Abstract
Purpose
To quantitatively evaluate cone-beam CT (CBCT) in target volume definition in an offline image guidance environment.
Methods and Materials
Fifteen patients each with 5 helical CTs (HCT) and 8 CBCTs were included. A single physician manually delineated prostate and seminal vesicles (SVs) on each CT. The clinical target volume (CTV) was prostate for low risk group (G1), plus SVs for intermediate risk group (G2). The internal target volumes (ITVs) on CBCT (ITVCBCT) was constructed and compared with ITVHCT. The following comparisons were performed: CTV and ITV volumes in HCT and CBCT; similarity of ITVs using overlap index (OI); surface differences between ITVs; quality assurance of ITVCBCT using CTV from weekly CBCT; and dosimetric evaluations of ITVHCT coverage on plans from ITVCBCT.
Results
There was no statistical significant difference of CTV or ITV volumes. The ITV OIs were 91%/88% for G1/G2 patients. They improved significantly with 1-2 mm margins. Therefore, the ITVs were mostly within 2 mm. The CTVs from weekly CBCT had >95% overlap with ITVCBCT. The ITV dose differences (D95, and Dmean) were <0.3%.
Conclusions
It is feasible to use CBCT for target definition in offline image guidance, thereby eliminating the separate helical CT scan process.
Keywords: Image-guided radiation therapy, Prostate Cancer, Cone-beam CT, Online and Offline Image Guidance, Margin
1 Introduction
Prostate cancer is the second most frequently diagnosed cancer and the third leading cause of death from cancer in men. Fractionated external beam radiation therapy represents one of the primary treatment modalities for patients with localized prostate cancer. The clinical target volume (CTV) includes prostate gland for low risk group patients, plus seminal vesicles (SVs) for intermediate risk group patients (1-3). Both organs are located between the bladder and the rectum. The position and shape of the CTV is affected by physiological changes in the bladder and rectum filling, which can vary from day to day. To account for these variations and ensure adequate dose coverage to the CTV, a margin is usually added to CTV to form the planning target volume (PTV) in treatment planning, which in turn may limit the dose that the CTV can be prescribed to and may also increases the doses to the surrounding organs-at-risk (OARs). In an effort to improve the accuracy and precision of dose delivery without increasing margins, we have developed an offline adaptive radiotherapy (ART) process over the past decade (4, 5). The ART is a treatment feedback control process that optimizes an individual patient treatment using the patient specific information measured during the treatment course. Specifically, a population margin of 1 cm is used to expand the initial CTV to PTV for the first 5 fractions of radiation treatment, during which four (4) additional helical CT (HCT) scans of the patient are performed, the CTV on each daily HCT is delineated, the combination of these CTVs and the one from planning HCT are used to form the patient specific internal target volume (ITV), which account for interfractional organ motion and shape change. A non-uniform margin, derived from the measurements of the setup errors from the first week treatments to account for the residual of systematic setup error prediction and compensation of random setup error, is added to the ITV to form the patient-specific PTV in the modified plan for the remainder treatment fractions. During daily treatment, patient setup is not corrected, therefore there is no negative effect in delivery efficiency, the analyses and decision making are performed offline. The ART has been shown to reduce the effective margins of the PTV and improve the local control on these patients (6, 7).
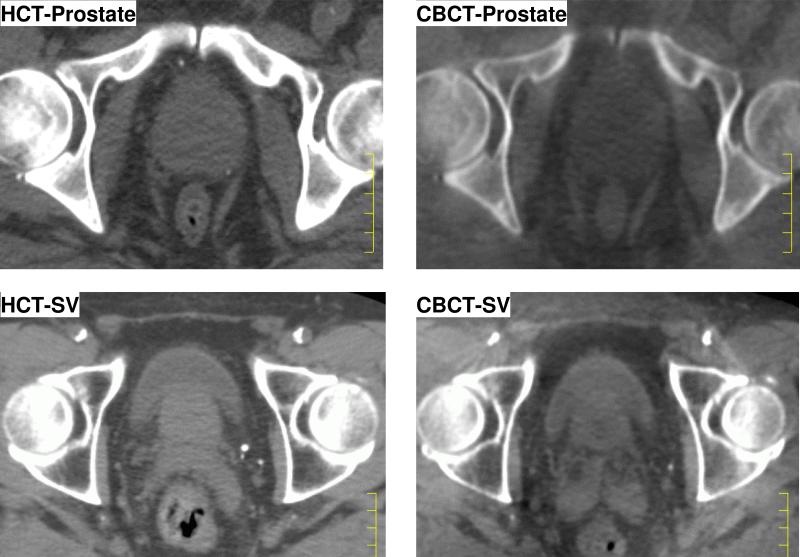
Recently medical linear accelerators capable of integrated kV cone beam CT (CBCT) have become available (8), thus providing the opportunity to localize target volumes while the patient is on the treatment table, just before or after each treatment delivery. The issue has been raised whether the daily HCT can be replaced with CBCT in order to improve the efficiency of workflow. The CBCT has excellent contrast between bone, soft tissue and air cavity, and is ideally used for target localization and image guidance. However, due to the lack of scatter rejection, the contrast between soft tissues, which is very important for pelvic region, is sub-optimal. Therefore, the online image guidance currently practiced with CBCT usually involves implanted radio-opaque markers. The uncertainties of directly using CBCT without markers remain to be investtigated. Fig. 1 shows the axial slice at different locations for HCT and CBCT for the same patient. The purpose of this study is to perform a comprehensive evaluation of CBCT in the target definition in an offline image guidance environment, without implanted markers.
Figure 1.
Comparison of helical CT (HCT, left) and cone-beam CT (CBCT, right) image quality on axial slices. Top row: prostate level, bottom row: Seminal Vesicles (SV) level.
2 Methods and Materials
In this study, we selected 15 patients with localized prostate cancer treated using ART at our clinic. Each patient had 13 CTs: 1 planning HCT, 4 daily HCTs (from a separate CT scanner) and 4 CBCTs from an Elekta Synergy (Elekta, Crawley, UK) with Bow-Tie filter on the same days in the first week. In addition, 4 CBCTs performed weekly after the modified plan during the treatment course were also included for quality assurance purpose. All CBCTs were acquired just before or after each treatment fraction while the patient was still on the table. Slice thickness was 3 mm for HCTs. The CBCT scans were performed using 120 kVp, 40 mA, 40 ms per projection, and image panel offset at 11.5 cm, the collimators for kV X-ray was limited to 10 cm in the patient superior-inferior direction to reduce the scatter effect on the CBCT images. The maximum imaging dose was estimated to be 2.1 cGy at the skin surface with a typical 650 projections. The reconstructed CBCT had a spatial resolution of 1×1×1 mm3.
The CT image data set was transferred to the treatment planning system (Pinnacle3 v8.0, Philips Radiation Oncology System, Milpitas, CA) for further processing. All CTs of the same patient were rigidly registered to the planning CT based on bony structures to remove setup errors. The registrations were made with translations only. Therefore, only organ motion (rigid and deformation) and rotational component of setup errors were investigated in this study.
A single physician manually delineated the prostate and the SVs on each CT. The planning HCT was allowed to be used as reference for contouring on other CTs. The CTV was defined as the prostate gland for Group 1 (G1) low risk patients, plus SV for Group 2 (G2) intermediate risk patients. To minimize the influence from other patients, the contouring of the same patient on all CTs was completed in one session. In addition, to reduce the bias of single observer in delineating the CTV, the contours on reference HCT was not used directly such as being overlaid on the CBCT and modified using tools available in treatment planning system. Rather, each CTV on daily HCT and CBCT were delineated individually by hand. Once the CTV contour on reference HCT was completed, no change was allowed to be made on it.
Two internal target volumes (ITV) were constructed in treatment planning system: ITVHCT is the union of CTVs in planning HCT (CTV0) and in daily HCTs,
| (Eq. 1) |
The ITVHCT is the current standard in our ART planning process. Similarly, ITVCBCT is the union of CTV0 and daily CBCTs,
| (Eq. 2) |
We performed the following comparisons to evaluate the differences of target volumes between HCT and CBCT
CTV volume in daily HCT and CBCT for the first 4 days.
Volumes of ITVHCT and ITVCBCT.
- Similarity of ITVHCT and ITVCBCT using overlap index (OI),
The OI was computed also when margins of 1, 2, and 3 mm margins were added to ITVCBCT.(Eq. 3) Center of mass (COM) distances between CTV0, ITVHCT and ITVCBCT. One purpose of the ITV in ART is to correct the systematic bias of the target position that might exist in the initial planning CT. Such bias can be demonstrated using the distance between the center of mass (COM) of CTV0 and the ITVs, assuming ITVs are representation of the true CTV position during treatment course. The difference between COM of ITVHCT and ITVCBCT were also computed.
Surface differences between ITVHCT and ITVCBCT. For each point on the surface of ITVHCT, a corresponding point can be found on ITVCBCT which has the shortest distance, i,e., the closest point. The distribution of the distances were tallied to demonstrate the ITV shape differences. No COM correction were performed before this analysis.
To validate the effectiveness of the ART process, quality assurance were performed using weekly CT scans after the modified planning based on ITV, to ensure that the ITV created from a few samples of the CTs is representative of the CTVs during the treatment course. We further calculated the OI between the CTVi in weekly CBCT (i = 5, 6, 7, −8.) and the initial CTV0 and ITVCBCT.
We performed the following dosimetric evaluations. For each patient, an IMRT plan was generated based on ITVCBCT. Because ITV only accounted for the internal organ motion, additional margins were necessary to compensate for setup errors in ART. In offline ART, the systematic component of setup error was corrected, however, the random component of setup error and the residuals of predicting the systematic error were compensated by margins. This additional margin was in general non-uniform and patient-specific depending on the characteristics of the measured setup errors for that patient. We used the minimum value for this part of the study. The PTV was defined as the ITVCBCT with a margin of 2 mm in the anterior-posterior and the left-right direction and 3 mm (the slice thickness in planning HCT) in the cranial-caudal direction. A 5-beam 18 MV photon IMRT plan was generated with prescription dose of 81 Gy in 45 fractions. We evaluated the coverage of the ITVHCT and weekly CBCT CTVi, i = 5, 6, 7, −8. Specifically, we compared the minimum dose (D99 and D95) and mean dose Dmean. We want to point out that we used the minimum margin allowed in ART in this reference plan. If we used the specific margin for each patient, the difference in dose would be smaller. Therefore, out results reflected the worst case scenario.
3 Results
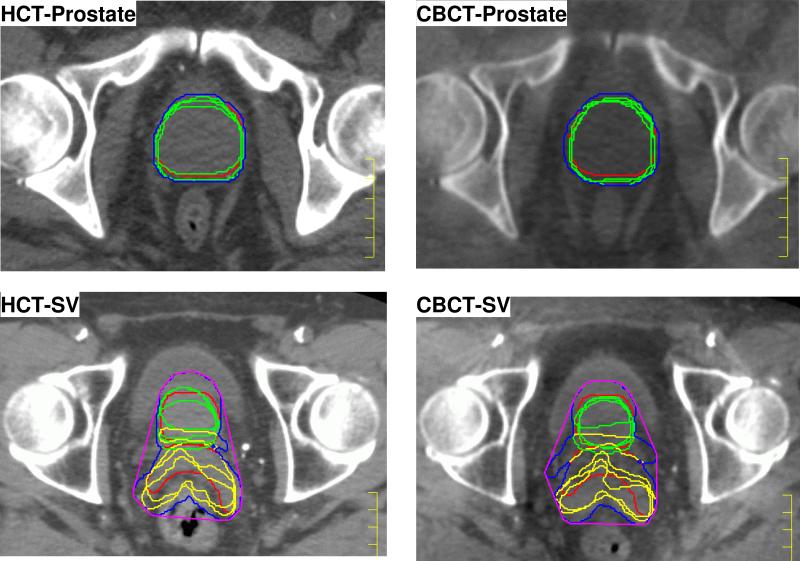
In one patient, the superior portion of SVs was not included within the field of view for CBCT, therefore, data from that patient was excluded from the G2 analyses. The contours and ITVs created for one patient in G1 and G2 are shown in Fig. 2, at the same CT slice as in Fig. 1. Due to the independent motion of SV relative to prostate, a larger variation in ITVs were expected for group 2 patients. The volume of CTVs are summarized in Table 1. There is no statistically significant difference between the CTV volumes defined on HCT or CBCT on same day.
Figure 2.
Comparison of contours drawn and ITVs created on HCT and CBCT at the same slices as in Fig. 1. Red: CTV0 (prostate and SV) on planning CT, Green = Prostatei in daily CT, Yellow: SVi in daily CT, Blue: ITV created with union operator, Purple: ITV created with convex hull.
Table 1.
Comparisons of CTV volume between HCT and CBCT. CTV = clinical target volume, HCT = helical CT, CBCT = cone-beam CT. The average ± standard deviation were computed from values for each patient (row 1 has 15 samples for Group 1 and 14 for Group 2) or each daily fractions (row 2-4 have 60/56 samples).
| Quantity | Group 1 | Group 2 |
|---|---|---|
| CTV0 Volume (cc) | 48.6±19.3 | 65.9±21.1 |
| Volume(CTVCBCT)/Volume(CTVHCT) | 1.00±0.08 | 0.98±0.08 |
| Volume(CTVHCT)/Volume(CTV0) | 0.98±0.06 | 0.97±0.04 |
| Volume(CTVCBCT)/Volume(CTV0) | 0.97±0.07 | 0.95±0.07 |
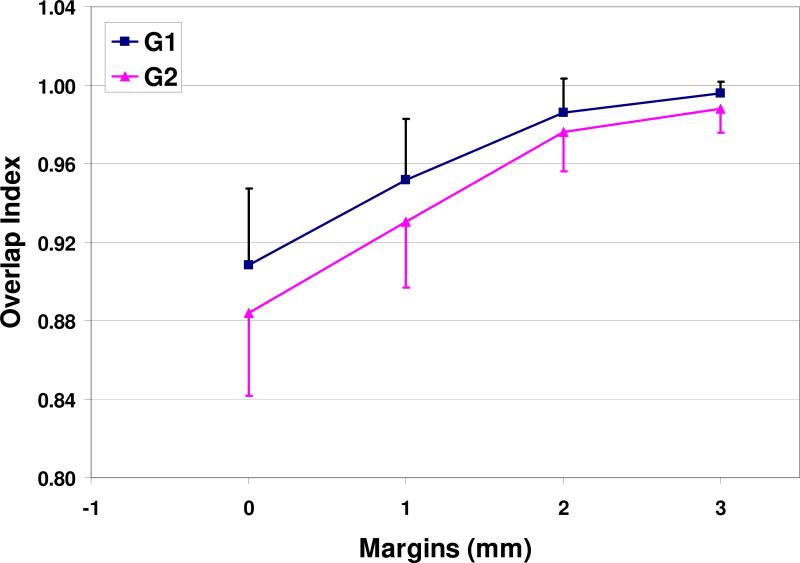
The volume and COM comparisons of ITVs are shown in Table 2. From CTV0 to ITV, the volume increases by 40% for G1 and 60% for G2, for either HCT or CBCT. The OI is (90.9±3.9)% for G1 and (88.4±4.2)% for G2 between the ITVHCT and ITVCBCT. Therefore, there are some differences between these two ITVs. To quantitatively evaluate the differences, a range of margins were added to ITVCBCT. The recomputed OIs improved significantly, as demonstrated in Fig. 3. Generally speaking, the OI is >95% with 1.5 mm margin and >98% with 2 mm margin. Therefore, we can conclude that the difference between these two ITVs are very small and mostly within 2 mm from each other.
Table 2.
Comparisons of ITV volumes, OIs and COM. CTV = clinical target volume, HCT = helical CT, CBCT = cone-beam CT. OI = overlap index. COM Diff = center of mass difference between ITVHCT and ITVCBCT. Values are average ± standard deviation.
| Index | Group 1 | Group 2 |
|---|---|---|
| Volume(ITVHCT)/Volume(CTV0) | 1.43±0.15 | 1.61±0.29 |
| Volume(ITVCBCT)/Volume(CTV0) | 1.42±0.10 | 1.59±0.24 |
| OI(ITVCBCT, ITVHCT) (%) | 90.9±3.9 | 88.4±4.2 |
| OI(CTV0, CTVWeeklyCBCT) (%) | 86.7±9.4 | 79.8±13.7 |
| OI(ITVCBCT, CTVWeeklyCBCT) (%) | 97.6±4.0 | 95.5±5.0 |
| COM Diff: Right-Left (mm) | 0.0±0.7 | 0.0±0.6 |
| COM Diff: Posterior-Anterior (mm) | −0.5±1.3 | −0.3±1.4 |
| COM Diff: Superior-Right (mm) | 0.0±1.1 | −0.3±1.4 |
Figure 3.
Overlap index with different ITV margins.
We compared the distance between the center of mass (COM) of CTV0 and subsequent ITVs. They are (2.5±1.3) mm between CTV0 and ITVHCT, (2.6±1.1) mm between CTV0 and ITVCBCT, and (1.7±0.8) mm between ITVHCT and ITVCBCT. This shows that the average correction of COM is 2.5 mm and the corrections by ITVHCT and ITVCBCT are in the similar directions and magnitudes. The COM difference between ITVs are < 1mm, as shown in Table 2.
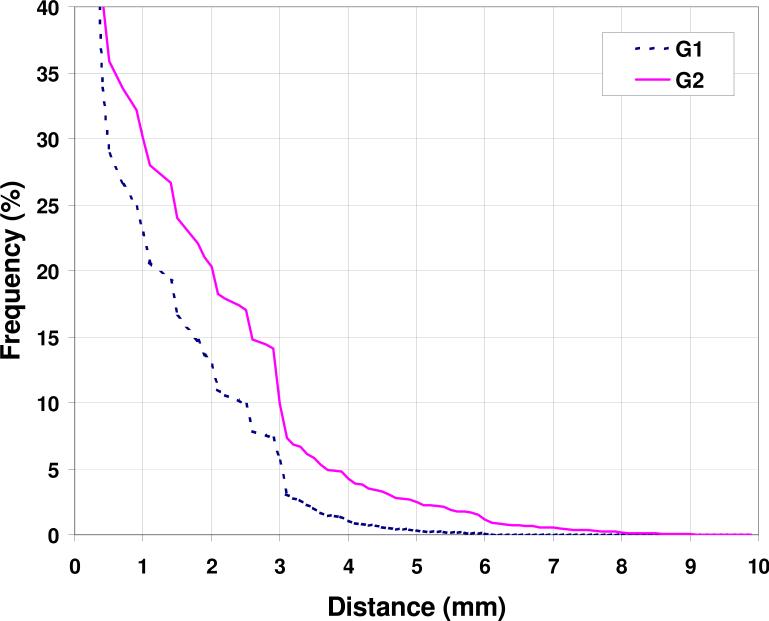
The surface differences between the two ITVs are demonstrated as cumulative histograms in Fig. 4 from all patients data. More than 77% surface points of ITVs of the same patient are within 1 mm from each other, and 87% within 2 mm for G1 patients. They are slightly worse at 71% and 83% for G2.
Figure 4.
Surface discrepancies between ITVHCT and ITVCBCT.
Quality assurance of ITV construction was evaluated by the calculating the OI between ITV and CTV from weekly CBCTs, they are also listed in Table 2. While the OI between CTV0 and the weekly CTVCBCT is only 87% and 80% for G1 and G2, they improved significantly to 98% and 96% for G1 and G2 when CTV0 was replaced by ITVCBCT in the calculation, demonstrating the validity of the ITV in offline ART.
The representative dosimetric endpoint for target volumes are summarized in Table 3. For G1 patients, the IMRT plan reached the prescription of 81 Gy to D99 of PTV with very small variations among patients. The homogeneity of the target coverage was also very good, with the Dmean at only 3.6% higher than D99. While the plans were generated using ITVCBCT, the coverage of ITVHCT was also excellent, the ratio of D99 was at 98.6%. The ratios for D95 and mean dose were closer to 100%. For G2 patients, the ITVCBCT shapes were less regular, therefore the plans were slightly worse, with D99 of PTV at 80.3 Gy and a larger variation among patients. Nevertheless, the coverage of ITVHCT was still excellent, with D99 ratio at 97.4% and higher ratios for D95 and Dmean. Therefore, we consider the dosimetric differences between ITVCBCT and ITVHCT small and negligible. We further looked at the dose indices of CTVs from the weekly CBCT after the modified plan, and observed excellent coverage, with almost 100% ratio for D99, D95 and Dmean.
Table 3.
Comparisons of ITV dose coverage. CTV = clinical target volume, HCT = helical CT, CBCT = cone-beam CT. D99 = Dose to 99% of volume. Similarly, D95 = Dose to 95% of the volume. Dmean = mean dose. Values are average ± standard deviation.
| Dose Index | Group 1 | Group 2 |
|---|---|---|
| PTV(ITVCBCT) D99 (Gy) | 81.0±0.0 | 80.3±2.3 |
| ITVCBCT D99 (Gy) | 81.7±0.3 | 82.8±1.0 |
| D99(ITVHCT)/D99(ITVCBCT) (%) | 98.6±4.6 | 97.4±5.1 |
| D99(CTVWeeklyCBCT)/D99(ITVCBCT) (%) | 100.0±1.5 | 100.2±1.5 |
| PTV(ITVCBCT) D95 (Gy) | 81.7±0.2 | 82.8±1.0 |
| ITVCBCT D95 (Gy) | 82.2±0.4 | 83.7±1.3 |
| D95(ITVHCT)/D95(ITVCBCT) (%) | 100.5±0.3 | 100.7±0.5 |
| D95(CTVWeeklyCBCT)/D95(ITVCBCT) (%) | 100.1±0.8 | 100.1±0.6 |
| PTV(ITVCBCT) Dmean (Gy) | 83.9±0.6 | 85.6±1.4 |
| ITVCBCT Dmean (Gy) | 84.1±0.7 | 86.0±1.5 |
| ITVHCT Dmean (Gy) | 84.1±0.7 | 85.7±1.4 |
| Dmean(CTVWeeklyCBCT)/Dmean(ITVCBCT) (%) | 100.1±0.3 | 100.1±0.3 |
4. Discussion
To date, the image guided radiotherapy for prostate cancer using CBCT is mostly practiced in online mode with implanted fiducial markers in the prostate gland (9). This is primarily due to the inability of directly visualizing prostate gland in online CBCT images. Although vast experience has been accumulated over the past few years in the implantation of fiducial markers with minimal risk to the patient, it is an invasive procedure and can be a deterrent for many patients for this treatment option.
In offline image guidance mode, implanted markers are not necessary, the CT images taken at different fractions are utilized to create a composite CTV, or ITV. The idea is that this ITV constructed from several measurements of the CTV during treatment are more representative of the CTV position and shape during treatment course than a single measurement at planning CT. In a recent paper (10), an alternative ART strategy using CBCT was reported, in which an average CTV position and shape instead of ITV was calculated based on the CBCTs acquired in a few initial fractions. This strategy may yield slightly different and smaller PTV, however, comparison of ART strategies is beyond the scope of this paper.
While the current image quality of CBCT is sub-optimal and not suitable for direct online image guidance, our results show that it is adequate for target definition in an offline environment. Several factors may be in the favor of offline ART. First, multiple CBCTs are used to define the target, instead of one, and the CTV0 is included in the ITVCBCT construction. As shown in Table 2, nearly 70% (1/1.42) of the ITVCBCT for G1 and 63% (1/1.59) for G2 are from CTV0. Second, the contouring are performed offline, the planning HCT was allowed to be used for reference when contouring was performed on subsequent CBCTs, the planner is not rushed to complete the delineation as in online image guidance. Third, the rotations and deformations of prostate can be included in the ITV, they are typically not corrected in online image guidance but compensated through margins.
In a recent paper, White et al presented inter-observer variability in contouring the prostate gland in CBCT (11). The variation of CTV volumes is slightly larger than previous studies on HCTs (12, 13). The mean standard deviation of COM among different observers for 5 patient CBCT images were 0.7, 1.8 and 2.8 mm in Right-Left, Posterior-Anterior, and Superior-Inferior direction, respectively. Our results shown in Table 2 is similar for RL axis, but much smaller in PA and SI directions. The key difference is that we used the ITV, the composite of several CTVs, in the calculation of COM instead of each individual CTV. This suggests that the use of multiple CBCTs, such as the offline ART presented in this study, is more accurate and consistent in defining the target positions than the online image guidance based on single CBCT.
The daily CBCT and HCT were taken on the same day and efforts were made to keep the patient setup the same, however, they were acquired at different times and patient internal anatomy may be slightly different, especially the content of bladder and rectum can change. Therefore, the difference observed in the daily CTV and subsequent ITVs includes both the target shape variations and the contouring uncertainties. Even though >10% of the surface points were more than 2 mm apart, as shown in Fig. 4, the dose coverage for the ITVs were equivalent.
There is a minimum margin in offline ART replanning, which is 2 mm in AP and RL and 3 mm in SI. This minimum margin is imposed to account for the CT resolution and sampling errors. We consider this minimum margin is adequate to compensate for the observed 1-2 mm difference between the ITVHCT and the ITVCBCT. As shown in the dosimetric study, the difference in dose is very small. Therefore, the additional margins are unnecessary to compensate these 1-2 mm differences.
In summary, we have quantitatively evaluated the use of CBCT in the target definition in an offline image-guidance protocol for prostate cancer. There is no significant difference between the internal target volume defined in CBCTs and HCTs. The ITVCBCT is within 1-2mm from ITVHCT, the dosimetric difference is even smaller. It is therefore feasible to use the CBCT in the offline ART for target definition, thereby eliminating the separate helical CT scan process, improving patient throughput, and reducing patient's waiting time.
Supplementary Material
Acknowledgement
We would like to thank Drs. Jian Liang and Yuwei Chi in assistance in data analyses. Portions of the studies were presented at the 50th ASTRO annual meeting, Sept., 2008, Boston, MA. This research is supported in part by Grants CA118037 from the National Cancer Institute. The contents are solely the responsibility of the authors and do not necessarily represent the official view of NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
5. References
- 1.Katcher J, Kupelian PA, Zippe C, et al. Indications for excluding the seminal vesicles when treating clinically localized prostatic adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 1997;37:871–876. doi: 10.1016/s0360-3016(96)00617-7. [DOI] [PubMed] [Google Scholar]
- 2.Kestin L, Goldstein N, Vicini F, et al. Treatment of prostate cancer with radiotherapy: should the entire seminal vesicles be included in the clinical target volume? Int J Radiat Oncol Biol Phys. 2002;54:686–697. doi: 10.1016/s0360-3016(02)03011-0. [DOI] [PubMed] [Google Scholar]
- 3.Marks LB, Anscher MS. Radiotherapy for prostate cancer: should the seminal vesicles be considered target? Int J Radiat Oncol Biol Phys. 1992;24:435–440. doi: 10.1016/0360-3016(92)91057-t. [DOI] [PubMed] [Google Scholar]
- 4.Yan D, Lockman D, Brabbins D, et al. An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:289–302. doi: 10.1016/s0360-3016(00)00608-8. [DOI] [PubMed] [Google Scholar]
- 5.Martinez AA, Yan D, Lockman D, et al. Improvement in dose escalation using the process of adaptive radiotherapy combined with three-dimensional conformal or intensity-modulated beams for prostate cancer. Int J Radiat Oncol Biol Phys. 2001;50:1226–1234. doi: 10.1016/s0360-3016(01)01552-8. [DOI] [PubMed] [Google Scholar]
- 6.Vargas C, Martinez A, Kestin LL, et al. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Brabbins D, Martinez A, Yan D, et al. A dose-escalation trial with the adaptive radiotherapy process as a delivery system in localized prostate cancer: analysis of chronic toxicity. Int J Radiat Oncol Biol Phys. 2005;61:400–408. doi: 10.1016/j.ijrobp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Jaffray DA, Siewerdsen JH, Wong JW, et al. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2002;53:1337–1349. doi: 10.1016/s0360-3016(02)02884-5. [DOI] [PubMed] [Google Scholar]
- 9.Letourneau D, Martinez AA, Lockman D, et al. Assessment of residual error for online cone-beam CT-guided treatment of prostate cancer patients. Int J Radiat Oncol Biol Phys. 2005;62:1239–1246. doi: 10.1016/j.ijrobp.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Nijkamp J, Pos FJ, Nuver TT, et al. Adaptive radiotherapy for prostate cancer using kilo-voltage cone-beam computed tomography: first clinical results. Int J Radiat Oncol Biol Phys. 2008;70:75–82. doi: 10.1016/j.ijrobp.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 11.White EA, Brock KK, Jaffray DA, et al. Inter-observer Variability of Prostate Delineation on Cone Beam Computerised Tomography Images. Clin Oncol. 2009;21:32–38. doi: 10.1016/j.clon.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Fiorino C, Reni M, Bolognesi A, et al. Intra- and inter-observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;47:285–292. doi: 10.1016/s0167-8140(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 13.Gao Z, Wilkins D, Eapen L, et al. A study of prostate delineation referenced against a gold standard created from the visible human data. Radiother Oncol. 2007;85:239–246. doi: 10.1016/j.radonc.2007.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.