Abstract
Oplopanax horridus or devil’s club is a herbal medicine distributed in North America. The constituents and pharmacological activities of O. horridus (OPH) are largely unknown. In this study, we assayed OPH stem and berry extracts using high performance liquid chromatography (HPLC). The anticancer potentials of extracts on different human cancer cell lines (SW-480, HCT-116, HT-29, MCF-7 and NSCLC) were determined by MTS method. The effect of stem extract on cancer cell cycle, expression of cyclin A, and apoptosis were assayed using flow cytometry. HPLC data showed that the composition of OPH stem extract is more complicated than the berry extract. The wavelength of maximum absorption of the major constituent in stem and berry is 196.0 nm and 201.9 nm, respectively. Compared to the berry extract, the stem extract showed significant potent antiproliferative effect on all the studied cell lines. The stem extract at 0.1 mg/ml arrested cancer cells in S- and G2/M-phases, and significantly induced expression of cyclin A. After treatment with 0.1 mg/ml of stem extract for 72 h, apoptotic cells were increased to 45.2%, while control was 9.6%. The cell cycle arrest and induction of apoptosis may play a critical role in cancer chemoprevention by Oplopanax horridus stem extract.
Keywords: Oplopanax horridus, devil’s club, HPLC analysis, cancer chemoprevention, cell cycle, apoptosis
1. Introduction
Colorectal cancer accounts for 10% of the overall cancer cases in the U.S. [1]. In late stage, treatment of this cancer generally employs chemotherapy using cytotoxic drugs and radiation therapy. Because this strategy is only moderately successful, novel approaches to the treatment of colorectal cancer are required [2]. Many effective anticancer drugs have been developed from botanical sources, and there is a significant untapped resource in herbal medicines, which needs to be investigated [3–5]. Botanicals could potentially have effective anticancer compounds that may be used alone or as adjuncts to existing chemotherapy to improve efficacy and reduce drug-induced toxicity [6, 7].
Oplopanax horridus (OPH), or devil’s club belongs to the genus Oplopanax, which consists of three species (O. elatus, O. japonicus and O. horridus). OPH is distributed from south-central Alaska to western Oregon and eastward to western Alberta and Montana. It is also found in Ontario and Michigan. Although this plant has been harvested and marketed widely as “Alaskan ginseng”, the chemical constituents and pharmacological activities of this herb is much different from ginseng. From the root of OPH, 5 polyynes were isolated and elucidated. The chemical constituent in other plant parts of OPH is unknown.
Previous pharmacological studies on OPH were limited. Several evaluations on anti-infection, anti-diabetes [8], and anti-TB of OPH were reported [9]. For the antimalignancy studies, in 2006, Tai et al., observed that the root bark extract of OPH showed antiproliferative effects on MCF7 and MDA-MB-468 human breast cancer cells, and K562 and HL60 human leukemia cells [10]. However, the effects of the stem and berry of OPH were not evaluated. In addition, the active concentration was not clear, and the antiproliferative mechanism of OPH was not observed.
In this study, we established a HPLC method for the analysis of stem and berry extracts of OPH. The UV spectra of representative constituents were obtained. The antiproliferative effects of OPH stem and berry extracts were tested using five human cancer cell lines, and the involved anticancer mechanisms including cell cycle arrest, expression of cyclin A and induction of apoptosis were evaluated.
2. Experiment
2.1. Plant
Oplopanax horridus (Araliaceae), stem bark and berry were obtained from Pacific Botanicals, LLC, which were gathered from Oregon, USA, and were authenticated by a Botanist. The voucher specimens were deposited in the Tang Center for Herbal Medical Research at the University of Chicago.
2.2. Extraction
Dried Oplopanax horridus (OPH) stem barks or berries were ground and extracted with 70% ethanol. The solvent of the extract solution was evaporated under vacuum. The dried extract was dissolved in water, and then extracted with water-saturated n-butanol. The n-butanol phase was evaporated under vacuum and then lyophilized to give the extracts.
2.3. HPLC analysis
The HPLC system was a Waters 2965 instrument with a 996 photodiode array detector (Milford, MA, USA). The separation was carried out on an Phenomenex Prodigy ODS(2) column (5 μ, 3.2×250 mm), with a guard column (5 μ, 3.2 ×7.5 mm) (Torrance, CA, USA). Acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 20% solvent A and 80% solvent B, was changed to 40% A for 15 min; to 80% A for 15 min; to 95% A for 5 min and held for 3 min; and finally changed to 20% A for 3 min and held for 8 min. The flow rate was 1.0 ml/min and the detection wavelength was set from 190 nm to 400 nm. All tested solutions were filtered through Millex 0.2-μm nylon membrane syringe filters before use.
2.4. Cell line and cell culture
The human colorectal cancer cell lines SW-480 (Leibovitz’s L-15), HCT-116 (McCoy’s 5A), HT-29 (McCoy’s 5A), MCF-7 breast cancer cells (RPMI-1640) and NSCLC non-small cell lung cancer cells (DMEM) were purchased from American Type Culture Collection (Manassas, VA, USA), and grown in the indicated media supplemented with 10% FBS and 50 IU penicillin/streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
2.5. Cell proliferation assay
OPH stem and berry extracts were dissolved in DMSO. Cancer cells were seeded in 96-well plates. After 1 day, various concentrations of extracts were added to the wells. The final concentration of DMSO was 0.5%. Controls were exposed to culture medium containing 0.5% DMSO without drugs. Cell proliferation was evaluated using an MTS assay according to the manufacturer’s instructions. Briefly, after 72 h treatment, the medium was replaced with 100 μl of fresh medium, 20 μl of MTS reagent (CellTiter 96 Aqueous Solution) in each well, and the plate was returned to the incubator for 1–2 h. A 60-μl aliquot of medium from each well was transferred to an ELISA 96-well plate and its absorbance at 490 nm was recorded [11, 12].
2.6. Cell cycle analysis
The SW-480 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed and the cells were treated with the OPH stem extract. The cells were incubated for 24, 48 and 72 h before harvesting. The cells were fixed gently with 80% ethanol in a freezer for 2 h and were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 300 μl of PBS containing 40 μg/ml PI and 0.1 mg/ml RNase, then incubated in a dark room for 20 min at room temperature and analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR, USA). For each measurement, at least 10,000 cells were counted [13].
2.7. Cyclin A assay
After 24, 48 and 72 h treatment with the OPH stem extract, the SW-480 cells were harvested in a similar manner to that used in the cell cycle assay. The cells were resuspended in 300 μl of PBS containing PI/RNase, and 20 μl of cyclin A-FITC was added to the cell suspension. Then, the cells were analyzed with a FACScan flow cytometer. For each measurement, at least 10,000 cells were counted [13].
2.8. Apoptosis assay
The SW-480 cells were seeded in 24-well tissue culture plates. After culturing for 1 day, the medium was changed and the OPH stem extract was added. After treatment for 24, 48 and 72 h, the cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then the culture medium containing 10% FBS (and floating cells) was added to inactivate the trypsin. After being pipetted gently, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and the cells were stained with annexin V-FITC and PI according to the manufacturer’s instructions. The cells were analyzed immediately after staining using a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted [12, 13].
2.9. Statistical analysis
Data are presented as mean ± standard deviation (SD). Student’s t-test was used for comparing two groups. The level of statistical significance was set at P<0.05.
3. Results
3.1. HPLC analysis of OPH stem and berry extracts
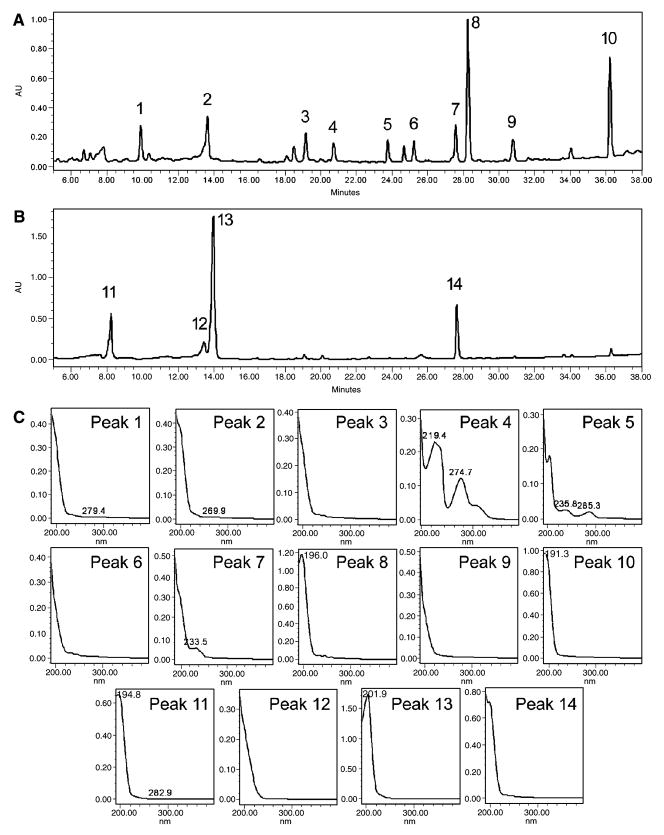
The chromatograms and spectra of representative constituents in OPH stem and berry extracts are shown in Fig. 1. From the chromatogram, peaks 8 and 10 are major constituents in the stem extract (Fig. 1A), and the wavelength of maximum absorption of the two peaks are 196.0 and 191.3 nm, respectively (Fig. 1C). The spectral information suggests that structures of the two compounds contain π-molecular orbital such as alkenes or alkynes. The spectra of other representative compounds in the stem extract are also shown in Fig. 1C. The absorption peaks at values of >230 nm were observed in peaks 4, 5 and 7. For the berry extract, the chromatogram is simpler than that of the stem extract, suggesting that there are fewer compounds in the berry extract (Fig. 1B). Peak 12 might be the same compound as peak 2, since the retention time of peak 12 (13.451 min) is similar with peak 2 (13.645 min), and the spectra of the two peaks are almost the same (Fig. 1C). Although the retention time of peak 14 (27.646 min) is very close to peak 7 (27.575 min), their UV spectra are much different. Thus, all the three major peaks in the berry extract (Peaks 11, 13 and 14) are different compounds from those in the stem extract. HPLC analysis suggests that the chemical composition of the stem extract is much different from the berry extract.
Fig. 1.
HPLC analysis of Oplopanax horridus stem and berry extracts. (A) HPLC chromatogram of stem extract recorded in 202 nm. (B) HPLC chromatogram of berry extract recorded in 202 nm. (C) UV spectra (190–400 nm) of peaks 1–14. Peak numbers are shown in (A) and (B).
3.2. Antiproliferative effects of OPH extracts on different cancer cell lines
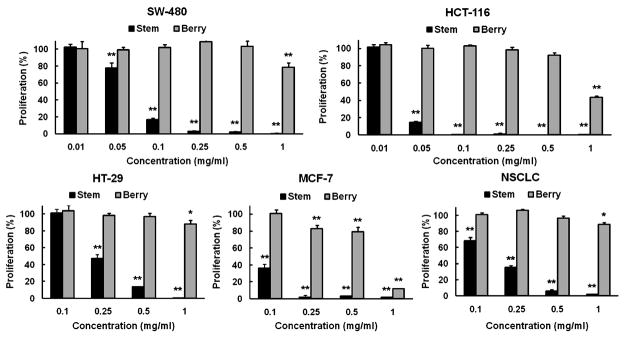
The cell growth inhibitory effects of OPH stem and berry extracts on five human cancer cell lines were determined. For the used cancer cells, three of them were colorectal cancer cell lines (SW-480, HCT-116 and HT-29), one was a breast cancer cell line (MCF-7), and one was a non-small cell lung cancer cell line (NSCLC). As shown in Fig. 2, the berry extract showed significant antiproliferative effects on MCF-7 cells at concentrations between 0.25–1 mg/ml, while only at 1 mg/ml, cell growth inhibitory effects were observed on the other four cell lines. On the other hand, stem extract showed antiproliferative effect on all the five cell lines. For the four cell lines except for HT-29, at as low as 0.1 mg/ml of treatment concentration, the stem extract showed significant antiproliferative effects. For the cell line HT-29, the antiproliferative effect of stem extract was significantly higher than that of berry extract. The most potent antiproliferative effects were observed on SW-480 and HCT-116 cell lines. At 0.05 mg/ml, the stem extract inhibited cell growth by 21.9% in the SW-480 cells and by 85.4% in the HCT-116 cells (both P<0.01 vs. untreated control). At 0.1 mg/ml, stem extract inhibited SW-480 cell growth by 85.1%, and almost completely inhibited HCT-116 cell growth (Fig. 2). Compared to berry extract, the stem extract showed much stronger antiproliferative effects on human cancer cells.
Fig. 2.
Effects of Oplopanax horridus stem and berry extracts on proliferation of different human cancer cell lines assayed by MTS method. Cell line used includes colorectal cancer (SW-480, HCT-116, HT-29), breast cancer (MCF-7) and non-small cell lung cancer cells (NSCLC). Cells were treated with 0.1–1 mg/ml of extract for 72 h. Since SW-480 and HCT-116 cells are more sensitive to stem extract, additional concentrations 0.01 and 0.05 mg/ml were also evaluated. * P<0.05; and ** P<0.01 vs. control (100%).
3.3. Effects of OPH stem extract on SW-480 cell cycle
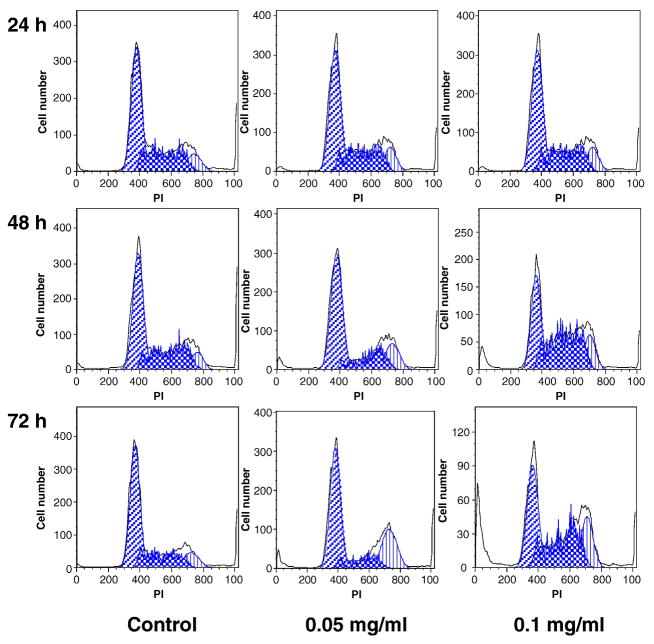
To explore the potential mechanism through which OPH stem extract inhibits cell growth, the cell cycle profile was assayed by flow cytometry after staining with propidium iodide (PI). Based on the MTS assay data, using SW-480 cells, significant antiproliferative effect was observed by treatment with 0.05 and 0.1 mg/ml of stem extract. Thus, we used the two treatment concentrations to evaluate the effect of stem extract on SW-480 cell cycle. As shown in Fig. 3, compared to the control, 24 h treatment did not change the cell cycle profile. However, after 48 and 72 h treatment, cell cycle profile was changed. At 0.05 mg/ml, although the percentage of cells in G1-phase was not changed, stem extract obviously reduced the percentage of S-phase and increased G2/M-phase cells. At 0.1 mg/ml, more significant changes were observed, but the cell cycle profiles were different with that of 0.05 mg/ml of treatment. After 72 h, compared to the control (58.9% of G1-phase, 27.4% of M-phase, and 12.9% of G2/M-phase), treatment with stem extract with 0.05 mg/ml, 55.6% of cells were in G1-phase, 15.5% of cells were in M-phase, and 27.8% of cells were in G2/M-phase; treatment with 0.1 mg/ml, 38.9% of G-phase, 40.1% of M-phase, and 20.8% of G2/M-phase (Fig. 3). The 0.1 mg/ml of stem extract treatment obviously decreases G1-phase cells, while increasing S- and G2/M-phase cells.
Fig. 3.
Cell cycle analysis using flow cytometry after propidium iodide (PI) staining. SW-480 cells were treated with 0.05 and 0.1 mg/ml of Oplopanax horridus stem extract for 24, 48 and 72 h.
3.4. Effects of OPH stem extract on expression of cyclin A
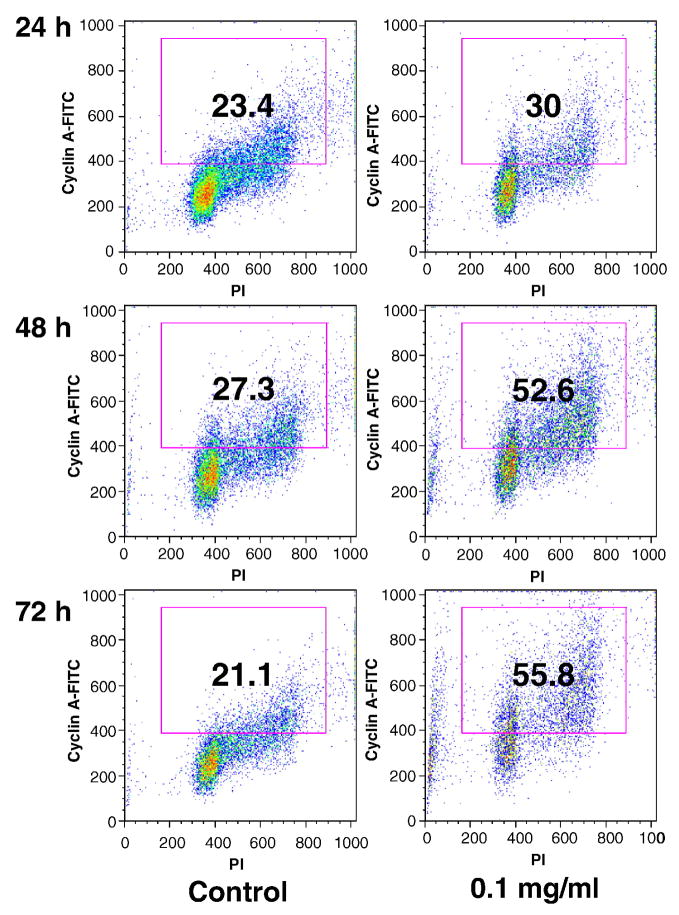
Since cell cycle progression is regulated by the cyclins and cyclin A is required for S-phase and passage through G2/M-phase, to observe additional information involved in the cell cycle regulations, the expression of cyclin A in SW-480 cancer cells was evaluated. At 24, 48 and 72 h, the percentage of cyclin A positive cells in the untreated control was 23.4%, 27.3% and 21.1%, respectively. After treatment with 0.1 mg/ml of stem extract for 24, 48 and 72 h, the proportion of cyclin A positive cells increased to 30.0%, 52.6% and 55.8%, respectively (Fig. 4). Treatment with OPH stem extract caused a marked increase in the expression of cyclin A on SW-480 cancer cells.
Fig. 4.
Cyclin A analysis of SW-480 cells using flow cytometry. After treatment with 0.1 mg/ml of Oplopanax horridus stem extract for 24, 48 and 72 h, cells were stained with cyclin A-FITC and propidium iodide (PI). The percentage of cyclin A positive cells is shown in the gate.
3.5. Apoptotic effects of OPH stem extract on SW-480 cells
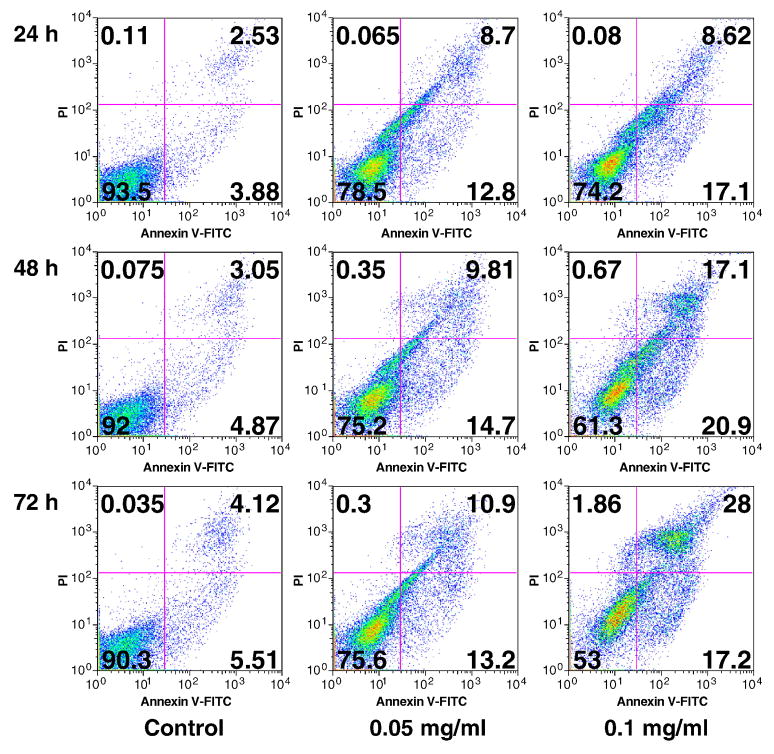
To further characterize the potential mechanism of OPH stem extract’s anticancer activity, we carried out an apoptotic assay by flow cytometry after staining with annexin V and PI (Fig. 5). Viable cells were negative for both annexin V and PI (lower left quadrant); early apoptotic cells were positive for annexin V and negative for PI (lower right quadrant); late apoptotic or necrotic cells displayed both positive annexin V and PI (upper right quadrant); non-viable cells which underwent necrosis were positive for PI and negative for annexin V (upper left quadrant). As shown in Fig. 5, at 24, 48 and 72 h, the summary of early and late apoptotic cells were 6.4–9.6%. Treatments with stem extract at 0.05 and 0.1 mg/ml obviously increased apoptotic cells, especially in the higher concentration. Treatment with 0.1 mg/ml of stem extract for 24, 48 and 72 h resulted in an increasing of apoptotic cells at 25.7%, 38.0% and 45.2%, respectively (Fig. 5). This result suggested that the antiproliferative effect of OPH stem extract was mediated by the induction of apoptosis.
Fig. 5.
Apoptosis assay using flow cytometry after annexin V-FITC and propidium iodide (PI) staining. SW-480 cells were treated with 0.05 and 0.1 mg/ml of Oplopanax horridus stem extract for 24, 48 and 72 h.
4. Discussion
Oplopanax horridus is one species in the genus Oplopanax (Torr. & A. Gray) Miq. Oplopanax is a very small genus in the family Araliaceae, consisting of three species of deciduous shrubs: O. elatus (Nakai) Nakai, O. japonicus (Nakai) Nakai and O. horridus (Sm.) Miq., the latter of which was investigated in this study.
O. elatus is distributed in Eastern Asia, including Northeast China, Korea and the Far East of Russia, The phytochemical studies of genus Oplopanax were focused on O. elatus. Several types of compounds were isolated from different plant parts of O. elatus, in which the representative compounds are saponins, which are believed to be bioactive constituents. Up to now, 28 triterpene glycosides were isolated and elucidated from O. elatus, of which 27 were identified from leaves. From O. elatus leaves, cirensenoside E, F, G, H [14]; cirensenoside I, J, K, L [15]; cirensenoside M, N [16]; cirensenoside O, P [17]; cirensenoside Q, R [18]; cirenshenoside S, T, U, V [19, 20]; 1H-cyclopenta[a]chrysene [21]; nipponoside B, saponins [161400-70-0] (CAS registration number), [161400-72-2], [161400-73-3] [22]; kalopanaxsaponin G, cussonoside A, saponins [136182-02-0], [136182-02-0] [23] were isolated and identified. In addition, daucosterol was isolated from the roots [24] and stems [25] of O. elatus. For the bioactivity observation, the effect of O. elatus on antiarthritis [26], antifungal, [27] anti-senility [28] and anti-psoriasis [29] were reported. Another species, O. japonicus is distributed in Japan. Two sesquiterpenes, oplopanone [30] and oplodiol [31], and four triterpene glycosides, oplopanaxoside A, B, C, D [32] were isolated and identified from this herb. We searched the database of PubMed and SciFinder Scholar, however, literature of pharmacological studies on O. japonicus was not found.
O. horridus, commonly known as devil’s club, is distributed from Alaska, British Columbia, southward to central Oregon and eastward to the southwestern Yukon, northwestern Alberta, Montana, and Idaho of USA and Canada. There are also several populations distributed in Michigan and Ontario.
Although it is a member of the family Araliaceae (ginseng family), O. horridus, also named Alaskan ginseng in the market, is much different from ginseng. Distributed in close geographical proximity to O. horridus, North American ginseng (Panax quinquefolius L.) is from another genus Panax L. in the family Araliaceae [12, 33]. Nearly all species in the Panax genus are important herbal medicines, which are erect perennial herbaceous plants, lacking aerial permanent woody stems. O. horridus is a deciduous understory shrub. The stems of O. horridus are upright to decumbent, covered with a dense armor of yellowish needle-like spines, which can cause severe skin irritation. The large palmately lobed leaves are spirally arranged on the stems. The flowers are small and whitish, borne in terminal pyramidal clusters, and ripen to shiny flattened, bright red berries [8].
The chemical constituent in O. horridus is different from ginseng. In ginseng plants, the major bioactive constituents are a group of triterpene glycosides, commonly referred to as ginsenosides [34]. The representative ginsenosides in ginseng plants such as ginsenosides Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2 and Rg3, are dammarane glycosides. The sapogenin of these ginsenosides are dammarane triterpenoids such as protopanaxadiol and protopanaxatriol [34, 35]. Up to now, 32 triterpene glycosides were isolated from O. elatus and O. japonicus, however, dammarane glycosides were not found in these two Oplopanax species. In addition, no triterpene glycosides were isolated and identified from O. horridus.
The identified compounds from O. horridus are polyynes and volatile compounds. Five polyynes were isolated from O. horridus: falcarinol; falcarindiol; oplopandiol; oplopandiol acetate; 9,17-octadecadiene-12,14-diyne-1,11,16-triol 1-acetate [9]. Based on GC/MS techniques, the composition of the essential oil of devil’s club was studied, and (E)-nerolidol was found to be the major constituent in both the stems (54.5%) and the roots (54.6%) [36].
Since there was a lack of standard, analytical studies on O. horridus was to investigate the HPLC fingerprint, which may reveal some information on the chemical constituent [10, 37]. However, previous reports only studied the root of O. horridus. Other plant parts were not assayed. Moreover, the structural information of major constituents was undetected.
In this study, we established a HPLC method to determine O. horridus stem and berry extract, and the UV spectra (190–400 nm) of representative peaks in chromatograms were assayed (Fig. 1). In the stem extract, more than ten constituents were detected. The wavelength of maximum absorption of the two major compounds are between 190–200 nm, suggesting they are not the identified polyynes isolated from O. horridus since those polyynes display UV absorbance with lambda max at approximately 206, 233, 245 nm and another lambda max at 260–270 nm [9, 38]. Compared to the stem extract, the constituents in the berry extract were fewer, and three major constituents in the berry extract were different from those of the stem extract. Although the UV spectra of peaks 5 and 7 showed maximum absorption at approximately 205 and 235 nm, lambda max at 245 and 265 nm were not found, therefore they are not polyynes [9, 38]. Data from HPLC assay suggest that polyynes are not major constituents in O. horridus stem and berry.
The reported traditional use of O. horridus is for the treatment of external and internal infections, headache, and diabetes [8]. The early pharmacognostic and pharmacological observations of O. horridus have been reviewed [8, 39, 40]. Modern pharmacological studies on O. horridus were limited. It was reported that O. horridus possesses antibacterial activity, especially anti-TB potentials [9]. Interestingly, the root bark extract of O. horridus showed antiproliferative effects on human breast and leukemia cell lines [10]. The botanical, phytochemical and pharmacological studies on O. horridus and the other two Oplopanax species are summarized in Table 1.
Table 1.
Phytochemical and pharmacological studies on the genus Oplopanax
| Oplopanax species | Major distribution | Phytochemistry | Pharmacology | ||
|---|---|---|---|---|---|
| Identified constituent | Study depth | Bioactivity | Study depth | ||
| O. elatus | Far East Russia Northeast China Korea |
28 triterpene glycosides | +++ | Antipsoriasis Antiarthritis Antifungus Antisenility |
++ |
| O. japonicus | Japan | 4 triterpene glycosides2 sesquiterpenes | ++ | − | − |
| O. horridus (OPH) | West Canada Northwest USA |
5 polyynes | + | Antibacteria Antidiabetes Antimalignancy |
+ |
In this study, using five human cancer cell lines, we compared the antiproliferative potential of O. horridus stem and berry extracts. The cancer cell line used includes three types of cancers: colon cancer (SW-480, HCT-116 and HT-29), breast cancer (MCF-7) and non-small cell lung cancer (NSCLC). The berry extract showed active cell inhibitory effect on MCF-7 cells. Compared to berry, the stem extract showed very significant antiproliferative effect on all the five cell lines, especially on the SW-480 and HCT-116 colorectal cancer cells. This cell type-selective inhibitory effect provided us evidence that O. horridus stem extract may have chemopreventive potential on human colorectal cancer. Furthermore, it is likely that active anti-colorectal cancer compounds could be isolated from O. horridus stem extract.
Since cancer chemoprevention studies on O. horridus are very limited [10], the involved cancer inhibitory mechanism is unknown. In this study, we observed O. horridus stem extract on cancer cell cycle. At lower treatment concentration, the stem extract mainly arrests cells in G2/M-phase, and does not influence the proportion of G1-phase. In higher treatment concentration, the stem extract arrests cells in S- and G2/M-phases, and obviously decreases the G1-phase fraction (Fig. 3). Since cyclins and cyclin-dependent kinases regulate cell cycle progression, and cyclin A is required in S- and G2/M-phases, we determined the expression of cyclin A in cancer cells. The fraction of cyclin A positive cells increased to 55.8% after treatment with 0.1 mg/ml of stem extract for 72 h; in untreated cells the fraction was only 21.1% (Fig. 4). The accumulation of cyclin A was critical to promote cell cycle arrest in S- and G2/M-phases.
In addition to cell cycle regulation, apoptosis is considered an important pathway in the inhibition of cancer cells by many anticancer agents [41, 42]. We assayed the induction of apoptosis by the stem extract on SW-480 cells. After treatment with 0.1 mg/ml of the stem extract for 24 h, 17.1% of cells were in early apoptosis and 8.6% in late apoptosis; for 48 h, 20.9% in early apoptosis, and 17.1% in late apoptosis; for 72 h, 17.2% in early apoptosis and 28% in late apoptosis. From 24 h to 72 h, the proportion of early apoptotic cells was relatively stable (17.1–0.9%), while interestingly, the late apoptotic cells were increased from 8.6% to 17.1%, then to 28% (Fig. 5). This result suggests that cell death induced by the stem extract was through early to late apoptosis, indicating that apoptosis plays a key role in the antiproliferation of O. horridus stem extract on cancer cells.
In summary, although the active constituents in O. horridus need to be identified, the HPLC analysis supplied original information of constituents in O. horridus stem and berry extract, including the essential structural information of detected constituents. Pharmacological data obtained from this study suggested that O. horridus stem extract possess cancer chemopreventive potential on different types of cancer cells. The mechanisms involved in cancer chemoprevention by O. horridus stem extract were cell cycle arrest and induction of apoptosis.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants AT003255, AT003441, AT004418 and 5P30DK042086.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. CA Cancer J Clin. 2008;58:71. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Venook A. Oncologist. 2005;10:250. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- 3.Suffredini IB, Paciencia ML, Varella AD, Younes RN. Fitoterapia. 2007;78:223. doi: 10.1016/j.fitote.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang CZ, Yuan CS. Am J Chin Med. 2008;36:1019. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PG, Dupuy Loo OA, Bonilla JA, Murillo R. Fitoterapia. 2008;79:428. doi: 10.1016/j.fitote.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Wang CZ, Fishbein A, Aung HH, Mehendale SR, Chang WT, Xie JT, et al. J Altern Complement Med. 2005;11:1059. doi: 10.1089/acm.2005.11.1059. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar S, Meeran SM, Katiyar N, Katiyar SK. Clin Cancer Res. 2009;15:821. doi: 10.1158/1078-0432.CCR-08-1901. [DOI] [PubMed] [Google Scholar]
- 8.Lantz TC, Swerhun K, Turner NJ. HerbalGram. 2004;62:33. [Google Scholar]
- 9.Kobaisy M, Abramowski Z, Lermer L, Saxena G, Hancock REW, Towers GHN, et al. J Nat Prod. 1997;60:1210. doi: 10.1021/np970182j. [DOI] [PubMed] [Google Scholar]
- 10.Tai J, Cheung S, Cheah S, Chan E, Hasman D. J Ethnopharmacol. 2006;108:228. doi: 10.1016/j.jep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, et al. Planta Med. 2007;73:669. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, et al. J Agric Food Chem. 2006;54:9936. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 13.Wang CZ, Aung HH, Zhang B, Sun S, Li XL, He H, et al. Anticancer Res. 2008;28:2545. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang GS, Zhao CF, Xu JD, Murayama T, Shoji J. Chem Res Chin Univ. 1994;10:285. [Google Scholar]
- 15.Wang GS, Xu JD, Murayama T, Shoji J. Chin Sci Bull. 1994;39:1969. [Google Scholar]
- 16.Wang GS, Xu JD, Ma XL, Sun YX, Liu JZ, Murayama T, et al. Chem Res Chin Univ. 1997;13:34. [Google Scholar]
- 17.Wang G, Chen X, Xu J, Murayama T, Shoji J. Yaoxue Xuebao. 1996;31:940. [PubMed] [Google Scholar]
- 18.Wang G, Xu J, Tetsuya M, Shoji J. Zhongguo Zhongyao Zazhi. 1997;22:101. [PubMed] [Google Scholar]
- 19.Wang G, Xu J. Stud Plant Sci. 1999;6:52. [Google Scholar]
- 20.Wang G, Yang X, Xu J. Yaoxue Xuebao. 2004;39:354. [Google Scholar]
- 21.Wang G, Xu J. Zhongguo Yaoxue Zazhi. 1993;28:593. [Google Scholar]
- 22.Wang GS, Zhao CF, Xu JD, Murayama T, Miyakoshi M, Shoji J. Chem Res Chin Univ. 1994;10:185. [Google Scholar]
- 23.Wang G, Meng Q, Xu J, Chuenshan Z, Zhuanshi S. Zhongguo Yaoxue Zazhi. 1996;31:522. [Google Scholar]
- 24.Liu J, Wu G. J Chin Pharm Sci. 1993;2:168. [Google Scholar]
- 25.Zhang H, Wu G. J Chin Pharm Sci. 1996;5:112. [Google Scholar]
- 26.Zhang S, Wang K. Yaoxue Xuebao. 1980;15:81. [Google Scholar]
- 27.Mi HM, Li CG, Su ZW, Wang NP, Zhao JX, Jiang YG. Yaoxue Xuebao. 1987;22:549. [PubMed] [Google Scholar]
- 28.Fu YX, Ma ZL, Ma ZJ. Zhongguo Yiyuan Yaoxue Zazhi. 1995;7:122. [Google Scholar]
- 29.Dou DQ, Hu XY, Zhao YR, Kang TG, Liu FY, Kuang HX, et al. Nat Prod Res. 2009;23:334. doi: 10.1080/14786410802075806. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Minato H, Ishikawa M. Chem Commun. 1965:79. [Google Scholar]
- 31.Minato H, Ishikawa M. J Chem Soc. 1967;C:423. [Google Scholar]
- 32.Hirai Y, Murayama T, Miyakoshi M, Hirono S, Isoda S, Ideura N, et al. Nat Med. 1995;49:462. [Google Scholar]
- 33.Wang CZ, Mehendale SR, Yuan CS. Am J Chin Med. 2007;35:543. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen LP. Adv Food Nutr Res. 2009;55:1. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang CZ, Ni M, Sun S, Li XL, He H, Mehendale SR, et al. J Agric Food Chem. 2009;57:2363. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garneau FX, Collin G, Gagnon H, Jean FI, Strobl H, Pichette A. Flavour Fragrance J. 2006;21:792. [Google Scholar]
- 37.Gruber JW, Kittipongpatana N, Bloxton JD, Der Marderosian A, Schaefer FT, Gibbs R. J Chromatogr Sci. 2004;42:196. doi: 10.1093/chromsci/42.4.196. [DOI] [PubMed] [Google Scholar]
- 38.Bernart MW, Cardellina JH, 2nd, Balaschak MS, Alexander MR, Shoemaker RH, Boyd MR. J Nat Prod. 1996;59:748. doi: 10.1021/np960224o. [DOI] [PubMed] [Google Scholar]
- 39.Justice JW. Alaska Med. 1966;8:36. [PubMed] [Google Scholar]
- 40.Smith GW. J Ethnopharmacol. 1983;7:313. doi: 10.1016/0378-8741(83)90005-3. [DOI] [PubMed] [Google Scholar]
- 41.Shi P, Huang Z, Chen G. Am J Chin Med. 2008;36:805. doi: 10.1142/S0192415X08006259. [DOI] [PubMed] [Google Scholar]
- 42.Waxman DJ, Schwartz PS. Cancer Res. 2003;63:8563. [PubMed] [Google Scholar]