Abstract
African American (AA) race/ethnicity, lower body mass index (BMI), and higher insulin-like growth factor 1 (IGF-1) levels are associated with premenopausal breast cancer risk. This cross-sectional analysis investigated whether BMI or BMI at age 21 years contribute to racial differences in IGF-1, IGF-2, IGFBP-3, or free IGF-1. Participants included 816 white and 821 AA women between ages 40 and 79 years across a wide BMI range (18.5–40 kg/m2). Compared with white women, AA women had higher mean IGF-1 (146.3 vs. 134.4 ng/ml) and free IGF-1 (0.145 vs. 0.127) levels, and lower IGF-2 (1633.0 vs. 1769.3 ng/ml) and IGFBP-3 (3663.3 vs. 3842.5 ng/ml) levels (all p<0.01; adjusted for age, height, BMI, BMI at age 21, and menopause status). Regardless of race, IGF-1 and free IGF-1 levels sharply rose as BMI increased to 22–24 kg/m2, then declined thereafter, while IGF-2 and IGFBP-3 levels tended to rise with BMI. In contrast, BMI at age 21 was inversely associated with all IGF levels, but only among white women (p-interaction = 0.01). With the decline in IGF-1 with BMI at age 21 among whites, racial differences in IGF-1 significantly increased among women who were obese in early adulthood. In summary, BMI was associated with IGF-1 levels regardless of race/ethnicity, while obesity during childhood or young adulthood may have a greater impact on IGF-1 levels among white women. The effects of obesity throughout life on the IGF axis and racial differences in breast cancer risk require study.
Keywords: Insulin-like growth factor, obesity, race, breast cancer
Introduction
African American women are at greater risk for premenopausal breast cancer and hormone receptor negative (ER-/PR-) tumors, and have an overall poorer prognosis, compared to white women (Brinton et al., 2008; Hausauer et al., 2007; Pfeiffer et al., 2008). It is unclear why ER-/PR- tumors are more common among African American women (Brinton et al., 2008), however IGF-1 and free (unbound) IGF-1 levels are also associated with premenopausal breast cancer risk and therefore may have a role in breast tumor progression in the absence of ER or PR activation (Renehan et al., 2006).
IGF-1 production is stimulated by growth hormone (GH) and is a key mediator of mitogenic and anti-apoptotic activity (Gennigens et al., 2006). IGF binding protein-3 (IGFBP-3) regulates IGF-1 bioavailability and also has functional activity to induce or inhibit apoptosis independently of IGF-1 bioavailability. Analyses of data from NHANES III, Nurses Health Study II (NHS II), and the Multiethnic Cohort Study (MEC) found African-American women had higher IGF-1 levels and lower IGFBP-3 levels compared to white women (Berrigan et al., 2008; DeLellis et al., 2004; Pinheiro et al., 2005), consistent with higher IGF-1 bioavailability and a potential role for the IGF axis in premenopausal breast cancer risk among African American women.
Aside from race/ethnicity, IGF-1 levels may also be associated with obesity or energy availability. Several studies found an inverse association between body mass index (BMI) and IGF-1 levels in whites (Gram et al., 2006; Jernstrom et al., 2001; Henderson et al., 2006). In contrast, analysis of MEC data found BMI was not associated with IGF-1 levels among African Americans (Henderson et al., 2006). The prevalence of obesity is increasing nationally and is highest among African American women (Flegal et al., 2002), however it is unclear if race/ethnicity differences in the relationship between obesity and the IGF axis may contribute to differences in breast cancer risk between race/ethnicity groups.
Our goal was to investigate the role of obesity upon serum IGF-1, IGFBP-3, and IGF-2 levels in a large sample of white and African-American women to help clarify whether obesity contributes to racial differences in the IGF axis.
Materials and Methods
Participants
Women in this study were selected from members of the Southern Community Cohort Study, a prospective cohort investigation initiated in 2001 designed to identify the determinants of cancer incidence and mortality in a racially diverse population. Detailed methods of the Southern Community Cohort Study (SCCS) have been reported (Signorello et al., 2005), and study information is also available at www.southerncommunitystudy.org. Briefly, adult men and women visiting one of 71 community health centers located throughout the southeastern U.S were approached for recruitment. Potential participants were eligible if they were between 40 and 79 years of age and had not been under treatment for cancer (except nonmelanoma skin cancer) within the past year. All SCCS protocols have been approved by IRBs at Vanderbilt University Medical Center and Meharry Medical College in Nashville, TN.
In 2006, a project was developed within a stratified sample of SCCS female participants to investigate racial/ethnic differences in blood biomarkers potentially relevant to breast cancer risk. This sample included 1000 African American and 1000 white female participants randomly selected from female SCCS cohort members with frozen-stored blood and without a prior breast cancer diagnosis. Furthermore, sampling was stratified by categories of BMI (18.5–24.9, 25.0–29.9, 30.0–45.0), age (5-year intervals), and menopausal status Premenopausal women included women with at least one menstrual cycle within the past six months, while postmenopausal women were six months or more since their last cycle whether due to natural aging, hysterectomy with or without oophorectomy, or other causes.
Data and Sample Collection
All SCCS participants provided written informed consent prior to completing a comprehensive, in-person, baseline interview administered by a trained interviewer. The computer-assisted interview included demographics, weight and height, and a wide range of other potential cancer risk factors. Women reporting six or more months since their last menstrual cycle were asked why their periods had stopped. A blood sample was collected from participants at recruitment. Blood samples were refrigerated immediately after collection, and then shipped cold on that day to Vanderbilt University. Blood was processed and stored frozen at −80°C on average within 1.2 days (range 1–5 days) after collection.
Laboratory Assays
Serum concentrations of IGF-I, IGF-2, and IGFBP-3 were determined with the use of commercially available ELISA kits (DSL, Inc., Webster, TX). The calibrators used in the assays ranged between 4.5–640 ng/ml for IGF-I, 50–2000 ng/ml for IGF-II, and 2.5–100 ng/ml for IGFBP-3. For IGFBP-3 measurement, plasma samples were diluted at 1:100 in an assay buffer. The intra-assay CVs were 4.0%, 2.4%, and 1.8%, and inter-assay CVs were 6.5%, 7.4%, and 7.1%, respectively, for IGF-I, IGF-2, and IGFBP-3. Each assay has no cross-reaction with other members of the IGF family. Complete data for blood IGF values were available for 1984 study participants. The molar ratio of IGF-1 to IGFBP-3 representing an index of free IGF-1 was calculated as (IGF-1 (ng/ml) × 1.30)/(IGFBP-3 (ng/ml) × 0.36). We did not find an association between IGF markers and transport time, duration of blood storage in freezer, or the time between the last meal and blood collection.
Data Analyses
From the initial sample of 1984 participants with IGF data, we excluded women taking hormone replacement therapy (n=202), insulin for the treatment of diabetes (n=111), or missing weight at age 21 data (n=33). We further excluded one participant with an IGF-1 value more than 8 SDs from the group mean. The final study population included 1637 participants (816 white and 821 African American women). Wilcoxon signed rank and chi-square (χ2) tests were used to compare median values and categorical levels, respectively, between African American and white women.
We calculated mean IGF marker levels within white or African American participants after adjusting for age (5 year intervals), BMI (categorized at 2 unit intervals to accommodate nonlinear association), height in meters (continuous), menopausal status at blood collection (premenopausal, postmenopausal) and BMI at age 21 years (<20, 20–24, 25–29, and 30 or more) in a linear regression model. Additional control for time of day of blood collection or year of blood collection (storage time) did not affect our results. Tests for trend were performed by including the categorical variable for the covariate of interest as a continuous variable in the full model. Tests for interaction represented differences with race in the association between each IGF marker and another covariate, and were evaluated by the significance of a race × covariate cross-product term in a model that also included each main effect term and other listed factors. Distributions of all IGF markers approached a normal distribution, with low kurtosis and skewness, such that log transformation of the IGF data did not lead to normalization of these distributions. A Box-Cox procedure suggested raising values of IGF-1 and IGF-2 to the power 0.75 or transforming IGFBP-3 and free IGF1 values with a square root function. However, study results using either the natural scale for each IGF marker or the transformed variable were almost identical. We therefore report mean IGF levels, standard errors, and p-values from statistical tests using IGF values on the natural scale of each IGF marker.
RESULTS
Study Population
African American and white women were selected to have a similar age and BMI (Table 1). African American and white women also had a similar BMI at age 21 (Wilcoxon p=0.88, χ2 p=0.41) and height (Wilcoxon p=0.07, χ2 p=0.19). There were 714 postmenopausal women, including 383 women that were postmenopausal because of a clinical intervention such as surgery.
Table 1.
Study Population Characteristics
| White (n=816) | African American (n=821) | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | 40 – 44 | 301 | 36.9% | 311 | 37.9% |
| 45 – 49 | 211 | 25.9% | 219 | 26.7% | |
| 50 – 54 | 107 | 13.1% | 120 | 14.6% | |
| 55 – 59 | 79 | 9.7% | 69 | 8.4% | |
| 60 – 64 | 70 | 8.6% | 34 | 4.1% | |
| 65 – 69 | 25 | 3.1% | 39 | 4.8% | |
| 70 – 79 | 23 | 2.8% | 29 | 3.5% | |
| BMI | 18 – 19 | 35 | 4.3% | 19 | 2.3% |
| 20 – 24 | 169 | 20.7% | 196 | 23.9% | |
| 25 – 29 | 211 | 25.8% | 205 | 25.0% | |
| 30 – 34 | 205 | 25.1% | 209 | 25.5% | |
| 35 – 39 | 114 | 14.0% | 125 | 15.2% | |
| 40 – 45 | 82 | 10.1% | 67 | 8.2% | |
| BMI at age 21 yrs | 13 – 19 | 283 | 34.7% | 268 | 32.6% |
| 20 – 24 | 366 | 44.9% | 370 | 45.1% | |
| 25 – 29 | 103 | 12.6% | 125 | 15.2% | |
| 30 – 51 | 64 | 7.8% | 58 | 7.1% | |
| Height (meters) | 1.22 –1.49 | 29 | 3.6% | 24 | 2.9% |
| 1.50 –1.59 | 196 | 24.0% | 176 | 21.4% | |
| 1.60 –1.64 | 222 | 27.2% | 203 | 24.7% | |
| 1.65 – 1.69 | 191 | 23.4% | 229 | 27.9 | |
| 1.70 –1.96 | 178 | 21.8% | 189 | 23.0 | |
| Premenopausal | 458 | 56.1% | 465 | 56.6% | |
| Postmenopausal | 358 | 43.9% | 356 | 43.4% | |
| Natural menopause | 161 | 19.7% | 170 | 20.7% | |
| Surgical or other menopause | 197 | 24.2% | 186 | 22.7% | |
IGFs by menopausal status and race
Premenopausal women had consistently higher IGF levels than postmenopausal women after controlling for age and other factors. These differences in IGF levels associated with menopausal status were similar between African American and white women (p-interaction > 0.05 for all tests of a race by menopause interaction) (Table 2). Racial differences in IGF levels persisted among postmenopausal women regardless of whether they experienced a natural or a surgical menopause (all p-interactions > 0.05).
Table 2.
Adjusted IGF levels; by race/ethnicity and menopausal status
| White | African American | ||||
|---|---|---|---|---|---|
| All* | Mean | SE | Mean | SE | p |
| IGF-1 (ng/ml) | 134.5 | 2.9 | 146.8 | 2.9 | <0.01 |
| IGF-2 (ng/ml) | 1773.9 | 20.2 | 1639.7 | 20.4 | <0.01 |
| IGFBP-3 (ng/ml) | 3874.6 | 54.1 | 3703.0 | 54.5 | <0.01 |
| free IGF-1 | 0.126 | 0.002 | 0.144 | 0.002 | <0.01 |
| Premenopausal** | |||||
| IGF-1 (ng/ml) | 148.4 | 11.9 | 157.0 | 11.7 | 0.02 |
| IGF-2 (ng/ml) | 1897.2 | 82.7 | 1745.5 | 82.0 | <0.01 |
| IGFBP-3 (ng/ml) | 4183.3 | 212.6 | 3950.3 | 210.6 | <0.01 |
| free IGF-1 | 0.128 | 0.010 | 0.146 | 0.010 | <0.01 |
| Postmenopausal*** | |||||
| IGF-1 (ng/ml) | 128.2 | 3.9 | 144.7 | 3.9 | <0.01 |
| IGF-2 (ng/ml) | 1778.7 | 28.5 | 1666.9 | 28.6 | <0.01 |
| IGFBP-3 (ng/ml) | 3872.5 | 79.4 | 3771.4 | 79.6 | 0.25 |
| free IGF-1 | 0.119 | 0.003 | 0.140 | 0.003 | <0.01 |
| Surgical Menopause**** | |||||
| IGF-1 (ng/ml) | 126.7 | 5.2 | 147.4 | 5.4 | <0.01 |
| IGF-2 (ng/ml) | 1800.0 | 41.0 | 1723.9 | 42.6 | 0.09 |
| IGFBP-3 (ng/ml) | 3934.6 | 111.6 | 3951.6 | 115.9 | 0.89 |
| free IGF-1 | 0.117 | 0.004 | 0.136 | 0.004 | <0.01 |
| Natural Menopause**** | |||||
| IGF-1 (ng/ml) | 132.9 | 6.3 | 145.8 | 6.3 | 0.06 |
| IGF-2 (ng/ml) | 1764.9 | 42.3 | 1608.1 | 42.7 | <0.01 |
| IGFBP-3 (ng/ml) | 3846.5 | 119.9 | 3615.8 | 121.1 | 0.08 |
| free IGF-1 | 0.124 | 0.005 | 0.145 | 0.005 | <0.01 |
Mean IGF levels adjusted for
age, height, BMI, BMI at age 21 and menopausal status;
age, height, BMI, and BMI at age 21;
age, height, BMI, BMI at age 21, and reason for menopause;
age, height, BMI, and BMI at age 21
African American premenopausal and postmenopausal women had significantly higher IGF-1 levels, and significantly lower IGF-2 levels, compared to similar white women (Table 2). IGFBP-3 levels were higher among whites than African Americans, although differences in IGFBP-3 levels between white and African American women were statistically significant only among premenopausal women. Free IGF-1 levels were significantly higher among premenopausal and postmenopausal African American women compared to similar white women. Indeed, free-IGF levels were highest among premenopausal African American women, followed by postmenopausal African American women, premenopausal white women, and postmenopausal white women, respectively.
IGFs and BMI, by race and menopausal status
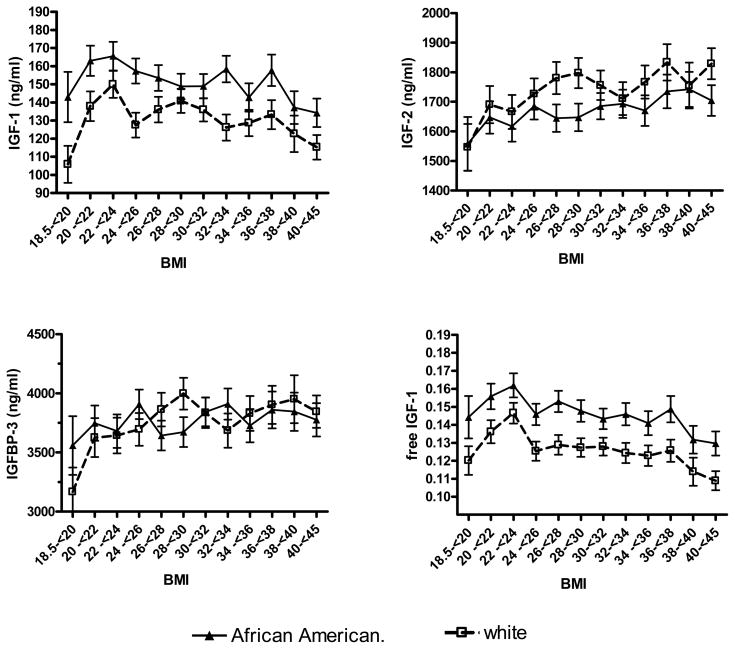
IGF-1 levels were higher among African American compared to white women across the range of BMI categories (Table 3 and Figure 1). However, the relationship between IGF-1 and BMI was similar between African American and white women (p-interactions > 0.05). The highest IGF-1 levels were found among participants with a BMI between 22 and 24 kg/m2, with a significant declining trend between BMI and IGF-1 thereafter within both race groups (p-trend<0.05). In contrast, IGF-2 levels tended to increase somewhat with BMI with African America and white women, while IGFBP-3 levels rose slightly (not significantly) as BMI increased beyond 20 kg/m2. Free IGF-1 levels across BMI categories followed the pattern established by IGF-1 for both African American and white women, with the highest free IGF-1 levels among African American women with a BMI between 22 kg/m2 and 24 kg/m2 and then significantly decreasing with a greater BMI. Further investigation of the association between IGF-1 or free IGF-1 with BMI found a similar pattern within premenopausal or postmenopausal women (Table 4), although IGF-1 levels were not lower among the six African American premenopausal women with a BMI less than 20 kg/m2.
Table 3.
Relationship between mean IGF levels with BMI or BMI at age 21, by race/ethnicity
| n | IGF-1 (ng/ml) | IGF-2 (ng/ml) | IGFBP-3 (ng/ml) | Free IGF-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | W | AA | W | AA | W | AA | W | AA | W | ||
| BMI | 18–19 | 19 | 35 | 133.3 | 114.1 | 1526.9 | 1638.4 | 3448.7 | 3445.4 | 0.139 | 0.119 |
| 20–24 | 196 | 169 | 154.4 | 145.6 | 1616.1 | 1748.1 | 3689.7 | 3821.1 | 0.151 | 0.137 | |
| 25–29 | 205 | 211 | 146.3 | 141.4 | 1641.3 | 1837.6 | 3649.9 | 4058.2 | 0.146 | 0.127 | |
| 30–34 | 209 | 205 | 149.9 | 131.9 | 1676.0 | 1783.7 | 3818.3 | 3897.4 | 0.144 | 0.123 | |
| 35–39 | 125 | 114 | 140.8 | 131.0 | 1736.6 | 1813.5 | 3858.6 | 3937.5 | 0.134 | 0.122 | |
| 40–45 | 67 | 82 | 134.0 | 114.9 | 1709.7 | 1856.2 | 3794.1 | 3918.2 | 0.129 | 0.106 | |
| p-trend | <0.01 | <0.01 | 0.12 | 0.05 | 0.61 | 0.34 | <0.01 | <0.01 | |||
| BMI age 21 | 12–19 | 268 | 283 | 147.0 | 144.5 | 1673.6 | 1881.8 | 3766.2 | 4174.7 | 0.144 | 0.126 |
| 20–24 | 370 | 366 | 147.9 | 133.7 | 1679.6 | 1791.0 | 3780.2 | 3843.5 | 0.143 | 0.126 | |
| 25–29 | 125 | 103 | 146.1 | 126.3 | 1701.3 | 1724.9 | 3759.9 | 3742.0 | 0.141 | 0.121 | |
| 30–45 | 58 | 64 | 149.1 | 119.3 | 1551.2 | 1697.4 | 3609.3 | 3735.4 | 0.148 | 0.115 | |
| p-trend | 0.59 | <0.01 | 0.63 | <0.01 | 0.99 | <0.01 | 0.89 | 0.06 | |||
IGF levels adjusted for age, height, menopausal status, and BMI or BMI at age 21 years. AA=African American, W=white
Figure 1.
IGF Levels by BMI and race
Test for trend (Af. Amer, white; respectively; where BMI > 22): IGF-1: p<0.01, p<0.01; IGF-2: p=0.15, p=0.06; IGFBP-3: p=0.66, p=0.40; free IGF1: p<0.01, p<0.01. adjusted for age, height, bmi at age 21, and menopausal status.
Table 4.
IGF-1 and Free-IGF levels and BMI, by race/ethnicity and menopausal status
| IGF-1 (ng/ml) | Free IGF-1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Premenopausal | Postmenopausal | Premenopausal | Postmenopausal | |||||
| BMI | AA | W | AA | W | AA | W | AA | W |
| 18–19 | 166.8 | 105.6 | 132.2 | 107.2 | 0.138 | 0.112 | 0.139 | 0.116 |
| 20–24 | 164.7 | 145.5 | 169.2 | 134.1 | 0.160 | 0.136 | 0.148 | 0.127 |
| 25–29 | 162.5 | 139.7 | 146.3 | 133.6 | 0.156 | 0.123 | 0.142 | 0.119 |
| 30–34 | 164.1 | 136.3 | 146.8 | 120.4 | 0.150 | 0.122 | 0.143 | 0.113 |
| 35–39 | 154.7 | 128.9 | 132.7 | 131.9 | 0.142 | 0.109 | 0.128 | 0.127 |
| 40–45 | 139.9 | 116.3 | 134.1 | 110.4 | 0.132 | 0.098 | 0.129 | 0.107 |
| BMI age 21 | ||||||||
| 12–19 | 155.8 | 140.0 | 143.2 | 142.2 | 0.143 | 0.117 | 0.142 | 0.126 |
| 20–24 | 159.7 | 133.0 | 145.8 | 125.2 | 0.146 | 0.122 | 0.140 | 0.121 |
| 25–29 | 158.9 | 121.8 | 141.3 | 117.9 | 0.147 | 0.114 | 0.130 | 0.117 |
| 30–45 | 160.8 | 120.1 | 143.9 | 106.3 | 0.150 | 0.113 | 0.140 | 0.108 |
Controlling for age, height, and BMI or BMI at age 21 years. Postmenopausal analysis also controlled for reason of menopause (surgical or natural). AA=African American, W=white
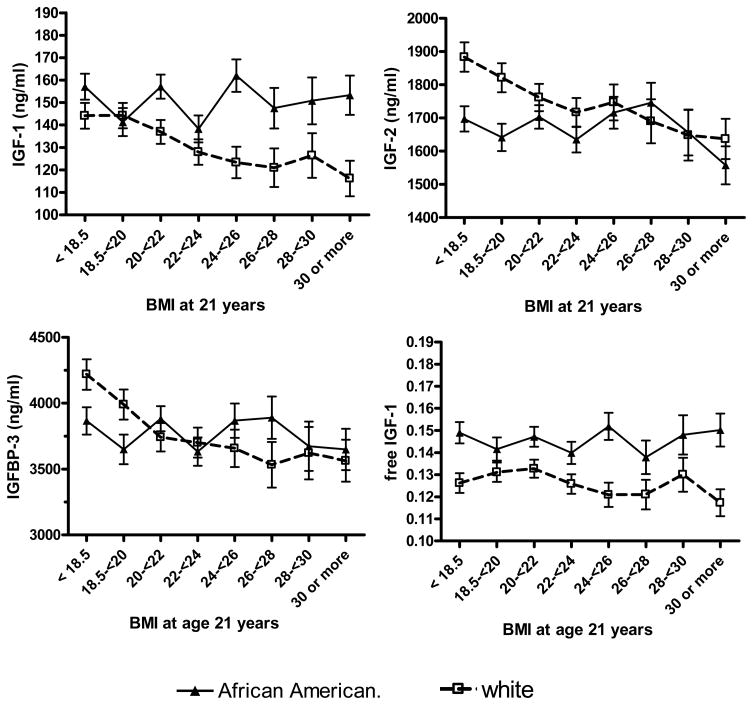
BMI at age 21 and height provide two metrics of body size during growth and development. BMI at age 21 was inversely associated with IGF-1, IGF-2, and IGFBP-3, but only among white women (Table 3). Thus, with increasing BMI at age 21, racial differences in IGF-2 and IGFBP-3 diminished, while racial differences in IGF-1 significantly increased (p-interaction = .01). Racial differences in free IGF-1 remained fairly constant across the range of BMI at age 21 (Figure 2). Inverse associations between IGF-1 and BMI at age 21 were consistent within premenopausal and postmenopausal white women (Table 4), and similar trends were observed with IGF-2 or IGFBP-3 (not shown). Height was not significantly associated with adjusted IGF levels (not shown).
Figure 2.
IGF levels by BMI at age 21 and race
Test for trend (Af. Amer, white; respectively): IGF-1: p=0.33, =0.81; IGF-2: p=0.38, p=0.71; IGFBP-3: p=0.18, p=0.83;free IGF1: p=0.98, p=0.53; adjusted for age, BMI, menopausal status, and BMI at age 21 years.
DISCUSSION
African America women are at greater risk for premenopausal breast cancer and ER-/PR- tumors compared to white women (Brinton et al., 2008; Hausauer et al., 2007). We found African American women had significantly higher IGF-1 and free IGF-1 levels, and lower IGF-2 and IGFBP-3 levels, compared to white women. Any explanation is speculative but may involve race/ethnicity differences in estrogen (Setiawan et al., 2006; Pinheiro et al., 2005) or vitamin D levels (Egan et al., 2008) that may in turn affect IGF levels (Lukanova et al., 2004; Janssen et al., 1998; Jorgensen et al., 2004; Rozen et al., 1997). While beyond the scope of this manuscript, differences in diet, physical activity, or reproductive history need to be considered in future analyses (Probst-Hensch et al., 2003; Holmes et al., 2002; Gapstur et al., 2004; McGreevy et al., 2007). Furthermore, perhaps one-third of circulating IGF-1 and two-thirds of IGF-2 and IGFBP-3 levels may be attributable to genetic factors (Harrela et al., 1996), and differences in the prevalence of these genetic determinants between race/ethnicity groups may contribute to race/ethnicity differences in circulating IGF levels (Jernström et al., 2001).
IGF-1 levels were sharply lower among women with a BMI less than 20 kg/m2, then rose before beginning a significant inverse trend starting with a BMI greater than 22 kg/m2. IGF-1 and free IGF-1 levels were lower with increasing BMI regardless of menopausal status. This is consistent with several prior analyses (Gram et al., 2006; Jernstrom et al., 2001; Lukanova et al., 2004), although studies of premenopausal white women have not always seen lower IGF-1 levels with a low (< 20 kg/m2) BMI (Schernhammer et al., 2007; Lukanova et al., 2004). IGF-1 levels are strongly affected by energy availability, and lean or fasting individuals in a state of relative energy deprivation may down-regulate GH receptor levels or increased resistance to GH signaling, decreasing IGF-1 synthesis (Clemmons and Underwood, 1991). This pathway may also affect IGFBP-3 and IGF-2, as we found the lowest IGFBP-3 and IGF-2 levels with a BMI less than 20 kg/m2. The pathophysiology linking obesity to lower GH and IGF-1 levels is not well understood but may involve a negative feedback loop by which lower GH secretion is mediated through decreased IGFBP-1 with visceral fat accumulation (Gram et al., 2006).
The Multiethnic Cohort Study reported obesity lowers IGF-1 levels in white but not African American participants (Henderson et al., 2006). However, we found that although African American women had higher IGF-1 levels, the associations between BMI on IGF levels were consistent with race/ethnicity. Two studies found ovulatory function did not mediate the association between BMI and premenopausal breast cancer (Michels et al., 2006; Palmer et al., 2007), and we might hypothesize that the protective association between BMI and premenopausal breast cancer risk may be attributable to the decrease in IGF-1 associated with obesity and that this protective effect could generalize across race/ethnicity. However, the literature regarding BMI and premenopausal breast cancer among African Americans is decidedly mixed (Hall et al., 2000; Mayberry, 1994; Zhu et al., 2005; Palmer et al., 2007). For example, Hall and colleagues analyzed data from the Carolina Breast Cancer Study and found a BMI greater than 30 kg/m2 was significantly associated with lower premenopausal breast cancer risk among whites (OR=0.46, 95% CI (0.26, 0.80)) but not African Americans (OR=0.89, 95% CI (0.38, 2.07)) (Hall et al., 2000). In contrast, Zhu and colleagues found BMI was associated with a nonsignificant increase in premenopausal breast cancer risk among African Americans (OR=2.49 (95% CI (0.82, 7.59)) (Zhu et al., 2005). Higher overall IGF-1 levels among African Americans may play a role, and reconciling the possible interactions between IGF levels, obesity, and race becomes more complicated if BMI affects tissue IGF-1 receptor expression and potential susceptibility to circulating IGF-1 levels (Suga et al., 2001). Biomarker assays in blood and in target tissue, in addition to body size measures, may be required.
BMI at age 21 years was inversely associated with all IGF markers levels in later life, but only among white women. The decline in IGF-1 among whites and the constant levels among African Americans with BMI at age 21 resulted in considerably lower IGF-1 levels for whites than African Americans for those who were obese in early adulthood. The reason is not clear but appears consistent with an analysis from the NHS II showing that BMI at age 18 was associated with reduced IGF-1 levels among white premenopausal women (Schernhammer et al., 2007). Our findings also raise the possibility that lower IGF-1 status may be implicated in a pre-menopausal breast cancer risk reduction with obesity among whites but not African Americans. The investigation of early-life obesity and breast cancer among African Americans is limited, with Zhu et al reporting no association between BMI at age 18 and premenopausal breast cancer while Palmer et al reporting early-life BMI was associated with a reduced risk among African Americans (Zhu et al., 2005; Palmer et al., 2007). BMI at age 21 also appeared to decrease the racial differences in IGF-2 and IGFBP-3 associated with steroid hormone activity and breast cancer risk (Figueroa et al., 1993; Gronbaek et al., 2004; Allen et al., 2005). Thus, the relevance of early-life obesity on race/ethnicity differences in IGF-1 in the context of decreasing race/ethnic differences in IGFBP-3 or IGF-2 remains to be investigated.
Strengths of this analysis include evaluation of a large number of African American and white women after excluding participants taking hormone replacement therapy or insulin. Furthermore, the range of BMI available for analyses extended beyond most studies. Rather than using only correlation coefficients to summarize IGF and BMI associations, we evaluated non-linear trends graphically over BMI values and controlled for potential confounders such as menopausal status and the reason for menopause. We excluded women with any cancer treatment within the past year, and lifetime cancer history was not significantly associated with any IGF level (all p>0.56). The women evaluated were all participants in the Southern Community Cohort Study and of generally similar socioeconomic status regardless of race. Hence, not only is the underlying study base population well defined, but also differences in education, income, or other attributes which sometimes exist in comparisons of African Americans and whites were minimized by the study design.
Our study has some limitations. Although temporal inference from BMI at age 21 to current IGF levels may be reasonable, the cross-sectional approach did not allow us to determine the temporal relationship between IGF markers and current BMI. BMI is based on self-reported data, although we have reported that self-reported BMI is correlated with blood leptin levels (Fowke et al., 2007). IGF measurements were based on a single serum sample stored at −80°C for up to four years, although IGF marker assays appear reliable when biospecimens are properly stored (Berrigan et al., 2007). Our IGF values are similar to those reported by MEC, NHS, NHANES and other research groups. Oral contraceptive use is associated with lower IGF-1 levels (Jernström et al., 2001), however we did not have data to exclude current oral contraceptive users from our analysis. If oral contraceptive use lowers IGF-1 among premenopausal women (Jernström et al., 2001) differences between pre- and postmenopausal women may be conservative. Generalization of our results to African American and white women will require confirmatory analyses in other studies. Our study also did not allow us to determine the combined effect of past and present obesity with the IGF axis on the risk of ER-/PR- breast cancer among African American women. Finding such relationship(s) requires further follow-up investigations in our cohort.
Conclusion
BMI was associated with IGF-1 levels regardless of race/ethnicity, while obesity during childhood or young adulthood may have a greater impact on IGF-1 levels among white women. The separate effects of obesity throughout life and the IGF axis on the risk of ER-/PR- breast cancer among African American women require investigation.
Acknowledgments
This project was supported by grant OP05-0927-DR1 from Susan G. Komen for the Cure. The Southern Community Cohort Study is supported by grant R01 CA92447 from the National Cancer Institute
Footnotes
Declarations of interest: - There are no conflicts of interest
Author contributions: JHF, CEM, MSB, WZ, and WJB developed the hypotheses and research approach. JHF conducted the analyses and was the primary author. HY supervised all IGF assays. QC and SC managed biospecimen and data repositories. All authors provided comments on an early draft.
References
- 1.Allen NE, Roddam AW, Allen DS, Fentiman IS, dos SS, Peto IJ, Holly JM, Key TJ. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92:1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrigan D, Potischman N, Dodd KW, Hursting SD, Lavigne J, Barrett JC, Ballard-Barbash R. Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2008 doi: 10.1016/j.ghir.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrigan D, Potischman N, Dodd KW, Nicar M, McQuillan G, Lavigne JA, Barrett JC, Ballard-Barbash R. Serum levels of insulin-like growth factor-I and insulin-like growth factor-I binding protein-3: quality control for studies of stored serum. Cancer Epidemiology Biomarkers Prevention. 2007;16:1017–1022. doi: 10.1158/1055-9965.EPI-07-0044. [DOI] [PubMed] [Google Scholar]
- 4.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent Trends in Breast Cancer Among Younger Women in the United States. J Natl Cancer Inst. 2008;100:1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clemmons DR, Underwood LE. Nutritional regulation of IGF-I and IGF binding proteins. Annual Review of Nutrition. 1991;11:393–412. doi: 10.1146/annurev.nu.11.070191.002141. [DOI] [PubMed] [Google Scholar]
- 6.DeLellis K, Rinaldi S, Kaaks RJ, Kolonel LN, Henderson B, Le Marchand L. Dietary and Lifestyle Correlates of Plasma Insulin-Like Growth Factor-I (IGF-I) and IGF Binding Protein-3 (IGFBP-3): The Multiethnic Cohort. Cancer Epidemiology Biomarkers Prevention. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 7.Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes and Control. 2008;19:527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa JA, Jackson JG, McGuire WL, Krywicki RF, Yee D. Expression of insulin-like growth factor binding proteins in human breast cancer correlates with estrogen receptor status. J Cell Biochem. 1993;52:196–205. doi: 10.1002/jcb.240520211. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Journal of the American Medical Association. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 10.Fowke JH, Matthews CM, Buchowski MS, Signorello LB, Chang SS, Cookson MS, Blot WJ. Association between prostate-specific antigen and leptin, adiponectin, HbA1c or C-peptide among African-American and Caucasian men. Prostate Cancer Prostatic Dis. 2007 doi: 10.1038/sj.pcan.4501022. [DOI] [PubMed] [Google Scholar]
- 11.Gapstur SM, Kopp P, Chiu BCH, Gann PH, Colangelo LA, Liu K. Longitudinal Associations of Age, Anthropometric and Lifestyle Factors with Serum Total Insulin-Like Growth Factor-I and IGF Binding Protein-3 Levels in Black and White Men: the CARDIA Male Hormone Study. Cancer Epidemiology Biomarkers Prevention. 2004;13:2208–2216. [PubMed] [Google Scholar]
- 12.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Tehard B, Clavel-Chapelon F, van Gils CH, van Noord PA, Peeters PH, Bueno-de-Mesquita HB, Nagel G, Linseisen J, Lahmann PH, Boeing H, Palli D, Sacerdote C, Panico S, Tumino R, Sieri S, Dorronsoro M, Quiros JR, Navarro CA, Barricarte A, Tormo MJ, Gonzalez CA, Overvad K, Paaske JS, Olsen A, Tjonneland A, Travis R, Allen N, bingham S, Khaw KT, Stattin P, Trichopoulou A, Kalapothaki V, Psaltopoulou T, Casagrande C, Riboli E, Kaaks R. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond) 2006;30:1623–1631. doi: 10.1038/sj.ijo.0803324. [DOI] [PubMed] [Google Scholar]
- 14.Gronbaek H, Flyvbjerg A, Mellemkjaer L, Tjonneland A, Christensen J, Sorensen HT, Overvad K. Serum insulin-like growth factors, insulin-like growth factor binding proteins, and breast cancer risk in postmenopausal women. Cancer Epidemiology Biomarkers Prevention. 2004;13:1759–1764. [PubMed] [Google Scholar]
- 15.Hall IJ, Newman B, Millikan RC, Moorman PG. Body size and breast cancer risk in black women and white women: the Carolina Breast Cancer Study. Am J Epidemiol. 2000;151:754–764. doi: 10.1093/oxfordjournals.aje.a010275. [DOI] [PubMed] [Google Scholar]
- 16.Harrela M, Koistinen H, Kaprio J, Lehtovirta M, Tuomilehto J, Eriksson J, Toivanen L, Koskenvuo M, Leinonen P, Koistinen R, Seppala M. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hausauer AK, Keegan TH, Chang ET, Clarke CA. Recent breast cancer trends among Asian/Pacific Islander, Hispanic, and African-American women in the US: changes by tumor subtype. Breast Cancer Res. 2007;9:R90. doi: 10.1186/bcr1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le ML. Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiology Biomarkers Prevention. 2006;15:2298–2302. doi: 10.1158/1055-9965.EPI-06-0344. [DOI] [PubMed] [Google Scholar]
- 19.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiology Biomarkers Prevention. 2002;11:852–861. [PubMed] [Google Scholar]
- 20.Janssen JA, Stolk RP, Pols HA, Grobbee DE, de Jong FH, Lamberts SW. Serum free IGF-I, total IGF-I, IGFBP-1 and IGFBP-3 levels in an elderly population: relation to age and sex steroid levels. Clin Endocrinol (Oxf) 1998;48:471–478. doi: 10.1046/j.1365-2265.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 21.Jernström H, Chu W, Vesprini D, Tao Y, Majeed N, Deal C, Pollak M, Narod SA. Genetic factors related to racial variation in plasma levels of insulin-like growth factor-1: Implications for premenopausal breast cancer risk. Molecular Genetics and Metabolism. 2001;72:144–154. doi: 10.1006/mgme.2000.3130. [DOI] [PubMed] [Google Scholar]
- 22.Jernstrom H, Deal C, Wilkin F, Chu W, Tao Y, Majeed N, Hudson T, Narod SA, Pollak M. Genetic and Nongenetic Factors Associated with Variation of Plasma Levels of Insulin-like Growth Factor-I and Insulin-like Growth Factor-binding Protein-3 in Healthy Premenopausal Women. Cancer Epidemiology Biomarkers Prevention. 2001;10:377–384. [PubMed] [Google Scholar]
- 23.Jorgensen JO, Christensen JJ, Krag M, Fisker S, Ovesen P, Christiansen JS. Serum insulin-like growth factor I levels in growth hormone-deficient adults: influence of sex steroids. Horm Res. 2004;62(Suppl 1):73–76. doi: 10.1159/000080762. [DOI] [PubMed] [Google Scholar]
- 24.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 25.Mayberry RM. Age-specific patterns of association between breast cancer and risk factors in black women, ages 20 to 39 and 40 to 54. Ann Epidemiol. 1994;4:205–213. doi: 10.1016/1047-2797(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 26.McGreevy K, Hoel B, Lipsitz S, Hoel D. Impact of nutreints on insulin-like growth factor-I, insulin-like growth factor binding protein-3, and their ratio in African American and white males. Public Health Nutrition. 2007;10:97–105. doi: 10.1017/S1368980007217999. [DOI] [PubMed] [Google Scholar]
- 27.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166:2395–2402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 28.Palmer JR, dams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A Prospective Study of Body Size and Breast Cancer in Black Women. Cancer Epidemiology Biomarkers Prevention. 2007;16:1795–1802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer RM, Mitani A, Matsuno RK, Anderson WF. Racial differences in breast cancer trends in the United States (2000–2004) J Natl Cancer Inst. 2008;100:751–752. doi: 10.1093/jnci/djn112. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial Differences in Premenopausal Endogenous Hormones. Cancer Epidemiology Biomarkers Prevention. 2005;14:2147–2153. doi: 10.1158/1055-9965.EPI-04-0944. [DOI] [PubMed] [Google Scholar]
- 31.Probst-Hensch NM, Wang H, Goh VHH, Seow A, Lee HP, Yu MC. Determinants of Circulating Insulin-like Growth Factor I and Insulin-like Growth Factor Binding Protein 3 Concentrations in a Cohort of Singapore Men and Women. Cancer Epidemiology Biomarkers Prevention. 2003;12:739–746. [PubMed] [Google Scholar]
- 32.Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer. 2006;13:273–278. doi: 10.1677/erc.1.01219. [DOI] [PubMed] [Google Scholar]
- 33.Rozen F, Yang XF, Huynh H, Pollak M. Antiproliferative action of vitamin D-related compounds and insulin- like growth factor-binding protein 5 accumulation. JNCI Journal of the National Cancer Institute. 1997;89:652–656. doi: 10.1093/jnci/89.9.652. [DOI] [PubMed] [Google Scholar]
- 34.Schernhammer ES, Tworoger SS, Eliassen AH, Missmer SA, Holly JM, Pollak MN, Hankinson SE. Body shape throughout life and correlations with IGFs and GH. Endocrine-Related Cancer. 2007;14:721–732. doi: 10.1677/ERC-06-0080. [DOI] [PubMed] [Google Scholar]
- 35.Setiawan VW, Haiman CA, Stanczyk FZ, Le Marchand L, Henderson BE. Racial/Ethnic Differences in Postmenopausal Endogenous Hormones: The Multiethnic Cohort Study. Cancer Epidemiology Biomarkers Prevention. 2006;15:1849–1855. doi: 10.1158/1055-9965.EPI-06-0307. [DOI] [PubMed] [Google Scholar]
- 36.Signorello LB, Hargreaves MK, Steinwandel MD, Zheng W, Cai Q, Schlundt DG, Buchowski MS, Arnold CW, McLaughlin JK, Blot WJ. Southern community cohort study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97:972–979. [PMC free article] [PubMed] [Google Scholar]
- 37.Suga K, Imai K, Eguchi H, Hayashi S, Higashi Y, Nakachi K. Molecular significance of excess body weight in postmenopausal breast cancer patients, in relation to expression of insulin-like growth factor I receptor and insulin-like growth factor II genes. Jpn J Cancer Res. 2001;92:127–134. doi: 10.1111/j.1349-7006.2001.tb01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu K, Caulfield J, Hunter S, Roland CL, Payne-Wilks K, Texter L. Body mass index and breast cancer risk in African American women. Ann Epidemiol. 2005;15:123–128. doi: 10.1016/j.annepidem.2004.05.011. [DOI] [PubMed] [Google Scholar]