Abstract
Adolescence is a time period when major changes occur in the brain with long-term consequences for behavior. One ramification is altered responses to drugs of abuse, but the specific brain mechanisms and implications for mental health are poorly understood. Here, we used a mouse model in which adolescents display dramatically reduced sensitivity to the acute locomotor stimulating effects of cocaine and methamphetamine. The goal was to identify key brain regions or circuits involved in the differential behavior. Male adolescent (PN 30–35) and young adult (PN 69–74) C57BL/6J mice were administered an intraperitoneal injection of cocaine (0, 15, 30 mg/kg) or methamphetamine (0, 2, 4 mg/kg) and euthanized 90 minutes later. Locomotor activity was monitored continuously in the home cage by video tracking. Immunohistochemical detection of Fos protein was used to quantify neuronal activation in 16 different brain regions. As expected, adolescents were less sensitive to the locomotor stimulating effects of cocaine and methamphetamine as indicated by a rightward shift in the dose response relationship. After a saline injection, adolescents showed similar levels of Fos as adults in all regions except the dorsal and lateral caudate where levels were lower in adolescents. Cocaine and methamphetamine dose dependently increased Fos in all brain regions sampled in both adolescents and adults, but Fos levels were similar in both age groups for a majority of regions and doses. Locomotor activity was correlated with Fos in several brain areas within adolescent and adult groups, and adolescents had a significantly greater induction of Fos for a given amount of locomotor activity in key brain regions including the caudate where they showed reduced Fos under baseline conditions. Future research will identify the molecular and cellular events that are responsible for the differential psychostimulant-induced patterns of brain activation and behavior observed in adolescent versus adult mice.
Keywords: adolescent, cocaine, methamphetamine, c-fos, locomotor stimulation, mice
The literature on acute locomotor stimulation from cocaine and amphetamines in animal models dates back more than 80 years (Tatum and Seevers, 1929). Key brain regions (e.g., caudate, nucleus accumbens), neural circuits (e.g., natural reward, basal ganglia, motor), and specific cellular and molecular events (e.g., dopamine neurotransmission, DARPP-32 signaling) have been identified that contribute to increased physical activity, arousal, reward and other behaviors induced by these drugs (Gold et al., 1989, Uhl et al., 2002, Rebec, 2006, Zachariou et al., 2006, Zombeck et al., 2008). However, it has proven much more difficult to identify the features that contribute to individual differences in sensitivity to locomotor responses or other behavioral effects (Volkow et al., 2002, Klein and Gulley, 2009). This is important because it has been argued that individual differences in sensitivity to initial drug experience are related to vulnerability for future drug abuse (Lambert et al., 2006).
Recently, an important gap in the literature is being filled that could make a significant contribution to the field. Several studies using rodent animal models have discovered that sensitivity to the acute locomotor response to psychostimulant drugs such as cocaine and amphetamines is strongly dependent on age. We previously reported that adolescent C57BL/6J mice (age range 30–35) are significantly less sensitive to the locomotor stimulating effects of cocaine and methamphetamine as compared to young adults (age range 60–67) (Zombeck et al., 2008). This general observation of reduced acute locomotor response has been observed in previous studies mostly using rats (Lanier and Isaacson, 1977, Laviola et al., 1995, Bolanos et al., 1998, Maldonado and Kirstein, 2005a, Frantz et al., 2007, Zakharova et al., 2009) but it is not always observed (Camarini et al., 2008, Parylak et al., 2008) and some studies show increased stimulation in adolescent rats as compared to adults (Catlow and Kirstein, 2005, Caster et al., 2007, Caster and Kuhn, 2009).
Many potential mechanisms could explain differential locomotor stimulation between ages. Developmental changes during adolescents include increased dopamine receptors in the caudate in adolescent rodents as compared to adults (Teicher et al., 1995, Tarazi et al., 1998, Tarazi et al., 1999), immature prefrontal cortex (Rosenberg and Lewis, 1995, Giedd et al., 1999), decreased white matter (Giedd, 2004), among others (for review see Spear, 2000). One method to refine the search for a mechanistic explanation is to examine the patterns of Fos activation that occur throughout the brain after an injection of drug in each age group. The idea is that neuronal activation, as indicated by Fos induction, will reflect behavior and therefore identify the key brain regions and circuits involved in differential behavioral responses (Rhodes et al., 2003, Rhodes et al., 2005, Zombeck et al., 2008). By virtue of knowing the key brain regions, information about the distribution of cell types, receptor signaling systems, and principle afferent and efferent connections in the regions becomes available from the literature. This can help refine hypotheses about specific cellular or molecular mechanisms underlying the behavioral difference between the age groups.
Only a few studies have compared Fos or other related molecular responses to psychostimulants in adolescent as compared to adult rodents. Caster and Kuhn (2009) found higher levels of c-fos gene expression in the caudate of adolescent (age 28 days) as compared to adult (65 days) Sprague Dawley rats in response to 10 mg/kg cocaine, but the reverse for 40 mg/kg. In this study, adolescents displayed greater locomotor stimulation than adults for the 10 mg/kg dose and the reverse for the 40 mg/kg dose. Another study found elevated ΔFosB expression in the nucleus accumbens and caudate of adolescent versus adult male CD-1 mice following chronic administration of cocaine (20 mg/kg/day for 7 days) or amphetamine (5 mg/kg/day for 7 days) (Ehrlich et al., 2002). ΔFosB accumulates after repeated administration of psychostimulants and is thought to mediate longer lasting transcriptional regulation that is directly induced from the immediate early gene responses that occur during the initial drug administration (Nestler et al., 2001). This would suggest that cells in the striatum may display a greater initial genomic response than adults for a given dose of drug associated with decreased sensitivity to the locomotor activating effects. However, Ehrlich et al. (2002) did not measure locomotor activity or immediate early gene responses, so this hypothesis requires further investigation. Consistent with the idea, Anderson et al. (2001) found a greater percentage of Fos positive cells in the striatum of adolescent (age 35 days) versus young adult (age 60) Sprague Dawley rats following acute amphetamine (1 or 5 mg/kg). However, lower c-fos mRNA levels were observed in the ventral caudate of adolescent compared to adult Sprague Dawley rats following two intravenous doses of cocaine (Cao et al., 2007).
Taken together the evidence reviewed above points to the striatum as a location where cellular or molecular differences occur in adolescents as compared to adults that could mediate reduced sensitivity to locomotor activating effects of psychostimulants between the age groups. That is not surprising given that cocaine and amphetamines increase dopamine in extracellular spaces and the striatum is a major site of dopamine innervation (Wise, 2002). But there are a number of remaining questions. First the direction of the difference in immediate early gene induction, i.e., whether adolescents show greater or reduced Fos response to the drugs as compared to adults, is not consistent between the studies. Second, to the best of our knowledge, none of the previous studies examined Fos induction from methamphetamine between adolescents and adults. Given current methamphetamine use (Winslow et al., 2007), and the potential differences during adolescence, we thought it would be important to investigate. Third, to our knowledge, none of the previous studies analyzed the Fos responses using locomotor activity as a covariate in the statistical analysis. We have found, as others have in previous studies, that level of locomotor activity is strongly correlated with Fos levels throughout the brain (Rhodes et al., 2005, Caster and Kuhn, 2009). Therefore, one of the aims of this study was to determine whether the differential Fos induction from cocaine and methamphetamine in adolescents as compared to adults could be explained merely based on the level of physical activity displayed by the animals. Fourth, other areas besides the striatum receive dopamine innervation, and cocaine and methamphetamine affect signaling of other neurotransmitters including serotonin (Cunningham and Callahan, 1994, Muller et al., 2003) and norepinephrine throughout the brain (Uhl et al., 2002). Moreover, many other brain regions are involved in the locomotor activating effects of cocaine and amphetamines besides the striatum (e.g., ventral pallidum, motor cortex). The adolescent brain significantly differs from adults in these brain areas as well and that could contribute to differential locomotor activity. Therefore, we examined 16 different regions throughout the brain that we hypothesized might be involved in the differential locomotor activating effects of cocaine and methamphetamine in adolescents as compared to adults.
As compared to adults, we predicted adolescents would have reduced levels of Fos in response to cocaine and methamphetamine in most brain areas because we expected Fos would reflect the reduced locomotor activity (Zombeck et al., 2009). After locomotor activity was removed as a covariate, we expected the differences in Fos between the age groups would no longer be apparent except in key brain regions involved in the differential behavior. We reasoned that reduced or enhanced signaling in response to the same stimulus in adolescents versus adults could modulate the motor circuit and contribute to reduced sensitivity to locomotor stimulation in adolescents.
Methods
Subjects
A total of 96 male C57BL/6J mice were used. The experiment was conducted in 4 separate batches consisting of 2 replicates for cocaine and 2 for methamphetamine. Each batch or replicate, consisted of 12 adults and 12 adolescents evenly distributed among the doses. After arrival from The Jackson Laboratory (Bar Harbor, ME), mice were housed in group of 4 for 6 days to habituate and then housed singly in custom-made acrylic home cages (18.5 cm × 33.5 cm × 16 cm) with clear plastic lids conducive for video tracking from above. Adolescent mice were 21 days old at arrival and tested at 30–35 days of age. Adult mice were 60 days old at arrival and tested at 69–74 days of age. All mice were housed on a 12:12 reverse light/dark cycle (lights off at 10 AM and on at 10 PM) with the room temperature maintained at 21 ± 1 °C. Free access to food and water was available at all times. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines.
Drug solutions
Cocaine hydrochloride or methamphetamine hydrochloride (Sigma Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline and was administered at a dose of 0, 15, 30 mg/kg or 0, 2, 4, mg/kg respectively via intraperitoneal injections in a volume of 10 ml/kg. Dose was chosen based on the literature (Azar et al., 1998, Vorhees et al., 2005, Zombeck et al., 2009) and was prepared according to the salt not the base form.
Locomotor activity
Locomotor activity was continuously recorded using Topscan software (Clever Sys Inc, Reston, VA, USA). Mice were video-tracked in custom-made home cages where they were acclimated for 3–8 days prior to any injections (see Subjects section). Recording began at the onset of the dark phase (i.e. active period). First, baseline locomotor activity was monitored for 1 hr. All mice then received a saline injection in order to measure the behavioral response to an injection. Activity was again measured for 1 hr after which an injection of cocaine (0, 15, 30 mg/kg) or methamphetamine (0, 2, 4 mg/kg) was administered. For each dose of each drug, 8 adolescent and 8 adult mice were sampled. Locomotor activity recorded for 1.5 hrs before animals were sacrificed by decapitation. Brains were quickly dissected and placed in 5% acrolein in phosphate buffered saline (PBS) solution overnight (Zombeck et al., 2008).
Immunohistochemistry
Following Zombeck et al. (2008), brains were transferred into 30% sucrose solution for 24 hours at 4 °C and then transferred into a fresh 30% sucrose solution for storage until sectioning. Brains were then sectioned (40 µm thick) using a cryostat. Sections were placed into a 24 well plate containing tissue cryoprotectant, then stored at −20 °C. Alternate sections were transferred into PBS, 24 hrs before beginning immunohistochemistry. Free-floating sections were pretreated with sodium borohydride (100 mg per 20 ml PBS) for 30 min, washed with PBS-X (PBS containing 0.2% v/v Triton X-100), and blocked with 6% v/v Normal Goat Serum (NGS) for 1 hr at room temperature. Sections were then incubated in rabbit antibody against c-Fos at a dilution of 1:20,000 (Calbiochem, San Diego, CA, USA) in PBS-X containing 2% NGS for 48 hrs at 5 °C. After primary incubation, sections were washed in PBS-X followed by incubation in secondary biotinylated antibody against rabbit immunoglobulin made in goat (Vector Labs, Burlingame, CA, USA) at a dilution of 1:500 in PBS-X with 2% NGS for 90 min at room temperature. The peroxidase method (ABC system, Vector Labs, Burlingam, CA, USA; 37 ul A, 37 ul B in 15 ml PBS-X) and diaminobenzidine (DAB) as chromogen enhanced with nickel chloride (Sigma, St. Louis, MO, USA) was used to visualize the antibody complex. The reaction was stopped by washing the sections in PBS. Sections were mounted onto subbed slides, allowed to air dry, and then were dehydrated and coverslipped using Permount (Sigma, St. Louis, MO, USA).
Image analysis
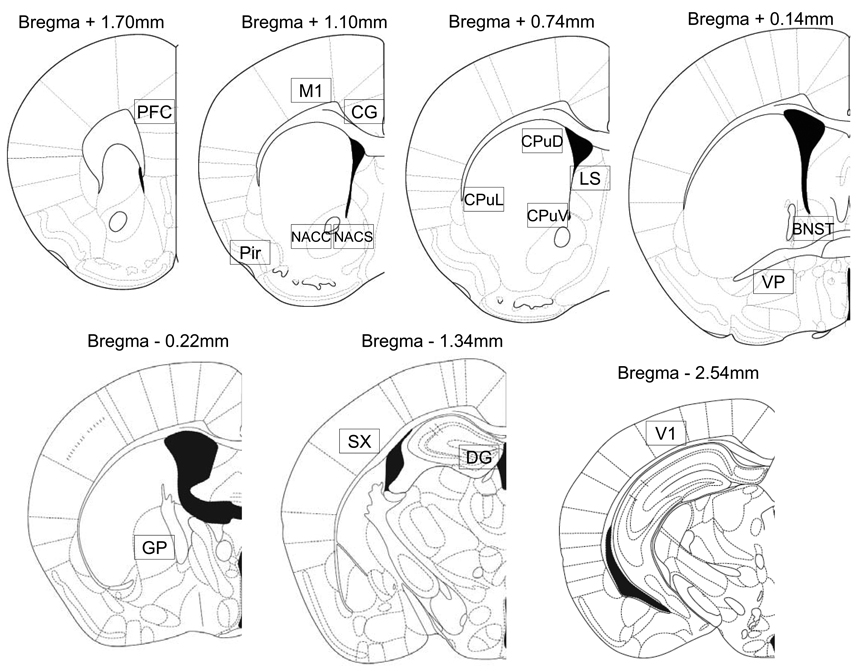
Following Zombeck et al. (2008), microscopic images of the sections were captured via a Zeiss Axiocam digital camera (Zeiss, Germany) interfaced to a personal computer. ImageJ software (NIH, Bethesda, MD) was used to automatically count Fos-positive cells at 100X total magnification within a frame (1.0 × 0.63 mm) placed at the locations shown in Figure 1 redrawn from Paxinos and Franklin (2001). For brain regions that were smaller than the frame, as was the case for the piriform cortex and the dentate gyrus, the region was outlined by hand and particles were counted only within the outlined structures. The counting was done unilaterally, in three alternate sections for each brain region, to obtain an average cell count per brain region for analysis.
Figure 1.
Locations where Fos positive cells were counted (boxes, shown roughly to scale, were 1 × 0.63 mm). Reprinted from The Mouse Brain in Stereotaxic coordinates, 2nd edition, Paxinos G and Franklin K, Figures 17, 22, 25, 30, 33, 42, 52, Copyright 2001, with permission from Elsevier. As noted, for the piriform cortex and the dentate gyrus, the nucleus was outlined by hand and particles were counted only within the outlined structures. Legend: PFC=prefrontal cortex, M1=motor cortex, Cg=cingulate cortex, NACC=nucleus accumbens core, NACS=nucleus accumbens shell, Pir=piriform cortex, CPuD=dorsal caudate, CPuL=lateral caudate, CPuV=ventral caudate, LS=lateral septum, BNST=bed nucleus of the stria terminalis, VP=ventral pallidum, GP=globus palidus, SX=somatosensory cortex, DG=dentate gyrus, V1=visual cortex.
Statistical analysis
Statistical analysis was preformed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Locomotor activity was analyzed two ways. First, total distance traveled was summed over each epoch; the 60 minutes before any injections were given, the 60 minutes after the saline injection, and the 90 minutes after the saline or drug injections. Baseline total distance traveled (before injections and after saline) was compared between adolescents and adults using an un-paired t-test. Distance following the drug injections was analyzed separately for each drug by two-way ANOVA with dose and age entered as the two factors. Second, locomotor activity was divided into 15 minute bins consisting of summed distance traveled over that period. Adolescents and adults were compared for baseline distance traveled over the 4 time increments (i.e., 1 hour period) following a saline injection using repeated measures analysis of variance with age and time (4 levels) as factors. Distance traveled after cocaine or methamphetamine administration was analyzed separately for each dose of drug over the 6 time increments (i.e., 90 min period) following drug injection using repeated measures analysis of variance with age and time (6 levels) as factors.
Number of Fos positive cells for each brain region was analyzed using analysis of variance with batch, age and dose as factors. Batch was included as a factor to eliminate differences in staining due to variance between immunohistochemisty runs (Zombeck et al., 2008). The Fos numbers were power transformed as needed to decrease skewness and kurtosis in the residual distribution. Least square means (adjusted for batch) and confidence intervals were back-transformed so that the means could be presented in the same units, number of Fos-positive cells, for all regions.
The relationship between Fos staining and locomotor activity was analyzed by analysis of covariance. This was done to determine whether Fos levels differ between adolescents and adults after accounting for the expected positive relationship between acute levels of physical activity and Fos observed in previous studies throughout the brain (Rhodes et al., 2005, Caster and Kuhn, 2009). In this model, Fos staining was analyzed as the response, locomotor activity summed over 90 minutes was the continuous predictor (covariate), and age was entered as a categorical factor. Separate tests were run for each dose. Otherwise, dose would strongly bias the correlation between physical activity and Fos because dose strongly influences both variables. Again, the Fos numbers were power transformed as needed to decrease skewness and kurtosis in the residual distribution. The difference between the least square means, adolescent minus adult, adjusted for locomotor activity, were back-transformed so that the difference could be presented in the same units, number of Fos-positive cells, for all regions.
Two different methods were used to correct for the multiple testing in this study. First we adjusted the cut off p-value so that the global false discovery rate for the entire study was less than or equal to 5% using Qvalue software (Storey, 2002, Rhodes et al., 2005). Second, we extracted the principle components (the linear combinations of Fos levels across all regions that explain 70% of the variation in the data), and analyzed those variables using the same strategy described above for analyzing Fos in individual regions.
Results
Locomotor activity
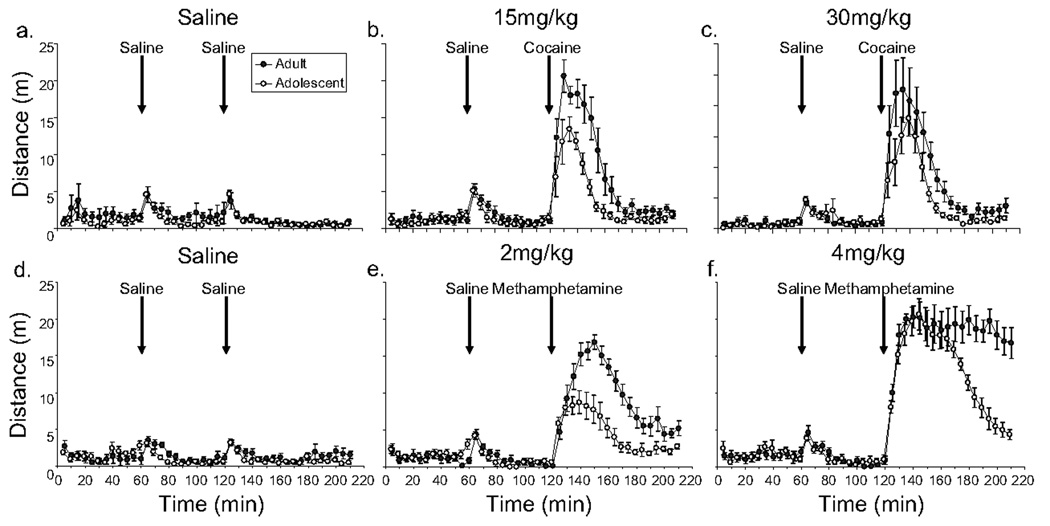
See Figure 2. Baseline locomotor activity summed over 60 minutes preceding injections and over the 60 minutes following the saline injection was not significantly different between adolescents and adults. A saline injection induced a small, brief increase in locomotor activity [time, F(3, 282)=163.2, P<0.0001; first 15 minutes following injection greater than following three time points, all P<0.0001], but no significant differences were observed between ages (i.e., the effect of age and age by time interaction were not significant).
Figure 2.
Reduced locomotor response to cocaine and methamphetamine in adolescent male C57BL/6J mice as compared to adults. Average distance traveled in 5 min bins (± SE) is plotted against time separately for adults (filled symbols) and adolescents (open symbols). Animals were given a saline injection at 60 min, and either saline or drug injection at 120 min. Data for the cocaine trials are shown on top and methamphetamine on the bottom. Each data point represents the average of 8 individuals. All graphs share the same x- and y-axis labels.
Both cocaine and methamphetamine increased the total distance traveled over the 90 minute period following injections as indicated by a main effect of dose [cocaine, F(2,42)=24.9, P<0.0001; methamphetamine, F(2,42)=133.6, P<0.0001]. Adolescents displayed reduced locomotor activity as compared to adults as shown by a main effect of age [cocaine, F(1,42)=10.0, P=0.003; methamphetamine, F(1,42)=23.9, P<0.0001]. The interaction between age and dose was significant for methamphetamine [F(2,42)=4.3, P=0.02] but not cocaine. To examine this difference in more detail, distance traveled in 15 minute bins following the drug injections was analyzed separately for each dose of each drug. The magnitude of the difference in locomotor activity between adolescents versus adults was similar at all time points for both doses of cocaine and for the 2 mg/kg dose of methamphetamine (i.e., the adolescent and adult curves were parallel following injection of cocaine or methamphetamine in Fig. 2b, c and e, and the interaction between age and time was not significant). However, the time-course of locomotor stimulation after 4 mg/kg, revealed an interesting difference between the age groups. Initially, during the first 45 minutes, both adolescents and adults displayed similar levels of locomotor activity, but the effect wore off between 45 to 90 minutes in adolescents whereas in adults, the high level of locomotor activity was maintained up to 90 minutes following the ip injection of 4 mg/kg (see Fig. 2f) [the interaction between age and time was significant, F(5,70)=8.4, P<0.0001].
Fos positive cells
Analysis of all the p-values collected from the tests reported in Table 1–Table 4 indicated that the standard cut off p-value, 0.05, would result in a global false discovery rate of 6 %. Qvalue calculated that if the cut off was set at 0.04 then the false discovery rate was 5%. Therefore, we considered a p-value less than or equal to 0.04 as evidence for statistical significance for the individual tests. For the results of the principle component analysis, we used the standard 0.05 cut off because the data were reduced across the 16 brain regions to 3 principle components.
Table 1.
Mean number of Fos positive cells and associated statistics after saline, 15 or 30 mg/kg cocaine.
| Brain Region | Least Squared Means | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Age | 0 | 15 | 30 | Dose | Age | ||
| Cortex | CG | adults | 75.0 (29.6, 120.4) | 122.4 (77.0, 467.9) | 157.0 (111.5, 202.4) | P<0.01 | p=0.97 |
| adolescents | 66.8 (21.4, 112.3) | 122.5 (73.9, 171.2) | 162.6 (117.2, 208.0) | ||||
| M1 | adults | 6.19 (2.9, 12.4) | 10.1 (5.0, 19.6) | 26.9 (14.2, 49.2) | p<0.01 | p=0.53 | |
| adolescents | 5.2 (2.5, 10.6) | 14.9 (7.2, 29.4) | 38.3 (20.7, 68.6) | ||||
| PFC | adults | 107.0 (56.0, 158.0) | 161.8 (110.8, 212.8) | 209.4 (154.8, 264.1) | p<0.01 | p=0.22 | |
| adolescents | 69.6 (18.5, 120.6) | 136.9 (82.3, 191.5) | 190.3 (135.7, 244.9) | ||||
| Pir | adults | 38.1 (17.4, 69.2) | 104.8 (63.9, 158.5) | 156.1 (99.1, 229.4) | p<0.01 | p=0.89 | |
| adolescents | 53.4 (28.8, 87.7) | 87.8 (51.4, 136.5) | 137.7 (88.7, 200.1) | ||||
| SX | adults | 14.9 (5.9, 34.7) | 80.0 (36.9, 164.4) | 75.0 (32.5, 162.3) | p<0.01 | p=0.04 | |
| adolescents | 6.19 (2.3, 15.5) | 35.8 (14.5, 81.9) | 41.1 (17.9, 88.5) | ||||
| V1 | adults | 24.2 (5.9, 65.2) | 105.0 (45.3, 205.5) | 134.4 (62.0, 251.8) | p<0.01 | p=0.77 | |
| adolescents | 16.8 (3.3, 49.9) | 127.3 (57.9, 240.7) | 106.8 (46.2, 208.3) | ||||
| Basal Ganglia | CPuD | adults | 19.2 (−54.5, 92.9) | 177.0 (103.3, 250.7) | 304.3 (225.4, 383.3) | p<0.01 | p=0.49 |
| adolescents | 8.8 (−64.9, 82.5) | 152.3 (73.4, 231.2) | 275.3 (201.6, 349.0) | ||||
| CPuL | adults | 4.8 (1.6, 12.0) | 19.4 (9.1, 37.5) | 60.3 (33.2, 102.6) | p<0.01 | p=0.04 | |
| adolescents | 0.9 (0.2, 2.9) | 17.9 (7.7, 36.6) | 30.4 (15.3, 55.7) | ||||
| CPuV | adults | 20.8 (−55.9, 97.5) | 176.7 (100.0, 253.4) | 296.8 (220.2, 373.5) | p<0.01 | p=0.84 | |
| adolescents | 17.3 (−59.4, 93.9) | 166.4 (84.3, 248.5) | 290.6 (213.9, 367.3) | ||||
| GP | adults | 1.1 (1.0, 1.2) | 1.2 (1.1, 1.3) | 1.4 (1.3, 1.5) | p<0.01 | p=0.60 | |
| adolescents | 1.0 (0.8, 1.1) | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.4) | ||||
| NACC | adults | 21.1 (10.6, 38.5) | 81.3, (48.7, 129.5) | 116.5 (72.4, 178.0) | p<0.01 | p=0.81 | |
| adolescents | 20.5 (10.3, 37.6) | 86.5 (49.8, 142.1) | 125.8 (78.8, 193.1) | ||||
| NACS | adults | 55.1 (30.1, 94.7) | 146.2 (89.2, 229.1) | 194.7 (122.3, 297.9) | p<0.01 | p=0.74 | |
| adolescents | 60.3 (33.2, 102.6) | 157.3 (96.7, 245.0) | 135.7 (66.8, 252.4) | ||||
| VP | adults | 32.7 (11.4, 54.1) | 42.0 (22.1, 61.9) | 52.0 (32.1, 71.9) | p=0.04 | p=0.95 | |
| adolescents | 28.8 (8.8, 48.7) | 34.1 (14.1, 54.0) | 62.2 (42.3, 82.2) | ||||
| Septum | BNST | adults | 72.2 (43.5, 112.1) | 86.4 (53.5, 131.3) | 93.4 (56.3, 144.8) | p=0.05 | p=0.23 |
| adolescents | 61.5 (36.0, 97.4) | 119.5 (77.7, 175.1) | 147.1 (98.3, 210.9) | ||||
| LS | adults | 62.7 (31.8, 93.5) | 75.0 (44.2, 105.9) | 114.0 (83.1, 144.8) | p<0.01 | p=0.96 | |
| adolescents | 29.2 (−1.7, 60.0) | 98.4 (65.5, 131.4) | 125.9 (92.9, 158.9) | ||||
| Hippocampus | DG | adults | 4.7 (2.5, 7.8) | 10.9 (7.1, 15.7) | 9.69 (6.2, 14.2) | p<0.01 | p=0.57 |
| adolescents | 3.8 (1.9, 6.6) | 8.6 (5.3, 12.8) | 10.6 (6.8, 15.3) | ||||
Note: Confident intervals are shown in parentheses after the means. The interaction between age and dose was not significant for any brain region and therefore these p-values are not shown. N=8 per group.
Table 4.
The difference in methamphetamine-induced Fos between adolescents and adults after correcting for locomotor activity.
| Brain Region | Statistics | |||||
|---|---|---|---|---|---|---|
| Dose | Locomotor Activity | Adolescents-Adults | Locomotor Activity | Age | ||
| Cortex | CG | 2 | + | −22.2 | p=0.86 | p=0.75 |
| 4 | + | 140.9 | p=0.07 | p<0.01 | ||
| M1 | 2 | + | 12.2 | p=0.32 | p=0.37 | |
| 4 | − | 20.6 | p=0.81 | p=0.38 | ||
| PFC | 2 | + | 8.3 | p=0.01 | p=0.99 | |
| 4 | + | 96.8 | p=0.61 | p=0.71 | ||
| Pir | 2 | + | 41.4 | p=0.70 | p=0.28 | |
| 4 | − | 37.8 | p=0.09 | p=0.11 | ||
| SX | 2 | + | −7.5 | p=0.29 | p=0.67 | |
| 4 | + | −9.2 | p=0.61 | p=0.71 | ||
| V1 | 2 | + | 173.2 | p=0.08 | p=0.11 | |
| 4 | − | 24.0 | p=0.09 | p=0.50 | ||
| Basal Ganglia | CPuD | 2 | + | 46.6 | p<0.01 | p=0.11 |
| 4 | + | 100.6 | p=0.60 | p=0.03 | ||
| CPuL | 2 | + | −3.2 | p=0.94 | p=0.90 | |
| 4 | # | 10.9 | p=0.61 | p<0.01 | ||
| CPuV | 2 | + | 14.5 | p=0.95 | p=0.64 | |
| 4 | − | 54.4 | p=0.05 | p=0.19 | ||
| GP | 2 | + | −7.0 | p=0.96 | p=0.42 | |
| 4 | − | −17.3 | p=0.16 | p=0.67 | ||
| NACC | 2 | + | −0.4 | p=0.36 | p=0.96 | |
| 4 | − | 9.2 | p=0.03 | p=0.45 | ||
| NACS | 2 | + | 41.7 | p=0.98 | p=0.24 | |
| 4 | + | 77.7 | p=0.16 | p<0.01 | ||
| VP | 2 | + | 4.0 | p=0.52 | p=0.36 | |
| 4 | + | 12.0 | p=0.66 | p=0.17 | ||
| Septum | BNST | 2 | + | −7.6 | p=0.79 | p=0.75 |
| 4 | − | 22.8 | p=0.95 | p=0.30 | ||
| LS | 2 | + | 12.0 | p=0.13 | p=0.59 | |
| 4 | + | 73.4 | p=0.40 | p=0.12 | ||
| Hippocampus | DG | 2 | + | −19.9 | p=0.27 | p=0.37 |
| 4 | − | 12.4 | p=0.64 | p=0.39 | ||
Note: In the locomotor activity column, the + or − sign indicates whether the correlation between locomotor activity and Fos was positive or negative.
a significant interaction between age and locomotor activity was observed for CPuL (p<0.01), adolescents showed a negative correlation between Fos and locomotor activity whereas adults showed a positive correlation. No other significant interactions between age and locomotor activity were observed. The column, Adolescents – Adults shows the difference in mean number of Fos cells at the average level of locomotor activity among both age groups.
Baseline differences
After a saline injection, Fos levels were similar in adolescents and adults in all regions except the dorsal and lateral caudate, where adolescents displayed significantly reduced Fos as compared to adults [dorsal caudate, F(1,26)=4.6, P=0.04; lateral caudate, F(1,26)=5.9, P=0.02]. Locomotor activity was significantly correlated with Fos in the globus pallidus, and similar trends were observed for the dentate gyrus (P=0.08), and nucleus accumbens core region (P=0.06).
Cocaine
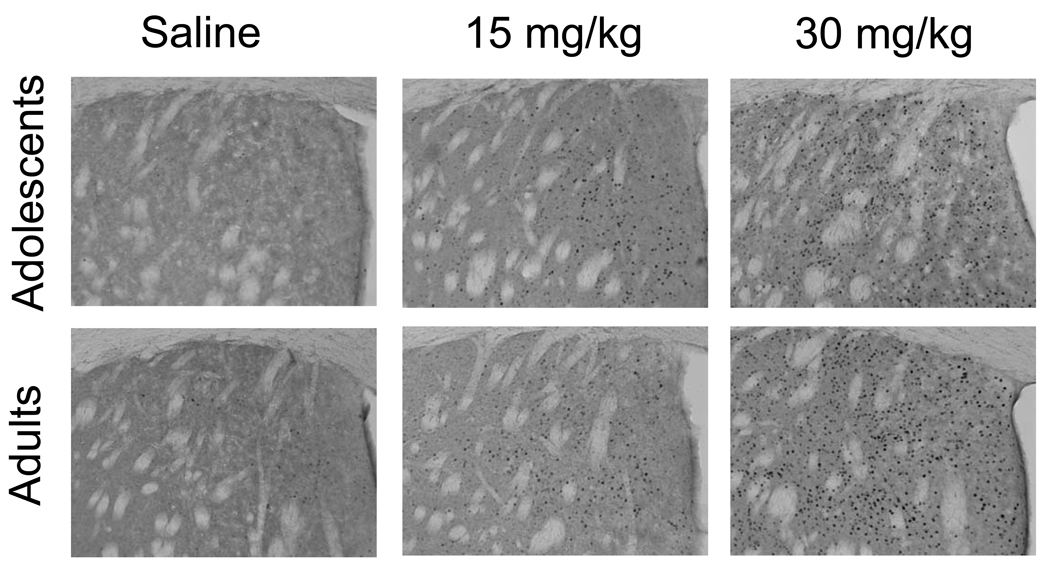
Cocaine increased Fos as indicated by a significant main effect of dose (see Fig. 3, Table 1). Inspection of the least square means in Table 1 shows a strong dose response for most regions. Levels of Fos were similar in adolescents and adults in all regions except the lateral caudate and the somatosensory cortex where adolescents displayed slightly lower Fos across all treatments including the baseline saline injection.
Figure 3.
Acute cocaine increases Fos in a dose-dependent fashion in adolescents and adults. Representative sections stained for Fos showing the dorsal caudate of adolescents and adults 90 min after an intraperitoneal injection of saline, 15, or 30 mg/kg cocaine. The dots represent Fos-positive nuclei, total magnification was 100×.
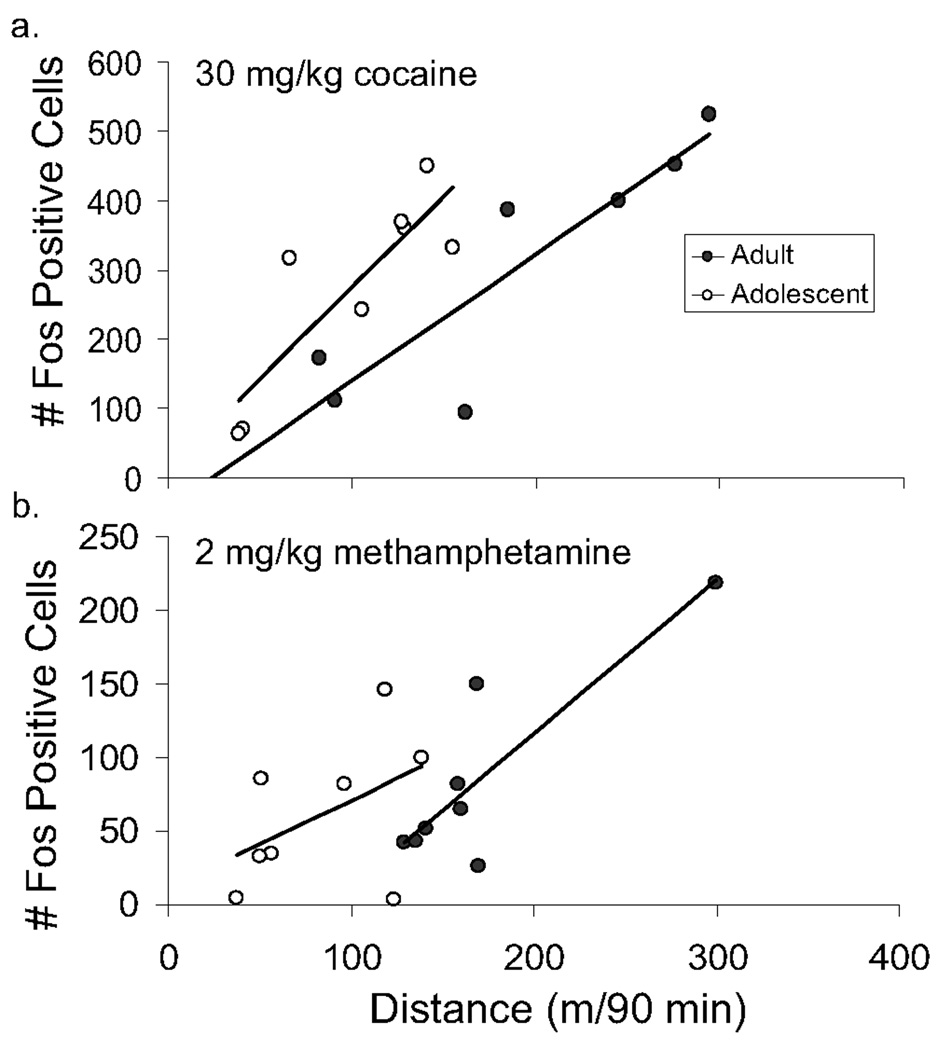
When Fos was analyzed with locomotor activity (distance traveled 90 min after drug injection up to the point of euthanasia) as a covariate, 3 of the 16 brain regions sampled showed a significant correlation between numbers of Fos positive cells and level of locomotor activity after the 30 mg/kg dose: the cingulate cortex, dorsal caudate, and dentate gyrus (Table 2). After the 15 mg/kg dose only 1 region showed a significant correlation, the motor cortex. After correcting for differences in locomotor activity among subjects using analysis of covariance, the dorsal caudate and the bed nucleus of the stria terminalis showed significant differences between the age groups, with adolescents showing greater Fos in this region as compared to adults for a given level of locomotor activity after the 30 mg/kg dose (Fig. 4a, Table 2).
Table 2.
The difference in cocaine-induced Fos between adolescents and adults after correcting for locomotor activity.
| Brain Region | Statistics | |||||
|---|---|---|---|---|---|---|
| Dose | Locomotor Activity | Adolescents-Adults | Locomotor Activity | Age | ||
| Cortex | CG | 15 | − | −38.8 | p=0.70 | p=0.69 |
| 30 | + | 69.6 | p=0.01 | p=0.07 | ||
| M1 | 15 | + | −13.0 | p=0.04 | p=0.20 | |
| 30 | + | 21.0 | p=0.45 | p=0.67 | ||
| PFC | 15 | + | −39.2 | p=0.92 | p=0.35 | |
| 30 | + | 48.0 | p=0.05 | p=0.56 | ||
| Pir | 15 | + | −20.7 | p=0.82 | p=0.46 | |
| 30 | + | −69.2 | p=0.91 | p=0.43 | ||
| SX | 15 | + | −4.1 | p=0.49 | p=0.35 | |
| 30 | + | −80.7 | p=0.18 | p=0.07 | ||
| V1 | 15 | + | 30.8 | p=0.64 | p=0.87 | |
| 30 | + | 48.1 | p=0.23 | p=0.73 | ||
| Basal Ganglia | CPuD | 15 | + | −12.8 | p=0.76 | p=0.82 |
| 30 | + | 197.7 | p<0.01 | p<0.01 | ||
| CPuL | 15 | + | −8.7 | p=0.87 | p=0.62 | |
| 30 | + | −11.8 | p=0.13 | p=0.73 | ||
| CPuV | 15 | + | 0.8 | p=0.97 | p=0.77 | |
| 30 | + | 170.1 | p=0.14 | p=0.29 | ||
| GP | 15 | + | 11.3 | p=0.53 | p=0.32 | |
| 30 | + | 4.2 | p=0.16 | p=0.68 | ||
| NACC | 15 | + | 18.9 | p=0.77 | p=0.94 | |
| 30 | + | 32.0 | p=0.68 | p=0.60 | ||
| NACS | 15 | + | 35.5 | p=0.69 | p=0.59 | |
| 30 | + | 40.8 | p=0.31 | p=0.83 | ||
| VP | 15 | + | 9.9 | p=0.36 | p=0.96 | |
| 30 | + | 32.2 | p=0.31 | p=0.19 | ||
| Septum | BNST | 15 | + | 31.0 | p=0.48 | p=0.54 |
| 30 | + | 111.9 | p=0.91 | p=0.04 | ||
| LS | 15 | − | 2.6 | p=0.23 | p=0.86 | |
| 30 | + | 23.7 | p=0.73 | p=0.48 | ||
| Hippocampus | DG | 15 | + | 1.1 | p=0.31 | p=0.85 |
| 30 | + | 6.5 | p=0.02 | p=0.10 | ||
Note: In the locomotor activity column, the + or − sign indicates whether the correlation between locomotor activity and Fos was positive or negative. The column Adolescents – Adults shows the difference in mean number of Fos cells at the average level of locomotor activity among both age groups. The interaction between age and locomotor activity was not significant for any brain region or dose and therefore these p-values are not shown.
Figure 4.
Increased Fos response from cocaine and methamphetamine in the dorsal caudate for a given level of locomotor activity in adolescents as compared to adults. Number of Fos positive cells in the dorsal caudate is plotted against distance traveled in the 90 minute period following an injection of either 30 mg/kg cocaine (top) or 2 mg/kg methamphetamine (bottom). Adolescents (open symbols) are shown separately from adults (filled symbols). The simple linear regression lines are shown separately for each age group. Both graphs share the same x-axis label.
The first principle component accounted for 50% of the variation in the data and was strongly correlated (Pearson’s r > 0.50) with number of Fos positive cells in 6 of the 16 brain regions (ventral caudate, dentate gyrus, globus pallidus, nucleus accumbens core and shell, and piriform cortex). Congruent with the results in Table 1 and Table 2 for most of the high loading brain regions, the first principle component showed significant effects for dose [F(2,28)=3.8, P=0.04] and locomotor activity [F(1,30)=4.2, P=0.05] but not age. The second and third principle components loaded on different regions but none of them showed significant effects of age. Together the first 3 components accounted for 70% of the variation in the data.
Methamphetamine
As with cocaine, methamphetamine significantly increased Fos in all regions (Table 3). Inspection of the least square means in Table 3 shows that the induction of Fos is strongly dose dependent for a majority of regions (Table 3). Levels of Fos were similar in adolescents and adults in all regions except the visual cortex and ventral pallidum where adolescents displayed slightly higher Fos across all treatments including the baseline saline injection.
Table 3.
Mean number of Fos positive cells and associated statistics after saline, 2, or 4 mg/kg methamphetamine.
| Brain Region | Least Squared Means | Statistics | |||||
|---|---|---|---|---|---|---|---|
| Age | 0 | 2 | 4 | Dose | Age | ||
| Cortex | CG | adults | 15.9 (4.8, 42.1) | 76.4 (32.6, 158.0) | 118.8 (52.5, 239.8) | p<0.01 | p=0.87 |
| adolescents | 14.2 (4.1, 38.2) | 63.0 (24.6, 138.8) | 178.3 (87.8, 331.6) | ||||
| M1 | adults | 6.1 (−14.8, 27.0) | 28.8 (7.9, 49.8) | 71.8 (50.9, 92.7) | p<0.01 | p=0.19 | |
| adolescents | 9.5 (−12.9, 31.9) | 48.2 (25.8, 70.6) | 89.9 (69.0, 110.9) | ||||
| PFC | adults | 11.5 (−38.3, 61.4) | 130.8 (87.9, 173.6) | 175.7 (129.9, 221.6) | p<0.01 | p=0.91 | |
| adolescents | 19.6 (−29.9, 68.1) | 70.9 (25.1, 116.8) | 220.7 (177.8, 263.5) | ||||
| Pir | adults | 40.3 (5.7, 74.9) | 85.0 (48.0, 122.1) | 97.0 (62.4, 131.6) | p<0.01 | p=0.17 | |
| adolescents | 25.8 (−11.3, 62.9) | 97.3 (62.7, 131.9) | 161.2 (126.6, 195.8) | ||||
| SX | adults | 2.4 (0.1, 13.1) | 19.0 (3.9, 55.8) | 95.0 (41.5, 184.3) | p<0.01 | p=0.45 | |
| adolescents | 17.3 (3.3, 52.0) | 24.5 (6.4, 63.4) | 66.3 (26.0, 137.7) | ||||
| V1 | adults | 7.1 (−96.6, 110.9) | 65.4 (−54.4, 185.2) | 268.5 (164.8, 372.3) | p<0.01 | p<0.01 | |
| adolescents | 15.0 (−88.7, 118.8) | 234.0 (123.0, 345.1) | 478.3 (374.6, 582.1) | ||||
| Basal Ganglia | CPuD | adults | 8.3 (−28.1, 44.7) | 85.1 (42.7, 121.5) | 138.2 (99.2, 177.2) | p<0.01 | p=0.54 |
| adolescents | 5.2 (−31.2, 41.6) | 61.4 (25.0, 97.8) | 193.2 (156.8, 229.6) | ||||
| CPuL | adults | 3.5 (0.8, 9.9) | 9.6 (3.4, 21.2) | 18.3 (7.4, 37.2) | p<0.01 | p=0.99 | |
| adolescents | 1.3 (0.2, 5.0) | 9.3 (2.9, 21.7) | 29.8 (14.7, 53.5) | ||||
| CPuV | adults | 26.8 (14.8, 45.5) | 103.7 (66.4, 156.0) | 175.2 (117.5, 254.6) | p<0.01 | p=0.30 | |
| adolescents | 24.1 (13.2, 41.4) | 125.8 (82.0, 186.6) | 259.6 (179.7, 365.7) | ||||
| GP | adults | 4.5 (1.9, 9.4) | 13.0 (6.5, 23.8) | 45.9 (27.1, 74.1) | p<0.01 | p=0.83 | |
| adolescents | 4.5 (1.7, 10.0) | 11.2 (5.5, 20.8) | 59.0 (35.7, 93.0) | ||||
| NACC | adults | 4.66 (1.5, 10.0) | 20.1 (10.4, 33.7) | 53.7 (37.3, 73.8) | p<0.01 | p=0.28 | |
| adolescents | 2.4 (0.5, 6.4) | 27.4 (16.3, 42.1) | 86.0 (63.9, 112.2) | ||||
| NACS | adults | 22.1 (−10.8, 54.9) | 75.3 (40.1, 110.4) | 134.8 (102.0, 167.7) | p<0.01 | p=0.07 | |
| adolescents | 34.2 (−1.0, 69.3) | 81.0 (45.8, 116.1) | 195.9 (163.0, 228.7) | ||||
| VP | adults | 5.4 (3.0, 9.0) | 10.1 (5.7, 16.4) | 38.1 (27.6, 51.1) | p<0.01 | p<0.01 | |
| adolescents | 12.8 (7.9, 19.7) | 14.3 (8.6, 22.2) | 50.8 (37.9, 66.6) | ||||
| Septum | BNST | adults | 25.7 (1.1, 50.2) | 93.0 (64.4, 121.6) | 74.5 (50.0, 99.1) | p<0.01 | p=0.41 |
| adolescents | 38.6 (7.5, 69.7) | 86.1 (59.8, 112.3) | 96.5 (70.3, 122.8) | ||||
| LS | adults | 33.0 (−4.5, 70.5) | 90.7 (53.2, 128.1) | 127.0 (86.2, 167.8) | p<0.01 | p=0.55 | |
| adolescents | 58.5 (13.1, 103.8) | 69.9 (29.1, 110.7) | 151.7 (116.7, 186.7) | ||||
| Hippocampus | DG | adults | 12.2 (6.1, 22.6) | 40.8 (23.8, 66.6) | 31.2 (16.6, 54.6) | p<0.01 | p=0.52 |
| adolescents | 11.9 (5.9, 22.0) | 20.0 (10.6, 35.0) | 39.9 (23.1, 65.2) | ||||
Note: Confident intervals are shown in parentheses after the means. The interaction between age and dose was not significant for any brain region and therefore these p-values are not shown. N=8 per group.
Locomotor activity was positively correlated with Fos in the dorsal caudate and the prefrontal cortex after the 2 mg/kg dose, and negatively correlated with Fos in the nucleus accumbens core region after the 4 mg/kg dose, but no other statistically significant locomotor correlations were detected. When locomotor activity was included as a covariate, the dorsal caudate showed a trend for increased Fos in adolescents as compared to adults for a given level of locomotor behavior in response to the 2 mg/kg dose (Table 4). This difference was greater and significant for the 4 mg/kg dose (Table 4). In a post hoc analysis for the 2 mg/kg dose, after removing one outlier (an adolescent animal that moved 123 meters yet displayed only 4 Fos cells, see Fig. 4b), the covariate, locomotor activity, remained significant [F(1,10)=18.9, P=0.002] , and age was statistically significant [F(1,10)=10.7, P=0.009] with adolescents showing greater Fos than adults for a given level of locomotor activity (Fig. 4b).
In addition to the dorsal caudate, 3 other brain regions showed elevated levels of Fos in adolescents as compared to adults for a given level of locomotor activity after the 4 mg/kg dose of methamphetamine: the lateral caudate, shell of the nucleus accumbens and cingulate cortex (Table 4). Of these, there was a significant interaction between age and locomotor activity in the lateral caudate, with adolescents showing a negative correlation between locomotor activity and number of Fos positive cells whereas adults showed a positive correlation.
The first principle component accounted for 58% of the variation in the data and was strongly correlated (Pearson’s r > 0.50) with number of Fos positive cells in 8 of the 16 brain regions (cingulate cortex, motor cortex, nucleus accumbens core and shell, prefrontal cortex, somatosensory cortex, visual cortex, and ventral pallidum). Congruent with results in Table 3 and Table 4, for most of the high loading brain regions, the first principle component showed significant effect of dose [F(2,24)=6.54, P=0.0054] and locomotor activity [F(1,26)=10.71, P=0.003] but not age. The second and third principle components loaded on different regions but none of them showed significant effects of age. Together the first 3 components accounted for 78% of the data.
Discussion
Despite significantly reduced locomotor response to cocaine and methamphetamine in adolescents as compared to adults (see Fig. 2), the Fos response was largely similar between the two age groups in the brain regions sampled (Fig. 3, Table 1 and Table 3). This was a surprise because in previous studies, we and others have found strong correlations between locomotor activity and Fos throughout the brain (Rhodes et al., 2005, Caster and Kuhn, 2009). Therefore, we predicted that the Fos response generally would be reduced in adolescents as compared to adults because their level of locomotor activity was reduced. In fact, the opposite was true. In a majority of regions adolescents tended to display greater Fos than adults for a given amount of locomotor activity (Table 2 and Table 4). After correcting for locomotor activity using analysis of covariance, the striatum stood out among all other brain areas as consistently showing significantly greater numbers of Fos cells for a given level of locomotor activity in adolescents as compared to adults (Fig. 4). This was not a result of baseline differences in Fos between the two age groups, because after a saline injection, adolescents had significantly fewer rather than greater numbers of Fos cells in the dorsal and lateral caudate as compared to adults. The number of Fos positive cells in the dorsal caudate in response to 30 mg/kg cocaine, and in the dorsal caudate, lateral caudate, and nucleus accumbens shell in response to 4 mg/kg methamphetamine were all significantly greater in adolescents than adults for a given level of locomotor activity (Table 2 and Table 4). Overall, these findings confirm previous studies that have identified the striatum as a location where molecular or developmental changes likely occur that mediate the reduced locomotor response to psychostimulants in adolescents.
Caster and Kuhn (2009) recently examined locomotor activity, c-fos and zif268 gene expression in various subregions of the striatum and cortex of Sprague Dawley rats after acute administration of 10 or 40 mg/kg cocaine. Our results are consistent with Caster and Kuhn (2009) in identifying the striatum as a key brain region involved in the differential locomotor response between age groups. However, the direction of the differences in locomotor behavior and induction of immediate early genes (i.e., whether adolescents displayed a greater or lesser response than adults) and the relationship between immediate early gene induction and locomotor activity were not consistent between the two studies. First, in Caster and Kuhn (2009), adolescent rats displayed greater locomotor activity than adults after 10 mg/kg and reduced locomotor activity after 40 mg/kg, whereas adolescent male C57BL/6J mice displayed reduced locomotor activity after 15, or 30 mg/kg cocaine and no significant locomotor stimulation in either age group from 5 mg/kg (Zombeck et al., 2009). Second, Caster and Kuhn (2009) found that striatal c-fos levels changed in parallel with locomotor activity between the age groups whereas we did not. In Caster and Kuhn (2009), c-fos levels were greater in adolescents than adults for the 10 mg/kg dose when adolescents were more physically active than adults, and greater in adults than adolescents for the 40 mg/kg dose when adults were more physically active than adolescents. Therefore, in Caster and Kuhn (2009), the differences in c-fos and zif268 between adolescents and adults could have been a reflection of the relationship between c-fos and locomotor activity, whereas in our study, analysis of covariance suggested that Fos is significantly greater in adolescent mice as compared to adults in the striatum for a given level of locomotor behavior (Fig. 4).
Only a few other studies besides Caster and Kuhn (2009) examined immediate early gene induction from cocaine or amphetamines in adolescents versus adults, but to the best of our knowledge none of these other studies measured locomotor activity. Our results are generally consistent with Ehrlich et al. (2002) who found greater ΔFosB expression in the caudate and nucleus accumbens after repeated administration of cocaine or amphetamine in adolescent versus adult CD-1 mice. Greater ΔFosB expression in the striatum is expected if Fos responses are greater during initial exposure to the drugs, because Fos contributes to the build up of the more stable transcription factor, ΔFosB. Results are also consistent with Anderson et al. (2001) who found slightly greater numbers of Fos-positive cells in the striatum of adolescent as compared to adult Sprague Dawley rats in response to 1 or 5 mg/kg amphetamine. On the other hand Kosofsky et al. (1995) and Cao et al. (2007) found reduced c-fos mRNA in the striatum of adolescent as compared to adult Sprague Dawley rats after i.p. 40 mg/kg cocaine (Kosofsky et al., 1995), or after two 100 µl intravenous injections of 750 µg/kg cocaine spaced 1 min apart (Cao et al., 2007). The explanation for the difference is not clear, but relatively large doses and varying routes of administration may have played a role. Additionally, knowing the level of locomotor behavior displayed by the animals before they were sampled in the different studies might help clarify some of the differences.
In our study, the results of the principle component analysis confirmed that the central (correlated) patterns of neural activation across the different brain regions significantly reflected locomotor activity but none of the first three principle components showed age differences. This result suggests that a large background pattern of neural activity during the test is unrelated to the age difference in locomotor behavior. Fos levels in the striatum, which differed significantly between the age groups in the individual tests, were also partially correlated with the principle components. That suggests only a subset of the signal represented by Fos in the striatum contributes to age differences in locomotor stimulation. One of the limitations of using the immunohistochemical detection of Fos to reflect neuronal activation, is that Fos labels many different types of neurons and even some glial cells that happen to be transcriptionally activated over a relatively large time window (e.g., 90 min) following drug administration (Nestler et al., 2001, Edling et al., 2007). In this analysis, cells were not labeled with other markers (i.e., double or triple labeled) because the goal was to first identify key brain regions or circuits implicated in the differential behavior.
Future studies will be needed to identify the phenotype of the cells in the striatum that display greater activation for a given level of locomotor stimulation in adolescents than adults. The majority of neurons in the striatum are GABAergic neurons that project to either the globus pallidus (internal or external segment) or substantia nigra. These projections are referred to as the direct, indirect, and striosomal pathways, each of which has implications for movement and can be individually identified with neuronal markers (e.g., dynorphin, enkephalin, substance P, etc.) (Graybiel, 1990).
Previous studies using adult rats tested in their home cage (i.e., as opposed to a novel environment), suggest that a majority of the Fos-positive cells in the striatum induced from acute cocaine or amphetamines contain dynorphin and express D1 as opposed to D2 receptors (Badiani et al., 1999, Uslaner et al., 2001, Ferguson et al., 2003, Gross and Marshall, 2009). A subset of these D1-expressing GABA/dynorphin neurons project back to the substantia nigra pars compacta and inhibit the dopamine output neurons (negative feedback). These neurons occur in discrete regions of the striatum called the striosomes that can be histologically differentiated from the surrounding regions, known as the matrix (Bolam et al., 1988, Graybiel et al., 1990). Increased Fos in striosomes in adolescents as compared to adults could explain reduced locomotor activity because that would suggest the dopamine output neurons were receiving stronger negative feedback. Alternatively, increased Fos in D1-expressing GABA/dynorphin neurons in the matrix that project to the internal portion of the globus pallidus and substantia nigra pars reticulata would not be expected to result in decreased locomotor activity because activation of this pathway increases motor activity via the ventral thalamus and cortical motor output neurons. Therefore, our current working hypothesis is that adolescents are less sensitive to the locomotor stimulating effects of cocaine and methampethamine in part because of greater negative feedback from the striatum back to dopamine output neurons.
Another interesting discovery was the difference in time course for locomotor stimulation in adolescents and adults in response to the 4 mg/kg dose of methamphetamine (Fig. 2f). The results show that for a high dose of methamphetamine, the effect on locomotor activity is initially the same for adolescents as adults, but wanes much more quickly in adolescents. This pattern was not apparent for any other dose of cocaine or methamphetamine that we tested. All the other doses showed a similar time course of locomotor stimulation and return to baseline between ages even if the amplitudes of stimulation were different. To the best of our knowledge we are the first to report this interesting difference for 4 mg/kg methamphetamine. In previous studies, we conducted a careful analysis of the concentrations of methamphetamine in the brain after a 4 mg/kg dose and found no statistically significant differences between the ages (Zombeck et al., 2009). One possibility for the rapid return to baseline in adolescents as compared to adults is that there is a ceiling effect preventing a greater peak response in adults. An alternative explanation is that the molecular, developmental or neurological changes in the brain that differentiate adults from adolescents in locomotor responses to psychostimulants are highly dynamic, capable of changing within the time-course of acute administration of the drug.
This study extends the current literature on psychostimulant induced locomotor activity in adolescents. Consistent with previous reports, adolescents stimulated less than adults to acute methamphetamine treatment (Zakharova et al., 2009, Zombeck et al., 2009). The literature for cocaine is mixed. Some studies are consistent with ours and have found attenuated stimulation to acute cocaine in adolescents as compared to adults (Laviola et al., 1995, Maldonado and Kirstein, 2005a, Frantz et al., 2007, Zombeck et al., 2009), while others have found no difference (Camarini et al., 2008, Parylak et al., 2008) or increased stimulation (Catlow and Kirstein, 2005, Caster et al., 2007, Caster and Kuhn, 2009) in adolescents. The cause for the discrepancies in the findings is unclear, however variations in age ranges within adolescents (Snyder et al., 1998), dose (Caster and Kuhn, 2009), route of administration, strain (McCarthy et al., 2004), and handling (Maldonado and Kirstein, 2005a, Maldonado and Kirstein, 2005b), may contribute.
Although it is possible that reduced locomotor stimulation in adolescents is a result of increased stereotypy (i.e., suggesting adolescents are more sensitive to the drugs), that is not likely for a number of reasons. First, we observed the mice during the tests, and although we did not record our observations in any formal way, we did not see evidence for increased stereotypy in any of the drug groups relative to saline controls. Moreover, that would not be expected because typically a higher dose of cocaine and methamphetamine is used to induce stereotypy in mice (Tolliver and Carney, 1994a, Tolliver and Carney, 1994b, Atkins et al., 2001, Schlussman et al., 2003, Tilley and Gu, 2008). Another reason is that both adolescents and adults increased activity at the higher doses of the drug relative to the medium doses. This suggests that both age groups are on the ascending limb of the dose response curve. Stereotypy is thought to contribute more to the descending limb of the curve (Shuster et al., 1977, Tolliver and Carney, 1994a).
In summary, results show that adolescent male C57BL/6J mice display greater Fos response to cocaine and methamphetamine in the striatum as compared to adults for a given level of locomotor activity. It is possible that the greater Fos reflects a greater negative feedback or inhibitory signal in adolescents. Future studies examining the phenotype of c-fos activated cells in the striatum and other brain regions of adolescents as compared to adults are needed to test these ideas.
Acknowledgements
We thank Weronika Brzezinska, Bryanna Close, Beth Dunn, Albert Cheng, and Andrew Revis for help with data collection. Thanks also to Dack Shearer, Donnell Parker, Reid McClure, Holly Fairfield, Eric Bialeschki, and Sheri Weidenburner for excellent animal care. This work was supported by grants from the National Institutes of Health, MH083807 and DA027487.
List of abbreviations
- PN
postnatal day
- IP
intraperitoneal
- PBS
phosphate buffered saline
- PBS-X
phosphate buffered saline containing Triton X
- NGS
normal goat serum
- DAB
diaminobenzidine
- PFC
prefrontal cortex
- M1
motor cortex
- Cg
cingulate cortex
- NACC
nucleus accumbens core
- NACS
nucleus accumbens shell
- Pir
piriform cortex
- CPuD
dorsal caudate
- CPuL
lateral caudate
- CPuV
ventral caudate
- LS
lateral septum
- BNST
bed nucleus of the stria terminalis
- VP
ventral pallidum
- GP
globus palidus
- SX
somatosensory cortex
- DG
dentate gyrus
- V1
visual cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–350. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Atkins AL, Helms ML, O'Toole LA, Belknap JK. Stereotypic behaviors in mice selectively bred for high and low methamphetamine-induced stereotypic chewing. Psychopharmacology (Berl) 2001;157:96–104. doi: 10.1007/s002130100774. [DOI] [PubMed] [Google Scholar]
- Azar MR, Acar N, Erwin VG, Barbato GF, Morse AC, Heist CL, Jones BC. Distribution and clearance of cocaine in brain is influenced by genetics. Pharmacol Biochem Behav. 1998;59:637–640. doi: 10.1016/s0091-3057(97)00471-1. [DOI] [PubMed] [Google Scholar]
- Badiani A, Oates MM, Day HE, Watson SJ, Akil H, Robinson TE. Environmental modulation of amphetamine-induced c-fos expression in D1 versus D2 striatal neurons. Behav Brain Res. 1999;103:203–209. doi: 10.1016/s0166-4328(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, Graybiel AM. Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: a combined Golgi and immunocytochemical study in cat and ferret. Neuroscience. 1988;24:853–875. doi: 10.1016/0306-4522(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Camarini R, Griffin WC, 3rd, Yanke AB, Rosalina dos Santos B, Olive MF. Effects of adolescent exposure to cocaine on locomotor activity and extracellular dopamine and glutamate levels in nucleus accumbens of DBA/2J mice. Brain Res. 2008;1193:34–42. doi: 10.1016/j.brainres.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32:2279–2289. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Caster JM, Kuhn CM. Maturation of coordinated immediate early gene expression by cocaine during adolescence. Neuroscience. 2009;160:13–31. doi: 10.1016/j.neuroscience.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacology (Berl) 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Kirstein CL. Heightened cocaine-induced locomotor activity in adolescent compared to adult female rats. J Psychopharmacol. 2005;19:443–447. doi: 10.1177/0269881105056518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. Neurobehavioral pharmacology of cocaine: role for serotonin in its locomotor and discriminative stimulus effects. NIDA Res Monogr. 1994;145:40–66. [PubMed] [Google Scholar]
- Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–340. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Sommer J, Canas E, Unterwald EM. Periadolescent mice show enhanced DeltaFosB upregulation in response to cocaine and amphetamine. J Neurosci. 2002;22:9155–9159. doi: 10.1523/JNEUROSCI.22-21-09155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-evoked c-fos mRNA expression in the caudate-putamen: the effects of DA and NMDA receptor antagonists vary as a function of neuronal phenotype and environmental context. J Neurochem. 2003;86:33–44. doi: 10.1046/j.1471-4159.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–126. [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl Acad Sci U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross NB, Marshall JF. Striatal dopamine and glutamate receptors modulate methamphetamine-induced cortical Fos expression. Neuroscience. 2009;161:1114–1125. doi: 10.1016/j.neuroscience.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Gulley JM. Reduced sensitivity to the locomotor-stimulant effects of cocaine is associated with increased sensitivity to its discriminative stimulus properties. Behav Pharmacol. 2009;20:67–77. doi: 10.1097/FBP.0b013e3283242fdd. [DOI] [PubMed] [Google Scholar]
- Kosofsky BE, Genova LM, Hyman SE. Postnatal age defines specificity of immediate early gene induction by cocaine in developing rat brain. J Comp Neurol. 1995;351:27–40. doi: 10.1002/cne.903510104. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–725. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Lanier LP, Isaacson RL. Early developmental changes in the locomotor response to amphetamine and their relation to hippocampal function. Brain Res. 1977;126:567–575. doi: 10.1016/0006-8993(77)90610-2. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Maldonado AM, Kirstein CL. Cocaine-induced locomotor activity is increased by prior handling in adolescent but not adult female rats. Physiol Behav. 2005a;86:568–572. doi: 10.1016/j.physbeh.2005.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado AM, Kirstein CL. Handling alters cocaine-induced activity in adolescent but not adult male rats. Physiol Behav. 2005b;84:321–326. doi: 10.1016/j.physbeh.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy LE, Mannelli P, Niculescu M, Gingrich K, Unterwald EM, Ehrlich ME. The distribution of cocaine in mice differs by age and strain. Neurotoxicol Teratol. 2004;26:839–848. doi: 10.1016/j.ntt.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Muller CP, Carey RJ, Huston JP. Serotonin as an important mediator of cocaine's behavioral effects. Drugs Today (Barc) 2003;39:497–511. doi: 10.1358/dot.2003.39.7.799442. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain Atlas in Sterotaxic Coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- Rebec GV. Behavioral electrophysiology of psychostimulants. Neuropsychopharmacology. 2006;31:2341–2348. doi: 10.1038/sj.npp.1301160. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Garland T, Jr, Gammie SC. Patterns of brain activity associated with variation in voluntary wheel-running behavior. Behav Neurosci. 2003;117:1243–1256. doi: 10.1037/0735-7044.117.6.1243. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ryabinin AE, Crabbe JC. Patterns of brain activation associated with contextual conditioning to methamphetamine in mice. Behav Neurosci. 2005;119:759–771. doi: 10.1037/0735-7044.119.3.759. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic "binge" cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–131. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Shuster L, Yu G, Bates A. Sensitization to Cocaine Stimulation in Mice. Psychopharmacology. 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Storey J. A direct approach to false discovery rates. Journal of the Royal Statistical Society. 2002;64:479–498. [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D-4-like receptors in rat forebrain regions: comparison with D-2-like receptors. Developmental Brain Research. 1998;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Tatum AL, Seevers MH. Experimental cocaine addiction. J Pharmacol Exp Ther. 1929;36 401-110. [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC. Evidence for Dopamine-Receptor Pruning between Adolescence and Adulthood in Striatum but Not Nucleus-Accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Tilley MR, Gu HH. Dopamine transporter inhibition is required for cocaine-induced stereotypy. Neuroreport. 2008;19:1137–1140. doi: 10.1097/WNR.0b013e3283063183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, Carney JM. Comparison of Cocaine and Gbr-12935 - Effects on Locomotor-Activity and Stereotypy in 2 Inbred Mouse Strains. Pharmacology Biochemistry and Behavior. 1994a;48:733–739. doi: 10.1016/0091-3057(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, Carney JM. Sensitization to stereotypy in DBA/2J but not C57BL/6J mice with repeated cocaine. Pharmacol Biochem Behav. 1994b;48:169–173. doi: 10.1016/0091-3057(94)90513-4. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Norton CS, Day HE, Watson SJ, Akil H, Robinson TE. Amphetamine and cocaine induce different patterns of c-fos mRNA expression in the striatum and subthalamic nucleus depending on environmental context. Eur J Neurosci. 2001;13:1977–1983. doi: 10.1046/j.0953-816x.2001.01574.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Winslow BT, Voorhees KI, Pehl KA. Methamphetamine abuse. Am Fam Physician. 2007;76:1169–1174. [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, Rhodes JS. Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav. 2008;93:637–650. doi: 10.1016/j.physbeh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]