHI0301) or HA antigen (0.04µg/well) diluted in PBS were used as capture reagents in 96-well polyvinylidene difluoride (Millipore, MSIPN4W50) microtiter plates. The plates were washed with RPMI medium containing 8% fetal bovine serum before adding PBMC and serial diluting (1:2) down the plate. The cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 2 hours before aspirating the cells and washing the plates with PBS-Tween. Alkaline phosphatase labeled anti-human IgG antibody (Jackson Fc Fragment Specific #109-055-008, 0.1µg/well) was added and incubated for 2 hours at room temperature. The plates were washed (PBS-Tween) before substrate (Vector AP substrate kit, Cat” SK-5300) is added to develop spots per manufacturer’s recommendations. ELISpot plates were analyzed using the CTL ImmunoSpot plate reader and counting software (Cellular Technology Limited, Cleveland, OH).
Tissue collection
Blood was drawn 21 days following primary and secondary immunizations from the submandibular vein. Blood was collected in 1.5ml conical tubes (Costar 3620) and allowed to coagulate. Samples were centrifuged and serum was collected and stored at −80°C.
Statistical Methods
For each condition, using the full responder cell dilution curve, the number of spots in the linear part of the curve was determined using Tukey’s median to control for outliers. This method is robust [28] and coincides with the median of a one-dimensional sample. Comparisons between groups and within individuals were assessed, respectively, using Wilcoxon Rank Sum and Signed Rank tests. Reported p values were two-sided at a minimum significance level of 0.05, though p ≤ 0.01 was routinely used as a cutoff.
Results
Patient populations
The University of Rochester Institutional Research Board approved all human subject protocols for this study. The subjects were individuals who had previously been vaccinated with two doses of baculovirus-expressed recombinant H5 hemagglutinin of the A/Hong Kong/156/97 (H5, Clade 0) vaccine in 1997 and 1998 [25]. Out of the 127 eligible subjects from the previous study, 37 were enrolled to receive a single 90 µg dose of inactivated subunit H5/VN/1203/04 vaccines, and of these, a total of 28 adults consented to donate peripheral blood samples for the present study. There were 18 females and 10 males, with an average age of 42.3. (range = 33.1–50.8) (Table 1).
Table 1.
Characteristics and prior H5 immunization history of the study population1
| Gender | No. | Average Age (Range) |
Previous recombinant H5/HK/156/97 vaccination and doses | |||
|---|---|---|---|---|---|---|
| Years | 25µg + 25µg | 45µg + 45µg | 90µg + 90µg | 90µg + 10µg | ||
| Male | 10 | 41.7 (34–50) |
4 | 2 | 3 | 1 |
| Female | 18 | 42.3 (33–51) |
4 | 4 | 5 | 5 |
Subjects who had participated in an earlier clinical study of experimental vaccination with recombinant HA H5/HK/156/97 and conducted in 1998 [12], were re-enrolled for the present study. In the earlier study, subjects received two doses of rH5 HA in the amounts indicated. The numbers of subjects in each dose category, age, and gender distributions are indicated.
Detection of HA specific MBC by IgG ELISPOT
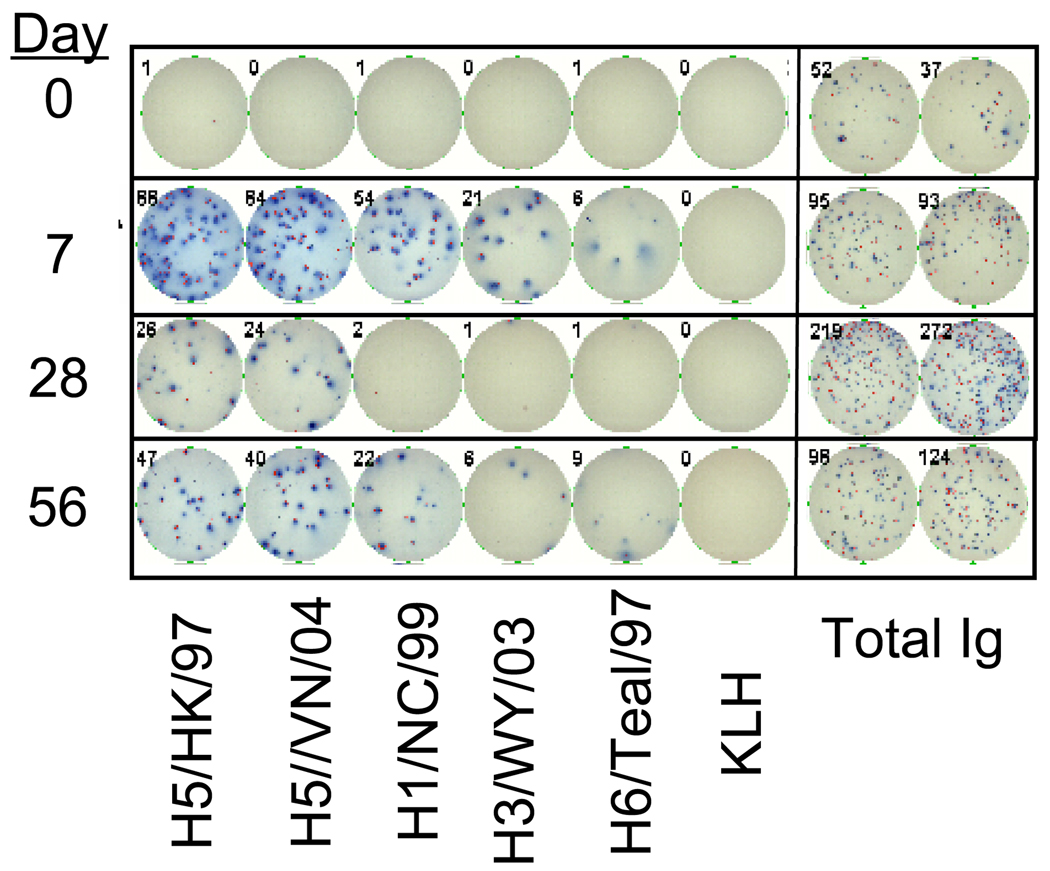
For the purposes of this study, MBC are defined as those cells that become antibody-secreting cells after in vitro stimulation using the method developed by Crotty et al. [24]. After a 6 day period of stimulation, the cells are collected and incubated on 96-well plates coated with a panel of recombinant HA proteins corresponding to H5/HK/156/97, H5/VN/1203/04, H1/New Caledonia/99, H3/Wyoming/03, and avian H6/Teal, or negative controls- keyhole limpet hemocyanin (KLH) or no antigen. Duplicate wells were coated with a human IgG capture antibody. All HA proteins were full-length, un-cleaved (HA0) products produced in insect cells and purified [25]. The HA specific responses are then reported as a frequency of the total IgG secreting population. A representative set of ELISPOT wells are depicted in Figure 1.
Figure 1. Memory B cell antibody-secreting assay against influenza hemagglutinin antigens.
PBMC were cultured as described in Material and Methods to reactivate MBC. The cells were then plated, at several dilutions, onto 96-well nitrocellulose dishes coated with 1µg/well of recombinant influenza HA antigens, KLH (negative control), or antibody against human IgG. After a 4-hour culture, the cells were washed off and the plates developed with a second anti-human IgG antibody conjugated to alkaline phosphatase, and TCIP/BCP substrate. Spots were counted on an ImmunoSpot automated plate reader. Plates were set up such that PBMC from all the time points from a single subject were on the same plate. The wells shown are from a single subject, though representative of all subjects responding in the assay.
The specificity of the HA B cell ELISPOT is demonstrated by considering the responses to the HA antigens compared to the controls. Two types of negative controls were included in all the plates. An avian rH6/Teal HA was used as a non-human influenza HA with the potential to reveal B cells cross-reacting to determinants shared by all influenza HAs. KLH was used as a non-HA protein control, and gave results similar to wells that were left uncoated (not shown). At day 0, considering the subject population as a whole, there was no difference in the MBC frequencies against either H5 HA, and the H1 and H3 HAs (Table 2 and Figure 2). In all subjects, and at all time points, the IgG spot forming responses to either H5 HA, or the human H1 and H3 HAs were greater than that to the unrelated H6 HA (p ≤ 0.1). In turn, MBC frequencies to the KLH or no-antigen controls were always lower (0–0.1%, p ≤ 0.01) than that to the H6 HA. MBC responses to another avian HA (H7) (not shown) were similar to H6 HA. Although we can not rule out that there is some unique (though low) reactivity to insect cell-produced, recombinant proteins used for the assay, these observations suggest that a low frequency (0.2–0.5%) of MBC cross-react with conserved features in non-human, avian HA proteins.
Table 2.
Frequencies1 of HA specific MBC before and after vaccination with HA/A/VN/1203/04
| Day | H5/HK/156/97 | H5/VN/1203/04 | H1/NC/99 | H3/WY/99 | H6/Teal/97 | control |
|---|---|---|---|---|---|---|
| 0 | 0.8 ± 1.5 | 0.8 ± 1.3 | 0.6 ± 1.1 | 1.4 ± 1.9 | 0.5 ± 1.1 | 0.1 ± 0.1 |
| 7 | 2.5 ± 3.42 | 2.3 ± 2.82 | 1.3 ± 1.8 | 1.3 ± 1.8 | 0.4 ± 0.5 | 0.0 ± 0.1 |
| 28 | 1.0 ± 1.5 | 1.3 ± 2.3 | 0.5 ± 0.6 | 0.7 ± 0.8 | 0.2 ± 0.4 | 0.0 ± 0.0 |
| 56 | 1.9 ± 2.2 | 1.7 ± 2.1 | 2.2 ± 3.6 | 1.7 ± 2.7 | 0.6 ± 1.3 | 0.1 ± 0.2 |
MBC IgG ELISPOT assays were conducted as described in Materials and Methods in plates coated with the indicated rHA proteins or a capture antibody for human IgG. The frequency of HA specific B cells is calculated by dividing the number of spots against each HA, by the number of total IgG spots at the same cell concentration. The resulting values were multiplied by 100 to give a percent HA/total IgG (100 × HA/total IgG = % HA specific). Averages and standard deviations were determined for the entire cohort of 28 subjects.
p < 0.01
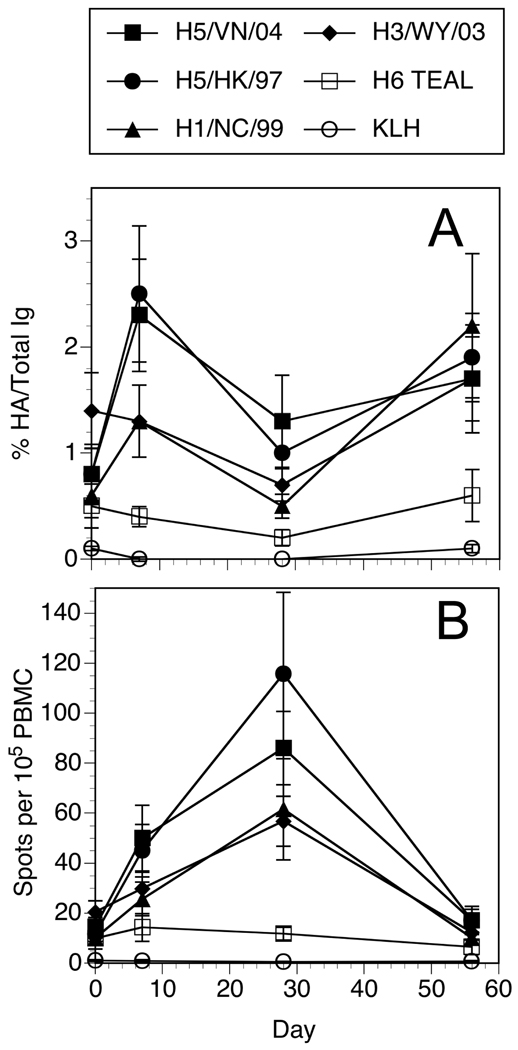
Figure 2. Average frequencies of influenza HA specific B cells from H5 re-vaccinated subjects.
Data from the B cell ELISPOT results of all the subjects for each HA or control antigen were averaged for each time point. Error bars indicate standard error of the mean. A. The data was normalized by taking the number of HA spots and dividing by the number of total IgG spots at a given dilution, then multiplied by 100 to give a value percent HA per Total IgG. B. The total number of HA spot forming cells collected from the cultures were calculated and divided by the number of PBMC originally placed into the mitogen cocktail at the start of the culture to give a frequency of specific cells per 105 PBMC.
Increase in H5 and H1 specific MBC after vaccination
One question was whether the frequencies of HA specific MBC increases after vaccination, and how specific these MBC are to the immunogen. The results are expressed as the frequency of antigen-specific per total IgG secreting cells to normalize the data for differences in cell recovery at the end of the culture period, and for changes in the overall MBC population [24]. At day 0, the frequency of circulating HA specific MBC is not significantly different (0.5 – 1.4%) from the background controls (KLH or no antigen) (0.1%). Analysis of the HA and IgG spot producing cells at 7, 28, and 56 days after vaccination indicated a significant (p ≤ 0.01) increase (from day 0) in the frequencies of H5/HK/156/97 and H5/VN/1203/04 HA-specific ASC at 7 days, but not at the other time points examined (Table 2). MBC frequencies to both H5 HAs were greater (p ≤ 0.1) than to H1 or H3 HA at day 7. In some subjects, there appeared to be a modest increase from day 0 to 7 in the H1 specific ASC frequencies, but this did not reach the level of significance (p = 0.17) when considering the cohort of subjects as a whole.
All of the cultures were started with a similar number of PBMC (~ 7 × 106). If one considers the number of spot forming cells detectable in the wells at the end of the culture period per 105 starting PBMC, there was a clear tendency for these values to peak at 28 days (not 7 days) after vaccination for both total IgG (not shown) and HA (Figure 2B). As a function of total IgG producing MBC, the frequencies of HA specific spots per total IgG were not highest at this same time point. This can be explained if there is an expansion or mobilization of MBC specific for antigens other than HA in the peripheral blood as a result of the immunization. These observations suggest that vaccination of H5 immune subjects results in significant, though transient changes in the frequencies of circulating MBC against the specific immunogen, as well as the drifted variant that preceded it but not to unrelated, contemporary human or distal avian HA.
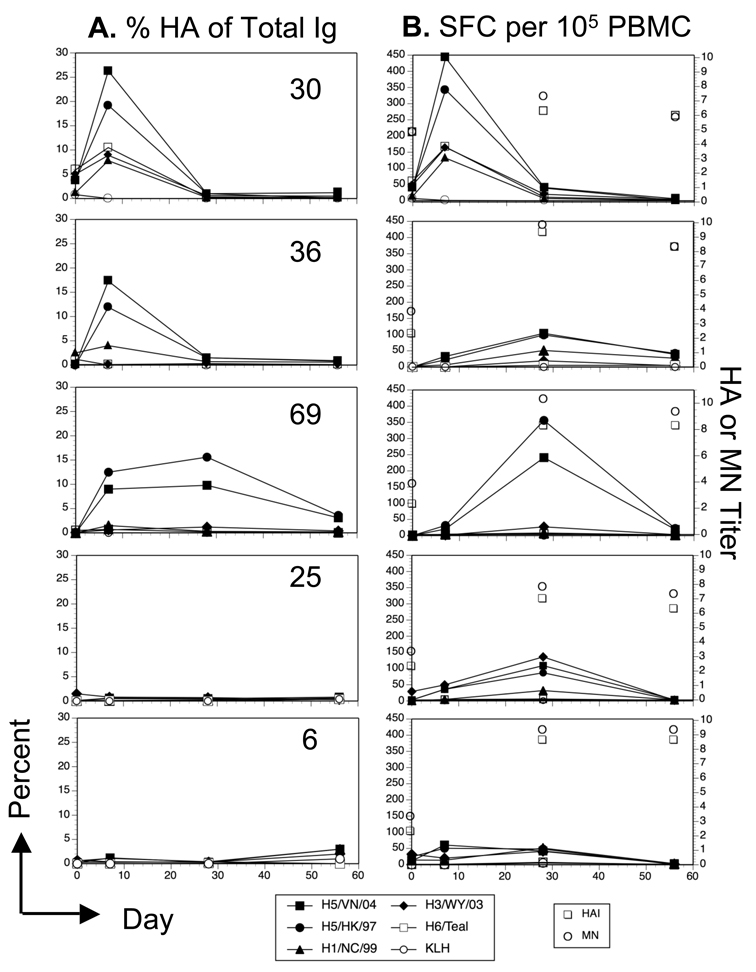
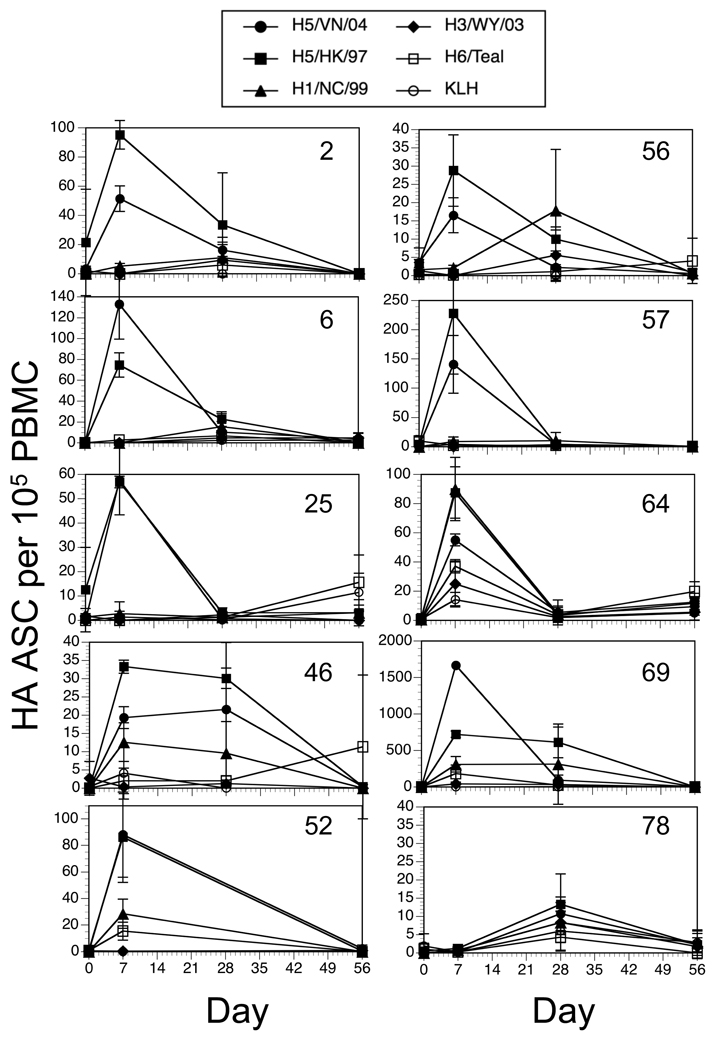
The re-vaccinated subjects did not exhibit a uniform pattern of responses over the course of the study. Figure 3 shows results for % HA/total IgG (Figure 3A) and HA specific per 105 PBMC (Figure 3B) for 5 individuals. While more subjects (12/28) gave responses similar to subjects 30 and 36 (Figure 3A), a small number (5/28) showed a prolonged response (subject 69), little response to vaccination (subject 25) (5/28), or a late rise in frequencies (subject 6) (6/28). The total HA MBC per 105 PBMC also exhibited some variation, though the majority of subjects (21/28) exhibited a peak at day 28 or day 7 (7/28) (Figure 3B).
Figure 3. Kinetics of the HA-specific MBC response versus the HAI and MN titers in 5 subjects.
The percent HA specific of the total IgG secreting B cells (A) and the frequency of HA specific B cells per 105 PBMC (B) from 5 individual subjects are shown to illustrate the variability in the pattern of responses. HAI and MN geometric mean titers (Log2) from days 0, 28, and 56 are indicated by the open symbols not connected by lines.
In spite of the detectable increases in H5 reactive MBC in a high proportion of the subjects, these responses did not correlate with serum neutralizing antibody. A high MBC frequency at 7 or 28 days did not predict a titer of 1:40 or greater (28 days after vaccination) (Figure 3B). Nor did a greater than 2-fold increase in H5 reactive MBC correlate with a four-fold increase in serum titers. In some subjects, a significant change in H5 specific MN or HAI activity in the serum occurred without obvious changes in the frequency of H5 reactive MBC. The most likely explanation is that in these subjects, the kinetics of the MBC response was different than the time points that were sampled, and it is possible that the actual peak of the response occurred at a time point not sampled. Alternatively, it suggests that serum antibody titer is not determined simply by the frequency of circulating MBC.
Drift variant vaccination increases the proportion of subjects with detectable circulating H5 HA specific MBC
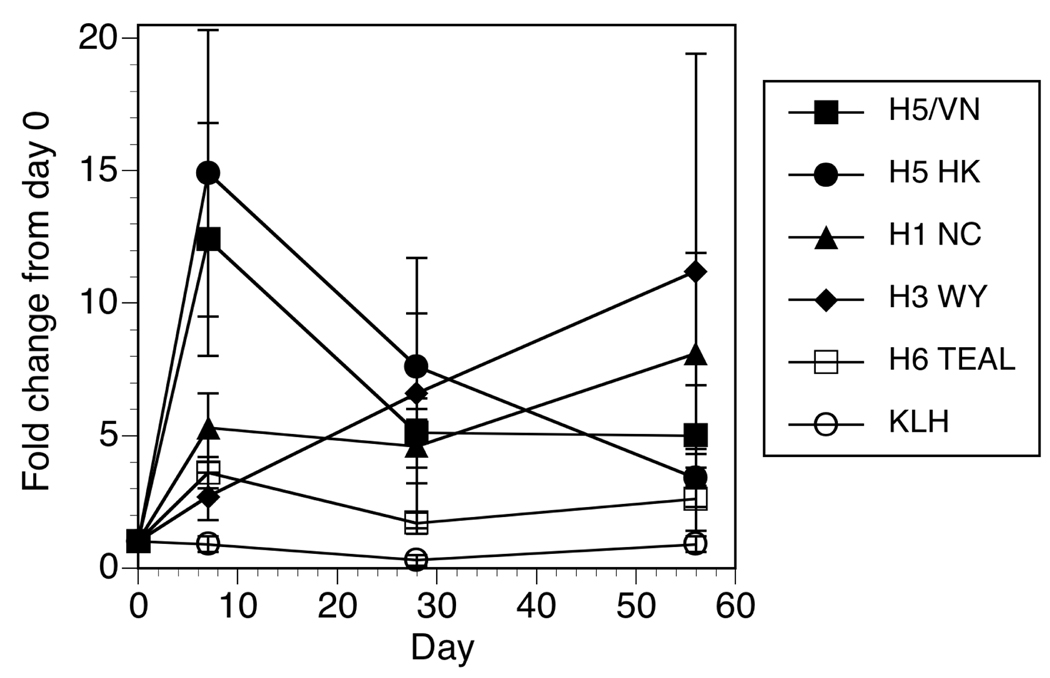
It is not known how MBC frequencies correlate with protection, or what magnitude of change would represent a “successful” boost in the response. In considering serum antibody titers against flu, the FDA considers a titer of greater than 1:40 as protective, with a 4-fold increase in titers from day 0 a successful vaccination [29]. In this same cohort, on day 28 after a single dose of H5/VN/1203/04 inactivated subunit vaccine, the geometric mean titer (GMT) of hemagglutination-inhibition antibody in primed subjects was 64.0 (95% confidence interval [CI], 37.8–108.5), with 68% responding (4-fold increase in antibody level to a titer of 1:40) [26]. In contrast, H5-naive subjects who received two 90-µg doses of H5/VN/1203/04 vaccine had a day 56 (28 days after the second dose) GMT of 27.7 (95% CI, 20.3–38.0), with only 43% responding [27]. Looking at the MBC responses in a similar way, we assessed the fold change in HA specific MBC frequencies from the start of the study. A significant (p ≤ 0.01) 12–14 fold mean increase over baseline was observed for both H5 HA proteins at 7 days after vaccination (Figure 4), but not at other time points. In proportion, 69% and 63% of the subjects had an at least 2-fold increase in MBC frequencies to H5/VN/1203/04 and H5/HK/156/97, respectively, at 7 days after vaccination. This compares to only 38% showing 2-fold or greater increases against H1, and 27% against H3 HAs. Note that we did observe a (not significant) trend for increased responses to H1 and H3 at the end of the study (day 56) (Figure 4), though it is possible this reflects natural exposure to seasonal influenza over the course of the study.
Figure 4. Fold increase in the frequency of HA reactive MBC.
The fold change from baseline in percent HA per Total IgG spots was determined by dividing the values at each time point by those measured at day 0. The mean ± standard error is plotted for the cohort of 28 subjects.
Frequencies of HA-specific Antibody Secreting Cells (ASC) increase after vaccination
Although these studies were designed to measure MBC from cryopreserved samples, recent work indicates that it is possible to measure frequencies of specific ASC directly from carefully frozen specimens [30]. After the completion of the MBC assays, ten subjects that had samples remaining were assayed for HA-specific ASC by direct (no restimulation) ELISPOT. PBMC were thawed and placed directly into plates coated with H1, H3, H5/HK, H5/VN, H6 HAs or bovine serum albumin (BSA), and cultured for 6 hours. Increases in specific ASC against both H5 variants could be observed in 9 of the 10 subjects at 7 days after vaccination (Figure 5). Considering the group of subjects as a whole, the increase in ASC were significant (p ≤ 0.01) at day 7 for both H5 HA, and approached significance (p = 0.02) at day 28 for H5/VN/04. There were no significant increases in the ASC frequencies against the H1, H3, or H6 HAs. Peak values were in the range of 30 to 1700 HA specific ASC per million PBMC (Figure 5). While higher values have been reported after vaccination or infection [30, 31], these are still in the range of what is observed from fresh PBMC. Therefore, like the MBC response, there is a peak around 7 days in ASC specific for the vaccine H5 antigen and a drifted variant.
Figure 5. Frequency and kinetics of ASC measured directly from the PBMC.
PBMC were thawed and plated, at several dilutions, onto 96-well nitrocellulose dishes coated with 1µg/well of recombinant influenza HA antigens, KLH (negative control), or antibody against human IgG. After a 4-hour culture, the cells were washed off and the plates developed with a second anti-human IgG antibody conjugated to alkaline phosphatase, and TCIP/BCP substrate. Spots were counted on an ImmunoSpot automated plate reader. Plates were set up such that PBMC from all the time points from a single subject were on the same plate. Each plot depicts the results from one of the 10 individual subjects tested.
Covariates of the MBC response to H5 HA
Some cofactors appeared to contribute to the success of the vaccination, defined arbitrarily as a significant increase in the frequency of specific MBC from baseline (d0) at any other time point in the study, while some obvious ones were not predictive. For example, regular, annual vaccination against seasonal influenza significantly correlated with the frequency of H5/VN/1203/04 responses at day 7. This suggests that annual vaccination may increase the number of HA cross-reactive MBC. There was no correlation at other time points however. Interestingly, a positive response to the original H5/HK/156/97 vaccination, in terms of rise in serum titer (>1:40 or 4-fold increase), did not predict whether the subjects would respond in the present re-vaccination with the H5/VN/1203/04 vaccine. Nevertheless, subjects who had received the 1997 based vaccine were more likely to respond to the 2004 drift variant in spite of the inability to detect responses during the H5/97 vaccine study. This suggests that prior immunization against H5 HA may produce MBC in the absence of the number of ASC needed to produce a positive serum IgG response. In fact in the present study, it was possible to detect a rise in HA-specific MBC in subjects that did not meet the criteria of a positive rise in serum HAI or MN activity. This suggests that in some subjects, vaccination can elicit a cellular immune response that is not apparent by measuring serum neutralizing antibody alone.
Discussion
The relatively recent development and adoption of spot-based assays of immune cells has facilitated the study of human immune responses [32, 33]. Significant value was added by the development of a simple, antigen-independent method to stimulate resting MBC to become cells that secrete IgG [24]. These approaches allow us to measure the circulating frequencies of both effector ASC as well as MBC [24, 30]. The method to reactivate MBC has been shown to cause the differentiation of CD27+ CD38+ (CD19+ CD3−) B cells into CD27+ CD38+++ ASC [24]. Existing ASC present at the start of the culture are lost during the culture period (Crotty, personal communication), and sorting of CD3− CD19+ cells by expression of CD27 and CD38 shows that the culture conditions induce antibody secretion by only the CD27+ CD38 low cells [9, 34]. In our hands, we cannot explicitly rule out carryover of ASC from the start of the culture; however, ASC frequencies were not high, making carryover less likely. What are less clear are the relationships between serum IgG, and the prevalence of ASC and MBC in the blood. This study and others show HA-specific ASC and MBC (as a function of total IgG secreting cells) increased in frequency at 7 days after vaccination [9]. In turn, the analogous MBC per million PBMC was highest at 28 days, consistent with the expansion or perisitence of a pool of circulating HA specific memory cells. However, we should note the difficulty in relating the frequency values to the actual numbers of antigen-specific cells in the blood using the MBC assay. Proliferation of selected cells during the 6-day MBC restimulation is not easily assessed, making it difficult to directly and accurately extrapolate the values back to the blood sample. Nevertheless, these observations support the concept that immunization with potential pandemic or emerging influenza HA protein in human subjects results in the development of both short and long term B cell mediated cellular immunity.
The generation of long-lived antigen-specific memory cells is a hallmark of successful vaccination [35]. However, few studies are available that evaluate existing cellular memory responses, or those elicited by vaccination. Fewer still compare one vaccination approach to another. Instead, evaluating the success of vaccination depends on measuring serum antibody levels. In the case of influenza, assays are available to measure total influenza specific antibody (ELISA), as well as biologically active antibodies that inhibit viral agglutination of red blood cells (HAI), or neutralization of live virus (microneutralization or plaque reduction assays). The latter are considered the “gold-standard” for influenza vaccines as they have been shown to correlate well with observed rates of protection from disease. One of the reasons these assays correlate better than the ELISA based approach is that the ELISA can detect antibodies to other determinants on the protein that, while antigenic, do not necessarily confer protection from infection. Interestingly, these non-neutralizing determinants tend to be more conserved among different hemagglutinin serotypes [36–39], and conserved neutralizing antigenic determinants in the HA protein have been described [40, 41].
The B cell ELISPOT assay is essentially analogous to the ELISA assay in that it will detect B cells secreting IgG whether or not those secreted antibodies will neutralize the virus or inhibit agglutination. On this basis, one could question the clinical relevance of the approach in studying vaccine related immune responses. However, it is important to consider that a general increase in the prevalence of a memory B cell population should correlate well with long-term persistence of an immune response. To be fair, there is insufficient data in the context of human diseases to know whether detecting these cellular responses can be predictive of future immune responses to modified vaccines or drifted viruses. It is also not known in humans how the presence of a memory B cell population affects subsequent immune responses to infection or vaccination with related, but not identical variants of the virus. One could imagine that the MBC could improve antigen presentation to CD4 T cells for example.
Immune responses in adults to experimental H5 influenza vaccines have been weak at best, even after two or three immunizations with up to 90µg of the same HA [26, 27, 42], with proportions of subjects mounting increased serum antibody to H5 after vaccination in the range of 40–55% [26, 27, 42]. Interestingly, in adult subjects receiving experimental H7 and H9 based subunit vaccines, the response rates were similarly low [43–45], though recent approaches with live attenuated candidates were more successful [46, 47]. For comparison, in children who are seronegative, two 7.5µg doses of TIV is effective at eliciting clinically significant serum antibody titers over 80% of recipients [48–50]. This suggests that inactivated subunit H5 influenza vaccines are poorly immunogenic in humans, though they may still be influenced by the immune status of the individual.
The success of seasonal influenza vaccines against H1 and H3 strains of the virus may be high because they depend on stimulating existing immunity to the viruses. Almost all individuals have been exposed to seasonal influenza virus by the age of 5 [51–55]. Adults have likely had several encounters with influenza of both subtypes, and it is likely that the strong immunity elicited by infection facilitates good immune responses to the low amounts of protein (15µg of each HA) administered without adjuvant in the present trivalent inactivated subunit vaccine (TIV).
On the other hand, rHA or inactivated subunit H5 vaccines administered experimentally to human subjects have a reputation of being poorly immunogenic, based largely on standard HAI and MN titers from serum samples. The data herein supports the notion that, although neutralizing or inhibitory antibodies are weak or undetectable, vaccination can elicit long-lived cellular immunity in human subjects. In a related study of H5 vaccination, Ali-Goji et al recently showed that subjects previously vaccinated with two doses of an experimental, recombinant protein vaccine against avian H5 influenza A/Hong Kong/156/97 subsequently mounted robust responses to an experimental egg-derived H5 vaccine based on a more recent influenza A/Vietnam/1203/04 strain of the virus [26]. Response rates determined by conventional HAI and MN assays were 86% or greater, with relatively high titers. This suggested that, although the response rates after administration of the H5/97 vaccine alone were much less than 80% [25], the vaccine may have established a component of long-lived memory that persisted. Therefore, the possibility exists that prior vaccination may have a clinical benefit, in spite of difficulty in detecting substantial neutralizing antibody responses.
The reasons that vaccination against H5, whether in recombinant or inactivated subunit forms, does not elicit significant amounts of neutralizing antibody, while viral infection does, are not known. It may be that conformational differences exist in the purified recombinant and inactivated subunit-derived proteins versus those produced by infected cells. In the range of antibodies that are elicited, only a small subset may have neutralizing activity. It may also be the case that there are aspects of how the HA antigen is presented during infection that competitively favors the production of neutralizing antibody, versus antibody to other determinants. Nevertheless, it seems that having B cells and antibody to other determinants should confer some protection from severe disease in animals, though it would not prevent infection.
We believe that the observations lend support the idea that the phenomenon of Original Antigenic Sin may not apply to protein vaccination [9], and is a unique feature of infection. The mechanisms that are unique to priming by infection and responsible for this effect are not known, but may explain the problem of extrapolating the concept of OAS to influenza vaccination (versus infection). Experimental HA drift variant immunization of naïve mice, for example, elicits antibodies that cross-reacted with HA of the same H5 subtype, but not across other H3 and H6 HA (data not shown), producing a similar quality of antibody responses seen in the human subjects. Unlike the mice however, the adult human subjects of the present study were not naïve to influenza, and had likely developed immunity to influenza through a history of both infection and vaccination. Unfortunately, it is not possible to resolve these issues within the context of the present study.
These studies support the observations reported for serum HAI and MN responses [26] that suggested some advantages to pre-pandemic vaccination with vaccine candidates closely related to the anticipated pandemic strain. A certain level of durable, long-lived cellular immunity is established and reactivated by HA protein or subunit immunization, and can develop in the absence of evidence of the serological response. These cell mediated immunity (CMI) assays compliment serum assays, though the predictive value to protection from infection remains to be established. It is worth considering the expansion of these types of clinical studies to further optimize the experimental design, look at responses to other types of immunization and infection, as well as determine whether or not they will be of use in predicting vaccine success or diagnosis of infection.
Acknowledgements
We would like to acknowledge the clinical staff in the University of Rochester Vaccine Research Unit and Processing Lab for their hard work and excellent service enrolling subjects, administering vaccine, collecting and processing the samples. We would also like to acknowledge the support of this work through HHS/NIH/NIAID under N01-AI25460, HHSN266200700008C, and N01-AI50020.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pereira MS. The effects of shifts and drifts on the epidemiology of influenza in man. Philos Trans R Soc Lond B Biol Sci. 1980;288(1029):423–432. doi: 10.1098/rstb.1980.0019. [DOI] [PubMed] [Google Scholar]
- 2.Webster RG, Govorkova EA. H5N1 influenza--continuing evolution and spread. N Engl J Med. 2006;355(21):2174–2177. doi: 10.1056/NEJMp068205. [DOI] [PubMed] [Google Scholar]
- 3.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuters. Chile finds H1N1 swine flu in turkeys. The Star. 2009 August 20; 2009. [Google Scholar]
- 5.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, Hayden FG, Nguyen DH, de Jong MD, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 6.Smith GJ, Naipospos TS, Nguyen TD, de Jong MD, Vijaykrishna D, Usman TB, et al. Evolution and adaptation of H5N1 influenza virus in avian and human hosts in Indonesia and Vietnam. Virology. 2006;350(2):258–268. doi: 10.1016/j.virol.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Fazekas de St G, Webster RG. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazekas de St G, Webster RG. Disquisitions on Original Antigenic Sin. II. Proof in lower creatures. J Exp Med. 1966;124(3):347–361. doi: 10.1084/jem.124.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrest HL, Khalenkov AM, Govorkova EA, Kim JK, Del Giudice G, Webster RG. Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine. 2009;27(31):4187–4195. doi: 10.1016/j.vaccine.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng X, Eisenbraun M, Xu Q, Zhou H, Kulkarni D, Subbarao K, et al. H5N1 vaccine-specific B cell responses in ferrets primed with live attenuated seasonal influenza vaccines. PLoS One. 2009;4(2):e4436. doi: 10.1371/journal.pone.0004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006;194(8):1040–1043. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- 13.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS One. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118(10):3478–3490. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Co MD, Orphin L, Cruz J, Pazoles P, Green KM, Potts J, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine. 2009;27(2):319–327. doi: 10.1016/j.vaccine.2008.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terajima M, Cruz J, Leporati AM, Orphin L, Babon JA, Co MD, et al. Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients. J Virol. 2008;82(18):9283–9287. doi: 10.1128/JVI.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Co MD, Orphin L, Cruz J, Pazoles P, Rothman AL, Ennis FA, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine. 2008;26(16):1990–1998. doi: 10.1016/j.vaccine.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J Immunol. 1999;162(12):7578–7583. [PubMed] [Google Scholar]
- 19.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176(10):6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 20.McElhaney JE, Hooton JW, Hooton N, Bleackley RC. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine. 2005;23(25):3294–3300. doi: 10.1016/j.vaccine.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 21.He XS, Holmes TH, Mahmood K, Kemble GW, Dekker CL, Arvin AM, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197(6):803–811. doi: 10.1086/528804. [DOI] [PubMed] [Google Scholar]
- 22.Eichelberger M, Golding H, Hess M, Weir J, Subbarao K, Luke CJ, et al. FDA/NIH/WHO public workshop on immune correlates of protection against influenza A viruses in support of pandemic vaccine development, Bethesda, Maryland, US, December 10–11, 2007. Vaccine. 2008;26(34):4299–4303. doi: 10.1016/j.vaccine.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Rimmelzwaan GF, McElhaney JE. Correlates of protection: novel generations of influenza vaccines. Vaccine. 2008;26 Suppl 4:D41–D44. doi: 10.1016/j.vaccine.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(1–2):111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, O'Brien D, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19(13–14):1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- 26.Goji NA, Nolan C, Hill H, Wolff M, Noah DL, Williams TB, et al. Immune responses of healthy subjects to a single dose of intramuscular inactivated influenza A/Vietnam/1203/2004 (H5N1) vaccine after priming with an antigenic variant. J Infect Dis. 2008;198(5):635–641. doi: 10.1086/590916. [DOI] [PubMed] [Google Scholar]
- 27.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354(13):1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Tyler DE. The influence function and maximum bias of Tukey's median. Ann Statistics. 2002;30(6):1737–1759. [Google Scholar]
- 29.Food & Drug Administration CfBR, editor. Guidance for Industry: Clinical Data Needed to Support the Licensure of Seasonal Inactivated Influenza Vaccines. Rockville, MD: Office of Communication, Training and Manufacturers Assistance (HFM-40); 2007. pp. 1–17. [Google Scholar]
- 30.Kyu SY, Kobie J, Yang H, Zand MS, Topham DJ, Quataert SA, et al. Frequencies of human influenza-specific antibody secreting cells or plasmablasts post vaccination from fresh and frozen peripheral blood mononuclear cells. J Immunol Methods. 2009;340(1):42–47. doi: 10.1016/j.jim.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malaspina A, Moir S, Orsega SM, Vasquez J, Miller NJ, Donoghue ET, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191(9):1442–1450. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 32.Lycke N. A sensitive method for the detection of specific antibody production in different isotypes from single lamina propria plasma cells. Scand J Immunol. 1986;24(4):393–403. doi: 10.1111/j.1365-3083.1986.tb02127.x. [DOI] [PubMed] [Google Scholar]
- 33.Czerkinsky CC, Nilsson LA, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65(1–2):109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 34.Crompton PD, Mircetic M, Weiss G, Baughman A, Huang CY, Topham DJ, et al. The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol. 2009;182(5):3318–3326. doi: 10.4049/jimmunol.0803596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrammert J, Miller J, Akondy R, Ahmed R. Human immune memory to yellow fever and smallpox vaccination. J Clin Immunol. 2009;29(2):151–157. doi: 10.1007/s10875-008-9267-3. [DOI] [PubMed] [Google Scholar]
- 36.Kaverin NV, Rudneva IA, Govorkova EA, Timofeeva TA, Shilov AA, Kochergin-Nikitsky KS, et al. Epitope mapping of the hemagglutinin molecule of a highly pathogenic H5N1 influenza virus by using monoclonal antibodies. J Virol. 2007;81(23):12911–12917. doi: 10.1128/JVI.01522-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaverin NV, Rudneva IA, Ilyushina NA, Varich NL, Lipatov AS, Smirnov YA, et al. Structure of antigenic sites on the haemagglutinin molecule of H5 avian influenza virus and phenotypic variation of escape mutants. J Gen Virol. 2002;83(Pt 10):2497–2505. doi: 10.1099/0022-1317-83-10-2497. [DOI] [PubMed] [Google Scholar]
- 38.Austin FJ, Webster RG. Antigenic mapping of an avian H1 influenza virus haemagglutinin and interrelationships of H1 viruses from humans, pigs and birds. J Gen Virol. 1986;67(Pt 6):983–992. doi: 10.1099/0022-1317-67-6-983. [DOI] [PubMed] [Google Scholar]
- 39.Yamada A, Brown LE, Webster RG. Characterization of H2 influenza virus hemagglutinin with monoclonal antibodies: influence of receptor specificity. Virology. 1984;138(2):276–286. doi: 10.1016/0042-6822(84)90351-9. [DOI] [PubMed] [Google Scholar]
- 40.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67(5):2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treanor JJ, Schiff GM, Couch RB, Cate TR, Brady RC, Hay CM, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006;193(9):1223–1228. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- 43.Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009;27(13):1889–1897. doi: 10.1016/j.vaccine.2009.01.116. [DOI] [PubMed] [Google Scholar]
- 44.Stephenson I, Nicholson KG, Gluck R, Mischler R, Newman RW, Palache AM, et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet. 2003;362(9400):1959–1966. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 45.Keitel WA, Atmar RL. Vaccines for pandemic influenza: summary of recent clinical trials. Curr Top Microbiol Immunol. 2009;333:431–451. doi: 10.1007/978-3-540-92165-3_21. [DOI] [PubMed] [Google Scholar]
- 46.Karron RA, Callahan K, Luke C, Thumar B, McAuliffe J, Schappell E, et al. A live attenuated H9N2 influenza vaccine is well tolerated and immunogenic in healthy adults. J Infect Dis. 2009;199(5):711–716. doi: 10.1086/596558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27(28):3744–3753. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldman S, Wright PF, Webster RG, Roberson PK, Mahoney J, Thompson J, et al. Use of influenza A virus vaccines in seronegative children: live cold-adapted versus inactivated whole virus. J Infect Dis. 1985;152(6):1212–1218. doi: 10.1093/infdis/152.6.1212. [DOI] [PubMed] [Google Scholar]
- 49.Daubeney P, Taylor CJ, McGaw J, Brown EM, Ghosal S, Keeton BR, et al. Immunogenicity and tolerability of a trivalent influenza subunit vaccine (Influvac) in high-risk children aged 6 months to 4 years. Br J Clin Pract. 1997;51(2):87–90. [PubMed] [Google Scholar]
- 50.Gonzalez M, Pirez MC, Ward E, Dibarboure H, Garcia A, Picolet H. Safety and immunogenicity of a paediatric presentation of an influenza vaccine. Arch Dis Child. 2000;83(6):488–491. doi: 10.1136/adc.83.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glezen WP, Taber LH, Frank AL, Gruber WC, Piedra PA. Influenza virus infections in infants. Pediatr Infect Dis J. 1997;16(11):1065–1068. doi: 10.1097/00006454-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Frank AL, Taber LH. Variation in frequency of natural reinfection with influenza A viruses. J Med Virol. 1983;12(1):17–23. doi: 10.1002/jmv.1890120103. [DOI] [PubMed] [Google Scholar]
- 53.Frank AL, Taber LH, Glezen WP, Paredes A, Couch RB. Reinfection with influenza A (H3N2) virus in young children and their families. J Infect Dis. 1979;140(6):829–836. doi: 10.1093/infdis/140.6.829. [DOI] [PubMed] [Google Scholar]
- 54.Johnson PR, Feldman S, Thompson JM, Mahoney JD, Wright PF. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. J Infect Dis. 1986;154(1):121–127. doi: 10.1093/infdis/154.1.121. [DOI] [PubMed] [Google Scholar]
- 55.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]