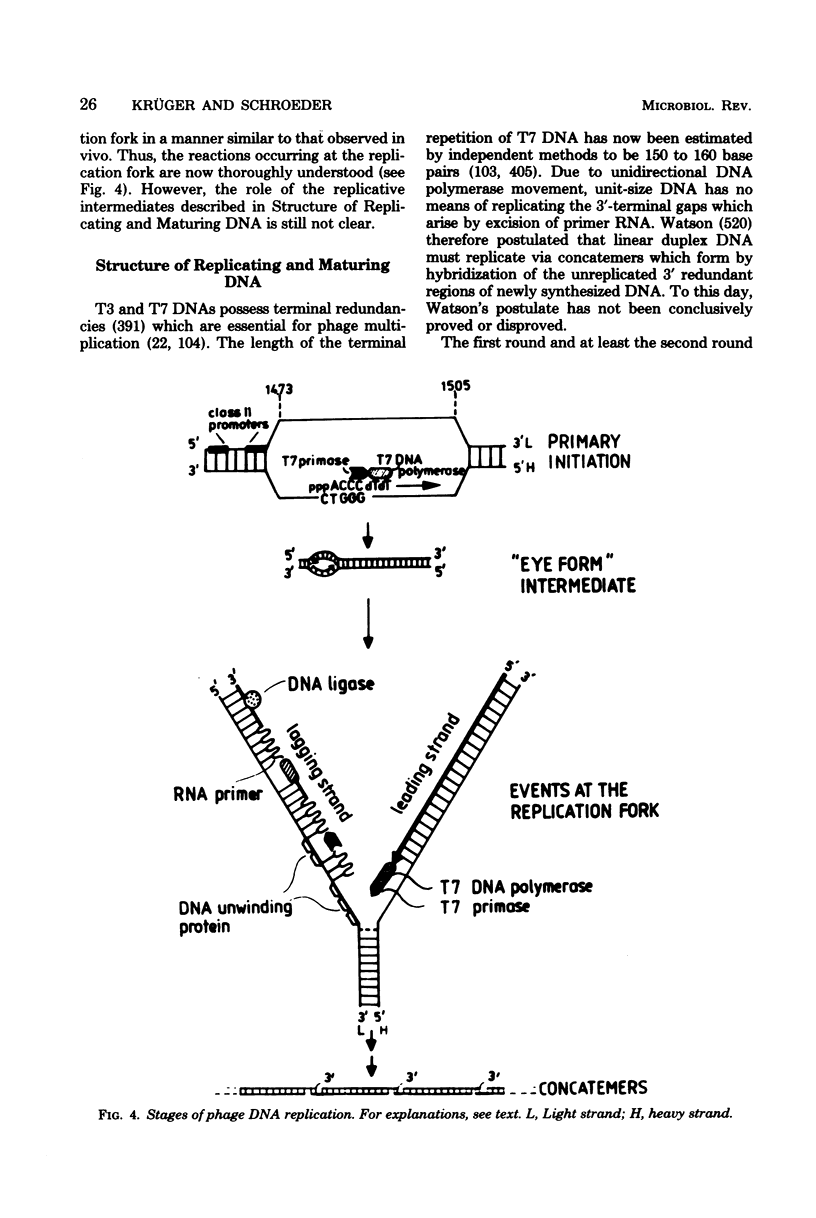
Full text
PDF





































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., WADE E. Classification of bacterial viruses; the relationship of two Serratia phages to coli-dysentery phages T3, T7, and D44. J Bacteriol. 1954 Sep;68(3):320–325. doi: 10.1128/jb.68.3.320-325.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Abraham K. A., Andersen K. J., Rognes A. Studies on deoxyribonucleic acid-dependent ribonucleic acid polymerase from Escherichia coli. Variations of the enzyme activity during growth. Biochem J. 1972 Sep;129(2):291–299. doi: 10.1042/bj1290291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R. L. Delayed methylation of DNA in developing sea urchin embryos. Nat New Biol. 1973 Jul 4;244(131):27–29. doi: 10.1038/newbio244027a0. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Adhya S., Sarkar P., Valenzuela D., Maitra U. Termination of transcription by Escherichia coli RNA polymerase: influence of secondary structure of RNA transcripts on rho-independent and rho-dependent termination. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1613–1617. doi: 10.1073/pnas.76.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler S., Modrich P. T7-induced DNA polymerase. Characterization of associated exonuclease activities and resolution into biologically active subunits. J Biol Chem. 1979 Nov 25;254(22):11605–11614. [PubMed] [Google Scholar]
- Alberts B., Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977 Oct 20;269(5630):655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Atkins J. F., Dunn J. J. Bacteriophage T3 and T7 early RNAs are translated by eukaryotic 80S ribosomes: active phage T3 coded S-adenosylmethionine cleaving enzyme is synthesized. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2752–2756. doi: 10.1073/pnas.73.8.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Kornberg A. A general priming system employing only dnaB protein and primase for DNA replication. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4308–4312. doi: 10.1073/pnas.76.9.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. DNA modification and restriction. Prog Nucleic Acid Res Mol Biol. 1974;14(0):1–37. doi: 10.1016/s0079-6603(08)60204-4. [DOI] [PubMed] [Google Scholar]
- Arber W. Promotion and limitation of genetic exchange. Science. 1979 Jul 27;205(4404):361–365. doi: 10.1126/science.377489. [DOI] [PubMed] [Google Scholar]
- Auer B., Günthert U., Wagner E. F., Schweiger M. Is the DNA of virus T7 methylated? J Gen Virol. 1979 Sep;44(3):609–613. doi: 10.1099/0022-1317-44-3-609. [DOI] [PubMed] [Google Scholar]
- Bailey J. N., Dembinski D. R., McAllister W. T. Derivation of a restriction map of bacteriophage T3 DNA and comparison with the map of bacteriophage T7 DNA. J Virol. 1980 Jul;35(1):176–183. doi: 10.1128/jvi.35.1.176-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister D., Glover S. W. Restriction and modification of bacteriophages by R+ strains of Escherichia coli K12. Biochem Biophys Res Commun. 1968 Mar 27;30(6):735–738. doi: 10.1016/0006-291x(68)90575-5. [DOI] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bautz E. K. Ferdinaud Springer Lecture: initiation of transcription by RNA polymerases of E. coli and phage T3. FEBS Lett. 1973 Oct 15;36(2):123–129. doi: 10.1016/0014-5793(73)80352-7. [DOI] [PubMed] [Google Scholar]
- Becker A., Lyn G., Gefter M., Hurwitz J. The enzymatic repair of DNA, II. Characterization of phage-induced sealase. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1996–2003. doi: 10.1073/pnas.58.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Golomb M., Chamberlin M. Isolation of recombinants between T7 and T3 bacteriophages and their use in vitro transcriptional mapping. J Virol. 1977 Feb;21(2):753–765. doi: 10.1128/jvi.21.2.753-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Hausmann R. Genetic map of bacteriophage T3. J Virol. 1973 Aug;12(2):417–419. doi: 10.1128/jvi.12.2.417-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Hausmann R. T3 times T7 phage crosses leading to recombinant RNA polymerases. Nature. 1974 Oct 11;251(5475):538–540. doi: 10.1038/251538a0. [DOI] [PubMed] [Google Scholar]
- Benbasat J. A., Burck K. B., Miller R. C., Jr Superinfection exclusion and lack of conservative transfer of bacteriophage T7 DNA. Virology. 1978 Jun 1;87(1):164–171. doi: 10.1016/0042-6822(78)90168-x. [DOI] [PubMed] [Google Scholar]
- Benzinger R., Enquist L. W., Skalka A. Transfection of Escherichia coli spheroplasts. V. Activity of recBC nuclease in rec+ and rec minus spheroplasts measured with different forms of bacteriophage DNA. J Virol. 1975 Apr;15(4):861–871. doi: 10.1128/jvi.15.4.861-871.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R. Transfection of Enterobacteriaceae and its applications. Microbiol Rev. 1978 Mar;42(1):194–236. doi: 10.1128/mr.42.1.194-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein C., Bernstein H. Coiled rings of DNA released from cells infected with bacteriophages T7 or T4 or from uninfected Escherichia coli. J Virol. 1974 Jun;13(6):1346–1355. doi: 10.1128/jvi.13.6.1346-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick M. D. Electron microscopic analysis of T7 deoxyribonucleic acid synthesized in vitro. J Biol Chem. 1974 May 10;249(9):2980–2984. [PubMed] [Google Scholar]
- Bishayee S., Yudelevich A., Maitra U. Specificity of RNA chain initiation by bacteriophage T7-induced RNA polymerase. Biochem Biophys Res Commun. 1976 Jan 26;68(2):626–633. doi: 10.1016/0006-291x(76)91191-8. [DOI] [PubMed] [Google Scholar]
- Bogdanova E. S., Gorlenko Z. M., Khourgess E. M. On the effect of bacteriophage infection on host RNA polymerase. Mol Gen Genet. 1974;133(3):261–272. doi: 10.1007/BF00267675. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Hayward R. S. New genes and promoters suggested by the DNA sequence near the end of the coliphage T7 early operon. Nucleic Acids Res. 1979 Dec 11;7(7):1931–1943. doi: 10.1093/nar/7.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C., Dubochet J. Electron microscopic localization of the binding sites of Escherichia coli RNA polymerase in the early promoter region of T7 DNA. Eur J Biochem. 1974 May 15;44(2):617–624. doi: 10.1111/j.1432-1033.1974.tb03519.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Inhibition of rifampicin-resistant RNA synthesis by rifampicin-RNA polymerase complexes. FEBS Lett. 1974 Sep 1;45(1):259–262. doi: 10.1016/0014-5793(74)80857-4. [DOI] [PubMed] [Google Scholar]
- Boyer H. W. DNA restriction and modification mechanisms in bacteria. Annu Rev Microbiol. 1971;25:153–176. doi: 10.1146/annurev.mi.25.100171.001101. [DOI] [PubMed] [Google Scholar]
- Braun V., Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43(0):89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Peebles C. L., Cozzarelli N. R. A topoisomerase from Escherichia coli related to DNA gyrase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6110–6114. doi: 10.1073/pnas.76.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel F., Davison J. Restriction insensitivity in bacteriophage T5. III. Characterization of EcoRI-sensitive mutants by restriction analysis. J Mol Biol. 1979 Mar 15;128(4):527–543. doi: 10.1016/0022-2836(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Brunovskis I., Burns R. O. Growth of coliphage T7 in Salmonella typhimurium. J Virol. 1973 May;11(5):621–629. doi: 10.1128/jvi.11.5.621-629.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunovskis I., Summers W. C. The process of infection with coliphage 17. VI. A phage gene controlling shutoff of host RNA synthesis. Virology. 1972 Nov;50(2):322–327. doi: 10.1016/0042-6822(72)90383-2. [DOI] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. I. In vivo transcription by Escherichia coli RNA polymerase. J Virol. 1973 Oct;12(4):882–886. doi: 10.1128/jvi.12.4.882-886.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräutigam A. R., Sauerbier W. Transcription unit mapping in bacteriophage T7. II. Proportionality of number of gene copies, mRNA, and gene product. J Virol. 1974 May;13(5):1110–1117. doi: 10.1128/jvi.13.5.1110-1117.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burck K. B., Miller R. C., Jr Marker rescue and partial replication of bacteriophage T7 DNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6144–6148. doi: 10.1073/pnas.75.12.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burck K. B., Scraba D. G., Miller R. C., Jr Electron microscopic analysis of partially replicated bacteriophage T7 DNA. J Virol. 1979 Nov;32(2):606–613. doi: 10.1128/jvi.32.2.606-613.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton W. G., Grabowy C. T., Sager R. Role of methylation in the modification and restriction of chloroplast DNA in Chlamydomonas. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1390–1394. doi: 10.1073/pnas.76.3.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi B., Reiser J., Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J Mol Biol. 1979 Feb 25;128(2):143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Tamanoi F., Richardson C. C., Studier F. W. Cloning of the T7 genome in Escherichia coli: use of recombination between cloned sequences and bacteriophage T7 to identify genes involved in recombination and a clone containing the origin of T7 DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):441–448. doi: 10.1101/sqb.1979.043.01.050. [DOI] [PubMed] [Google Scholar]
- Cantoni G. L. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- Carlson K. Intracellular fate of deoxyribonucleic acid from T7 bacteriophages. J Virol. 1968 Oct;2(10):1230–1233. doi: 10.1128/jvi.2.10.1230-1233.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson P. S. The use of protoplasts for genetic research. Proc Natl Acad Sci U S A. 1973 Feb;70(2):598–602. doi: 10.1073/pnas.70.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Cech C. L., McClure W. R. Characterization of ribonucleic acid polymerase-T7 promoter binary complexes. Biochemistry. 1980 May 27;19(11):2440–2447. doi: 10.1021/bi00552a023. [DOI] [PubMed] [Google Scholar]
- Center M. S. Bacteriophage T7 DNA synthesis in isolated DNA-membrane complexes. J Virol. 1973 Oct;12(4):847–854. doi: 10.1128/jvi.12.4.847-854.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S. Bacteriophage T7-induced endonuclease II. Purification and properties of the enzyme. J Biol Chem. 1972 Jan 10;247(1):146–156. [PubMed] [Google Scholar]
- Center M. S. Replicative intermediates of bacteriophage T7 deoxyribonucleic acid. J Virol. 1972 Jul;10(1):115–123. doi: 10.1128/jvi.10.1.115-123.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center M. S., Richardson C. C. An endonuclease induced after infection of Escherichia coli with bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1970 Dec 10;245(23):6285–6291. [PubMed] [Google Scholar]
- Center M. S. Role of gene 2 in bacteriophage T7 DNA synthesis. J Virol. 1975 Jul;16(1):94–100. doi: 10.1128/jvi.16.1.94-100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. Interaction between mutations of ribosomes and RNA polymerase: a pair of strA and rif mutants individually temperature-insensitive but temperature-sensitive in combination. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1157–1161. doi: 10.1073/pnas.74.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. R., Bandyopadhyay P., Huang H. H., Maitra U. Fidelity of in vitro transcription of T3 deoxyribonucleic acid by bacteriophage T3-induced ribonucleic acid polymerase and by Escherichia coli ribonucleic acid polymerase. J Biol Chem. 1974 Nov 10;249(21):6901–6909. [PubMed] [Google Scholar]
- Chakraborty P. R., Salvo R. A., Majumder H. K., Maitra U. Further characterization of bacteriophage T3-induced ribonucleic acid polymerase. Studies on the size of in vitro transcripts and interaction of T3 RNA polymerase with T3 DNA. J Biol Chem. 1977 Sep 25;252(18):6485–6493. [PubMed] [Google Scholar]
- Chakraborty P. R., Sarkar P., Huang H. H., Maitra U. Studies on T3-induced ribonucleic acid polymerase. 3. Purification and characterization of the T3-induced ribonucleic acid polymerase from bacteriophage T3-infected Escherichia coli cells. J Biol Chem. 1973 Oct 10;248(19):6637–6646. [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J Virol. 1974 Sep;14(3):509–516. doi: 10.1128/jvi.14.3.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., Ring J. Characterization of T7-specific ribonucleic acid polymerase. 1. General properties of the enzymatic reaction and the template specificity of the enzyme. J Biol Chem. 1973 Mar 25;248(6):2235–2244. [PubMed] [Google Scholar]
- Champoux J. J. Proteins that affect DNA conformation. Annu Rev Biochem. 1978;47:449–479. doi: 10.1146/annurev.bi.47.070178.002313. [DOI] [PubMed] [Google Scholar]
- Chao L., Speyer J. F. A new form of RNA polymerase isolated from Escherichia coli. Biochem Biophys Res Commun. 1973 Mar 17;51(2):399–405. doi: 10.1016/0006-291x(73)91271-0. [DOI] [PubMed] [Google Scholar]
- Cherny D. I., Aleksandrov A. A., Zarudnaya M. I., Kosaganov Y. N., Lazurkin Y. S., Beabealashvilli R. S., Svochkina L. P. Investigation of the binding of Escherichia coli RNA polymerase to DNA from bacteriophages T2 and T7 by kinetic formaldehyde method and electron microscopy. Eur J Biochem. 1977 Sep 15;79(1):309–317. doi: 10.1111/j.1432-1033.1977.tb11811.x. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Price P., Pedrinan L., Acs G. Correlation between hypomethylation of DNA and expression of globin genes in Friend erythroleukemia cells. Eur J Biochem. 1977 Nov 15;81(1):53–61. doi: 10.1111/j.1432-1033.1977.tb11926.x. [DOI] [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E. The role of Zn(II) in transcription by T7 RNA polymerase. Biochem Biophys Res Commun. 1974 Sep 23;60(2):641–648. doi: 10.1016/0006-291x(74)90289-7. [DOI] [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- Conley R. R., Pigiet V. In vivo distribution of phosphothioredoxin and thioredoxin in Escherichia coli. J Biol Chem. 1978 Aug 25;253(16):5568–5572. [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Reed R., Taylor F. R., Jackson M. B. Properties and biosynthesis of cyclopropane fatty acids in Escherichia coli. J Bacteriol. 1979 Apr;138(1):118–121. doi: 10.1128/jb.138.1.118-121.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Dausse J. P. Localization of Escherichia coli RNA polymerase initiation sites in T7 DNA early promoter region. FEBS Lett. 1975 Feb 1;50(2):214–218. doi: 10.1016/0014-5793(75)80491-1. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Horaist M. Existence and possible roles of transcriptional barriers in T7 DNA early region as shown by electron microscopy. Nature. 1975 Jul 24;256(5515):288–292. doi: 10.1038/256288a0. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Rho, a factor causing the modulation of early T7 genes transcription. Biochimie. 1974;56(5):693–701. doi: 10.1016/s0300-9084(74)80040-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. The functions of rho in T7-DNA transcription in vitro. Eur J Biochem. 1973 Jun 15;35(3):517–526. doi: 10.1111/j.1432-1033.1973.tb02868.x. [DOI] [PubMed] [Google Scholar]
- Dausse J. P., Sentenac A., Fromageot P. Interaction of RNA polymerase from Escherichia coli with DNA. Analysis of T7 DNA early-promoter sites. Eur J Biochem. 1975 Sep 15;57(2):569–578. doi: 10.1111/j.1432-1033.1975.tb02332.x. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Davison J., Brunel F. Restriction insensitivity in bacteriophage T5 I. Genetic characterization of mutants sensitive to EcoRI restriction. J Virol. 1979 Jan;29(1):11–16. doi: 10.1128/jvi.29.1.11-16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison J., Brunel F. Restriction insensitivity in bacteriophage T5. II. Lack of EcoRI modification in T5+ and T5ris mutants. J Virol. 1979 Jan;29(1):17–20. doi: 10.1128/jvi.29.1.17-20.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd UV-induced alleviation of K-specific restriction of bacteriophage lambda. J Virol. 1977 Mar;21(3):1249–1251. doi: 10.1128/jvi.21.3.1249-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wyngaert M., Hinkle D. C. Involvement of DNA gyrase in replication and transcription of bacteriophage T7 DNA. J Virol. 1979 Feb;29(2):529–535. doi: 10.1128/jvi.29.2.529-535.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini M., Halegoua S., Inouye M. Lysozymes from bacteriophages T3 and T5. J Virol. 1975 Aug;16(2):459–461. doi: 10.1128/jvi.16.2.459-461.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWyngaert M. A., Hinkle D. C. Bacterial mutants affecting phage T7 DNA replication produce RNA polymerase resistant to inhibition by the T7 gene 2 protein. J Biol Chem. 1979 Nov 25;254(22):11247–11253. [PubMed] [Google Scholar]
- DeWyngaert M. A., Hinkle D. C. Characterization of the defects in bacteriophage T7 DNA synthesis during growth in the Escherichia coli mutant tsnB. J Virol. 1980 Feb;33(2):780–788. doi: 10.1128/jvi.33.2.780-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M, Fano U. Bacteriophage-Resistant Mutants in Escherichia Coli. Genetics. 1945 Mar;30(2):119–136. doi: 10.1093/genetics/30.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. DNA gyrase and DNA unwinding. Nature. 1979 Jul 19;280(5719):196–198. doi: 10.1038/280196a0. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Goldberg E. B. Phage-coded protein prevents restriction of unmodified progeny T4 DNA. Nature. 1976 Apr 1;260(5550):454–456. doi: 10.1038/260454a0. [DOI] [PubMed] [Google Scholar]
- Dharmgrongartama B., Mahadik S. P., Srinivasan P. R. Modification of RNA polymerase after T3 phage infection of Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2845–2849. doi: 10.1073/pnas.70.10.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doy C. H., Gresshoff P. M., Rolfe B. G. Biological and molecular evidence for the transgenosis of genes from bacteria to plant cells. Proc Natl Acad Sci U S A. 1973 Mar;70(3):723–726. doi: 10.1073/pnas.70.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiseikelmann B., Eichenlaub R., Wackernagel W. The effect of differential methylation by Escherichia coli of plasmid DNA and phage T7 and lambda DNA on the cleavage by restriction endonuclease MboI from Moraxella bovis. Biochim Biophys Acta. 1979 May 24;562(3):418–428. doi: 10.1016/0005-2787(79)90105-9. [DOI] [PubMed] [Google Scholar]
- Dreiseikelmann B., Steger U., Wackernagel W. Length determination of the terminal redundant regions in the DNA of phage T7. Mol Gen Genet. 1980 Apr;178(1):237–240. doi: 10.1007/BF00267236. [DOI] [PubMed] [Google Scholar]
- Dreiseikelmann B., Wackernagel W. The terminal redundant regions of bacteriophage T7 DNA: their necessity for phage production studied by the infectivity of T7 DNA after modification by various exonucleases. Mol Gen Genet. 1978 Feb 27;159(3):321–328. doi: 10.1007/BF00268269. [DOI] [PubMed] [Google Scholar]
- Dressler D. The recent excitement in the DNA growing point problem. Annu Rev Microbiol. 1975;29:525–559. doi: 10.1146/annurev.mi.29.100175.002521. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Anderson C. W., Atkins J. F., Bartelt D. C., Crockett W. C. Bacteriophages T7 and T3 as model systems for RNA synthesis and processing. Prog Nucleic Acid Res Mol Biol. 1976;19:263–273. doi: 10.1016/s0079-6603(08)60924-1. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., McAllister W. T., Bautz E. K. Transcription in vitro of T3 DNA by Escherichia coli and T3 RNA polymerases. Analysis of the products in cell-free protein-synthesizing system. Eur J Biochem. 1972 Sep 25;29(3):500–508. doi: 10.1111/j.1432-1033.1972.tb02014.x. [DOI] [PubMed] [Google Scholar]
- Dunn J. J. RNase III cleavage of single-stranded RNA. Effect of ionic strength on the fideltiy of cleavage. J Biol Chem. 1976 Jun 25;251(12):3807–3814. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Effect of RNAase III, cleavage on translation of bacteriophage T7 messenger RNAs. J Mol Biol. 1975 Dec 15;99(3):487–499. doi: 10.1016/s0022-2836(75)80140-9. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Processing transcription, and translation of bacteriophage T7 messenger RNAs. Brookhaven Symp Biol. 1975 Jul;(26):267–276. [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs are generated by site-specific cleavages. Proc Natl Acad Sci U S A. 1973 May;70(5):1559–1563. doi: 10.1073/pnas.70.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. The transcription termination site at the end of the early region of bacteriophage T7 DNA. Nucleic Acids Res. 1980 May 24;8(10):2119–2132. doi: 10.1093/nar/8.10.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Kievitt K. D. Bacteriophage proteins associated with the bacterial membrane after bacteriophage T7 infection. J Virol. 1977 May;22(2):561–567. doi: 10.1128/jvi.22.2.561-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin B., Lautenberger J. A., Linn S. Host-controlled modification and restriction of bacteriophage T7 by escherichia coli B. J Virol. 1973 Jun;11(6):1020–1023. doi: 10.1128/jvi.11.6.1020-1023.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskridge R. W., Weinfeld H., Paigen K. Susceptibility of different coliphage genomes to host-controlled variation. J Bacteriol. 1967 Mar;93(3):835–844. doi: 10.1128/jb.93.3.835-844.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D. K. Host range mutants and semitemperate mutants of bacteriophage T3. Virology. 1957 Jun;3(3):527–553. doi: 10.1016/0042-6822(57)90008-9. [DOI] [PubMed] [Google Scholar]
- FRASER D., DULBECCO R. A genetic analysis of the factors controlling the h character in bacteriophage T3. Cold Spring Harb Symp Quant Biol. 1953;18:15–17. doi: 10.1101/sqb.1953.018.01.004. [DOI] [PubMed] [Google Scholar]
- Fröhlich B., Powling A., Knippers R. Formation of concatemeric DNA in bacteriophage T7-infected bacteria. Virology. 1975 Jun;65(2):455–468. doi: 10.1016/0042-6822(75)90051-3. [DOI] [PubMed] [Google Scholar]
- Fuchs E. The formation of bacteriophage T7 and T3 lysozymes from inactive precursors. FEBS Lett. 1977 Aug 1;80(1):27–29. doi: 10.1016/0014-5793(77)80399-2. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Miyazaki J. I., Minagawa T. In vitro packaging of phage T3 DNA. Virology. 1978 Jun 15;87(2):394–400. doi: 10.1016/0042-6822(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Fujisawa H., Yamagishi M., Minagawa T. In vitro formation of the concatemeric DNA of bacteriophage T3 and its biological activity in the in vitro packaging reaction. Virology. 1980 Mar;101(2):327–334. doi: 10.1016/0042-6822(80)90448-1. [DOI] [PubMed] [Google Scholar]
- GEOBEL W. F., JESAITIS M. A. The somatic antigen of a phage-resistant variant of Phase II Shigella sonnei. J Exp Med. 1952 Nov;96(5):425–438. doi: 10.1084/jem.96.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLD M., HAUSMANN R., MAITRA U., HURWITZ J. THE ENZYMATIC METHYLATION OF RNA AND DNA. 8. EFFECTS OF BACTERIOPHAGE INFECTION ON THE ACTIVITY OF THE METHYLATING ENZYMES. Proc Natl Acad Sci U S A. 1964 Aug;52:292–297. doi: 10.1073/pnas.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gefter M., Hausmann R., Gold M., Hurwitz J. The enzymatic methylation of ribonucleic acid and deoxyribonucleic acid. X. Bacteriophage T3-induced S-adenosylmethionine cleavage. J Biol Chem. 1966 May 10;241(9):1995–2006. [PubMed] [Google Scholar]
- Geier G. E., Modrich P. Recognition sequence of the dam methylase of Escherichia coli K12 and mode of cleavage of Dpn I endonuclease. J Biol Chem. 1979 Feb 25;254(4):1408–1413. [PubMed] [Google Scholar]
- Geier M. R., Merril C. R. Lambda phage transcription in human fibroblasts. Virology. 1972 Mar;47(3):638–643. doi: 10.1016/0042-6822(72)90553-3. [DOI] [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Roberts R. J. Two sequence-specific endonucleases from Moraxella bovis. J Mol Biol. 1977 Jul;114(1):169–179. doi: 10.1016/0022-2836(77)90290-x. [DOI] [PubMed] [Google Scholar]
- Glickman B., van den Elsen P., Radman M. Induced mutagenesis in dam- mutants of Escherichia coli: a role for 6-methyladenine residues in mutation avoidance. Mol Gen Genet. 1978 Jul 25;163(3):307–312. doi: 10.1007/BF00271960. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schiess W. The fate of a bacterial plasmid in mammalian cells. Mol Gen Genet. 1975 Jun 19;138(3):213–223. doi: 10.1007/BF00269348. [DOI] [PubMed] [Google Scholar]
- Gold M., Gefter M., Hausmann R., Hurwitz J. Methylation of DNA. J Gen Physiol. 1966 Jul;49(6):5–28. doi: 10.1085/jgp.49.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. J. T7- and T3-specific RNA polymerases: characterization and mapping of the in vitro transcripts read from T3 DNA. J Virol. 1977 Feb;21(2):743–752. doi: 10.1128/jvi.21.2.743-752.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. A preliminary map of the major transcription units read by T7 RNA polymerase on the T7 and T3 bacteriophage chromosomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):760–764. doi: 10.1073/pnas.71.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. Characterization of T7-specific ribonucleic acid polymerase. IV. Resolution of the major in vitro transcripts by gel electrophoresis. J Biol Chem. 1974 May 10;249(9):2858–2863. [PubMed] [Google Scholar]
- Gordon R. L., Humphries P., McConnell D. J. Restriction enzyme cleavage mapping of T7 virus early region. Mol Gen Genet. 1978 Jul 4;162(3):329–339. doi: 10.1007/BF00268859. [DOI] [PubMed] [Google Scholar]
- Grasso R. J., Paigen K. Loss of host-controlled restriction and modification of phage lambda in Escherichia coli K12 previously infected with UV-irradiated coli-phage dT3. Virology. 1969 May;38(1):191–194. doi: 10.1016/0042-6822(69)90145-7. [DOI] [PubMed] [Google Scholar]
- Gravchev M. A., Zaichikov E. F., Kravchenko V. V., Pletnev A. G. Vydelenie promotornykh i terminatornogo fragmentov DNK faga T7 is gidrolizata, poluchennogo deivistviem éndonukleazy restrikstii BsuR. Dokl Akad Nauk SSSR. 1978;239(2):475–478. [PubMed] [Google Scholar]
- Grippo P., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. J Biol Chem. 1971 Nov 25;246(22):6867–6873. [PubMed] [Google Scholar]
- Gómez B., Nualart L. Requirement of the bacteriopahge T7 0.7 gene for phage growth in the presence of the Col 1b factor. J Gen Virol. 1977 Apr;35(1):99–106. doi: 10.1099/0022-1317-35-1-99. [DOI] [PubMed] [Google Scholar]
- Haberman A. The bacteriophage P1 restriction endonuclease. J Mol Biol. 1974 Nov 15;89(4):545–563. doi: 10.1016/0022-2836(74)90035-7. [DOI] [PubMed] [Google Scholar]
- Hadi S. M., Bickle T. A., Yuan R. The role of S-adenosylmethionine in the cleavage of deoxyribonucleic acid by the restriction endonuclease from Escherichia coli K. J Biol Chem. 1975 Jun 10;250(11):4159–4164. [PubMed] [Google Scholar]
- Hadi S. M., Bächi B., Shepherd J. C., Yuan R., Ineichen K., Bickle T. A. DNA recognition and cleavage by the EcoP15 restriction endonuclease. J Mol Biol. 1979 Nov 5;134(3):655–666. doi: 10.1016/0022-2836(79)90372-3. [DOI] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on efficiency of translation of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):793–804. doi: 10.1128/jvi.26.3.793-804.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Effect of RNase III on the size of bacteriophage T7 lysozyme mRNA. J Virol. 1978 Jun;26(3):783–792. doi: 10.1128/jvi.26.3.783-792.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Young E. T. Preparative polyacrylamide gel electrophoresis of ribonucleic acid. Identification of multiple molecular species of bacteriophage T7 lysozyme messenger ribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3394–3400. doi: 10.1021/bi00713a033. [DOI] [PubMed] [Google Scholar]
- Hagen F., Young E. T. Regulation of synthesis of bacteriophage T7 lysozyme mRNA. Virology. 1973 Sep;55(1):231–241. doi: 10.1016/s0042-6822(73)81026-8. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Lipopolysaccharide-deficient, bacteriophage-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):98–108. doi: 10.1128/jb.127.1.98-108.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattman S., Brooks J. E., Masurekar M. Sequence specificity of the P1 modification methylase (M.Eco P1) and the DNA methylase (M.Eco dam) controlled by the Escherichia coli dam gene. J Mol Biol. 1978 Dec 15;126(3):367–380. doi: 10.1016/0022-2836(78)90046-3. [DOI] [PubMed] [Google Scholar]
- Hausmann R. Bacteriophage T7 genetics. Curr Top Microbiol Immunol. 1976;75:77–110. doi: 10.1007/978-3-642-66530-1_3. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Bacteriophage T3- and T7-directed deoxyribonucleases. J Virol. 1968 Mar;2(3):265–266. doi: 10.1128/jvi.2.3.265-266.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., LaRue K. Variations in sedimentation patterns among deoxyribonucleic acids synthesized after infection of Escherichia coli by different amber mutants of bacteriophage T7. J Virol. 1969 Feb;3(2):278–281. doi: 10.1128/jvi.3.2.278-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R. Sedimentation analysis of phage T7-directed DNA synthesized in the presence of a dominant conditional lethal phage gene. Biochem Biophys Res Commun. 1968 May 23;31(4):609–615. doi: 10.1016/0006-291x(68)90522-6. [DOI] [PubMed] [Google Scholar]
- Hausmann R. Synthesis of an S-adenosylmethionine-cleaving enzyme in T3-infected Escherichia coli and its disturbance by co-infection with enzymatically incompetent bacteriophage. J Virol. 1967 Feb;1(1):57–63. doi: 10.1128/jvi.1.1.57-63.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland D., Nygaard A. P. Selected genes of T7 DNA associated with the Escherichia coli membrane following phage infection. FEBS Lett. 1975 Jan 15;50(1):13–16. doi: 10.1016/0014-5793(75)81029-5. [DOI] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Jovanovich S., Sauerbrier W. Early gene expression in bacteriophage T7. I. In vivo synthesis, inactivation, and translational utilization of early mRNA's. J Virol. 1976 Feb;17(2):642–658. doi: 10.1128/jvi.17.2.642-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Rahmsdorf H. J., Pai S. H., Schweigher M. Translational control induced by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1088–1092. doi: 10.1073/pnas.71.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P., Schweiger M., Sauerbier W. Host- and phage-RNA polymerase mediated synthesis of T 7 lysozyme in vivo. Mol Gen Genet. 1971;112(2):152–160. doi: 10.1007/BF00267492. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. "Host shutoff" function of bacteriophage T7: involvement of T7 gene 2 and gene 0.7 in the inactivation of Escherichia coli RNA polymerase. J Virol. 1977 Dec;24(3):736–745. doi: 10.1128/jvi.24.3.736-745.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. I protein: bacteriophage T7-coded inhibitor of Escherichia coli RNA polymerase. J Virol. 1977 Dec;24(3):746–760. doi: 10.1128/jvi.24.3.746-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbach B. A., Nakada D. Inactive complex formation between E. coli RNA polymerase and inhibitor protein purified from T7 phage infected cells. Nature. 1975 Nov 27;258(5533):354–357. doi: 10.1038/258354a0. [DOI] [PubMed] [Google Scholar]
- Hesselbach B. A., Yamada Y., Nakada D. Isolation of an inhibitor protein of E. coli RNA polymerase from T7 phage infected cell. Nature. 1974 Nov 1;252(5478):71–74. doi: 10.1038/252071b0. [DOI] [PubMed] [Google Scholar]
- Hiebsch R., Center M. S. Intracellular organization of bacteriophage T7 DNA: analysis of parenteral bacteriophage T7 DNA-membrane and DNA-protein complexes. J Virol. 1977 May;22(2):540–547. doi: 10.1128/jvi.22.2.540-547.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillenbrand G., Morelli G., Lanka E., Scherzinger E. Bacteriophage T7 DNA primase: a multifunctional enzyme involved in DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):449–459. doi: 10.1101/sqb.1979.043.01.051. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C. Evidence for direct involvement of T7 RNA polymerase bacteriophage DNA replication. J Virol. 1980 Apr;34(1):136–141. doi: 10.1128/jvi.34.1.136-141.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Purification and properties of the gene 4 protein of bacteriophage T7. J Biol Chem. 1975 Jul 25;250(14):5523–5529. [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Requirements for deoxyribonucleic acid synthesis and characterization of the product. J Biol Chem. 1974 May 10;249(9):2974–2980. [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Pfenning-Yeh M., Ponta H., Herrlich P., Schweiger M. A virus-specified mechanism for the prevention of multiple infection--T7- and T3-mutual and superinfection exclusion. Mol Gen Genet. 1976 Dec 22;149(3):243–249. doi: 10.1007/BF00268524. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Holmgren A., Ohlsson I., Grankvist M. L. Thiroedoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978 Jan 25;253(2):430–436. [PubMed] [Google Scholar]
- Hopper J. E., Ko G., Young E. T. Comparative analysis of the in vivo and in vitro expression of bacteriophage T7 messenger RNAs during infection of Escherichia coli. J Mol Biol. 1975 Jun 5;94(4):539–554. doi: 10.1016/0022-2836(75)90320-4. [DOI] [PubMed] [Google Scholar]
- Hori K., Mark D. F., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. Characterization of the exonuclease activities of the gene 5 protein and the reconstituted polymerase. J Biol Chem. 1979 Nov 25;254(22):11598–11604. [PubMed] [Google Scholar]
- Hori K., Mark D. F., Richardson C. C. Deoxyribonucleic acid polymerase of bacteriophage T7. Purification and properties of the phage-encoded subunit, the gene 5 protein. J Biol Chem. 1979 Nov 25;254(22):11591–11597. [PubMed] [Google Scholar]
- Hotchkiss R. D. Models of genetic recombination. Annu Rev Microbiol. 1974;28(0):445–468. doi: 10.1146/annurev.mi.28.100174.002305. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physicochomecial studies on interactions between DNA and RNA polymerase. Isolation and mapping of a T7 DNA fragment containing the early promoters for Escherichia coli RNA polymerase. Biochemistry. 1976 Dec 28;15(26):5776–5783. doi: 10.1021/bi00671a014. [DOI] [PubMed] [Google Scholar]
- Humphries P., Gordon R. L., McConnell D. J., Connolly P. Endonuclease R. Hind fragments of T7 DNA. Virology. 1974 Mar;58(1):25–31. doi: 10.1016/0042-6822(74)90138-x. [DOI] [PubMed] [Google Scholar]
- Humphries P., McConnel D. J., Connolly P. Interaction of Escherichia coli DNA-dependent RNA polymerase and endonuclease R. Hind fragments of T7 DNA. Biochim Biophys Acta. 1974 Sep 27;366(1):70–78. doi: 10.1016/0005-2787(74)90319-0. [DOI] [PubMed] [Google Scholar]
- Hyman R. W., Brunovskis I., Summers W. C. A biochemical comparison of the related bacteriophages T7, phiI, phiII, W31, H, and T3. Virology. 1974 Jan;57(1):189–206. doi: 10.1016/0042-6822(74)90120-2. [DOI] [PubMed] [Google Scholar]
- Ihler G. M., Thomas C. A., Jr Equal incorporation of both parental bacteriophage T7 deoxyribonucleic acid strands into intracellular concatemeric deoxyribonucleic acid. J Virol. 1970 Dec;6(6):877–880. doi: 10.1128/jvi.6.6.877-880.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Arnheim N., Sternglanz R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J Biol Chem. 1973 Oct 25;248(20):7247–7252. [PubMed] [Google Scholar]
- Issinger O. G., Beier H., Hausmann R. In vivo and in vitro "phenotypic mixing" with amber mutants of phages T3 and T7. Mol Gen Genet. 1973 Mar 27;122(1):81–88. doi: 10.1007/BF00337976. [DOI] [PubMed] [Google Scholar]
- Issinger O. G., Falk H. Comparative studies on the structural proteins of T3 and T7 phages. Arch Virol. 1976;52(3):217–231. doi: 10.1007/BF01348019. [DOI] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. I. Involvement of DNA gyrase in bacteriophage T7 DNA replication. Nature. 1977 Nov 3;270(5632):78–80. doi: 10.1038/270078a0. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., James A. P. Single-cell studies on the carrier state of bacteriophage IKe, a virus specific for conjugative plasmids of the N incompatibility and conjugative group. Can J Microbiol. 1978 Dec;24(12):1595–1601. doi: 10.1139/m78-255. [DOI] [PubMed] [Google Scholar]
- Jensen H. B., Pryme I. F. The separation of a gene 3.5 directed activity and a lytic activity ("lysozyme") in T7 infected E. coli B cells. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1117–1123. doi: 10.1016/s0006-291x(74)80094-x. [DOI] [PubMed] [Google Scholar]
- Jovin T. M. Recognition mechanisms of DNA-specific enzymes. Annu Rev Biochem. 1976;45:889–920. doi: 10.1146/annurev.bi.45.070176.004325. [DOI] [PubMed] [Google Scholar]
- Kadesch T. R., Williams R. C., Chamberlin M. J. Electron microscopic studies of the binding of Escherichia coli RNA polymerase to DNA. I. Characterization of the non-specific interactions of holoenzyme with a restriction fragment of bacteriophage T7 DNA. J Mol Biol. 1980 Jan 5;136(1):65–78. doi: 10.1016/0022-2836(80)90366-6. [DOI] [PubMed] [Google Scholar]
- Kadesch T. R., Williams R. C., Chamberlin M. J. Electron microscopic studies of the binding of Escherichia coli RNA polymerase to DNA. II. Formation of multiple promoter-like complexes at non-promoter sites. J Mol Biol. 1980 Jan 5;136(1):79–93. doi: 10.1016/0022-2836(80)90367-8. [DOI] [PubMed] [Google Scholar]
- Kan N. C., Lautenberger J. A., Edgell M. H., Hutchison C. A., 3rd The nucleotide sequence recognized by the Escherichia coli K12 restriction and modification enzymes. J Mol Biol. 1979 May 15;130(2):191–209. doi: 10.1016/0022-2836(79)90426-1. [DOI] [PubMed] [Google Scholar]
- Kao P. C., Regan J. D., Volkin E. Fate of homologous and heterologous DNAs after incorporation into human skin fibroblasts. Biochim Biophys Acta. 1973 Sep 28;324(1):1–13. doi: 10.1016/0005-2787(73)90245-1. [DOI] [PubMed] [Google Scholar]
- Karu A. E., Sakaki Y., Echols H., Linn S. The gamma protein specified by bacteriophage gamma. Structure and inhibitory activity for the recBC enzyme of Escherichia coli. J Biol Chem. 1975 Sep 25;250(18):7377–7387. [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Mapping of class II promoter sites utilized in vitro by T7-specific RNA polymerase on bacteriophage T7 DNA. J Virol. 1979 Jan;29(1):196–208. doi: 10.1128/jvi.29.1.196-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Kaya K. M., Chamberlin M. J. Escherichia coli RNA polymerase-rifampicin complexes bound at promoter sites block RNA chain elongation by Escherichia coli RNA polymerase and T7-specific RNA polymerase. Biochemistry. 1978 Dec 26;17(26):5798–5804. doi: 10.1021/bi00619a029. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Saitoh T., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. IX. Growth-dependent variations in the synthesis rate, content and distribution of RNA polymerase. Mol Gen Genet. 1979 Jul 13;174(2):107–116. doi: 10.1007/BF00268348. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Smith H. O. A restriction enzyme from Hemophilus influenzae. II. J Mol Biol. 1970 Jul 28;51(2):393–409. doi: 10.1016/0022-2836(70)90150-6. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Gene 6 exonuclease of bacteriophage T7. I. Purification and properties of the enzyme. J Biol Chem. 1972 Jan 10;247(1):305–310. [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Gene 6 exonuclease of bacteriophage T7. II. Mechanism of the reaction. J Biol Chem. 1972 Jan 10;247(1):311–318. [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Packaging and maturation of DNA of bacteriophage T7 in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3545–3549. doi: 10.1073/pnas.71.9.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. The involvement of genes 3,4,5 and 6 in genetic recombination in bacteriophage T7. Virology. 1975 May;65(1):281–285. doi: 10.1016/0042-6822(75)90031-8. [DOI] [PubMed] [Google Scholar]
- Kiefer M., Neff N., Chamberlin M. J. Transcriptional termination at the end of the early region of bacteriophages T3 and T7 is not affected by polarity suppressors. J Virol. 1977 May;22(2):548–552. doi: 10.1128/jvi.22.2.548-552.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. Mechanismen der wirtskontrollierten Modifikation des Phagen T1. Z Vererbungsl. 1965;96(4):346–363. [PubMed] [Google Scholar]
- Kleppe G., Jensen H. B., Pryme I. F. Purification and characterization of the lytic enzyme N-acetylmuramyl-L-alanine amidase of bacteriophage T7. Eur J Biochem. 1977 Jun 15;76(2):317–326. doi: 10.1111/j.1432-1033.1977.tb11598.x. [DOI] [PubMed] [Google Scholar]
- Koller T., Kübler O., Portmann R., Sogo J. M. High resolution physical mapping of specific binding sites of Escherichia coli RNA polymerase on the DNA of bacteriophage T7 . J Mol Biol. 1978 Mar 25;120(1):121–131. doi: 10.1016/0022-2836(78)90298-x. [DOI] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Masamune Y., LeClerc J. E., Richardson C. C. Gene 4 protein of bacteriophage T7. Purification physical properties, and stimulation of T7 DNA polymerase during the elongation of polynucleotide chains. J Biol Chem. 1978 Jan 25;253(2):566–573. [PubMed] [Google Scholar]
- Kolodner R., Richardson C. C. Gene 4 protein of bacteriophage T7. Characterization of the product synthesized by the T7 DNA polymerase and gene 4 protein in the absence of ribonucleoside 5'-triphosphates. J Biol Chem. 1978 Jan 25;253(2):574–584. [PubMed] [Google Scholar]
- Kolodner R., Richardson C. C. Replication of duplex DNA by bacteriophage T7 DNA polymerase and gene 4 protein is accompanied by hydrolysis of nucleoside 5'-triphosphates. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1525–1529. doi: 10.1073/pnas.74.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Regulation of initiation of DNA replication. Annu Rev Genet. 1979;13:355–391. doi: 10.1146/annurev.ge.13.120179.002035. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Aspects of DNA replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):1–9. doi: 10.1101/sqb.1979.043.01.003. [DOI] [PubMed] [Google Scholar]
- Korsten K. H., Tomkiewicz C., Hausmann R. The strategy of infection as a criterion for phylogenetic relationships of non-coli phages morphologically similar to phage T7. J Gen Virol. 1979 Apr;43(1):57–73. doi: 10.1099/0022-1317-43-1-57. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M., Steitz J. A. Nucleotide sequences of the 5' and 3' termini of bacteriophage T7 early messenger RNAs synthesized in vivo: evidence for sequence specificity in RNA processing. J Mol Biol. 1974 Nov 15;89(4):767–776. doi: 10.1016/0022-2836(74)90051-5. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Rosenberg M. The isolation and characterization of bacteriophage T7 messenger RNA fragments containing an RNase III cleavage site. Nucleic Acids Res. 1976 Oct;3(10):2411–2426. doi: 10.1093/nar/3.10.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger D. H., Presber W., Hansen S., Rosenthal H. A. Biological functions of the bacteriophage T3 SAMase gene. J Virol. 1975 Aug;16(2):453–455. doi: 10.1128/jvi.16.2.453-455.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Chernin L. S., Hansen S., Rosenthal H. A., Goldfarb D. M. Protection of foreign DNA against host-controlled restriction in bacterial cells. I. protection of F' plasmid DNA by preinfection with bacteriophages T3 or T7. Mol Gen Genet. 1978 Feb 7;159(1):107–110. doi: 10.1007/BF00401754. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Gola G., Weisshuhn I., Hansen S. The ocr gene function of bacterial viruses T3 and T7 prevents host-controlled modification. J Gen Virol. 1978 Oct;41(1):189–192. doi: 10.1099/0022-1317-41-1-189. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Presber W., Rudolph M., Scholz D., Rosenthal H. A. Wirtsbereichsverhalten der Bakteriophagen T3 und T7 an Escherichia coli K12-Stämmen. Z Allg Mikrobiol. 1979;19(7):473–480. doi: 10.1002/jobm.3630190704. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C. Host-dependent modification of bacterial virus T3 affecting its adsorption ability. Virology. 1980 Apr 30;102(2):444–446. doi: 10.1016/0042-6822(80)90111-7. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C., Presber W. Host-dependent modification of bacteriophage T7 and SAMase-negative T3 derivatives affecting their adsorption ability. Mol Gen Genet. 1977 May 20;153(1):107–110. doi: 10.1007/BF01036002. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Schroeder C. Virus adaptation to host cells: the non-classical modification of phage T3. Z Allg Mikrobiol. 1980;20(8):495–502. [PubMed] [Google Scholar]
- Krüger D. H., Presber W., Hansen S., Rosenthal H. A. Different restriction of bacteriophages T3 and T7 by P1-lysogenic cells and the role of the T3-coded SAMase. Z Allg Mikrobiol. 1977;17(8):581–591. doi: 10.1002/jobm.3630170802. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Presber W., Mann W., Rosenthal H. A. Untersuchungen an T3-Phagen. 3. Uber einen unterschiedlichen Nährstoffmangeleffekt auf die Vermehrung der Phagen T3 und T7. Acta Biol Med Ger. 1972;29(4):477–482. [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C., Hansen S., Rosenthal H. A. Active protection by bacteriophages T3 and T7 against E. coli B- and K-specific restriction of their DNA. Mol Gen Genet. 1977 May 20;153(1):99–106. doi: 10.1007/BF01036001. [DOI] [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. In vitro packaging of UV radiation-damaged DNA from bacteriophage T7. J Virol. 1977 Sep;23(3):509–516. doi: 10.1128/jvi.23.3.509-516.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B., Abdel-Monem M., Hoffmann-Berling H. DNA helicases. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):63–67. doi: 10.1101/sqb.1979.043.01.011. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Complementary specificity of restriction endonucleases of Diplococcus pneumoniae with respect to DNA methylation. J Mol Biol. 1977 Jul;114(1):153–168. doi: 10.1016/0022-2836(77)90289-3. [DOI] [PubMed] [Google Scholar]
- Landy A., Ruedisueli E., Robinson L., Foeller C., Ross W. Digestion of deoxyribonucleic acids from bacteriophage T7, lambda, and phi 80h with site-specific nucleases from Hemophilus influenzae strain Rc and strain Rd. Biochemistry. 1974 May 7;13(10):2134–2142. doi: 10.1021/bi00707a022. [DOI] [PubMed] [Google Scholar]
- Langman L., Paetkau V. Purification and structures of recombining and replicating bacteriophage T7 DNA. J Virol. 1978 Feb;25(2):562–569. doi: 10.1128/jvi.25.2.562-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C. Methylation-dependent DNA synthesis in Escherichia coli mediated by DNA polymerase I. J Bacteriol. 1979 Jan;137(1):44–50. doi: 10.1128/jb.137.1.44-50.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. RNA polymerase of Escherichia coli. Curr Top Microbiol Immunol. 1978;83:37–91. doi: 10.1007/978-3-642-67087-9_2. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Kan N. C., Lackey D., Linn S., Edgell M. H., Hutchison C. A., 3rd Recognition site of Escherichia coli B restriction enzyme on phi XsB1 and simian virus 40 DNAs: an interrupted sequence. Proc Natl Acad Sci U S A. 1978 May;75(5):2271–2275. doi: 10.1073/pnas.75.5.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawhorne L., Kleber I., Mitchell C., Benzinger R. Transfection of Escherichia coli spheroplasts. II. Relative infectivity of native, denatured, and renatured lambda, T7, T5, T4, and P22 bacteriophage DNAs. J Virol. 1973 Oct;12(4):733–740. doi: 10.1128/jvi.12.4.733-740.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Richardson C. C. Gene 2 protein of bacteriophage T7: purification and requirement for packaging of T7 DNA in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4852–4856. doi: 10.1073/pnas.76.10.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt J. C., Schechtman L. M., Ts'o P. O., Borenfreund E., Bendich A. The attachment and penetration of T7 DNA/phage in Syrian hamster embryonic cells. Biochim Biophys Acta. 1976 Jun 18;435(2):167–183. doi: 10.1016/0005-2787(76)90248-3. [DOI] [PubMed] [Google Scholar]
- Lee M., Miller R. C., Jr T7 exonuclease (gene 6) is necessary for molecular recombination of bacteriophage T7. J Virol. 1974 Nov;14(5):1040–1048. doi: 10.1128/jvi.14.5.1040-1048.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Linn S. The 1978 Nobel prize in physiology or medicine. Science. 1978 Dec 8;202(4372):1069–1071. doi: 10.1126/science.362532. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Bacillus subtilis RNA polymerase and its modification in sporulating and phage-infected bacteria. Adv Enzymol Relat Areas Mol Biol. 1976;44:165–185. doi: 10.1002/9780470122891.ch5. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A., Summers W. C. A restriction fragment analysis of the T7 left-early region. Virology. 1975 Dec;68(2):360–373. doi: 10.1016/0042-6822(75)90279-2. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A., Summers W. C. Localization of RNA polymerase binding sites on T7 DNA. Virology. 1976 May;71(1):278–290. doi: 10.1016/0042-6822(76)90112-4. [DOI] [PubMed] [Google Scholar]
- MAEKELAE O., MAEKELAE P. H., SOIKKELI S. SEX-SPECIFICITY OF THE BACTERIOPHAGE T7. Ann Med Exp Biol Fenn. 1964;42:188–195. [PubMed] [Google Scholar]
- Mahadik S. P., Dharmgrongartama B., Srinivasan P. R. An inhibitory protein of Escherichia coli RNA polymerase in bacteriophage T3-infected cells (core polymerase-sigma factor-host polymerase-phage polymerase-initiation). Proc Natl Acad Sci U S A. 1972 Jan;69(1):162–166. doi: 10.1073/pnas.69.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadik S. P., Dharmgrongartama B., Srinivasan P. R. Regulation of host ribonucleic acid synthesis in bacteriophage T3-infected cells. Properties of an inhibitory protein of Escherichia coli ribonucleic acid polymerase. J Biol Chem. 1974 Mar 25;249(6):1787–1791. [PubMed] [Google Scholar]
- Maitra U., Huang H. H. Initiation, release, and reinitiation of RNA chains by bacteriophage-T3-induced polymerase from T3 DNA templates (E. coli-guanosine triphosphate terminus-purified polymerase). Proc Natl Acad Sci U S A. 1972 Jan;69(1):55–59. doi: 10.1073/pnas.69.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U. Induction of a new RNA polymerase in Escherichia coli infected with bacteriophage T3. Biochem Biophys Res Commun. 1971 Apr 16;43(2):443–450. doi: 10.1016/0006-291x(71)90773-x. [DOI] [PubMed] [Google Scholar]
- Maitra U., Salvo R. A., Chakraborty P. R. Specificity of ribonucleic acid chain initiation by bacteriophage T3-induced ribonucleic acid polymerase. J Biol Chem. 1974 Sep 25;249(18):5835–5839. [PubMed] [Google Scholar]
- Majumder H. K., Bishayee S., Chakraborty P. R., Maitra U. Ribonuclease III cleavage of bacteriophage T3RNA polymerase transcripts to late T3 mRNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4891–4894. doi: 10.1073/pnas.74.11.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder H. K., Maitra U., Rosenberg M. Termination of transcription by bacteriophage T3 RNA polymerase: homogeneous 3'-terminal oligonucleotide sequence of in vitro T3 RNA polymerase transcripts. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5110–5113. doi: 10.1073/pnas.76.10.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino O., Kawamura J., Saito H., Ikeda Y. Inactivation of restriction endonuclease bamNx after infection with phage phi NR2. Nature. 1979 Jan 4;277(5691):64–66. doi: 10.1038/277064a0. [DOI] [PubMed] [Google Scholar]
- Male C. J., Christensen J. R. Synthesis of messenger ribonucleic acid after bacteriophage T1 infection. J Virol. 1970 Dec;6(6):727–737. doi: 10.1128/jvi.6.6.727-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. I. An assay for the rate and extent of ribonucleic acid chain initiation. J Biol Chem. 1974 May 25;249(10):2995–3001. [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamune Y., Frenkel G. D., Richardson C. C. A mutant of bacteriophage T7 deficient in polynucleotide ligase. J Biol Chem. 1971 Nov 25;246(22):6874–6879. [PubMed] [Google Scholar]
- Masker W. E., Kuemmerle N. B., Allison D. P. In vitro packaging of bacteriophate T7 DNA synthesized in vitro. J Virol. 1978 Jul;27(1):149–163. doi: 10.1128/jvi.27.1.149-163.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Kuemmerle N. B. In vitro recombination of bacteriophage T7 DNA damaged by UV radiation. J Virol. 1980 Jan;33(1):330–339. doi: 10.1128/jvi.33.1.330-339.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro VI. Synthesis of biologically active T7 DNA. J Mol Biol. 1976 Feb 5;100(4):557–567. doi: 10.1016/s0022-2836(76)80045-9. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. V. Synthesis of intact chromosomes of bacteriophage T7. J Mol Biol. 1976 Feb 5;100(4):543–556. doi: 10.1016/s0022-2836(76)80044-7. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Hybridization mapping of restriction fragments from the early region of bacteriophage T7 DNA. Virology. 1977 Oct 15;82(2):275–287. doi: 10.1016/0042-6822(77)90003-4. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Roles of the early genes of bacteriophage T7 in shutoff of host macromolecular synthesis. J Virol. 1977 Sep;23(3):543–553. doi: 10.1128/jvi.23.3.543-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister W. T., Küpper H., Bautz E. K. Kinetics of transcription by the bacteriophage-T3 RNA polymerase in vitro. Eur J Biochem. 1973 May 2;34(3):489–501. doi: 10.1111/j.1432-1033.1973.tb02785.x. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., McCarron R. J. Hybridization of the in vitro products of bacteriop&hage T7 RNA polymerase to restriction fragments of T7 DNA. Virology. 1977 Oct 15;82(2):288–298. doi: 10.1016/0042-6822(77)90004-6. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Wu H. L. Regulation of transcription of the late genes of bacteriophage T7. Proc Natl Acad Sci U S A. 1978 Feb;75(2):804–808. doi: 10.1073/pnas.75.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J. Control sites in the sequence at the beginning of T7 gene 1. Nucleic Acids Res. 1979 Aug 10;6(11):3491–3503. doi: 10.1093/nar/6.11.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell D. J. The DNA sequence at the T7 C promoter. Nucleic Acids Res. 1979 Feb;6(2):525–544. doi: 10.1093/nar/6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R. Bacteriophage interactions with higher organisms. Trans N Y Acad Sci. 1974 Mar;36(3):265–272. doi: 10.1111/j.2164-0947.1974.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Geier M. R., Petricciani J. C. Bacterial virus gene expression in human cells. Nature. 1971 Oct 8;233(5319):398–400. doi: 10.1038/233398a0. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Assymetric annealing of an RNA linked DNA molecule isolated during the initiation of bacteriophage T7 DNA replication. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1082–1086. doi: 10.1016/0006-291x(72)90323-3. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr, Lee M. The role of bacteriophage T7 exonuclease (gene 6) in genetic recombination and production of concatemers. J Mol Biol. 1976 Feb 25;101(2):223–234. doi: 10.1016/0022-2836(76)90374-0. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Jr Replication and molecular recombination of T-phage. Annu Rev Microbiol. 1975;29:355–376. doi: 10.1146/annurev.mi.29.100175.002035. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr Transcription of the early region of bacteriophage T7: characterization of the in vivo transcripts. J Mol Biol. 1974 Mar;83(3):289–304. doi: 10.1016/0022-2836(74)90281-2. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Jr Transcription of the early region of bacteriophage T7: specificity and selectivity in the in vitro initiation of RNA synthesis. J Mol Biol. 1974 Mar;83(3):305–331. doi: 10.1016/0022-2836(74)90282-4. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Miyazaki J. I., Fujisawa H., Minagawa T. Biological activity of purified bacteriophage T3 prohead and proheadlike structures as precursors for in vitro head assembly. Virology. 1978 Dec;91(2):283–290. doi: 10.1016/0042-6822(78)90376-8. [DOI] [PubMed] [Google Scholar]
- Miyazaki J. I., Ryo Y., Fujisawa H., Minagawa T. Mutation in bacteriophage T3 affecting host cell lysis. Virology. 1978 Aug;89(1):327–329. doi: 10.1016/0042-6822(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 Deoxyribonucleic acid replication in vitro. A protein of Escherichia coli required for bacteriophage T7 DNA polymerase activity. J Biol Chem. 1975 Jul 25;250(14):5508–5514. [PubMed] [Google Scholar]
- Modrich P., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication invitro. Bacteriophage T7 DNA polymerase: an an emzyme composed of phage- and host-specific subunits. J Biol Chem. 1975 Jul 25;250(14):5515–5522. [PubMed] [Google Scholar]
- Molineux I. J., Friedman S., Gefter M. L. Purification and properties of the Escherichia coli deoxyribonucleic acid-unwinding protein. Effects on deoxyribonucleic acid synthesis in vitro. J Biol Chem. 1974 Oct 10;249(19):6090–6098. [PubMed] [Google Scholar]
- Molineux I. J., Gefter M. L. Properties of the Escherichia coli in DNA binding (unwinding) protein: interaction with DNA polymerase and DNA. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3858–3862. doi: 10.1073/pnas.71.10.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Blumberg D. D., Malamy M. H. T7 protein synthesis in F' episome-containing cells: assignment of specific proteins to three translational groups. J Virol. 1974 Feb;13(2):386–393. doi: 10.1128/jvi.13.2.386-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D. Restriction endonucleases, simian virus 40, and the new genetics. Science. 1979 Nov 23;206(4421):903–909. doi: 10.1126/science.228393. [DOI] [PubMed] [Google Scholar]
- Nathans D., Smith H. O. Restriction endonucleases in the analysis and restructuring of dna molecules. Annu Rev Biochem. 1975;44:273–293. doi: 10.1146/annurev.bi.44.070175.001421. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of bacterial growth, RNA, and protein synthesis. Annu Rev Microbiol. 1978;32:393–432. doi: 10.1146/annurev.mi.32.100178.002141. [DOI] [PubMed] [Google Scholar]
- Nierman W. C., Chamberlin M. J. Studies of RNA chain initiation by Escherichia coli RNA polymerase bound to T7 DNA. Direct analysis of the kinetics and extent of RNA chain initiation at T7 promoter A1. J Biol Chem. 1979 Aug 25;254(16):7921–7926. [PubMed] [Google Scholar]
- Nikolskaya I. I., Lopatina N. G., Chaplygina N. M., Debov S. S. The host specificity system in Escherichia coli SK. Mol Cell Biochem. 1976 Nov 30;13(2):79–87. doi: 10.1007/BF01837057. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Condit R. C. Translational Mapping of Bacteriophage T7 RNAs synthesized in vitro by purified T7 RNA polymerase. J Mol Biol. 1975 Oct 15;98(1):57–67. doi: 10.1016/s0022-2836(75)80101-x. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Conlon S. W., Summers W. C. Purification and physical characterization of T7 RNA polymerase from T7-infected Escherichia coli B. Biochemistry. 1974 Sep 10;13(19):3904–3912. doi: 10.1021/bi00716a014. [DOI] [PubMed] [Google Scholar]
- Nossal N. G. A T4 bacteriophage mutant which lacks deoxyribonucleic acid polymerase but retains the polymerase-associated nuclease. J Biol Chem. 1969 Jan 10;244(1):218–220. [PubMed] [Google Scholar]
- Nossal N. G., Hershfield M. S. Nuclease activity in a fragment of bacteriophage T4 deoxyribonucleic acid polymerase induced by the amber mutant am B22. J Biol Chem. 1971 Sep 10;246(17):5414–5426. [PubMed] [Google Scholar]
- Oakley J. L., Coleman J. E. Structure of a promoter for T7 RNA polymerase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4266–4270. doi: 10.1073/pnas.74.10.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley J. L., Pascale J. A., Coleman J. E. T7 RNA polymerase: conformation, functional groups, and promotor binding. Biochemistry. 1975 Oct 21;14(21):4684–4691. doi: 10.1021/bi00692a019. [DOI] [PubMed] [Google Scholar]
- Oakley J. L., Strothkamp R. E., Sarris A. H., Coleman J. E. T7 RNA polymerase: promoter structure and polymerase binding. Biochemistry. 1979 Feb 6;18(3):528–537. doi: 10.1021/bi00570a023. [DOI] [PubMed] [Google Scholar]
- Oen H., Wu C. W., Haas R., Cole P. E. T7 deoxyribonucleic acid directed, rapid-turnover, single-step addition reactions catalyzed by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1979 Sep 18;18(19):4148–4155. doi: 10.1021/bi00586a015. [DOI] [PubMed] [Google Scholar]
- Oey J. L., Strätling W., Knippers R. A DNA polymerase induced by bacteriophage T7. Eur J Biochem. 1971 Dec 10;23(3):497–504. doi: 10.1111/j.1432-1033.1971.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Araki H., Tsujimoto Y. Recombination intermediates formed in the extract from T7-infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1033–1041. doi: 10.1101/sqb.1979.043.01.112. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherichia coli. III. Detection of intact primer RNA. Nucleic Acids Res. 1979 Nov 24;7(6):1621–1633. doi: 10.1093/nar/7.6.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontell M. P., Nakada D. Rescue of abortive T7 gene 2 mutant phage infection by rifampin. J Virol. 1980 May;34(2):438–445. doi: 10.1128/jvi.34.2.438-445.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachl C. A., Yound E. T. The size and messenger RNA activity of bacteriophage T7 late transcripts synthesized in vivo. J Mol Biol. 1978 Jun 15;122(1):69–101. doi: 10.1016/0022-2836(78)90109-2. [DOI] [PubMed] [Google Scholar]
- Pachl C. A., Young E. T. Detection of polycistronic and overlapping bacteriophage T7 late transcripts by in vitro translation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):312–316. doi: 10.1073/pnas.73.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacumbaba R. P., Center M. S. Studies on an endonuclease activity associated with bacteriophage T7 DNA-membrane complex. J Virol. 1974 Dec;14(6):1380–1387. doi: 10.1128/jvi.14.6.1380-1387.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacumbaba R., Center M. S. Partial purification and properties of a bacteriophage T7 inhibitor of the host exonuclease V activity. J Virol. 1975 Nov;16(5):1200–1207. doi: 10.1128/jvi.16.5.1200-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Bradley R., Scraba D., Miller R. C., Jr Folded, concatenated genomes as replication intermediates of bacteriophage T7 DNA. J Virol. 1977 Apr;22(1):130–141. doi: 10.1128/jvi.22.1.130-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Miller R. C., Jr The origin of nascent single-stranded fragments in replicating TM DNA. J Mol Biol. 1975 Nov 15;98(4):719–737. doi: 10.1016/s0022-2836(75)80006-4. [DOI] [PubMed] [Google Scholar]
- Pai S. H., Rahmsdorf H. J., Ponta H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Protein kinase of bacteriophage T7. 2. Properties, enzyme synthesis in vitro and regulation of enzyme synthesis and activity in vivo. Eur J Biochem. 1975 Jun 16;55(1):305–314. doi: 10.1111/j.1432-1033.1975.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Palchaudhuri S., Maas W. K. Physical mapping of genes on the F plasmid of Escherichia coli responsible for inhibition of growth of female-specific bacteriophages. J Bacteriol. 1977 Nov;132(2):740–743. doi: 10.1128/jb.132.2.740-743.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Cloning and localization of the in vitro functional origin of replication of bacteriophage T7 DNA. J Biol Chem. 1979 Jun 25;254(12):5555–5561. [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Identification, cloning and characterization of three late promoters at 14.6, 14.8 and 15.9% of T7 DNA. J Mol Biol. 1979 Nov 25;135(1):91–109. doi: 10.1016/0022-2836(79)90342-5. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Recognition and initiation site for four late promoters of phage T7 is a 22-base pair DNA sequence. Nature. 1979 Jul 5;280(5717):35–39. doi: 10.1038/280035a0. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Speyer J. F. Mutants of T7 bacteriophage inhibited by lambda prophage. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3642–3646. doi: 10.1073/pnas.72.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Speyer J. F. Order of injection of T7 bacteriophage DNA. J Virol. 1973 Jun;11(6):1024–1026. doi: 10.1128/jvi.11.6.1024-1026.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson K. A., Warner H. R., Anderson D. L., Snustad D. P. Analysis of nuclear disruption and binding of intermediates in host DNA breakdown to membranes after infection of Escherichia coli with bacteriophages T4 and T7. J Virol. 1973 May;11(5):806–809. doi: 10.1128/jvi.11.5.806-809.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. G., Hayward R. S. Dinucleotide sequences in the regions of T7 DNA coding for termination of early transcription. Eur J Biochem. 1974 Oct 1;48(1):199–208. doi: 10.1111/j.1432-1033.1974.tb03757.x. [DOI] [PubMed] [Google Scholar]
- Peters G. G., Hayward R. S. Transcriptional termination in vitro: the 3'-terminal sequence of coliphage T7 "early" RNA. Biochem Biophys Res Commun. 1974 Nov 27;61(2):809–816. doi: 10.1016/0006-291x(74)91029-8. [DOI] [PubMed] [Google Scholar]
- Pfennig-Yeh M. L., Ponta H., Hirsch-Kauffmann M., Rahmsdorf H. J., Herrlich P., Schweiger M. Early T7 gene expression: rates of RNA synthesis and degradation, protein kinase dependent termination of transcription, and efficiency of translation. Mol Gen Genet. 1978 Oct 30;166(2):127–140. doi: 10.1007/BF00285915. [DOI] [PubMed] [Google Scholar]
- Pigiet V., Conley R. R. Isolation and characterization of phosphothioredoxin from Excherichia coli. J Biol Chem. 1978 Mar 25;253(6):1910–1920. [PubMed] [Google Scholar]
- Ponta H., Altendorf K. H., Schweiger M., Hirsch-Kaufmann M., Pfennig-Yeh M. L., Herrlich P. E. coli membranes become permeable to ions following T7-virus-infection. Mol Gen Genet. 1976 Dec 8;149(2):145–150. doi: 10.1007/BF00332882. [DOI] [PubMed] [Google Scholar]
- Ponta H., Orätzel M., Pfennig-Yeh M., Hirsch-Kauffmann M., Schweiger M. Membrane alteration induced by T7 virus infection. FEBS Lett. 1977 Feb 1;73(2):207–209. doi: 10.1016/0014-5793(77)80982-4. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7. Isolation of a new control protein and mechanism of action. Mol Gen Genet. 1974;134(1):29–38. doi: 10.1007/BF00332810. [DOI] [PubMed] [Google Scholar]
- Ponta H., Rahmsdorf H. J., Pai S. H., Hirsch-Kauffmann M., Herrlich P., Schweiger M. Control of gene expression in bacteriophage T7: transcriptional controls. Mol Gen Genet. 1974;134(4):281–297. doi: 10.1007/BF00337463. [DOI] [PubMed] [Google Scholar]
- Portmann R., Sogo J. M., Koller T., Zillig W. Binding sites of E. coli RNA polymerase on T7 DNA as determined by electron microscopy. FEBS Lett. 1974 Sep 1;45(1):64–67. doi: 10.1016/0014-5793(74)80811-2. [DOI] [PubMed] [Google Scholar]
- Powling A., Knippers R. Recombination of bacteriophage T7 in vivo. Mol Gen Genet. 1976 Nov 24;149(1):63–71. doi: 10.1007/BF00275961. [DOI] [PubMed] [Google Scholar]
- Powling A., Knippers R. Some functions involved in bacteriophage T7 genetic recombination. Mol Gen Genet. 1974;134(2):173–180. doi: 10.1007/BF00268418. [DOI] [PubMed] [Google Scholar]
- Prehm P., Jann B., Jann K., Schmidt G., Stirm S. On a bacteriophage T3 and T4 receptor region within the cell wall lipopolysaccharide of Escherichia coli B. J Mol Biol. 1976 Feb 25;101(2):277–281. doi: 10.1016/0022-2836(76)90377-6. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess H., Koller B., Hess B., Delius H. Electron microscopic mapping and sequence analysis of the terminator for the early message of E. coli phage T7. Mol Gen Genet. 1980 Apr;178(1):27–34. doi: 10.1007/BF00267209. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Herrlich P., Pai S. H., Schweiger M., Wittmann H. G. Ribosomes after infection with bacteriophage T4 and T7. Mol Gen Genet. 1973 Dec 31;127(3):259–271. doi: 10.1007/BF00333766. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Pai S. H., Ponta H., Herrlich P., Roskoski R., Jr, Schweiger M., Studier F. W. Protein kinase induction in Escherichia coli by bacteriophage T7. Proc Natl Acad Sci U S A. 1974 Feb;71(2):586–589. doi: 10.1073/pnas.71.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R. N., Pereira M. G. Behavior of a hybrid F' ts114 lac+, his+ factor (F42-400) in Klebsiella pneumoniae M5a1. J Bacteriol. 1975 Sep;123(3):792–805. doi: 10.1128/jb.123.3.792-805.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner D. The interaction bacterial and phage proteins with immobilized Escherichia coli RNA polymerase. J Mol Biol. 1974 Sep 15;88(2):373–383. doi: 10.1016/0022-2836(74)90488-4. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Horiuchi K., Zinder N. D. Nucleotide sequence of the recognition site for the restriction-modification enzyme of Escherichia coli B. Proc Natl Acad Sci U S A. 1978 May;75(5):2266–2270. doi: 10.1073/pnas.75.5.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Goren D., Friedman J. Studies on the biological role of DNA methylation: inhibition of methylation and maturation of the bacteriophage phichi174 by nicotinamide. Nucleic Acids Res. 1975 Oct;2(10):1967–1974. doi: 10.1093/nar/2.10.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisbig R. R., Woody A. Y., Woody R. W. Spectroscopic analysis of the interaction of Escherichia coli DNA-dependent RNA polymerase with T7 DNA and synthetic polynucleotides. J Biol Chem. 1979 Nov 25;254(22):11208–11217. [PubMed] [Google Scholar]
- Remes B., Elseviers D. Adenosine 5'-triphosphate leakage does not cause abortive infection of bacteriophage T7 in male Escherichia coli. J Bacteriol. 1980 Aug;143(2):1054–1056. doi: 10.1128/jb.143.2.1054-1056.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Gefter M. L. A DNA-binding protein induced by bacteriophage T7. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1846–1850. doi: 10.1073/pnas.70.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Gefter M. L. A deoxyribonucleic acid-binding protein induced by bacteriophage T7. Purification and properties of the protein. J Biol Chem. 1974 Jun 25;249(12):3843–3850. [PubMed] [Google Scholar]
- Reuter M., Krüger D. H., Scholz D., Rosenthal H. A. Schutz zellfremder DNA vor wirtskontrollierter Restriktion in Bakterienzellen. II. Schutz des Plasmids pSF2124 durch die ocr+ Genfunktion der Bakteriophagen T3 und T7. Z Allg Mikrobiol. 1980;20(5):345–354. doi: 10.1002/jobm.3630200506. [DOI] [PubMed] [Google Scholar]
- Richardson C. C., Romano L. J., Kolodner R., LeClerc J. E., Tamanoi F., Engler M. J., Dean F. B., Richardson D. S. Replication of bacteriophage T7 DNA by purified proteins. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):427–440. doi: 10.1101/sqb.1979.043.01.049. [DOI] [PubMed] [Google Scholar]
- Richter D., Herrlich P., Schweiger M. Phage DNA directed enzyme synthesis in vitro system from yeast mitochondria. Nat New Biol. 1972 Jul 19;238(81):74–76. doi: 10.1038/newbio238074a0. [DOI] [PubMed] [Google Scholar]
- Ritchie D. A., Thomas C. A., Jr, MacHattie L. A., Wensink P. C. Terminal repetition in non-permuted T3 and T7 bacteriophage DNA molecules. J Mol Biol. 1967 Feb 14;23(3):365–376. doi: 10.1016/s0022-2836(67)80111-6. [DOI] [PubMed] [Google Scholar]
- Robb S. M., Woods D. R., Robb F. T., Struthers J. K. Rifampicin-resistant mutant supporting bacteriophage growth on stationary phase Achromobacter cells. J Gen Virol. 1977 Apr;35(1):117–123. doi: 10.1099/0022-1317-35-1-117. [DOI] [PubMed] [Google Scholar]
- Roberts L., Sheldon R., Sadowski P. D. Genetic recombination of bacteriophage T7 DNA in vitro. IV. Asymmetry of recombination frequencies caused by polarity of DNA packaging. Virology. 1978 Aug;89(1):252–261. doi: 10.1016/0042-6822(78)90057-0. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases. CRC Crit Rev Biochem. 1976 Nov;4(2):123–164. doi: 10.3109/10409237609105456. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction endonucleases: a new role in vivo? Nature. 1978 Feb 9;271(5645):502–502. doi: 10.1038/271502a0. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Dunn J. J. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Pathways of recombination of bacteriophage T7 DNA in vitro. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1023–1032. doi: 10.1101/sqb.1979.043.01.111. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Zillig W. Studies on the transcription complex of Escherichia coli RNA polymerase. Eur J Biochem. 1977 Oct 3;79(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11822.x. [DOI] [PubMed] [Google Scholar]
- Romano L. J., Richardson C. C. Characterization of the ribonucleic acid primers and the deoxyribonucleic acid product synthesized by the DNA polymerase and gene 4 protein of bacteriophage T7. J Biol Chem. 1979 Oct 25;254(20):10483–10489. [PubMed] [Google Scholar]
- Romano L. J., Richardson C. C. Requirements for synthesis of ribonucleic acid primers during lagging strand synthesis by the DNA polymerase and gene 4 protein of bacteriophage T7. J Biol Chem. 1979 Oct 25;254(20):10476–10482. [PubMed] [Google Scholar]
- Rosa M. D. Four T7 RNA polymerase promoters contain an identical 23 bp sequence. Cell. 1979 Apr;16(4):815–825. doi: 10.1016/0092-8674(79)90097-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Simon M. N., Studier F. W., Roberts R. J. Survey and mapping of restriction endonuclease cleavage sites in bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):907–915. doi: 10.1016/0022-2836(79)90519-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Kramer R. A., Steitz J. A. T7 early messenger RNAs are the direct products of ribonuclease III cleavage. J Mol Biol. 1974 Nov 15;89(4):777–782. doi: 10.1016/0022-2836(74)90052-7. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D. An endodeoxyribonuclease induced after infection with phage T7 bearing amber mutations in genes 3, 5, and 6. Can J Biochem. 1972 Sep;50(9):1016–1023. doi: 10.1139/o72-140. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D. Genetic recombination of bacteriophage T7 DNA in vitro. II. Further properties of the in vitro recombination-packaging reaction. Virology. 1977 May 1;78(1):192–202. doi: 10.1016/0042-6822(77)90091-5. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Kerr C. Degradation of Escherichia coli B deoxyribonucleic acid after infection with deoxyribonucleic acid-defective amber mutants of bacteriophage T7. J Virol. 1970 Aug;6(2):149–155. doi: 10.1128/jvi.6.2.149-155.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D. Suppression of a mutation in gene 3 of bacteriophage T7 (T7 endonuclease I) by mutations in phage and host polynucleotide ligase. J Virol. 1974 Jan;13(1):226–229. doi: 10.1128/jvi.13.1.226-229.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Genetic recombination of bacteriophage T7 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Mar;73(3):692–696. doi: 10.1073/pnas.73.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Kitchin R. Selective silencing of eukaryotic DNA. Science. 1975 Aug 8;189(4201):426–433. [PubMed] [Google Scholar]
- Saigo K. Polar DNA ejection in bacteriophage T7. Virology. 1975 May;65(1):120–127. doi: 10.1016/0042-6822(75)90012-4. [DOI] [PubMed] [Google Scholar]
- Saito H., Tabor S., Tamanoi F., Richardson C. C. Nucleotide sequence of the primary origin of bacteriophage T7 DNA replication: relationship to adjacent genes and regulatory elements. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3917–3921. doi: 10.1073/pnas.77.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y., Karu A. E., Linn S., Echols H. Purification and properties of the gamma-protein specified by bacteriophage lambda: an inhibitor of the host RecBC recombination enzyme. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2215–2219. doi: 10.1073/pnas.70.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W. A. DNA sequencing techniques. Annu Rev Biochem. 1974;43(0):923–965. doi: 10.1146/annurev.bi.43.070174.004423. [DOI] [PubMed] [Google Scholar]
- Salvo R. A., Chakraborty P. R., Maitra U. Studies on T3-induced ribonucleic acid polymerase. IV. Transcription of denatured deoxyribonucleic acid preparations by T3 ribonucleic acid polymerase. J Biol Chem. 1973 Oct 10;248(19):6647–6654. [PubMed] [Google Scholar]
- Satta G., Debbia E., Pruzzo C., Calegari L. The peculiar behaviour of coliphage P1vir mutants on restricting hosts. Microbios. 1978;22(88):93–102. [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Calegari L. Lysogenic conversion in Klebsiella pneumoniae: system which requires active immunity regulation for expression of the conversion phenomenon. J Virol. 1978 Dec;28(3):786–794. doi: 10.1128/jvi.28.3.786-794.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Schweiger M., Herrlich P. Control of gene function in bacteriophage Tr. 3. Preventing the shutoff of early enzyme synthesis. J Virol. 1971 Nov;8(5):613–618. doi: 10.1128/jvi.8.5.613-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Herrlich P., Schweiger M., Schuster H. The early region of the DNA of bacteriophage T7. Eur J Biochem. 1972 Feb 15;25(2):341–348. doi: 10.1111/j.1432-1033.1972.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Herrlich P., Schweiger M. Transcription of T3 and T7 early genes by T3 and T7 RNA polymerases. Mol Gen Genet. 1972;118(1):67–77. [PubMed] [Google Scholar]
- Scherzinger E., Klotz G. Studies on bacteriophage T7 DNA synthesis in vitro. II. Reconstitution of the T7 replication system using purified proteins. Mol Gen Genet. 1975 Dec 1;141(3):233–249. doi: 10.1007/BF00341802. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Lanka E., Hillenbrand G. Role of bacteriophage T7 DNA primase in the initiation of DNA strand synthesis. Nucleic Acids Res. 1977 Dec;4(12):4151–4163. doi: 10.1093/nar/4.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Lanka E., Morelli G., Seiffert D., Yuki A. Bacteriophage-T7-induced DNA-priming protein. A novel enzyme involved in DNA replication. Eur J Biochem. 1977 Feb;72(3):543–558. doi: 10.1111/j.1432-1033.1977.tb11278.x. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Lauppe H. F., Voll N., Wanke M. Recombinant plasmids carrying promoters, genes and the origin of DNA replication of the early region of bacteriophage T7. Nucleic Acids Res. 1980 Mar 25;8(6):1287–1305. doi: 10.1093/nar/8.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F. In vitro studies on the role of phage T7 gene 4 product in DNA replication. Mol Gen Genet. 1974;135(1):73–86. doi: 10.1007/BF00433903. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F. Initiation of the replication of single-stranded DNA by concerted action of phage T7 RNA and DNA polymerases. Eur J Biochem. 1974 Jul 1;46(1):179–187. doi: 10.1111/j.1432-1033.1974.tb03610.x. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Litfin F., Jost E. Stimulation of T7 DNA polymerase by a new phage-coded protein. Mol Gen Genet. 1973 Jul 2;123(3):247–262. doi: 10.1007/BF00271243. [DOI] [PubMed] [Google Scholar]
- Scherzinger E., Seiffert D. Studies on bacteriophage T7 DNA synthesis in vitro. I. Resolution of the T7 replication system into its components. Mol Gen Genet. 1975 Dec 1;141(3):213–232. doi: 10.1007/BF00341801. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Schleicher M., Bautz E. K.F. Stability of phage T7 gene I mRNA in E. coli cells infected with wild type phage and with gene I amber mutants. FEBS Lett. 1972 Dec 1;28(2):139–141. doi: 10.1016/0014-5793(72)80695-1. [DOI] [PubMed] [Google Scholar]
- Scholz D., Hansen S., Rosenthal H. A. Untersuchungen an Ts-Phagen. IV. Isolierung temperatursensitiver Mutanten des Phagen T3 und ihre partielle Charakterisierung. Z Allg Mikrobiol. 1973;13(5):425–437. [PubMed] [Google Scholar]
- Scholz D., Meissner C., Rosenthal H. A. Nutzung von Gen5 und Gen6 ts-Mutanten des Phagen T3 für die Anzeige von Hemmstoffen der DNS-Synthese. Z Allg Mikrobiol. 1979;19(9):653–661. doi: 10.1002/jobm.3630190907. [DOI] [PubMed] [Google Scholar]
- Schweiger M., Gold L. M. Bacteriophage T4 DNA-dependent in vitro synthesis of lysozyme. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1351–1358. doi: 10.1073/pnas.63.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Krämer R., Zillig W., Cudny H. On the initiation of transcription by DNA-dependent RNA polymerase from Escherichia coli. Eur J Biochem. 1973 Dec 17;40(2):367–373. doi: 10.1111/j.1432-1033.1973.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Seki T., Okazaki T. RNA-linked nascent DNA pieces in phage T7-infected Escherchia coli. II. Primary structure of the RNA portion. Nucleic Acids Res. 1979 Nov 24;7(6):1603–1619. doi: 10.1093/nar/7.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seroka K., Wackernagel W. In vivo effects of recBC DNase, exonuclease I, and DNA polymerases of Escherichia coli on the infectivity of native and single-stranded DNA of bacteriophage T7. J Virol. 1977 Mar;21(3):906–912. doi: 10.1128/jvi.21.3.906-912.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P. Complexes between bacteriophage T7 capsids and T7 DNA. Virology. 1974 May;59(1):89–107. doi: 10.1016/0042-6822(74)90208-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fibrous projections from the core of a bacteriophage T7 procapsid. J Supramol Struct. 1979;11(3):321–326. doi: 10.1002/jss.400110307. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Serwer P., Pichler M. E. Electrophoresis of bacteriophage T7 and T7 capsids in agarose gels. J Virol. 1978 Dec;28(3):917–928. doi: 10.1128/jvi.28.3.917-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Okazaki T. RNA-linked nascent DNA pieces in T7 phage-infected Escherichia coli cells. I. Role of gene 6 exonuclease in removal of the linked RNA. Mol Gen Genet. 1977 Sep 9;154(3):263–267. doi: 10.1007/BF00571281. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Okazaki T. T7 gene 6 exonuclease has an RNase H activity. Nucleic Acids Res. 1978 Nov;5(11):4245–4261. doi: 10.1093/nar/5.11.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. Nucleotide sequence of the three major early promoters of bacteriophage T7. Nucleic Acids Res. 1979;6(5):1895–1907. doi: 10.1093/nar/6.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siegel P. J., Schaechter M. The role of the host cell membrane in the replication and morphogenesis of bacteriophages. Annu Rev Microbiol. 1973;27:261–282. doi: 10.1146/annurev.mi.27.100173.001401. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Simmon V. F., Lederberg S. Degradation of bacteriophage lambda deoxyribonucleic acid after restriction by Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):161–169. doi: 10.1128/jb.112.1.161-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]
- Skare J., Niles E. G., Summers W. C. Localization of the leftmost initiation site for T7 late transcription, in vivo and in vitro. Biochemistry. 1974 Sep 10;13(19):3912–3916. doi: 10.1021/bi00716a015. [DOI] [PubMed] [Google Scholar]
- Skavronskaia A. G., Aleshkin G. I., Demkin V. V. Osobennosti modifikatsii-restriktsii bakteriofagov lambda i P1 u shtammov Escherichia coli, kontroliruemykh faktorom R124. Genetika. 1979;15(10):1719–1723. [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Nucleotide sequence specificity of restriction endonucleases. Science. 1979 Aug 3;205(4405):455–462. doi: 10.1126/science.377492. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Wilcox K. W. A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol. 1970 Jul 28;51(2):379–391. doi: 10.1016/0022-2836(70)90149-x. [DOI] [PubMed] [Google Scholar]
- Sommer R., Schaller H. Nucleotide sequence of the recognition site of the B-specific restriction modification system in E. coli. Mol Gen Genet. 1979 Jan 11;168(3):331–335. doi: 10.1007/BF00271504. [DOI] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P., Bickle T. A. A novel bacteriophage defence mechanism: the anti-restriction protein. Nature. 1979 Mar 1;278(5699):30–34. doi: 10.1038/278030a0. [DOI] [PubMed] [Google Scholar]
- Spoerel N., Herrlich P. Colivirus-T3-coded S-adenosylmethionine hydrolase. Eur J Biochem. 1979 Apr 2;95(2):227–233. doi: 10.1111/j.1432-1033.1979.tb12957.x. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. An expanded transcriptional map of T7 bacteriophage. Reading of minor T7 promoter sites in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1977 Jun 5;112(4):577–601. doi: 10.1016/s0022-2836(77)80165-4. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. Transcription of T7 DNA containing modified nucleotides by bacteriophage T7 specific RNA polymerase. J Biol Chem. 1978 Jul 25;253(14):4951–4959. [PubMed] [Google Scholar]
- Staudenbauer W. L., Scherzinger E., Lanka E. Replication of the colicin E1 plasmid in extracts of Escherichia coli: uncoupling of leading strand from lagging strand synthesis. Mol Gen Genet. 1979;177(1):113–120. doi: 10.1007/BF00267260. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Bryan R. A. Two ribosome binding sites from the gene 0-3 messenger RNA of bacteriophages T7. J Mol Biol. 1977 Aug 25;114(4):527–543. doi: 10.1016/0022-2836(77)90176-0. [DOI] [PubMed] [Google Scholar]
- Strome S., Young E. T. Chemical and functional quantitation of gene 0.3 messenger RNA during T7 infection. J Mol Biol. 1980 Feb 5;136(4):417–432. doi: 10.1016/0022-2836(80)90398-8. [DOI] [PubMed] [Google Scholar]
- Strome S., Young E. T. Translational control of the expression of bacteriophage T7 gene 0.3. J Mol Biol. 1978 Oct 15;125(1):75–93. doi: 10.1016/0022-2836(78)90255-3. [DOI] [PubMed] [Google Scholar]
- Strome S., Young E. T. Translational discrimination against bacteriophage T7 gene 0.3 messenger RNA. J Mol Biol. 1980 Feb 5;136(4):433–450. doi: 10.1016/0022-2836(80)90399-x. [DOI] [PubMed] [Google Scholar]
- Strothkamp R. E., Oakley J. L., Coleman J. E. Promoter melting by T7 ribonucleic acid polymerase as detected by single-stranded endonuclease digestion. Biochemistry. 1980 Mar 18;19(6):1074–1080. doi: 10.1021/bi00547a005. [DOI] [PubMed] [Google Scholar]
- Strätling W., Knippers R. Function and purification of gene 4 protein of phage T7. Nature. 1973 Sep 28;245(5422):195–197. doi: 10.1038/245195a0. [DOI] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic mapping of a mutation that causes ribonucleases III deficiency in Escherichia coli. J Bacteriol. 1975 Oct;124(1):307–316. doi: 10.1128/jb.124.1.307-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Hausmann R. Integration of two sets of T7 mutants. Virology. 1969 Nov;39(3):587–588. doi: 10.1016/0042-6822(69)90106-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Simon M. N., Dunn J. J. Genetic and physical mapping in the early region of bacteriophage T7 DNA. J Mol Biol. 1979 Dec 25;135(4):917–937. doi: 10.1016/0022-2836(79)90520-5. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Electron microscopy of DNA: determination of absolute molecular weights and linear density. Mol Gen Genet. 1977 Sep 9;154(3):299–303. doi: 10.1007/BF00571286. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Brunovskis I., Hyman R. W. The process of infection with coliphage T7. VII. Characterization and mapping of the major in vivo transcription products of the early region. J Mol Biol. 1973 Mar 5;74(3):291–300. doi: 10.1016/0022-2836(73)90374-4. [DOI] [PubMed] [Google Scholar]
- Summers W. C. Regulation of RNA metabolism of T7 and related phages. Annu Rev Genet. 1972;6:191–202. doi: 10.1146/annurev.ge.06.120172.001203. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Transcription of late phage RNA by T7 RNA polymerase. Nature. 1970 Dec 19;228(5277):1160–1162. doi: 10.1038/2281160a0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Takanami M. Isolation of DNA segments containing promoters from bacteriophage T3 DNA. Biochemistry. 1974 Dec 17;13(26):5388–5394. doi: 10.1021/bi00723a022. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Saito H., Richardson C. C. Physical mapping of primary and secondary origins of bacteriophage T7 DNA replication. Proc Natl Acad Sci U S A. 1980 May;77(5):2656–2660. doi: 10.1073/pnas.77.5.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson B. J., Domingo E., Warner R. C. Pseudolysogeny of Azotobacter phages. Virology. 1980 Apr 30;102(2):267–277. doi: 10.1016/0042-6822(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Transfection of restrictionless Escherichia coli by bacteriophage T7 dna: effect of in vitro erosion of DNA by gamma exonuclease. J Mol Biol. 1976 Aug 25;105(4):603–609. doi: 10.1016/0022-2836(76)90238-2. [DOI] [PubMed] [Google Scholar]
- Travers A. RNA polymerase specificity and the control of growth. Nature. 1976 Oct 21;263(5579):641–646. doi: 10.1038/263641a0. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. EcoRI-sensitive mutation of T7 phage. Mol Gen Genet. 1977 Jan 18;150(2):221–223. doi: 10.1007/BF00695402. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. Intermediates in genetic recombination of bacteriophage T7 DNA. Biological activity and the roles of gene 3 and gene 5. J Mol Biol. 1978 Nov 5;125(3):255–273. doi: 10.1016/0022-2836(78)90402-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. Intermediates in genetic recombination of bacteriophage T7 DNA. J Mol Biol. 1977 Jan 25;109(3):423–426. doi: 10.1016/s0022-2836(77)80021-1. [DOI] [PubMed] [Google Scholar]
- Venner H., Reinert H. Possible role of methylated DNA bases for the transcription of the genetic information. Z Allg Mikrobiol. 1973;13(7):613–624. doi: 10.1002/jobm.3630130711. [DOI] [PubMed] [Google Scholar]
- Vetter D., Roberts L., Jackowski J. B., Sadowski P. D. Location of the ss--mutation of bacteriophage T7 in genes 10, the structural gene for the major capsid protein. J Virol. 1977 Jun;22(3):694–701. doi: 10.1128/jvi.22.3.694-701.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachopoulou P. J., Sadowksi P. D. Genetic recombination of bacteriophage T7 DNA in vitro III. A physical assay for recombinant DNA. Virology. 1977 May 1;78(1):203–215. doi: 10.1016/0042-6822(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Vovis G. F., Lacks S. Complementary action of restriction enzymes endo R-DpnI and Endo R-DpnII on bacteriophage f1 DNA. J Mol Biol. 1977 Sep 25;115(3):525–538. doi: 10.1016/0022-2836(77)90169-3. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Hermanns U. Inhibition of exonuclease V after infection of E. coli by bacteriophage T7. Biochem Biophys Res Commun. 1974 Sep 23;60(2):521–527. doi: 10.1016/0006-291x(74)90271-x. [DOI] [PubMed] [Google Scholar]
- Watanabe I., Sakurai T., Furuse K., Ando A. "Pseudolysogenization" by RNA phage Q beta. Microbiol Immunol. 1979;23(11):1077–1083. doi: 10.1111/j.1348-0421.1979.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli. Annu Rev Biochem. 1978;47:1163–1191. doi: 10.1146/annurev.bi.47.070178.005503. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Williams L., Meynell G. G. Female-specific phages and F-minus strains of Escherichia coli K12. Mol Gen Genet. 1971;113(3):222–227. doi: 10.1007/BF00339542. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Chamberlin M. J. Electron microscope studies of transient complexes formed between Escherichia coli RNA polymerase holoenzyme and T7 DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3740–3744. doi: 10.1073/pnas.74.9.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel G. The effect of U.V. light on formation and synthetic capacity of DNA-membrane complexes from T7-infected cells of E. coli. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Feb;25(2):105–111. doi: 10.1080/09553007414550121. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Bacteriophage T7 DNA replication. An electron microscopic study of the growing point and the role of the T7 gene 4 protein in the formation of DNA fragments. J Biol Chem. 1979 Oct 25;254(20):10490–10495. [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Regions of single-stranded DNA in the growing points of replicating bacteriophage T7 chromosomes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2682–2686. doi: 10.1073/pnas.69.9.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakada D. Early to late switch in bacteriophage T7 development: no translational discrimination between T7 early messenger RNA and late messenger RNA. J Mol Biol. 1976 Jan 5;100(1):35–45. doi: 10.1016/s0022-2836(76)80032-0. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakada D. Translation of T7 RNA in vitro without cleavage by RNase III. J Virol. 1976 Jun;18(3):1155–1159. doi: 10.1128/jvi.18.3.1155-1159.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Silnutzer J., Nakada D. Accumulation of bacteriophage T7 head-related particles in an Escherichia coli mutant. J Virol. 1979 Jul;31(1):209–219. doi: 10.1128/jvi.31.1.209-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Silnutzer J., Nakada D. Mutant of Escherichia coli which blocks T7 bacteriophage assembly: accumulation of short T7 DNA. J Mol Biol. 1978 May 5;121(1):95–111. doi: 10.1016/0022-2836(78)90264-4. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Chemical stability of bacteriophage T7 early mRNA. J Virol. 1975 Dec;16(6):1683–1687. doi: 10.1128/jvi.16.6.1683-1687.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Early to late switch in bacteriophage T7 development: functional decay of T7 early messenger RNA. J Mol Biol. 1974 Oct 25;89(2):293–303. doi: 10.1016/0022-2836(74)90520-8. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Whitaker P. A., Nakada D. Functional instability of T7 early mRNA. Nature. 1974 Mar 22;248(446):335–338. doi: 10.1038/248335a0. [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Fujisawa H., Yamagishi H., Minagawa T. Purification of gene 6 product of bacteriophage T3 and its role in vitro DNA packaging. Virology. 1980 Jan 30;100(2):382–389. doi: 10.1016/0042-6822(80)90529-2. [DOI] [PubMed] [Google Scholar]
- Yang F., Montoya A. L., Merlo D. J., Drummond M. H., Chilton M. D., Nester E. W., Gordon M. P. Foreign DNA sequences in crown gall teratomas and their fate during the loss of the tumorous traits. Mol Gen Genet. 1980;177(4):707–714. doi: 10.1007/BF00272683. [DOI] [PubMed] [Google Scholar]
- Young R. J., Smith G. The end groups of T7 mRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):952–959. doi: 10.1016/0006-291x(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Yuan R., Reiser J. Steps in the reaction mechanism of the Escherichia coli plasmid P15-specific restriction endonuclease. J Mol Biol. 1978 Jul 15;122(4):433–445. doi: 10.1016/0022-2836(78)90420-5. [DOI] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]
- Zechel K. Initiation of DNA synthesis by RNA. Curr Top Microbiol Immunol. 1978;82:71–112. doi: 10.1007/978-3-642-46388-4_3. [DOI] [PubMed] [Google Scholar]
- Zillig W., Fujiki H., Blum W., Janeković D., Schweiger M., Rahmsdorf H., Ponta H., Hirsch-Kauffmann M. In vivo and in vitro phosphorylation of DNA-dependent RNA polymerase of Escherichia coli by bacteriophage-T7-induced protein kinase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2506–2510. doi: 10.1073/pnas.72.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zin'kovskaia G. G., Berdyshev G. D., Vaniushin B. F. Tkanespetsificheskoe umen'shenie izmenenie kharaktera metilirovaniia DNK krupnogo rogatogo skota pri starenii. Biokhimiia. 1978 Oct;43(10):1883–1892. [PubMed] [Google Scholar]


