Abstract
Little is known about CRF expression and regulation in the rat colon compared to the brain. We investigated CRF gene expression, cellular location, and regulation by endotoxin and corticosterone in the male rat colon at 6 h after intraperitoneal (ip) injection. CRF mRNA level, detected by reverse transcription polymerase chain reaction (RT-PCR) was 2.3-fold higher in the distal than proximal colon and 4.4-fold higher in the proximal colonic submucosa plus muscle layers than in mucosa. CRF immunoreactivity was located in the epithelia, lamina propria and crypts, and co-localized with tryptophan hydroxylase, a marker for enterochromaffin (EC) cells, and in enteric neurons. Lipopolysaccharide (LPS, 100 μg/kg, ip) increased defecation by 2.9-fold and upregulated CRF mRNA by 3.5-fold in the proximal and 2.1-fold in the distal colon while there was no change induced by corticosterone as monitored by quantitative PCR. LPS-induced increased CRF mRNA expression occurred in the submucosa plus muscle layers (2.5-fold) and the mucosa of proximal colon (1.9-fold). LPS increased significantly CRF immunoreactivity in the submucosal and myenteric plexuses of proximal and distal colon compared to saline groups. These results indicate that in rats, CRF is expressed in both proximal and distal colon and more prominently in enteric neurons of the submucosa plus muscle layers and subject to upregulation at the gene and protein levels by LPS through corticosteroid independent pathways. These data suggests that colonic CRF may be part of the local effector limb of the CRF1 receptor mediated colonic alterations induced by acute stress.
Keywords: Corticotropin releasing factor, enterochromaffin cells, enteric nervous system, lipopolysaccharide, colon, stress, defecation, corticosterone
1. Introduction
CRF, characterized by Vale et al. in 1981 as a novel 41-amino-acid hypothalamic peptide [44], was established to mediate stress-evoked activation of the hypothalamo-pituitary-adrenal (HPA) axis through the activation of pituitary CRF1 receptor [4,10]. Subsequently, activation of CRF-CRF1 receptor signaling was shown to participate in various components of the adaptive response to stressful events [4,10]. In particular, CRF injected centrally recapitulates stress-related colonic functional alterations (increased motility, permeability, secretion, transit, bacterial translocation, secretion and hypersensitivity to colorectal distension) and CRF1 receptor antagonists alleviate acute stress-induced colonic responses in conscious rodents [40].
In addition to the centrally mediated CRF actions, recent experimental and clinical studies indicate that peripheral injection of CRF or CRF1 agonists mimic the effects of acute stress on the colon as shown by the increase in myenteric neuronal activity, motility, transit, mucus secretion, macromolecular permeability and visceral pain [7,11,25,26,36,46,47,55]. These actions of exogenously administered CRF into the circulation or isolated colon in rats or colonic biopsy specimens in humans are largely mediated by the activation of CRF1 receptor prominently located in myenteric neurons and immune cells [9,22,29,34,46,55]. The relevance of the peripheral activation of CRF receptor signaling is also supported by the dampening of acute stress-induced stimulation of colonic secretory motor function by peripheral injection of peptide CRF receptor antagonists with poor brain penetrance in rats and humans [39,41,50]. However, compared to the brain, less is known on CRF gene expression and CRF localization and its regulation by stressors in the rat colon. So far, CRF mRNA has been reported to be expressed in the mice and rat ileum [24,51], rat cecum [45] and human colonic mucosa [18] and rapidly upregulated in rat and mice ileum in response to toxin A that was perfused in the ileal loop [24,51].
Therefore in the present study, we investigated CRF expression at the gene and protein levels in the proximal and distal colon in naïve rats and in response to peripheral injection of lipopolysaccharide (LPS) and corticosterone. Gene expression was assessed by reverse transcription-polymerase chain reaction (RT-PCR) and real time quantitative PCR [53]. The cellular location and identity (endocrine vs neuronal) of CRF positive cells were characterized using immunohistochemistry and double immunostaining with tryptophan hydroxylase (TPH) as an enterochromaffin cell marker [21,54] and Hu C/D as a neuronal marker [27] in the whole thickness tissue sections. CRF immunoreactivity change in response to LPS was also evaluated using digital computer-assisted image analysis in whole mount preparation of enteric plexuses (submucosal and myenteric).
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (265–297 g, Harlan Laboratory, San Diego, CA) were group housed under controlled conditions (22–24°C, lights on from 6:00 AM to 6:00 PM) with free access to standard rodent chow (Prolab RMH 2500, PMY Nutrition International, Brentwood, MO) and tap water. Studies were conducted under the approved protocols of the Department of Veterans Affairs Animal Component of Research Veterans Affairs Greater Los Angeles Healthcare System (04012-06 and 9906-820). All experiments were performed between 9:00 AM and 1:00 PM to avoid confounding variables of diurnal rhythm influence on the HPA axis under basal and stress conditions.
2.2. CRF expression in the colon of naïve rats
2.2.1. CRF mRNA expression in the proximal and distal colon by RT-PCR
Three naive rats were decapitated and proximal (1 cm from cecum) and distal (2 cm to the anus) segments of the colon were collected. In the proximal colon segment, half of it was separated into mucosa and submucosa plus muscle layer as previously described [55]. All colonic samples were snapped frozen in dry ice and stored at −70° C until used. Total RNA was extracted using RNA-Bee™ (TEL-TEST, Friendswood, TX), following manufacturer's recommended protocol. RNA pellets were resuspended in DEPC-treated water and further digested with DNase I for 60 min at 37°C (Promega, Madison, WI). Total RNA (5 μg) was denatured at 65°C for 5 min and used to synthesize first-strand cDNA in a total volume of 20 μl reaction by ThermoScript™ RT-PCR system (Invitrogen, CA). cDNA (1μl) was amplified for CRF directly by the polymerase chain reaction (PCR) with 33 cycles at 92°C for 40 s, 57°C for 40 s, 72°C for 2 min, and a final extension step at 72°C for 5 min using specific CRF primers for rat (Table 1). PCR for the house-keeping gene, rat acidic ribosome protein (ARP) served as the internal control as in our previous study [55]. PCR products were separated by 1% agarose gel electrophoresis, and visualized with ethidium bromide. The gel images were acquired by Kodak EDAS 290 system and quantitative densitometry was performed with NIH Image software (Scion Corporation, Frederick, MD). In all samples, the intensity of the bands was normalized to that of ARP in each sample respectively and results were expressed as the fold change in reference to the control group. Then the PCR product corresponding to the predicted CRF was extracted with QIA quick Gel Extraction Kit (QIAGEN, Hilden, Germany). The DNA fragment was inserted into pCR2.1 vector and transformed into bacterial competent cells (TA Cloning kit, Invitrogen). The plasmid with positive insert was sequenced in both directions to confirm its identity of rat CRF using the Big Dye Terminator (ver. 3) in Cycle Sequencing System (Applied Biosystems, Foster City, CA).
Table 1.
Sequences of oligonucleotide primers used for RT-PCR and real-time quantitative PCR
| cDNA | Direction | Primer (5'–3') | Size (bp) |
|---|---|---|---|
| for RT-PCR | |||
| rCRF | Sense | TGATCCGCATGGGTGAAGAATACTTCCTC | 394 |
| Antisense | CCCGATAATCTCCATCAGTTTCCTGTTGCTG | ||
| rARP | Sense | GTTGAACATCTCCCCCTTCTC | 402 |
| Antisense | ATGTCCTCATCGGATTCCTCC | ||
| for real time PCR | |||
| rCRF | Sense | TCTCTCTGGATCTCACCTTCCACC | 77 |
| Antisense | AGCTTGCTGAGCTAACTGCTCTGC | ||
| rGAPDH | Sense | AGACAGCCGCATCTTCTTGT | 142 |
| Antisense | TGATGGCAACAATGTCCACT | ||
r: rat
2.2.2. CRF immunohistochemistry in the whole thickness sections of proximal colon and double labeling with tryptophan hydroxylase or Hu C/D
The proximal colon was collected from 3 naïve rats, fixed in formalin, embedded in paraffin and sectioned at 5 m thickness. Immunohistochemistry was performed using avidin-boiotin-peroxidase technique. Sections were deparaffinized in xylene and hydrated in descending grades of ethanol. After washing 2 times (5 min each) in phosphate buffer saline (PBS), slides were placed in a plastic Coplin jar filled with 10 mM citrate buffer (pH 6.0), boiled for 8 min, and followed by cooling to room temperature. Sections were washed twice in PBS. Endogenous peroxidase activity was blocked by incubation for 30 min with 0.3% hydrogen peroxide in PBS at room temperature. Slides were incubated overnight at 4°C with rabbit anti-rat/human CRF antibody C70 (1:20,000, gift from Dr. Wylie W Vale, Clayton Foundation Laboratories, Salk Institute, San Diego, CA) diluted in PBS containing 0.3% Triton X-100, followed by incubation with biotinylated donkey anti-goat IgG (1:1000; Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. Sections were subsequently processed for avidin-biotin-peroxidase procedure using diaminobenzidine as a chromogen, and then counterstained with hematoxylin. Immunohistochemical control was routinely performed following the same procedures, except that the primary antibody was pre-absorbed with CRF antigen peptide (20 μg/ml, rat CRF, gift from Jean Rivier, Clayton Foundation Laboratories, Salk Institute).
For the double immunostaining, sections were deparaffinized in xylene, hydrated in descending grades of ethanol and an antigen retrieval procedure was conducted as described above, then sections were washed three times at 10-min intervals with PBS, and incubated in 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) in 0.3% Triton-X 100/PBS for 30 min at room temperature followed by two-overnight incubations with the mixture of rabbit anti-rat CRF antibody (1:5,000, gift from Dr. Wylie Wale) and mouse anti-TPH (1:40, Novocastra Lab Ltd., Newcastles, UK), or mouse anti-Hu C/D (1:200, Molecular probes, Eugene, OR), a neuronal marker. Sections were washed with PBS three times at 10-min intervals and incubated for 2 h at room temperature with the mixture of FITC-conjugated donkey anti-rabbit IgG and Rhodamine Red™-X-conjugated donkey anti-mouse IgG (1:100. Jackson ImmunoResearch Laboratories, Inc, West Grove, PA). After three washes in PBS, sections were counterstained with 4, 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich, Saint Louis, MO), mounted on slides with anti-fade mounting media (Vector Laboratory Inc., Burlingame, CA) and visualized by standard fluorescence microscopy. The double labeling of CRF/TPH was assessed in at least 20 TPH immunoreactive (ir) positive cells for each rat and expressed in percentage of the total number of TPH positive cells respectively. The means of the values from each animal were used to calculate the group mean.
2.3 Effect of intraperitoneal injection of LPS on CRF gene and protein expression in rat colon
Freely fed rats (n=6/group) were single housed the day before and thereafter treatment. Rats were subjected to ip injection (0.3 ml) of either vehicle (endotoxin-free saline) or LPS (Escherichia coli serotype O26: B6; code 3755, lot 37H4095; 100 μg/kg BW). The dose was selected based on our previous studies showing alterations of upper gut propulsive motor function and hormone release [49]. Fecal pellet output was monitored 75 min after ip injection. Both groups were euthanized by decapitation 6 h after the ip injection to assess changes in CRF expression in the proximal and distal segments of the colon.
2.3.1. Colonic CRF gene expression assessed by real time quantitative PCR
The proximal (1 cm from cecum) and distal (2 cm to the anus) segments of colon were collected and a half piece of each proximal colonic sample was separated into mucosa and submucosa plus muscle layers as previously described [55]. All samples were processed for RNA extraction and cDNA synthesis as described above. Real time quantitative PCR for CRF was performed in triplicates using DNA Engine Opticon® 2 Detection System interfaced to the Opticon MONITOR™ Analysis Software version 2.01(MJ Research Inc., Waltham, MA) in a 25 μl reaction volume. The optimized reaction contained 12.5 μl of SYBR® Premix Ex Taq™ (Perfect Real Time) (Takara Mirus Bio Inc., Madison, WI), 0.5 μl each of oligonucleotide primers (10 μM), 1 μl of the cDNA synthesis reaction, and 10.5 μl of H2O. Selected forward (f) and reverse (r) primers listed in Table 1 were used for rat CRF. The house keeping genes, rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also analyzed as internal controls. Thermal conditions were as follows: 95 °C for 4 min followed by 40 cycles of 95 °C for 10 s, 58 °C for 10 s; 72 °C for 20 s with a final extension cycle of 10 min at 72 °C. The specificity of the amplification reaction was determined by performing a melting curve analysis of the PCR fragments. For each gene, a standard curve was prepared by serial dilution of a gel-purified PCR product. The standard curve, spanning seven decades, was used to verify that crossing-point values (Ct) of all samples were within the exponential range of the PCR reaction and to calculate final expression levels. The amount of target gene was subsequently divided by the gene amount of rat GAPDH to obtain a normalized target gene values and presented as fold change in reference to the control group.
2.3.2. Colonic CRF immunoreactivity in the whole mount preparation of enteric plexuses
The proximal and distal colons collected from vehicle- and LPS-treated groups were opened along the mesenteric border. The tissue was pinned flat and fixed overnight in 0.1 M sodium phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde and 14% picric acid. After being rinsed in 0.01 M PBS, the tissue samples were processed for longitudinal muscle/myenteric plexus (LMMP) and submucosal whole-mount preparations as described previously [31,55]. Briefly, the mucosa was scraped off, the submucosa was collected and the circle muscle was carefully removed using fine forceps. LMMP whole-mount preparation included the myenteric plexus adhering to the longitudinal muscle. To avoid regional differences, in each rat, two pieces of preparation (~0.5 cm2) of proximal or distal colon were dissected from the segment 1 cm from the cecum or 2 cm rostral to anus respectively. After being washed three times at 10-min intervals with PBS, free floating submucosa and LMMP whole mounts of proximal and distal colon were incubated in 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) in 0.3% Triton-X 100/PBS for 30 min at room temperature followed by one overnight incubation with the mixture of rabbit anti CRF antibody (1:5,000, gift from Dr. Wylie Wale) and mouse anti-Hu C/D (1:200, Molecular probes, Eugene, OR). Whole-mount preparations were washed with PBS three times at 10-min intervals and incubated for 2 h at room temperature with the mixture of FITC-conjugated donkey anti-rabbit IgG and Rhodamine Red™-X-conjugated donkey anti-mouse IgG (1:100. Jackson ImmunoResearch Laboratories, Inc, West Grove, PA). After washing three times in PBS, preparations were mounted on the slides with anti-fade mounting media (Vector Laboratory Inc., Burlingame, CA). To minimize variability in staining, immunohistochemical detection in the whole mount preparations from all animals were processed simultaneously as a single batch. A negative control was performed with pre-absorbed primary antibody as described above. The intensity of CRF immunoreactivity was evaluated using a digital computer-assisted method. Briefly, the images of 15 submucosal or myenteric ganglia best representing CRF immunostaining in the preparations were captured from each rat using a fluorescence microscope (Zeiss Axioskop 2 plus) coupled to a color digital camera (Hamamatsu, C4742-95). The images were analyzed with the image software (NIH ImageJ 1.42 bundled with 32-bit Java 1.6.0_10). All fluorescent images were digitally converted into a grey scale image before starting the analysis. The sum of the gray values of all the pixels within one ganglion was measured and then divided by the number of pixels per ganglion. The results are expressed as average optical density (AOD) per ganglion. Background intensity was also analyzed using the same steps and then subtracted from that of the ganglion of interest.
2.4. Effect of corticosterone on CRF gene expression in proximal colon
Rats (n=4/group) received successive subcutaneous (sc, 0.2 ml) injections of either vehicle (11% ethanol-containing saline), or corticosterone (125 μg/rat, Sigma-Aldrich Co, St. Louis, MO) at 0, 30, 60, 120, and 180 min. Such a regimen of administration was previously reported to induce similar 6-h area under the curve of circulating corticosterone as that achieved by LPS at a low dose (50 μg/kg, intravenous) [17]. All groups were euthanized by decapitation at 6 h after the first injection and the proximal colon was harvested and CRF gene expression measured by real time quantative PCR as described above.
2.5. Statistical analysis
All data are expressed as mean ± SE. Statistical analysis of the difference between two groups was performed by two-tailed student's t-test. Multiple group comparisons were performed with one-way ANOVA followed by Duncan's test. A P value <0.05 was considered statistically significant.
3. Results
3.1. CRF mRNA expression in the proximal and distal colon and proximal colonic mucosa vs submucosa plus muscle layers in naïve male rats
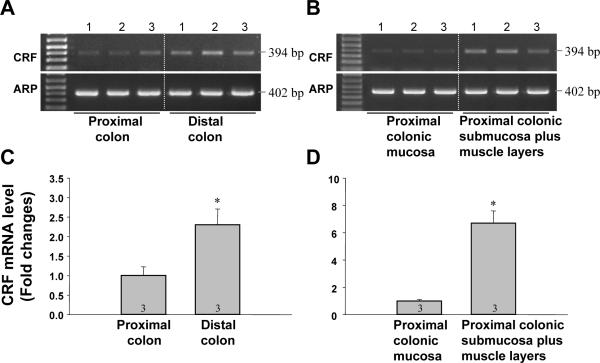
A single band for the PCR product of rat CRF was yielded at the predicted size (394 bp) in the samples of the proximal and the distal colon as well as in both mucosa and submucosa plus muscle layers of the proximal colon in naïve rats (Figs. 1A,B). The intensity of the bands for CRF was stronger in the distal colon than in the proximal colon (Fig. 1A). Furthermore, the bands in the submucosa plus muscle layers were stronger than in the mucosa (Fig. 1B). Bands for the housekeeping gene ARP were detected in all samples with the predicted size (402 bp) and had similar intensity (Figs. 1 A, B). Densitometric analysis showed that CRF mRNA level is significantly 2.3-fold higher in the distal than the proximal colon (Fig. 1C). In the proximal colon, CRF mRNA level was 4.9-fold more elevated in the submucosa plus muscle layers than in the mucosa (Fig. 1D). PCR products for CRF were sequenced and found to be identical to the corresponding sequence of rat CRF.
Fig. 1.
CRF gene expression in naive rat colon. A, B. Gel images of RT-PCR for CRF in the proximal and the distal colon (A) and in the mucosa and the submucosa plus muscle layers of the proximal colon layers (B). C, D. Quantitative analysis of PCR product for CRF in the proximal and the distal colon (C) and in the mucosa and the submucosa plus muscle layers (D). In all samples, the intensity of the band was normalized to that of ARP in the same sample and results are expressed as the fold change in reference to the proximal colon (C) or mucosa (D). Data are presented as the mean ± SEM. * P<0.05 vs proximal colon (C) or mucosa (D).
3.2. Endocrine and neuronal cellular localization of CRF immunoreactivity in whole thickness section of the proximal colon in naïve male rats
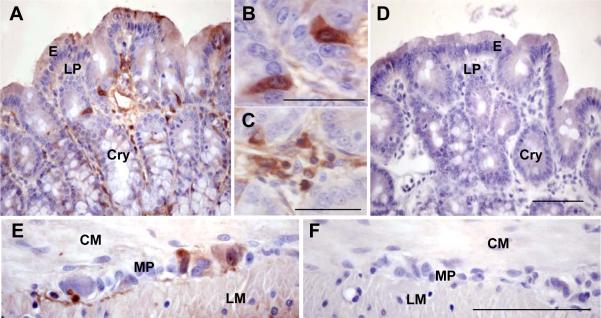
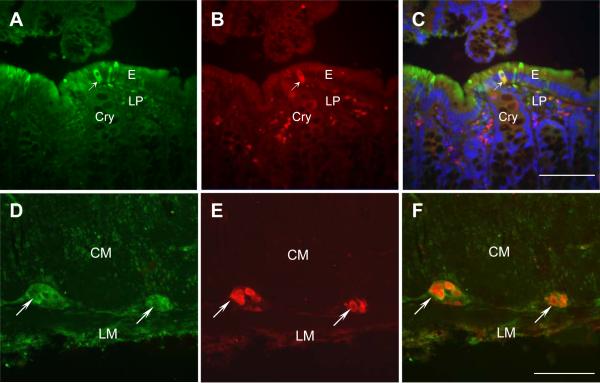
CRF immunoreactivity was revealed in individual cells scattered in the mucosa mainly in cells located in the epithelia (E), crypts (Cry) and lamina propria (LP) in proximal colon of all naïve rats investigated (Figs. 2A, B, C). In addition, CRF immunoreactivity was also found in myenteric plexus (MP) (Fig. 2E). In the control slides in which the primary antibody was pre-absorbed with antigen peptide, no CRF immunoreactivity was detected in the mucosa and myenteric plexus (Figs. 2D, F). To characterize the endocrine and neuronal identity of CRF positive cells, double staining of CRF and marker antibodies for TPH to identify EC cells in the epithelium and Hu D/C for myenteric neurons was conducted in the tissue sections from the proximal colon in naïve rats (Fig. 3). CRF/TPH double-labeled cells represent 98.9±4.4% of TPH ir cells in the mucosal epithelia (Figs. 3A–C) and CRF immunoreactivity was detected in all myenteric ganglia labeled by Hu C/D without CRF specific staining in muscle layers (Figs. 3D–F), suggesting that almost all EC cells in mucosal epithelia and all ganglia in myenteric plexuses were CRF ir positive.
Fig. 2.
CRF immunohistochemistry in the whole thickness tissue sections of proximal colon in a naïve rat. A. CRF immunoreactivity was located in cells scattered in the epithelia (E), crypts (Cry) and lamina propria (LP). B, C. Inserts show a higher magnifications of CRF immunoreactive positive cells in the crypts (B) and lamina propria (C). E. CRF immunoreactivity was also found in myenteric plexus (MP), but not in the circle muscle (CM) and longitudinal muscle (LM). D, F. Pre-absorption of primary antibody by antigen peptide: no CRF immunoreactivity was seen in the mucosa (D) and myenteric plexuses (F). Bar: 100 μm and 50 μm in the inserts.
Fig. 3.
Double labeling of CRF and enterochromaffin (EC) cell marker tryptophan hydroxylase (TPH) or neuronal marker Hu C/D in the whole thickness tissue sections of proximal colon in a naïve rat. A. CRF staining in the mucosa. B. TPH staining in the mucosa. C. The merged image of A and B showing the co-localization of CRF and TPH. The arrow points to a cell double-labeled by CRF and TPH. D. CRF staining in a myenteric ganglion. E. Hu C/D staining in the myenteric ganglia. F. The merged image of D and E showing the co-localization of CRF and Hu C/D. The arrow points to the double-labeled ganglia by CRF and Hu C/D. E: epithelia, Cry: crypts, LP: lamina propria, CM: circle muscle, LM: longitudinal muscle. Bar: 100 μm.
3.3. LPS increases CRF mRNA expression in rat colon
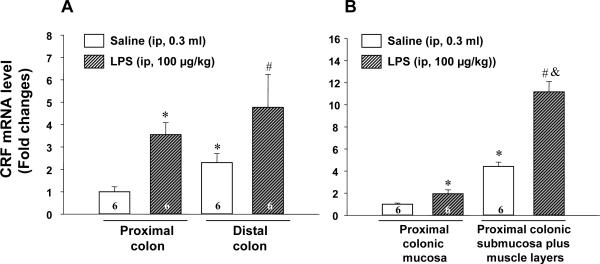
Real time PCR was performed using the standard curve method to detect the regulation of CRF mRNA expression in rat colon in response to LPS and corticosterone. The melt curve was obtained by plotting the negative first derivative values of the fluorescence intensity as a function of temperature, which resulted in a single narrow peak. CRF mRNA level monitored by real time PCR was significantly higher in the distal than proximal colon and higher in the submucosa plus muscle layers than mucosa in the proximal colon in ip saline-treated rats (Fig. 4) as we observed in naïve rats using RT-PCR (Fig. 3). LPS injection (100 μg/kg, ip) increased CRF mRNA in the proximal colon by 3.5-fold (3.5±0.5 vs 1.0±0.2 in saline group, p<0.05) and distal colon by 2.1-fold (4.7±1.5 vs 2.3±0.4 in saline group, p<0.05) as monitored 6-h after injection (Fig. 4A). LPS-induced increase in CRF mRNA expression in the proximal colon was more prominent in the submucosa plus muscle layers (11.2±0.9 vs 4.4±0.4 in saline group, p<0.001) than the mucosa (1.9±0.4 vs 1.0±0.1 in saline group, p<0.05) (Fig. 4B). By contrast, five repeated subcutaneous injections of corticosterone (125 μg/kg/injection sc) over 3 h had no effect on CRF mRNA expression in the proximal colon as monitored 6 h after the initial injection in male rats compared with vehicle group (0.9±0.2 vs 1.0±0.2, p=0.75) (data not shown).
Fig. 4.
LPS upregulated CRF gene expression in the rat proximal and distal colon (A) and in proximal colonic mucosa and submucosa plus muscle layers (B). Tissues were collected 6 h after ip LPS (100 μg/kg) or saline injection. For each sample, the amount of CRF and GAPDH mRNA were determined by real-time quantitative PCR from the appropriate standard curves. CRF mRNA level was subsequently divided by GAPDH mRNA to obtain a normalized target value and data were represented as the fold changes in reference to saline group. The number of rats was indicated at the bottom of each column. A. * p<0.05 vs. proximal colon of saline-treated rats. # p<0.05 vs distal colon of saline-treated rats. B. * p<0.05 vs. proximal colonic mucosa of saline-treated rats. # p<0.05 vs proximal colonic mucosa of LPS-treated rats. & p<0.05 vs proximal colonic submucosa plus muscle layers of saline-treated rats.
3.3. LPS increases CRF immunoreactivity in the enteric plexus of proximal and distal colon
Whole-mount preparation allowed us to better visualize as a 3-dimensional picture, the neuronal networks branching out and interconnections within the enteric ganglia in the proximal and distal colon. CRF immunoreactivity was found occasionally in the neurons of the proximal (Fig. 5A) and distal colon (Fig. 5B) and mainly in nerve terminals shown as varicosity around neurons and running between ganglia (Figs. 6 and 7). The stronger immunostaining was found in the whole mount samples of either submucosal or myenteric plexus in rats injected ip with LPS (100 μg/kg) compared with saline (Figs. 6 and 7). The immunostaining was totally blocked by using antigen peptide absorption in submucosal (Figs. 6M–O) and myenteric (Figs. 7M–O) whole mount preparations. Semi-quantitative analysis revealed that the AOD of CRF immunoreactivity is significantly higher in LPS-treated rats than saline group in submucosal plexus as well as in myenteric plexus in both the proximal and distal colon (Table 2). In saline group, although there is trend showing higher AOD values in the submucosal or myenteric plexuses of the distal compared with proximal colon, this did not reach significance (Table 2). In the same groups of rats, the ip injection of LPS significantly increased fecal pellet output compared to ip saline group (number /75 min: 4.7±1.0 vs 1.2±0.7 respectively; p<0.05).
Fig. 5.
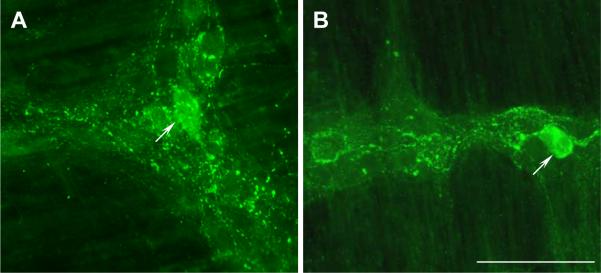
CRF immunostaining in the whole mount preparation of myenteric plexus in the proximal (A) and the distal colon (B) in a saline-treated rat. Colon was harvested 6-h after ip injection of saline. CRF immunoreactivity was mainly located in nerve terminals shown as varicosity around neurons and running between ganglia. CRF ir positive neurons were also observed (pointed by arrows). Bar: 100 μm.
Fig. 6.
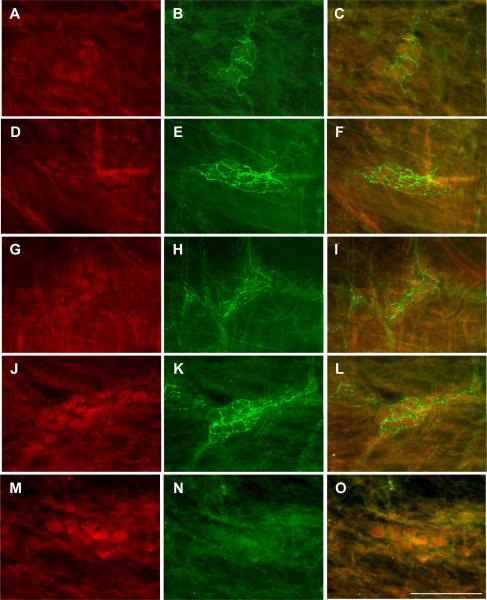
CRF immunoreactivity in the whole mount preparation of colonic submucosal plexus identified by Hu C/D in rats that received an ip injection of LPS (100 μg/kg) or saline. A, D, G, J, M: Hu C/D; B, E, H, K: CRF; C, F, I, L, O: merged images. A–C: proximal colon in saline group. D–F: proximal colon in LPS group. G–I: distal colon in saline group. J–L: distal colon LPS group. M–O: Control immunoreactivity in the proximal colonic submucosal plexus with antibody pre-absorbed by CRF antigen peptide. No CRF immunostaining was seen in Figs. 6N and 6O. Bar: 100 mμ.
Fig. 7.
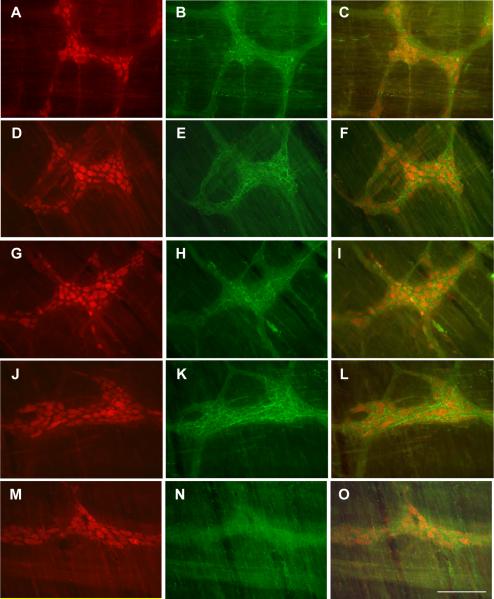
CRF immunoreactivity in the whole mount preparation of colonic myenteric plexus identified by Hu C/D in rats that received ip injection of LPS (100 μg/kg) or saline. A, D, G, J, M: Hu C/D; B, E, H, K: CRF; C, F, I, L, O: merged images. A–C: proximal colon in saline group. D–F: proximal colon in LPS group. G–I: distal colon in saline group. J–L: distal colon in LPS group. M–O: Control immunoreactivity in the proximal colonic myenteric plexus with antibody pre-absorbed by CRF antigen peptide. No CRF immunostaining was seen in Figs. 6N and 6O. Bar: 100 mμ.
Table 2.
Semi quantitative analysis of CRF immunostaining in the colonic enteric plexus1
| CRF immunoreactivity1 (Average optical density/ganglion) |
|||
|---|---|---|---|
| Saline | LPS2 | ||
| Colon | |||
| Proximal | Submucosal plexus | 19.4±0.5 | 24.4±1.3* |
| Myenteric plexus | 18.3±0.9 | 22.9±1.8* | |
| Distal | Submucosal plexus | 21.2±1.6 | 26.3±2.1* |
| Myenteric plexus | 20.2±1.8 | 24.7±1.5* | |
CRF immunostaining was evaluated using NIH ImageJ software within 15 ganglia of submucosal and myenteric plexuses for each animal. The intensity of the immunoreactivity was expressed as average optical density (AOD) per ganglion. The mean from each animal was used to calculate the group mean. Mean ± SEM of 6 rats.
p<0.05 vs saline.
Non fasted rats were injected ip with saline or LPS (100 μg/kg) and 6 h later, animals were euthanized and colonic samples were harvested and processed for CRF immunohistochemistry.
4. Discussion
In the present study, CRF gene expression was detected in the rat colon with a 2.3-fold higher expression in the distal than proximal colon in naïve male rats as detected by RT-PCR and confirmed by sequencing. In addition, we found that CRF gene was expressed at a 4.4-fold higher level in the submucosa plus muscle layers compared to mucosa in the proximal colon. The higher expression in the distal vs proximal colon and proximal submyenteric plus muscle layers vs mucosa in the proximal colon was confirmed by real time PCR in a control group injected ip with saline. Of functional relevance, CRF is known to display preferential affinity for CRF1 receptor [14] and the CRF gene expression pattern is consistent with that of CRF1 receptor previously reported to be prevalent in submucosa plus muscle layers compared with the mucosa in the rat proximal colon [55]. Taken together these data established CRF gene expression in the rat colon expending to rodents CRF gene expression that was so far reported only in colonic mucosal biopsies of healthy human subjects [18,19,34]. It also described for the first time differential f CRF expression in the rat colon with levels in the distal>proximal segments and submucosa plus muscle layers>mucosa layer.
Next, we examined in the whole thickness tissue sections of rat proximal colon, the localization and distribution of CRF peptide by immunohistochemistry. Consistent with results of higher expression of CRF gene by RT-PCR in the submucosa plus muscle layers, we detected strong CRF immunoreactivity in all ganglia of myenteric plexus in proximal colon. Whole-mount preparation technique revealed that CRF immunoreactivity was mainly located in nerve terminals shown as varicosity around neurons and running between ganglia in proximal and distal colon. Due to the absence of treatment with colchicine to block axonal transport only few CRF positive neurons were observed as previously reported in myenteric and submucosal plexuses of rat distal colon [22]. A previous report in whole mount preparation of myenteric ganglia from guinea pig proximal colon treated with colchicine, indicates that the majority of CRF positive neurons exhibits Dogiel I morphology (oval or round cell body with single long process and small short lamellar dendrites) and co-localizes with excitatory cholinergic/substance P motor neurons [28]. Whole thickness histological sections and whole mount preparations without colchicine treatment in the present study precluded us to identify with certainty whether CRF labeled neurons in rat proximal and distal colon also display Dogiel I morphology and what percentage they represented from the total myenteric neurons in the whole mount preparation. Irrespectively, these data expend the identification of CRF immunoreactivity to proximal and distal colonic myenteric and submucosal neurons that was previously established in rats within the cecal plexus of the external muscle layer [45], nerve fibers of submucosal plexus in the ileum, jejunum and duodenum [24, 52] and submucosal/myenteric neurons of distal colon [22].
In the proximal colonic mucosa, we also found strong CRF immunoreactivity in individual cells located in the epithelia, crypts and lamina propria. Likewise, in the distal colon of female Wistar rats, one previous study showed that preproCRF labeling in enterocytes and weaker immunreactivity in colonic bottom crypts and submucosal cells although their phenotypes were not identified [5]. In the present study, double labeling showed that all TPH labeled cells are CRF ir positive in the epithelia. Immunohistological localization of TPH, the initial and rate limiting enzyme in 5-HT biosynthesis [48], has been previously established to characterize 5-HT producing cells in the rat gastrointestinal tract [54]. Therefore the present results indicate that EC cells are one endocrine source of CRF in the rat proximal colon. Consistent with these findings, it has been reported that CRF and 5-HT coexist in mucosal cells from colonic biopsies of healthy subjects [18]. We also found some cells labeled by CRF but not by TPH in the epithelia, the phenotype of which still remains to be identified. In the lamina propria, CRF ir cells are likely to encompasses immune cells as immunostaining for CRF was localized in monocytes in normal human colonic mucosa [19]. Taken together these data indicate the expression of CRF immunoreactivity in neuronal, endocrine (EC cells) and lamina propria cells of the rat proximal colon mucosa under basal conditions.
Previous studies established that stress, particularly immunological stress influences CRF gene expression in the hypothalamus [43]. In particular, peripheral injection of LPS, known to induce the innate defense immune response to Gram-negative bacteria infection [32], activates CRF gene expression and CRF release in the hypothalamus leading to the stimulation of the HPA axis and rise in circulating levels of corticosterone in rats [38, 43].Therefore, we assessed whether proximal and distal colonic CRF gene expression is also upregulated by LPS. Real time quantitative PCR detection of CRF gene expression showed that LPS significantly increased CRF mRNA level by 3.5 and 2.1-fold in the proximal and distal colon respectively as monitored 6-h after injection. LPS-induced increase CRF mRNA expression in the proximal colon occurred in the mucosa and more prominently in submucosa plus muscle layers. By contrast, repeated subcutaneous injections of corticosterone did not alter CRF gene expression in the proximal colon at 6 h after the first injection. Such a regimen of administration was previously established to reproduce the 6-h area under the curve of plasma corticosterone concentration induced by a low dose LPS [17]. It has been well documented that there are regional differences in the regulation of CRF mRNA by glucocorticoids in the central nervous system [16,30] while less is known in the periphery. Although it remains to be further ascertained, the present data suggest that CRF gene expression in the proximal colon is not responsive to corticosterone and that LPS induced up-regulation of CRF expression is likely independent from the activation of the HPA. A potential direct action of LPS through its receptor is suggested by the abundant expression of Toll like receptor 4 (TLR4) in the rat colon where TLR4 immunoreactivity has been localized in crypt bottom epithelial cells, mononuclear cells and lymphocytes in lamina propria [37;42]. In addition, TLR4 is expressed in approximately 35% of all myenteric neurons in rat small intestine [2]. Therefore, LPS can regulate CRF expression via TLR4 on epithelial cells, immune cells and myenteric neurons. Alternatively LPS-induced release of interleukin-1, interleukin-6, and tumor necrosis factor alpha [12,13] may provide other mechanisms through which cytokine occurrence may modulate colonic CRF expression as shown in the hypothalamus [43]. We previously reported that alterations of gut hormone release by ip LPS at a similar dose is mediated by activation of interleukin-1 receptor in rats [49]. Other studies showed that acute perfusion of mice ileal loop with toxin A results in marked increase in CRF mRNA signal within 60 min [51] and cecal injection of peptidoglycan-polysaccharide from Streptococcus pyogenes increases CRF mRNA in the rat cecum in the chronic phase of inflammation [45]. Collectively these data indicate that the local CRF gene expression in the intestine is upregulated in response to acute administration of enterotoxin or Gram negative bacteria.
We next assessed whether the increased CRF gene expression in the proximal and distal colon induced by LPS translates to changes in protein expression. CRF is a small bioactive peptide (4.7 kDa) and it would be likely detected in the brain by Western, given the prominent expression of the peptide in the rat brain [8] and the low amount of interfering substances. However, in peripheral tissues such as the colon, the combination of low levels of expression and the low molecular weight of the peptide made it less amendable to be detected by Western blotting method. Therefore to achieve the detection of CRF at the protein level induced by LPS, CRF immunostaining was performed in whole mount preparation of enteric plexus because we detected higher CRF mRNA level in submucosa plus muscle layers than mucosa and CRF staining was selectively located in the enteric plexus. The evaluation of immunoreactivity with digital computer-assisted image analysis demonstrated a significant higher CRF immunoreactivity in rats treated with LPS than saline indicative that CRF protein level was increased in the colonic enteric plexus consistent with the data yielded by real time PCR. These results suggest that CRF gene upregulation induced by an immune stressor is likely to be associated with increase protein translation. Previous studies showed that an increase in CRF gene transcriptional activity in the hypothalamus in response to immune challenge [23] that needs to be also assessed in the colon. In the present study, LPS injected ip at a low dose (100 μg/kg) that we previously reported to inhibit gastric emptying in rats [49] also stimulated colonic propulsive motor function as shown by the significant increased in defecation. The gut motor responses to LPS under these conditions of administration are similar to those induced by peripheral injection of CRF, well established to inhibit gastric while stimulating colonic transit in rats [6,41]. The upregulation of CRF expression in the colon may have a bearing with the stimulation of functional motor changes which will need to be further ascertained.
Conclusion
CRF is expressed at the gene and protein levels in proximal and distal colon of naïve rats and is upregulated by LPS injected peripherally at a low dose. These findings along with the described prominent CRF1 receptor expression in rat colon [9,55], provide molecular/anatomical support for an important role for CRF/CRF1 coupling in the colon as part of the local effector limb of the CRF1 receptor mediated colonic motor response to stress [40] and established proinflammatory role [1,20,51]. CRF immunoreactivity was localized in submucosal and myenteric plexuses, the enterochromaffin 5-HT synthesizing cells in the epithelia, and in cells yet to be characterized in the lamina propria. CRF endogenously produced at these sites can exert CRF1 receptor paracrine or autocrine action and thereby serve to facilitate the colonic responses to stress. It is well known that serotonin (5-HT) released from EC cells in response to chemical or mechanical stimuli affects gastrointestinal motility and local injection of CRF in the colon stimulates colonic motor function [40]. Thus, CRF might be co-released with 5-HT from EC cells in response to acute stress to stimulate the colonic motor function which is supported by the blockade of stress- and peripheral injection of CRF-induced propulsive motor function by CRF, 5-HT3 and 5-HT4 receptor antagonists [3,15,33,35].
Acknowledgements
We thank Drs. Wylie W. Vale and Jean Rivier (Salk institute, San Diego, CA) for the generous donation of the rabbit anti-CRF antiserum, and rat CRF respectively and Ms Honghui Liang for technical assistance. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, grants R01 DK-57238 (YT), VA Career Scientist Award and Merit Award (YT), DK-41301 (Animal Core, YT), P01-DK 33506 (C. Pothoulakis, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Anton PM, Gay J, Mykoniatis A, Pan A, O'Brien M, Brown D, Karalis K, Pothoulakis C. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci U S A. 2004;101:8503–8508. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arciszewski MB, Sand E, Ekblad E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul Pept. 2008;146:218–223. doi: 10.1016/j.regpep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- [3].Ataka K, Kuge T, Fujino K, Takahashi T, Fujimiya M. Wood creosote prevents CRF-induced motility via 5-HT3 receptors in proximal and 5-HT4 receptors in distal colon in rats. Auton Neurosci. 2007;133:136–145. doi: 10.1016/j.autneu.2006.11.002. [DOI] [PubMed] [Google Scholar]
- [4].Bale TL, Vale WW. CRF and CRF receptor: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- [5].Barreau F, Cartier C, Leveque M, Ferrier L, Moriez R, Laroute V, Rosztoczy A, Fioramonti J, Bueno L. Pathways involved in gut mucosal barrier dysfunction induced in adult rats by maternal deprivation: corticotrophin-releasing factor and nerve growth factor interplay. J Physiol. 2007;580:347–356. doi: 10.1113/jphysiol.2006.120907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience. 1996;73:1029–1047. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- [7].Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- [8].Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chatzaki E, Crowe PD, Wang L, Million M, Taché Y, Grigoriadis D. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- [10].Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- [11].Fukudo S, Nomura T, Hongo M. Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Givalois L, Dornand J, Mekaouche M, Solier MD, Bristow AF, Ixart G, Siaud P, Assenmacher I, Barbanel G. Temporal cascade of plasma level surges in ACTH, corticosterone, and cytokines in endotoxin-challenged rats. Am J Physiol. 1994;267:R164–R170. doi: 10.1152/ajpregu.1994.267.1.R164. [DOI] [PubMed] [Google Scholar]
- [13].Hamann L, El Samalouti V, Ulmer AJ, Flad HD, Rietschel ET. Components of gut bacteria as immunomodulators. Int J Food Microbiol. 1998;41:141–154. doi: 10.1016/s0168-1605(98)00047-6. [DOI] [PubMed] [Google Scholar]
- [14].Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- [15].Hirata T, Keto Y, Nakata M, Takeuchi A, Funatsu T, Akuzawa S, Sasamata M, Miyata K. Effects of serotonin 5-HT(3) receptor antagonists on CRF-induced abnormal colonic water transport and defecation in rats. Eur J Pharmacol. 2008;587:281–284. doi: 10.1016/j.ejphar.2008.03.040. [DOI] [PubMed] [Google Scholar]
- [16].Imaki T, Nahan J-L, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kageyama K, Bradbury MJ, Zhao L, Blount AL, Vale WW. Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology. 1999;140:5651–5658. doi: 10.1210/endo.140.12.7223. [DOI] [PubMed] [Google Scholar]
- [18].Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–865. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- [19].Kawahito Y, Sano H, Mukai S, Asai K, Kimura S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Corticotropin releasing hormone in colonic mucosa in patients with ulcerative colitis. Gut. 1995;37:544–551. doi: 10.1136/gut.37.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kiank C, Taché Y, Larauche M. Stress-related modulation of inflammation in experimental models of bowel disease and post-infectious irritable bowel syndrome: Role of corticotropin-releasing factor receptors. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kidd M, Modlin IM, Eick GN, Champaneria MC. Isolation, functional characterization, and transcriptome of Mastomys ileal enterochromaffin cells. Am J Physiol Gastrointest Liver Physiol. 2006;291:G778–G791. doi: 10.1152/ajpgi.00552.2005. [DOI] [PubMed] [Google Scholar]
- [22].Kimura T, Amano T, Uehara H, Ariga H, Ishida T, Torii A, Tajiri H, Matsueda K, Yamato S. Urocortin I is present in the enteric nervous system and exerts an excitatory effect via cholinergic and serotonergic pathways in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2007;293:G903–G910. doi: 10.1152/ajpgi.00066.2007. [DOI] [PubMed] [Google Scholar]
- [23].Kresse AE, Million M, Saperas E, Taché Y. Colitis induces CRF expression in hypothalamic magnocellular neurons and blunts CRF gene response to stress in rats. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1203–G1213. doi: 10.1152/ajpgi.2001.281.5.G1203. [DOI] [PubMed] [Google Scholar]
- [24].la Fleur SE, Wick EC, Idumalla PS, Grady EF, Bhargava A. Role of peripheral corticotropin-releasing factor and urocortin II in intestinal inflammation and motility in terminal ileum. Proc Natl Acad Sci U S A. 2005;102:7647–7652. doi: 10.1073/pnas.0408531102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Larauche M, Gourcerol G, Wang L, Pambukchian K, Brunnhuber S, Adelson DW, Rivier J, Million M, Taché Y. Cortagine, a CRF1 agonist, induces stresslike alterations of colonic function and visceral hypersensitivity in rodents primarily through peripheral pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G215–G227. doi: 10.1152/ajpgi.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lembo T, Plourde V, Shui Z, Fullerton S, Mertz H, Taché Y, Sytnik B, Munakata J, Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- [27].Lin Z, Gao N, Hu HZ, Liu S, Gao C, Kim G, Ren J, Xia Y, Peck OC, Wood JD. Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea-pig small intestine. Neurogastroenterol Motil. 2002;14:197–204. doi: 10.1046/j.1365-2982.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- [28].Liu S, Gao N, Hu HZ, Wang X, Wang GD, Fang X, Gao X, Xia Y, Wood JD. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J Comp Neurol. 2006;494:63–74. doi: 10.1002/cne.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang GD, Xia Y, Wood JD. Expression of type 1 corticotropin-releasing factor receptor in the guinea pig enteric nervous system. J Comp Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- [30].Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- [31].Miampamba M, Yang H, Sharkey KA, Taché Y. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G979–G991. doi: 10.1152/ajpgi.2001.280.5.G979. [DOI] [PubMed] [Google Scholar]
- [32].Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- [33].Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induce defecation in rats. Am J Physiol. 1998;274:G827–G831. doi: 10.1152/ajpgi.1998.274.5.G827. [DOI] [PubMed] [Google Scholar]
- [34].Muramatsu Y, Fukushima K, Iino K, Totsune K, Takahashi K, Suzuki T, Hirasawa G, Takeyama J, Ito M, Nose M, Tashiro A, Hongo M, Oki Y, Nagura H, Sasano H. Urocortin and corticotropin-releasing factor receptor expression in the human colonic mucosa. Peptides. 2000;21:1799–1809. doi: 10.1016/s0196-9781(00)00335-1. [DOI] [PubMed] [Google Scholar]
- [35].Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1037–G1044. doi: 10.1152/ajpgi.00419.2006. [DOI] [PubMed] [Google Scholar]
- [36].Nozu T, Kudaira M. Corticotropin-releasing factor induces rectal hypersensitivity after repetitive painful rectal distention in healthy humans. J Gastroenterol. 2006;41:740–744. doi: 10.1007/s00535-006-1848-4. [DOI] [PubMed] [Google Scholar]
- [37].Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- [38].Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol. 1995;7:501–525. doi: 10.1111/j.1365-2826.1995.tb00788.x. [DOI] [PubMed] [Google Scholar]
- [39].Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Taché Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Mot. 2004;16(Suppl 1):1–6. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- [42].Tomita M, Ohkubo R, Hayashi M. Lipopolysaccharide transport system across colonic epithelial cells in normal and infective rat. Drug Metab Pharmacokinet. 2004;19:33–40. doi: 10.2133/dmpk.19.33. [DOI] [PubMed] [Google Scholar]
- [43].Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- [44].Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- [45].van Tol EA, Petrusz P, Lund PK, Yamauchi M, Sartor RB. Local production of corticotropin releasing hormone is increased in experimental intestinal inflammation in rats. Gut. 1996;39:385–392. doi: 10.1136/gut.39.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wallon C, Soderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann N Y Acad Sci. 2009;1165:206–210. doi: 10.1111/j.1749-6632.2009.04030.x. [DOI] [PubMed] [Google Scholar]
- [47].Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, Perdue MH, Soderholm JD. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57:50–58. doi: 10.1136/gut.2006.117549. [DOI] [PubMed] [Google Scholar]
- [48].Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- [49].Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St Pierre DH, Taché Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- [50].Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- [51].Wlk M, Wang CC, Venihaki M, Liu J, Zhao D, Anton PM, Mykoniatis A, Pan A, Zacks J, Karalis K, Pothoulakis C. Corticotropin-releasing hormone antagonists possess anti-inflammatory effects in the mouse ileum. Gastroenterology. 2002;123:505–515. doi: 10.1053/gast.2002.34783. [DOI] [PubMed] [Google Scholar]
- [52].Wolter HJ. Corticotropin-releasing factor is contained within perikarya and nerve fibres of rat duodenum. Biochem Biophys Res Commun. 1984;122:381–387. doi: 10.1016/0006-291x(84)90486-8. [DOI] [PubMed] [Google Scholar]
- [53].Wu SV, Yuan P-Q, Wang L, Peng YL, Chen C-Y, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology. 2007;148:1675–1687. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yu PL, Fujimura M, Okumiya K, Kinoshita M, Hasegawa H, Fujimiya M. Immunohistochemical localization of tryptophan hydroxylase in the human and rat gastrointestinal tracts. J Comp Neurol. 1999;411:654–665. doi: 10.1002/(sici)1096-9861(19990906)411:4<654::aid-cne9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [55].Yuan PQ, Million M, Wu SV, Rivier J, Taché Y. Peripheral corticotropin releasing factor (CRF) and a novel CRF1 receptor agonist, stressin1-A activate CRF1 receptor expressing cholinergic and nitrergic myenteric neurons selectively in the colon of conscious rats. Neurogastroenterol Motil. 2007;19:923–936. doi: 10.1111/j.1365-2982.2007.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]