Abstract
Introduction
Despite known health risks, nicotine use remains high, especially in populations diagnosed with mental illnesses, including anxiety disorders and Post-Traumatic Stress Disorder (PTSD). Smoking in these populations may relate to the effects of nicotine on emotional memories. The current study examined the effects of nicotine administration on the extinction of conditioned fear memories.
Methods
C57BL/6J mice were trained with 2 white noise conditioned stimulus (CS; 30 s, 85 dB)-foot shock (2 s, 0.57 mA) pairings. Extinction sessions consisted of 6 presentations of the CS (60 s) across multiple days. Mice were either tested in an AAA design, in which all stages occurred in the same context, or in an ABA design to identify if context changes alter extinction. Saline or nicotine was administered 5 minutes before training and/or extinction.
Results
In the AAA design, nicotine administration before training did not alter extinction. Nicotine administered prior to extinction sessions enhanced extinction and nicotine administered before training and extinction decreased extinction. In the ABA design, nicotine administered before extinction enhanced extinction and blocked context renewal of conditioned fear, while nicotine administered during training and extinction did not alter extinction but enhanced the context renewal of conditioned fear.
Conclusions
Nicotine has a differential effect on extinction of fear conditioning depending on when it is administered. Administration during extinction enhances extinction whereas administration during training and extinction may strengthen contextual fear memories and interfere with extinction.
Keywords: Addiction, Learning, Extinction, PTSD, Anxiety, Acetylcholine
Introduction
In response to recognized health risks associated with smoking, the number of current adult smokers in the United States has decreased steadily over the last decade (CDC, 2008), yet rates of smoking remain high in populations with psychiatric disorders, including the population suffering from post-traumatic stress disorder (PTSD) (Breslau et al. 2004b). The relationship between PTSD and smoking is bidirectional (Fu et al. 2007); initiation of smoking and increases in smoking rates both occur after the development of PTSD (Breslau et al. 2003; Breslau et al. 2004b), and nicotine dependence before trauma increases the likelihood that PTSD will develop (Koenen et al. 2005). In addition, patients with PTSD that were smokers prior to trauma reported greater symptoms than non-smoking patients (Beckham et al. 2008). Although correlational studies suggest a bidirectional relationship between nicotine use and PTSD, no study has directly compared the effects of nicotine use during a fear-evoking event to nicotine use after a fear-evoking event on maintenance of fear memories.
PTSD is a disorder of emotional dysregulation that is also characterized as a stress-induced fear circuitry disorder (Shin and Handwerger 2009), in which sufferers continue to re-experience trauma-related anxiety and distress after the cessation of the trauma itself (Hopper et al. 2007). In other words, this maladaptive response does not decrease or extinguish over time. Fear conditioning and fear extinction are well studied associative learning paradigms at both the behavioral and neural levels (Burgos-Robles et al. 2007; Maren and Quirk 2004; Mueller et al. 2008; Quirk et al. 2006) that have been used to study PTSD and anxiety-related disorders (for review, Amstadter et al. 2009; Foa et al. 1992; Quirk et al. 2007; Quirk and Mueller 2008; Rasmusson and Charney 1997). In fear conditioning, animals form an association between a previously-neutral conditioned stimulus (CS) and a fear-related unconditioned stimulus (US). Responding to the CS persists after the initial conditioning session (Fendt and Fanselow 1999; LeDoux 2000), just as anxiety or fear persists in PTSD (for review, Pitman 1997). Conversely, repeated presentations of the CS without the US extinguishes fear responses elicited by the CS (Brooks and Bouton 1993) and may represent a model for healthy emotional regulation because the fear response is attenuated in the continued absence of the fear-related stimuli. Therefore, deficits in extinction of fear responses may reflect abnormal emotional regulation similar to that seen with PTSD.
Extinction is influenced by many factors including contextual information. For example, contextual associations with the original conditioning event can delay extinction or reinstate responding in subjects that underwent extinction (Bouton et al. 2006; Ji and Maren 2007; Neumann and Longbottom 2008). Variables that strengthen contextual associations may disrupt extinction. Nicotine can enhance contextual learning when administered at training and testing (Gould 2003; Gould and Higgins 2003; Gould and Wehner 1999). This is in line with a large body of literature that suggests that nicotine has pro cognitive effects (Kenney and Gould 2008a; Levin et al. 2006). Therefore, nicotine could disrupt extinction through enhancing contextual associations. However, extinction involves new learning (Quirk et al. 2006); thus it is possible that nicotine could enhance extinction through direct effects on the neurobiological substrates of extinction independent of effects on contextual information.
The current experiments compared the effects of nicotine administration prior to training and extinction, training only, or extinction only to determine if different nicotine administration time points produce divergent effects on extinction. Furthermore, to determine whether nicotine modulates extinction by producing or altering contextual cues, we examined the effects of nicotine on extinction in an AAA design, in which training and all of the extinction sessions were conducted in the same context; and in an ABA renewal design, in which training was conducted in one context and then five extinction sessions were conducted in a novel context, with a final extinction session conducted in the training context to examine renewal of conditioned fear. It was hypothesized that nicotine administered at extinction only would enhance extinction but nicotine administration at both training and extinction would interfere with extinction by strengthening contextual fear associations.
Experimental Procedures
Subjects
Subjects consisted of male and female C57BL/6J mice 8–12 weeks of age. Mice were purchased from Jackson Laboratories (Bar Harbor, ME). All subjects were group-housed and provided ad libitum access to food and water. The colony room was maintained on a 12 h light/dark cycle (lights on at 07:00) and all behavioral and pharmacological procedures were performed between 08:00 and 18:00. All behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Apparatus
Extinction sessions occurred in two contexts, designated A and B, to examine whether the effects of nicotine administration on extinction are modulated by contextual cues. All mice were conditioned in one of four identical chambers (26.5 × 20.4 × 20.8 cm) housed in sound-attenuating boxes (Context A). The chamber walls and ceiling were composed of clear Plexiglas and the floor was a grid of metal bars (0.20 cm diameter) spaced 1.0 cm apart and connected to a shock generator and scrambler (Med Associates, St. Albans, VT, Model ENV-414). Each box had a speaker for CS presentation mounted directly above the Plexiglas chamber and a ventilation fan attached to the side of the box to provide background noise and air exchange. A 4-watt light bulb was mounted next to the speaker to illuminate each box. CS and US presentations were controlled by a PC running LabView software (National Instruments, Austin, TX).
Context B differed from the conditioning context in size, composition, and olfactory cues. Mice were tested in one of four identical chambers (20.3 × 22.9 × 17.8 cm) housed in sound-attenuating boxes (Med Associates, St Albans, VT). Each chamber consisted of stainless steel sides with a clear Plexiglas front, back, and top and an opaque white plastic floor. In the left wall of each chamber, a light and speaker were mounted for illumination and CS presentation, respectively. A ventilation fan was attached to the side of each box to provide background noise and air exchange. A paper towel with vanilla extract on it was placed under the floor of each conditioning chamber to provide a novel odor. A noise generator (Grason-Stadler, West Concord, MA, Model 901B) was used for CS presentation.
Behavioral Procedures
For all experiments, the behavioral index was freezing; defined as the absence of visible movement except for respiration. A time-sampling procedure was employed for measuring freezing. Every 10 s, each mouse was observed for 1 s and judged as either freezing or active. Observations were converted to percent time freezing and graphed as average percent time freezing.
Fear conditioning was based upon the paradigm described in Gould and Wehner (1999). During fear conditioning, mice were trained with two co-terminating CS (30 s, 85-dB white noise)-US (2 s, 0.57-mA foot shock) pairings separated by 120 s. After the second CS-US presentation, mice remained in the chamber for 30 s. The conditioning session lasted 5.5 min.
Approximately 24 hr after conditioning, mice began the extinction training. Each extinction session consisted of a 120 s pre-cue period followed by six CS (60 s, 85-dB white noise) presentations. The longer CS presentation during extinction (60 s) compared to training (30 s) was used to ensure the detection of potential differences in freezing levels between groups. The CS presentations were separated by five, 120 s inter-stimulus intervals (ISI), with a 60 s post-cue period following the final CS presentation. Freezing behavior was recorded throughout the entire 19 min session. Freezing behavior observed during CS presentations was categorized as ‘cued freezing’ while freezing behavior observed during the other periods was categorized as ‘non-cued freezing’.
Drugs and Administration
Nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline. In all groups, nicotine (0.09 mg/kg, reported as freebase weight) or saline was administered by intraperitoneal (ip) injection either one minute prior to a session or immediately after a session. Drug treatment was counterbalanced within cages and between run pairs. The 0.09 mg/kg dose of nicotine enhances contextual fear conditioning (for review, Gould 2006) and produces plasma nicotine levels comparable to the levels seen in smokers (Benowitz et al. 1989; Davis et al. 2005; Henningfield and Keenan 1993).
Experimental Conditions
Two main experimental conditions were used to examine the effects of nicotine administration on extinction: in the AAA design, mice were conditioned in Context A on day 0. For days 1–6, mice received extinction training in Context A. In the ABA design, mice were conditioned in Context A on day 0. For days 1–5, mice received extinction training in Context B. On day 6, mice were returned to Context A for a final extinction session to measure context-induced renewal of conditioned fear.
AAA Design
The effects of nicotine administration prior to extinction only in the AAA design were examined first. Mice received no injections prior to conditioning and either saline or nicotine injections prior to daily extinction sessions. Subjects in this experiment consisted of both male and female C57BL/6J mice. In all further experiments, subjects were male C57BL/6J mice, as no difference was found in cued extinction between sexes.
To determine whether the effect of nicotine on extinction was due to mice being in different drug states at training versus extinction (i.e., nicotine present at one phase but not the other), we administered nicotine at training only in the AAA design. Mice received saline or nicotine before training, and no injections before extinction sessions. If nicotine provides interoceptive contextual information, the absence of nicotine during extinction could possibly facilitate extinction because shifts in context between training and extinction sessions can facilitate extinction (Neumann and Longbottom 2008).
A study by Bouton, Kenney and Rosengard (1990) showed that rats that underwent extinction after administration of a benzodiazepine displayed drug-state-dependent renewal when tested in the original nondrug state. In order to examine whether nicotine produces a similar effect or if nicotine just decreases freezing but does not alter extinction, mice were conditioned on day 0 in Context A and then underwent three days of extinction in Context A. All mice received saline injections prior to conditioning. Subjects were randomly placed into one of three groups that differed in drug administration during extinction. One group received daily injections of saline for all three days of extinction (SSS), the second group received daily injections of nicotine (NNN), and the final group received nicotine injections for the first two days of extinction and a saline injection on day three (NNS).
In the final AAA design experiment, we investigated the effects of nicotine on extinction when nicotine was administered during both conditioning and extinction in the AAA design. Nicotine or saline was administered before each session.
ABA Design
The possibility that the nicotine enhancement of extinction is altered when the extinction context differs from the conditioning context was investigated by using an ABA design. If nicotine enhances extinction by altering contextual cues, then robust changes in contextual information between training and extinction may obscure the effects of nicotine on extinction. Saline or nicotine was injected before each extinction session. The final day of extinction occurred in the training context in order to examine context renewal of conditioned fear.
Finally, the effects of nicotine administered during conditioning as well as extinction in the ABA design were evaluated. Nicotine or saline was administered before the training session and before each extinction session and the last day of extinction occurred in the training context.
Statistical Analysis
Freezing rates across days were examined using repeated measures ANOVA. Cued and non-cued freezing rates were analyzed separately. Post-hoc comparisons were made using planned comparison t-tests at α = 0.05. All statistical analyses were carried out using SPSS 16.0.
Results
Effects of nicotine administration on AAA extinction when administered at extinction only
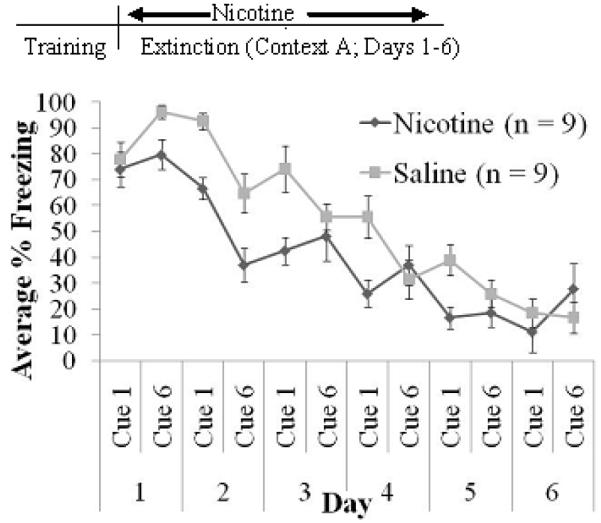
To examine the effects of nicotine administered at extinction only on extinction of cued and non-cued freezing, mice were conditioned on day 0, and then nicotine (n = 9) or saline (n = 9) was administered prior to each of six daily extinction sessions. There were significant effects of extinction day on cued freezing, F(5,80)=155.294 p<0.001, and on non-cued freezing, F(5,80)=10.229 p<0.001 (Figure 1A & B). For cued freezing, each day was significantly different from all other days (p<0.05) with the exception that day 5 and day 6 were not significantly different from each other. For non-cued freezing, the only significant differences were between day 1 and day 5 (p<0.05) and between day 1 and day 6 (p<0.05). A significant effect of drug was observed in cued freezing, F(1,14)=37.480 p<0.001, but not non-cued freezing. Additionally, a day by drug interaction was observed for cued freezing, F(3,49)=4.103 p<0.01. Post-hoc tests for cued freezing revealed significant differences between nicotine-treated and saline-treated animals on day 1, day 2, and day 3 of extinction (p<0.001). Thus, nicotine administration at extinction enhanced between-day extinction to the cue without affecting non-cued freezing.
Fig. 1.
Nicotine administration during extinction only in the AAA design. Nicotine enhanced between-day cued extinction (A) without affecting non-cued extinction (B). In addition, freezing at trial 1 of Day 1 was similar between groups, demonstrating that all subjects began extinction at the same freezing level (C) (mean ± S.E.M.; * = p < 0.05 compared to saline group).
Additionally, since the difference in cued freezing levels on day 1 may have been due to differences in levels of initial conditioning (i.e. nicotine-treated mice started extinction at a lower level of conditioning), we analyzed freezing levels during the first cue presentation on day 1 of extinction using an independent samples t-test (Figure 1C). No significant difference was observed, indicating that all subjects began extinction training at the same level of freezing.
In order to determine if nicotine was selectively enhancing either between-session extinction (i.e., decreased freezing between the last cue on day 1 and the first cue on day 2) or within-session extinction (i.e., decreased freezing between the first cue on day 1 and the last cue on day 1), we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. There was a significant effect of time, F(10,140)=3.70 p<0.01, but no effect of group; there was also a significant time by group interaction, F(10,140)=3.04 p<0.05. Planned comparison t-tests revealed that the nicotine group had a higher rate of extinction between day 3 and day 4 and again between day 4 and day 5 compared to saline controls (p<0.05), while saline controls had a higher rate of extinction within day 4 and within day 6 compared to the nicotine-treated group (p<0.05; Figure 2). Thus, nicotine administered at extinction enhanced between-session extinction but did not enhance within-session extinction.
Fig. 2.
Nicotine administration during extinction only in the AAA design. The nicotine-treated group showed greater extinction than the saline-treated group between Day 3 and Day 4 and between Day 4 and Day 5. Saline controls showed greater extinction than the nicotine-treated group within Day 4 and within Day 6 (mean± S.E.M.).
No significant effect of sex on cued freezing was observed; however, a significant effect of sex was observed for non-cued freezing, F(1,14)=5.05 p<0.05. No interactive effects were observed for sex with day or drug for either cued or non-cued freezing. For the effect of sex on non-cued freezing, females (nicotine n = 4, saline n = 5; mean percent ± S.E.M.; 19.25 ± 2.25) showed a significantly higher rate of freezing than males (nicotine n = 5, saline n = 4;13.06 ± 1.41). However, the main focus of this study was on the effects of nicotine on cued freezing; since there was no effect of sex on cued freezing, all subsequent experiments utilized male C57BL/6J mice as subjects.
In the previous experiments, nicotine was administered prior to each session. To test if nicotine administration after extinction altered the behavior, mice received injections of nicotine (n = 8) or saline (n = 8) immediately after each extinction session. There were significant effects of day on cued freezing, F(5, 70) = 48.382 p<0.001, and non-cued freezing, F(5, 70) = 3.289 p<0.01. However, no interactive effects or between-group differences were observed.
Effects of nicotine administration on AAA extinction when administered at conditioning only
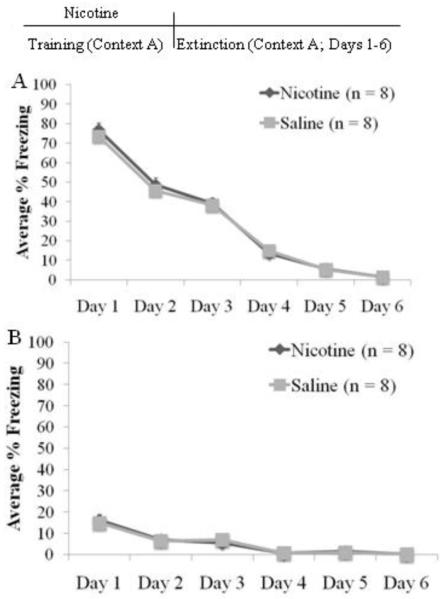
To determine whether the effects of nicotine on extinction were due to nicotine providing drug-state-dependent cues that provided different contextual information between training and extinction, we administered nicotine (n = 8) or saline (n = 8) before training but not before the extinction sessions in the AAA paradigm. Significant differences in freezing across extinction days were seen for cued freezing, F(5,75)=136.8 p<0.001, and non-cued freezing, F(5,75)=81.6 p<0.001, but there was neither an effect of drug nor a drug by day interaction for either cued or non-cued freezing (Figure 3). For cued freezing, freezing on day 1 was significantly higher than all other days (p<0.05), freezing on days 2 and 3 was similar but significantly higher than freezing on days 4–6 (p<0.05), and freezing on day 4 was similar to freezing on day 5 but significantly higher than freezing on day 6 (p<0.05). Finally, freezing on days 5 and 6 was similar. Thus, cued freezing decreased progressively across days with no differences between drug treatment groups, demonstrating that nicotine administered at training only does not alter subsequent extinction. For non-cued freezing, only freezing on day 1 was significantly higher than the other days (p<0.05).
Fig. 3.
Nicotine administration during training only in the AAA design. There was no effect of nicotine on cued (A) or on non-cued freezing (B) (mean ± S.E.M.).
In order to determine if nicotine was selectively altering either between-session extinction or within-session extinction, we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. There was a significant effect of time, F(10,140)=13.64 p<0.001, but no effect of group and no time by group interaction. Thus, nicotine administered at conditioning only failed to alter between-session extinction and within-session extinction.
Effects of a shift in nicotine administration on nicotine enhancement of extinction
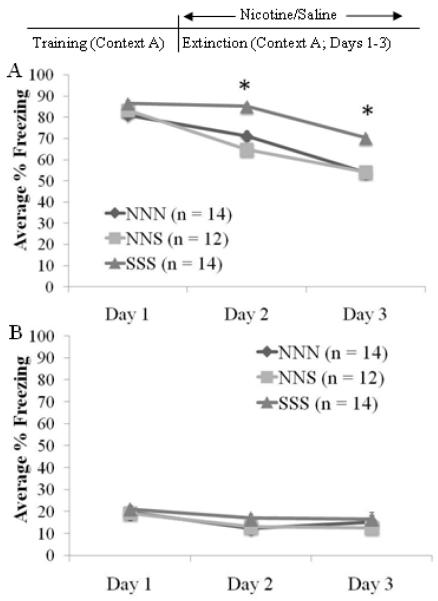
One possible explanation for the enhancement of extinction when nicotine was administered during extinction only is that nicotine decreased freezing. If this is the case, then removal of nicotine during extinction should produce a renewal of conditioned responses. If no renewal is seen, this would suggest that nicotine is facilitating extinction and not altering the expression of conditioned fear. To test this, mice received either nicotine prior to each of three extinction session (NNN; n = 14), saline prior to each extinction session (SSS; n = 14), or nicotine on days 1 and 2 of extinction and then saline on the final day (NNS; n = 12).
A significant effect of extinction day was observed for both cued freezing, F(2,74)=191.305 p<0.001, and non-cued freezing, F(2,74)=9.474 p<0.005 (Figure 4). A significant effect of drug group was observed for cued, F(2,37)=25.082 p<0.001, but not non-cued freezing. In addition, a day by group interaction was observed for cued, F(4,72)=9.432 p<0.001, but not non-cued freezing. For cued freezing, post-hoc tests indicated that the SSS group was significantly different from both the NNN and the NNS groups on days 2 and 3 (p<0.001), while the NNN and NNS groups were not significantly different from each other at any point. For non-cued freezing, post-hoc tests revealed that freezing on day 1 was significantly higher than freezing on day 3 (p<0.05) but there were no other differences. These results suggest that nicotine is enhancing extinction and not altering expression of conditioned fear as the NNN and NNS groups displayed enhanced extinction and the NNS group did not display increased conditioned responding in the absence of nicotine.
Fig. 4.
A shift in nicotine administration during three days of extinction in the AAA design does not reinstate conditioned responding. Mice received either saline on all three days of extinction (SSS), nicotine on all three days (NNN), or nicotine on the first two days and saline on the third (NNS). The NNN and NNS groups displayed enhanced extinction but there was no renewal of conditioned fear in the NNS group on day 3 (A). There were no drug effects in non-cued extinction (B) (mean ± S.E.M.; * = p < 0.05 compared to saline group).
In order to determine if nicotine was selectively enhancing either between-session extinction or within-session extinction, we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. There was a significant effect of time, F(4,148)=49.36 p<0.001, and an effect of group, F(2,37)=15.51 p<0.001; there was also a significant time by group interaction, F(8,144)=4.19 p<0.001. Planned comparison t-tests revealed that both the NNN and NNS groups had a higher rate of extinction between day 1 and day 2 compared to saline controls (p<0.05), while the saline group and the NNN group had a higher rate of extinction within day 3 compared to the NNS group (p<0.05), and the NNN group had a higher rate of extinction between day 2 and day 3 compared to the NNS group. Thus, nicotine again enhanced between-session extinction.
Effects of nicotine administration on AAA extinction when administered throughout conditioning and extinction
Research has shown that when nicotine is administered at both training and testing, contextual fear conditioning is enhanced (Gould and Higgins 2003; Gould and Wehner 1999). In this experiment, we tested whether nicotine effects on contextual learning would alter extinction. Nicotine (n = 10) or saline (n = 9) was administration during conditioning and extinction in an AAA extinction design. As before, there was a significant effect of extinction day on cued, F(5,85)=46.204 p<0.001, as well as non-cued, F(5,85)=6.373 p<0.005, freezing (Figure 5). Post-hoc tests demonstrated for cued freezing that days 1–3 were not significantly different but that all three days were significantly different from days 4–6 (p<0.05); days 4–6 were not significantly different from each other. For non-cued freezing, the only significant difference was between day 1 and day 6 (p<0.05). A significant effect of drug was observed for non-cued, F(1,17)=10.995 p<0.005, freezing but not for cued freezing; however, a day by drug interaction was observed for cued, F(5,85)=7.643 p<0.001, but not non-cued freezing. Post-hoc tests for cued freezing indicated no significant difference between the nicotine- and saline-treated groups on days 1–5 but a significant difference on day 6 (p<0.001), nicotine-treated mice showed less extinction. These results show that when nicotine is administered prior to conditioning as well as extinction it delays cued extinction instead of enhancing it. Additionally, post-hoc tests for non-cued freezing demonstrated differences between groups on days 1 and 5 (p<0.05); when nicotine was administered prior to conditioning as well as extinction, the amount of non-cued freezing increased. These results suggest that nicotine effects on contextual learning could delay extinction, however, the delay might be due to prior exposure to nicotine rather than nicotine enhancing contextual associations. To test this possibility, nicotine (n = 8) or saline (n = 8) was administered immediately after training and before each extinction session. A significant effect of day was observed for cued freezing, F(5, 70) = 72.455 p<0.001, and non-cued freezing, F(5, 70) = 6.659 p<0.001, but no interactive effects or between-group differences were observed. Therefore, nicotine must be administered prior to training and each extinction session to delay cued extinction.
Fig. 5.
Nicotine administration during training and extinction in the AAA design. Nicotine prior to conditioning as well as extinction delayed cued extinction (A) and increased non-cued freezing (B) (mean ± S.E.M.; * = p < 0.05 compared to saline group).
In order to determine if nicotine was selectively altering either between-session extinction or within-session extinction, we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. There was a significant effect of time, F(10,120)=3.24 p<0.01, and an effect of group, F(1,12)=8.60, p<0.05, but no time by group interaction. Planned comparison t-tests revealed that the nicotine group had a higher rate of extinction between day 4 and day 5 compared to the saline group (p<0.05), while saline controls had a higher rate of extinction within day 5 compared to the nicotine-treated group (p<0.05). Thus, nicotine administered throughout conditioning and extinction enhanced between-session extinction, even though overall extinction was delayed in this group, but did not enhance within-session extinction in the AAA design.
Effects of nicotine administration on ABA extinction
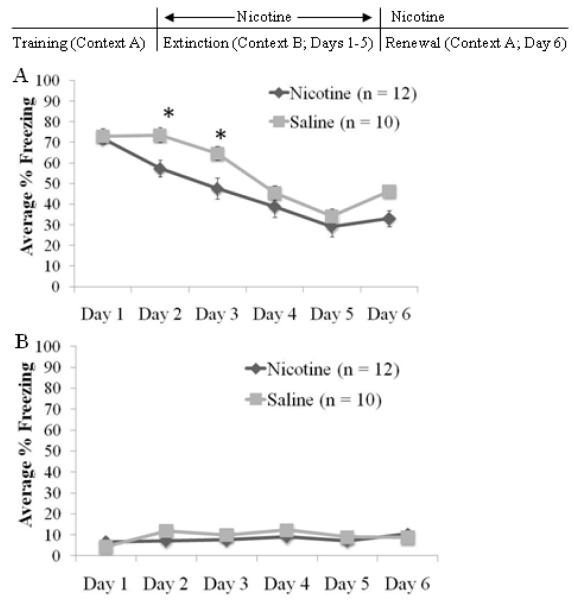
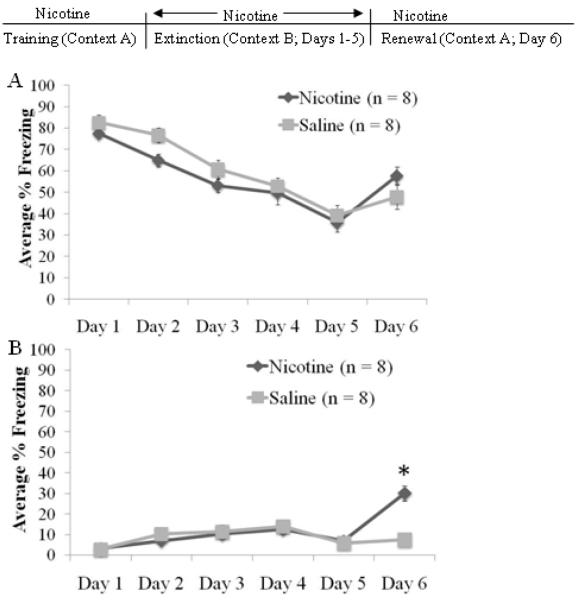
To test if the enhancement of extinction by nicotine would remain when the extinction context differed from the conditioning context, we utilized an ABA extinction design in which nicotine (n = 12) or saline (n = 10) was administered throughout extinction.
There was a significant effect of extinction day for cued, F(5,100)=57.954 p<0.001, but not non-cued freezing levels (Figure 6). Post-hoc tests demonstrated decreases in cued freezing between day 1 and days 3–5 (p<0.05), between day 2 and days 4–6 (p<0.05), and between day 3 and days 4–5 (p<0.05). There was no significant difference between day 5 and day 6. A significant drug effect was observed for cued, F(1,20)=5.303 p<0.05, but not non-cued freezing. There was also a day by drug interaction for cued freezing, F(5,100)=2.35 p<0.05. The nicotine-treated animals froze significantly less than saline controls on days 2 and 3 (p<0.05) but there were no other significant differences. These results indicate that the enhancement of extinction by nicotine remains when extinction occurs in an altered context. If context renewal occurred, then day 6 should have significantly more freezing than day 5. There was a significant difference in freezing between days 5 and 6 in the saline group (p<0.01) but not in the nicotine group. These results suggest that when nicotine is administered during extinction only and continued through renewal, nicotine enhances extinction independent of the context and also attenuates the context renewal of conditioned fear.
Fig. 6.
Nicotine administered during extinction in the ABA design. Nicotine administered during extinction in an altered context enhanced between-day cued extinction and blocked context-dependent renewal of conditioned fear on Day 6 (A) without altering non-cued extinction (B) (mean ± S.E.M.; * = p < 0.05 compared to saline group).
In order to determine if nicotine was selectively enhancing either between-session extinction or within-session extinction, we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. To avoid the confound of renewal on day 6, we examined only days 1–5. There was no significant effect of time or of group; there was a significant time by group interaction, F(8,136)=3.04 p<0.05. Planned comparison t-tests revealed that the nicotine group had a higher rate of extinction between day 1 and day 2 compared to saline controls (p<0.05), while saline controls had a higher rate of extinction within day 5 compared to the nicotine-treated group (p<0.05). Thus, nicotine administered at extinction enhanced between-session extinction in both the AAA and ABA design.
Effects of nicotine administration on ABA extinction and renewal when administered throughout conditioning and extinction
When nicotine was administered at training and extinction in the AAA design, extinction was delayed. We wanted to test if the same result would occur when the context was changed at extinction. Nicotine (n = 8) or saline (n = 8) was administered prior to conditioning as well as extinction in the ABA extinction paradigm. Analysis of days 1–6 showed a significant effect of day for both cued, F(5,70)=66.918 p<0.001, and non-cued, F(5,70)=10.887 p<0.001, freezing (Figure 7). For cued freezing, days 1–2 were significantly different from days 3–6 (p<0.05), and days 3–4 and 6 were significantly different from day 5 (p<0.05). Day 6 (when the animals were tested in the original training context) was significantly higher than day 5 (p<0.05), demonstrating renewal of conditioned fear. For non-cued freezing, the only significant differences were between day 1 and day 4 (p<0.05) and between day 6 and days 1–2 (p<0.05). Neither a significant drug effect nor a drug by day interaction was observed for cued freezing but there was a significant drug by day interactive effect for non-cued freezing, F(5,70)=24.82 p<0.001. The saline group demonstrated significantly lower non-cued freezing than the nicotine group on day 6 (p<0.05). These results indicate that when nicotine is administered prior to both conditioning and extinction in an altered context, neither the enhancing nor the inhibiting effect of nicotine is observed on cued freezing. In addition, post-hoc tests demonstrated that nicotine-treated animals froze significantly more during the non-cued period on day 6 (renewal) compared to day 5 (p<0.001). Because mice were tested in the training context on day 6 (context A), this increase in freezing may be due to stronger contextual memories of the training context in the nicotine-treated group.
Fig. 7.
Nicotine administered during training and extinction in the ABA design. Nicotine prior to both conditioning and extinction did not alter extinction of cued (A) or non-cued freezing (B). Nicotine prior to both conditioning and extinction enhanced context renewal of conditioned fear (mean ± S.E.M.; * = p < 0.05 compared to saline group).
In order to determine if nicotine was selectively altering either between-session extinction or within-session extinction, we analyzed the differences between groups using group and time (difference scores between cues) as independent variables in a repeated measures ANOVA. There was no significant effect of time or group, and no time by group interaction. Thus, nicotine administered throughout conditioning and extinction failed to alter between-session extinction and within-session extinction.
Discussion
The current study demonstrated that the effects of nicotine on the extinction of fear conditioning depend upon when nicotine was administered and on the context during extinction. Nicotine enhanced extinction when administered only at extinction and this appeared to be a long-lasting change as the enhanced extinction remained after nicotine administration ceased. Nicotine delayed extinction, however, when administered at both training and extinction. This delay of extinction was dependent on the consistency of contextual information between training and extinction. Nicotine administered at both training and extinction delayed extinction if the context was the same at both stages; however, if the context changed between training and extinction, no effect of nicotine was seen. In addition to altering extinction, nicotine altered context renewal of conditioned responding during extinction. Nicotine blocked renewal when administered at extinction only but facilitated renewal when administered at both training and extinction. These findings are in contrast to the effects of other drugs, such as the partial NMDA receptor agonist d-cycloserine and the α2 noradrenergic receptor antagonist yohimbine, that enhance extinction but fail to alter renewal of conditioned fear (Morris and Bouton 2007; Woods and Bouton 2006); but this is not surprising because different classes of drugs act on different neural systems.
The ability of nicotine to enhance extinction in both the AAA design and the ABA design suggests that nicotine may facilitate extinction of fear memories when administered during the extinction period, regardless of changes in context. Alternative interpretations include that nicotine is altering freezing or that nicotine is providing an interoceptive contextual cue that facilitates extinction. This second alternative interpretation is based on the possibility that contextual shifts between training and extinction could facilitate extinction (Neumann and Longbottom 2008). Findings from the current study and past studies, however, argue against these alternative interpretations. First, if nicotine is decreasing freezing but not actually altering extinction, removal of nicotine should increase freezing; this was not seen (Figure 4a). This supports previous studies demonstrating that nicotine administered at recall (i.e., testing) only has no effect on contextual or cued fear conditioning (Gould and Higgins 2003; Gould and Wehner 1999). Second, if nicotine administration is facilitating extinction through interoceptive cues that provide a shift in contextual information between training and extinction, then administration of nicotine at training only should also facilitate extinction; this was not seen. Third, if nicotine administered during extinction provides interoceptive contextual cues that facilitate extinction, then removal of those cues, i.e. cessation of nicotine administration, should result in a renewal of conditioned responding; this was not seen. Thus, nicotine may enhance the learning that underlies extinction of conditioned fear.
One important question is what aspect of extinction learning is nicotine enhancing. Nicotine enhance extinction within sessions or enhance the consolidation of extinction between sessions. To analyze this, we examined within-session effects between nicotine and saline treated mice by comparing changes between trial 1 and trial 6 for each session and between-session effects by comparing changes between trial 6 of the previous session with trial 1 of the next session. Nicotine produced a greater change between sessions, suggesting that nicotine enhances consolidation of the extinction learning. Interestingly, we found that nicotine administered after an extinction session did not enhance extinction. Whereas this result could argue against the conclusion that nicotine is enhancing consolidation, prior results from our laboratory support a role for nicotine in enhancing consolidation. Previous research found that nicotine administered at training but not at consolidation enhanced contextual fear conditioning (Gould and Higgins 2003). These effects likely occur through an interaction with NMDA receptor-mediated calcium signaling (Gould and Lewis 2005). We recently found, however, that these effects of nicotine administration at training result in changes in cell-signaling processes involved in consolidation of the nicotine-enhanced memory (Kenney et al. 2009). Nicotine and training interacted to increase expression of the JNK1 gene during consolidation and inhibition of the JNK1 protein during consolidation, but not during acquisition or retrieval, blocked the nicotine enhancement of learning. While the cell-signaling cascades involved in the effects of nicotine on extinction are currently unknown, it is possible that a similar effect is occurring such that nicotine administration before each extinction session leads to a change in cell signaling that results in enhanced consolidation.
In contrast to the effects of nicotine when administered at extinction only, the effects of nicotine on extinction when administered at training and extinction appear to involve contextual processes. When nicotine was administered at both training and extinction in the same context, extinction of responding to the auditory cue was decreased, that is fear responding persisted. In addition, responding to contextual information during the non cued period of extinction was elevated in the group that received nicotine throughout training and extinction. This suggests that administration of nicotine during training and extinction increased the strength of contextual fear associations, which supports other studies showing that nicotine enhances contextual conditioning when administered at training and recall (Gould 2003; Gould and Higgins 2003; Gould and Wehner 1999; Kenney and Gould 2008b). It is unlikely this effect was the result of a generalized increase in freezing due to a nonspecific increase in anxiety because numerous studies have shown that nicotine increases context-specific freezing but not freezing in an alternate context (Davis and Gould 2006; 2007; Davis et al. 2007; Davis et al. 2006; Gould and Higgins 2003; Gulick and Gould 2008; Kenney and Gould 2008b).
Further support for the contention that nicotine administration during training and extinction strengthens contextual fear-related memories comes from the ABA study where nicotine was administered throughout all phases of conditioning and extinction. Nicotine did not alter extinction when administered throughout the study as long as extinction was assessed in the altered context. When mice were returned to the original context, however, nicotine-treated mice showed a large renewal effect during the non-cued period, which reflected context-evoked freezing. This suggests that nicotine use at the time of a traumatizing event may sensitize individuals to the development of context-based fear memories, and continued use of nicotine after the event may facilitate the persistence of these memories.
Based on the present results, we propose that nicotine directly enhances extinction but when nicotine is administered at both training and extinction, nicotine strengthens contextual associations formed during training. These enhanced contextual associations can interfere with extinction when extinction occurs in the same context as used for training. One other study has examined the role of nicotine in the extinction of fear conditioning. Tian and colleagues (2008) found that previous exposure to chronic nicotine (two weeks prior to training and extinction) impaired fear extinction. While it is difficult to exactly match patterns of smoking with nicotine administration in animals, these findings support previous studies demonstrating correlations between nicotine dependence prior to trauma and increased likelihood of the development of PTSD (Koenen et al. 2005), and between nicotine use after trauma and decreased symptoms of PTSD (Breslau et al. 2003; Breslau et al. 2004a). That is, prior nicotine use may strengthen the formation of maladaptive fear associations that could contribute to PTSD but initiation of nicotine use after PTSD may facilitate adaptive learning strategies such as extinction of fear responses. Together, these findings suggest that tobacco use may be an important factor to consider when treating PTSD.
An open-ended question for future studies is what brain regions nicotine acts to alter extinction. Nicotine administered at both fear conditioning and extinction may delay extinction by enhancing contextual fear conditioning. Previous work has shown that nicotine enhances contextual fear conditioning through direct effects in the hippocampus (Davis et al. 2007). Whether nicotine acts in the hippocampus to alter extinction is unknown. It is possible that the enhancement of extinction when administered at extinction only and the disruption of extinction when administered before conditioning and extinction in the same context involve different brain regions as recent work has shown that the effects of nicotine on contextual fear conditioning and on ethanol-induced deficits in fear conditioning involve different brain regions with the latter involving the cingulate cortex (Gulick and Gould 2009). Thus, the ability of nicotine to enhance extinction may involve an area other than the hippocampus. One area that may warrant investigation for involvement in the effects of nicotine on extinction is the medial prefrontal cortex as this area was been shown to be critically involved in extinction of conditioned fear and is altered in patients with PTSD (Quirk et al. 2006).
Acknowledgements
The authors would like to thank David J. Bucci, Ph.D., Dartmouth University; Brian P. Marx, Ph.D., National Center for Posttraumatic Stress Disorder; and Joseph R. Troisi II, Ph.D., Saint Anselm College for valuable comments on a draft of this manuscript. This work was supported by National Institute for Drug Abuse (NIDA) Grant DA017949 (T.J.G.). DSW was supported by a NIDA diversity supplement (DA024787-01A1S1). All research complies with current US laws for the care and use of laboratory animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear Conditioning as a Model for Future Research. Psychiatr Ann. 2009;39:358–367. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Wiley MT, Miller SC, Dennis MF, Wilson SM, McClernon FJ, Calhoun PS. Ad lib smoking in post-traumatic stress disorder: an electronic diary study. Nicotine Tob Res. 2008;10:1149–57. doi: 10.1080/14622200802123302. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., 3rd Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–87. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Kenney FA, Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behav Neurosci. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–94. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Daily smoking and the subsequent onset of psychiatric disorders. Psychol Med. 2004a;34:323–33. doi: 10.1017/s0033291703008869. [DOI] [PubMed] [Google Scholar]
- Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004b;55:69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Process. 1993;19:77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- CDC 2008 http://www.cdc.gov/nchs/data/nhis/earlyrelease/200803_08.pdf.
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–52. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–52. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–7. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–5. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–60. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: an animal model. Psychol Bull. 1992;112:218–38. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Fu SS, McFall M, Saxon AJ, Beckham JC, Carmody TP, Baker DG, Joseph AM. Post-traumatic stress disorder and smoking: a systematic review. Nicotine Tob Res. 2007;9:1071–84. doi: 10.1080/14622200701488418. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine produces a within-subject enhancement of contextual fear conditioning in C57BL/6 mice independent of sex. Integr Physiol Behav Sci. 2003;38:124–32. doi: 10.1007/BF02688830. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–57. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lewis MC. Coantagonism of glutamate receptors and nicotinic acetylcholinergic receptors disrupts fear conditioning and latent inhibition of fear conditioning. Learn Mem. 2005;12:389–98. doi: 10.1101/lm.89105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–9. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008;196:483–95. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits through different effects at nicotinic receptors. Neuropsychopharmacology. 2009;34:2167–79. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–50. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–25. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Florian C, Portugal GS, Abel T, Gould TJ. Involvement of Hippocampal Jun-N Terminal Kinase Pathway in the Enhancement of Learning and Memory by Nicotine. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.153. Epub Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008a;38:101–21. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behav Neurosci. 2008b;122:1158–65. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005;62:1258–65. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–52. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci. 2007;121:501–14. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–75. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DL, Longbottom PL. The renewal of extinguished conditioned fear with fear-relevant and fear-irrelevant stimuli by a context change after extinction. Behav Res Ther. 2008;46:188–206. doi: 10.1016/j.brat.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Overview of biological themes in PTSD. Ann N Y Acad Sci. 1997;821:1–9. doi: 10.1111/j.1749-6632.1997.tb48264.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–43. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Martinez KG, Nazario Rodriguez LL. Translating findings from basic fear research to clinical psychiatry in Puerto Rico. P R Health Sci J. 2007;26:321–8. [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson AM, Charney DS. Animal models of relevance to PTSD. Ann N Y Acad Sci. 1997;821:332–51. doi: 10.1111/j.1749-6632.1997.tb48290.x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009 doi: 10.1002/jts.20442. Epub Sep 9. [DOI] [PubMed] [Google Scholar]
- Tian S, Gao J, Han L, Fu J, Li C, Li Z. Prior chronic nicotine impairs cued fear extinction but enhances contextual fear conditioning in rats. Neuroscience. 2008;153:935–43. doi: 10.1016/j.neuroscience.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–62. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]