Abstract
The observed high incidence of smoking amongst depressed individuals has led to the hypothesis of ‘self medication” with nicotine in some of these patients. The inbred Wistar-Kyoto (WKY) rats exhibit depressive-like characteristics as evidenced by exaggerated immobility in the forced swim test (FST). One aim of this study was to investigate whether nicotine may have an antidepressant-like effect in these animals. Moreover, because of human postmortem studies indicating a reduction of the hippocampus volume in depressed patients, it was of interest to determine whether such an anatomical anomaly may also be manifested in WKY rats and whether it would be affected by chronic nicotine treatment. Adult female WKY and their control Wistar rats were administered nicotine consecutively (0.2 mg/kg, ip, once or twice daily for 14 days) and their activity in an open field, as well as their immobility in FST were assessed either 15 min or 18 hr after the last injection. Another set of animals was treated twice daily with 0.2 mg/kg nicotine for 14 days and sacrificed on day 15 for stereological evaluation of the hippocampal volume. When tested 15 min after the last injection, once or twice daily nicotine exacerbated the immobility in the FST in WKY rats only. When tested 18 hr after the last injection, only twice daily nicotine treatment resulted in less immobility in the FST in WKY rats. Open field locomotor activity was not affected by any nicotine regimen. WKY rats had significantly less hippocampal volume (approximately 20%) than Wistar rats which was not altered by nicotine. These findings further validate the use of WKY rats as an animal model of human depression and signify the importance of inherent genetic differences in final behavioral outcome of nicotine.
Keywords: WKY rats, Depression, Hippocampus, Nicotine, Forced Swim Test, Stereology
INTRODUCTION
It is well documented that the incidence of smoking is much higher in various neuropsychiatric disorders including depression compared to general population (see reviews: Glassman, 1993; Quattrocki et al., 2000). Although common genetic risk factors may contribute to the co-morbid condition of nicotine dependence and depression (Kendler et al., 1993; Fu et al., 2007; Lyons et al., 2008), a number of preclinical as well as controlled human studies have reported an antidepressant-like effect of nicotine (Semba et al., 1998, Djuric et al., 1999; Tizabi et al., 1999, 2000; Ferguson et al., 2000; McClernon et al., 2006). Moreover, depressed patients are less likely to quit smoking compared to non-depressed individuals (Hughes, 2007; Leventhal et al., 2008; Thorndike et al., 2008). Thus, it has been postulated that at least a subpopulation of depressed patients may be smoking to self medicate with tobacco’s nicotine (Cook et al., 2007; Moreno-Coutino et al., 2007; Spring et al., 2008). Although it might be difficult to conceptualize a true animal model of human depression, various rat models with construct and predictive validity have been introduced. Previously, it was reported that Flinders Sensitive Line (FSL) rats exhibit depressive-like characteristics compared to their control the Flinders Resistant Line (FRL) rats. FSL rats are so designated because of their sensitivity to the toxic effects of the organophosphate cholinesterase inhibitors. However, it was later discovered that these inbred rats show remarkable immobility in the forced swim test (FST), reflective of their helplessness and hence depressive-like characteristic (see review: Overstreet et al., 2005). Furthermore, injection or oral administration of nicotine resulted in an antidepressant-like effect in this model (Djuric et al., 1999; Tizabi et al., 1999, 2000).
Wistar Kyoto (WKY) rats represent another putative animal model of depression as these genetically inbred rats also exhibit exaggerated immobility in the FST and are prone to develop stress-induced ulcer or anxiety-like characteristics (Soderpalm, 1989; Paré and Redei, 1993; Pini et al., 1997; Getachew et al., 2008). Moreover, WKY rats have sleep pattern disturbances similar to that observed in depressed individuals (Paré and Redei, 1993). However, unlike FSL rats, WKY rats do not respond to clinically effective selective serotonin uptake inhibitors (SSRIs) whereas both lines respond to tricyclic antidepressants (Griebel et al., 1994; Lahmame et al., 1997; Lopez-Rubalcaava and Lucki, 2000; Tejani-Butt et al., 2003; Getachew et al., 2008). Hence, WKY rats may represent a class of treatment resistant depressive individuals with unique underlying biological bases. One aim of this study was to investigate whether nicotine may also act as an antidepressant in this model.
Although the etiologies of neuropsychiatric and neurodegenerative disorders may be distinct, recent studies suggest that some neuropsychiatric disorders could also involve impairments of structural and functional plasticity in discrete brain regions albeit to a lesser extent than in neurodegenerative disorders (Manji et al., 2003; Fossati et al., 2004). Noteworthy in this regard is the finding of hippocampal volume reduction in depressed humans and volume recovery after antidepressant treatment (Sheline et al., 2003; Czeh and Lucassen, 2007). Thus, another aim of this study was to investigate whether WKY rats show a hippocampal volume reduction, and whether chronic nicotine treatment would affect the hippocampal volume in these rats. Overall our hypotheses were: 1. nicotine would exert an antidepressant-like effect in WKY rats and that this effect would be observable at least a day after the last chronic injection; 2. WKY rats would exhibit a reduction in hippocampal volume and that this reduction would be normalized by chronic nicotine administration.
MATERIALS AND METHODS
Animals
Age matched adult female WKY and Wistar rats (Harlan Laboratories, Indianapolis, IN) were used throughout the study. We selected female rats because of higher prevalence of depression in women, although there have been fewer studies in female than in male rodents (Herzog et al., 2009). Moreover, parallel to what is seen in human population (Kessler et al., 1993; Meagher and Murray, 1997), the female WKY rats show a higher level of depressive-like behavior compared to the male rats of the same strain (Paré and Redei, 1993). Animals were housed in groups of four in standard polypropylene shoebox cages (42 × 20.5 × 20 cm) on hardwood chip bedding (alpha-dry) in a room designated for female rats. Animals had access to food (Harlan Tek Lab) and water ad libitum. The room was maintained at 24–26°C at 51 – 66% relative humidity, on a 12-h reversed light/dark cycle (lights on at 19.00 hr). The reversal of time cycle was to allow convenient measurement of the behavior in active (dark) phase of the light cycle. All experiments were carried out in accordance with NIH guidelines as approved by the Institutional Animal Care and Use Committee.
To acclimate the subjects to housing conditions, animals arrived one week prior to testing. During this period, they were gentled once daily in order to minimize any stress that might result from routine handling. Behaviors were evaluated in the early part of the dark phase between 09:00 A.M. and 12:00 P.M using a red light as source of illumination. Different groups of rats were used for various chronic studies. A total of 160 rats (80 Wistar and 80 WKY) were used. Each experiment consisted of 4 groups (8 rats/group).
Nicotine bitartrate, purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) was dissolved in saline and injected intraperitoneally (i.p.) in a volume of 1ml/kg. The reported dose is indicative of pure nicotine base.
Drug Treatment
Initially, a pilot study was undertaken to determine the effects of two acute doses of nicotine (0.1 and 0.2 mg/kg, ip) on locomotor activity and forced swim test. These doses were chosen based on our previous studies with other rat models of depression (Tizabi et al., 1999; Tyler et al., 2000,) with the intention of determining the minimal effective dose of nicotine on a behavioral parameter in WKY rats.
One set of adult female WKY and Wistar rats was treated with nicotine consecutively (0.2 mg/kg, ip, once or twice daily for 14 days) and their locomotor activity in an open field followed by their immobility in the forced swim test was assessed 15 min after the last injection. These two doses of nicotine (0.2 and 0.4 mg/kg/day) were chosen based on reports in the literature and our own previous studies with Flinders Sensitive (FSL) and Fawn-Hooded rats (Matta et al., 2007; Tizabi et al., 1999, 2000, 2009). For once daily treatment the injections were carried out at 10.00 hr and for twice daily treatments the injections were carried out at 10.00 and 16.00 hr.
Another set of animals was treated exactly as above; however, the behavioral tests were carried out 18 hr after the last injection when no blood nicotine would be detectable. A third set of animals was treated twice daily with 0.2 mg/kg nicotine, however, 18 hour after the last injection these animals were perfused as described below for stereological evaluation of the hippocampal volume. Controls in all cases received saline.
These experimental protocols are summarized in the chart below.
Acute pilot studies
Nicotine 0.1 and 0.2 mg/kg
Chronic studies
Nicotine 0.2 mg/kg once daily for 14 days, behavioral tests 15 min after the last injection. Nicotine 0.2 mg/kg once daily for 14 days, behavioral tests 18 hr after the last injection. Nicotine 0.2 mg/kg twice daily for 14 days, behavioral tests 15 min after the last injection. Nicotine 0.2 mg/kg twice daily for 14 days, behavioral tests 18 hr after the last injection. Nicotine 0.2 mg/kg twice daily for 14 days, animals were sacrificed18 hr after the last injection for histological studies.
Behavioral Testing
The locomotor activity test was always carried out first for 10 min and was followed immediately by the forced swim test for 5 min. The tests were performed alternately on each rat from a different strain and a different treatment group. For all behavioral tests the animals were moved from the housing units to the testing room in their home cage at least one hour before the test to acclimate them to the environment.
Locomotor Activity (LCA) Monitoring
Locomotor activity was measured for each animal during a 10 minute period immediately preceding the swim test. An open-field activity monitoring cage (27 × 27 × 20.3 cm, Med Associates, Inc., St. Albans, VT) was used to assess activity. Ambulatory counts representing the number of infrared beam interruptions were recorded.
Forced Swim Test (FST)
The method of Porsolt et al. (1977) with modification by Detke et al. (1995) was used to assess the immobility of the rats as a measure of their helplessness or depressive-like behavior. Rats were placed individually in a round Pyrex cylinder pool measuring 18 cm in diameter and 60 cm in height for 5 min. The cylinder was filled with 30 cm water (25±1 °C) to ensure that animals could not touch the bottom of the container with their hind paws or their tails (Lucki 1997). The animal’s FST activity was video recorded for subsequent analysis. Fresh water was used for each FST in every animal.
A time sampling scoring technique was used whereby the predominant behavior in each 5-s period of the 300-s test was recorded (Detke et al., 1995, Getachew et al., 2007; Tizabi et al., 2009). Inactivity (immobility) and swimming were distinguished as mutually exclusive behavioral states. Swimming behavior was defined as movement throughout the cylinder. Immobility was defined when no additional activity was observed other than that required to keep the rat's head above the water.
It is of relevance to note that most behavioral tests assessing the antidepressant effects of drugs use the model where the rats are exposed to 15 min pretest 18–24 hr before the actual test (Porsolt et al., 1977, Overstreet et al., 2005). However, the WKY rats as well as other genetically selected models of depression (e.g. FSL and Fawn-Hooded rats) have the distinct advantage of exhibiting the depressive-like characteristic without the need for the pretest (Overstreet et al., 2005; Tizabi et al., 1999, 2000, 2009). Thus, these models along with their appropriate controls are used to evaluate the neurobiological substrates of depression or antidepressant effects of various drugs including nicotine (Tejani-Butt et al., 2003; Getachew et al., 2008; Tizabi et al., 2009).
Volume Determination
A. Histology
Rats were deeply anesthetized with sodium pentobarbital (100 mg/kg) and transcardially perfused with approximately 200 ml phosphate-buffered saline (PBS) to flush out the blood, followed by the fixation solution (4% paraformaldehyde in 0.1 M PBS, pH 7.4). Each animal received approximately 150 ml of the fixaton solution via manual injection into the right atrium and through the aorta. The descending aorta was clamped to optimize fixation of the brain. The brains were post-fixed in the 4% paraformaldehyde fixative overnight and were then transferred to a 30% sucrose phosphate buffer solution until they sank at which point were frozen in CO2/isopentane and stored at −80°C until sectioning. Each brain was serially sectioned in the coronal plane on a sliding freezing microtome. Sections were cut at an instrument setting of 50 µm ansd sampled in a systematic-random manner, i.e., with random start in the first ten sections, then systematic for every tenth section. Thus, the first section was chosen randomly from the first 10 sections followed by collection of every tenth section such that a total of 8–12 sections per brain were obtained. This approach ensures that all parts of the hippocampus or the sampling region have an equal chance of being sampled. At the microscopic level, this systematic random sampling is achieved using a motorized stage that steps through the hippocampus on each section in a systematic-random manner. For estimation of the hippocampal volume, which included neuronal and molecular layers of dentate gyrus (DG) and CA1-4 regions, sampling was carried out through the entire left dorsal and ventral hippocampus. A similar approach was applied to sampling every tenth section of the total sections through complete left hemisphere for estimation of total hemispheric volume. Sampled sections were stained using routine cresyl violet for Nissl substance and cover-slipped for microscopic visualization.
B. Stereology
Using computer-assisted stereology, volumes for tissue sections were estimated using the Cavalieri principle with point counting (Gunderson and Jensen, 1987; Mouton et al., 2002; O'Neil et al., 2007), as detailed previously for brain and hippocampus volumes in rodent brains (Manaye et al., 2007).
Briefly, the Cavalieri's (Gunderson et al., 1999) principle was applied to analyze the left hemispheric and hippocampal volumes. Every 10th section was selected through the entire structure (total of 10–12 section per region per brain). The reference space on each section was outlined under low power magnification (4x, NA=1.0) using a Nikon 800 Microscope with stereoinvestigator software by Microbrightfield Inc (MicroBrightField, Williston, VT). The relevant equation for volume estimation was:
where, Vref = reference volume (hippocampal formation or hemishphere, in mm3)
ΣAreaslices = area on slice or section, in mm2
Average thicknessslices= mean slice thickness, mm.
In order to capture the majority of variability within- and between-rats for each group, and achieve normalization, data for each brain was collected at a high level of stringency, i.e., the coefficient of error (CE<.0.1) was less than one-half of the biological variability (Gundersen et al., 1999).
Statistical Analysis
All behavioral and stereological data were analyzed using two-way analysis of variance (ANOVA), followed by Tukey’s post hoc test when significant main effects were indicated. All analyses were two-tailed and P<0.05 was considered significant.
RESULTS
In the pilot studies it was revealed that fifteen min after the higher nicotine dose (0.2 mg/kg) but not the lower dose (0.1 mg/kg) there was an increase in immobility in the forced swim test (FST) in WKY rats only. Neither dose had any effect on locomotor activity (LCA) in either strain. On the basis of these findings, the chronic studies were conducted with 0.2 mg/kg nicotine administered once or twice daily.
As expected, WKY rats consistently showed a decrease in open field LCA, F(1, 28) = 16.9, p < 0.01 and exaggerated immobility in the FST, F(1, 28) = 12.8, p < 0.01 compared to Wistar rats (Figs 1). This trend was evident in all the studies with similar statistical significance (Fig 2–Fig 4).
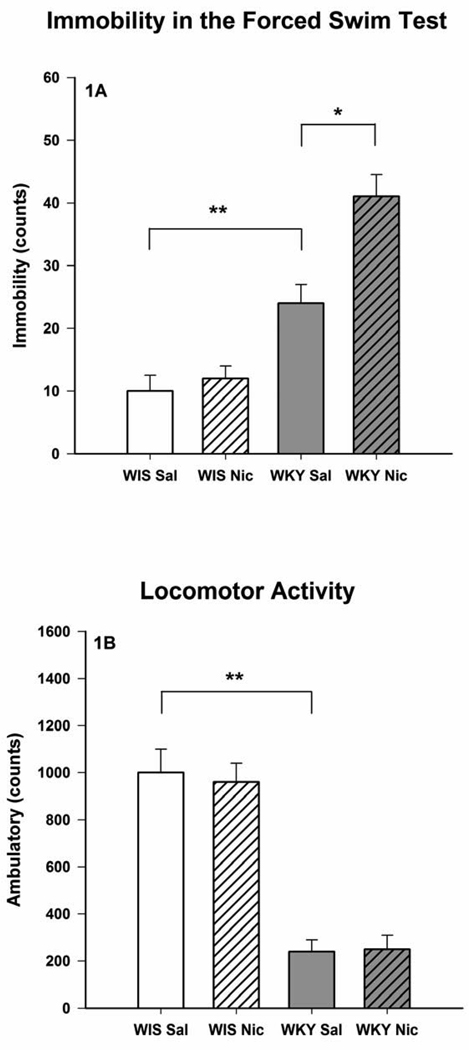
Fig. 1.
Effects of chronic nicotine treatment on the forced swim test (1A) and open field locomotor activity (1B) of female Wistar and WKY rats. The animals were treated once daily with 0.2 mg/kg nicotine for 14 consecutive days and were evaluated for LCA and FST, 15 min after the last injection. Values are mean ± SEM. N=8/group, *p<0.05, **p<0.01
Fig. 2.
Effects of chronic nicotine treatment on the forced swim test (2A) and open field locomotor activity (2B) of female Wistar and WKY rats. The animals were treated once daily with 0.2 mg/kg nicotine for 14 consecutive days and were evaluated for LCA and FST, 18 h after the last injection. Values are mean ± SEM. N=8/group, **p<0.01
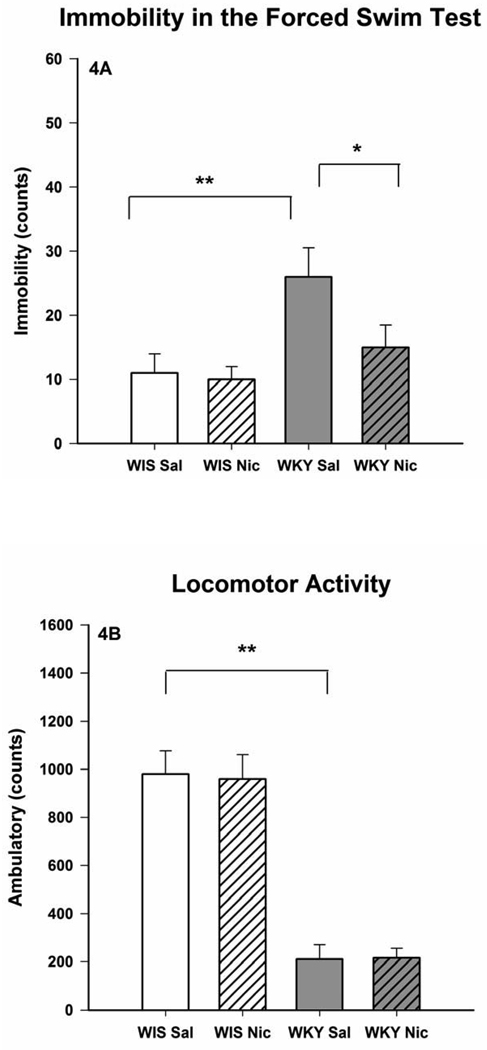
Fig. 4.
Effects of chronic nicotine treatment on the forced swim test (4A) and open field locomotor activity (4B) of female Wistar and WKY rats. The animals were treated twice daily with 0.2 mg/kg nicotine for 14 consecutive days and were evaluated for LCA and FST, 18 h after the last injection. Values are mean ± SEM. N=8/group, *p<0.05, **p<0.01
Administration of nicotine (0.2 mg/kg) once daily for 14 days resulted in an increase in immobility in the FST in WKY rats only when tested 15 min after the last injection (Fig 1A), but did not affect LCA (Fig 1B). Consistent with the selective effect of nicotine on FST in WKY rats, there was a significant interaction effect [F(1,28) = 11.56, p < 0.01]. However, no effect of nicotine on FST or LCA was detected when these behaviors were evaluated 18 hr after the last injection (Figs 2A and 2B).
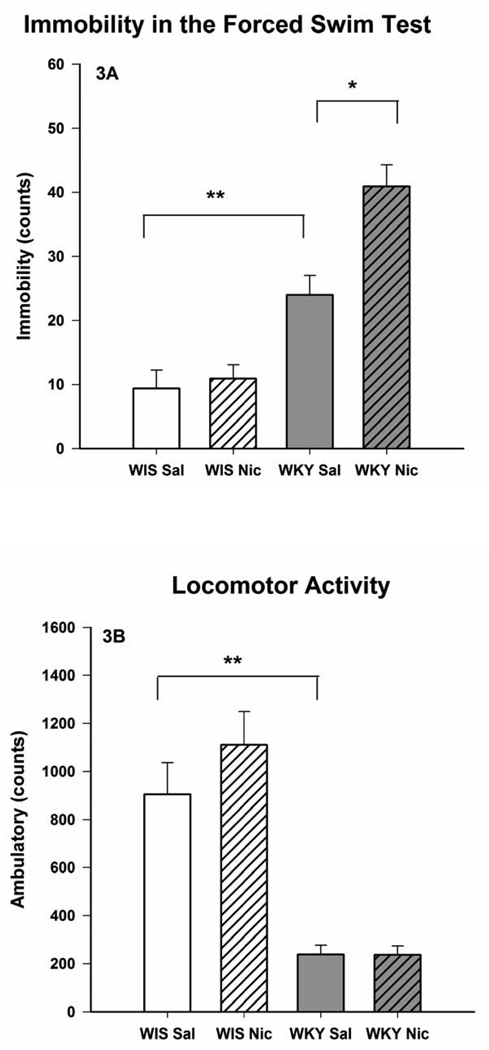
Administration of nicotine (0.2 mg/kg) twice daily for 14 days also resulted in an increase in immobility in the FST, F(1, 28) = 6.5, p < 0.05 in WKY rats only (Fig 3A) without any effect on LCA (Fig 3B) when tested 15 min after the last injection. However, when the behavioral tests were carried out 18 hr after the last injection a significant reduction in the FST immobility was observed in WKY rats only, F(1, 28) = 5.9, p < 0.05 (Fig 4A). This selective effect in WKY rats was reflected in significant interactions [F(1, 28) = 6.0, p < 0.05 for test after 15 min, and F(1, 28) = 6.9, p < 0.05 for test after 18 hr]. Again, open field LCA was not affected by nicotine in either strain (Fig 4B).
Fig. 3.
Effects of chronic nicotine treatment on the forced swim test (3A) and open field locomotor activity (3B) of female Wistar and WKY rats. The animals were treated twice daily with 0.2 mg/kg nicotine for 14 consecutive days and were evaluated for LCA and FST, 15 min after the last injection. Values are mean ± SEM. N=8/group, *p<0.05, **p<0.01
Interestingly, the effects of nicotine on FST in WKY rats when tested 15 min after the last injection were very similar to what had been observed in the pilot study with acute (0.2 mg/kg) nicotine. Moreover, nicotine-withdrawn WKY rats for 18 hrs had very similar scores to saline-treated Wistar rats. Wistar rats were not affected by nicotine withdrawal in this test (Fig 1–Fig 4). The effects of 18 hr nicotine withdrawal (0.2 mg/kg twice daily) on FST immobility in WKY rats had disappeared when the animals were tested a week later (data not shown).
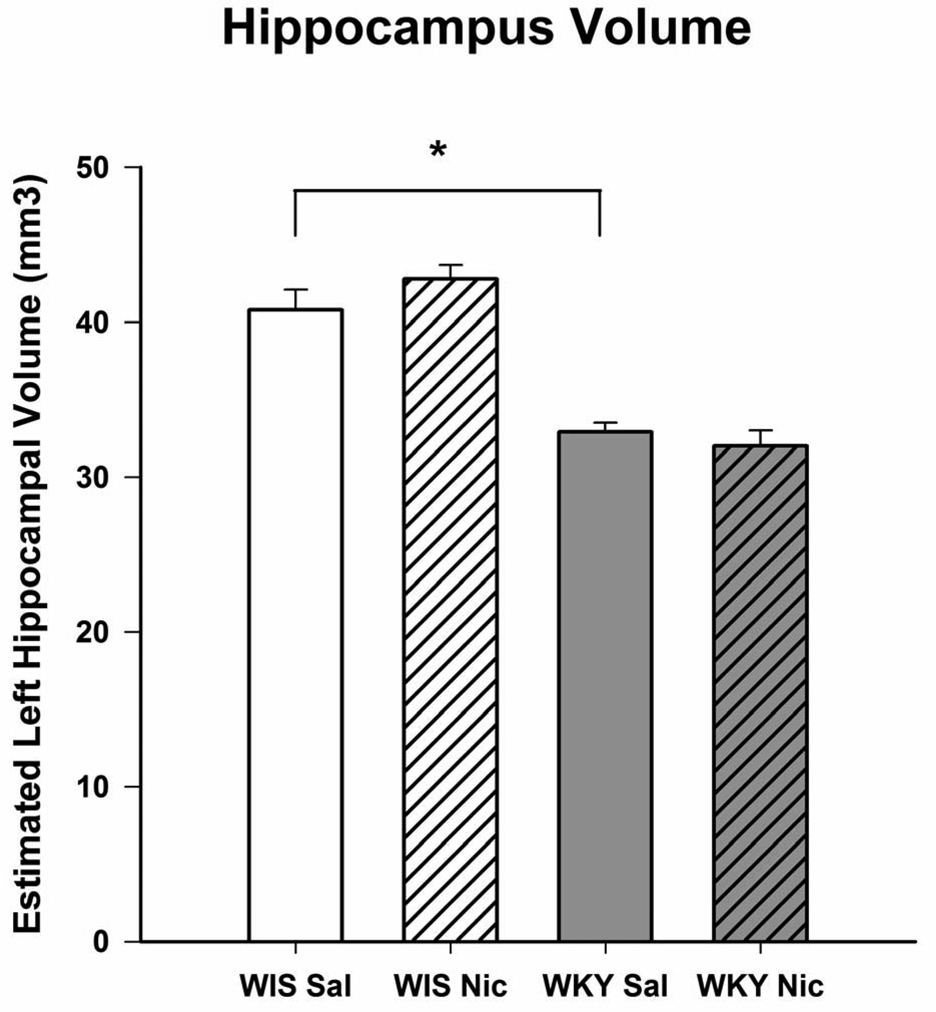
Fig 5 depicts the hippocampal volume and the effects of chronic nicotine treatment. There was a significant reduction (approximately 20%) in the hippocampal volume of WKY rats compared to Wistar rats, F(1, 28) = 6.2, p < 0.05. The total hemispheric volume of WKY rats (622 ± 14.0 mm3) was very close to that of Wistar rats (640 ± 18.4 mm3), suggesting that the observed reduction was specific to the hippocampus, and not due to a decrease in total brain volume. There was no detectable effect of nicotine on hippocampal volume. The measurement of hippocampal volume was carried out only following nicotine withdrawal because this was the instance when an antidepressant effect of nicotine in WKY was observed.
Fig. 5.
The total left hippocampal volume in Wistar and WKY rats following 14 consecutive days of daily treatment with nicotine or saline. Rats were injected twice daily with 0.2 mg/kg nicotine and were perfused for histological studies 18 h after the last injection. N=8/group, *p<0.05
A repeat of the hippocampal volume determination in a set of naïve female Wistar and WKY rats yielded almost an identical result to that observed in saline-treated Wistar and WKY rats (data not shown).
DISCUSSION
The results of this study indicate that WKY rats, a putative animal model of depression may show further exacerbation of their depressive-like characteristic or an antidepressant-like response to nicotine depending on temporal evaluation of the behavior post nicotine administration. Thus, an exacerbation of the depressive-like behavior may be obtained when nicotine is present in plasma or an antidepressant-like effect may be manifested during drug withdrawal. These findings are in contrast to previous reports where an antidepressant-like effect of nicotine in other animal models of depression (e.g., FSL and Fawn-Hooded rats) was observed regardless of such temporal consideration (Djuric et al., 1999; Tizabi et al., 1999, 2000, 2009). These differences are unlikely to be due to gender effect or estrous cycle variations as studies with female Fawn-Hooded rats using a similar housing condition or female FSL rats did not show any “depressogenic” effect of nicotine (Djuric et al., 1999; Tizabi et al., 2009).
Although the majority of human studies indicate an antidepressant-like effect of nicotine and exacerbation of the depressive symptoms following smoking cessation, WKY rats may represent a unique population where withdrawal and not the presence of nicotine may result in antidepressant-like effect. These individuals would be unlikely to develop addiction to nicotine or if they did, an antidepressant-like effect may be reported upon nicotine withdrawal. Curiously, at least one recent human study indicates alleviation of the depressive-like symptom upon smoking cessation in smokers with threshold or subthreshold depressive disorders (Blalock et al., 2008). Moreover, there are reports of antidepressant effects of nicotinic antagonists but not agonists in mice (Rabenstein et al., 2006; Andreasen et al., 2009a). Indeed, a “depressogenic” effect of nicotine in some mouse strains has recently been reported (Hayase, 2007, 2008; Andreasen and Redrobe, 2009b). Thus, depending on the species, strain, gender and the behavioral test, differential outcome of nicotine effect may be observed (Tizabi et al., 1999, 2000, 2009; Andreasen and Redrobe, 2009b).
It is known that nicotinic receptor may be desensitized upon chronic nicotine administration and can recover following termination of exposure (Buccafusco et al., 2008; Yu et al., 2009). It is also possible that some of the nicotinic receptor subtypes may become supersensitive following withdrawal from nicotine. Such super-sensitivity can render the receptors more responsive to the endogenous ligand acetylcholine (Buisson and Bertrand, 2002) which in case of WKY strain may lead to normalization of neurotransmitters or circuitries involved in mood regulation and hence result in an antidepressant-like effect. Alternatively, a delicate balance between receptor subtype activation and desensitization in discrete brain circuitries in WKY rats may be responsible for the observed effects (Picciotto et al., 2008). Interestingly, WKY rats which appear to experience a depressogenic effect of nicotine upon its administration and antidepressant-like effect upon its withdrawal, do exhibit significant reduction in nicotine self-administration (De La Garza II, 2005), which may be related to a deficit in nicotine reward in these animals (Rauhut et al., 2008).
The effects of nicotine in the FST appear to be independent of its effects on general locomotor activity as this parameter was not consistently affected by nicotine treatment. However, the very low baseline locomotor activity (LCA) in WKY rats might be responsible for the high degree of immobility of these rats in the FST. Moreover, LCA in WKY rats might have been at a floor level where further decrease in this parameter could not be detected despite an increase in FST immobility. It is also of relevance to note that the effect of nicotine on open field locomotor activity is quite varied and depends on a number of factors including dose of nicotine, the duration of the treatment, the testing paradigm, strain and sex (Stolerman, 1990; DiFranza and Wellman, 2007; Andreasen and Redrobe, 2009b). That nicotine did not have any behavioral effects in Wistar rats is most likely due to genetic differences between this strain and WKY rats. However, at this point contribution of differential nicotine pharmacokinetics in these two strains cannot be ruled out as pharmacokinetic differences in nicotine between various rat strains have been observed (Sziraki et al., 2001). Moreover, it would be difficult to observe any antidepressant-like effect of nicotine in Wistar rats using the forced swim test without the pretest due to their low level of immobility. However, no “depressogenic” effect of nicotine in Wistar rats was observed either. This together with our previous findings where nicotine did not affect the FST in control strains for FSL or Fawn-Hooded rats (Tizabi et al., 1999, 2009), strengthen the contention that inherent genetic differences are critical determinants of final behavioral outcome of nicotine.
The differential responses of various individuals or various rat strains to nicotine may be reflective of inherent differences in nicotinic receptor distribution and/or differential responses of these receptors to nicotine (see reviews Fowler et al., 2008; Tizabi, 2007). Furthermore, nicotinic receptor stimulation may result in release of a variety of neurotransmitters including biogenic amines as well as glutamate (Wonnacott, 1997). As these neurotransmitters are considered to be major players in mood regulation (see reviews: Charney, 1998; Maeng and Zarate, 2007; Mathew et al., 2008; McNally et al., 2008; Nutt, 2008), differential release of such neurotransmitters by nicotine in different population may contribute to the diverse behavioral outcome of nicotine. In addition, differential distribution of brain derived neurotrophic factor (BDNF) and nicotinic interaction with this system can play a role in observed behavioral differences as a role for BDNF in mood regulation and interaction of nicotine with this system has been documented (Rot et al., 2009; Son and Winzer-Serhan, 2009).
The data also provides further justification for use of WKY rats as a suitable animal model of depression as these rats similar to depressed humans show a reduction of hippocampal volume compared to Wistar rats. However, unlike the reported study in humans where some antidepressants may normalize the volume reduction of the depressed patients (Sheline et al., 2003; Czeh and Lucassen, 2007) we did not observe any effect of nicotine on hippocampal volume in this study. Although a neuroprotective effect of nicotine in various in-vitro and in-vivo studies have been reported (Tizabi et al., 2003, 2004, 2005; Copeland et al., 2005, 2007; Toborek et al., 2006, Picciotto and Zoli, 2008; Quik et al., 2008; Das and Tizabi, 2009), the lack of nicotine effect on hippocampal volume in WKY rats might not be so surprising given that the WKY rats actually show an exacerbation of their depressive-like behavior when tested 15 min after nicotine administration. Hence, it would be unlikely that volume recovery would occur during the short (18 hr) withdrawal from nicotine. Alternatively, it might be suggested that hippocampal volume recovery is not a requisite for antidepressant effectiveness. Recent findings indicate that not only depression subtype, but also the chronicity of depression might be important factors associated with loss of hippocampal or amygdalar volumes in depression (Keller et al., 2008). Thus, it would be of significant interest to evaluate the hippocampal and amygdalar volume in various animal models of depression at basal as well as following long term nicotine or other antidepressant treatments.
CONCLUSION
In summary, nicotine may exacerbate or alleviate the depressive-like characteristics of WKY rats depending on temporal evaluation of the behavior post nicotine administration. Moreover, loss of hippocampal volume in this strain further validates their use as an animal model of human depression. The findings also signify the importance of inherent genetic differences in final behavioral outcome of nicotine.
Acknowledgment
Supported by NIH/NIGMS (2 SO6 GM08016-39)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 2009a;23:797–804. doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests depend on strain and test, but not sex. Behav Pharm. 2009b;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- Blalock JA, Robinson JD, Wetter DW, Schreindorfer LS, Cinciripini PM. Nicotine withdrawal in smokers with current depressive disorders undergoing intensive smoking cessation treatment. Psychol Addict Behav. 2008;22:122–128. doi: 10.1037/0893-164X.22.1.122. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exp Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–136. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;14:11–14. [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson's disease. Neurotox Res. 2005;8:289–293. doi: 10.1007/BF03033982. [DOI] [PubMed] [Google Scholar]
- Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis glial changes and apoptosis implicated? Psychiatry Clin Neurosci. 2007;257:250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotoxicity Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- De La Garza R., II Wistar Kyoto rats exhibit reduced sucrose pellet reinforcement behavior and intravenous nicotine self-administration. Pharmacol Biochem Behav. 2005;82:330–337. doi: 10.1016/j.pbb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effects of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology. 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- Fossati P, Radtchenko A, Boyer P. Neuroplasticity: from MRI to depressive symptoms. Eur Neuropsychopharm. 2004;14:503–510. doi: 10.1016/j.euroneuro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Lyons MJ, Tsuang MT, True WR, Eisen SA. Common genetic risk of major depression and nicotine dependence: the contribution of antisocial traits in a United States veteran male twin cohort. Twin Res Hum Genet. 2007;10:470–478. doi: 10.1375/twin.10.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008;91:97–103. doi: 10.1016/j.pbb.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. Am J Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT uptake inhibitors differentially modulates emotional responses in anxiety models in rodents. Psychopharmacology. 1994;113:463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Gunderson HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hayase T. Chronologically overlapping occurrences of nicotine-induced anxiety-and depression-related behavioral symptoms: effects of anxiolytic and cannabinoid drugs. BMC Neurosci. 2007;8:76–85. doi: 10.1186/1471-2202-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T. Nicotine (NC)-induced "depressive" behavioral symptoms and effects of antidepressants including cannabinoids. J Toxicol Sci. 2008;33:555–564. doi: 10.2131/jts.33.555. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Corbach BCS, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, Fuchs E. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: etiology, animal models, epidemiology, and significance: a subjective review. Nicotine Tob Res. 2007;9:329–339. doi: 10.1080/14622200701188927. [DOI] [PubMed] [Google Scholar]
- Keller J, Shen L, Gomez RG, Garrett A, Solvason HB, Reiss A, Schatzberg AF. Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165:872–880. doi: 10.1176/appi.ajp.2008.07081257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neal MC, MacLean CL, Heath AC, Eaves LJ, Kessler RC. Smoking and major depressions: a causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J.Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Lahmame A, del Arco C, Pazos A, Yritia M, Amario A. Are Wistar-Kyoto rats a genetic model of depression resistant to antidepressants? Eur J Pharmacol. 1997;337:115–123. doi: 10.1016/s0014-2999(97)01276-4. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcaava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharm. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, Grant MD, Rende R, Eisen S, Eaves L, Tsuang MT. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine Tob Res. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr. Psychiatry. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Paul WC, O'Neil JN, Huang SY, Tao X, De-Liang L, Tizabi Y, Ottinger MA, Ingram DK, Mouton PR. Neuropathological quantification of dtg APP/PS1: neuroimaging, stereology, and biochemistry. Age. 2007;29:87–96. doi: 10.1007/s11357-007-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff KA, Gray N, Zarate CA, Charney DS. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiat. 2003;15:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Manji HK, Charney DS. Novel drugs and therapeutic targets for severe mood disorders. Neuropsychopharm. 2008;33:2080–2092. doi: 10.1038/sj.npp.1301652. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology. 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- Meagher D, Murray D. Depression. Lancet. 1997;349 Suppl 1:sI17–sI20. doi: 10.1016/s0140-6736(97)90006-4. [DOI] [PubMed] [Google Scholar]
- Moreno-Coutiño A, Calderón-Ezquerro C, Drucker-Colín R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res. 2007;9:389–396. doi: 10.1080/14622200701188901. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain Res. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. 2008;69:4–7. [PubMed] [Google Scholar]
- O'Neil JN, Mouton PR, Tizabi Y, Ottinger MA, Lei DL, Ingram DK, Manaye KF. Catecholaminergic neuronal loss in locus coeruleus of aged female dtg APP/PS1 mice. J Chem Neuroanat. 2007;34:102–107. doi: 10.1016/j.jchemneu.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Friedman E, Mathe AA, Yadid G. The Flinders Sensitive Line rat: a selectively bred putative animal model of depression. Neurosci Biobehav. 2005;29:739–759. doi: 10.1016/j.neubiorev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Paré WP, Redei E. Sex differences and stress response of WKY rats. Physiol Behav. 1993;54:1179–1185. doi: 10.1016/0031-9384(93)90345-g. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le PM, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Quattrocki E, Baird A, Yurgelun-Todd D. Biological aspects of the link between smoking and depression. Harv Rev Psychiatry. 2000;8:99–110. [PubMed] [Google Scholar]
- Quik M, O'Leary K, Tanner CM. Nicotine and Parkinson's disease: implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- aan het Rot M, Mathew SJ MD, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba J, Mataki C, Yamada S, Nankai M, Toru M. Antidepressant-like effects of chronic nicotine on learned helplessness paradigms in rats. Biol Psychiatry. 1998;43:389–391. doi: 10.1016/s0006-3223(97)00477-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiat. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Soderpalm B. The SHR exhibits less "anxiety" but increased sensitivity to the anticonflict effect of clonidine compared to normotensive controls. Pharmacol Toxicol. 1989;65:381–386. doi: 10.1111/j.1600-0773.1989.tb01193.x. [DOI] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases mRNA expression of neurotrophic factors in the postnatal rat hippocampus. Brain Res. 2009;179:159–165. doi: 10.1016/j.brainres.2009.04.046. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, Vanderveen J, Hedeker D. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology. 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Behavioural pharmacology of nicotine: implications for multiple brain nicotinic receptors. Ciba Found Symp. 1990;152:3–16. doi: 10.1002/9780470513965.ch2. discussion 16–22. [DOI] [PubMed] [Google Scholar]
- Sziraki, Lipovac MN, Hashim A, Sershen H, Allen D, Cooper T, Czobor P, Lajtha A. Differences in nicotine-induced dopamine release and nicotine pharmacokinetics between Lewis and Fischer 344 rats. Neurochem Res. 2001;26:609–617. doi: 10.1023/a:1010979018217. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Paré WP. Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharm Biol Psychiat. 2003;27:7–14. doi: 10.1016/s0278-5846(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres-Finnerty N, Rigotti NA. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Arch Intern Med. 2008;168:186–191. doi: 10.1001/archinternmed.2007.60. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Getachew B, Rezvani AH, Hauser SR, Overstreet DH. Antidepressant-like effects of nicotine and reduced nicotinic receptor binding in the Fawn-hooded rat, an animal model of co-morbid depression and alcoholism. Prog Neuropsychopharm Biol Psychiat. 2009;33:398–402. doi: 10.1016/j.pnpbp.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y. Nicotine and nicotinic system in hypoglutamatergic models of schizophrenia. Neurotox Res. 2007;12:233–246. doi: 10.1007/BF03033907. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res. 2005;7:319–322. doi: 10.1007/BF03033888. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Smoot DT, Taylor RE. Nicotine inhibits ethanol-induced toxicity in cultured cortical cells. Neurotox Res. 2004;6:311–316. doi: 10.1007/BF03033441. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res. 2003;5:409–415. doi: 10.1007/BF03033151. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: Involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E, Janowsky DS, Kling MA. Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology. 1999;142:193–199. doi: 10.1007/s002130050879. [DOI] [PubMed] [Google Scholar]
- Toborek M, Son KW, Pudelko A, King-Pospisil K, Wylegala E, Malecki A. ERK 1/2 signaling pathway is involved in nicotine-mediated neuroprotection in spinal cord neurons. J Cell Biochem. 2007;100:279, 292. doi: 10.1002/jcb.21013. [DOI] [PubMed] [Google Scholar]
- Tyler KY, Edwards E, Copeland RL, Jr, Tizabi Y. Effects of nicotine in the congenital learned helplessness model of depression. Soc Neuroscience Abst. 2000;26(1):1004. [Google Scholar]
- Yu KD, Liu Q, Wu J, Lukas RJ. Kinetics of desensitization and recovery from desensitization for human alpha4beta2-nicotinic acetylcholine receptors stably expressed in SH-EP1 cells. Acta Pharmacol Sin. 2009;30:805–817. doi: 10.1038/aps.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]