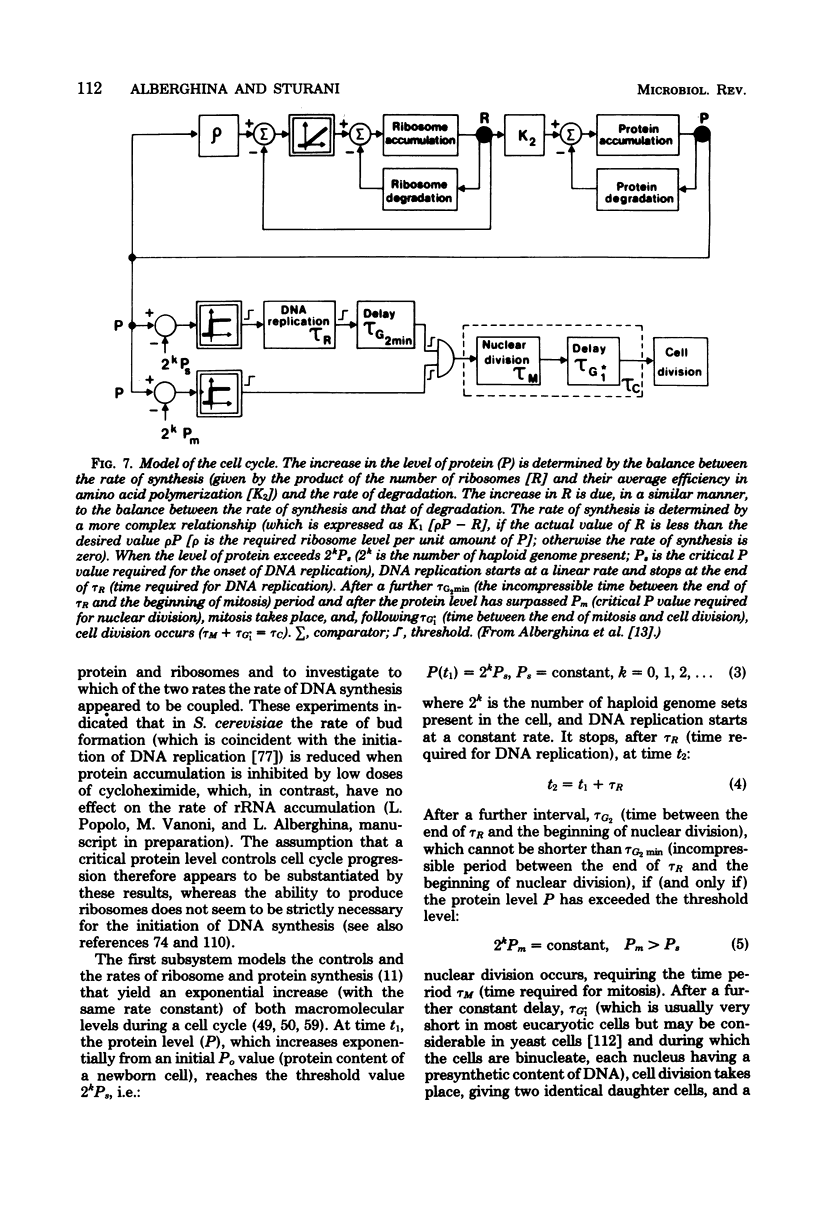
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberghina F. A. A model for the regulation of growth in mammalian cells. J Theor Biol. 1975 Dec;55(2):533–545. doi: 10.1016/s0022-5193(75)80100-7. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A. Growth regulation in Neurospora crassa. Effects of nutrients and of temperature. Arch Mikrobiol. 1973 Feb 5;89(2):83–94. doi: 10.1007/BF00425025. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A., Guarnieri D. Change of the cytochrome oxidase level during exponential growth in Neurospora crassa. Experientia. 1975 Aug 15;31(8):914–915. doi: 10.1007/BF02358848. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A., Schiaffonati L., Zardi L., Sturani E. Lack of guanosine tetraphosphate accumulation during inhibition of RNA synthesis in Neurospora crassa. Biochim Biophys Acta. 1973 Jun 23;312(2):435–439. doi: 10.1016/0005-2787(73)90389-4. [DOI] [PubMed] [Google Scholar]
- Alberghina F. A., Sturani E., Gohlke J. R. Levels and rates of synthesis of ribosomal ribonucleic acid, transfer ribonucleic acid, and protein in Neurospora crassa in different steady states of growth. J Biol Chem. 1975 Jun 25;250(12):4381–4388. [PubMed] [Google Scholar]
- Alberghina F. A., Trezzi F., Signorini R. C. The biogenesis of mitochondria in Neurospora crassa: ultrastructural changes induced by nutrients. Cell Differ. 1974 Mar;2(6):307–317. doi: 10.1016/0045-6039(74)90009-8. [DOI] [PubMed] [Google Scholar]
- Alberghina L., Mariani L. Analysis of a cell cycle model for Escherichia coli. J Math Biol. 1980 Jun;9(4):389–398. doi: 10.1007/BF00276501. [DOI] [PubMed] [Google Scholar]
- Alberghina L., Mariani L., Martegani E. Analysis of a model of cell cycle in eukaryotes. J Theor Biol. 1980 Nov 7;87(1):171–188. doi: 10.1016/0022-5193(80)90226-x. [DOI] [PubMed] [Google Scholar]
- Barford J. P., Hall R. J. Estimation of the length of cell cycle phases from asynchronous cultures of Saccharomyces cerevisiae. Exp Cell Res. 1976 Oct 15;102(2):276–284. doi: 10.1016/0014-4827(76)90043-4. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Maaloe O. The effects of fusidic acid on growth, ribosome synthesis and RNA metabolism in Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):541–561. doi: 10.1016/0022-2836(74)90234-4. [DOI] [PubMed] [Google Scholar]
- Boehlke K. W., Friesen J. D. Cellular content of ribonucleic acid and protein in Saccharomyces cerevisiae as a function of exponential growth rate: calculation of the apparent peptide chain elongation rate. J Bacteriol. 1975 Feb;121(2):429–433. doi: 10.1128/jb.121.2.429-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock C. J. DNA synthesis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1970 Apr;60(1):16–26. doi: 10.1016/0014-4827(70)90484-2. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. Continuous protein synthesis is required to maintain the probability of entry into S phase. Cell. 1977 Sep;12(1):311–317. doi: 10.1016/0092-8674(77)90209-4. [DOI] [PubMed] [Google Scholar]
- Brooks R. F. The cytoplasmic origin of variability in the timing of S phase in mammalian cells. Cell Biol Int Rep. 1979 Dec;3(9):707–716. doi: 10.1016/0309-1651(79)90075-4. [DOI] [PubMed] [Google Scholar]
- Buckel P., Böck A. Lack of accumulation of unusual guanosine nucleotides upon amino acid starvation of two eukaryotic organisms. Biochim Biophys Acta. 1973 Sep 28;324(1):184–187. doi: 10.1016/0005-2787(73)90262-1. [DOI] [PubMed] [Google Scholar]
- Buhler J. M., Iborra F., Sentenac A., Fromageot P. Structural studies on yeast RNA polymerases. Existence of common subunits in RNA polymerases A(I) and B(II). J Biol Chem. 1976 Mar 25;251(6):1712–1717. [PubMed] [Google Scholar]
- Bull A. T., Trinci A. P. The physiology and metabolic control of fungal growth. Adv Microb Physiol. 1977;15:1–84. doi: 10.1016/s0065-2911(08)60314-8. [DOI] [PubMed] [Google Scholar]
- Cammarano P., Felsani A., Romeo A., Alberghina F. M. Particle weights of active ribosomal subunits from Neurospora crassa. Biochim Biophys Acta. 1973 May 18;308(3):404–411. doi: 10.1016/0005-2787(73)90333-x. [DOI] [PubMed] [Google Scholar]
- Carlin R. K. The poly(A) segment of mRNA: (1) Evolution and function and (2) The evolution of viruses. J Theor Biol. 1978 Apr 6;71(3):323–338. doi: 10.1016/0022-5193(78)90163-7. [DOI] [PubMed] [Google Scholar]
- Carter B. L., Dawes I. W. Synthesis of two DNA-dependent RNA polymerases in yeast. Exp Cell Res. 1975 May;92(2):253–258. doi: 10.1016/0014-4827(75)90378-x. [DOI] [PubMed] [Google Scholar]
- Carter B. L., Jagadish M. N. Control of cell division in the yeast Saccharomyces cerevisiae cultured at different growth rates. Exp Cell Res. 1978 Mar 15;112(2):373–383. doi: 10.1016/0014-4827(78)90220-3. [DOI] [PubMed] [Google Scholar]
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Miller M. R., Lehtomaki D. M., Pardee A. B. Comparison of DNA replication and repair enzymology using permeabilized baby hamster kidney cells. J Biol Chem. 1979 Aug 10;254(15):6904–6908. [PubMed] [Google Scholar]
- Chattopadhyay S. K., Kohne D. E., Dutta S. K. Ribosomal RNA genes of Neurospora: isolation and characterization. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3256–3259. doi: 10.1073/pnas.69.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin H. J., Sauer B. L., Munkres K. D. Respiration of wild type and extrachromosomal mutants of Neurospora crassa. J Bacteriol. 1973 Dec;116(3):1314–1321. doi: 10.1128/jb.116.3.1314-1321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. A unifying model for the G1 period in prokaryotes and eukaryotes. Nature. 1979 Jul 5;280(5717):17–19. doi: 10.1038/280017a0. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Costantini M. G., Zippel R., Sturani E. Levels of the ribonucleoside triphosphates and rate of RNA synthesis in Neurospora crassa. Biochim Biophys Acta. 1977 Jun 17;476(4):272–278. doi: 10.1016/0005-2787(77)90291-x. [DOI] [PubMed] [Google Scholar]
- Cox R. A., Peden K. Organization of the ribosomal ribonucleic acid gene cluster of Neurospora crassa by means of restriction endonucleases and cloning in bacteriophage lambda [proceedings]. Biochem Soc Trans. 1978;6(6):1230–1232. doi: 10.1042/bst0061230. [DOI] [PubMed] [Google Scholar]
- Craig N. Effect of reduced temperatures on protein synthesis in mouse L cells. Cell. 1975 Apr;4(4):329–335. doi: 10.1016/0092-8674(75)90153-1. [DOI] [PubMed] [Google Scholar]
- Craig N. Regulation of translation in rabbit reticulocytes and mouse L-cells; comparison of the effects of temperature. J Cell Physiol. 1975 Dec;87(2):157–166. doi: 10.1002/jcp.1040870204. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Das H. K., Goldstein A. Limited capacity for protein synthesis at zero degrees centigrade in Escherichia coli. J Mol Biol. 1968 Jan 28;31(2):209–226. doi: 10.1016/0022-2836(68)90440-3. [DOI] [PubMed] [Google Scholar]
- DeCarlo R. R., Somberg E. W. The effect of essential amino acid deprivation on macromolecular synthesis and nucleotide pool sizes in Neurospora crassa. Arch Biochem Biophys. 1974 Nov;165(1):201–212. doi: 10.1016/0003-9861(74)90156-8. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes P. A. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977 Apr;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Grant W. D., Pritchard R. H., Sudbery P. E., Wheals A. E. The regulation of cell size and the control of mitosis. J Theor Biol. 1975 Mar;50(1):213–244. doi: 10.1016/0022-5193(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978 Sep;115(2):317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Fantes P., Nurse P. Control of cell size at division in fission yeast by a growth-modulated size control over nuclear division. Exp Cell Res. 1977 Jul;107(2):377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The regulation of synthesis of Krebs cycle enzymes in Neurospora by catabolite and end product repression. Eur J Biochem. 1970 Apr;13(3):548–553. doi: 10.1111/j.1432-1033.1970.tb00959.x. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Fraser R. S., Carter B. L. Synthesis of polyadenylated messenger RNA during the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1976 Jun 14;104(1):223–242. doi: 10.1016/0022-2836(76)90010-3. [DOI] [PubMed] [Google Scholar]
- Fraser R. S. Turnover of polyadenylated messenger RNA in fission yeast. Evidence for the control of protein synthesis at the translational level. Eur J Biochem. 1975 Dec 15;60(2):477–486. doi: 10.1111/j.1432-1033.1975.tb21026.x. [DOI] [PubMed] [Google Scholar]
- Free S. J., Rice P. W., Metzenberg R. L. Arrangement of the genes coding for ribosomal ribonucleic acids in Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1219–1226. doi: 10.1128/jb.137.3.1219-1226.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H., Lu P., Rich A. Ribosomal subunits produced by cold sensitive initiation of protein synthesis. Nature. 1969 Aug 30;223(5209):909–913. doi: 10.1038/223909a0. [DOI] [PubMed] [Google Scholar]
- Fukuda R., Taketo M., Ishihama A. Autogenous regulation of RNA polymerase beta subunit synthesis in vitro. J Biol Chem. 1978 Jul 10;253(13):4501–4504. [PubMed] [Google Scholar]
- Gallant J., Shell L., Bittner R. A novel nucleotide implicated in the response of E. coli to energy source downshift. Cell. 1976 Jan;7(1):75–84. doi: 10.1016/0092-8674(76)90257-9. [DOI] [PubMed] [Google Scholar]
- Germershausen J., Goodman D., Somberg E. W. 5' Cap methylation of homologous poly A(+) RNA by a RNA (guanine-7) methyltransferase from Neurospora crassa. Biochem Biophys Res Commun. 1978 Jun 14;82(3):871–878. doi: 10.1016/0006-291x(78)90864-1. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Howell E. M., Li J. B., Martel S. B., Prouty W. F. Physiological significance of protein degradation in animal and bacterial cells. Fed Proc. 1974 Apr;33(4):1112–1120. [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F. Diadenosine 5',5'''-P1,P4-tetraphosphate triggers initiation of in vitro DNA replication in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):371–375. doi: 10.1073/pnas.75.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Grummt I., Mayer E. Ribosome biosynthesis is not necessary for initiation of DNA replication. Eur J Biochem. 1979 Jun;97(1):37–42. doi: 10.1111/j.1432-1033.1979.tb13083.x. [DOI] [PubMed] [Google Scholar]
- Grummt F., Grummt I. Studies on the role of uncharged tRNA in pleiotypic response of animal cells. Eur J Biochem. 1976 Apr 15;64(1):307–312. doi: 10.1111/j.1432-1033.1976.tb10302.x. [DOI] [PubMed] [Google Scholar]
- Hager G. L., Holland M. J., Rutter W. J. Isolation of ribonucleic acid polymerases I, II, and III from Saccharomyces cerevisiae. Biochemistry. 1977 Jan 11;16(1):1–8. doi: 10.1021/bi00620a001. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R. S., Tittawella I. P., Scaife J. G. Evidence for specific control of RNA polymerase synthesis in Escherichia coli. Nat New Biol. 1973 May 2;243(122):6–9. [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Howell N., Zuiches C. A., Munkres K. D. Mitochondrial biogenesis in Neurospora crassa. I. An ultrastructural and biochemical investigation of the effects of anaerobiosis and chloramphenicol inhibition. J Cell Biol. 1971 Sep;50(3):721–736. doi: 10.1083/jcb.50.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Phillips S. L. Turnover of polyadenylate-containing ribonucleic acid in Saccharomyces cerevisiae. J Bacteriol. 1976 Feb;125(2):595–600. doi: 10.1128/jb.125.2.595-600.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Ehrhardt C. W., Lorincz A., Carter B. L. Regulation of cell size in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):1–5. doi: 10.1128/jb.137.1.1-5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Kessel M., Rosenberger R. F. Regulation and timing of deoxyribonucleic acid synthesis in hyphae of Aspergillus nidulans. J Bacteriol. 1968 Jun;95(6):2275–2281. doi: 10.1128/jb.95.6.2275-2281.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khym J. X., Jones M. H., Lee W. H., Regan J. D., Volkin E. On the question of compartmentalization of the nucleotide pool. J Biol Chem. 1978 Dec 25;253(24):8741–8746. [PubMed] [Google Scholar]
- Killander D., Zetterberg A. A quantitative cytochemical investigation of the relationship between cell mass and initiation of DNA synthesis in mouse fibroblasts in vitro. Exp Cell Res. 1965 Oct;40(1):12–20. doi: 10.1016/0014-4827(65)90285-5. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Kuriyama Y., Luck D. J. Ribosomal RNA synthesis in mitochondria of Neurospora crassa. J Mol Biol. 1973 Feb 5;73(4):425–437. doi: 10.1016/0022-2836(73)90091-0. [DOI] [PubMed] [Google Scholar]
- Leick V. Growth rate dependency of protein and nucleic acid composition of Tetrahymena pyriformis and the control of synthesis of ribosomal and transfer RNA. C R Trav Lab Carlsberg. 1967;36(7):113–126. [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- Lindgren A., Westermark B. Reset of the pre-replicative phase of human glia cells in culture. Exp Cell Res. 1977 Apr;106(1):89–93. doi: 10.1016/0014-4827(77)90244-0. [DOI] [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- Lucas M. C., Jacobson J. W., Giles N. H. Characterization and in vitro translation of polyadenylated messenger ribonucleic acid from Neurospora crassa. J Bacteriol. 1977 Jun;130(3):1192–1198. doi: 10.1128/jb.130.3.1192-1198.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M. C., Vanderhoef L. N. Characterization of 5.8S ribosomal ribonucleic acid in Neurospora crassa. J Bacteriol. 1975 Nov;124(2):736–739. doi: 10.1128/jb.124.2.736-739.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Oliver S. G., McLaughlin C. S. The regulation of RNA synthesis in yeast II: Amino acids shift-up experiments. Mol Gen Genet. 1977 Dec 30;158(2):117–122. doi: 10.1007/BF00268303. [DOI] [PubMed] [Google Scholar]
- López S., Gancedo J. M. Effect of metabolic conditions on protein turnover in yeast. Biochem J. 1979 Mar 15;178(3):769–776. doi: 10.1042/bj1780769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martegani E., Alberghina L. Intracellular protein degradation in Neurospora crassa. J Biol Chem. 1979 Aug 10;254(15):7047–7054. [PubMed] [Google Scholar]
- Martegani E., Levi M., Trezzi F., Alberghina L. Nuclear division cycle in Neurospora crassa hyphae under different growth conditions. J Bacteriol. 1980 Apr;142(1):268–275. doi: 10.1128/jb.142.1.268-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martegani E., Popolo L., Alberghina L., Sturani E. Reduction of ribosome activity and synthesis of stable RNA in Neurospora crassa. Biochim Biophys Acta. 1980 Dec 11;610(2):318–330. doi: 10.1016/0005-2787(80)90013-1. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E., McCalley B. Synthesis of polyadenylic acid-containing ribonucleic acid during the germination of Neurospora crassa conidia. J Bacteriol. 1976 Jan;125(1):174–180. doi: 10.1128/jb.125.1.174-180.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkes P. E. Messenger ribonucleoprotein complexes isolated by oligodeoxythymidylate-cellulose chromatography from Neurospora crassa polysomes. J Bacteriol. 1977 Jul;131(1):240–246. doi: 10.1128/jb.131.1.240-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M., Darzynkiewicz Z., Baserga R. DNA synthesis and cell division in a mammalian cell mutant temperature sensitive for the processing of ribosomal RNA. Exp Cell Res. 1980 Jan;125(1):241–249. doi: 10.1016/0014-4827(80)90208-6. [DOI] [PubMed] [Google Scholar]
- Muto A. Control of ribosomal RNA synthesis in Escherichia coli. IV. Frequency of transcription of ribosomal RNA genes as a function of growth rate. Mol Gen Genet. 1978 Aug 4;164(1):39–44. doi: 10.1007/BF00267596. [DOI] [PubMed] [Google Scholar]
- NYGAARD O. F., GUTTES S., RUSCH H. P. Nucleic acid metabolism in a slime mold with synchronous mitosis. Biochim Biophys Acta. 1960 Feb 26;38:298–306. doi: 10.1016/0006-3002(60)91245-2. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. A. A control acting over the initiation of DNA replication in the yeast Schizosaccharomyces pombe. J Cell Sci. 1979 Apr;36:155–168. doi: 10.1242/jcs.36.1.155. [DOI] [PubMed] [Google Scholar]
- Nazario M., Evans J. A. Physical and kinetic studies of arginyl transfer ribonucleic acid ligase of Neurospora. A sequential ordered mechanism. J Biol Chem. 1974 Aug 10;249(15):4934–4936. [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975 Aug 14;256(5518):547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McLaughlin C. S. The regulation of RNA synthesis in yeast. I: Starvation experiments. Mol Gen Genet. 1977 Jul 20;154(2):145–153. doi: 10.1007/BF00330830. [DOI] [PubMed] [Google Scholar]
- Ord M. J. The synthesis of DNA through the cell cycle of Amoeba proteus. J Cell Sci. 1968 Dec;3(4):483–491. doi: 10.1242/jcs.3.4.483. [DOI] [PubMed] [Google Scholar]
- POWELL E. O. An outline of the pattern of bacterial generation times. J Gen Microbiol. 1958 Apr;18(2):382–417. doi: 10.1099/00221287-18-2-382. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., McLaughlin C. S., Nierlich D. P. Half life of yeast messenger RNA. Nature. 1976 Mar 4;260(5546):70–72. doi: 10.1038/260070a0. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Stringent control of intracellular proteolysis in Escherichia coli. J Bacteriol. 1973 Dec;116(3):1253–1257. doi: 10.1128/jb.116.3.1253-1257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut B. S., Turnock G. Coordination of macromolecular synthesis in the slime mould Physarum polycephalum. Mol Gen Genet. 1975;137(3):211–225. doi: 10.1007/BF00333017. [DOI] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J. 1965 Oct;97(1):284–297. doi: 10.1042/bj0970284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O. Effect of growth rate on the macromolecular composition of Prototheca zopfii, a colorless alga which divides by multiple fission. J Bacteriol. 1973 Jan;113(1):203–211. doi: 10.1128/jb.113.1.203-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premakumar R., Sorger G. J., Gooden D. Stability of messenger RNA for nitrate reductase in Neurospora crassa. Biochim Biophys Acta. 1978 Jun 22;519(1):275–278. doi: 10.1016/0005-2787(78)90080-1. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H., Zaritsky A. Effect of thymine concentration on the replication velocity of DNA in a thymineless mutant of Escherichia coli. Nature. 1970 Apr 11;226(5241):126–131. doi: 10.1038/226126a0. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Zamecnik P. C. Presence of diadenosine 5',5''' -P1, P4-tetraphosphate (Ap4A) in mamalian cells in levels varying widely with proliferative activity of the tissue: a possible positive "pleiotypic activator". Proc Natl Acad Sci U S A. 1976 Nov;73(11):3984–3988. doi: 10.1073/pnas.73.11.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron A., Prescott D. M. The timing of DNA synthesis in Amoeb proteus. Exp Cell Res. 1969 Aug;56(2):430–434. doi: 10.1016/0014-4827(69)90035-4. [DOI] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- Russell P. J., Hammett J. R., Selker E. U. Neurospora crassa cytoplasmic ribosomes; ribosomal ribonucleic acid synthesis in the wild type. J Bacteriol. 1976 Aug;127(2):785–793. doi: 10.1128/jb.127.2.785-793.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Neurospora crassa conidial germination: role of endogenous amino acid pools. J Bacteriol. 1975 Oct;124(1):232–242. doi: 10.1128/jb.124.1.232-242.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian J., Mian F., Halvorson H. O. Effect of the growth rate on the level of the DNA-dependent RNA polymerases in Saccharomyces cerevisiae. FEBS Lett. 1973 Aug 15;34(2):159–162. doi: 10.1016/0014-5793(73)80782-3. [DOI] [PubMed] [Google Scholar]
- Sebastian J., Takano I., Halvorson H. O. Independent regulation of the nuclear RNA polymerases I and II during the yeast cell cycle. Proc Natl Acad Sci U S A. 1974 Mar;71(3):769–773. doi: 10.1073/pnas.71.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel B. L., Somberg E. W. Characterization of Neurospora crassa polyadenylated messenger ribonucleic acid: structure of the 5'-terminus. Arch Biochem Biophys. 1978 Apr 15;187(1):108–112. doi: 10.1016/0003-9861(78)90012-7. [DOI] [PubMed] [Google Scholar]
- Serna L., Stadler D. Nuclear division cycle in germinating conidia of Neurospora crassa. J Bacteriol. 1978 Oct;136(1):341–351. doi: 10.1128/jb.136.1.341-351.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn A., Horowitz N. H. A study of transfer ribonucleic acid in Neurospora. I. The attachment of amino acids and amino acid analogs. Biochemistry. 1969 Jan;8(1):295–303. doi: 10.1021/bi00829a042. [DOI] [PubMed] [Google Scholar]
- Sheldon R., Jurale C., Kates J. Detection of polyadenylic acid sequences in viral and eukaryotic RNA(polu(U)-cellulose columns-poly(U) filters-fiberglass-HeLa cells-bacteriophage T4). Proc Natl Acad Sci U S A. 1972 Feb;69(2):417–421. doi: 10.1073/pnas.69.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields R., Brooks R. F., Riddle P. N., Capellaro D. F., Delia D. Cell size, cell cycle and transition probability in mouse fibroblasts. Cell. 1978 Oct;15(2):469–474. doi: 10.1016/0092-8674(78)90016-8. [DOI] [PubMed] [Google Scholar]
- Shields R. Further evidence for a random transition in the cell cycle. Nature. 1978 Jun 29;273(5665):755–758. doi: 10.1038/273755a0. [DOI] [PubMed] [Google Scholar]
- Shilo B., Riddle V. G., Pardee A. B. Protein turnover and cell-cycle initiation in yeast. Exp Cell Res. 1979 Oct 15;123(2):221–227. doi: 10.1016/0014-4827(79)90462-2. [DOI] [PubMed] [Google Scholar]
- Shilo B., Shilo V., Simchen G. Cell-cycle initiation in yeast follows first-order kinetics. Nature. 1976 Dec 23;264(5588):767–770. doi: 10.1038/264767a0. [DOI] [PubMed] [Google Scholar]
- Shulman R. W., Sripati C. E., Warner J. R. Noncoordinated transcription in the absence of protein synthesis in yeast. J Biol Chem. 1977 Feb 25;252(4):1344–1349. [PubMed] [Google Scholar]
- Shulman R. W., Warner J. R. Ribosomal RNA transcription in a mutant of Saccharomyces cerevisiae defective in ribosomal protein synthesis. Mol Gen Genet. 1978 May 3;161(2):221–223. doi: 10.1007/BF00274191. [DOI] [PubMed] [Google Scholar]
- Silverman R. H., Atherly A. G. The search for guanosine tetraphosphate (ppGpp) and other unusual nucleotides in eucaryotes. Microbiol Rev. 1979 Mar;43(1):27–41. doi: 10.1128/mr.43.1.27-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjold A. C., Juarez H., Hedgcoth C. Relationships among deoxyribonucleic acid, ribonucleic acid, and specific transfer ribonucleic acids in Escherichia coli 15T - at various growth rates. J Bacteriol. 1973 Jul;115(1):177–187. doi: 10.1128/jb.115.1.177-187.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L. Adenine nucleotide levels in Neurospora, as influenced by conditions of growth and by metabolic inhibitors. J Bacteriol. 1973 May;114(2):752–766. doi: 10.1128/jb.114.2.752-766.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. W., Rees D. C., Orchard P. P., Slayman C. L. Generation of adenosine triphosphate in cytochrome-deficient mutants of Neurospora. J Biol Chem. 1975 Jan 25;250(2):396–408. [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon S. L., Matchett W. H. Inhibition of aminoacyl-transfer ribonucleic acid synthetases and the regulation of amino acid biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1977 Mar;129(3):1303–1312. doi: 10.1128/jb.129.3.1303-1312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John A. C., Conklin K., Rosenthal E., Goldberg A. L. Further evidence for the involvement of charged tRNA and guanosine tetraphosphate in the control of protein degradation in Escherichia coli. J Biol Chem. 1978 Jun 10;253(11):3945–3951. [PubMed] [Google Scholar]
- St John A. C., Goldberg A. L. Effects of reduced energy production on protein degradation, guanosine tetraphosphate, and RNA synthesis in Escherichia coli. J Biol Chem. 1978 Apr 25;253(8):2705–2711. [PubMed] [Google Scholar]
- Steele G. C., Trinci A. P. Effect of temperature and temperature shifts on growth and branching of a wild type and a temperature sensitive colonial mutant (Cot 1) of Neurospora crassa. Arch Microbiol. 1977 May 13;113(1-2):43–48. doi: 10.1007/BF00428578. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Yanishevsky R. M. Entry into S phase is inhibited in two immortal cell lines fused to senescent human diploid cells. Exp Cell Res. 1979 Apr;120(1):155–165. doi: 10.1016/0014-4827(79)90546-9. [DOI] [PubMed] [Google Scholar]
- Sturani E., Costantini M. G., Martegani E., Alberghina L. Level and turnover of polyadenylate-containing ribonucleic acid in Neurospora crassa in different steady states of growth. Eur J Biochem. 1979 Aug 15;99(1):1–7. doi: 10.1111/j.1432-1033.1979.tb13224.x. [DOI] [PubMed] [Google Scholar]
- Sturani E., Costantini M. G., Zippel R., Alberghina F. A. Regulation of RNA synthesis in Neurospora crassa. An analysis of a shift-up. Exp Cell Res. 1976 May;99(2):245–252. doi: 10.1016/0014-4827(76)90580-2. [DOI] [PubMed] [Google Scholar]
- Sturani E., Magnani F., Alberghina F. A. Inhibition of ribosomal RNA synthesis during a shift-down transition of growth in Neurospora crassa. Biochim Biophys Acta. 1973 Aug 24;319(2):153–164. doi: 10.1016/0005-2787(73)90006-3. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. IV. Accumulation of intermediates in mutants defective in the subunit assembly. J Mol Biol. 1976 Apr 5;102(2):297–310. doi: 10.1016/s0022-2836(76)80055-1. [DOI] [PubMed] [Google Scholar]
- Tan S. T., Marzluf G. A. Multiple intracellular peptidases in Neurospora crassa. J Bacteriol. 1979 Mar;137(3):1324–1332. doi: 10.1128/jb.137.3.1324-1332.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez de Iñon M. T., Leoni P. D., Torres H. N. RNA polymerase activities in Neurospora crassa. FEBS Lett. 1974 Feb 1;39(1):91–95. doi: 10.1016/0014-5793(74)80024-4. [DOI] [PubMed] [Google Scholar]
- Thuriaux P., Nurse P., Carter B. Mutants altered in the control co-ordinating cell division with cell growth in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1978 May 3;161(2):215–220. doi: 10.1007/BF00274190. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Turian G. Multiple DNA-dependent RNA polymerases of Neurospora. Experientia. 1974 Nov 15;30(11):1236–1238. doi: 10.1007/BF01945157. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Trinci A. P. A study of the kinetics of hyphal extension and branch initiation of fungal mycelia. J Gen Microbiol. 1974 Mar;81(1):225–236. doi: 10.1099/00221287-81-1-225. [DOI] [PubMed] [Google Scholar]
- Trinci A. P. The hyphal growth unit of wild type and spreading colonial mutants of Neurospora crassa. Arch Mikrobiol. 1973 May 24;91(2):127–136. doi: 10.1007/BF00424756. [DOI] [PubMed] [Google Scholar]
- Tyson C. B., Lord P. G., Wheals A. E. Dependency of size of Saccharomyces cerevisiae cells on growth rate. J Bacteriol. 1979 Apr;138(1):92–98. doi: 10.1128/jb.138.1.92-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. Ribosomal RNA synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):227–242. doi: 10.1016/0022-2836(72)90279-3. [DOI] [PubMed] [Google Scholar]
- Unger M. W., Hartwell L. H. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc Natl Acad Sci U S A. 1976 May;73(5):1664–1668. doi: 10.1073/pnas.73.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Hager G. L., Weinberg F., Rutter W. J. Molecular structure of yeast RNA polymerase III: demonstration of the tripartite transcriptive system in lower eukaryotes. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1024–1028. doi: 10.1073/pnas.73.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keulen H., Retèl J. Transcription specificity of yeast RNA polymerase A. Highly specific transcription in vitro of the homologous ribosomal transcription units. Eur J Biochem. 1977 Oct 3;79(2):579–588. doi: 10.1111/j.1432-1033.1977.tb11842.x. [DOI] [PubMed] [Google Scholar]
- Viotti A., Bagni N., Sturani E., Alberghina F. A. Magnesium and polyamine levels in Neurospora crassa mycelia. Biochim Biophys Acta. 1971 Aug 19;244(2):329–337. doi: 10.1016/0304-4165(71)90234-0. [DOI] [PubMed] [Google Scholar]
- WEIJER D. L. KARYOKINESIS OF SOMATIC NUCLEI OF NEUROSPORA CRASSA. I. THE CORRELATION BETWEEN CONIDIAL RADIOSENSITIVITY AND THEIR KARYOKINETIC STAGE. Can J Genet Cytol. 1964 Dec;6:383–392. doi: 10.1139/g64-049. [DOI] [PubMed] [Google Scholar]
- WEIJER J., KOOPMANS A., WEIJER D. L. KARYOKINESIS OF SOMATIC NUCLEI OF NEUROSPORA CRASSA: 3. THE JUVENILE AND MATURATION CYCLES (FEULGEN AND CRYSTAL VIOLET STAINING). Can J Genet Cytol. 1965 Mar;7:140–163. doi: 10.1139/g65-020. [DOI] [PubMed] [Google Scholar]
- Waldron C., Jund R., Lacroute F. Evidence for a high proportion of inactive ribosomes in slow-growing yeast cells. Biochem J. 1977 Dec 15;168(3):409–415. doi: 10.1042/bj1680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron C., Jund R., Lacroute F. The elongation rate of proteins of different molecular weight classes in yeast. FEBS Lett. 1974 Sep 15;46(1):11–16. doi: 10.1016/0014-5793(74)80323-6. [DOI] [PubMed] [Google Scholar]
- Waldron C., Lacroute F. Effect of growth rate on the amounts of ribosomal and transfer ribonucleic acids in yeast. J Bacteriol. 1975 Jun;122(3):855–865. doi: 10.1128/jb.122.3.855-865.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron C. Synthesis of ribosomal and transfer ribonucleic acids in yeast during a nutritional shift-up. J Gen Microbiol. 1977 Jan;98(1):215–221. doi: 10.1099/00221287-98-1-215. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. Yeast has a true stringent response. Nature. 1978 Sep 28;275(5678):338–339. doi: 10.1038/275338a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Whatley S. A., Hill B. T. The relationship between RNA content, cell volume and growth potential in ageing human embryonic mesenchymal cells. Cell Biol Int Rep. 1979 Nov;3(8):671–683. doi: 10.1016/0309-1651(79)90098-5. [DOI] [PubMed] [Google Scholar]
- ZALOKAR M. Kinetics of amino acid uptake and protein synthesis in Neurospora. Biochim Biophys Acta. 1961 Jan 29;46:423–432. doi: 10.1016/0006-3002(61)90573-x. [DOI] [PubMed] [Google Scholar]


