Abstract
Hepatitis E virus (HEV) is a small, non-enveloped, single-strand, positive-sense RNA virus of approximately 7.2 kb in size. HEV is classified in the family Hepeviridae consisting of four recognized major genotypes that infect humans and other animals. Genotypes 1 and 2 HEV are restricted to humans and often associated with large outbreaks and epidemics in developing countries with poor sanitation conditions, whereas genotypes 3 and 4 HEV infect humans, pigs and other animal species and are responsible for sporadic cases of hepatitis E in both developing and industrialized countries. The avian HEV associated with Hepatitis-Splenomegaly syndrome in chickens is genetically and antigenically related to mammalian HEV, and may represent a new genus in the family. There exist three open reading frames in HEV genome: ORF1 encodes non-structural proteins, ORF2 encodes the capsid protein, and the ORF3 encodes a small phosphoprotein. ORF2 and ORF3 are translated from a single bicistronic mRNA, and overlap each other but neither overlaps ORF1. Due to the lack of an efficient cell culture system and a practical animal model for HEV, the mechanisms of HEV replication and pathogenesis are poorly understood. The recent identification and characterization of animal strains of HEV from pigs and chickens and the demonstrated ability of cross-species infection by these animal strains raise potential public health concerns for zoonotic HEV transmission. It has been shown that the genotypes 3 and 4 HEV strains from pigs can infect humans, and vice versa. Accumulating evidence indicated that hepatitis E is a zoonotic disease, and swine and perhaps other animal species are reservoirs for HEV. A vaccine against HEV is not yet available.
1. Introduction
Hepatitis E accounts for a significant proportion of enterically transmitted form of viral hepatitis in humans (Purcell and Emerson, 2001). The disease is an important public health problem in many developing countries of Asia and Africa, and is also endemic in many industrialized countries including the United States and several European countries (Meng, 2008). Although the overall mortality of hepatitis E is less than 1% in the general population, it can reach up to 28% in infected pregnant women (Purcell and Emerson, 2001). The causative agent, hepatitis E virus (HEV), is transmitted primarily via the fecal-oral route through contaminated water or food. In industrialized countries, acute cases of hepatitis E were reported in travelers returning from endemic regions although sporadic cases have also been reported in patients with no known epidemiological risk factors (Clemente-Casares et al., 2003). In developing countries with poor sanitation conditions, rare outbreaks of acute hepatitis E in more explosive epidemic form are generally associated with fecal contamination of drinking water (Arankalle et al., 1995; Purcell and Emerson, 2001).
The recent discoveries of animal strains of HEV, swine hepatitis E virus (swine HEV) from pigs in 1997 (Meng et al., 1997) and avian hepatitis E virus (avian HEV) from chickens in 2001 (Haqshenas et al., 2001), and the existence of other animal species that are seropositive for IgG anti-HEV (Meng, 2006; Meng and Halbur, 2006; Meng et al., 2008), have broadened the host ranges and diversity of the virus. Accumulating evidences indicate that hepatitis E is a zoonotic disease, and domestic pigs, wild boars and maybe other animal species are reservoirs for HEV. The ubiquitous nature of the virus in domestic pigs and wild boars as well as in other animal species raises public health concern for zoonosis and food safety.
2. History
2.1. Human HEV
In 1980, seroepidemiological studies of waterborne epidemics of hepatitis originally thought to be caused by hepatitis A virus (HAV) in India revealed that the patients were negative for antibodies to HAV, thus providing evidence for the existence of a new form of enterically-transmitted viral hepatitis which was later designated as hepatitis E (Purcell and Emerson, 2001). Successful transmission of viral hepatitis E to a human volunteer via fecal-oral route with a stool sample collected from a hepatitis E patient was demonstrated in 1983, and virus-like particles were detected in the stool of the infected volunteer (Balayan et al., 1983). However, the causative agent, hepatitis E virus (HEV), was not identified until 1990 when its complete genome was cloned and sequenced (Tam et al., 1991). Thus far HEV still could not be efficiently propagated in cell culture system, and this has greatly hindered our understanding of its biology and pathogenesis.
2.2. Swine HEV
Balayan et al (1990) reported an experimental infection of domestic swine with an Asian strain of human HEV, and thus providing the first evidence of HEV infections in pigs. However, a retrospective study revealed that the virus infecting the pigs in that study was not a human HEV but rather a strain of swine origin (Lu et al., 2004). Subsequently, two independent laboratories in the United States failed to reproduce HEV infections in pigs using a well-characterized genotype 1 Asian strain (Sar-55) and a genotype 2 Mexican strain (Mex-14) of human HEV (Meng, 2003). Detections of HEV antibodies and RNA were also reported from pigs in Nepal in 1995, although the identity of the virus infecting the Nepalese pigs was not known (Clayson et al., 1995). In 1997, the first animal strain of HEV, swine HEV, was identified and characterized from pigs in the United States (Meng et al., 1997). The authors serendipitously found out that the majority of adult pigs in the United States were positive for IgG anti-HEV, suggesting that the pigs were exposed to a HEV-related agent. To identify the agent responsible for the seropositivity in pigs, a prospective study was conducted in a commercial swine farm in Illinois (Meng et al., 1997). Twenty piglets born to both IgG anti-HEV seronegative and seropositive sows in a swine farm were monitored for more than 5 months for evidence of HEV infection. By 21 weeks of age, 16 of the 20 piglets monitored in this prospective study had seroconverted to IgG anti-HEV, and subsequently a novel virus genetically and antigenically closely related to human HEV, designated swine HEV, was molecularly cloned and characterized from the naturally infected piglets. Koch's postulates were fulfilled as specific-pathogen-free (SPF) pigs were experimentally infected with swine HEV, and the same virus was recovered from experimentally-infected pigs (Meng et al., 1998).
2.3. Avian HEV
Payne et al (1999) reported a HEV-related virus associated with big liver and spleen disease (BLS) in chickens from Australia. The BLS disease affects commercial broiler breeder flocks and causes decreased egg production and slight increase in mortality. Based upon the sequence of a 523 bp fragment of the BLS virus (BLSV), it was found that BLSV shared approximately 62% nucleotide sequence identity with human HEV (Payne et al., 1999). In the United States and Canada, a disease known as Hepatitis-Splenomegaly (HS) Syndrome with no known cause was also reported. Bacteria could not be routinely isolated from affected livers, and toxins or bacterins were ruled out as the cause of the HS syndrome. Haqshenas et al (2001) first isolated and characterized a virus genetically and antigenically related to human HEV from bile samples of chickens with HS Syndrome in the United States. Based upon the similar genomic organization and significant sequence identities with human and swine HEVs, the novel virus in chickens is designated as avian HEV to distinguish it from the mammalian HEV. Avian HEV shared approximately 80% nucleotide sequence identity with the Australian BLSV (Haqshenas et al., 2001), suggesting that BLS in Australia and HS syndrome in North America are likely caused by variant strains of the same virus.
3. The Infectious Agent
3.1. Virion properties
HEV is an icosahedral, non-enveloped, spherical virus particle with a diameter of approximately 32-34 nm in size. The open reading frame 2 (ORF2) encodes the only known structural protein, the capsid protein (Purcell and Emerson, 2001). It has been demonstrated that a N-terminally-truncated capsid protein containing amino acid residues 112 to 660 can self-assemble into empty virus-like particles when expressed in baculovirus (Li et al., 2005a). The buoyant density of HEV virions is reportedly 1.35 to 1.40 g/cm3 in CsCL, and 1.29 g/cm3 in potassium tartrate and glycerol. HEV virion is sensitive to low-temperature storage and iodinated disinfectants (Purcell and Emerson, 2001). Recently, Emerson et al (2005) showed that HEV virion is more heat labile than is HAV: HAV was only 50% inactivated at 60°C for 1 hr but was almost totally inactivated at 66°C; in contrast, HEV was about 50% inactivated at 56°C and almost totally inactivated (96%) at 60°C. It has been demonstrated that the in vivo infectivity of HEV present in commercial pig livers is completely inactivated by adequate cooking such as frying or boiling the contaminated pig livers for 5 minutes, however incubation of the HEV-contaminated pig liver homogenates at 56C for 1 hour did not abolish the virus infectivity (Feagins et al., 2008a). It is believed that HEV is resistant to inactivation by acidic and mild alkaline conditions in the intestinal tract, and thus facilitating the fecal-oral route of transmission (Purcell and Emerson, 2001).
3.2. Classification
Based upon its superficial similarity in morphology and genomic organization to that of caliciviruses, HEV was originally classified in the family Caliciviridae (Purcell and Emerson, 2001). However, subsequent studies showed that HEV does not share significant sequence homology or codon usage with caliciviruses. For example, at the 5' end of the viral genome, HEV has a Cap structure (Kabrane-Lazizi et al., 1999a) whereas caliciviruses contains a VPg. Therefore, HEV was officially declassified from the family Caliciviridae, and was placed in a new genus Hepevirus of the proposed family Hepeviridae (Emerson et al., 2004). Currently there are 4 recognized major genotypes of HEV within the genus Hepevirus (Meng, 2008; Okamoto, 2007): genotype 1 (primarily Burmese-like Asian strains of human HEV), genotype 2 (a Mexican strain of human HEV and recent African strains of human HEV) (Nicand et al., 2005), genotype 3 (human HEV strains from sporadic cases in industrialized countries, and swine HEV strains from pigs in both developing and industrialized countries), and genotype 4 (variant strains of human HEV from sporadic cases in Asia, and swine HEV strains from pigs in both developing and industrialized countries). The avian HEV from chickens is genetically very divergent from mammalian HEV strains sharing only approximately 50% nucleotide sequence identity, and thus avian HEV likely represents a yet-to-be-named separate genus within the family Hepeviridae (Meng et al., 2008). Despite the extensive nucleotide sequence variations between mammalian HEV and avian HEV, however, it appears that there exists a single serotype (Guo et al., 2006; Meng, 2006).
3.3. Genome organization
The genome of mammalian HEV is a single-stranded, positive-sense, RNA molecule of approximately 7.2 kb consisting of a short 5' noncoding region (NCR), three ORFs (ORFs 1, 2 and 3), and a 3' NCR (Tam et al., 1991). ORF2 overlaps ORF3, but neither overlaps with ORF1. The ORF1 at the 5' end encodes non-structural polyproteins. Based upon computer predictions and analogy with other positive-strand RNA viruses, numerous functional domains including methyltransferases, papain-like cystein proteases, helicases and RNA-dependent RNA polymerases were identified in the ORF1 (Huang et al., 2007). The ORF2 encodes the capsid protein that contains immunogenic epitopes, induces neutralizing antibodies, and is the target for vaccine development (Meng, 2008). The ORF3 encodes a small cytoskeleton-associated phosphoprotein with unknown function (Zafrullah et al., 1997). The N-terminus of ORF3 has a cysteine-rich region, binds to HEV RNA, and enters into a complex with the capsid protein. The C-terminal region of the ORF3 protein is a multifunctional domain, and may be involved in HEV virion morphogenesis and viral pathogenesis. Monoclonal antibodies raised against the ORF3 protein can capture HEV particles in culture supernatant and serum but not those in feces suggesting that the ORF3 protein is present on the surface of HEV virions newly-released from infected cells (Takahashi et al., 2008). ORF3 antigen has been used in a serological assay to detect HEV antibodies (Herremans et al., 2007).
Avian HEV is morphologically and genetically related to the mammalian HEV. The genome of avian HEV is only about 6.6 kb in length, which is approximately 600 bp shorter than that of mammalian HEV. Although avian HEV shares only about 50% nucleotide sequence identity with mammalian HEV across the entire genome, the genomic organization and functional motifs are relatively conserved between avian and mammalian HEV (Haqshenas et al., 2001; Huang et al., 2004). Antigenic epitopes in the capsid protein that are unique to avian HEV as well as common between mammalian HEV and avian HEV have been identified (Guo et al., 2006). Phylogenetic analyses indicated that avian HEV is distinct from mammalian HEV, and thus likely belongs to a separate genus within the family (Meng, 2008). Additional sequences of avian HEV isolates from different geographic regions of the world will help more definitively classify avian HEV as a separate genus (Peralta et al., 2008).
3.4. Replication strategy
The mechanisms of HEV replication, transcription and translation are largely unknown due to the lack of an efficient cell culture system for HEV. A bicistronic subgenomic mRNA was identified by using an in vitro HEV replicon system, and found to encode both ORF2 and ORF3 proteins (Graff et al., 2006). Subgenomic mRNAs were also detected in HEV-infected liver tissues (Purcell and Emerson, 2001). The ORF3 gene contains a cis-reactive element and is translated from the third in-frame AUG codon in the ORF1/ORF2 intergenic region (Huang et al., 2007). It has been demonstrated that the ORF3 encodes a protein that is essential for virus infectivity in vivo (Huang et al., 2007), although the expression of ORF3 protein is not required for virus replication, virion assembly or infection of liver cells in vitro (Emerson et al., 2006). The translation and posttranslational processes of the ORF1 polyprotein remain largely unknown (Meng, 2008). When expressed in baculovirus, the ORF1 polyprotein was reportedly processed into smaller proteins that correlate with predicted functional domains in ORF1. However, when the ORF1 polyprotein was expressed in bacterial or mammalian expression system, no processing of the ORF1 polyprotein was detected. A proline-rich hypervariable region within the ORF1 of HEV is found to be dispensable for HEV replication both in vitro and in vivo (Pudupakam et al., 2009), although the biological and pathological significance of this region is not yet known.
4. Diseases in Animals and Man
4.1. Epidemiology
In humans, hepatitis E is endemic in both industrialized and developing countries worldwide. Epidemics, however, only occurs in developing countries of Asia, Africa and in Mexico. The epidemics are usually associated with HEV-contaminated drinking water, whereas the risk factors associated with endemic cases are often difficult to identify although contaminated shellfish, animal meats and direct contacts with infected animals have been linked to sporadic cases of acute hepatitis E in both developing and industrialized countries (Meng, 2003, 2006; Purcell and Emerson, 2001). The prevalence of IgG anti-HEV is very high in some developing countries such as Egypt with more than 70% of the general population positive for IgG anti-HEV (Stoszek et al., 2006). Surprisingly, IgG anti-HEV prevalence in some industrialized countries is much higher than expected: for example, in some regions of the United States, up to 30% of the normal blood donors were positive for IgG anti-HEV (Drobeniuc et al., 2001; Meng et al., 2002). The source of the relatively high seropositivity in individuals from industrialized countries is unknown but zoonotic transmissions through contacts with animals or consumption of contaminated animal meats are suspected (Meng, 2006). The disease has a higher attack rate in young adults, although the seroprevalence is age-dependent and appears to increase with ages (Arankalle et al., 1995).
In pigs, swine HEV infection is widespread in swine farms worldwide from both developing and industrialized countries, regardless of whether HEV is endemic in the respective human populations (Meng, 2003; Meng and Halbur, 2006). The infection in pigs generally occurs at about 2 to 3 months of age. Infected pigs generally have a transient viremia lasting for 1 to 2 weeks, and shed viruses in feces for about 3 to 7 weeks. The majority of adult pigs including sows and boars, although mostly positive for IgG anti-HEV, are free of virus shedding. Sequence analyses of swine HEV isolates identified thus far revealed that there exist at least two genotypes of swine HEV worldwide, genotypes 3 and 4, both of which are known to cause sporadic cases of hepatitis E in humans (Meng, 2003; Meng and Halbur, 2006). Like human HEV, the transmission route for swine HEV is presumably fecal-oral. Feces from infected pigs contain large amount of infectious virus, and are likely the main source of virus for transmission. It is believed that pigs acquire infection through direct contact with infected pigs or through ingestion of feces-contaminated feed or water (Bouwknegt et al., 2008). However, experimental reproduction of swine HEV infection in pigs via the oral route of inoculation has been difficult (Kasorndorkbua et al., 2004; Bouwknegt et al., 2008), even though pigs can be readily infected with swine HEV via the intravenous route of inoculation. Other route(s) of transmission cannot be ruled out. Since feces from infected pigs contain large amounts of virus and thus, swine manure and feces could contaminate irrigation or coastal waters, and thus leading to possible contamination of produce or shellfish (Meng and Halbur, 2006). HEV strains of swine origins have been detected in sewage water, and consumption of contaminated shellfish has been implicated in sporadic cases of acute hepatitis E (Meng and Halbur, 2006).
In chickens, HS syndrome associated with avian HEV infection was first reported in western Canada in 1991, but the disease has now been reported in eastern Canada and the United States (Meng et al., 2008). BLS associated with avian HEV infection has been reported from chickens in Australia, and serological evidence of avian HEV infection was also reported in the United Kingdom and Spain (Peralta et al., 2008). Leghorn hens in cages are typically affected although the disease has also been recognized in broiler breeder hens. In the United States, avian HEV infection is enzootic in chicken flocks. A recent serosurvey of 1,276 chickens of different ages and breeds from 76 different flocks in five states showed that approximately 71% chicken flocks and 30% chickens in the United States were positive for antibodies to avian HEV (Huang et al., 2002). Like human and swine HEV, the seroprevalence in chickens appears to be age-dependant: approximately 17% of young chickens of less than 18 weeks of age and 36% of adult chickens were positive for avian HEV-specific antibodies (Huang et al., 2002). Transmission of avian HEV within and between chicken flocks appears to occur readily. In a prospective study of natural avian HEV infection in a chicken flock, all 14 chickens monitored in the study were seronegative at 12 weeks of age. The first chicken became seroconverted at 13 weeks of age, and by 21 weeks of age, all 14 chickens in the flock had seroconverted (Sun et al., 2004a). Like mammalian HEVs, the transmission route for avian HEV is presumably fecal-oral, and experimental avian HEV infection has been successfully reproduced via oronasal route inoculation of SPF chickens (Billam et al., 2005), although other routes of transmission cannot be ruled out. In addition to chickens, young turkeys experimentally inoculated with avian HEV also became infected. However, attempts to experimentally infect rhesus monkeys and mice with avian HEV were unsuccessful (Meng et al., 2008). There is no known carrier or vector implicated in the transmission of avian HEV, although rodents in the chicken farms might serve as a mechanical carrier (Sun and Meng, unpublished data).
4.2. Clinical signs and pathological lesions
The histopathological lesions are similar in humans, pigs and chickens infected by different strains of HEV, although the manifestation of clinical diseases varies in different species.
In humans, not all HEV-infected individuals develop overt clinical disease. For examples, a significant proportion of individuals in many industrialized countries are seropositive for HEV antibodies, although these individuals had no known history of hepatic diseases (Drobeniuc et al., 2001; Meng et al., 2002). It is known that hepatitis E is a dose-dependant disease: patients exposed to higher doses of HEV often develop clinical symptoms of hepatitis E whereas patients exposed to lower doses of the virus generally had only subclinical infections (Purcell and Emerson, 2001). The incubation period ranges from 2 weeks to 2 months. Patients generally have jaundice, anorexia, and hepatomegaly, and approximately 50% of the patients will also have abdominal pains and tenderness, nausea, vomiting and fever (Purcell and Emerson, 2001). The disease in many industrialized countries is sporadic in nature, although rare outbreaks do occur in developing countries. A unique feature of HEV infection is the observed high mortality during pregnancy in women. However, under experimental conditions, pregnant sows infected with swine HEV at various stages of gestation had no clinical signs of hepatitis or elevation of liver enzymes (Meng and Halbur, 2006). Similarly, pregnant rhesus monkeys experimentally infected with human HEV did not manifest more severe hepatitis than the non-pregnant monkeys either (Tsarev et al., 1995). The observed severe and fulminant hepatitis in infected pregnant women could not be experimentally reproduced in pregnant pigs or rhesus monkeys experimentally infected with HEV.
In pigs, swine HEV infection is ubiquitous essentially in all swine-producing regions worldwide (Meng, 2003; Meng and Halbur, 2006). Pigs naturally infected with swine HEV are asymptomatic. In a prospective study of naturally-infected piglets in a farm from the United States, gross pathological lesions were not detected in the liver or 18 other tissues and organs in 4 pigs necropsied during early stages of swine HEV infection (Meng et al., 1997), although all four piglets had microscopic evidence of hepatitis characterized by mild to moderate multifocal and periportal lymphoplasmacytic hepatitis with mild focal hepatocellular necrosis. Similar to natural infections, pigs experimentally infected with swine HEV and a genotype 3 strain of human HEV had no clinical signs (Halbur et al., 2001), although the infected pigs did have mildly-to-moderately enlarged hepatic and mesenteric lymph nodes from 7 to 55 days post inoculation (DPI). Histological lesions including mild-to-moderate multifocal lymphoplasmacytic hepatitis, and focal hepatocellular necrosis were also observed.
In chickens, the morbidity and mortality of HS syndrome (or BLS) associated with avian HEV infection in the field are relatively low, although subclinical infections of avian HEV are very common in chicken flocks in the United States and perhaps in other countries as well (Huang et al., 2002; Sun et al., 2004a: Peralta et al., 2008). HS syndrome is characterized by above-normal mortality in broiler breeder hens and laying hens of 30-72 week of age, with the highest incidence occurring between 40-50 weeks of age. Prior to death, clinical sign is generally not recognized in chickens with HS syndrome. In some (but not all) cases, a drop in egg production of up to 20% was observed. Weekly mortality increases to approximately 0.3% for several weeks during the middle of the production period and may sometimes exceed 1.0%. Similar to HS syndrome, the clinical signs for BLS in Australia also vary from subclinical infection to egg drops that may reach 20% with up to 1% mortality per week over a period of 3-4 weeks (Meng et al., 2008). Affected flocks in the United States, Canada and Europe appear to have milder or subclinical infections compared to those in Australia. Dead chickens associated with avian HEV infection usually have regressive ovaries, red fluid in the abdomen, and enlarged liver and spleen. Livers are enlarged with hemorrhage and may have subcapsular hematomas. Spleens from affected birds are mild to severely enlarged. Microscopically, liver lesions varied from multifocal hemorrhage to extensive areas of necrosis and hemorrhage and infiltration of heterophils and mononuclear inflammatory cells around portal triads (Meng et al., 2008). Under experimental conditions, gross lesions characteristic of HS syndrome including subcapsular hemorrhages and slightly enlarged right intermediate lobe of the livers were reproduced in approximately one-fourth of the SPF chickens experimentally infected with avian HEV (Billam et al., 2005). Foci of lymphocytic periphlebitis and phlebitis were the characteristic histological lesions in livers. There was no significant elevation of serum levels of liver enzymes AST, albumin/globulin (A/G) ratios or bile acids. However, LDH levels behaved differently over time (Billam et al., 2005).
4.3. Hepatitis E as a zoonotic disease
Hepatitis E is now considered as a zoonotic disease, and domestic pigs and wild boars are reservoirs for HEV (Meng, 2006). Pig farmers, swine veterinarians and other pig handlers in both developing and industrialized countries have been shown to be at increased risk of HEV infection. Meng et al (2002) tested a total of 465 swine veterinarians for IgG anti-HEV prevalence using recombinant capsid antigens from swine HEV and a genotype 1 strain of human HEV. Among the 295 swine veterinarians from 8 U.S. States from which 400 age- and geography-matched normal U.S. blood donors were available, approximately 23% (swine HEV antigen) or 27% (human HEV antigen) of swine veterinarians were positive for IgG anti-HEV compared to only 17% (swine HEV antigen) or 18% (human HEV antigen) in normal blood donors. Swine veterinarians in the U.S. were 1.51 times (swine HEV antigen, p=0.03) and 1.46 times (human HEV antigen, p=0.06) more likely to be anti-HEV positive than normal U.S. blood donors. Veterinarians who reported having needle sticks while performing procedures on pigs were about 1.9 times more likely to be seropositive than those who did not. Also, individuals from traditionally major swine states appear to be more likely seropositive than those from traditionally non-swine States: for example, subjects from Minnesota, a major swine State, are approximately 5-6 times more likely to be seropositive than those from Alabama, which is traditionally not a major swine State. Drobeniuc et al (2001) also determined the IgG anti-HEV prevalence in 264 swine farmers and 255 control subjects in Moldova, and found that approximately 51% of swine farmers were positive for IgG anti-HEV, whereas only 25% of control subjects with no occupational exposure to swine were seropositive. Withers et al (2002) reported that swine workers in North Carolina had a 4.5-fold higher IgG anti-HEV prevalence rate (10.9%) than the control subjects (2.4%).
Sporadic and cluster cases of acute hepatitis E due to the consumption of raw or undercooked pig livers have been reported in patients from Japan. It has been shown that approximately 2% of the pig livers sold in local grocery stores in Japan (Yazaki et al., 2003) and 11% in the United States (Feagins et al., 2007) were positive for swine HEV RNA. Most importantly, the contaminating virus in commercial pig livers sold in local grocery stores from the United States remains fully infectious when inoculated into pigs (Feagins et al., 2007). The virus sequences recovered from pig livers in grocery stores are closely related, or identical in some cases, to the viruses recovered from human hepatitis E patients in Japan (Yazaki et al., 2003). Besides domestic pigs, IgG anti-HEV is also found to be highly prevalent in the wild boar populations (de Deus et al., 2008; Michitaka et al., 2007), and sequences of genotype 3 HEV strains have been detected from wild boars (de Deus et al.; 2008; Sonoda et al., 2004; Takahashi et al., 2004). Importantly, sporadic cases of hepatitis E have been reported in patients who consumed wild boar meats (Li et al., 2005b; Masuda et al., 2005; Matsuda et al., 2003). These data provided compelling evidence that hepatitis E is a zoonotic disease, and both domestic and wild swine are the reservoirs.
In addition to swine and chickens, IgG anti-HEV has also been detected in a number of other animal species including rodents, cattle, cats, deer, horses, and dogs(Meng, 2006, 2008). Unfortunately, the source of HEV seropositivity in these animal species, with the exception of domestic and wild swine, chickens, deer and mongoose (Nakamura et al., 2006; Tei et al., 2003), could not be identified, since virus was either not recovered from these species or the recovered virus could not be sequenced to confirm its identity. Also, genotype 1 HEV sequences indistinguishable from Egyptian isolates of human HEV were reportedly detected from horses in Egypt (Saad et al., 2007), although independent confirmation of genotype 1 HEV infection in horses is still lacking. A relatively high prevalence of HEV antibody has been detected in rats, suggesting that there is a widespread infection of rats by a HEV-related agent. Rodents may play a role in HEV transmission (Kabrane-Lazizi et al., 1999b), as they are frequently found in contact with humans both in urban and rural settings. It has been reported that human populations with occupational exposure to wild animals have an increased risk of zoonotic infection. Karetnyi et al (1999) tested 87 field workers of the Iowa Department of Natural Resources (DNR) and 332 normal blood donors for prevalence of HEV antibodies, and found that the DNR workers had higher HEV antibody prevalence than normal blood donors (P<0.05). Transmissions of hepatitis E from a pet cat and a pet pig to its human owners have also been reported (Kuno et al., 2003; Renou et al., 2007). In Japan, a cluster of 4 cases of acute hepatitis E were definitively linked to the consumption of raw deer meats in two families (Tei et al., 2003). The HEV sequence amplified from the leftover frozen deer meat was 99.7 to 100% nucleotide sequence identical to the viruses recovered from the four human patients. Taken together, these evidences strongly indicate that pigs are likely not the only animal reservoirs for HEV.
4.4. Cross-species infections by HEV
It has been demonstrated that rhesus monkeys and a chimpanzee can be experimentally infected with swine HEV (Meng et al., 1998). The infected rhesus monkeys seroconverted to IgG anti-HEV 4 weeks postinoculation, developed viremia, shed virus in feces, and had elevation of serum liver enzymes isocitrate dehydrogenase and alanine aminotransferase. Microscopic lesions of viral hepatitis characterized by focal necroinflammatory changes were also observed in liver biopsies. The chimpanzee inoculated with swine HEV also became infected as evidenced by the detection of swine HEV RNA in feces of the inoculated chimpanzee, and seroconversion to IgG anti-HEV at 6 weeks postinoculation, although both rhesus monkeys and the chimpanzee infected with swine HEV remained clinically normal. Conversely, it has been shown that SPF swine experimentally inoculated with a genotype 3 human HEV (Halbur et al., 2001) and a genotype 4 human HEV (Feagins et al., 2008b) rapidly became infected as evidenced by viremia and seroconvertion to IgG anti-HEV within 2 weeks post-inoculation.
Besides pigs, experimental interspecies transmissions of HEV have also been reported in other animal species. Usmanov et al (1994) has reported experimental infections of lambs with two human HEV isolates, and the inoculated lambs reportedly developed clinical signs of hepatitis and shed virus in feces. Wistar rats experimentally inoculated with a human HEV also became infected (Maneerat et al., 1996): HEV RNA was detected in feces and sera, and HEV antigen was detected in the liver and several other tissues of the inoculated rats. However, independent studies to confirm the rat transmission results with a genotype 1 human HEV, a genotype 3 swine HEV, a genotype 4 swine HEV, and an avian HEV were unsuccessful (Meng, 2003, 2006; Sun and Meng, unpublished data). Although a HEV-related agent of rat origin can be successfully transmitted in laboratory rats, the identity of the virus could not be determined (Emerson and Purcell, personal communication). It is likely that the virus infecting the rats is genetically very different from the known strains of HEV. Like mammalian HEV, the avian HEV can also cross species barriers and infect turkeys (Sun et al., 2004b). However, under experimental conditions, avian HEV failed to infect two rhesus monkeys (Huang et al., 2004), suggesting that chickens are likely not a reservoir for HEV, although more studies are needed to further evaluate the zoonotic potential of avian HEV. The expanded host ranges of HEV and its ability to infect across species (Table 1) raise additional concern for zoonotic infection of HEV.
Table 1.
Host range and cross-species infection of the hepatitis E viruses (HEV) under natural and experimental conditions
| HEV strains | Natural hosts | Experimental hosts |
|---|---|---|
| Genotype 1 | Humans | Non-human primates, rats, lambs |
| Genotype 2 | Humans | Non-human primates |
| Genotype 3 | Humans, pigs, deer, mongoose, horse (?) | Non-human primates, pigs |
| Genotype 4 | Humans, pigs | Non-human primates, pigs |
| Avian HEV | Chickens | Turkeys, chickens |
5. Diagnosis
The clinical symptoms of human hepatitis E could not be distinguished from other types of acute viral hepatitis, and thus accurate diagnosis of hepatitis E must rely on laboratory tests. HEV is a difficult virus to work with since it can not be efficiently propagated in cell culture. Currently, the diagnosis of HEV infection is primarily based on PCR and ELISA. Commercial ELISA reagents and test kits are available in many countries.
The diagnosis of HEV infection in pigs must depend upon RT-PCR and serology, since swine HEV infection is subclinical in swine herds. The recombinant human HEV capsid antigen cross-reacted well with antibodies to swine HEV in ELISA assay, and has been used to detect anti-HEV antibody in swine (Meng et al., 1997, 1998, 2002). In addition, the capsid protein of a genotype 3 swine HEV has been expressed and used in an ELISA to detect anti-HEV in pigs. Unfortunately, there is no specific test that could distinguish infections by swine HEV and human HEV. Since genotypes 3 and 4 swine and human HEV are genetically indistinguishable, a differential diagnostic assay for swine HEV is not possible or necessary. Sensitive and specific RT-PCR assays have also been developed for the detection of swine HEV from infected pigs (Jothikumar et al., 2006; Meng and Halbur, 2006), however, the specificity of the RT-PCR assays in detecting swine HEV in pigs from different geographic regions is not known. The preferred sample for RT-PCR diagnosis is fecal materials from infected pigs since viremia in pigs is transient (Meng et al., 1997).
A presumptive diagnosis of avian HEV infection in chickens can be made on the basis of clinical signs and characteristic pathological lesions of HS syndrome. Virus particles of 30-35 nm may be detected in bile of chickens with HS syndrome by EM. Since the majority of chickens infected by avian HEV infection are subclinical, laboratory tests are needed to make a definitive diagnosis of avian HEV infection. Embryonic chicken eggs can be experimentally infected with avian HEV via intravenous inoculation, however, virus isolation with chicken embryos is not practical due to the technical difficulty and high mortality associated with the intravenous inoculation procedure. Currently, the diagnosis of avian HEV infection is primarily based on detection of the virus RNA by RT-PCR or detection of antibodies by ELISA. A truncated version of the avian HEV capsid protein has been expressed and used in an ELISA to detect avian HEV antibodies in chickens (Huang et al., 2002; Sun et al., 2004a). Avian HEV-specific RT-PCR assays have also been developed for the detection of avian HEV infections in chickens (Sun et al., 2004a). However, the specificity of the RT-PCR assays in detecting avian HEV strains in chickens from different geographic regions is not known, since avian HEV strains identified from chickens in different geographic regions are genetically heterogenic.
6. Prevention and Control
A vaccine against HEV in humans is not yet available, although the experimental recombinant HEV vaccines appear to be very promising (Shrestha et al., 2007). In the absence of a vaccine, preventive measures such as practice good hygiene and avoid drinking water of unknown purity or consuming raw or undercooked pig livers are necessary to minimize the risk of HEV infection. Swine HEV has the ability to infect and cause hepatitis in humans especially in high risk groups such as pig handlers, therefore, effective prevention of swine HEV infection in pigs may prevent its transmission to humans, and thus reduce the risk of pork safety concern. A simple measure to prevent HEV infection for pig handlers is to wash hands thoroughly with soap and water after handling pigs. Although swine HEV is non-pathogenic in pigs, it is not known if concurrent infections of swine HEV with other swine pathogens could have any synergistic effects. Therefore, it will still be advantageous for the swine industry to develop a vaccine against swine HEV infections in pigs. Avian HEV apparently is not a risk for zoonotic human infection. Implementation of strict biosecurity in chicken farms may limit the spread of virus, although development of a vaccine against avian HEV will help protect chickens from developing HS syndrome or BLS.
7. Conclusions
HEV is an important but extremely understudied human pathogen. The discoveries of swine HEV from pigs, and the demonstrated ability of swine HEV to infect across species and the recovery of viruses resembling swine HEV from human patients with acute hepatitis E raise a serious concern for zoonotic HEV infection. The discovery of avian HEV from chickens and the existence of several other animal species that are positive for HEV antibodies suggest that, in addition to pigs, there likely exist other animal reservoirs for HEV. Therefore, HEV poses a potential public health risk for zoonosis and food safety (especially for pork products). The inability to efficiently propagate HEV in cell cultures greatly hinders our understanding of the biology and pathogenesis of the virus and slows the progress of developing an affordable vaccine against HEV.
8. Future Prospects
The existence of numerous animal species that are seropositive for IgG anti-HEV suggest that these animal species are infected by a HEV-related agent(s). Unfortunately, thus far only swine HEV from pigs and avian HEV from chickens have been fullycharacterized. Failure to identify HEV from these animal species based upon the known sequences of mammalian and avian HEV strains suggest that these animal species may harbor their own strains of HEV that are genetically distinct from the known strains.Future studies to genetically identify and experimentally characterize the agent(s) responsible for the seropositivity in these animal species are warranted. Additional research on the natural history and ecology of HEV will help understand the magnitude and significance of the HEV zoonotic risk. The relatively high prevalence of HEV antibody in individuals from industrialized countries suggests that the occurrence of hepatitis E in these countries may be underestimated, since sporadic cases of acute hepatitis E may go undiagnosed. Therefore, it is important to fully assess the extent and impact of HEV infections in industrialized countries. Although the current experimental vaccines appear to be promising, it will be important to evaluate the efficacies of the vaccines against the emerging strains of HEV, especially those animal strains with zoonotic potential. Development of vaccines against the animal strains of HEV may minimize the risks of zoonotic transmission and increase food safety.
Figure 1.
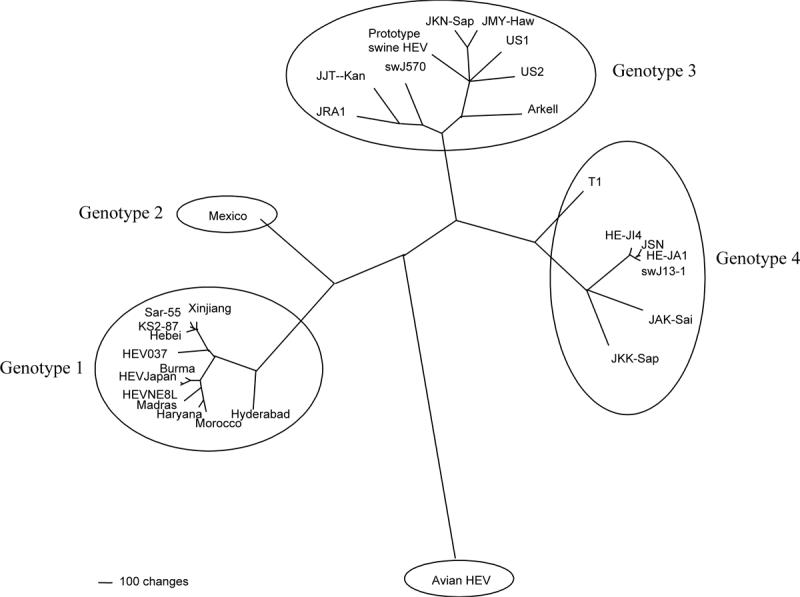
A phylogenetic tree based on the complete genomic sequences of 30 human, swine, and avian HEV strains. Genotypes 1 and 2 HEV are restricted to humans, whereas genotypes 3 and 4 HEV infect both humans and swine. The avian HEV likely belongs to a separate species within the family. A scale bar, indicating the number of character state changes, is proportional to the genetic distance. Modified with permission by the Society for General Microbiology from Huang et al (2004).
Acknowledgments
9. Conflict of Interest Statement The author's research on the hepatitis E virus is funded by grants from the National Institutes of Health (R01 AI074667, and R01 AI050611). The author is an inventor for patents on avian hepatitis E virus (U.S. patent number 7,005,130) and on swine hepatitis E virus (U.S. patent number 6,432,408). There is no other apparent conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arankalle VA, Tsarev SA, Chadha MS, Alling DW, Emerson SU, Banerjee K, Purcell RH. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J. Infect. Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, Poleschuk VF. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- Balayan MS, Usmanov RK, Zamyatina DI, Karas FR. Brief report: experimental hepatitis E infection in domestic pigs. J. Med. Virol. 1990;32:58–59. doi: 10.1002/jmv.1890320110. [DOI] [PubMed] [Google Scholar]
- Billam P, Huang FF, Sun ZF, Pierson FW, Duncan RB, Elvinger F, Guenette DK, Toth TE, Meng XJ. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J. Virol. 2005;79:3429–3437. doi: 10.1128/JVI.79.6.3429-3437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwknegt M, Frankena K, Rutjes SA, Wellenberg GJ, de Roda Husman AM, van der Poel WH, de Jong MC. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet. Res. 2008;39:40. doi: 10.1051/vetres:2008017. [DOI] [PubMed] [Google Scholar]
- Clayson ET, Myint KS, Snitbhan R, Vaughn DW, Innis BL, Chan L, Cheung P, Shrestha MP. Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J. Infect. Dis. 1995;172:927–933. doi: 10.1093/infdis/172.4.927. [DOI] [PubMed] [Google Scholar]
- Clemente-Casares P, Pina S, Buti M, Jardi R, MartIn M, Bofill-Mas S, Girones R. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 2003;9:448–454. doi: 10.3201/eid0904.020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Deus N, Peralta B, Pina S, Allepuz A, Mateu E, Vidal D, Ruiz-Fons F, Martín M, Gortázar C, Segalés J. Epidemiological study of hepatitis E virus infection in European wild boars (Sus scrofa) in Spain. Vet. Microbiol. 2008;129:163–70. doi: 10.1016/j.vetmic.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Drobeniuc J, Favorov MO, Shapiro CN, Bell BP, Mast EE, Dadu A, Culver D, Iarovoi P, Robertson BH, Margolis HS. Hepatitis E virus antibody prevalence among persons who work with swine. J. Infect. Dis. 2001;184:1594–1597. doi: 10.1086/324566. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Anderson D, Arankalle VA, Meng XJ, Purdy M, Schlauder GG, Tsarev SA. Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus Taxonomy, VIIIth Report of the ICTV. Elsevier/Academic Press; London: 2004. pp. 851–855. [Google Scholar]
- Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J. Infect. Dis. 2005;192:930–933. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Nguyen H, Torian U, Purcell RH. ORF3 protein of hepatitis E virus is not required for replication, virion assembly, or infection of hepatoma cells in vitro. J. Virol. 2006;80:10457–10464. doi: 10.1128/JVI.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J. Gen. Virol. 2007;88:912–917. doi: 10.1099/vir.0.82613-0. [DOI] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Guenette DK, Halbur PG, Meng XJ. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Intl. J. Food Microbiol. 2008a;123:32–37. doi: 10.1016/j.ijfoodmicro.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins AR, Opriessnig T, Huang YW, Halbur PG, Meng XJ. Cross-species infection of specific-pathogen-free pigs by a genotype 4 strain of human hepatitis E virus. J. Med. Virol. 2008b;80:1379–1386. doi: 10.1002/jmv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J, Torian U, Nguyen H, Emerson SU. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006;80:5919–5926. doi: 10.1128/JVI.00046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zhou EM, Sun ZF, Meng XJ, Halbur PG. Identification of B-cell epitopes in the capsid protein of avian hepatitis E virus (avian HEV) that are common to human and swine HEVs or unique to avian HEV. J. Gen. Virol. 2006;87:217–223. doi: 10.1099/vir.0.81393-0. [DOI] [PubMed] [Google Scholar]
- Halbur PG, Kasorndorkbua C, Gilbert C, Guenette DK, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J. Clin. Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 2001;82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- Herremans M, Bakker J, Duizer E, Vennema H, Koopmans MP. Use of serological assays for diagnosis of hepatitis E virus genotype 1 and 3 infections in a setting of low endemicity. Clin. Vaccine Immunol. 2007;14:562–8. doi: 10.1128/CVI.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 2004;85:1609–1618. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- Huang FF, Haqshenas G, Shivaprasad HL, Guenette DK, Woolcock PR, Larsen CT, Pierson FW, Elvinger F, Toth TE, Meng XJ. Heterogeneity and seroprevalence of a newly identified avian hepatitis E virus from chickens in the United States. J. Clin. Microbiol. 2002;40:4197–4202. doi: 10.1128/JCM.40.11.4197-4202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, Opriessnig T, Halbur PG, Meng XJ. Initiation at the third in-frame AUG codon of open reading frame 3 of the hepatitis E virus is essential for viral infectivity in vivo. J. Virol. 2007;81:3018–3026. doi: 10.1128/JVI.02259-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothikumar N, Cromeans TL, Robertson BH, Meng XJ, Hill VR. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Kabrane-Lazizi Y, Meng XJ, Purcell RH, Emerson SU. Evidence that the genomic RNA of hepatitis E virus is capped. J. Virol. 1999a;73:8848–50. doi: 10.1128/jvi.73.10.8848-8850.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabrane-Lazizi Y, Fine JB, Elm J, Glass GE, Higa H, Diwan A, Gibbs CJ, Jr, Meng XJ, Emerson SU, Purcell RH. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am J Trop Med Hyg. 1999b;61:331–5. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- Karetnyi YV, Gilchrist MJ, Naides SJ. Hepatitis E virus infection prevalence among selected populations in Iowa. J. Clin. Virol. 1999;14:51–55. doi: 10.1016/s1386-6532(99)00037-2. [DOI] [PubMed] [Google Scholar]
- Kasorndorkbua C, Guenette DK, Huang FF, Thomas PJ, Meng XJ, Halbur PG. Routes of transmission of swine hepatitis E virus in pigs. J. Clin. Microbiol. 2004;42:5047–5052. doi: 10.1128/JCM.42.11.5047-5052.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno A, Ido K, Isoda N, Satoh Y, Ono K, Satoh S, Inamori H, Sugano K, Kanai N, Nishizawa T, Okamoto H. Sporadic acute hepatitis E of a 47-year-old man whose pet cat was positive for antibody to hepatitis E virus. Hepatol. Res. 2003;26:237–242. doi: 10.1016/s1386-6346(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Li TC, Takeda N, Miyamura T, Matsuura Y, Wang JC, Engvall H, Hammar L, Xing L, Cheng RH. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J. Virol. 2005a;79:12999–13006. doi: 10.1128/JVI.79.20.12999-13006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, Kurata Y, Ishida M, Sakamoto S, Takeda N, Miyamura T. Hepatitis E virus transmission from wild boar meat. Emerg. Infect. Dis. 2005b;11:1958–60. doi: 10.3201/eid1112.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Drobeniuc J, Kobylnikov N, Usmanov RK, Robertson BH, Favorov MO, Margolis HS. Complete sequence of a Kyrgyzstan swine hepatitis E virus (HEV) isolated from a piglet thought to be experimentally infected with human HEV. J. Med. Virol. 2004;74:556–562. doi: 10.1002/jmv.20214. [DOI] [PubMed] [Google Scholar]
- Maneerat Y, Clayson ET, Myint KS, Young GD, Innis BL. Experimental infection of the laboratory rat with the hepatitis E virus. J. Med. Virol. 1996;48:121–128. doi: 10.1002/(SICI)1096-9071(199602)48:2<121::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Masuda J, Yano K, Tamada Y, Takii Y, Ito M, Omagari K, Kohno S. Acute hepatitis E of a man who consumed wild boar meat prior to the onset of illness in Nagasaki, Japan. Hepatol. Res. 2005;31:178–83. doi: 10.1016/j.hepres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J. Infect. Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Wiseman B, Elvinger F, Guenette DK, Toth TE, Engle RE, Emerson SU, Purcell RH. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002;40:117–122. doi: 10.1128/JCM.40.1.117-122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Top. Microbiol. Immunol. 2003;278:185–216. doi: 10.1007/978-3-642-55541-1_7. [DOI] [PubMed] [Google Scholar]
- Meng XJ. Hepatitis E as a zoonosis. In: Thomas H, Zuckermann A, Lemon S, editors. Viral Hepatitis. 3rd edition Blackwell Publishing Ltd; Oxford, U.K.: 2006. pp. 611–623. [Google Scholar]
- Meng XJ, Halbur PG. Swine hepatitis E virus. In: Straw BE, et al., editors. Diseases of Swine. 9th Edition Blackwell Publishing Press; 2006. pp. 537–545. [Google Scholar]
- Meng XJ. Hepatitis E virus (hepevirus) In: Mahy BWJ, van Regenmortel MHV, editors. Encyclopedia of Virology. 3rd edition 5 vols. Elsevier; Oxford: 2008. pp. 377–383. [Google Scholar]
- Meng XJ, Shivaprasad HL, Payne C. Hepatitis E virus infections. In: Saif M, et al., editors. Diseases of Poultry. 12th Edition Blackwell Publishing Press; 2008. pp. 443–452. [Google Scholar]
- Michitaka K, Takahashi K, Furukawa S, Inoue G, Hiasa Y, Horiike N, Onji M, Abe N, Mishiro S. Prevalence of hepatitis E virus among wild boar in the Ehime area of western Japan. Hepatol. Res. 2007;37:214–20. doi: 10.1111/j.1872-034X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Takahashi K, Taira K, Taira M, Ohno A, Sakugawa H, Arai M, Mishiro S. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: Demonstration of anti-HEV antibodies and a full-genome nucleotide sequence. Hepatol. Res. 2006;34:137–40. doi: 10.1016/j.hepres.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Nicand E, Armstrong GL, Enouf V, Guthmann JP, Guerin JP, Caron M, Nizou JY, Andraghetti R. Genetic heterogeneity of hepatitis E virus in Darfur, Sudan, and neighboring Chad. J. Med. Virol. 2005;77:519–21. doi: 10.1002/jmv.20487. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007;127:216–228. doi: 10.1016/j.virusres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet. Microbiol. 1999;68:119–125. doi: 10.1016/s0378-1135(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Peralta B, Biarnés M, Ordóñez G, Porta R, Martín M, Mateu E, Pina S, Meng XJ. Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Vet. Microbiol. 2008 doi: 10.1016/j.vetmic.2008.12.010. 2008 Dec 13. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pudupakam RS, Huang YW, Opriessnig T, Halbur PG, Pierson FW, Meng XJ. Deletions of the hypervariable region (HVR) in open reading frame 1 of hepatitis E virus do not abolish virus infectivity: evidence for attenuation of HVR deletion mutants in vivo. J. Virol. 2009;83:384–95. doi: 10.1128/JVI.01854-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. Hepatitis E virus. In: Knipe D, Howley P, Griffin D, Lamb R, Martin M, Roizman B, et al., editors. Fields Virology. 4th edition Lippincott:Williams and Wilkins; Philadelphia, PA: 2001. pp. 3051–3061. [Google Scholar]
- Renou C, Cadranel JF, Bourlière M, Halfon P, Ouzan D, Rifflet H, Carenco P, Harafa A, Bertrand JJ, Boutrouille A, Muller P, Igual JP, Decoppet A, Eloit M, Pavio N. Possible zoonotic transmission of hepatitis E from pet pig to its owner. Emerg. Infect. Dis. 2007;13:1094–6. doi: 10.3201/eid1307.070063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MD, Hussein HA, Bashandy MM, Kamel HH, Earhart KC, Fryauff DJ, Younan M, Mohamed AH. Hepatitis E virus infection in work horses in Egypt. Infect Genet Evol. 2007;7:368–73. doi: 10.1016/j.meegid.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr, Thapa GB, Thapa N, Myint KS, Fourneau M, Kuschner RA, Shrestha SK, David MP, Seriwatana J, Vaughn DW, Safary A, Endy TP, Innis BL. Safety and efficacy of a recombinant hepatitis E vaccine. N. Engl. J. Med. 2007;356:895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Abe M, Sugimoto T, Sato Y, Bando M, Fukui E, Mizuo H, Takahashi M, Nishizawa T, Okamoto H. Prevalence of hepatitis E virus (HEV) Infection in wild boars and deer and genetic identification of a genotype 3 HEV from a boar in Japan. J. Clin. Microbiol. 2004;42:5371–4. doi: 10.1128/JCM.42.11.5371-5374.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH, Strickland GT. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans. R. Soc. Trop. Med. Hyg. 2006;100:89–94. doi: 10.1016/j.trstmh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Dunlop A, Huang FF, Pierson FW, Toth TE, Meng XJ. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J. Gen. Virol. 2004a;85:693–700. doi: 10.1099/vir.0.19582-0. [DOI] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Huang FF, Billam P, Pierson FW, Toth TE, Meng XJ. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J Clin Microbiol. 2004b;42:2658–62. doi: 10.1128/JCM.42.6.2658-2662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–5. doi: 10.1016/j.virol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamada K, Hoshino Y, Takahashi H, Ichiyama K, Tanaka T, Okamoto H. Monoclonal antibodies raised against the ORF3 protein of hepatitis E virus (HEV) can capture HEV particles in culture supernatant and serum but not those in feces. Arch. Virol. 2008;153:1703–13. doi: 10.1007/s00705-008-0179-6. [DOI] [PubMed] [Google Scholar]
- Tam AW, Smith MM, Guerra ME, Huang CC, Bradley DW, Fry KE, Reyes GR. Hepatitis E virus (HEV): molecular cloning and sequencing of the fulllength viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, Rippy MK, Zack P, Shapiro M, Purcell RH. Experimental hepatitis E in pregnant rhesus monkeys: failure to transmit hepatitis E virus (HEV) to offspring and evidence of naturally acquired antibodies to HEV. J. Infect. Dis. 1995;172:31–7. doi: 10.1093/infdis/172.1.31. [DOI] [PubMed] [Google Scholar]
- Usmanov RK, Balaian MS, Dvoǐnikova OV, Alymbaeva DB, Zamiatina NA, Kazachkov IuA., Belov VI. An experimental infection in lambs by the hepatitis E virus. Vopr. Virusol. 1994;39:165–168. [PubMed] [Google Scholar]
- Withers MR, Correa MT, Morrow M, Stebbins ME, Seriwatana J, Webster WD, Boak MB, Vaughn DW. Antibody levels to hepatitis E virus in North Carolina swine workers, non-swine workers, swine, and murids. Am. J. Trop. Med. Hyg. 2002;66:384–388. doi: 10.4269/ajtmh.2002.66.384. [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be foodborne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J. Virol. 1997;71:9045–9053. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


