Abstract
Background
The incidence of non-AIDS-defining malignancies (non-ADM) is reported as unchanged or increasing in the HAART era. Whether incidence of non-ADM is significantly higher in HIV-infected than in HIV-uninfected patients remains unclear.
Methods
Incidence rates of malignancies were calculated in a cohort of veterans in care for HIV-infected and age, race, and gender-matched uninfected patients from 1997 to 2004. For HIV-infected patients CD4 counts closest to first observation date were compared between those with and without cancer.
Results
33,420 HIV-infected and 66,840 HIV-uninfected patients were followed for a median of 5.1 and 6.4 years. The Incidence rate ratio [IRR] of HIV-infected to HIV-uninfected was 1.6 (1260 vs. 841/100,000 person-years; 95% CI: 1.5–1.7). IRR for individual cancers was highest for anal cancer (14.9; CI: 10.1–22.1). Among HIV-infected patients, median CD4 counts were lower for those with non-ADM (249 vs. 270, p=0.02), anal cancer (154 vs. 270; p<0.001), and Hodgkin’s (217 vs. 270; p=0.03). Prostate cancer was associated with a higher CD4 count (310 vs. 270; p<0.001).
Conclusions
In the HAART era, the incidence of non-ADMs is higher among HIV-infected than HIV-uninfected patients, adjusting for age, race, and gender. Some non-ADMs do not appear to be associated with significantly lower CD4 counts.
Keywords: AIDS-defining malignancies, Non-AIDS-defining malignancies, Incidence, HAART
Introduction
Prior to HAART, certain AIDS-defining malignancies (ADMs: non-Hodgkins lymphoma, Kaposi’s sarcoma and invasive cervical carcinoma) were found to be highly correlated with the degree of immunosuppression. With use of HAART and the resulting improved survival1, 2, the incidence of ADMs has declined3, 4. Yet, the incidence of malignancies not known to be associated with immunosuppression (non-AIDS-defining malignancies or non-ADMs 5) have been found to be significantly more common than in the general population 3, 5–7. Recent studies suggest that the incidence of selected non-ADMs has increased in the HAART era 6, 8–12, but these studies have not included matched cohorts of HIV-positive and HIV-negative patients.
We used data from the United States Veterans Affairs Healthcare System to compare rates of non-ADMs between HIV-infected and matched HIV-uninfected patients receiving care during the period of 1997 through 2004 (HAART era), and determine the association of non-ADM incidence with the degree of immunosuppression at baseline among HIV-infected patients. The purpose of this study is to determine whether HIV infection is associated with an increase in the risk of non-ADMs, and to determine whether this risk is associated with the patients’ level of immunosuppression. A better characterization of the incidence of non-ADMs, and their risk factors will likely allow for identification and implementation of screening and preventive measures specific to this population, as well as improvement of the outcomes of patients with HIV and cancer.
Methods
Use of VA national administrative data was approved by the VA Pittsburgh Healthcare System and University of Pittsburgh institutional review boards (Pittsburgh, PA). VA electronic medical record data from patient treatment files and outpatient clinic files were used. 13 We adapted an algorithm developed by Fasciano et al 13 using International Classification of Diseases, ninth revision (ICD-9-CM) diagnostic codes to identify patients with a first HIV or AIDS diagnosis from October 1997 to September 2004. Our modifications required patients to have one or more inpatient or two or more outpatient HIV-related codes rather than a single inpatient or outpatient HIV-related codes. Our program searched for the following ICD-9-CM codes: AIDS (042), asymptomatic HIV (V08), and related Diagnostic Related Group codes (488–490). We have previously demonstrated that requiring at least two outpatient codes or one inpatient code results in substantial improvement in positive predictive value (88% vs. 69%) without a substantial decrement in sensitivity, specificity, or negative predictive value14. For each HIV-positive veteran identified, we identified two HIV-negative patients matched on age, sex, race, geographic location, and in care in the same year. This methodology is described in full elsewhere 15. Subjects were followed from the date of their first HIV-related diagnostic code, or their matching date. Subjects were censored at their date of death, or last encounter within the VA before September 2004.
We searched for diagnostic codes for cancers in inpatient files from October 1990 and in outpatient files from October 1996 through September 2004. We using ICD-9-CM site-specific cancer codes as identified by Surveillance Epidemiology and End Results (SEER)16. Cancers were included if the veteran had one or more inpatient or two or more outpatient cancer diagnoses of the same type. Patients with cancer diagnosis prior to their HIV diagnosis or match date were excluded from analyses for that particular cancer. For the overall category of non-ADM we included all cancers except lymphoma, Kaposi’s sarcoma, cervical carcinoma, skin, and ill-defined cancers. We excluded skin and ill-defined cancers because of concern of overlap with other cancer categories.. The non-ADMs we examined individually are: anal, lung, melanoma, prostate, Hodgkins, and liver. For comparison purposes, we also incude the ADM: Kaposi’s Sarcoma, Non-Hodgkin’s lymphoma (NHL), and cervical carcinoma.
CD4 data were available for HIV-infected veterans who were also enrolled in the Immunology Case Registry (ICR). The ICR is a VA registry designed to collect data on all HIV-infected veterans in care15 . Of the VACS Virtual Cohort HIV-infected veterans, 86% were also enrolled in the ICR. Additionally, the CD4 dataset ends at February 2003, but our identification date goes until September 2004. Of HIV-infected veterans in the ICR and with a first observation date prior to February 2004, 86% (22,725) have a baseline CD4 value. We created a variable for number of clinic visits during the first year of observation. Primary care visits and infectious disease subspecialty visits were included in this category. We include infectious disease visits because that is where HIV-infected patients receive much of their “general” care. Multiple clinic visits on the same day were counted as only one visit.
Because hepatitis C virus and alchol use are known cancer risk factors 17, we describe the prevalence for HIV-infected and uninfected veterans in the VACS Virtual Cohort. We considered alcohol abuse or dependence present if there were one or more inpateint or two or more outpateint ICD-9 diagnosis codes. Hepatits C virus was considered present if there were one or more inpatient or two or more outpatient ICD-9 diagnosis codes or a positive laboratory value for hepatitis C virus.
Analyses
Demographic characteristics, observation time, fiscal year identified in cohort, number of clinic visits, hepatitis C infection, and alcohol abuse and dependence were described for the HIV-infected and uninfected veterans. Incidence rates (IRs) per 100,000 person years of specific non-ADMs, ADMs, and total non-ADMs were calculated and compared between HIV-infected and uninfected patients. Time at risk (person-years) was calculated from date of enrollment into the cohort to date of cancer diagnosis or last documented clinic visit or date of death. HIV-infected compared with HIV-uninfected age-, race-, and gender-adjusted incidence rate ratios (IRRs) and 95% confidence intervals (CI) were calculated using Poisson regression analysis. As a sub-analysis, IRs and IRRs by year of entering the cohort (two year groupings) were also calculated for total non-ADMs. We also ran the poisson models limiting follow-up time to a maximum of two years.
Median CD4 cell counts closest to first observation date were compared between those with and without cancer, using Wilcoxon rank-sum tests, to determine the association of immunosuppression with rates of malignancies in HIV-infected patients. CD4 count at first observation was used to allow an analysis of immunodeficiency as a risk factor for cancer. If counts at cancer diagnosis were used, there would be no means of comparing counts between those with and without cancer. Comparing counts at diagnosis among those with cancer and counts at baseline for those who did not develop cancer would be inherently biased because, all else equal, CD4 counts decrease with time. Further, the cancer itself may affect the CD4 cell count, making the value obtained at or immediately after a cancer diagnosis difficult to interpret.
Results
A total of 33,420 HIV-infected and 66,840 age-, gender-, race/ethnicity- and location- matched HIV-uninfected patients were identified in the VACS Virtual Cohort. Of those, 32,942 (98.6%) of HIV-infected and 64,966 (97.2%) of HIV-uninfected had greater than zero days of observation time. Almost half of the HIV-infected and uninfected subjects (46.4% and 46.5%) were identified in fiscal year 1997, the majority representing patients already in care, with slightly decreasing numbers of subjects identified for most of the subsequent years. Years subsequent to 1997 represent patients new to HIV care in the VA system, not necessarily newly infected. The mean age of the patients were 45.8 (SD: 10.0) in the HIV-infected group and 46.1 (SD: 10.1) in the HIV-uninfected group. The proportion of males in each group was 97.8. Race/ethnicity was similar between groups. Overall, 32% were White, 43% African-American, 8% Hispanic, and 17%, other/unkown. The median follow-up time was 5.1 years for the HIV-infected patients and 6.4 years for the HIV-uninfected patients. HIV-infected patients had a median of 5 visits (Interquartile Range(IQR): 3–8) in the year following identification in cohort compared to a median of 2 visits (IQR: 1–3) for HIV-uninfected patients. Alcohol abuse or dependence was similar between groups (21% for HIV-infected vs. 20% for HIV-uninfected). However, Hepatits C infection was more common among HIV-infected veterans (36% vs. 12%; Table 1).
Table 1.
Characteristics of VACS Virtual Cohort
| HIV-infected (n=32,942) |
HIV-uninfected (n=64,966) |
|
|---|---|---|
| Age, mean in years (SD) | 45.8 (10.0) | 46.1 (10.1) |
| Female, No. (%) | 724 (2.2) | 1,430 (2.2) |
| Race/Ethnicity, No (%) | ||
| White | 10,665 (32.4) | 21,141 (32.5) |
| Black | 14,163 (43.0) | 28,260 (43.5) |
| Hispanic | 2,472 (7.5) | 4,961 (7.6) |
| Other/Unknown | 5,642 (17.1) | 10,604 (16.3) |
| Year Identified (%) | ||
| 1997 | 15,298 (46.4) | 30,235 (46.5) |
| 1998 | 3,605 (10.9) | 7,115 (11.0) |
| 1999 | 2,850 (8.7) | 5,649 (8.7) |
| 2000 | 2,393 (7.3) | 4,786 (7.4) |
| 2001 | 2,361 (7.2) | 4,661 (7.2) |
| 2002 | 2,239 (6.8) | 4,380 (6.7) |
| 2003 | 2,307 (7.0) | 4,473 (6.9) |
| 2004 | 1,889 (5.7) | 3,667 (5.6) |
| Median observation time in years | 5.1 | 6.4 |
| Median number of visits during year after first observation date (Interquartile Range) | 5 (3–8) |
2 (1–3) |
| Hepatitis C positive (%) | 35.8 | 11.6 |
| Alcohol abuse or dependence (%) | 21.1 | 19.6 |
In Poisson models adjusted for gender, race/ethnicity, and age, IRRs indicate that HIV-infected veterans were more likely to have anal, lung, melanoma, Hodgkins, and liver cancer than HIV-uninfected veterans. Likelihood of prostate cancer was similar between HIV-infected and HIV-uninfected patients (IRR: 1.0; CI: 0.9–1.1).
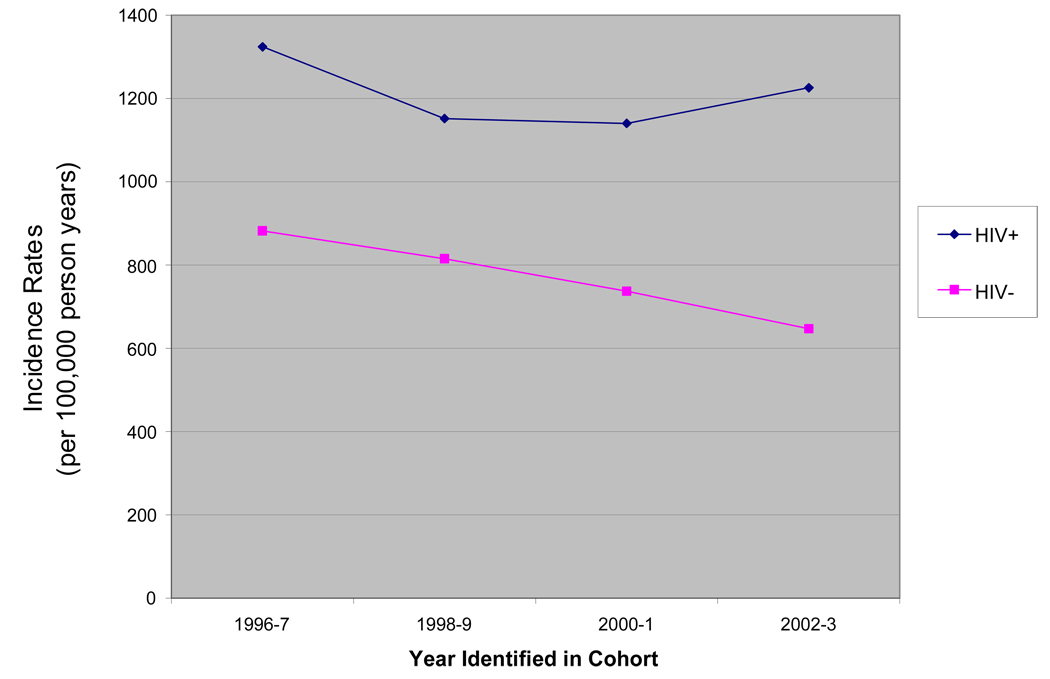
A total of 2128 and 3142 non-ADMs were diagnosed among the HIV-infected and unifected groups, respectively. IRs (per 100,000 person-years) were 1260.5 among HIV-infected and 841.8 among HIV-uninfected. The non-ADMs HIV-infected to uninfected IRR was 1.6 (95% CI: 1.5–1.7) (Table 2). IRs of non-ADMs were also compared for consecutive two-year periods. There was statistically significant decline in the rate of non-ADMs among the HIV-uninfected patients from 1998–99 to 2000–01 (p=.003). Conversely, rates on non-ADMs remained stable over time among the HIV-infected group (Figure 1). HIV-infected to uninfected IRRs for each two-year period (from 1996–1997 to 2002–2003) were statistically significant, and increased from 1998–1999 and onward: 1996–1997 IRR: 1.6; 95% CI 1.5–1.7; 1998–1999 IRR: 1.5; 95% CI: 1.3 – 1.7; and 2002–2003 IRR: 2.0; 95% CI: 1.6 – 2.6. Patterns were similar for IRRs using a maximum of two years of follow-up time (data not shown).
Table 2.
IRs and IRRs of Cancers and CD4 Counts for HIV+ Patients
| HIV+ to HIV− | Median CD4 in HIV+ |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cancer | Frequency | Incidence Rate1 | IRR3 | Cancer | ||||
| Type | HIV+ | HIV− | HIV+ | HIV− | (95% CI) | Yes | No | P-value |
| Anal | 195 | 29 | 111.2 | 7.4 | 14.9 (10.1–22.1) | 156 | 270 | <0.001 |
| Lung | 504 | 604 | 287.9 | 155.5 | 2.0 (1.8–2.2) | 246 | 269 | 0.2 |
| Melanoma | 96 | 124 | 54.6 | 31.9 | 1.7 (1.3–2.3) | 267 | 268 | 0.3 |
| Prostate | 441 | 1041 | 259.8 | 277.9 | 1.0 (0.9–1.1) | 311 | 266 | <.001 |
| Hodgkin’s | 135 | 62 | 76.9 | 15.9 | 4.9 (3.6–6.6) | 217 | 269 | 0.03 |
| Liver | 172 | 144 | 97.8 | 36.9 | 2.8 (2.2–3.5) | 271 | 268 | 0.3 |
| All non-ADM2 | 2128 | 3142 | 1260.5 | 841.8 | 1.6 (1.5–1.7) | 249 | 270 | .02 |
| Kaposi’s Sarcoma | 661 | 7 | 382.3 | 1.80 | 209.8 (99.6–441.8) | 160 | 272 | <.001 |
| NHL | 691 | 196 | 398.1 | 50.4 | 8.02 (6.84–9.40) | 171 | 272 | <.001 |
| Cervical | 12 | 2 | 292.5 | 22.8 | 12.8 (2.9–57.3) | 154 | 340 | .03 |
per 100,000 person-years
includes all cancers except KS, lymphoma, cervical, skin, and ill-defined
Incidence Rate Ratios (IRR) and 95% Confidence Intervals (CI) adjusted for age, race, and gender using Poisson models
Figure 1.
Incidence Rates of Non-ADM, by Year Identified in Cohort
Compared to HIV-infected patients without cancer the median CD4 counts were lower for patients with non-ADM (249 vs. 270, p=0.02), anal cancer (156 vs. 270; p<0.001), and Hodgkin’s (217 vs. 269; p=0.03). For all other non-ADM, there was no statistically significant difference between the median CD4 counts of cancer patients and non-cancer patients, with the notable exception of prostate cancer which was associated with a higher CD4 count (311 vs. 266; p<0.001).
We also report the IRs, IRRs, and CD4 counts for the ADM. ADM have higher IRs as expected. Among HIV-infected pateints, CD4 counts are statistically significantly lower for patients with each ADM than for those without (Table 2).
Discussion
In the HAART era, the incidence of non-ADMs is significantly higher among HIV-infected than age-, race-, and gender-matched HIV-uninfected patients. After a median follow-up time of over 5 years, the IRR of all non-ADM among HIV-infected patients was 1.6 (95% CI: 1.5–1.7). This 60% higher rate of non-ADM among HIV-infected patients was consistent for two time-periods representing early and more recent HAART eras (1996–1997 to 2002–2003, respectively). Further, cancers other than those strongly asssociated with HCV infection (hepatoma) or men who have sex with men (anal cancer) demonstrated significantly increased IRR when HIV infected patients were compared with demographically similar uninfected patients.
Patients with non-ADMs tend to have somewhat lower CD4 counts than non-cancer patients. This effect appears to be largely driven by anal cancers which not only have a significantly higher IRR (in HIV-infected vs. HIV-uninfected patients), but appear to be significantly associated with immunosuppression in our cohort, whith the median CD4 count of anal cancer patients being only 58% of that of non-cancer patients. Both the very high IRR and the association of immunosuppression make the epidemiology of this malignancy more similar to that of ADM than non-ADM . Indeed, the biology of anal cancer – with its strong association with Human Papilloma Virus (HPV) infection – is very similar to that of cervical cancer, which is included among ADMs. Prostate cancer patients had higher median CD4 count than non-cancer patients with HIV infection. This result, consistent with previous observations of a high mean CD4 count in HIV patients with prostate cancer,18 is intriguing, but it should also be noted that prostate cancer was equally common among HIV infected and uninfected subjects.
Congruent to our findings, a previously published analysis of linked population-based AIDS and cancer registry data from 11 geographically diverse areas in the United States 7 concluded that selected non-ADM, including Hodgkin’s disease, lung cancer, soft tissue malignancies, lip and testicular cancer were potentially associated with immunosuppression. Consistent with this observation, an interesting study has recently been published comparing cancer incidence in population-based cohort studies of people with HIV/AIDS and solid organ transplantation (another immunosuppressed population in which epidemiologic studies have also found a wide range of cancers) 19. The authors interpreted their finding of similar spectra and a similar pattern of increased risk of cancer in the two populations as strongly suggesting that immune deficiency is the likely underlying cause.
However, with the exception of anal cancer and Hodgkin’s disease, an association has not been consistently found between advancing immunosuppression and the development of non-ADM (Table 2) among HIV infected patients4, 5, 7, 20. Indeed, even if patients with non-ADMs tend to have slightly lower CD4 counts than non-cancer patients in some cohorts, these malignancies do not appear to share the same association with degree of immunosuppression as the AIDS-defining malignancies 4, 7. One possible explanation of the increased risk is that, despite improved immune function on HAART, cancer immune surveillance is still inadequate in patients with HIV.
It is possible that despite the matching, the current findings reflect an overrepresentation of traditional cancer risk factors among the HIV-infected patients, especially viral co-infections (HCV and HPV) and smoking status. Several studies have described an increased prevalence and recurrence of both cervical HPV infection and invasive cervical cancer among HIV-1 positive women compared to HIV-1 negative patients, especially among those with low CD4 cell counts21, 22. The prevalence of oral, anal, and cervical HPV infection in HIV-positive individuals compared with HIV-negative individuals increases with progressively lower CD4+ levels, as does incident high-grade intraepithelial neoplasia (CIN) 22–25. While we do not have the rates of HPV infection among HIV-infected and uninfected patients in the VACS Virtual Cohort, it could be inferred by the proportion of men who have sex with men in both groups in the VACS Ongoing Study Cohort, (47.5% and 6.4% respectively) that the HPV infection rates would be higher in HIV-infected patients.
An excess risk of traditionally smoking-related cancers is seen in HIV-infected patients 3, 4, 6, 7. Importantly, Crothers et al., 26 found (through a self-administered questionnaire) nearly equivalent rates of current or past smoking history in 1,014 HIV-infected and 713 HIV-uninfected veterans enrolled in the Veterans Aging Cohort 5 Site Study in 2001 and 2002 (75% vs. 76%). Regardless, after controlling for smoking, rates of lung cancer are higher in HIV-infected than uninfected patients 27. Consistent with a prior analysis of by McGinnis et al.17, we find that rates of alcohol abuse and dependence are similar in among HIV-infected and uninfected patients in the VACS Virtual Cohort (21% and 20% respectively), but HCV co-infection is more common among HIV-infected than uninfected patients (36% vs. 12%).
A possible limitation to our study is that our findings may be explained by ascertainment bias. Specifically, the HIV-infected patients were seen more often than uninfected patients and therefore may have had more opportunity to be have a diagnosis of cancer made and recorded resulting in the HIV infected patients appearing to have higher rates of cancer when the rates were not truly different. We think this is unlikely. First, if ascertainment bias explained our results, we would have expected a more uniform effect across cancers. Instead only a handful of cancers were more common and, among HIV infected individuals, these cancers were more common among more immunosuppressed individuals. Second, cancer rates among the uninfected comparators in our cohort are higher than those reported by SEER for the general population 16, 17. This suggests that, if anything, our estimate of rates among the controls may be upwardly biased. Specifically, controls had to have a reason to seek medical care—one of which might be symptoms of cancer. Thus, our estimate of relative rates comparing HIV infected individuals to HIV uninfected demographic controls receiving care in the VA may instead be a conservative estimate of the true difference in rates. Finally, others have also found increased rates of specific non-ADMs among those with HIV infection 4, 6, 12. There remains the strong possibility that HIV itself and/or HAART use have an oncogenic potential, which would be likely to be more evident as survival of HIV patients lengthens, and treatment duration is prolonged 28 29.
There was an increase in IRR of non-ADM over time in our cohort, with the highest rates being recorded in the later years of the follow-up period. All patients were observed exclusively in the HAART era (1997 through 2004), and HAART use among participants of the VACS is estimated at 80% 26, 30. Data on use of HAART by individual patients in the cohort is not included in this analysis, and the potential impact of HAART on the incidence of malignancies was not evaluated. However, other studies have shown significantly higher incidence of non-ADMs in the HAART era, compared to the pre-HAART era 4, 8, 11, 27, 31. We have shown that all studies that failed to show an increase in non-ADM rates in the HAART era had an average follow-up time of less than 1 year per patient in the HAART era. When follow-up time in the HAART era was greater than 1 year per patient, an increase in rates was oberved (author reply to Dal Maso et al 32).
The strengths of our study include a thorough description of the VACS cohort, including ascertainment of the rates of several cancer risk factors, including smoking, substance abuse and HCV co-infection, which cannot be ascertained in population-based comparison groups 3, 7, 12 3, 4, 6; matching, and a long median follow-up time, compared to some previous studies which allows more lag time for detection of cancer incidences 3, 6, 32. In addition, for ascertainment of patient diagnoses (HIV and malignancy diagnoses) we used a validated method requiring at least 2 outpatient and one inpatient diagnostic codes, which maximizes the positive predictive value of the identification algorithm 15. Our major limitations include the uneven distribution of some cancer risk factors (such as HCV infection) within the two groups and the overrepresentation of males making it difficult to adequately evaluate rates of female-specific cancers.
In conclusion, after adjusting for age, race and gender, the incidence of non-ADMs is significantly higher among HIV-infected than HIV-uninfected patients in the HAART era. This pattern is consistent throughout the observation period and is not only true of cancers associated with HCV infection or homosexual orientation. These trends warrant a high index of suspicion for malignancies among HIV providers, and a renewed focus on understanding the mechanisms underlying the increased rates.
Acknowledgments
Funding Sources: the National Institute on Alcohol and Alcohol Abuse 52U10 AA 13566); the Veterans Health Administration; the VHA Office of Research and Development; and the VHA Public Health Strategic Health Care Group.
Footnotes
- Hot Topics in HIV (Data included in the review). ICAAC/IDSA 2008,; Washington, DC, October 24–24, 2008
- Bedimo R, et al. Incidence of Non-AIDS-Defining Malignancies in HIV-Infected Vs. Non-Infected Veterans in The HAART Era: Impact of Immunosuppression. XVII International AIDS Conference, Mexico City, August 2–8, 2008. Abstract MOPE0243
- Bedimo R, et al. (Partial Data) Incidence of Non-AIDS-Defining Malignancies in HIV-Infected Vs. Non-Infected Veterans in The HAART Era: Impact of Immunosuppression. ICCAC 2007, Abstract H-1721; Chicago, IL, September 17–20, 2007.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs
Contributor Information
Roger J. Bedimo, VA North Texas Health Care System and University of Texas Southwestern Medical Center Dallas, TX.
Kathleen A. McGinnis, VA Pittsburgh Healthcare System, Pittsburgh, PA.
Melinda Dunlap, UT Southwestern Medical Center, Dallas, TX.
Maria C. Rodriguez-Barradas, Michael E. DeBakey VA Medical Center, and Baylor College of Medicine, Houston, TX.
Amy C. Justice, Yale University School of Medicine, West Haven, CT..
References
- 1.Mocroft A, Brettle R, Kirk O, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. Aids. 2002 Aug 16;16(12):1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000 Nov 15;92(22):1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 4.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005 Mar 16;97(6):425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 5.Mbulaiteye SM, Biggar RJ, Goedert JJ, Engels EA. Immune deficiency and risk for malignancy among persons with AIDS. J Acquir Immune Defic Syndr. 2003 Apr 15;32(5):527–533. doi: 10.1097/00126334-200304150-00010. [DOI] [PubMed] [Google Scholar]
- 6.Herida M, Mary-Krause M, Kaphan R, et al. Incidence of non-AIDS-defining cancers before and during the highly active antiretroviral therapy era in a cohort of human immunodeficiency virus-infected patients. J Clin Oncol. 2003 Sep 15;21(18):3447–3453. doi: 10.1200/JCO.2003.01.096. [DOI] [PubMed] [Google Scholar]
- 7.Frisch M, Biggar RJ, Engels EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. Jama. 2001 Apr 4;285(13):1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 8.Bedimo R, Chen RY, Accortt NA, et al. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989–2002. Clin Infect Dis. 2004 Nov 1;39(9):1380–1384. doi: 10.1086/424883. [DOI] [PubMed] [Google Scholar]
- 9.Patel P, Novak RM, Tong T. Incidence of Non-AIDS-defining Malignancies in the HIV Outpatient Study. Paper presented at: 11th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2004; San Francisco. [Google Scholar]
- 10.Pantanowitz L, Schlecht HP, Dezube BJ. The growing problem of non-AIDS-defining malignancies in HIV. Curr Opin Oncol. 2006 Sep;18(5):469–478. doi: 10.1097/01.cco.0000239886.13537.ed. [DOI] [PubMed] [Google Scholar]
- 11.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. Aids. 2006 Aug 1;20(12):1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 13.Fasciano NJ, Cherlow AL, Turner BJ, Thornton CV. Profile of Medicare beneficiaries with AIDS: application of an AIDS casefinding algorithm. Health Care Financ Rev. 1998 Spring;19(3):19–38. [PubMed] [Google Scholar]
- 14.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: A comparison of disease measurement strategies. Med Care. 2006 Aug;44(8 Suppl 2):S52–S60. doi: 10.1097/01.mlr.0000228003.08925.8c. [DOI] [PubMed] [Google Scholar]
- 15.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a "virtual" cohort using the National VA Health Information System. Med Care. 2006 Aug;44(8 Suppl 2):S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 16.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 17.McGinnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular carcinoma and non-Hodgkin's lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol. 2006 Nov 1;24(31):5005–5009. doi: 10.1200/JCO.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 18.Pantanowitz L, Bohac G, Cooley TP, Aboulafia D, Dezube BJ. Human immunodeficiency virus-associated prostate cancer: clinicopathological findings and outcome in a multi-institutional study. BJU Int. 2008 Apr 2; doi: 10.1111/j.1464-410X.2008.07474.x. [DOI] [PubMed] [Google Scholar]
- 19.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul 7;370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 20.Vilchez RA, Finch CJ, Jorgensen JL, Butel JS. The clinical epidemiology of Hodgkin lymphoma in HIV-infected patients in the highly active antiretroviral therapy (HAART) era. Medicine (Baltimore) 2003 Mar;82(2):77–81. doi: 10.1097/00005792-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Branca M, Garbuglia AR, Benedetto A, et al. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003 Jun;14(6):417–425. doi: 10.1258/095646203765371321. [DOI] [PubMed] [Google Scholar]
- 22.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000 Jun 15;151(12):1148–1157. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 23.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001 Feb 1;183(3):383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 24.Volkow P, Rubi S, Lizano M, et al. High prevalence of oncogenic human papillomavirus in the genital tract of women with human immunodeficiency virus. Gynecol Oncol. 2001 Jul;82(1):27–31. doi: 10.1006/gyno.2001.6244. [DOI] [PubMed] [Google Scholar]
- 25.Hagensee ME, Cameron JE, Leigh JE, Clark RA. Human papillomavirus infection and disease in HIV-infected individuals. Am J Med Sci. 2004 Jul;328(1):57–63. doi: 10.1097/00000441-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006 Nov;130(5):1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 27.Kirk GD, Merlo C, O'Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007 Jul 1;45(1):103–110. doi: 10.1086/518606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyagol J, Leucci E, Onnis A, et al. The Effects of HIV-1 Tat Protein on Cell Cycle during Cervical Carcinogenesis. Cancer Biol Ther. 2006 Jun;5(6):684–690. doi: 10.4161/cbt.5.6.2907. [DOI] [PubMed] [Google Scholar]
- 29.Carter MM, Torres SM, Cook DL, Jr, et al. Relative mutagenic potencies of several nucleoside analogs, alone or in drug pairs, at the HPRT and TK loci of human TK6 lymphoblastoid cells. Environ Mol Mutagen. 2007 Apr–May;48(3–4):239–247. doi: 10.1002/em.20282. [DOI] [PubMed] [Google Scholar]
- 30.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006 Aug;44(8 Suppl 2):S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bower M, Powles T, Nelson M, et al. HIV-related lung cancer in the era of highly active antiretroviral therapy. Aids. 2003 Feb 14;17(3):371–375. doi: 10.1097/00002030-200302140-00011. [DOI] [PubMed] [Google Scholar]
- 32.Dal Maso L, Tirelli U, Polesel J, Franceschi S. Trends in cancer incidence rates among HIV-infected patients. Clin Infect Dis. 2005 Jul 1;41(1):124–126. doi: 10.1086/430832. author reply 126–127. [DOI] [PubMed] [Google Scholar]