Abstract
Objective
Limited endothelial cell (EC) coverage and anastomotic intimal hyperplasia contribute to thrombosis and failure of prosthetic grafts. Lipid accumulation and lipid oxidation are associated with decreased EC migration and intimal hyperplasia. The goal of this study was to assess the ability of antioxidants to improve graft healing in hypercholesterolemic animals.
Methods
Rabbits were placed in one of four groups: chow plus N-acetylcysteine (NAC), chow plus probucol, chow with 1% cholesterol plus NAC, or chow with 1% cholesterol plus probucol. After two weeks, 12 cm long, 4 mm internal diameter expanded polytetrafluoroethylene grafts were implanted in the abdominal aorta. Six weeks after implantation, the grafts were removed and analyzed for cholesterol content, EC coverage, anastomotic intimal thickness, and the cellular composition of the neointima. Plasma samples were obtained to assess systemic oxidative stress. The data was compared with previously reported data from animals on chow and chow with 1% cholesterol diets.
Results
Prosthetic grafts from rabbits on a chow with 1% cholesterol diet had significantly greater anastomotic intimal thickening and lower EC coverage than grafts from rabbits on a chow diet. In hypercholesterolemic rabbits, antioxidant therapy decreased global oxidative stress as evidenced by a 40% decrease in plasma thiobarbituric acid reactive substances. In rabbits on the chow with 1% cholesterol diet, NAC decreased intimal hyperplasia at the proximal anastomosis by 29% and significantly increased graft EC coverage from 46% to 71% (P = .03). Following a similar pattern, probucol decreased intimal hyperplasia by 43% and increased graft EC coverage to 53% in hypercholesterolemic rabbits.
Conclusions
Global oxidative stress and anastomotic intimal hyperplasia are increased and endothelialization of prosthetic grafts is significantly reduced in rabbits on a high cholesterol diet. Antioxidant treatment improves EC coverage and decreases intimal hyperplasia. Reducing oxidative stress may promote healing of prosthetic grafts.
Clinical Relevance
Hypercholesterolemia is associated with an increased inflammatory response, elevated oxidative stress, and increased intimal hyperplasia following stent or vein graft placement in animal models and in humans. Reduced endothelialization is seen following stent or graft placement in hypercholesterolemic animals, and reduced EC growth and patency of EC-seeded grafts is found in humans with elevated serum lipid levels. Our results suggest that antioxidants are effective in reducing this pathologic response and improving graft healing.
Keywords: endothelial cell, migration, intimal hyperplasia, antioxidant, prosthetic vascular graft, oxidative stress
INTRODUCTION
Peripheral arterial disease (PAD) is a devastating disorder with a major impact on quality of life. In the United States, more than 8 million individuals age 40 and older have PAD.1 Because the population is aging, the number Americans afflicted with PAD and the number of peripheral vascular interventions performed each year will increase. Intervention may take the form of angioplasty, endarterectomy, or autologous or prosthetic bypass graft. The long-term patency after any intervention is compromised by the thrombogenicity of the prosthetic material or injured luminal surface, development of intimal hyperplasia (IH), and limitation of inflow or outflow due to extension and/or progression of atherosclerotic disease.
After implantation of a prosthetic graft, macrophages infiltrate the graft, lipids are deposited, endothelial cells (ECs) and smooth muscle cells (SMCs) migrate from the adjacent artery, and extracellular matrix is produced. In the inflammatory response to graft placement, macrophages are activated and produce reactive oxygen species (ROS). Excessive ROS production overwhelms the endogenous antioxidant defense mechanisms and leads to the oxidation of macromolecules such as DNA, proteins, carbohydrates, and lipids.2 Lipids that accumulate in the graft, particularly at the anastomoses,3 are oxidized. Lipid oxidation products, but not native lipids or lipoproteins, cause cellular dysfunction in vitro, including inhibition of EC migration,4 stimulation of SMC proliferation,5 and increased platelet-derived growth factor (PDGF) and collagen production by SMCs,6, 7 which could impair graft healing in vivo. Studies have confirmed that prosthetic graft material induces monocytes to oxidize low-density lipoprotein (LDL),8 and lipid oxidation products accumulate in vascular grafts in vivo.9 In a previous rabbit study, hypercholesterolemia impaired prosthetic graft healing and was associated with increased anastomotic IH and macrophage infiltration and decreased endothelialization of the graft.10 Clinical reviews suggest that elevated serum cholesterol decreases graft patency in humans.11, 12
The mechanism by which hypercholesterolemia adversely affects graft healing may be attributable, in part, to oxidative stress. Oxidized LDL (oxLDL) inhibits EC migration by several mechanisms including stimulation of ROS production by ECs.13 The inhibitory effects of oxLDL on EC migration in vitro can be reversed with superoxide dismutase and NAD(P)H oxidase inhibitors.13 SMC proliferation is mediated, in part, by SMC PDGF receptor activation in response to ROS, and antioxidant treatment decreases PDGF signaling and SMC proliferation in vitro.14 We postulate that hypercholesterolemia adversely affects prosthetic graft healing in vivo by increasing oxidative stress, and this oxidative stress inhibits EC migration and stimulates SMC proliferation. In the present study, we evaluate the ability of N-acetylcysteine (NAC), a cell-permeable antioxidant with direct and indirect antioxidant activity,15, 16 and probucol, an antioxidant with weak lipid-lowering activity, to block the adverse effects of hypercholesterolemia on prosthetic graft healing.
METHODS
Graft implantation and removal
Adult New Zealand white rabbits (3.5 to 4.5 kg, Myrtle’s Rabbitry, Thompsons Station, TN) were randomized to one of four groups: 1) chow diet NIH-09 containing 2.4% fat (chow; Zeigler Brothers, Inc., Gardners, PA) plus NAC (250 mg/kg/day) (MP Biomedicals, LLC, Solon, OH), 2) high cholesterol (HC) diet, consisting of NIH-09 containing 2.4% fat supplemented with 1% (wt/wt) cholesterol, plus NAC, 3) chow plus probucol (280 mg/kg/day) (Daiichi Pharmaceuticals Co., LTD, Tokyo, Japan), or 4) HC plus probucol. Rabbits on chow and HC diets, without drug therapy, utilized during an overlapping time period, but previously reported, were used for comparison.10 NAC was dissolved in apple juice (pH 7.0–7.4) and administered by oral gavage once daily. Probucol was mixed in applesauce and administered orally once daily. The animal study protocol was approved by the Institutional Animal Care and Use Committee, and all procedures and care complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, 1996).
Rabbits were placed on the assigned diet and medication two weeks prior to graft placement. At graft implantation, anesthesia was induced with ketamine hydrochloride (40 mg/kg) and xylazine (20 mg/kg). The rabbit was intubated, mechanically ventilated, and maintained on 1.25–1.5% isofluorane throughout the procedure. Prior to graft placement, the rabbits were anticoagulated with heparin sodium (150 Units/kg). Expanded polytetrafluoroethylene (ePTFE) grafts (W. L. Gore & Associates, Flagstaff, AZ), 10–12 cm long and 4 mm internal diameter, were anastomosed end-to-end to the abdominal aorta between the celiac axis and the superior mesenteric artery and end-to-side to the infrarenal aorta just proximal to the aortic bifurcation, as previously described.10
For six weeks after implantation, the rabbits were maintained on the assigned diet and medication. No anticoagulants or anti-platelet agents were administered after implantation. At six weeks, the rabbits were anesthetized and anticoagulated with heparin sodium (200 Units/kg). An infusion catheter was inserted into the proximal descending aorta and the graft was flushed as previously described.10 The grafts from half the rabbits were perfusion-fixed with 4% paraformaldehyde and processed for immunohistochemistry and scanning electron microscopy (SEM), as previously described.10 The remaining grafts were removed for lipid extraction and quantitation.
Plasma biochemical assays
Plasma was procured at the time of graft implantation and at the conclusion of the study to measure cholesterol, high-density lipoprotein (HDL) and thiobarbituric acid reactive substances (TBARS). After the addition of butylated hydroxytoluene (BHT) 227 μmol/L, the plasma was stored under N2 at −80° C until analyzed.
Total plasma cholesterol concentration was determined using a cholesterol oxidase method (Infinity Cholesterol Reagent, Thermo Fisher Scientific, Inc., Waltham, MA). To determine the HDL level, the low-density/very low-density lipoprotein fraction was precipitated using magnesium chloride and dextran,17 and the supernatant containing the HDL fraction was quantitated using a cholesterol oxidase method. Lipid peroxidation was measured as TBARS.18 To prevent oxidation during assay, aliquots of 100 μL of plasma were transferred to tubes containing 1 μmol of BHT. To each sample, 500 μL of 1% thiobarbituric acid and 500 μL of 25% trichloroacetic acid were added. The samples were incubated at 90°C for 60 minutes, centrifuged at 820 × g for 20 minutes, and then TBARS levels quantified using a spectrofluorometric method (λex = 515 nm, λem = 553 nm) with a standard curve prepared using malondialdehyde.
Tissue lipid extraction
Lipids from the graft and aorta were extracted as previously described.10 Briefly, the aorta and graft were cut into segments containing the descending thoracic aorta, proximal anastomosis, mid-graft, distal anastomosis, and distal abdominal aorta. The tissue was minced and the lipids were extracted using chloroform, methanol and water in a 2:2:1.8 ratio.19 After phase separation, the organic layer was removed and dried under N2.
Tissue cholesterol content
Total cholesterol from graft and aortic segments was measured by HPLC.10, 20 The extracted samples were re-suspended in ethanol and 10 μL aliquots were added to 200 μL of PBS containing β-sitosterol (5 μg; Sigma, St. Louis, MO) as an internal standard. Cholesterol oxidase (0.1 U) and cholesterol esterase (0.1 U) was added to 50 μL of reaction mixture (150 mmol/L NaPO4, 30 mmol/L sodium taurocholate, 1 mmol/L polyethylene glycol) to determine total cholesterol. The sterols were extracted using NaCl (4 mol/L, 250 μL) and acetonitrile (500 μL). After phase separation, the supernatant was analyzed using reverse-phase HPLC (Nova-Pak C18 3.9×75 mm, Waters Corp., Milford, MA). Methanol:acetonitrile (1:1) was used as the eluent at a flow rate of 1 mL/min with detection at 235 nm.
Morphologic Assessment of Grafts
After removal, graft and aortic segments were processed as previously described.10 Briefly, the grafts were fixed overnight in 4% paraformaldehyde and divided longitudinally, with one half used for SEM and the other half for immunohistochemistry. SEM samples were dehydrated, dried, sputter-coated with gold, and examined using JSM-6930 microscope (JEOL, Peabody, MA). Endothelial coverage was analyzed using SEM and confirmed by immunohistochemistry as previously described, and reported as a percentage (endothelialized surface area/total luminal surface area × 100). 10 Each area was examined under low-power magnification (×15), then high-power magnification (×1500) was used to verify the presence or absence of ECs.
To assess for IH, the tissue segments were embedded in paraffin, sectioned longitudinally, and stained with hematoxylin and eosin. The proximal anastomotic region was imaged and the neointima was measured, by an observer blinded to the treatment group, at the thickest point between 1.5 and 2.5 mm from the end of the graft to avoid suture line distortion, as previously described.10 The results were reported as the ratio of intimal (I) to graft (G) thickness (I/G ratio). Normalizing the graft thickness mathematically corrected for any mild tissue angulation that occurred during the embedding process.
Immunostaining for Macrophages and Nitrotyrosine
The presence of macrophages or nitrotyrosine was assessed by immunohistochemistry as previously described.10 Antibody to RAM-11 (1:100, DAKO, Glostrup, Denmark) or human nitrotyrosine (1:200, Cayman Chemical, Ann Arbor, MI) was used to identify macrophages or nitrotyrosine, respectively. Diaminobenzidine (DAB Kit, Vector Laboratories) was used to visualize an avidin-conjugated secondary antibody (ABC Kit, Vector Laboratories, Burlingame, CA).
Macrophage accumulation within the graft and neointima was quantitated by identifying cells positive for RAM-11. Positively-stained and non-stained cells were counted in three 0.0625 mm2 areas in distinct regions of the graft by two observers blinded to treatment group. The data were expressed as total cells or percentage of macrophages in a 0.0625 mm2 area of graft or neointima. Nitrotyrosine was used as a marker of peroxynitrite generation, which forms when nitric oxide reacts with superoxide. Sections of tissue from all dietary groups were processed simultaneously to minimize variations in staining technique. Three observers blinded to the treatment group qualitatively scored the nitrotyrosine staining on a scale from 1 to 5, and the scores were averaged.
Statistical Analysis
Results were represented as the mean ± standard error (SE) of the mean. Data were evaluated by Chi-square test, student t-test, or analysis of variance (ANOVA) followed by Tukey’s posthoc multiple comparison using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA). P ≤ .05 was considered statistically significant.
RESULTS
A total of 73 rabbits were included in this study, including 36 rabbits on chow (n = 18) and HC (n = 18) diets implanted during an overlapping time period, but reported previously,10 and 37 rabbits allocated to the additional four groups. Rabbits were assigned to a treatment group two weeks prior to graft implantation and maintained on the assigned diet and medication until six weeks after placement. An equal number of rabbits were initially assigned to each study group, but some rabbits developed respiratory ailments prior to graft implantation and were excluded from further analysis. In the chow plus NAC group (n = 7), the surgical mortality was 14% with one rabbit euthanized for failure to thrive, defined as weight loss greater than 25% of the pre-operative weight. In the chow plus probucol group (n = 11), the surgical mortality was 27%. Two rabbits died intraoperatively and one was euthanized due to hind-limb paralysis. In the HC plus NAC group (n = 10), the surgical mortality was 30%, with three rabbits euthanized for failure to thrive. In the HC plus probucol group (n = 9), the surgical mortality was 11% with one rabbit euthanized for hind-limb paralysis. Although the operative mortality varied among the groups, the mortality was within an acceptable range for this model. The differences were not statistically significant. No mortalities were due to graft occlusion. Data from grafts in rabbits on chow (n = 18) and HC (n = 18) diets were included for comparison.10
The preoperative and postoperative data for all study groups are summarized in Table I. The rabbit ages and weights were similar across all groups. The HC diet increased plasma cholesterol levels more than 20-fold at the time of graft implantation and more than 30-fold at six weeks after implantation, as compared to the chow diet. NAC and probucol did not affect the plasma cholesterol at graft implantation or removal. NAC had no effect on HDL levels, but probucol treatment resulted in a 35% decrease in HDL levels in rabbits on a chow diet (P < .01) and a 49% decrease in HDL levels in rabbits on the HC diet (P < .001).
Table I.
Pre-operative and post-operative data, presented as mean ± standard error
| Weight (kg) | Plasma Cholesterol (mg/dL) | ||||||
|---|---|---|---|---|---|---|---|
| Group | No. | Age (months) | At implant | At removal | Total at implant | Total at removal | HDL at removal |
| Chow, controla | 13 | 8.1 ± 0.5 | 3.8 ± 0.1 | 3.9 ± 0.1 | 34.0 ± 4.4b | 18.2 ± 3.0b | 37.1 ± 3.5 |
| Chow + NAC | 6 | 7.8 ± 0.5 | 4.3 ± 0.2 | 4.4 ± 0.2 | 33.7 ± 5.3c | 33.0 ± 5.1c | 39.3 ± 2.5 |
| Chow + Probucol | 8 | 7.2 ± 0.6 | 4.2 ± 0.1 | 4.3 ± 0.2 | 31.3 ± 4.8d | 25.8 ± 3.6d | 24.2 ± 2.7e |
| High Cholesterola | 13 | 8.4 ± 0.4 | 4.0 ± 0.1 | 3.9 ± 0.1 | 689.4 ± 30 | 1087.7 ± 116.5 | 45.5 ± 2.9 |
| HC + NAC | 7 | 7.5 ± 0.3 | 4.1 ± 0.2 | 3.7 ± 0.1 | 954.2 ± 198.7 | 1118.9 ± 94.2 | 36.8 ± 2.9 |
| HC + Probucol | 8 | 8.2 ± 0.4 | 4.4 ± 0.1 | 4.0 ± 0.1 | 993.1 ± 90.1 | 1257.7 ±131.8 | 23.4 ± 3.2f |
HC = High Cholesterol
Data from reference 10
Significant differences (P < .05) are designated as:
Between chow control group and all high cholesterol groups
Between chow + NAC group and all high cholesterol groups
Between chow + probucol group and all high cholesterol groups
Between chow control group and chow + probucol group
Between high cholesterol and high cholesterol + probucol group
Tissue cholesterol content
Tissue lipids were extracted from the descending thoracic aorta, proximal anastomosis, mid-graft, distal anastomosis, and distal abdominal aorta and quantitated. Aortic and graft cholesterol content was significantly increased in rabbits on a HC diet compared to chow (Fig. 1A, B).10 NAC and probucol did not affect the aortic or graft tissue cholesterol levels in normocholesterolemic rabbits. In hypercholesterolemic rabbits, NAC and probucol significantly reduced the cholesterol in the proximal aorta, and NAC decreased the cholesterol at the proximal anastomosis, but neither drug affected the cholesterol of the mid-graft, distal anastomosis, or distal aorta (Fig. 1, A, B).
Fig. 1.
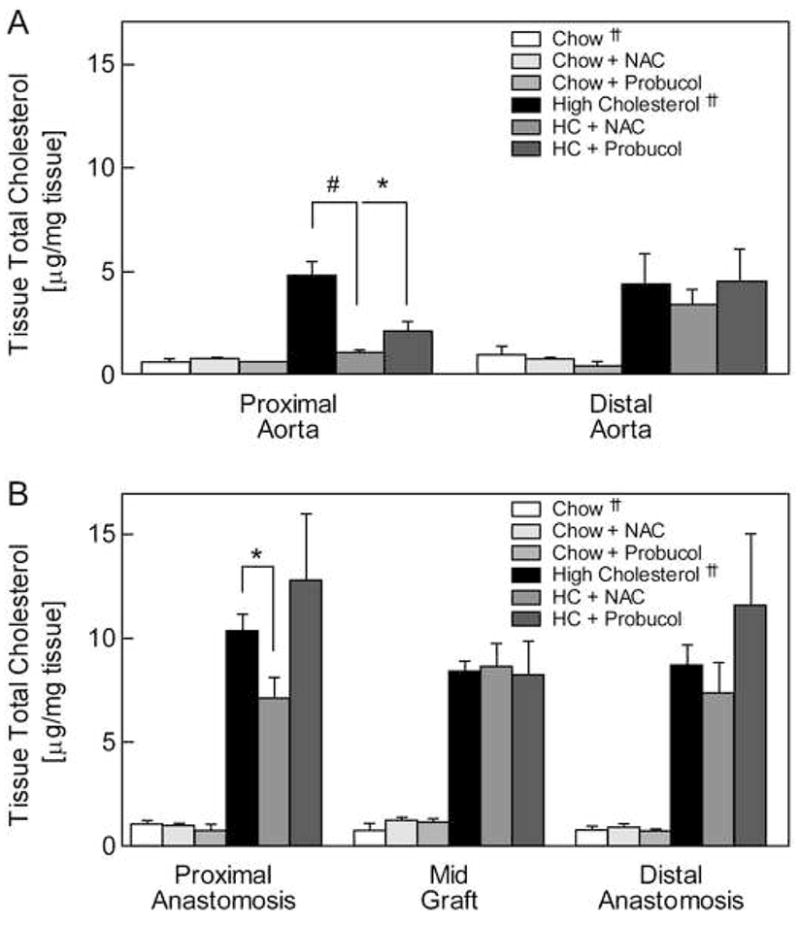
Tissue cholesterol content. Lipids were extracted from aortic (A) and graft (B) samples and tissue cholesterol levels were determined using high-performance liquid chromatography. Levels are reported as μg/mg tissue (wet weight) and expressed as the mean ± standard error for each tissue segment and rabbit group: chow (n = 3), chow plus NAC (n = 2), chow plus probucol (n = 3), high cholesterol (HC, n = 3), HC plus NAC (n = 3), and HC plus probucol (n = 3). * P < .05, # P < .005. †† Data from reference 10
Plasma TBARS levels
TBARS levels were determined in plasma samples obtained at graft removal as a measure of global oxidative stress. TBARS in the chow group was significantly lower than in the HC group (P = .03), and antioxidants did not further decrease TBARS levels in chow-fed animals (Fig. 2). In hypercholesterolemic animals, TBARS levels were 1.04 ± 0.40 nmol MDA/mL in the HC group and were decreased to 0.61 ± 0.08 nmol MDA/mL (P = .1) and 0.61 ± 0.07 nmol MDA/mL (P = .1) by NAC and probucol, respectively (Fig. 2). The trend indicates decreased global oxidative stress with NAC and probucol treatment, and the TBARS levels in all hypercholesterolemic rabbits receiving antioxidants (NAC or probucol) were significantly lower than in the HC group (P = .05).
Fig. 2.
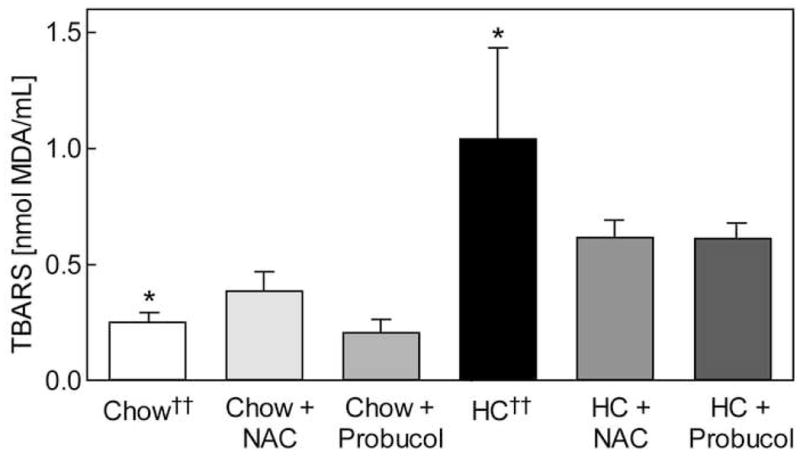
Plasma thiobarbituric acid reactive substances (TBARS). Plasma TBARS levels were determined six weeks post-operatively and reported as nmol MDA/mL. Values are expressed as the mean ± standard error for each rabbit group: chow (n = 7), chow plus NAC (n = 6), chow plus probucol (n = 8), high cholesterol (HC, n = 6), HC plus NAC (n = 7), and HC plus probucol (n = 8). * P < .05 between chow and HC groups. †† Data from reference 10
Endothelialization of ePTFE grafts
SEM was used to determine EC coverage of grafts implanted for six weeks. As reported previously, hypercholesterolemia significantly decreased EC coverage compared to chow.10 NAC did not affect endothelial coverage but probucol decreased endothelialization in normocholesterolemic rabbits (Fig. 3, A). In hypercholesterolemic rabbits, NAC significantly improved endothelialization of ePTFE grafts from 46% ± 7% in HC rabbits to 71% ± 9% in HC + NAC rabbits (P = .03). Probucol treatment showed a trend toward improved endothelialization of ePTFE grafts in hypercholesterolemic rabbits with coverage of 53% ± 7% (Fig. 3, A). Overall, the EC coverage of ePTFE grafts in hypercholesterolemic rabbits receiving antioxidant therapy was significantly higher than in the HC group (P = .05).
Fig. 3.
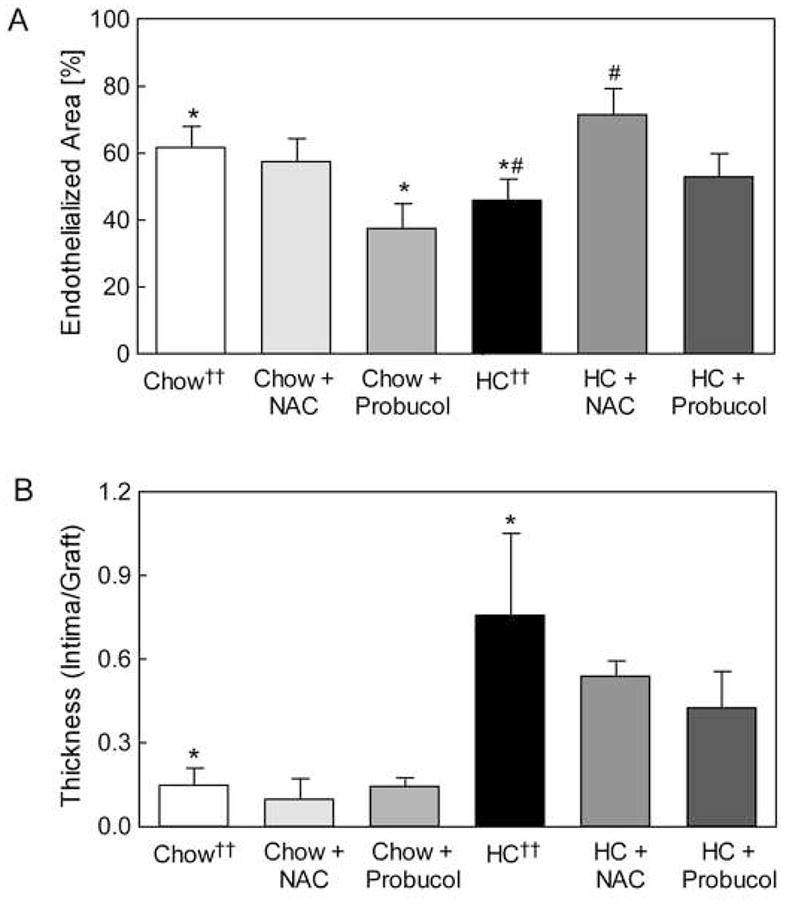
Endothelialization of expanded polytetrafluoroethylene (ePTFE) grafts. Graft endothelialization and total luminal area were determined by scanning electron microscopy. Endothelialization (A) was reported as the percentage of vascular graft covered by endothelium relative to the total luminal area of the graft and expressed as the mean ± standard error for each group: chow (n = 10), chow plus NAC (n = 4), chow plus probucol (n = 5), high cholesterol (HC, n = 11), HC plus NAC (n = 4), and HC plus probucol (n = 5). * P < .05 between chow and chow plus probucol or HC groups, # P < .05 between HC and HC + NAC groups. Anastomotic intimal hyperplasia (B) was measured on histological sections between 1.5 mm and 2.5 mm from the graft end and expressed as the mean ± standard error for each group: chow (n = 7), chow plus NAC (n = 4) chow plus probucol (n = 5), high cholesterol (HC, n = 4), HC plus NAC (n = 4), and HC plus probucol (n = 5). * P < .05 between chow and HC groups. †† Data from reference 10
Anastomotic intimal hyperplasia
Anastomotic IH was measured in hematoxylin and eosin-stained tissue sections of the proximal anastomosis. IH was significantly lower in the chow group than the HC group (P < .05), and antioxidants did not further decrease the IH (Fig. 3, B). NAC and probucol decreased the I/G ratio in hypercholesterolemic rabbits from 0.76 ± 0.29 in the HC group,10 to 0.54 ± 0.05 and 0.43 ± 0.13 (Fig. 3, B), respectively. The data suggest that hypercholesterolemic rabbits receiving antioxidant therapy may have less development of IH than the HC group (P = .1).
Macrophage accumulation
Macrophage infiltration and total cellularity of the prosthetic graft and neointima were assessed using tissue sections stained for RAM-11 and counterstained with hematoxylin. The total number of cells in the graft or neointima was not significantly different between groups, except for increased graft cells in the chow plus NAC group (Table II). The percentage of macrophages in the graft and neointima was significantly lower in the chow group than in the HC group (P < .05),10 and antioxidant therapy did not further decrease macrophage infiltration. In hypercholesterolemic rabbits, NAC decreased the percentage of macrophages in the graft (P = .06), but probucol did not alter the macrophage infiltration (Table II) (Fig. 4).
Table II.
Cellularity and macrophage accumulation of the neointima and graft, expressed as mean ± standard error
| Neointima | Graft | ||||
|---|---|---|---|---|---|
| Group | No. | Total Cellsb | % Macrophagec | Total Cellsb | % Macrophagec |
| Chow, control †† | 6 | 163 ± 19 | 0 ± 0.3d | 146 ± 30 | 0 ± 0.2g |
| Chow + NAC | 3 | 142 ± 13 | 0 ± 0.0 | 300 ± 50f | 1 ± 0.4h |
| Chow + Probucol | 5 | 184 ± 28 | 2 ± 1.1e | 235 ± 42 | 4 ± 2.0i |
| High Cholesterol †† | 5 | 195 ± 20 | 26 ± 9.9 | 159 ± 28 | 30 ± 6.0 |
| High Cholesterol + NAC | 4 | 130 ±21 | 23 ± 2.9 | 192 ± 16 | 18 ± 2.0j |
| High Cholesterol + Probucol | 5 | 133 ± 11 | 16 ± 4.1 | 186 ± 31 | 28 ± 3.6 |
Data from reference 10
Total cell count per 0.0625 mm2
Percent of total number of cells identified as macrophages per 0.0625 mm2
Significant difference between chow control group and all hypercholesterolemic groups (P < .05)
Significant difference between chow + probucol all hypercholesterolemic groups (P < .05)
Significant difference between chow control and chow + NAC group (P < .05)
Significant difference between chow control and all hypercholesterolemic groups (P < .05)
Significant difference between chow + NAC and all hypercholesterolemic groups (P < .05)
Significant difference between chow + probucol and all hypercholesterolemic groups (P < .05)
Difference between high cholesterol and high cholesterol + NAC (P = .06)
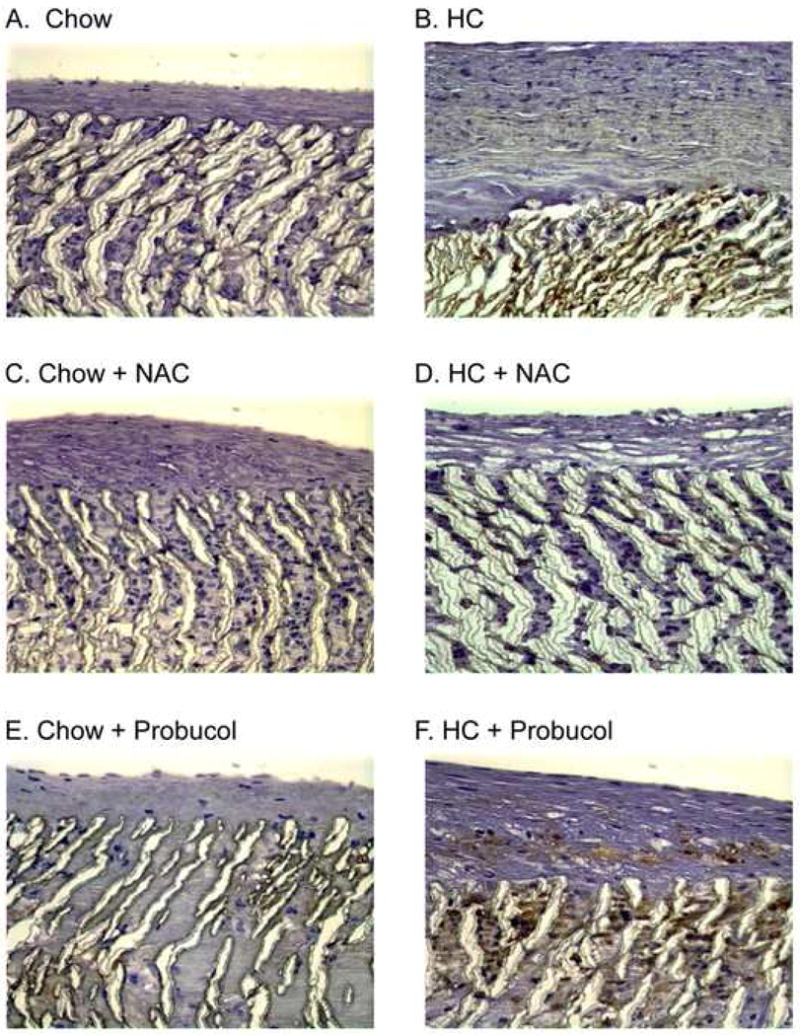
Fig. 4.
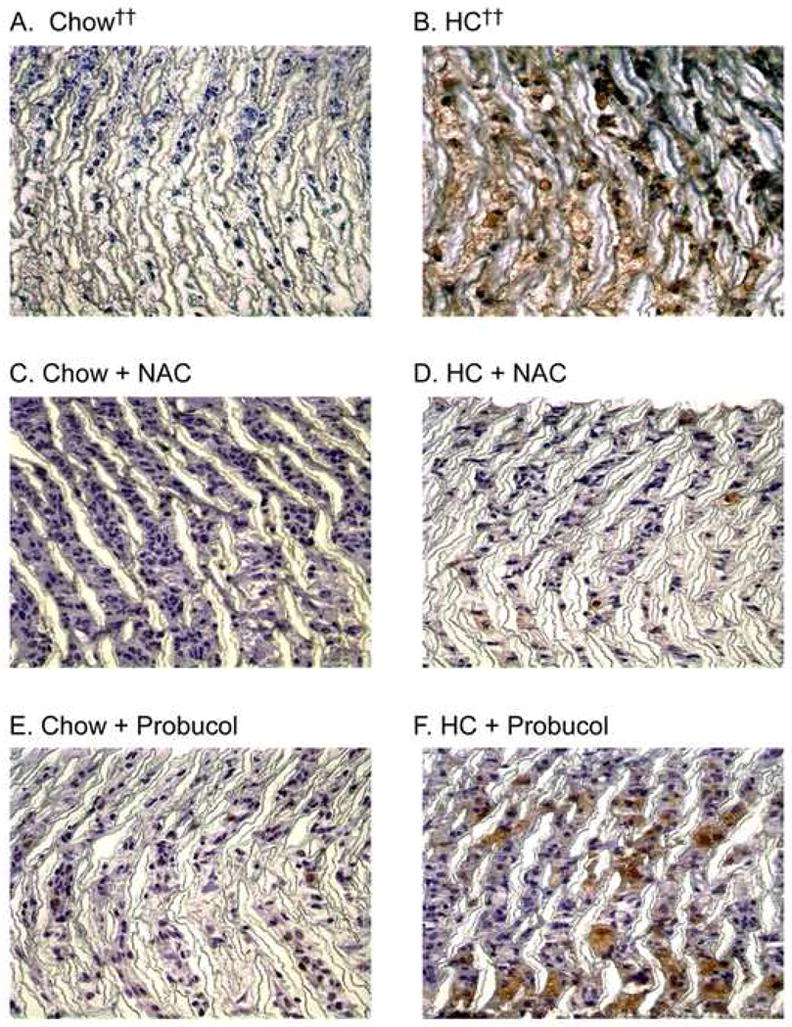
Macrophage infiltration. Following perfusion-fixation with 4% paraformaldehyde, graft sections were processed for immunohistochemistry and stained using mouse anti-rabbit macrophage antibody (RAM-11). Representative sections are shown from each rabbit group (A) chow diet (n = 6), (B) high cholesterol diet (HC, n = 5), (C) chow plus NAC (n = 3), (D) HC plus NAC (n = 4), (E) chow plus probucol (n = 5), (F) HC plus probucol (n = 5). Original magnification ×100. †† Data from reference 10
Nitrotyrosine staining
Nitrotyrosine is the stable end-product of tissue nitration by peroxynitrite, a potent oxidant. As a result, nitrotyrosine can be used is an indicator of tissue oxidative stress. The nitrotyrosine staining in the graft and neointima was decreased in the chow group compared to the HC group, and antioxidants did not further decrease the nitrotyrosine staining. NAC, but not probucol, qualitatively decreased nitrotyrosine staining in grafts from hypercholesterolemic rabbits (Fig. 5). The graft nitrotyrosine scores from blinded observers were 1.90 ± 0.64, 0.33 ± 0.17, and 2.45 ± 0.94 in the HC, HC + NAC, and HC + probucol group, respectively (P = .06 for HC compared to HC + NAC). The nitrotyrosine scores for the neointima followed a similar pattern. NAC reduced the amount of nitrotyrosine present in the graft and neointima of hypercholesterolemic rabbits, but probucol had no effect.
Fig. 5.
Nitrotyrosine staining. Following perfusion-fixation with 4% paraformaldehyde, graft sections were processed for immunohistochemistry and stained using mouse anti-human nitrotyrosine antibody. Representative sections are shown from rabbits fed (A) regular chow diet (n = 3), (B) high cholesterol diet (HC, n = 5), (C) chow plus NAC (n = 2), (D) HC plus NAC (n = 3), (E) chow plus probucol (n = 5), (F) HC plus probucol (n = 5). Original magnification ×100.
DISCUSSION
Prosthetic graft healing is impaired by hypercholesterolemia in vivo.3, 10 The mechanism by which hypercholesterolemia impairs graft healing remains unclear, but oxidized lipids in the graft may play a causative role. Lipid oxidation products accumulate in prosthetic grafts,9 and the adverse effects of oxidized lipids on SMC proliferation and EC migration have been demonstrated in vitro. OxLDL stimulates SMC proliferation and enhances SMC collagen production.5, 7 OxLDL also inhibits EC migration, but this can be reversed by treating cultured cells with antioxidants.13 α-Tocopherol, a chain-breaking antioxidant, improved graft healing in hypercholesterolemic rabbits, but the mechanism was unclear because α-tocopherol also decreased tissue cholesterol content.10 Evidence suggests that two-electron redox reactions are important in atherogenesis, but α-tocopherol is most protective against one-electron oxidation reactions.21, 22 NAC and probucol are antioxidants that have been shown to reduce intimal hyperplasia and coronary artery restenosis rates in both animal and human studies.23, 24 This study was undertaken to evaluate the role of oxidative stress in prosthetic graft healing in hypercholesterolemic animals by using these two potent antioxidants.
NAC and probucol have different mechanisms of antioxidant action. NAC has direct and indirect antioxidant activity due to its sulfhydryl group.15, 16 NAC directly inactivates ROS and hypochlorite by conjugation or reduction to form NAC radicals.15 NAC also increases glutathione formation, and glutathione is quantitatively the most important intracellular antioxidant. Glutathione can react with peroxynitrite, preventing its accumulation and protecting against nitrosative stress.25 Peroxynitrite causes cellular damage through peroxidation of membrane lipids, protein denaturation, and direct DNA damage.25 In addition, NAC inhibits NF-κB binding to the promoter region of several proinflammatory cytokines,26 including TNF-α, IL-1β, IL-2, IL-6, IL-8, and MCP-1.27 Decreased proinflammatory cytokine levels lead to decreased macrophage and neutrophil recruitment and activation.26 NAC inhibits NF-κB expression and leads to decreased SMC proliferation and neointimal formation.28 Probucol’s antioxidant activity is mediated by the heme oxygenase-1 pathway, as opposed to direct free radical scavenging.22 Evidence from mouse and rabbit models indicates the sulfur component of probucol is critical for protection from ROS-induced atherogenesis.22
Overall, the results suggest that NAC and probucol therapy is associated with a decrease in oxidative stress in hypercholesterolemic rabbits. In the present study, the effectiveness of antioxidant therapy is assessed by plasma TBARS levels and tissue nitrotyrosine staining. Plasma TBARS levels decreased by approximately 42% in hypercholesterolemic rabbits treated with an antioxidant, suggesting decreased global oxidative stress. NAC also decreases nitrotyrosine staining in grafts of hypercholesterolemic rabbits, indicating decreased local oxidative stress. The decreased peroxynitrite formation is likely due to increased glutathione formation and decreased macrophage accumulation in grafts of NAC-treated rabbits. Probucol does not show a similar decrease in nitrotyrosine staining, possibly due to the low sensitivity of this qualitative analysis and probucol’s different mechanism of action.
Hypercholesterolemia decreases graft endothelialization 10 and in this study, NAC significantly improves graft endothelialization in hypercholesterolemic rabbits, and probucol shows a potential trend toward improved graft endothelialization. The decreased endothelialization by probucol in chow rabbits may be due decreased HDL, which has been associated with probucol treatment.29, 30 In vitro studies have shown that HDL stimulates EC migration,31 and in vivo studies have shown decreased endothelialization after arterial injury in apolipoprotein A-I deficient mice with markedly decreased HDL levels.32 In our study, rabbits on the chow diet or HC diet have a significant decrease in HDL levels with probucol treatment. Thus, potentially promigratory effects of probucol’s antioxidant activity may be counteracted by the adverse effects of HDL lowering.
Our previous study showed that hypercholesterolemia increased IH,10 and the present study demonstrates that NAC and probucol are associated with a trend toward decreased anastomotic IH in hypercholesterolemic rabbits. These data are consistent with previous studies showing decreased IH with NAC or probucol after arterial injury in rabbits,29, 33–35 and following coronary angioplasty in humans.23, 24 Our probucol results are inconsistent with a previous report by Baumann et al.36 that showed no change in intimal thickening with probucol treatment, but significantly decreased number of SMCs and macrophages. Our study shows no difference in cellularity suggesting that the decrease in IH with antioxidants is due to a proportional decrease in cell accumulation and extracellular matrix deposition. OxLDL increases PDGF production that can result in SMC proliferation and increased collagen production.6, 7, 14 Both NAC and probucol reduce PDGF signaling by SMC and subsequently inhibit IH in animal models of arterial injury.14, 37
NAC and probucol have beneficial effects on graft healing in our hypercholesterolemic rabbits, but NAC is more effective than probucol. The antioxidants decreased systemic oxidative stress, but only NAC showed decreased local oxidative stress levels. Given the trend toward decreased oxidative stress levels without significant change in plasma cholesterol levels, the effects of NAC and probucol on graft healing are potentially due to their antioxidant activity. The mechanism for decreased tissue cholesterol deposition at the proximal anastomosis in hypercholesterolemic rabbits treated with NAC or probucol is unclear because not all segments of tissue were affected in the same manner. The difference may be related to decreased inflammatory infiltrate and improved endothelialization, or may be associated with increased deposition at the distal anastomosis due to turbulent blood flow. The differences in graft healing between NAC and probucol groups may be due to their distinct mechanisms of action, as well as the HDL-lowering effect of probucol, which would be expected to negatively affect graft endothelialization. Despite different mechanisms of action, NAC and probucol are associated with beneficial effects on prosthetic graft healing in hypercholesterolemia, suggesting the potential importance of their antioxidant activity.
Hypercholesterolemia leads to impaired graft healing in our rabbit model, and elevated serum lipids adversely affect EC growth and graft patency in humans.38 In the rabbit model, antioxidant therapy ameliorated the reduced endothelialization and increased IH due to hypercholesterolemia. Extrapolation of these findings to humans should be done with caution because of differences in graft healing. Analysis of ePTFE grafts removed from patients shows ECs only in the first few millimeters from the anastomoses,39 but ePTFE grafts seeded with venous ECs prior to implantation have endothelium lining the mid portion of these grafts years later.38 Furthermore, patency of EC seeded grafts is significantly improved compared with unseeded ePTFE grafts, and surface thrombogenicity is reduced.40 The current study shows that altering the environment into which a graft is placed has a dramatic effect on healing. This raises the possibility that long-term modification of oxidative stress might promote optimal graft healing in humans with resultant improvement in patency.
Acknowledgments
This project was supported by Grant Numbers HL41187, HL64357, and F32HL090205 from the National Heart, Lung, Blood Institute.
Footnotes
Competition of interest: nil
Presented as the American Vascular Association (AVA) Resident Research Prize Paper at the Sixty-third annual meeting of the Society for Vascular Surgery, Denver, CO (2009).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. American Journal of Preventive Medicine. 2007;32:328–33. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Yao E-H, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Current Pharmaceutical Biotechnology. 2006;7:101–8. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 3.Baumann DS, Doblas M, Daugherty A, Sicard G, Schonfeld G. The role of cholesterol accumulation in prosthetic vascular graft anastomotic intimal hyperplasia. J Vasc Surg. 1994;19:435–45. doi: 10.1016/s0741-5214(94)70070-2. [DOI] [PubMed] [Google Scholar]
- 4.Murugesan G, Chisolm GM, Fox PL. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993;120:1011–9. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992;111:143–7. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- 6.Absood A, Furutani A, Kawamura T, Graham LM. Differential PDGF secretion by graft and aortic SMC in response to oxidized LDL. Am J Physiol Heart Circ Physiol. 2002;283:H725–H32. doi: 10.1152/ajpheart.00060.2002. [DOI] [PubMed] [Google Scholar]
- 7.Absood A, Furutani A, Kawamura T, Graham LM. A comparison of oxidized LDL-induced collagen secretion by graft and aortic SMCs: role of PDGF. Am J Physiol Heart Circ Physiol. 2004;287:H1200–H6. doi: 10.1152/ajpheart.00228.2004. [DOI] [PubMed] [Google Scholar]
- 8.van Aalst JA, Pitsch RJ, Absood A, Fox PL, Graham LM. Mechanism of Dacron-activated monocytic cell oxidation of low density lipoprotein. J Vasc Surg. 2000;31:171–80. doi: 10.1016/s0741-5214(00)70079-6. [DOI] [PubMed] [Google Scholar]
- 9.van Aalst JA, Fox PL, Graham LM. Lipid oxidation products in implanted vascular grafts. Surg Forum. 1998;49:324–6. [Google Scholar]
- 10.Miyazaki K, Colles SM, Graham LM. Impaired graft healing due to hypercholesterolemia is prevented by dietary suplementation with α-tocopherol. J Vasc Surg. 2008;48:986–93. doi: 10.1016/j.jvs.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campeau L, Enjalbert M, Lespérance J, Bourassa MG, Kwiterovich P, Wacholder S, et al. The relation of risk factors to the development of atherosclerosis in saphenous-vein bypass grafts and the progression of disease in the native circulation. N Engl J Med. 1984;311:1329–32. doi: 10.1056/NEJM198411223112101. [DOI] [PubMed] [Google Scholar]
- 12.AbuRahma AF, Robinson PA, Stuart SP, Witsberger TA, Stewart WA, Boland JP. Polytetrafluoroethylene grafts in infrainguinal arterial revascularization. Factors affecting outcome Arch Surg. 1993;128:417–22. doi: 10.1001/archsurg.1993.01420160055008. [DOI] [PubMed] [Google Scholar]
- 13.van Aalst JA, Zhang D-M, Miyazaki K, Colles SM, Fox PL, Graham LM. Role of reactive oxygen species in inhibition of endothelial cell migration by oxidized low-density lipoprotein. J Vasc Surg. 2004;40:1208–15. doi: 10.1016/j.jvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Kappert K, Sparwel J, Sandin Å, Seiler A, Siebolts U, Leppänen O, et al. Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol. 2006;26:2644–51. doi: 10.1161/01.ATV.0000246777.30819.85. [DOI] [PubMed] [Google Scholar]
- 15.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Boil Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 16.Witko-Sarsat V, Gausson V, Nguyen A-T, Touam M, Drüeke T, Santangelo F, et al. AOPP-induced activation of human nuetrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 17.McNamara JR, Huang C, Massov T, Leary ET, Warnick GR, Rubins HB, et al. Modification of the dextran-Mg2+ high-density lipoprotein cholesterol precipitation method for use with previously frozen plasma. Clin Chem. 1994 Feb;40(2):233–9. [PubMed] [Google Scholar]
- 18.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–6. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 20.Goh EH, Krauth DK, Colles SM. Analysis of cholesterol and desmosterol in cultured cells without organic solvent extraction. Lipids. 1990;25:738–41. doi: 10.1007/BF02544043. [DOI] [PubMed] [Google Scholar]
- 21.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. PhysiolRev. 2004;84:1381–478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 22.Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–27. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tardif J-C, Côté G, Lespérance J, Bourassa M, Lambert J, Doucet S, et al. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. N Engl J Med. 1997;337:365–72. doi: 10.1056/NEJM199708073370601. [DOI] [PubMed] [Google Scholar]
- 24.Daido H, Kuwabara Y, Yokoi H, Nishikawa H, Takatsu F, Nakata Y, et al. Effect of probucol on repeat revascularization rate after percutaneous transluminal coronary angioplasty (from the Probucol Angiplasty Restenosis Trial [PART]) Am J Cardiol. 2000;86:550–2. doi: 10.1016/s0002-9149(00)01013-4. [DOI] [PubMed] [Google Scholar]
- 25.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Ciccolo A, Centorrino T, et al. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15:1187–200. doi: 10.1096/fj.00-0526hyp. [DOI] [PubMed] [Google Scholar]
- 26.Geudens N, Van De Wauwer C, Neyrinck AP, Timmermans L, Vanhooren HM, Vanaudenaerde BM, et al. N-acetyl cysteine pre-treatment attenuates inflammatory changes in the warm ischemic murine lung. Journal of Heart and Lung Transplantation. 2007;26:1326–32. doi: 10.1016/j.healun.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kB. Annu Rev Cell Biol. 1994;10:405–55. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi K, Takahata H, Kitagawa N, Kitange G, Kaminogo M, Shibata S. N-acetylcysteine inhibited nuclear factor-kB expression and the intimal hyperplasia in rat carotid arterial injury. Neurol Res. 2001;23:731–8. doi: 10.1179/016164101101199252. [DOI] [PubMed] [Google Scholar]
- 29.Lau AK, Leichtweis SB, Hume P, Mashima R, Hou JY, Chaufour X, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation. 2003;107:2031–6. doi: 10.1161/01.CIR.0000062682.40051.43. [DOI] [PubMed] [Google Scholar]
- 30.Johansson J, Olsson AG, Bergstrand L, Elinder LS, Nilsson S, Erikson U, et al. Lowering of HDL2b by probucol partly explains the failure of the drug to affect femoral atherosclerosis in subjects with hypercholesterolemia. A Probucol Quantitative Regression Swedish Trial (PQRST) report. Arterioscler Thromb Vasc Biol. 1995;15:1049–56. doi: 10.1161/01.atv.15.8.1049. [DOI] [PubMed] [Google Scholar]
- 31.Murugesan G, Sa G, Fox PL. High-density lipoprotein stimulates endothelial cell movement by a mechanism distinct from basic fibroblast growth factor. Circ Res. 1994;74:1149–56. doi: 10.1161/01.res.74.6.1149. [DOI] [PubMed] [Google Scholar]
- 32.Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 33.Mass H, Pirazzi B, Gonzalez P, Collazo V, Fitzovich D, Avakian E. N-acetylcysteine diminishes injury induced by balloon angioplasty of the carotid artery in rabbits. Biochem Biophys Res Commun. 1995;215:613–8. doi: 10.1006/bbrc.1995.2508. [DOI] [PubMed] [Google Scholar]
- 34.Ghigliotti G, Mereto E, Eisenberg PR, Martelli A, Orsi P, Sini D, et al. N-acetyl-cysteine reduces neointimal thickening and procoagulant activity after balloon-induced injury in abdominal aortae of New Zealand White rabbits. Thromb Haemost. 2001;85:724–9. [PubMed] [Google Scholar]
- 35.Tanous D, Bräsen JH, Choy K, Wu BJ, Kathir K, Lau A, et al. Probucol inhibits in-stent thrombosis and neointimal hyperplasia by promoting re-endothelialization. Atherosclerosis. 2006;189:342–9. doi: 10.1016/j.atherosclerosis.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 36.Baumann DS, Doblas M, Schonfeld G, Sicard GA, Daugherty A. Probucol reduces the cellularity of aortic intimal thickening at anastomotic regions adjacent to prosthetic grafts in cholesterol-fed rabbits. Arterioscler Thromb. 1994;14:162–7. doi: 10.1161/01.atv.14.1.162. [DOI] [PubMed] [Google Scholar]
- 37.Miyauchi K, Aikawa M, Tani T, Nakahara K, Kawai S, Nagai R, et al. Effect of probucol on smooth muscle cell proliferation and dedifferentiation after vascular injury in rabbits: Possible role of PDGF. Cardiovasc Drugs Ther. 1998;12:251–60. doi: 10.1023/a:1007761631674. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–62. doi: 10.1016/j.jvs.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 39.Formichi MJ, Guidoin RG, Jausseran JM, Awad JA, Johnston KW, King MW, et al. Expanded PTFE prostheses as arterial substitutes in humans: Late pathological findings in 73 excised grafts. Annals of Vascular Surgery. 1998;2:14–27. doi: 10.1016/s0890-5096(06)60773-5. [DOI] [PubMed] [Google Scholar]
- 40.Meinhart J, Deutsch M, Zilla P. Eight years of clinical endothelial cell transplantation. Closing the gap between prosthetic grafts and vein grafts. ASAIO J. 1997 Sep–Oct;43(5):M515–21. [PubMed] [Google Scholar]


