Abstract
IMVAMUNE® is a Modified Vaccinia Ankara-based virus that is being developed as a safer 3rd generation smallpox vaccine. In order to determine the optimal dose for further development, a double-blind, randomized Phase II trial was performed testing three different doses of IMVAMUNE® in 164 healthy volunteers. All three IMVAMUNE® doses displayed a favourable safety profile, with local reactions as the most frequent observation. The 1×108 TCID50 IMVAMUNE® dose induced a total antibody response in 94% of the subjects following the first vaccination and the highest peak seroconversion rates by ELISA (100%) and PRNT (71%). This IMVAMUNE® dose was considered to be optimal for the further clinical development of this highly attenuated poxvirus as a safer smallpox vaccine.
Keywords: Smallpox, vaccination, MVA, vaccinia
1. Introduction
Following a long and coordinated worldwide vaccination campaign using a variety of vaccinia virus strains, such as Lister-Elstree or New York City Board of Health (Dryvax®), the WHO officially declared the eradication of smallpox in 1980 [1]. As people have not been vaccinated against smallpox for the last 30 years, the majority of the world's population is highly susceptible to this deadly disease. This has raised fears that through an act of bioterrorism, variola virus, the causative agent of smallpox, may be released back into the general population and has led to discussions of re-introducing smallpox vaccination and/or stockpiling vaccines. The original or 1st generation smallpox vaccines were crudely manufactured on the skin of animals that has led to the development of 2nd generation smallpox vaccines, which are still based on the same vaccinia virus strains, but are manufactured using modern sterile techniques [2].
While traditional smallpox vaccines have been shown to be highly effective in eradicating smallpox, their general use is restricted, because of rare but severe side effects, particularly in immune compromised individuals [3;4]. Indeed, due to contraindications associated with the use of these vaccines, it has been estimated that approximately 25% of the U.S. population should be excluded from prophylactic vaccination using conventional smallpox vaccines [5;6]. Furthermore, unexpectedly high incidences for developing myopericarditis were observed following vaccination with conventional smallpox vaccines in clinical trials [7] and recent studies using 1st and 2nd generation smallpox vaccines showed an incidence of 10.38 and 5.73 cases of myopericarditis for every thousand vaccinations [8]. Therefore, there is a need for a safer or 3rd generation smallpox vaccine for the general population, including large risk populations currently contraindicated for the current smallpox vaccines, namely people infected with HIV or diagnosed with atopic dermatitis.
Modified Vaccinia Ankara (MVA) is an attenuated poxvirus created by more than 500 serial passages of Chorioallantois vaccinia Ankara virus (CVA) in chicken embryo fibroblast (CEF) cells [9] and was used in more than 120,000 vaccinees for priming prior to administration of a conventional smallpox vaccine in a two-step protocol used in the 1970's in Germany [10;11]. MVA-BN® (trade name IMVAMUNE®) has been derived from the licensed MVA used in Germany by additional passages and limiting dilutions in CEF cells under serum-free conditions and has been shown not to replicate in human cells and can be safely administered to severely immune compromised animals [12;13]. During the attenuation in CEF cells, six major deletions have occurred in the MVA genome compared to CVA and further deletions, insertions and mutations have also been detected in 122 of the remaining 195 open reading frames of MVA that presumably contribute to the highly restriction host range of MVA compared to vaccinia virus [14]. Despite the severe host cell restriction of MVA to avian cells, MVA has demonstrated to induce a comparable efficacy to various lethal poxvirus challenges in mice and non-human primate models as traditional smallpox vaccines [15–22]. Earlier Phase I studies have also demonstrated that IMVAMUNE® was safe, well tolerated and immunogenic [23–25].
In this article we present the results of a double-blind, randomized, Phase II dose-finding study evaluating three different doses of IMVAMUNE® in 164 healthy volunteers. The study was designed to evaluate the safety and immunogenicity induced by IMVAMUNE® doses between 2×107 and 1×108 TCID50, in order to determine an optimal dose in terms of safety and the magnitude of the humoral and cell mediated immune responses for the further clinical development of IMVAMUNE® as a third generation smallpox vaccine.
2. Methods
2.1. Vaccine
The IMVAMUNE® smallpox vaccine was produced by IDT Biologika GmbH (Dessau-Roßlau, Germany) according to current Good Manufacturing Practice at a nominal titre of 2 × 108 TCID50/ml and was provided by Bavarian Nordic A/S (Kvistgaard, Denmark) as a freeze-dried product stored under refrigerated conditions. The vaccine was reconstituted in 0.5 ml sterile water for injection to a minimal dose of 1 × 108 TCID50. The two lower doses of 2 × 107 and 5 × 107 TCID50 were prepared by dilutions of the reconstituted vaccine.
2.2. Study design and subjects
The study was designed as a double-blind, randomized, Phase II dose-finding study comparing three different doses of IMVAMUNE® to determine the optimal dose in terms of safety and immunogenicity. The trial was performed in a monocentric setting. The study protocol was approved by the responsible independent ethics committee and all volunteers provided a signed consent prior to vaccination. Screening of subjects included determination of vaccinia titre by ELISA, and only healthy male and female subjects between 18 to 30 years of age who were confirmed to be vaccinia-naïve were selected for study participation. In order to obtain 150 evaluable records, a total of 165 subjects were randomly assigned to one of the three treatment groups and were immunized twice with IMVAMUNE® in a four week interval. Each immunization consisted of one subcutaneous injection of 0.5 ml IMVAMUNE® in the non-dominant upper arm. Study-specific assessments were conducted at screening and on Days 0, 28, 42 and 84.
2.3. Safety assessments
Safety measures such as physical examinations, laboratory parameters and adverse events were assessed regularly throughout the trial to obtain data on safety and reactogenicity. Occurrence, relationship and intensity of any unsolicited adverse event (AE) that was spontaneously reported by the subject at any time were recorded and documented at each visit during the 12 week study period. Solicited AE constituted pre-defined, expected local reactions (erythema, swelling, induration and pain) and general symptoms (body temperature, headache, chills, nausea and fatigue). All solicited AE were assessed for occurrence, intensity and duration by the subjects and recorded by completing a diary card for the 7 day period following each vaccination. Unsolicited AE were defined as all events reported by the subject within a 30-day period after vaccination not solicited in the diary card. All unsolicited and solicited AEs were analysed according to predefined scales.
2.4. Vaccinia specific Enzyme-linked immunosorbent assay (ELISA)
Immunoglobulin subclass G (IgG) responses to vaccinia virus were measured using an automated direct ELISA on a Biomek FX (Beckman Coulter, Krefeld, Germany). ELISA plates (96-well TRANSP MAXIS plates; Nunc, Wiesbaden, Germany) were coated overnight at 4°C with 100 μl (7.5 μg/ml) of MVA antigen in coating buffer (200 mM Na2CO3, pH 9.6). Following coating all subsequent steps of the ELISA was automated. The plates were blocked with 200 μl of PBS-FCS 5 % (PAA Laboratories, Linz, Austria) for 30 min at room temperature, washed and heat-inactivated (56 C for 30 min) test sera was titrated in duplicate using 2-fold serial dilutions starting at 1:50. The plates were incubated for 1 h at room temperature, washed and incubated again for 1 h with the detection antibody goat anti-human IgG-HRP (Sigma, Taufkirchen, Germany) before development with 3,3',5,5'-Tetramethylbenzidine substrate (Sigma).
The Optical Density (OD) value was measured at 492 nm with a reference wavelength of 405 nm. The antibody titres were calculated by linear regression and defined as the serum dilution that resulted in an optical density of 0.35. A titre of ≥ 50 was the lowest reliably detectable antibody level and if this titre was reached, the subject was considered seropositive. A titre of < 50 was considered seronegative and was given an arbitrary value of 1. Geometric mean titres (GMTs) were calculated using the antilogarithm of the mean of the log10 titre transformation. The percentages of seropositive subjects were expressed as a percent (%) seroconversion rate, which was determined for all study visits.
2.5. Plaque reduction neutralization test (PRNT)
Vaccinia-specific neutralising antibody levels were measured using a PRNT assay. Sera were heat-inactivated (56 C for 30 min) and serially diluted (starting dilution 1:20) in DMEM (Invitrogen, Karlsruhe, Germany) media containing 100 IU penicillin, 100 μg/ml streptomycin and incubated with approximately 100 plaque forming units (pfu) of the vaccinia virus strain IHD-J for 2 hrs at 37 °C in 5% CO2. Following incubation, the sera were added to duplicate wells of a 24-well tissue culture plate containing a confluent monolayer of Vero cells (Vero E6, ATCC: CRL-1586, pre-seeded for 24 hrs) and incubated for 24–30 hrs at 37 °C with 5% CO2. Monolayers were stained with crystal violet in order to visualize plaques and the neutralising titre was determined as the dilution resulting in a 50% reduction of total plaques. GMTs were calculated using the antilogarithm of the mean of the log10 titre transformations. A PRNT value ≥ 20 was considered seropositive, while titres <20 were assigned an arbitrary value of 1 and considered seronegative.
2.6. Intracellular cytokine staining (ICS) by flow cytometry
Vaccinia-specific T cell immunogenicity was measured by ICS of Interferon-gamma (IFN-γ) producing cells using cryopreserved peripheral blood mononuclear cells (PBMCs). T cell responses for both CD4+ and CD8+ T cells were measured by flow cytometry using a semi-automated ICS assay. Cryopreserved PBMCs were thawed and cultured at 2 × 107/ml with IMVAMUNE® at a multiplicity of infection of 10 and co-stimulated with anti-CD28 (final concentration 0.3 μg/ml, BD Bioscience) at 37 °C with 5% CO2 for 16–24 hrs. After the first 2 hrs of incubation, secretion of intracellular cytokines was blocked by addition of 5 μg/ml of Brefeldin A (Sigma). Cells were mixed and returned to the incubator. The cells were loaded onto a progressive automated line, an integrated, semi-automated unit for cell staining, permeabilized, washed and detected using flow cytometry (Beckman Coulter). The cells were surface stained with a cocktail of antibodies to CD3, CD4, CD8 and CD69 (Beckman Coulter), fixed, permeabilized and stained with IFN-γ antibodies. After staining, the cells were washed using hollow fibre technology, re-suspended and loaded onto a Beckman Coulter FC500 analyzer. Non-stimulated cells were used as controls. These cells were treated equally but without the addition of IMVAMUNE®. The non-stimulated cells were stained with isotype-matched control antibodies for specificity and also with the test antibodies to obtain the non-stimulated or baseline value of IFN-γ production of T cells. Data were analysed using Cytomics RXP software. A lymphocyte gate was defined on forward and side scatter and confirmed for CD3 positive status with a second gate. T cells were defined with gates for CD8+ or CD4+ T cells (CD3+ and CD4+ or CD3+ and CD8+). T cells were considered positive for IFN-γ production when the cells were positive for the activation marker CD69 and intracellular IFN-γ. The percentage of CD4+ or CD8+ T cells minus the non-stimulated background values was calculated. A positive vaccination response was defined as a value greater than the clinical relevance limit (CRL, defined as 2 standard deviations of the baseline responses, minus any clear outliers). For CD8+ T cells the CRL threshold was 0.25% and for CD4+ T cells 0.14%.
2.7. Statistical methods
The dose response was shown using linear regression on the log of the titres against the log of the dose for day 28 (4 weeks after the first vaccination) and day 42 (two weeks after the second vaccination) using Pearson correlation. A possible dose-response was also analysed by non-parametric correlation analysis (Spearman correlation) between dose and titre data. The correlation coefficient of the ELISA and PRNT titres were calculated. For the safety analysis, adverse events were summarized using frequency tables.
3. Results
3.1. Study population
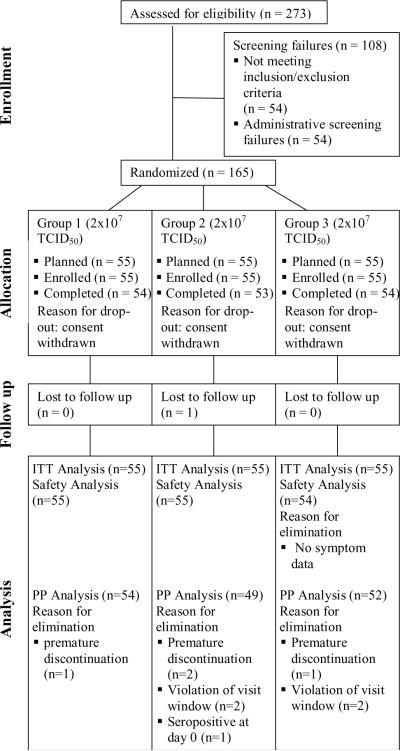
A total of 165 subjects were randomized and all received at least one dose of IMVAMUNE®. One subject was removed from the safety analysis due to missing safety data (subject diary). Ten subjects did not fulfil the criteria for inclusion in the per-protocol (PP) population, either due to premature discontinuation (no second vaccination, n = 4), or violation of visit schedule (n = 5) or positive serology status before the first vaccination (n = 1). Consequently, the analysis of immune responses induced by the various doses of IMVAMUNE® was performed on the 155 subjects of the PP set (Group 1: n=54, Group 2: n=49, Group 3: n=52, Figure 1).
Figure 1. Disposition of subjects and cohorts analyzed.
Out of 273 screened volunteers, 165 subjects were assessed as being eligible for enrolment and allocated to the three study groups. In total, ten subjects were excluded from the per-protocol analysis e.g. due to premature study discontinuation, violation of visit window and seropositivity at study start.
3.2 Subject demographics
The arithmetic mean values together with the standard deviation (SD) of the safety population (n = 164) were as follows: age (23.2 ± 3.0 years), height (170.7 ± 8.8 cm) and weight (67.3 ± 14.4 kg). More women than men were enrolled into the study with 64% of the total safety population. However, demographics were similar between the safety population and the PP set and no statistically significant differences in demographics were detected between the three dose groups (data not shown).
3.3. Safety and reactogenicity
A total of 164 subjects were included in the safety analysis. Comparing the three treatment groups, IMVAMUNE® was well tolerated at all dose levels. There were no deaths and no vaccine related serious adverse events throughout the study. Analysis of vital signs and laboratory parameters revealed no differences between the groups (data not shown). Only minor laboratory value deviations from normal were observed, but none were assessed to be clinically significant or relevant. One 27 year old woman became pregnant during the study and based on the regular pregnancy tests foreseen in the protocol, the earliest time point of conception was 17 days, or longer, after having received the second vaccination with IMVAMUNE®. The pregnancy progressed to term without complications and the mother delivered a healthy, normal baby.
The majority of subjects (≥ 96.4%) in each group experienced at least one solicited or unsolicited adverse event (Table 1). Erythema and pain at the injection site were the most prevalent solicited local symptoms recorded in the subject diary (Table 2). In general, subjects classified most solicited events to be mild or moderate in severity (Grade 1 or 2), although 21.3% of subjects reported injection site erythema ≥ 50 mm (Grade ≥ 3 intensity), thus representing the most prevalent Grade 3 solicited adverse event in all groups. The frequency of injection site erythema and injection site swelling increased with higher doses, however for all general symptoms no clear dose dependency was seen (Table 2). For all groups fatigue and headache, followed by myalgia, nausea and chills, were the most prevalent solicited general adverse events (Table 2). As is often seen with injected vaccines, the number of adverse events reported after the second vaccination was lower compared to the first immunization (Table 1).
Table 1.
Overall incidence of adverse events (solicited and unsolicited) according to per vaccination and per subject analysis, reported during the 30-day follow-up visit after vaccination
| Vaccination Period | Treatment Group [TCID50] | N | Any Solicited or Unsolicited Adverse Event n(%) | Any Unsolicited Adverse Event n(%) |
|---|---|---|---|---|
| 1 | 2×107 | 55 | 53 (96.4) | 23 (41.8) |
| 5×107 | 55 | 53 (96.4) | 23 (41.8) | |
| 1×108 | 54 | 53 (98.1) | 27 (50.0) | |
| total | 164 | 159 (97.0) | 73 (44.5) | |
| 2 | 2×107 | 55 | 48 (87.3) | 20 (36.4) |
| 5×107 | 55 | 49 (89.1) | 21 (38.2) | |
| 1×108 | 54 | 51 (94.4) | 22 (40.7) | |
| total | 164 | 148 (90.2) | 63 (38.4) |
Table 2.
Solicited or unsolicited adverse events with a frequency of ≥ 5% in at least one group regardless of causal relationship to study vaccine (safety population)
| Adverse events | Group la (N=55) n (%) | Group 2b (N=55) n (%) | Group 3c (N=54) n (%) | Total (N=164) n (%) |
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| Nausea | 11(20.0) | 5 (9.1) | 12 (22.2) | 28 (17.1) |
| ≥ Grade 3d | 1 (1.8) | 1 (1.8) | 1 (1.9) | 3 (1.8) |
| General disorders and administration site conditions | ||||
| Injection site erythema | 47 (85.5) | 49 (89.1) | 52 (96.3) | 148 (90.2) |
| ≥ Grade 3 (≥ 50 mm) | 7 (12.7) | 13 (23.6) | 15 (27.8) | 35 (21.3) |
| Injection site pain | 48 (87.3) | 44 (80.0) | 53 (98.1) | 145 (88.4) |
| ≥ Grade 3 | 0 (0.0) | 3 (5.5) | 2 (3.7) | 5 (3.0) |
| Injection site induration | 43 (78.2) | 41 (74.5) | 43 (79.6) | 127 (77.4) |
| ≥ Grade 3 (≥ 50 mm) | 3 (5.5) | 6 (10.9) | 5 (9.3) | 14 (8.5) |
| Injection site swelling | 38 (69.1) | 41 (74.5) | 44 (81.5) | 123 (75.0) |
| ≥ Grade 3 (≥ 50 mm) | 4 (7.3) | 7 (12.7) | 9 (16.7) | 20 (12.2) |
| Injection site pruritus | 9 (16.4) | 8 (14.5) | 14 (25.9) | 31 (18.9) |
| ≥ Grade 3e | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Injection site inflammation | 2 (3.6) | 1 (1.8) | 3 (5.6) | 6 (3.7) |
| ≥ Grade 3e | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fatigue | 27 (49.1) | 20 (36.4) | 26 (48.1) | 73 (44.5) |
| ≥ Grade 3f | 1 (1.8) | 2 (3.6) | 2 (3.7) | 5 (3.0) |
| Chills | 2 (3.6) | 3 (5.5) | 5 (9.3) | 10 (6.1) |
| ≥ Grade 3g | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Infections and infestations | ||||
| Nasopharyngitis | 4 (7.3) | 9 (16.4) | 4 (7.4) | 17 (10.4) |
| ≥ Grade 3e | 1 (1.8) | 1 (1.8) | 0 (0.0) | 2 (1.2) |
| Musculoskeletal and connective tissue disorders | ||||
| Myalgia | 10 (18.2) | 9 (16.4) | 14 (25.9) | 33 (22.1) |
| ≥ Grade 3h | 1 (1.8) | 1 (1.8) | 0 (0.0) | 2 (1.2) |
| Nervous system disorders | ||||
| Headache | 30 (54.5) | 24 (43.6) | 22 (40.7) | 76 (46.3) |
| ≥ Grade 3i | 3 (5.5) | 2 (3.6) | 3 (5.6) | 8 (4.9) |
Group 1: 2×107TCID50
Group2: 5×107TCID50
Group 3: 1×108TCID5O
Grading used for nausea: 0 = None; 1 = Able to eat; 2 = Oral intake significantly decreased; 3 = No significant intake, requiring i.v. fluids
Grading used for unsolicited AEs (injection site pruritus, injection site inflammation, nasopharyngitis): 0 = No adverse event; 1 = An adverse event which is easily tolerated by the patient, causing minimal discomfort and not interfering with everyday activities; 2 = An adverse event which is sufficiently discomforting to interfere with normal everyday activities; 3 = An adverse event which prevents normal, everyday activities. (Such an adverse event would, for example, prevent attendance at work and would necessitate the administration of corrective therapy.)
Grading used for Fatigue: 0 = None; 1 = Increased over baseline, but not altering normal activities; 2 = Moderate (decrease in performance status by 1 ECOG level) or causing difficulty performing some activities; 3 = Severe (decrease in performance status by ≥ 2 ECOG levels) or loss of ability to perform some activities; 4 = Bedridden or disabling
Grading used for chills: 0 = None; 1 = Mild, requiring symptomatic treatment or non-narcotic medication; 2 = Severe and/or prolonged, requiring narcotic medication; 3 = Not responsive to narcotic medication
Grading used for myalgia: 0 = None; 1 = Mild pain not interfering with function; 2 = Moderate pain: pain or analgesics interfering with function, but not interfering with activities of daily living; 3 = Severe pain: severely interfering with daily life activities; 4 = Disabling
Grading used for headache: 0 = None; 1 = Mild pain not interfering with function; 2 = Moderate pain: pain or analgesics interfering with function, but not interfering with daily life activities; 3 = Severe pain: severely interfering with daily life activities; 4 = Disabling
More than a third of adverse events classified as unsolicited were other local injection site reactions not mentioned in the diary card, mainly pruritus. Table 2 presents all solicited or unsolicited adverse events with a frequency of ≥ 5% in at least one group, regardless of causal relationship to study vaccine. Only two unsolicited AEs (diarrhoea NOS and flatulence) were assessed to be possibly related to vaccine and of Grade 3 (preventing daily activities). All other Grade 3 events were assessed as not related. All patients with unsolicited adverse events recovered without sequelae within the 30-day follow-up period after vaccination with one exception, which was a subject in Group 2 that reported dental damage due to an accident that occurred three days before the end of the study period. Two non-vaccine related serious adverse events were reported during this study. One volunteer experienced a fracture of the right forearm (Grade 3, preventing normal daily activities) four days after the first vaccination. Hospitalization was not required and the subject recovered without sequelae 61 days following the event. The second serious adverse event occurred 37 days after the second vaccination in a subject hospitalized due to intermittent abdominal pain lasting 2 days and 14 hrs (Grade 3, definition see above). Both serious adverse events did not lead to premature discontinuation of the study and both subjects recovered without sequelae.
3.2. Immune responses
3.2.1. Total antibody responses to vaccinia virus measured by ELISA
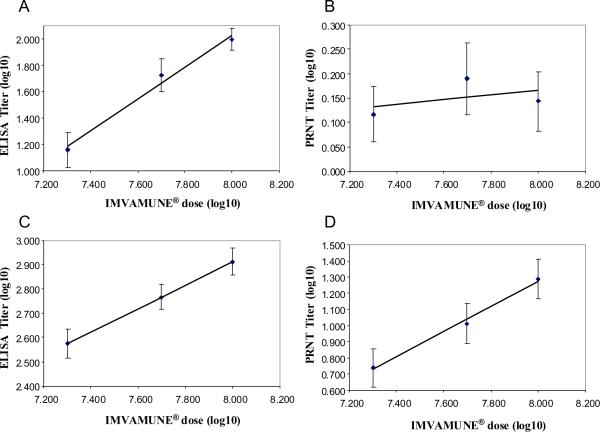
Following a single vaccination with IMVAMUNE® the majority of the subjects had seroconverted by ELISA, regardless of the vaccine dose. In the low dose group (2×107 TCID50) 59.3% of subjects had seroconverted 28 days after the first vaccination, whereas in the middle (5×107 TCID50) and high (1×10850 TCID50) dose groups 81.6% and 94.2% seroconverted, respectively (Table 3). By Day 42, two weeks post the secondary vaccination with IMVAMUNE®, all subjects in Groups 1 to 3 had seroconverted by ELISA and this level was maintained until the end of the active study phase (Day 84) in the two higher dose groups. Whereas in the lowest dose group, the antibody responses in three subjects were no longer detectable by ELISA, resulting in a seroconversion rate of 94.2% on Day 84. There was a significant linear relationship between the vaccine dose and the ELISA titres induced by IMVAMUNE® following a single (Day 28; p < 0.0001) and booster (Day 42; p < 0.0001) vaccination (Figure 2A and 2C). From an undetectable baseline titre in all subjects (given an arbitrary value of 1), the ELISA GMT increased to 14.4, 53.2 and 98.5 within the three study groups four weeks after the first IMVAMUNE® vaccination (Table 3). These responses were significantly boosted two weeks post the secondary vaccination with IMVAMUNE® with a 27-, 11- or 8-fold increase in antibody titre in the groups vaccinated with IMVAMUNE® doses of 2×107 TCID50, 5×107 TCID50 and 1×108 TCID50, respectively. Although the largest increase in antibody titre was observed in the lowest dose group, a clear dose effect was observed with the highest GMT of 813.8 being recorded in the high dose group (1×108 TCID50) compared to only a GMT of 377.2 in group 1 (2×107 TCID50). Although by Day 84, there was a 60–64% decline in the total antibody response in all groups, a clear linear relationship between vaccine dose and ELISA titre remained, with GMT of 134, 228 and 324 in the low, middle and high dose IMVAMUNE® groups, respectively.
Table 3.
ELISA and PRNT seroconversion rates and GMT (PP population, N = 155)
| ELISA | PRNT | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Day | Na | n (%)b | GMTc | 95% CI (LLe - ULf) | n (%)b | GMTc | 95% CI (LLe-ULf) |
| Group 1 2×107 TCID50 | 0 | 54 | 0 (0%) | 1.0 | NA | 0 (0%) | 1.00 | NA |
| 28 | 54 | 32 (59.3%) | 14.4 | 7.7 – 26.8 | 4 (7.4%) | 1.31 | 1.01 – 1.70 | |
| 42 | 54 | 54 (100%) | 377.2 | 288.3 – 493.5 | 23 (42.6%) | 5.51 | 3.17 – 9.59 | |
| 84 | 54 | 51 (94.4%) | 134.3 | 91.1 – 198.2 | 10 (18.5%) | 1.94 | 1.31 – 2.85 | |
| Group 2 5×107 TCID50 | 0 | 49 | 0 (0%) | 1.00 | NA | 0 (0%) | 1.00 | NA |
| 28 | 49 | 40 (81.6%) | 53.2 | 29.9 – 94.9 | 6 (12.2%) | 1.55 | 1.10 – 2.19 | |
| 42 | 49 | 49 (100%) | 583.6 | 461.6 – 737.9 | 29 (59.2%) | 10.31 | 5.78 – 18.40 | |
| 84 | 49 | 49 (100%) | 227.8 | 176.4 – 294.1 | 11 (22.4%) | 2.32 | 1.47 – 3.66 | |
| Group 3 1×108 TCID50 | 0 | 52 | 0 (0%) | 1.0 | NA | 0 (0%) | 1.00 | NA |
| 28 | 52 | 49 (94.2%) | 98.5 | 67.6 – 143.7 | 5 (9.6%) | 1.39 | 1.05 – 1.85 | |
| 42 | 52 | 52 (100%) | 813.8 | 628.7 – 1053.3 | 37 (71.2%) | 19.43 | 11.05 – 34.16 | |
| 84 | 52 | 52 (100%) | 323.6 | 246.8 – 424.3 | 15 (28.8%) | 2.94 | 1.81 – 4.76 | |
N = number of subjects with samples for antibody response; PP = per protocol
n (%) = number (percentage) of seropositive subjects (PRNT titres ≥ 20, ELISA titres ≥ 50)
GMT = Geometric Mean Titre
CI = confidence interval
LL = lower limit
UL = upper limit
Figure 2. Correlation between the dose of IMVAMUNE® and ELISA and PRNT antibody titres.
Subjects were vaccinated on Day 0 and 28 with three different doses of IMVAMUNE® (2×107, 5×107 and 1×108 TCID50) and blood was taken at various intervals and analysed for total and neutralising antibody responses to vaccinia by ELISA and PRNT respectively. The log10 of three different IMVAMUNE® doses was plotted against the log10 of the ELISA (Day 28: 1A, Day 42: 1C) and PRNT titres (Day 28: 1B, Day 42: 1D).
3.2.2. Vaccinia virus neutralising antibody responses measured by PRNT
Four weeks after the first vaccination (Day 28), the immune responses measured by PRNT were only measurable in 7–12% of all subjects without any vaccine dose response being observed (Table 3, Figure 2 B). However, after the second vaccination seroconversion rates increased to 42.6%, 59.2% and 71.2% in the low, middle and high IMVAMUNE® dose groups. On Day 42, two weeks post the secondary vaccination, a significant linear relationship between IMVAMUNE® dose and neutralising antibody titres was observed (Figure 2 D), with the highest GMT response recorded in the high dose group with a GMT of 19.43, followed by the middle (10.31) and low dose groups (5.51, Table 3). By Day 84, only a minor population still had detectable neutralising antibodies and there appeared to be no clear dose effect.
3.2.3. Cellular immune response measured by ICS
Cellular immune responses were analysed using ICS detecting vaccinia-specific IFN-γ producing CD4+ and CD8+ activated (CD69+) T cells following an overnight stimulation with vaccinia virus (Table 4). The number of subjects with a positive CD4+ response did not reveal a clear dose relationship with IMVAMUNE® post vaccination. Fourteen days after the second vaccination with IMVAMUNE®, 15.4% to 18.5% of subjects had a detectable CD3+, CD69+, CD4+ response independent of the IMVAMUNE® dose and the highest CD4+ T cell response rate was recorded in the lowest dose group (2×107 TCID50). The percentage of subjects with a CD4+ T cell response in the lowest and highest dose groups decreased at Day 84 to approximately 9%, while the middle dose group had the highest responders (16.3%), which had remained at the same level recorded 6 weeks earlier on Day 42. CD4+ responses were measured as high as 0.1% in individual subjects following vaccination with IMVAMUNE® (data not shown) and while there was no dose effect with regards to the number of subjects with a CD4+ response and the vaccine dose, a significant correlation between the magnitude of the mean CD4+ T cell response over time could be detected (p < 0.0001) in the low and high dose groups (Table 4). However, the CD4+ T cell response had already peaked with a mean response of 0.09% after the first vaccination with the middle dose of IMVAMUNE®.
Table 4.
Response rates of Vaccinia-specific IFN-γ production measured by ICS (CD4+ and CD8+ T cells) in the PP population
| CD4+ | CD8+ | |||||
|---|---|---|---|---|---|---|
| Group | Day | Na | Meanb (%) | Responders n (%)c | Meanb (%) | Responders n (%)c |
| Group 1 | 0 | 54 | 0.0543 | 4 (7.4%) | 0.0540 | 2 (3.7%) |
| 28 | 54 | 0.0744 | 5 (9.3%) | 0.1828 | 10 (18.5%) | |
| 42 | 54 | 0.0805 | 10 (18.5%) | 0.5108 | 25 (46.3%) | |
| 84 | 54 | 0.0538 | 5 (9.3%) | 0.2692 | 16 (29.6%) | |
| Group 2 | 0 | 49 | 0.0506 | 4 (8.2%) | 0.0963 | 5 (10.2%) |
| 28 | 49 | 0.0891 | 8 (16.3%) | 0.2355 | 19 (38.8%) | |
| 42 | 49 | 0.0672 | 8 (16.3%) | 0.4095 | 25 (51.0%) | |
| 84 | 49 | 0.0697 | 8 (16.3%) | 0.3693 | 22 (44.9%) | |
| Group 3 | 0 | 52 | 0.0263 | 1 (1.9%) | 0.3039 | 3 (5.8%) |
| 28 | 52 | 0.0512 | 4 (7.7%) | 0.1887 | 14 (26.9%) | |
| 42 | 52 | 0.0730 | 8 (15.4%) | 0.6525 | 27 (51.9%) | |
| 84 | 52 | 0.0729 | 5 (9.6%) | 0.3602 | 28 (53.8%) | |
N = total number of subjects analysed.
The mean percentage of all CD3+CD69+CD4+ and CD3+CD69+CD8+ responses was determined. In all cases for the calculation of the mean, n = N, except for Group 1/ Day 0 where n = 53.
Number (percentage) of subjects per group showing a positive response. An individual titre above or at the assay cut-off value of 0.14% for CD4+ and 0.25% for CD8+ T cells was counted as positive response.
As expected, the peak CD3+, CD69+, CD8+ T cell response rate to vaccinia virus was 2 to 3 times higher than those recorded for the CD4+ T cells, although again there was no clear relationship to the dose of IMVAMUNE® (Table 4). Four weeks post the primary vaccination with IMVAMUNE® 38.5% of the people vaccinated with 5 × 107 TCID50 IMVAMUNE® recorded a CD8+ T cell response, which represented the highest response rate compared to the low (18.5%) or high (26.9%) dose groups. Two weeks post the booster vaccination, a 2-fold increase in the numbers of people with a CD8+ T cell response was observed in the low and high dose groups, although the number of subjects with a positive CD8+ T cell response was comparable in all three dose groups with responder rates between 46–52%. These CD8+ response rates remained elevated 6 weeks later on Day 84 in the middle and high dose groups, although there was a more pronounced decline in responders (29.6%) in the low dose group. As with the CD4+ responses, a significant correlation between the magnitudes of the mean CD8+ T cell response over time could be detected (p < 0.0001) in all three IMVAMUNE® dose groups. The mean response peaked in all three groups two weeks post the secondary vaccination (day 42) with mean CD8+ responses of 0.5%, 0.4% and 0.7% for the low, middle and high dose groups respectively (Table 4). However, some individuals recorded CD8+ T cell responses as high as 2.2% following the booster vaccination with IMVAMUNE®, which is about 20 times higher than their maximal CD4+ response.
3.2.4 Correlation of ELISA and PRNT analyses
A correlation analysis of the total antibody responses measured by ELISA versus the neutralising antibodies measured by PRNT titres (both log10 transformed) was performed for all study visits. This revealed that the ELISA and PRNT titres were highly significantly correlated at all time points with correlation coefficients between 0.41 and 0.67 (p < 0.0001).
4. Discussion
As smallpox has been eradicated the future licensure of a new generation smallpox vaccine will have to rely, in part, on the demonstration of efficacy in animals [26]. As such, the determination of a relevant clinical dose will also have to take into consideration the efficacious dose established in suitable animal models. In this study, the highest dose of IMVAMUNE® (1×108 TCID50) was shown to be the most optimal dose evaluated for further development in terms of the timing and magnitude of the immune response and has confirmed the findings from two previous smaller Phase I studies that also evaluated various doses of IMVAMUNE®, ranging from 1×106 TCID50 to 1×108 TCID50 [23;24]. Importantly, this dose of MVA has also been shown to be optimal in various animal species (unpublished data) and is the dose that has been used to demonstrate a comparable immunogenicity and efficacy of MVA in comparison to traditional smallpox vaccines in various mouse and non-human primate challenge models [15–18].
The study has also demonstrated that all doses of IMVAMUNE® were well tolerated and confirmed the favourable safety profile demonstrated in other clinical studies evaluating this 3rd generation smallpox vaccine [23–25]. No serious adverse events related to the vaccine occurred and there was no clear correlation between the dose of IMVAMUNE® and any of the general adverse events that were recorded. The most frequently recorded symptoms were local adverse reactions following the vaccination(s), which occurred more frequently using the highest dose of IMVAMUNE®. Local reactions such as redness, swelling and pain are commonly associated with other vaccines, particularly those administered subcutaneously [27] and in this study were generally assessed by the subjects to be mild to moderate and resolved within 3 to 4 days post vaccination. Therefore, even the highest dose of IMVAMUNE® displayed an acceptable safety profile, which supported the conclusion that this dose is favourable in terms of safety and immunogenicity.
The growing amount of clinical safety data available for IMVAMUNE® strongly suggest that IMVAMUNE® has a favourable safety profile compared to 1st or 2nd generation smallpox vaccines. Indeed, a third of the subjects vaccinated in a trial with Dryvax® missed work, school, sleep or recreational activities, due to the severity of very common side effects, such as myalgia, malaise, headache, nausea and lymph node pain associated with the traditional smallpox vaccine [8;28;29]. In contrast, no subject discontinued this study due to a vaccine-related AE or suffered any significant impairment of daily activities after the first or second vaccination with IMVAMUNE®. Moreover, many of the severe side effects associated with traditional smallpox vaccines, such as auto-inoculation, progressive vaccinia, generalized vaccinia and eczema vaccinatum are all associated with the replicating nature of the various vaccinia virus strains [1]. Given that IMVAMUNE® fails to replicate in human cells [12;13], none of these side effects are expected, or have been observed in any clinical studies evaluating IMVAMUNE® [25]. The reasons for the reported high rates of myopericarditis following vaccination with 1st and 2nd generation vaccines remains unclear, but may be associated with a non-infectious pathogenesis of inflammation [30;31]. While no incidences of myopericarditis were reported in this study, cardiac events were not specifically monitored. Encouragingly, in other clinical studies evaluating IMVAMUNE®, cardiac function has been assessed by monitoring ECG and troponin I levels and no clinical abnormalities have been reported following vaccination with IMVAMUNE® [25; 32].
There was a clear dose response in terms of humoral immunity induced by IMVAMUNE® as measured by both ELISA and PRNT. Moreover, the total antibodies and neutralising antibodies were shown to be highly correlated at all time points analysed, indicating that the ELISA and PRNT assays, while measuring different aspects of humoral immunity, detected overlapping antibody responses. As has been reported in other clinical studies evaluating either IMVAMUNE® or Dryvax® [23;24], the total antibody response is higher, both in terms of the number of responders and in the magnitude of the response, compared to neutralising titres. This probably reflects that the PRNT is only measuring a proportion of the functional antibodies involved in the direct neutralization of vaccinia virus in vitro, while in contrast the ELISA quantifies all (IgG) antibodies, some of which will have other functions other than direct neutralization of the virus. These include the clearance of infected cells by complement fixation and antibody directed cellular cytotoxicity by NK cells, which have been shown to play an important role in protection from poxviruses [33;34]. Moreover, the PRNT is known to be highly variable [35] in contrast to an ELISA that is more reproducible and accurate [36], which will also contribute to the differences measured by the two assays.
Although a clear dose response was recorded for the neutralizing antibody responses, the PRNT seroconversion rates following vaccination with traditional smallpox vaccines have been reported to be at least 90% [29], which is higher than the rates reported following the booster vaccination even with the optimal dose of IMVAMUNE® in the current study. However, this appears to be more related to the PRNT assay used in the present study, because the optimal dose of IMVAMUNE® has been shown to induce comparable neutralizing antibody responses when compared directly to Dryvax® in a Phase I study, using a different PRNT assay [24]. Indeed, a prime-boost vaccination with IMVAMUNE® has been shown to be as effective, if not superior, as the standard Dryvax® vaccination regime at eliciting variola virus-neutralizing responses in the clinical [37].
The role the individual arms of the immune response play in the robust protection against poxviruses remains unclear and no single immune correlate has been established. While there are numerous reports demonstrating the important role antibodies play in protection [19;38], the protection of B cell deficient mice using MVA, suggests an important role for the cell mediated arm of the immune response [20]. Indeed, there is ample evidence both from genetically engineered and immune suppressed mice that protection from poxviruses requires both arms of the adaptive immune response for a robust protection from poxviruses [20;39]. Therefore, it was important to investigate both the humoral and cell mediated responses induced by IMVAMUNE® in the current trial. In contrast to the humoral immunity induced by IMVAMUNE®, the cell mediated response did not show a clear dose response, presumably due to the high variability of cell based biological assays. However, similar responder rates of 50 to 60% reported in the current study for IMVAMUNE® have been reported using flow cytometry following vaccination with 1st or 2nd generation smallpox vaccines [29]. Furthermore, in a direct clinical comparison to traditional smallpox vaccines, the peak antibody and cell mediated immune responses induced by IMVAMUNE® were shown to be comparable to those induced by Dryvax®, a smallpox vaccine with a known protective efficacy against smallpox [24].
An interesting result from this study was that a single vaccination with the optimal dose of IMVAMUNE® resulted in a 94% seroconversion by the first time point analysed post vaccination. This supports the earlier observation of high antibody responder rates within 2 weeks post vaccination with IMVAMUNE® [24], while peak immune responses with traditional smallpox vaccines are only observed 4 weeks post vaccination [24;28;29]. In animal models this earlier induction of immunity by MVA has been shown to translate into inducing a faster protective response compared to traditional smallpox vaccines in mice [21;22] and non-human primates [18]. Moreover, recently MVA has shown to have a superior post-exposure protection in immune suppressed animals that succumbed to the vaccinations with Dryvax® [15], providing further data supporting the efficacious properties of IMVAMUNE®.
In conclusion this study has demonstrated that IMVAMUNE® is well tolerated in healthy subjects and based on the timing and the magnitude of the immune response, coupled with the optimal efficacy in non-human primates [16], the higher dose investigated was shown to be optimal for further clinical development. The findings from this clinical study support the safety, immunogenicity and efficacy of IMVAMUNE® from previous clinical and animal studies, which indicate that IMVAMUNE® represents a good candidate as an alternative 3rd generation smallpox vaccine.
Acknowledgement
We thank Drs' Gerry Kovacs and Robert Johnson for many helpful discussions on developing IMVAMUNE® and for comments to this manuscript. The study was co-funded by Bavarian Nordic A/S and National Institute for Allergy and Infectious Diseases, National Institute for Health under contract N01-AI-30016. We wish to gratefully acknowledge the study participants that devoted their time and effort to this research endeavour.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest This clinical trial was co-financed by Bavarian Nordic A/S, Denmark and National Institute for Allergy and Infectious Diseases, National Institute for Health under contract N01-AI-30016.
References
- [1].Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. WHO. Geneva, Switzerland: 1988. Smallpox and its eradication. whqlibdoc.who.int/smallpox/9241561106.pdf. [Google Scholar]
- [2].Monath TP, Caldwell JR, Mundt W, Fusco J, Johnson CS, Buller M, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)--a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8(Suppl 2):S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med. 1969;281(22):1201–8. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- [4].Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. JAMA. 1970;212(3):441–4. [PubMed] [Google Scholar]
- [5].Grabenstein JD, Winkenwerder W., Jr. US military smallpox vaccination program experience. JAMA. 2003;289(24):3278–82. doi: 10.1001/jama.289.24.3278. [DOI] [PubMed] [Google Scholar]
- [6].Kemper AR, Davis MM, Freed GL. Expected adverse events in a mass smallpox vaccination campaign. Eff Clin Pract. 2002;(2):84–90. [PubMed] [Google Scholar]
- [7].Arness MK, Eckart RE, Love SS, Atwood JE, Wells TS, Engler RJ, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160(7):642–51. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- [8].ACAM2000 Vaccines and Related Biological Products Advisory Committee (VRBPAC) Briefing Document. 2007 April; http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4292B2-00-index.htm. 2007.
- [9].Mayr A, Hochstein-Mintzel V, Stickl H. Passage History: Abstammung, Eigenschaften und Verwendung des attenuierten Vaccina-Stammes MVA. Infection. 1975;3(1):6–14. [Google Scholar]
- [10].Mayr A, Stickl H, Muller HK, Danner K, Singer H. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author's transl)] Zentralbl Bakteriol [B] 1978;167(5–6):375–90. [PubMed] [Google Scholar]
- [11].Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, Holzner A. [MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author's transl)] Dtsch Med Wochenschr. 1974;99(47):2386–92. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- [12].Suter M, Meisinger-Henschel C, Tzatzaris M, Hülsemann V, Lukassen S, Wulff NH, et al. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of viruses that determine the biological properties of each strain. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.05.095. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [13].Jones T. IMVAMUNE, an attenuated modified vaccinia Ankara virus vaccine for smallpox infection. Curr Opin Mol Ther. 2008;10(4):407–17. [PubMed] [Google Scholar]
- [14].Meisinger-Henschel C, Schmidt M, Lukassen S, Linke B, Krause L, Konietzny S, et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J Gen Virol. 2007;88(Pt 12):3249–59. doi: 10.1099/vir.0.83156-0. [DOI] [PubMed] [Google Scholar]
- [15].Samuelsson C, Hausmann J, Lauterbach H, Schmidt M, Akira S, Wagner H, et al. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118(5):1776–84. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stittelaar KJ, van Amerongen G, Kondova I, Kuiken T, van Lavieren RF, Pistoor FH, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(12):7845–51. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(6979):182–5. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- [18].Earl PL, Americo JL, Wyatt LS, Espenshade O, Bassler J, Gong K, et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A. 2008;5;105(31):10889–94. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Law M, Putz MM, Smith GL. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J Gen Virol. 2005;86(Pt 4):991–1000. doi: 10.1099/vir.0.80660-0. [DOI] [PubMed] [Google Scholar]
- [20].Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101(13):4590–5. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Staib C, Suezer Y, Kisling S, Kalinke U, Sutter G. Short-term, but not post-exposure, protection against lethal orthopoxvirus challenge after immunization with modified vaccinia virus Ankara. J Gen Virol. 2006;87(Pt 10):2917–21. doi: 10.1099/vir.0.82068-0. [DOI] [PubMed] [Google Scholar]
- [22].Mateo L, Chaplin P. Use of a modified poxvirus for the rapid induction of immunity against a poxvirus or other infectious agents. 2006. inventors. WO 2006/089690 A1. [Google Scholar]
- [23].Vollmar J, Arndtz N, Eckl KM, Thomsen T, Petzold B, Mateo L, et al. Safety and immunogenicity of IMVAMUNE, a promising candidate as a third generation smallpox vaccine. Vaccine. 2006;24(12):2065–70. doi: 10.1016/j.vaccine.2005.11.022. [DOI] [PubMed] [Google Scholar]
- [24].Frey SE, Newman FK, Kennedy JS, Sobek V, Ennis FA, Hill H, et al. Clinical and immunologic responses to multiple doses of IMVAMUNE (Modified Vaccinia Ankara) followed by Dryvax challenge. Vaccine. 2007;25(51):8562–73. doi: 10.1016/j.vaccine.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kennedy JS, Greenberg RN. IMVAMUNE: modified vaccinia Ankara strain as an attenuated smallpox vaccine. Expert Rev Vaccines. 2009;8(1):13–24. doi: 10.1586/14760584.8.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].21 CFR Parts 314 (subpart I) and 601 (subpart H) New Drug and Biological Drug Products; Evidence Needed to Demonstrate Effectiveness of New Drugs When Human Efficacy Studies Are Not Ethical or Feasible. Food and Drug Administration, HHS; 2002. [PubMed] [Google Scholar]
- [27].Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- [28].Frey SE, Couch RB, Tacket CO, Treanor JJ, Wolff M, Newman FK, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346(17):1265–74. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- [29].Frey SE, Newman FK, Kennedy JS, Ennis F, Abate G, Hoft DF, et al. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax® in healthy vaccinia-naive adults. Vaccine. 2008;27(10):1637–44. doi: 10.1016/j.vaccine.2008.11.079. [DOI] [PubMed] [Google Scholar]
- [30].Cassimatis DC, Atwood JE, Engler RM, Linz PE, Grabenstein JD, Vernalis MN. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43(9):1503–10. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- [31].Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44(1):201–5. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [32].Sano J, Chaitman BR, Swindle J, Frey SE. Electrocardiography screening for cardiotoxicity after modified vaccinia Ankara vaccination. Am J of Med. 2009;122(1):79–84. doi: 10.1016/j.amjmed.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Benhnia MR, McCausland MM, Moyron J, Laudenslager J, Granger S, Rickert S, et al. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J Virol. 2009;83:1201–15. doi: 10.1128/JVI.01797-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Parker AK, Parker S, Yokoyama WM, Corbett JA, Buller RM. Induction of natural killer cell responses by ectromelia virus controls infection. J Virol. 2007;81:4070–9. doi: 10.1128/JVI.02061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manischewitz J, King LR, Bleckwenn NA, Shiloach J, Taffs R, Merchlinsky M, et al. Development of a novel vaccinia-neutralization assay based on reporter-gene expression. J Infect Dis. 2003;188(3):440–8. doi: 10.1086/376557. [DOI] [PubMed] [Google Scholar]
- [36].Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21(6):1249–73. doi: 10.1016/s0731-7085(99)00244-7. [DOI] [PubMed] [Google Scholar]
- [37].Damon IK, Davidson WB, Hughes CM, Olson VA, Smith SK, Holman RC, et al. Evaluation of smallpox vaccines using variola neutralization. J Gen Virol. 2009;90(Pt 8):1962–66. doi: 10.1099/vir.0.010553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- [39].Lustig S, Maik-Rachline G, Paran N, Melamed S, Israely T, Erez N, et al. Effective post-exposure protection against lethal orthopoxviruses infection by vaccinia immune globulin involves induction of adaptive immune response. Vaccine. 2009;27(11):1691–1699. doi: 10.1016/j.vaccine.2009.01.038. [DOI] [PubMed] [Google Scholar]