Abstract
We have previously shown that leaky junctions associated with dying or dividing cells are the dominant pathway for LDL transport under convective conditions, accounting for more than 90% of the transport [1]. To explore the role of apoptosis in the leaky junction pathway, TNFα and cycloheximide (TNFα/CHX) were used to induce an elevated rate of apoptosis in cultured bovine aortic endothelial cell (BAEC) monolayers and the convective fluxes of LDL and water were measured. Treatment with TNFα/CHX induced a 18.3-fold increase in apoptosis and a 4.4-fold increase in LDL permeability. Increases in apoptosis and permeability were attenuated by treatment with the caspase inhibitor Z-VAD-FMK. Water flux increased by 2.7-fold after treatment with TNFα/CHX, and this increase was not attenuated by treatment with Z-VAD-FMK. Immunostaining of the tight junction protein ZO-1 showed that TNFα/CHX treatment disrupts the tight junction in addition to inducing apoptosis. This disruption is present even when Z-VAD-FMK is used to inhibit apoptosis, and likely accounts for the increase in water flux. We found a strong correlation between the rate of apoptosis and the permeability of BAEC monolayers to LDL. These results demonstrate the potential of manipulating endothelial monolayer permeability by altering the rate of apoptosis pharmacollogicaly. This has implications for the treatment of atherosclerosis.
Keywords: LDL permeability, water flux, leaky junctions, endothelial cells, apoptosis
Introduction
Atherosclerosis is a disease of the large arteries in which fatty lesions develop within the blood vessel walls. The initiating event in atherosclerosis is the transport of lipid, specifically low density lipoprotein (LDL), into the subendothelial space [2]. Elevated permeability of vascular endothelium to LDL is characteristic of focal sites within the vasculature that are prone to the development of atherosclerosis [3]. In animal studies, lesion prone areas have been detected consistently before plaques become visible by their uptake of the protein-binding dye Evans Blue [4]. These lesion prone (blue) areas have also been shown to exhibit an enhanced permeability to albumin, LDL and cholesterol [5-7]. Despite the documented association of enhanced LDL permeability with the formation of atherosclerotic plaques, current strategies for the treatment of atherosclerosis focus on lowering LDL concentration in the blood (statins [8-10]), not controlling endothelial permeability to LDL.
There are three potential pathways for transport of macromolecules, such as LDL, across the endothelium (reviewed in Tarbell [11]): transcytosis in vesicles, paracellular transport through the breaks in the tight junction strand, and the so-called “leaky junction” pathway associated with cells undergoing mitosis or apoptosis. Weinbaum et al. [12] proposed that these leaky junctions were the primary pathway for LDL transport. Using a quantitative model, they showed that a small fraction of cells in the process of dying or dividing could account for a significant increase in permeability. Previous in vivo[13-15] studies have supported the importance of the leaky junction pathway in LDL and albumin transport across the endothelium. Lin et al. [14] examined the leakage of Evans Blue-albumin (EBA) in association with dying cells in the rat aorta using IgG as a marker for cell death. They found that while frequency of dying cells was low (0.48%) a large fraction of these (63%) were associated with EBA leakage spots, accounting for 37% of all leakage sites.
Indirect evidence supporting the importance of apoptosis in atherogenesis comes from observations that hemodynamic factors usually associated with the development of atherosclerosis, have also been shown to affect endothelial cell apoptosis. Atherosclerotic plaques tend to be localized in regions of low shear stress and disturbed flow [16]. Irregular flow conditions have been shown to increase apoptosis rates [17], while increases in shear stress have been shown to reduce the rate of endothelial cell apoptosis [18, 19].
In a recent study, we used an in vitro model consisting of bovine aortic endothelial cells (BAEC) plated onto porous, polyester filters to measure the fluxes of water, albumin and LDL through BAEC monolayers [1]. The results of that study showed that leaky junctions are the dominant transport pathway for LDL under convective conditions, when the pressure differential across the monolayer drives a physiological level of water flux. The leaky junctions accounted for more than 90% of the LDL transport. The vesicular pathway accounted for the remainder (9.1%) of the LDL transport. These results were consistent with in vivo observations that vesicles do not contribute significantly to LDL uptake in arteries [20, 21]. In the present study, the same in vitro model was used to probe the role of endothelial apoptosis in LDL transport under convective conditions and to assess the potential for reducing LDL transport by reducing the rate of apoptosis. BAEC monolayers were treated with a combination of tumor necrosis factor alpha (TNFα) and cycloheximide (CHX) to induce an elevated rate of apoptosis, and with the caspase inhibitor Z-VAD-FMK to inhibit apoptosis. LDL flux was measured using an automated fluorometer system and water flux was measured using a bubble tracker apparatus. BAEC monolayers were assayed to determine apoptosis rates as well as to determine the effects of treatment on the tight junction protein, ZO-1. We observed that the rate of LDL transport across the endothelium was proportional to the apoptosis rate.
Methods
Cell Culture
Bovine aortic endothelial cells (BAECs) were purchased from VEC Technologies, Inc. (Rensselaer, NY) and grown in minimum essential medium (MEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 1% l-glutamine and 1% penicillin-streptomycin. For transport experiments, cells were plated onto fibronectin-coated Transwell membranes (Corning, Acton, MA) at a density of 1.25 × 105 cells/cm2. Experiments were carried out on monolayers 4-6 days post-plating.
Induction, Inhibition and Measurement of Apoptosis
BAEC monolayers were incubated with tumor necrosis factor alpha (TNFα; 20ng/mL) and cycloheximide (CHX; 3μg/mL) in 10% FBS-MEM for 3.5 hrs as described in Polunovsky et al [22]. To inhibit apoptosis, some monolayers were simultaneously treated with the pan-caspase inhibitor Z-VAD-FMK (Calbiochem, La Jolla, CA) at concentrations of 50 or 100μM. The inducer was then removed, and the monolayers were allowed to recover for 20 hrs in the presence or absence of the inhibitor (recovery phase). In preliminary studies, monolayers that were not allowed to recover after the induction phase appeared disrupted after application of 10cm-H2O pressure gradient for 1hr, while control monolayers appeared intact and confluent. The recovery time allowed treated monolayers to be pressurized without disruption while maintaining an elevated rate of apoptosis. The reason that monolayers that were not allowed to recover did not hold up well under pressure was hypothesized to be an effect of TNFα on junction proteins (see Discussion below).
Apoptosis rates were determined using the Vybrant Apoptosis assay kit #2 from Molecular Probes (Eugene, OR) following manufacturer's instructions. This assay uses the high affinity binding of annexin V to phosphatidylserine to identify apoptotic cells. Propidium iodide (PI) was used to identify necrotic cells. The apoptosis rate is defined as the number of apoptotic cells in a field divided by the total number of cells in the field, and presented as a percent.
For co-staining of apoptosis and junction proteins, the In situ cell death detection kit (TMR red) from Roche (Indianapolis, IN) was used. This assay uses the TdT-mediated dUTP nick end labeling (TUNEL) technique to identify apoptotic cells. Cells were first stained for ZO-1 as indicated below, re-fixed with 4% paraformaldehyde for 1hr, permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2min, then incubated with the TUNEL reaction mixture for 1hr at 37 °C. Cells were then washed 3X with PBS and imaged under a fluorescent microscope.
Immunostaining of Junction Proteins
BAEC monolayers were grown in the same manner as for transport experiments. Monolayers were washed 2X with PBS, fixed in 1% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 in PBS for 10 min and blocked with 10% bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS for 1 hr. After washing with PBS, the monolayers were incubated with primary antibody for ZO-1 (Zymed, San Francisco, CA) for 3 hrs at room temperature or overnight at 4°C. The monolayers were then washed 5X with PBS and incubated with secondary antibody, Alexa Fluor 488 donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA), for 1 hr at room temperature. The monolayers were washed 4X in PBS before imaging.
All imaging was conducted with a Nikon Eclipse TE2000-E microscope. Images were taken with a Photometrics Cascade 650 CCD camera from Roper Scientific (Tucson, AZ).
Measurement of solute and water flux
The flux of human LDL tagged with 1,1′-dioctadecyl – 3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-LDL) (Biomedical Technologies, Stoughton, MA) was measured using an automated fluorometer system previously developed in our laboratory [23], consisting of eight transport chambers, each of which was connected to a laser excitation source and an emission detector via optical fibers arranged in a 45° configuration. Excitation light at 532 nm was provided by a 10-mW Crystal Laser. The emission fibers were connected to a Model D48 photon counting detector from C&L Instruments (Hummelstown, PA). The FluorMeasure acquisition software and Model PC-DAQ control card (both from C&L Instruments) were used to control the laser exciter and detector, and for data acquisition.
A Transwell filter containing the BAEC monolayer was sealed within each chamber to form a luminal (top) and abluminal (bottom) compartment. The abluminal compartment was connected to a fluid reservoir via tygon tubing. The reservoir could be lowered to apply a hydrostatic pressure differential across the monolayer. Both the luminal compartment and abluminal reservoir were continuously supplied with 5% CO2-95% air to maintain the medium at physiological pH. The experimental apparatus, excluding the laser source and the emission detector were housed inside a temperature-controlled Plexiglas box maintained at 37°C.
Each filter was rinsed twice with experimental medium (1% BSA in phenol red-free MEM) prior to inserting into the chambers. A solution of 5μg/mL DiI-LDL was added to the luminal compartment. Each transport experiment consisted of a one hour equilibration period, followed by application of a 10-cm H2O pressure differential (to drive convective flux) and data collection for one hour, and finally two hours of data collection under diffusive conditions (no pressure differential).
The water flux was measured simultaneously with solute flux in two of the eight chambers. These two chambers were connected via tygon tubing and borosilicate glass tubing to an abluminal reservoir. When a 10-cm H2O differential pressure was applied, the water flux (Jv) was measured by tracking the position of an air bubble that was inserted into the glass tubing. The bubble was monitored using a spectrophotometer which followed the air-liquid interface in the glass tube.
Calculation of Permeability (Pe or Po) and water flux (Jv) values
The fluorescence intensity in the abluminal compartment was recorded for 10 seconds of every minute for the duration of the experiment. The intensity was converted to concentration by means of a calibration curve, and the permeability was calculated by
where Pe (Po) is the convective (diffusive) permeability, ΔCa/Δt is the change in abluminal concentration with respect to time, Va is the fluid volume in the abluminal compartment, Cl is the concentration in the luminal compartment, and A is the area of the filter.
The bubble displacement data was used to compute Jv values using the following equation
where Δd/Δt is the bubble displacement per unit time, and F is a tube calibration factor (fluid volume per unit length of tubing).
Statistical Analysis
Permeability and water flux values were normalized with respect to control and presented as mean ± SEM. Data sets were analyzed for statistical significance by a Student's t-test and p<0.05 was considered statistically significant. Where multiple comparisons were made, the Bonferroni correction was used. The Bonferroni correction gives a conservative significance level of p/m, where m is the number of comparisons. For example, when comparing four groups p<0.05 is replaced with p<0.05/6 or 0.008.
Results
Effect of TNFα/CHX and Z-VAD-FMK on apoptosis rate and LDL permeability
BAEC monolayers were incubated with the apoptosis inducers TNFα/CHX for 3.5 hrs in the presence or absence of the inhibitor Z-VAD-FMK then allowed to recover for 20 hrs without the inducer as described in the Methods. The baseline apoptosis rate was 0.3% ± 0.036%. TNFα/CHX-treated monolayers had an apoptosis rate of 5.9% ± 0.356%, an 18.3-fold increase over baseline. While the inhibitor had no effect on the baseline rate of apoptosis (p>0.6 vs control, data not shown), it did inhibit TNFα/CHX-induced apoptosis in a dose-dependent manner. When the cells were treated with 50μM and 100μM Z-VAD-FMK, the apoptosis rate was increased by 9.2-fold and 5.6-fold, respectively (Figure 1A). Necrotic cells were occasionally found, usually in an annular area midway between the center and the edge of a filter. However, the number and pattern of necrotic cells were not affected by treatment with TNFα/CHX or with the caspase inhibitor. Instead, we found that careful handling of the filter during the apoptosis assay eliminated necrotic cells.
Figure 1.
Effect of TNFα/CHX and Z-VAD-FMK on (A) apoptosis rate and (B) convective LDL permeability (Pe). BAEC monolayers were treated for 3.5hrs followed by a 20-hr recovery period. Control monolayers had an average Pe of 2.07 × 10−6 ± 3.18 × 10−7 cm/s. Mean ± SEM shown. *p<0.008 vs control, **p<0.008 vs 50μM Z-VAD-FMK, +p<0.008 vs TNFα/CHX.
The diffusive permeability (Po) of control monolayers was 2.6 × 10−7 ± 3.7 × 10−8 cm/s, similar to our previous measurements [1]. There was no significant difference between the Po of control and treated monolayers (data not shown). Figure 1B shows the effect of TNFα/CHX and Z-VAD-FMK on the convective permeability (Pe) of BAEC monolayers to LDL. Control monolayers had a baseline Pe of 2.07 × 10−6 ± 3.18 × 10−7 cm/s. Treatment with TNFα/CHX alone increased the permeability by 4.4-fold. Addition of Z-VAD-FMK attenuated the increase in permeability to 3.5-fold when 50μM Z-VAD-FMK was added, and to 2.2-fold when 100μM Z-VAD-FMK was added.
BAEC monolayers were incubated with CHX alone to control for the effects of protein inhibition on apoptosis and LDL permeability. At the concentration and duration used in this study, CHX had no significant effect on apoptosis rate (0.21% ± 0.029%, p>0.048 vs. control) or on LDL permeability (1.47 × 10−6 ± 1.69 × 10−7 cm/s, p>0.012 vs. control).
Effect of TNFα/CHX and Z-VAD-FMK on water flux (Jv)
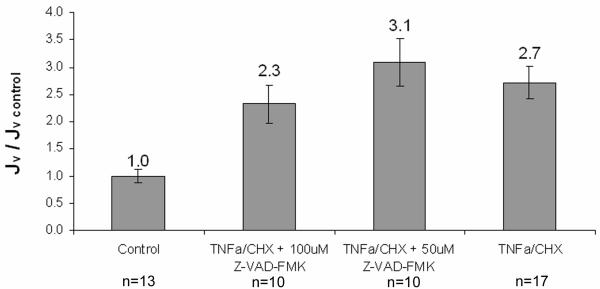
The water flux of treated monolayers was measured to assess the effect of TNFα/CHX treatment on paracellular transport through the breaks in the tight junction strand, the main pathway for water transport[1]. Control monolayers had a Jv of 5.05 × 10−6 ± 4.41 × 10−7 cm/s. Figure 2 shows that treatment with TNFα/CHX increased Jv by a factor of 2.7. Treatment with the caspase inhibitor Z-VAD-FMK did not significantly attenuate the increase in Jv at any concentration. Treatment with CHX alone had no significant effect on the Jv (3.53 × 10−6 + 2.06 × 10−7 cm/s, p>0.010 vs. control).
Figure 2.
Effect of TNFα/CHX and Z-VAD-FMK on water flux (Jv). BAEC monolayers were treated for 3.5hrs followed by a 20-hr recovery period. Control monolayers had an average Jv of 4.78 × 10−6 ± 3.86 × 10−7 cm/s. Mean ± SEM shown. All conditions are statistically different (p<0.008) from control, but not from each other.
Effect of TNFα/CHX and Z-VAD-FMK on tight junction proteins
The results of water flux measurements suggest that TNFα/CHX may be affecting the paracellular pathway through the breaks in the tight junction strand in ways unrelated to the induction of apoptosis. Since previous studies [24-26] have shown that TNFα disrupts the junctional complex of endothelial cells independent of its apoptotic effects, we performed immunostaining of the tight junction protein ZO-1 in order to assess junctional integrity under the treatment conditions of this study.
BAEC monolayers were incubated with TNFα/CHX in the presence or absence of ZVAD-FMK for 3.5 hrs then allowed to recover for 20 hrs in the presence or absence of Z-VAD-FMK. The monolayers were then exposed to a 10-cm H2O pressure gradient for 1 hr, as in the transport experiments, before being stained for ZO-1 as indicated in the methods. Figure 3 shows representative photomicrographs of ZO-1 immunostating.
Figure 3.
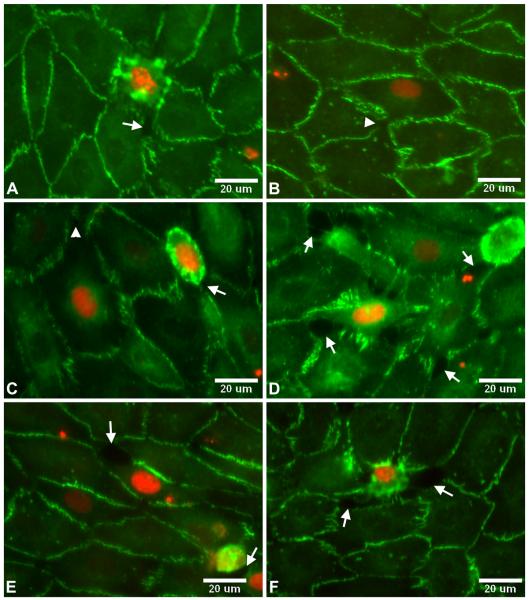
ZO-1 immunostaining of BAEC monolayers after treatment with TNFα/CHX in the presence or absence of the caspase inhibitor Z-VAD-FMK for 3.5hrs followed by a 20-hr recovery period. A) control. B) TNFα/CHX. C) TNFα/CHX + 100μM Z-VAD-FMK. Arrows denote detaching cells.
Immunoreactivity in treated and untreated monolayers is concentrated at the cell-cell border, with negligible cytoplasmic staining. Control monolayers show a broad, continuous band of ZO-1 staining around the perimeter of each cell (Figure 3A). The staining appears predominantly in spikes that are oriented perpendicular to the perimeter of the cell. Monolayers treated with TNFα/CHX show a narrower and weaker band of ZO-1 around the perimeter with numerous discontinuitites (Figure 3B). Monolayers treated with TNFα/CHX + 100μM Z-VAD-FMK also show a narrow and discontinuous band of staining around the perimeter (Figure 3C). Several cells appear to be detaching from the treated monolayers (arrows in Figures 3B and 3C). Gaps between cells are visible around or near the detaching cells. These gaps likely account for the increased permeability of treated monolayers.
BAEC monolayers immunostained for ZO-1 were co-stained for apoptosis using the TUNEL technique as described in the methods to assess whether detaching cells were apoptotic. Representative photomicrographs from control and treated monolayers are shown in Figure 4. The nucleus in TUNEL positive (apoptotic) cells stained red as the TMR-labelled dUTP incorporated into damaged DNA. Apoptotic cells were found in all monolayers. In most cases, the apoptotic cell was found to be balling up and detaching from the monolayer, creating gaps between it and its neigboring cells (arrows in Figures 4A, C, D, E and F). In other cases, the apoptotic cell was still attached and spread with small gaps beginning to form (arrowheads in Figures 4B and 4C). There was no distinguishable difference in the TUNEL staining between control and treated monolayers, aside from the frequency of TUNEL postitive cells found.
Figure 4.
Co-localization of endothelial cell detachment and gap formation with apoptosis. BAEC monolayers were immunostained for ZO-1 (green) and apoptotic cells were identified using the TUNEL method (red). A) and B) control. C) and D) TNFα/CHX. E) and F) TNFα/CHX + 100μM Z-VAD-FMK. Arrows denote gaps around apoptotic cells that are balling up and detaching from the monolayer. Arrowheads denote small gaps starting to form around apoptotic cells that are still attached and spread.
Discussion
The aims of this study were to test the hypothesis that apoptosis rates dictate, in part, the permeability of endothelial monolayers to LDL under physiologically relevant (convective) conditions, and to demonstrate that inhibition of apoptosis results in a reduction of LDL permeability. We were able to show this by inducing an elevated rate of apoptosis with TNFα/CHX and then bringing the rate back down with the caspase inhibitor Z-VAD-FMK. TNFα is a proinflammatory ligand which has been shown to induce apoptosis [22] and endothelial barrier dysfunction [24, 25] in vascular endothelial cells. Cycloheximide (CHX) is an inhibitor of protein synthesis which can either potentiate or attenuate TNFα induced apoptosis, depending on the timing of exposure in relation to TNFα treatment. Polunovsky et al. [22] studied the kinetics and synergy of apoptosis induction by TNFα/CHX in bovine aortic endothelial cells in vitro. They found that CHX could potentiate TNFα-induced apoptosis if it was added either alongside or after the addition of TNFα, but not if it was added before TNFα. On our BAEC monolayers TNFα/CHX induced between 2 and 5 times more apoptosis than TNFα alone at the same concentration and duration (data not shown). The rate of apoptosis induced by TNFα/CHX in Polunovsky et al. was five times higher than in the present study (31% vs 5.9%), but it should be noted that the cells in that study were in the log phase of growth when treated, whereas our cells were confluent monolayers with well developed junctions and low, stable growth rates. Previous studies have shown that junctional adhesive proteins transfer intercellular signals that negatively modulate cell proliferation and sensitivity to apoptotic stimuli [27, 28].
The application of a 10cm-H2O hydrostatic pressure gradient necessitated a 20-hr recovery period in which TNα/CHX was removed from the media. Monolayers that were not allowed to recover did not hold up well to the pressure gradient, appearing disrupted after its application (not shown). Monolayers that were allowed to recover for 20 hours remained intact and confluent after application of the pressure gradient (Figures 3 and 4). The apoptosis rate remained elevated after the recovery period on cells treated with the apoptosis inducers (Figure 1A). The recovery period is likely to be allowing cells to reestablish their junctions enough to withstand the hydrostatic pressure gradient.
TNFα/CHX-treated monolayers were immunostained for the tight junction protein ZO-1. Despite the 20-hr recovery period, a decreased immunoreactivity is clear for treated monolayers (Figures 3B and 3C). In addition to inducing apoptosis, TNFα has been shown to disrupt the junctional complex in epithelial [29, 30] and endothelial [24-26] cells. McKenzie and Ridley [24] showed that TNFα induced a fragmented distribution of ZO-1, loss of occludin from the cell junctions and down-regulation of occludin levels in HUVECs after 24 hr treatment with TNFα. These morphological changes occurred on the same timescale as an increase in permeability of 42 kDa dextran. While apoptosis levels were not measured in McKenzie and Ridley, their results suggest that the TNFα–induced increase in 42 kDa dextran permeability was partly due to the loss of tight junction proteins [24].
CHX could also be altering the endothelial cell junction by its inhibition of protein synthesis. We measured the apoptosis, LDL permeability and water flux of BAEC monolayers treated with CHX alone at the same concentration and duration used when the cells were treated with the combination of TNFα and CHX. We found no effect of CHX on apoptosis, LDL permeability or water flux. The fact that CHX did not increase water flux suggests that it also has no effect on ZO-1 distribution, since water transport occurs primarily though the breaks in the tight junction strand [1]. Therefore, it is likely that the reduction in ZO-1 is due to the effects of TNFα that have been previously described.
The main limitations of this study are: 1) it was performed in vitro and 2) shear stress was not applied. Endothelial cells in vivo are constantly exposed to mechanical forces as a result of the blood flow. These forces have been shown to affect endothelial function[31, 32]. Physiological levels of steady shear have been shown to have several atheroprotective effects on the endothelium, some directly affecting its permeability, while disturbed flow seems to have an atherogenic effect on the endothelium (see [33] for a review). Steady shear reduces cell proliferation [34] and apoptosis [18, 19], both of which would reduce permeability through the leaky junction pathway. In contrast, disturbed flow such as that occurring in branch points and bifurcations in arteries increases cell turnover (mitosis[34] and apoptosis[17]) and activates sterol regulatory element binding proteins (SREBPs), a family of transcription factors involved in receptor-mediated LDL uptake and metabolism [35]. Liu et al. showed that activation of SREBPs by shear led to a nearly 2-fold increase in 125I-LDL binding to BAECs in vitro. While increased LDL binding through the LDL receptor could potentially increase transendothelial transport, several in vivo studies [20, 21] as well as our previous in vitro study [1] have shown that vesicles contribute only a small fraction of the total transport of LDL under convective conditions. Finally, disturbed flow could induce conformational changes that may alter the cell-cell junctions, thus affecting permeability [33]. BAECs exposed to disturbed flow had discontinuous VE-cadherin staining, indicating the presence of gaps in the cell-cell junction [36].
The apoptosis levels induced in the present study are similar to those observed around human atherosclerotic plaques by Tricot et al.[37] This group measured endothelial cell apoptosis upstream and downstream of the plaque stenosis and found a preferential occurrence of apoptosis in the downstream part of the plaque, where shear levels are low. The apoptosis rate was 2.7% in the upstream part of the plaque, and 18.8% in the downstream part. Our maximum apoptosis value was 5.9%.
The decrease in apoptosis obtained with Z-VAD-FMK treatment had no significant effect on water flux (Figure 2). This observation can be explained by the fact that water transport occurs primarily through the breaks in the tight junction strand and not through leaky junctions[1]. As shown in Figure 3C, the discontinuities in ZO-1 staining persist, like the increase in Jv, even after treatment with Z-VAD-FMK. Therefore, it seems that the increase in water flux caused by TNFα/CHX treatment is primarily the result of an overall increase in the area of the breaks (as evidenced by discontinuous ZO-1 staining) rather than an increase in individual leaky junctions resulting from increased apoptosis.
On the other hand, it is unlikely that LDL (22nm diameter) would pass through an altered endothelial junction with discontinuities in the tight junction strand because the widest part of the junction is expected to be about 20nm [38]. Most of the cell-cell junction, i.e. both tight and adherens junctions need to be disrupted to create a large pore that would allow the passage of LDL molecules. This kind of disruption is not evident in our treated monolayers except in places where a cell appears to be detaching from the layer (arrows in Figures 3B and 3C). These cells are likely to be apoptotic as they exhibit the same morphology as cells identified as apoptotic using the annexin V assay. To confirm this, we co-stained monolayers for ZO-1 and TUNEL. The results (Figure 4) show gaps forming around or near TUNEL positive (apoptotic) cells as they detach from their neighboring cells.
We conclude that the increase in LDL permeability in response to TNFα/CHX is due to leaky junctions created by individual apoptotic cells, and not to a global effect of TNFα on the junctions. We are, therefore, able to show a correlation between apoptosis and LDL permeability in Figure 5 (obtained by cross-plotting the data in Figures 1A and 1B). The Pearson Product Moment Correlation coefficient was calculated to be 0.72, indicating a strong correlation between LDL permeability and apoptosis. These results demonstrate the potential to manipulate endothelial LDL permeability by altering the rate of apoptosis. Because the flux of LDL into the arterial wall (J) is related to its concentration in the blood (Cl) and the endothelial permeability (Pe) by the simple relationship
it is clear, for example, that reducing Pe by 50% (by reducing the apoptosis rate by 50% - Figure 4) would reduce the flux, J, as effectively as reducing Cl by 50%. This has obvious implications in the treatment of atherosclerosis.
Figure 5.
Correlation between the normalized LDL permeability and apoptosis rate. The Pearson Product Moment Correlation coefficient was calculated to be 0.72.
Acknowledgements
This study was supported by NIH Grant HL 57093.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancel LM, Fitting A, Tarbell JM. In vitro study of LDL transport under pressurized (convective) conditions. Am J Physiol Heart Circ Physiol. 2007;293:H126–132. doi: 10.1152/ajpheart.01188.2006. [DOI] [PubMed] [Google Scholar]
- 2.Schwenke DC, Carew TE. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. I. Focal increases in arterial LDL concentration precede development of fatty streak lesions. Arteriosclerosis. 1989;9:895–907. doi: 10.1161/01.atv.9.6.895. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen LB, Nordestgaard BG, Stender S, et al. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits. Arterioscler Thromb. 1992;12:1402–1409. doi: 10.1161/01.atv.12.12.1402. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz CJ, Kelley JL, Nerem RM, et al. Pathophysiology of the atherogenic process. Am J Cardiol. 1989;64:23G–30G. doi: 10.1016/0002-9149(89)90952-1. [DOI] [PubMed] [Google Scholar]
- 5.Somer JB, Schwartz CJ. Focal 3 H-cholesterol uptake in the pig aorta. Atherosclerosis. 1971;13:293–304. doi: 10.1016/0021-9150(71)90073-6. [DOI] [PubMed] [Google Scholar]
- 6.Bell FP, Somer JB, Craig IH, et al. Patterns of aortic Evans blue uptake in vivo and in vitro. Atherosclerosis. 1972;16:369–375. doi: 10.1016/0021-9150(72)90084-6. [DOI] [PubMed] [Google Scholar]
- 7.Fry DL, Herderick EE, Johnson DK. Local intimal-medial uptakes of 125I-albumin, 125ILDL, and parenteral Evans blue dye protein complex along the aortas of normocholesterolemic minipigs as predictors of subsequent hypercholesterolemic atherogenesis. Arterioscler Thromb. 1993;13:1193–1204. doi: 10.1161/01.atv.13.8.1193. [DOI] [PubMed] [Google Scholar]
- 8.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 9.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 11.Tarbell JM. Mass transport in arteries and the localization of atherosclerosis. Annu Rev Biomed Eng. 2003;5:79–118. doi: 10.1146/annurev.bioeng.5.040202.121529. [DOI] [PubMed] [Google Scholar]
- 12.Weinbaum S, Tzeghai G, Ganatos P, et al. Effect of Cell Turnover and Leaky Junctions on Arterial Macromolecular Transport. American Journal of Physiology. 1985;248:H945–H958. doi: 10.1152/ajpheart.1985.248.6.H945. [DOI] [PubMed] [Google Scholar]
- 13.Chien S, Lin SJ, Weinbaum S, et al. The role of arterial endothelial cell mitosis in macromolecular permeability. Adv Exp Med Biol. 1988;242:59–73. doi: 10.1007/978-1-4684-8935-4_8. [DOI] [PubMed] [Google Scholar]
- 14.Lin S-J, Jan K-m, Chien S. Role of Dying Endothelial Cells in Transendothelial Macromolecular Transport. Arteriosclerosis. 1990;10:703–709. doi: 10.1161/01.atv.10.5.703. [DOI] [PubMed] [Google Scholar]
- 15.Lin S-J, Jan K-M, Schuessler G, et al. Enhanced Macromolecular Permeability of Aortic Endothelial Cells in Association with Mitosis. Atherosclerosis. 1988;73:223–232. doi: 10.1016/0021-9150(88)90045-7. [DOI] [PubMed] [Google Scholar]
- 16.Frangos SG, Gahtan V, Sumpio B. Localization of atherosclerosis: role of hemodynamics. Arch Surg. 1999;134:1142–1149. doi: 10.1001/archsurg.134.10.1142. [DOI] [PubMed] [Google Scholar]
- 17.Freyberg MA, Kaiser D, Graf R, et al. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun. 2001;286:141–149. doi: 10.1006/bbrc.2001.5314. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Haendeler J, Rippmann V, et al. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399:71–74. doi: 10.1016/s0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- 19.Cho A, Mitchell L, Koopmans D, et al. Effects of changes in blood flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ Res. 1997;81:328–337. doi: 10.1161/01.res.81.3.328. [DOI] [PubMed] [Google Scholar]
- 20.Wiklund O, Carew TE, Steinberg D. Role of the low density lipoprotein receptor in penetration of low density lipoprotein into rabbit aortic wall. Arteriosclerosis. 1985;5:135–141. doi: 10.1161/01.atv.5.2.135. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren BI, Carlsson O, Venturoli D, et al. Transvascular passage of macromolecules into the peritoneal cavity of normo- and hypothermic rats in vivo: active or passive transport? J Vasc Res. 2004;41:123–130. doi: 10.1159/000077131. [DOI] [PubMed] [Google Scholar]
- 22.Polunovsky VA, Wendt CH, Ingbar DH, et al. Induction of Endothelial Cell Apoptosis by TNFα: Modulation by Inhibitors of Protein Synthesis. Experimental Cell Research. 1994;214:584–594. doi: 10.1006/excr.1994.1296. [DOI] [PubMed] [Google Scholar]
- 23.Antonetti DA, Wolpert EB, DeMaio L, et al. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677. doi: 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 25.Petrache I, Birukova A, Ramirez SI, et al. The Role of the Microtubules in Tumor Necrosis Factor-α-Induced Endothelial Cell Permeability. American Journal of Respiratory Cell and Molecular Biology. 2003;28:574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 26.Wojciak-Stothard B, Entwistle A, Garg R, et al. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 27.Spagnuolo R, Corada M, Orsenigo F, et al. Gas1 is induced by VE-cadherin and vascular endothelial growth factor and inhibits endothelial cell apoptosis. Blood. 2004;103:3005–3012. doi: 10.1182/blood-2003-07-2459. [DOI] [PubMed] [Google Scholar]
- 28.Bazzoni G, Dejana E, Lampugnani MG. Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol. 1999;11:573–581. doi: 10.1016/s0955-0674(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 29.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory Cytokines Disrupt Epithelial Barrier Function by Apoptosis-Independent Mechanisms. The Journal of Immunology. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 30.Gitter AH, Bendfeldt K, Schulzke J-D, et al. Leaks in the epithelial barrier caused by spontaneous and TNF-α-induced single-cell apoptosis. The FASEB Journal. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 31.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30:430–446. doi: 10.1114/1.1467924. [DOI] [PubMed] [Google Scholar]
- 33.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83:131–151. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Chen BP, Lu M, et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler Thromb Vasc Biol. 2002;22:76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 36.Miao H, Hu YL, Shiu YT, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 37.Tricot O, Mallat Z, Heymes C, et al. Relation Between Endothelial Cell Apoptosis and Blood Flow Direction in Human Atherosclerotic Plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 38.Adamson RH, Michel CC. Pathways through the intercellular clefts of frog mesenteric capillaries. J Physiol. 1993;466:303–327. [PMC free article] [PubMed] [Google Scholar]