Summary
Methamphetamine (MA) abuse has reached epidemic proportions in the United States. Users of MA report dramatic increases in sexual drive that have been associated with increased engagement in risky sexual behavior leading to higher rates of sexually transmitted diseases and unplanned pregnancies. The ability of MA to enhance sexual drive in females is enigmatic since related psychostimulants like amphetamine and cocaine appear not to affect sexual drive in women, and in rodents models, amphetamine has been reported to be inhibitory to female sexual behavior. Examination of MA’s effects on female sexual behavior in an animal model is lacking. Here, using a rodent model, we have demonstrated that MA enhanced female sexual behavior. MA (5mg/kg) or saline vehicle was administered once daily for three days to adult ovariectomized rats primed with ovarian steroids. MA treatment significantly increased the number of proceptive events and the lordosis response compared to hormonally-primed, saline controls. The effect of MA on the neural circuitry underlying the motivation for sexual behavior was examined using Fos immunoreactivity. In the medial amygdala and the ventromedial nucleus of the hypothalamus, nuclei implicated in motivated behaviors, ovarian hormones and MA independently enhance the neuronal activation, but more striking was the significantly greater activation induced by their combined administration. Increases in dopamine neurotransmission may underlie the MA/hormone mediated increase in neuronal activation. In support of this possibility, ovarian hormones significantly increased tyrosine hyroxylase (the rate limiting enzyme in dopamine synthesis) immunoreactivity in the medial amygdala. Thus our present data suggest that the interactions of MA and ovarian hormones leads to changes in the neural substrate of key nuclei involved in mediating female sexual behaviors, and these changes may underlie MA’s ability to enhance these behaviors.
Keywords: proceptive behavior, lordosis, neuronal activation, tyrosine hydroxylase, dopamine neurotransmission
Introduction
Methamphetamine (MA) is a highly addictive psychostimulant drug of abuse. One of the most striking effects of MA in anecdotal and clinical self-report studies is an increase in sexual thoughts, behaviors, and activities, which have the consequences of increased rates of sexually transmitted diseases and unplanned pregnancies (Rawson et al., 2002; Semple et al., 2004a; Semple et al., 2004b; Mansergh et al., 2006). Additionally, studies of sexual behavior often focus on male users of MA even though there is no sex difference in the effects of MA (Rawson et al., 2002; Semple et al., 2004a). The ability of MA to enhance sexual drive in women is intriguing as two related psychostimulants, cocaine (Rawson et al., 2002) and amphetamine (Käll and Nilsonne, 1995), have been reported to increase sexual activities in male, but not female, users. Moreover, studies examining the effects of cocaine and amphetamine in rodent models of female sexual behavior have resulted in ambiguous and contradictory findings. To our knowledge there have been no animal studies examining the effects of MA on sexual behavior in the female rat.
In female rodents, sexual behavior is an intrinsic process modulated by the actions of the ovarian steroid hormones, estradiol and progesterone (P), and the incentive properties of the stimulus male (Beach, 1942). Female sexual behavior is characterized by a motivational and a reflexive component. Motivation for sexual behavior in the female rat can be quantified by the occurrence of proceptive behaviors (hopping, darting and ear wiggling), which solicit a male’s attention (Beach, 1976; Erskine, 1989; Pfaus et al., 2003), and paced mating behavior, a more naturalistic paradigm in which the female controls the sexual interactions (Erskine 1989; Pfaus et al, 2003). The lordosis response is a reflexive dorsoflexion of the spine adopted by a sexually receptive female. It has been suggested that the degree or intensity of the curvature is proportional to the level of sexual motivation elicited by the female (Erskine, 1989; Pfaff and Ågmo, 2002; Pfaus et al., 2003),
The neurotransmitter dopamine is an important modulator of many motivated behaviors, including the motivation to seek out sexual partners (Auger, 2001; Becker et al., 2001). Recent evidence suggests that the mesolimbic dopamine system mediates the motivation or the ‘wanting’ of a natural and/or hedonic stimulus by attributing salience to it, as opposed to mediating the pleasure or ‘liking’ of a reward (Ikemoto and Panksepp, 1999; Berridge, 2007; Salamone et al., 2007). Dopamine is released into the striatum and nucleus accumbens (nuclei part of the mesolimbic pathway) of female rats prior to engaging in sexual behavior (Mermelstein and Becker, 1995; Pfaus et al., 1995; Becker et al., 2001; Jenkins and Becker, 2003), making this neurotransmitter a good candidate for regulating sexual motivation. In fact, ovarian hormones have been reported to increase DA synthesis and evoked release in the striatum (Becker and Rudrick, 1999). Thus, when one considers that MA, by reversing monoamine reuptake transporters, results in the liberation of synaptic terminal dopamine (Fleckenstein et al., 2000), it is temping to speculate that an interaction between ovarian hormones and MA may result in enhanced dopamine signaling in nuclei mediating female sexual motivation and behavior.
We tested whether MA affects sexual behavior in hormonally-primed female rats. A typical pattern of psychostimulant use tends to be multiple doses over a short time period. Therefore, we administered MA or saline vehicle once a day for three days. At the same time, all animals received ovarian hormones. Here, we report that MA enhances both the proceptive and receptive component of female sexual behaviors. Additionally, in a sexually naïve cohort, we identified the medial amygdala (MeA) as a potential site of ovarian hormone and MA interactions, and this interaction may involve an increase in catecholamine levels.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (9 – 12 wks) were purchased from Charles River Laboratories (Kingston, NY) and housed in the Laboratory Animal Facility of the Bressler Research Building at the University of Maryland School of Medicine under a reversed 12 h: 12 h dark: light cycle (lights off at 1000 h) with food and water available ad libitum. Animals were bilaterally ovariectomized (OVX) under Ketamine: Acepromazine (80 mg: 2.5 mg/kg) anesthesia. Following surgery, animals were allowed a 10 day recovery period. All procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Hormones and Methamphetamine Treatment
17-b-estradiol benzoate (EB) and P were obtained from Sigma-Aldrich (St. Louis, MO, USA). The EB (10µg/0.1ml) and P (500µg/0.1ml) were dissolved in sesame oil for subcutaneous (SC) administration. MA (Sigma-Aldrich) was dissolved in sterile saline vehicle to a concentration of 5mg/ml for intraperitoneal (IP) injection. Forty-eight hours before the start of the experimental procedure (e.g. behavioral testing or tissue collection), the animals were hormonally primed with 5µg EB followed by 10µg EB 24 h later. Four hours prior to testing, the rats were injected with P (dose specified in Table 1). During the three days of hormonal priming, rats received a daily injection of MA (5mg/kg/day) or saline vehicle in a repeated administration paradigm designed to model frequent drug use within a short time period (Figure 1). Injections of the hormones and MA were administered between 0900 and 1000 h.
Table 1.
Experimental Treatment Groups. Delineation of experimental treatment groups, number of animals used and the experimental endpoints.
| Experiment | Hormone Administration (0900h) |
Drug Treatment | Endpoint (1300h) |
||
|---|---|---|---|---|---|
| D10 | D11 | D12 | D10-D12 | ||
| Dose Response |
EB (5µg) |
EB (10µg) |
P (200µg) |
MA (1, 3, or 5 mg/kg; n=7 each) or saline (n=7) |
Sexual Behavior Testing |
| Hormone Requirement |
EB (5µg) |
EB (10µg) |
Oil | MA (5 mg/kg; n=5) or saline (n=7) |
Sexual Behavior Testing |
| EB (5µg) |
EB (10µg) |
P (200µg) |
MA (5 mg/kg; n=7) or saline (n=7) |
||
| EB (5µg) |
EB (10µg) |
P (250µg) |
MA (5 mg/kg; n=6) or saline (n=6) |
||
| EB (5µg) |
EB (10µg) |
P (500µg) |
MA (5 mg/kg; n=10) or saline (n=8) |
||
| Stereotyped Behavior |
EB (5µg) |
EB (10µg) |
P (500µg) |
MA (5 mg/kg; n=4) or saline (n=4) |
Rate Stereotyped Behavior (also at 0930 and 1130h) |
| Oil | Oil | Oil | MA (5 mg/kg; n=4) or saline (n=4) |
||
| Hyperactivity | EB (5µg) |
EB (10µg) |
P (500µg) |
MA (5 mg/kg; n=8) or saline (n=8) |
Open Field Testing |
| Oil | Oil | Oil | MA (5 mg/kg; n=7) or saline (n=7) |
||
| Fos | EB (5µg) |
EB (10µg) |
P (500µg) |
MA (5 mg/kg; n=8) or saline (n=8) |
Brain Collection |
| Oil | Oil | Oil | MA (5 mg/kg; n=7) or saline (n=7) |
||
| Tyrosine Hydroxylase |
EB (5µg; n=6) |
EB (10µg) |
P (500µg) |
Brain Collection | |
| Oil (n=6) |
Oil | Oil | |||
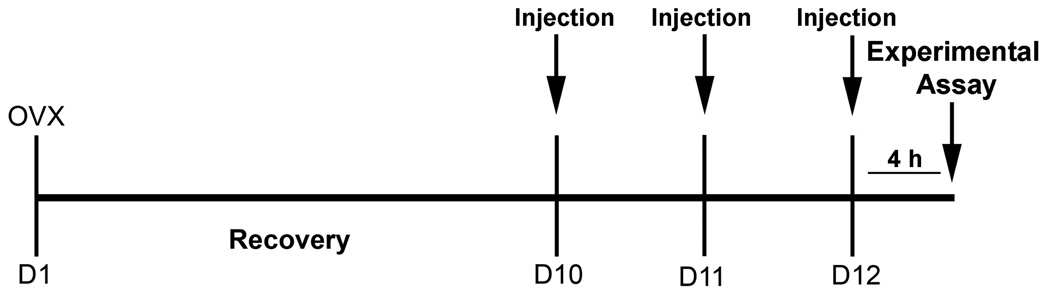
Figure 1.
General Experimental Timeline. Rats were ovariectomized (OVX; D1) and allowed to recover. At this time, rats were randomly assigned to treatment groups. All injections occurred on D10, D11, and D12 between 0900h and 1000h. On the last day of experimentation (D12), experimental assays (either sexual behavior, open field behavior, or perfusions for immunocytochemistry) were performed 4 h after injections.
Sexual Behavior
One cohort of animals was used to determine the optimal dose of MA (see Dose Response Experiment in Table 1). The doses of MA used yield plasma concentrations within the low micromolar range, which is within the range of human use (Melega et al., 2007). Another cohort was used to investigate the level of sexual receptivity required for MA-facilitated sexual behavior (see Hormone Requirement Experiment in Table 1).
Behavioral testing was conducted under dim red light in the dark phase of the light cycle between 1300 and 1600 h, approximately 4 h after the last MA administration. Experimental females were placed in a 50×38×25 cm Plexiglas observation chamber with a sexually experienced male. Each behavioral trial was recorded by a video camera and was completed when 10 mounts were received or 15 min had elapsed. The investigator remained at a consistent location approximately 0.5 m away from the observation chambers during all trials. An experimenter blind to treatment groups scored the sexual behaviors. Quantitative and qualitative parameters of the stereotypic, reflexive posture of lordosis were scored. The lordosis quotient (LQ) was defined as the number of lordoses divided by the number of mounts multiplied by 100. The lordosis intensity score (LS) assessed the degree of spine curvature which was measured on a scale from 0 to 3 (0 was no response and 3 was a complete dorsoflexion of the spine (Hardy and Debold, 1971)). The number of proceptive events occurring in the first 10 min of testing was quantified. A proceptive event was defined as a hop, a dart or a series of hopping and darting followed by a stop.
Stereotyped Behavior and Hyperactivity
Moderate to high doses (3 to 10mg/kg) of MA induce persistent, repetitive behaviors and purposeless movements (stereotypy) and hyperactivity (Segal and Kuczenski, 1997). As motor movements are key components in mating behavior, it was important to test whether MA treatment resulted in an overall increase in locomotor activity. A controlled time-course of stereotyped behaviors was conducted in a separate cohort of animals (see Stereotyped Behavior Experiment in Table 1). Stereotyped behavior was assessed in the animal’s home cage. It is known that stereotyped behavior is induced by MA (3mg/kg) shortly after injection and begins to wane 2 h after injection (Milesi-Halle et al., 2007). Therefore, we made observations under dim red light between 0915 and 0930 h, 1130 and 1145 h, and 1300 and 1315 h approximately 15, 150, and 240 min after the administration of MA. The observation made 240 min after MA injection represents the time point at which sexual behavior testing occurred. The investigator remained at a consistent location approximately 0.5 m away from the outside wall of the home cages for all observational periods. A video camera was mounted above the home cages, recording the activity of the animal for 5 min. The animal was observed for 20 sec and then 10 sec were allowed to record the score. This procedure was continued until the 5 min had elapsed. For each 20-sec observational period, the animal was assigned a score using the rating scale of Ellinwood and Balster (1974). These ratings were averaged to yield a single value per animal.
To further explore the effects of MA on locomotor activity, a separate cohort of animals was used for open-field testing (see Hyperactivity Experiment in Table 1). Open field-testing began 4 h after the last administration of MA to assess locomotor behavior at the same time at which sexual behavior testing occurred. Behavior was assessed in a large Plexiglas box (120×80×40 cm) with the floor divided into 30 squares (20 cm long and 16 cm wide). Testing was conducted under dim red light between 1300 and 1500 h. The investigator remained at a consistent location approximately 0.5 m away from the outside wall of the open field apparatus for all trials. A video camera was mounted above the open field box, recording each trial. Each rat was placed into the open field arena with its nose facing the wall and allowed to freely investigate the arena for 5 min. The number of grid crossings was quantified. Between trials, the open field box was thoroughly cleaned with 70% ethanol.
Fos immunoreactivity (Fos-ir) and Quantification
A separate cohort of animals was used for Fos analysis to determine patterns of neuronal activation by ovarian hormones and MA (see Fos Experiment in Table 1). The animals did not undergo behavioral testing, rather, 4 h after the last injection, they were transcardially perfused under sodium pentobarbital anesthesia (65 mg/kg, IP) with phosphate buffered saline (PBS; 0.1M, pH 7.4), followed by 4% paraformaldehyde in PBS. Following perfusion, the brains were removed, stored overnight in 4% paraformaldehyde in PBS at 4ºC followed by cryoprotection in 30% sucrose in PBS. After cryoprotection, the brains were frozen on dry ice and stored at −80ºC until processed for immunocytochemistry. Brains were sectioned (30µm) in the coronal plane in a cryostat and stored in a cryoprotectant solution (ethylene glycol/glucose in phosphate buffer) until processed for Fos-ir. Cohorts containing sections from all treatment groups were processed for Fos immunocytochemistry using a rabbit polyclonal antibody (1:250000; Oncogene Sciences, MA, USA) following standard protocols (Hadjimarkou et al., 2008).
The number of Fos-positive cells was counted with the aid of the Neurolucida software (MicroBrightField, Colchester, VT), which allows quantification of cell numbers in three-dimensional space. A standardized contour specific to the nuclei examined was used to demarcate the counting areas. Slides were anatomically matched and numerically coded so that the investigator conducting the analysis was blind to the experimental group. Sections containing the NAcc (core and shell), basolateral amygdala (BLA), central amygdala (CeM) and medial amygdala (MeA), bed nucleus of the stria terminalis (BNST), medial preoptic area (mPOA), and the ventromedial nucleus of the hypothalamus (VMN) were analyzed. Three brain sections (in series) separated by 120µm were used. In the event that three sections from the appropriate brain region could not be obtained, the animal was excluded from that region’s analysis. The placement and size of the counting contours were in accordance with previously defined parameters (Hosokawa and Chiba, 2007). Briefly, the counting contour area was 0.16mm2 for the NAcc core and shell, BLA, BNST, and mPOA and 0.09mm2 for the MeA, VMN, and CeM. Both sides of the bilateral nuclei were included in the analysis resulting in six counting contours per region. From these six contours an average Fos-positive cell number per section for each region was derived.
Tyrosine Hydroxylase immunoreactivity and Quantification
A separate cohort of animals was used for tyrosine hydroxylase (TH) immunocytochemistry (see Tyrosine Hydroxylase Experiment in Table 1). Brains were collected and processed as described above. Sections from both treatment groups were processed for immunocytochemistry using a rabbit polyclonal antibody (1:100000; Pel-freeze Biologicals; Rogers, AR) following standard protocols (Hadjimarkou et al., 2008).
Like the Fos quantification, the standardized contour specific for the VMN and MeA was used to demarcate the counting areas in anatomically matched and numerically coded slides. With the aid of the Neurolucida software, the length of the TH-positive process within that counting frame was measured in three sections. The number of these TH-positive processes per section did not differ among the treatment groups. The number of varicosities, or swellings of the process, that may represent release sites along the TH-positive process was also counted. The length measurements and varicosity counts were averaged to yield a single value per animal.
Statistical analyses
Results are expressed as means ± SEM. The distribution of data did not deviate significantly from normality except for the LQ in the dose response experiment. In this case, a Kruskal Wallis, nonparametric one-way ANOVA was performed. Student’s t-tests were used for two-group comparisons. When appropriate, a one-way ANOVA followed by Newman-Keuls comparisons or a two-way ANOVA with hormone and drug treatment as independent measures, followed by Bonferroni t-test post hoc comparisons was used. This is indicated where appropriate. All statistical tests were conducted using the GraphPad Prism program (San Diego, CA, USA) on a Macintosh Duo-core computer.
Results
The Effects of Methamphetamine on Female Sexual Behavior
Dose Response of Methamphetamine
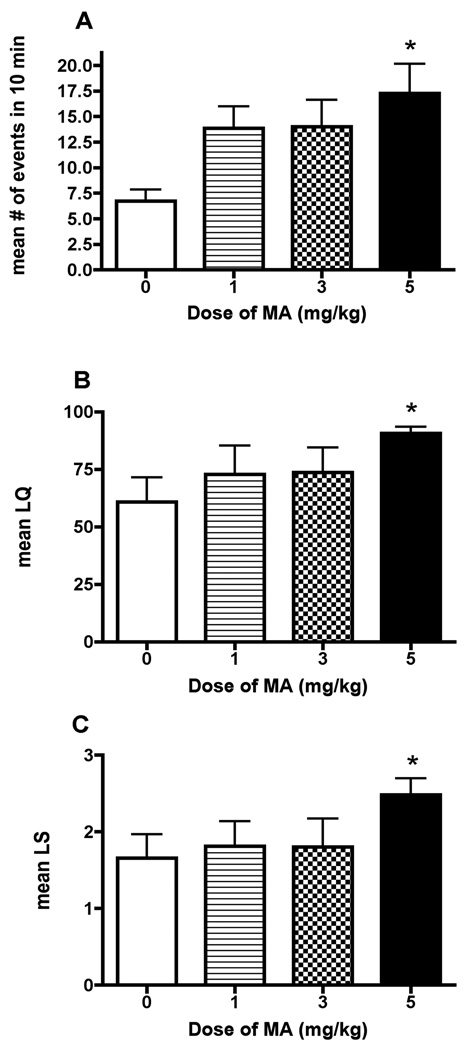
To determine the optimal dose of MA, rats received daily injections of one of three doses of MA (1, 3, and 5mg/kg) or saline vehicle for 3 days. There was a significant main effect of MA on the proceptive behavior [F(4) = 3.35, p<0.05; Figure 2A], the LQ [Kruskal Wallis statistic(4) = 9.63, p<0.05; Figure 2B], and the LS [F(4) = 3.402, p<0.05; Figure 2C]. Post hoc analyses revealed that females exposed to 5mg/kg/day of MA exhibited an increased LQ (p<0.05), a higher LS (p<0.05) and a greater number of proceptive behaviors (p<0.05). Based on these results, all subsequent experiments were conducted using the 5mg/kg dose of MA.
Figure 2.
Effects of different doses of MA on (A) proceptive behavior and (B, C) the lordosis response. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 (10µg) prior to the day of behavioral testing. Four hours before the start of behavioral testing, animals received an injection of 200µg P. MA (either 1, 3, or 5 mg/kg/day) or saline vehicle was co-administered on each day of the hormonal priming. A one-way ANOVA followed by a Newman-Keuls comparison indicated a significant effect of the dose of MA administered. Treatment with 5mg/kg/day of MA increased the number of proceptive events that occurred within 10 min, the LQ and LS, compared to the saline-treated controls (*p<0.05) Data are represented as means ± SEM. (n = 7 animals in each group).
Hormone Requirements
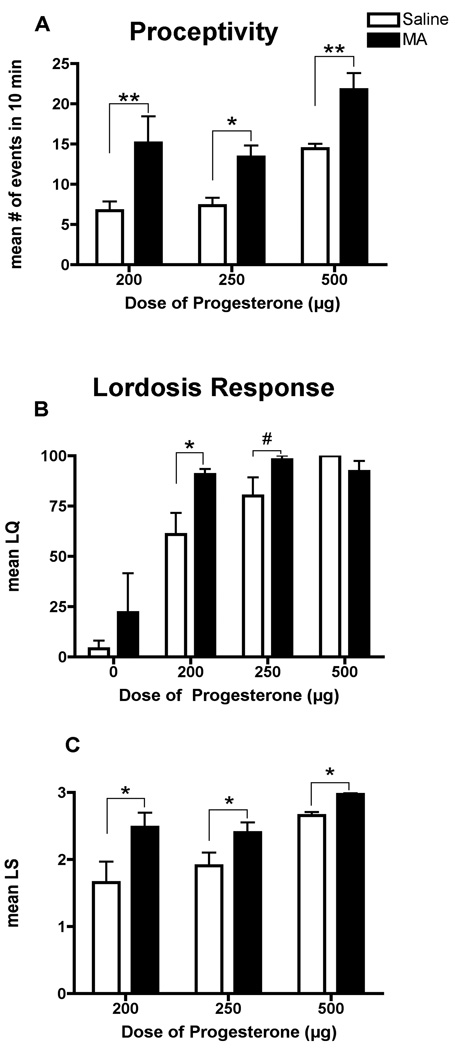
As expected, there was a significant main effect of the dose of P on sexual behaviors as measured by proceptive events [F(2,36) = 10.72, p<0.005], the LQ [F(2, 43) = 38.12, p<0.005], and the LS [F(2, 35) = 11.14, p<0.005]. The lower doses of P (200 and 250µg) did not elicit a high degree of sexual receptivity as measured by proceptive events (p<0.05) and lordosis responses (p<0.05) in the saline-treated animals when compared to a dose of P (500µg) that is known to induce behavioral estrus (Figure 3A). Overall, there was a significant effect of MA treatment on female sexual behavior as measured by proceptive events [F(1,35) = 18.62, p<0.0001; Figure 3A] and the LS [F(1,35) = 13.41, p<0.001; Figure 3C]. Proceptive events were significantly increased following MA treatment in all groups (p<0.05). Similarly, MA treatment increased the LS in females receiving P, compared to their respective saline-treated controls (p<0.05). However, there was a significant interaction of the dose of P administered and MA treatment on the LQ [F (3,43) = 6.242, p<0.05; Figure 3B]. The combination of MA and the lowest dose of P had a significant effect on the LQ, compared to the saline-treated controls (p<0.05). Additionally, there was a strong trend in the LQ toward a significant increase in the animals treated with a combination of 250 µg P and MA (p=0.059). It should be noted that at 250 and 500 µg of P, the LQ responses was near or at a maximal response, respectively, making it numerically impossible to detect a significant increase. Finally, no quantifiable proceptive behaviors were observed in the EB-only (no P) condition. MA administration to EB-only primed rats yielded mean LQ of 22% (±19.56) compared to the oil-treated controls (4% ±4). Of the animals that had a lordosis response, there was no significant difference in the mean LS between the MA-treated animals (1.00 ±0.63) and saline-treated controls (0.88 ±0.44).
Figure 3.
Effects of ovarian hormones and MA on (A) proceptive behaviors and (B, C) the lordosis response. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 h (10µg) prior to the day of behavior testing. Four hours before the start of the behavioral testing animals received an injection of oil vehicle or either 200µg, 250µg or 500µg of P. MA (5mg/kg) was co-administered on each day of the hormonal priming. A two-way ANOVA followed by Bonferroni t-tests indicated a significant interaction between the MA and the dose of P administered. MA treatment increased the number of proceptive events that occurred within 10 min in animals administered all doses of P, compared to the respective saline-treated controls (*p<0.05, **p<0.01). MA treatment increased the LQ in animals administered 200µg of P (*p<0.05) but only led to a strong trend towards an increase in animals administered 250µg of P (#p=0.06), compared to the respective saline-treated controls. Finally, rats treated with EB-only were not considered sexually receptive as they did not display any proceptive behaviors and the LQ was less than 25% (80% or greater represents a highly receptive female), suggesting that P administration is necessary to see MA-induced facilitation of sexual behavior. Data are represented as means ± SEM. (n = 5 to 10 animals in each group).
Stereotyped Behavior and Hyperactivity
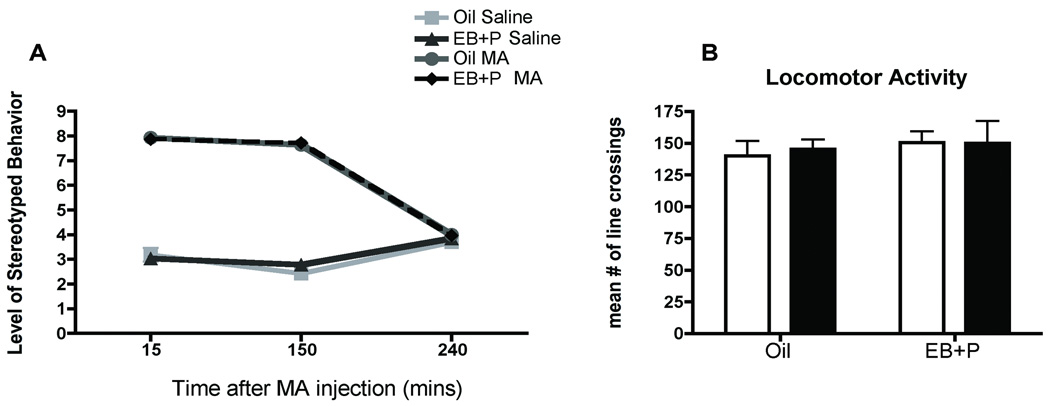
There was a significant interaction of MA treatment and the time following administration [F(6,50) = 46.61, p<0.0001; Figure 4A]. Post hoc analyses revealed that MA treatment induced stereotyped behavior at both 15 (p<0.001) and 150 min (p<0.001) post-MA injection, compared to saline controls, but by 240 min after injection, no stereotyped behavior was observed. Moreover, 240 min post administration (the time point at which sexual behavior was tested), there was no evidence of a generalized increase in locomotion or hyperactivity due to MA [F(1,26) = 0.92, p = 0.8; Figure 4B]. Hormonal-priming did not affect the psychomotor stimulant effects of MA on stereotyped behavior or hyperactivity as measured by the open field. Our current findings that locomotor activity returned to baseline by 4 hrs after MA exposure are supported by earlier studies describing the motor stimulant effects of MA (Edgar and Seidel, 1997; Riviere et al., 1999).
Figure 4.
Effect of ovarian hormones and MA on locomotor behaviors (A) Effects of ovarian hormones and MA on stereotyped behaviors. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 h (10µg) prior to the day of behavior testing. Four hours before the start of the behavioral testing animals received an injection of oil vehicle or 500µg of P. MA (5mg/kg) was co-administered on each day of the hormonal priming. Rats were observed at 15 min, 150 min and 240 min post-MA injection for 5 min each to assess the level of stereotyped behavior. A two-way ANOVA followed by Bonferroni t-tests indicated a significant interaction between the MA and the time following administration. MA treatment induced stereotyped behavior at both 15 (***p<0.001) and 150 min (***p<0.001) post-MA injection compare to saline controls, but by 240 min after injection no stereotyped behaviors were observed. (n = 4 to 6 animals in each group). (B) Effects of ovarian hormones and MA on locomotion. Treatment groups were analogous to those described above. At the start of the test, female rats were placed in open field arenas and allowed to freely move around the arena for 5 min while the number of grid crossings was recorded. A two-way ANOVA indicated no significant effects of MA or EB+P on locomotor activity in the open field-testing (p=0.45). Data are represented as means ± SEM. (n = 7 to 8 animals in each group).
Fos Immunoreactivity
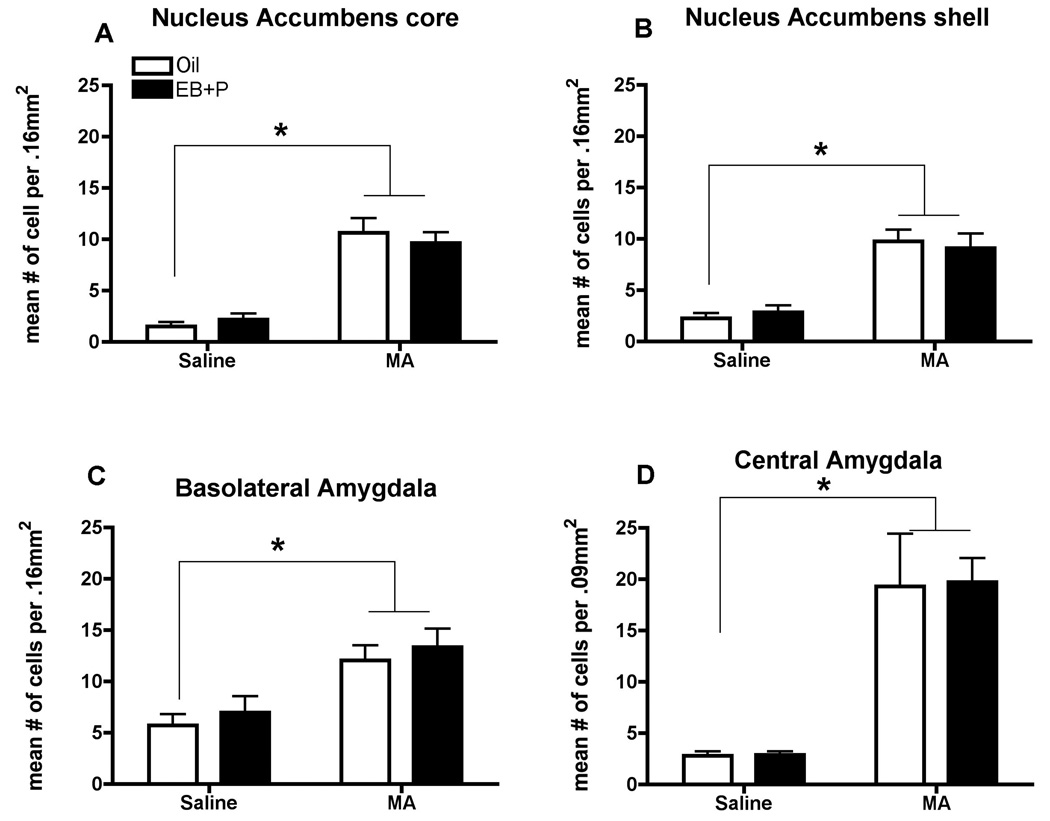
There was a significant main effect of MA administration on the number of neurons expressing Fos in both the core [F(1,24) = 76.49, p<0.0001; Figure 5A] and shell [F(1,24) = 46.32; p<0.0001; Figure 5B] of the NAcc, a major brain region regulating reward, but there was no effect of hormonal status. In the BLA and CeM, which are implicated in relaying sensory information to induce motivation (Masco and Carrer, 1984; Newman, 1999), there was a significant main effect of MA administration on the number of neurons expressing Fos [F(1,24) = 17.76, p<0.001, F(1,24) = 34.83, p<0.0001 respectively; Figure 5C, D], but there was no effect of hormonal treatment. In the BNST, a region implicated in both reward and motivation for sex (Masco and Carrer, 1984; Newman, 1999), there was a strong trend for MA administration to increase the neuronal activation [F(1,24) = 12.09, p=0.069; data not shown], but there was no effect of hormonal treatment (oil & saline 13.79, ± 3.12; oil & MA 26.52 ± 5.93; EB +P & saline 20.08 ± 2.57; EB+P & MA 23.71 ± 5.13).
Figure 5.
Effects of ovarian hormones and MA on Fos-immunoreactivity (ir) in the Nucleus Accumbens (A) core and (B) shell, the Basolateral (C) and Central (D) Nuclei of the Amgydala. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 h (10µg) prior to the day of collection. Four hours before the collection animals, animals received an injection of oil vehicle or 500µg P. MA (5mg/kg) was co-administered on each day of the hormonal priming. A two-way ANOVA followed by Bonferroni t-tests indicated a significant effect of MA treatment on Fos-ir compared to saline-controls (*p<0.05), but not of hormones. Data are represented as means ± SEM. (n = 7 to 8 animals in each group).
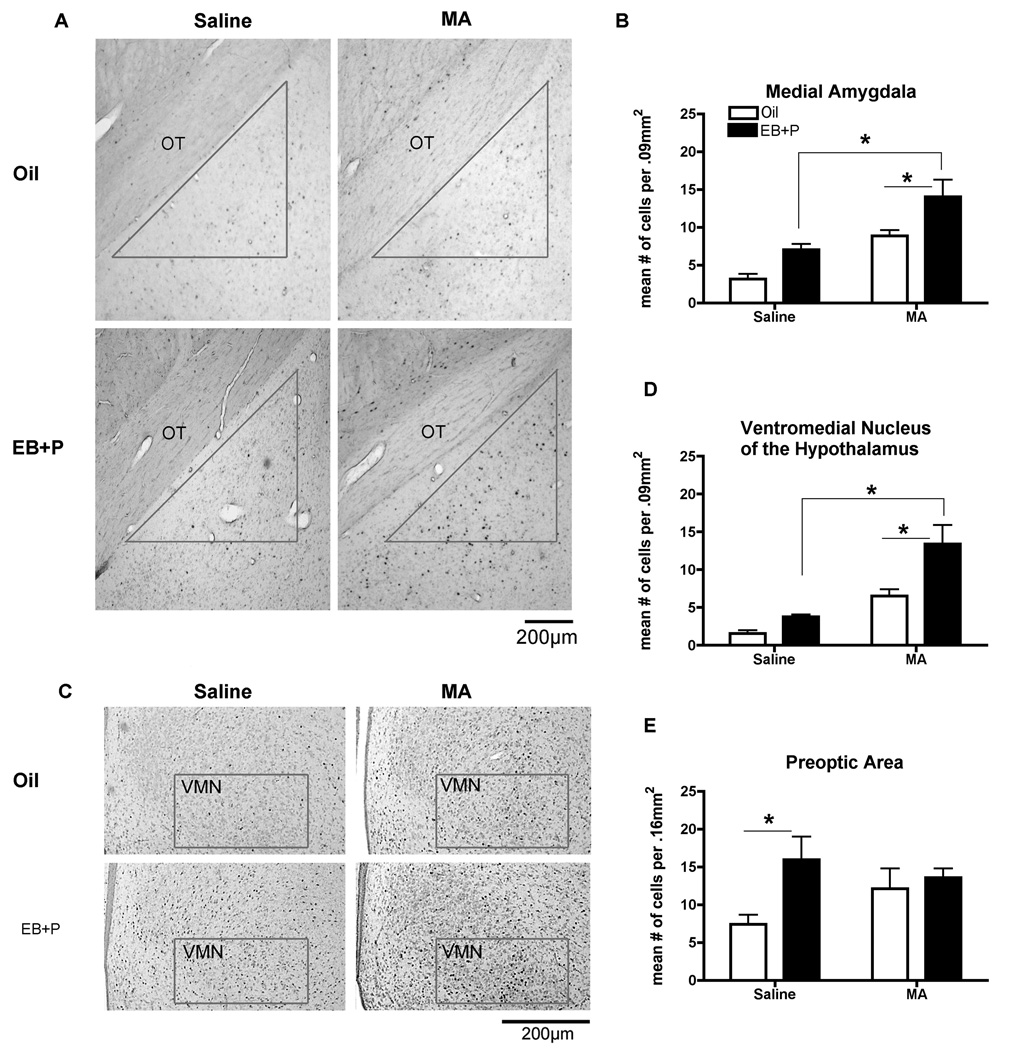
In contrast, there was an effect of both ovarian hormones and MA treatment in the MeA, a brain region implicated in sexual behavior (Erskine, 1989; Polston et al., 2001; Lehmann and Erskine, 2005). There was a significant main effect of MA administration [F(1,18) = 15.85, p<0.01; Figure 6A, B] and ovarian hormones [F(1,18) = 7.386, p<0.05; Figure 6A, B] on the Fos-immunoreactivity in the MeA. Individually, MA (p<0.05) and ovarian hormones (p<0.05) significantly increased Fos-immunoreactivity, compared to the oil, saline-treated controls. Animals treated with both ovarian hormones and MA expressed significantly more Fos-positive cells compared to all treatment groups (p<0.05; Figure 6B). The VMN showed a pattern of activation similar to that of the MeA in which there was a main effect of MA [F(1,24) = 43.39, p<0.0001; Figure 6C, D], and ovarian hormones [F(1,24)=10.45, p<0.01; Figure 6C, D]. Post hoc analyses revealed that individually MA (p<0.05) and ovarian hormone (p<0.05) treatment significantly increased Fos-immunoreactivity with an even greater increase in the presence of both ovarian hormones and MA (p<0.05; Figure 6D). This pattern of activation was specific to the MeA and the VMN. In the mPOA, which also projects to the VMN and receives innervation from the MeA, there was only a significant main effect of ovarian hormones [F(1,24) = 5.169, p<0.05; Figure 6E] on the number of neurons expressing Fos.
Figure 6.
Effect of ovarian hormones and MA on Fos-immunoreactivity (ir) in the (A) the Medial Amgydala and (B) Ventromedial Nucleus of the Hypothalamus. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 h (10µg) prior to the day of collection. Four hours before the collection, animals received an injection of oil vehicle or 500µg P. MA (5mg/kg) was co-administered on each day of the hormonal priming. The photomicrographs represent the Fos-ir in the medial amygdala (A) and the ventromedial nucleus of the hypothalamus (B). The contour represents the counting area used to quantify the number of Fos-positive cells. OT, optic tract. Scale bar: 200 µm. A two-way ANOVA followed by Bonferroni t-tests indicated the combination of EB+P and MA significantly increases Fos-ir compared to either oil control and MA treatments or EB+P and saline controls (*p<0.05) in both brain regions. (n = 7 to 8 animals in each group). (C) Effect of ovarian hormones on Fos-ir in the medial preoptic area. A two-way ANOVA followed Bonferroni t-tests indicated that EB+P and saline-treatment significantly increased Fos-ir compared to the oil and saline-control (*p<0.05). Data are represented as means ± SEM. (n = 7 to 8 animals in each group).
Tyrosine Hydroxylase Immunoreactivity
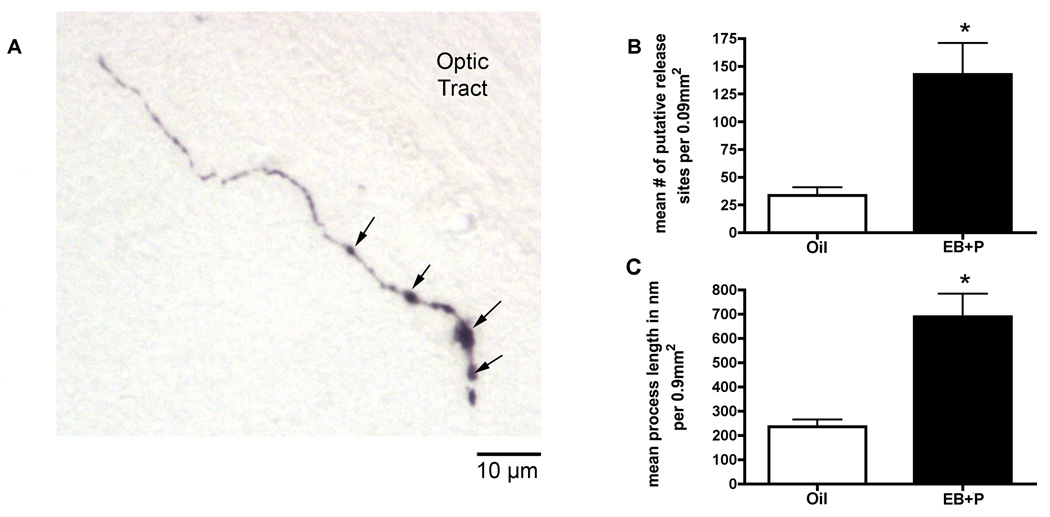
Estradiol has been reported to increase the protein expression of TH in the ventral tegmental area (Bao, 2003), which projects to the MeA (Gray, 1999; Pitkänen, 2000). To test whether ovarian hormones increased TH protein levels in the MeA and VMN, TH-immunoreactivity was quantified. In the MeA and VMN, no TH cell bodies were present as expected. Both nuclei contained TH-positive axonal processes (Figure 7A). The length and number of visible varicosities, which may represent putative neurotransmitter release sites, were quantified as a measure of TH protein expression. Ovarian hormones increased the number of TH-positive varicosities [t(10)=3.723, p<0.01; Figure 7B] and the length of TH-positive processes [t(10)=4.589, p<0.01; Figure 7C], compared to oil vehicle controls. There was no significant difference in TH-immunoreactivity in the VMN (data not shown).
Figure 7.
Effects of ovarian hormones on tyrosine hydroxylase (TH) immunoreactivity (ir) in the medial amygdala. Adult ovariectomized Sprague-Dawley rats were primed with EB 48 h (5µg) and 24 h (10µg) prior to the day of collection or given oil vehicle. Four hours before the collection animals received an injection of oil vehicle or 500µg of P. (A) The photomicrograph represents TH-positive axonal process in the medial amygdala (MeA). There are no cell bodies positive for TH in the MeA, so (B) the number of varicosities, which may represent putative release sites of dopamine, and (C) the length of the axonal processes were quantified. The arrows indicate varicosities as putative release sites. Approximately 20 processes per section were counted. Data represent bilateral counts in three sections that were averaged to yield a single value per animal. A t-test indicated that EB+P treatment significantly increased TH-ir compared to the oil -control (*p<0.05). Data are represented as means ± SEM. (n = 6 animals in each group).
Discussion
We report here that administration of MA (5mg/kg/day for 3 days) to hormonally-primed female rats enhanced proceptive behaviors and the lordosis response as quantified by LQ and LS. MA did not increase stereotypy or locomotor behavior at the time of sexual behavior testing, suggesting that the increase in female sexual behavior was not due to a generalized effect on locomotor activity caused by the stimulatory effects of MA. The pattern of neuronal activation indicates that the VMN and MeA may underlie the enhanced sexual behavior by MA, and the increase in TH in the MeA suggests that this region could be a site of convergence for the hormone and drug actions.
Female receptivity as measured by lordosis is dependent upon estradiol while P in combination with estradiol will facilitate the response. In contrast, activation of neural progesterone receptors is necessary for proceptive behaviors (Beach, 1942; Whalen, 1974; Blaustein, 2008). Overall, MA treatment enhanced female sexual behavior in EB-primed female only when P was present. Specifically, MA increased the number of proceptive events displayed in females treated with all doses of P (200, 250 and 500 µg). Remarkably, in animals primed with the lower doses of P, MA increased the number of proceptive events to the level of saline-treated animals primed with 500 µg. Similarly, MA also increased the LQ in animals treated with a low dose of P to that of a highly receptive female. These data suggest that the actions of MA on female sexual behavior may be mediated through a convergence of the dopamine and P signaling pathways.
Dopamine signaling through the D1 subclass of receptors has been shown to facilitate sexual receptivity (i.e., lordosis responses) via progesterone receptors (Mani et al., 1994; Apostolakis et al., 1996; Mani et al., 1996; Auger et al., 1997; Meredith et al., 1998; Mani et al., 2000; Mani, 2001; Auger, 2004). It is thought that phosphorylation of the progesterone receptor underlies dopamine’s ability to increase lordosis behavior in the absence of P (Auger et al., 1997; Mani, 2001). The progesterone receptor in vitro can be transcriptionally activated by dopamine, in the absence of its ligand, by phosphorylation of its different serine sites (Denner et al., 1990; Power et al., 1991; Bai et al., 1997). Because activation of neural progesterone receptors is crucial for the expression of proceptive behaviors (Beach, 1942; Blaustein, 2008), our data suggest that the combination of MA and ovarian hormones has an additive effect on progesterone receptor activation leading to an enhancement of female sexual behaviors.
It was anticipated that the combination of ovarian hormones and MA would activate key nuclei in motivational and sexual circuitries that may underlie the increase in sexual behavior following MA treatment. The pattern of neuronal activation of the NAcc, BLA, and CeM suggests that while these areas are involved in MA-induced reward, they are not necessarily involved in mediating the MA-induced increase in sexual behavior. In the MeA and the VMN, there was an additive effect of both MA and ovarian hormones on neuronal activation. This pattern of neuronal activation may implicate the MeA and VMN as brain regions mediating the MA-induced increase in the sexual behavior. It is known that the VMN is involved in the expression of female sexual behavior, however the role of the MeA is less understood.
The MeA is a steroid concentrating region that receives dopaminergic transmission from the ventral tegmental area (Gray, 1999; Pitkänen, 2000) and projects to the VMN (Erskine, 1989; Polston et al., 2001; Lehmann and Erskine, 2005). The MeA has been implicated in the control of paced mating behavior (Erskine, 1989; Erskine, 1993; Polston and Erskine, 1995; Polston et al., 2001; Lehmann and Erskine, 2005) making this nucleus a good candidate region for regulating sexual motivation and modulating the output sexual behavior. In support of a modulatory role, when the projections from the MeA to the VMN are severed, female rats display significantly fewer lordosis responses (Masco and Carrer, 1984) and lesions of the MeA do not disrupt sexual behavior (Guarraci et al., 2004).
The increase in TH-immunoreactivity in the MeA in the presence of ovarian hormones suggests a potential mechanism by which the combination of ovarian hormones and MA enhance female sexual behavior. Actions of MA are generally thought to target the dopamine system. Briefly, MA enters the synaptic terminals via the dopamine reuptake transporters where it inhibits packing of dopamine into synaptic vesicles. (Fukui et al., 2003). However, TH, the rate-limiting enzyme in the production of dopamine, is not affected and continues to synthesize dopamine, resulting in an increase in unpackaged dopamine within the synaptic terminal (Fleckenstein et al., 2000). As the dopamine reuptake transporters are concentration dependent, the rising concentration of dopamine within the terminal results in a reversal of the transporters (Fukui et al., 2003), and an increase in extracellular dopamine. In the presence of MA, a consequence of an ovarian steroid-mediated increase in TH could be a further increase in dopamine availability and signaling in the MeA. As TH protein expression in the VMN does not change, the projections from the MeA may trans-synaptically excite neurons in the VMN in the presence of MA.
We have found that systemic administration of MA enhances both receptive and proceptive sexual behaviors in females and these enhancements are dependent upon P. Similarly, acute intracerebroventricular administration of cocaine, which blocks the reuptake of dopamine, also facilitates lordosis is a P dependent manner (Apostolakis et al., 1996) Surprisingly, acute administration of amphetamine, which acts similarly to MA, reduces both proceptive and receptive behaviors in a dose- and time-dependent manner (Michanek and Meyerson, 1977b; Michanek and Meyerson, 1977a; Michanek, 1979) and increases both the female’s avoidance of the male and the number of escapes following a sexual interaction (Guarraci and Clark, 2003; Ellingsen and Agmo, 2004). These observations suggest that MA may have a distinct action not shared by amphetamine on the processes underlying female sexual behavior. In fact, caffeine (a psychostimulant with a distinctly different mechanism of action from MA or amphetamine) shortens the return latency in a paced mating paradigm, which is indicative of enhanced sexual motivation (Guarraci and Benson, 2005). Taken together, these studies suggest that different classes of psychostimulants may preferentially enhance natural reward pathways. At present, the neural mechanisms by which psychostimulants such as cocaine, amphetamine and caffeine affect sexual motivation and behavior are unclear. A comparative examination into the effects of the stimulants on the MeA and VMN may provide a better understanding of their differences.
In conclusion, our current data suggest that the combination of MA and ovarian hormones activate key nuclei involved in female sexual behavior. This activation may be a unique action of MA; however, further investigation is needed to compare sites of actions of different psychomotor stimulant drugs, or drug/hormone interactions, in order to begin to elucidate potential pathways and mechanisms involved in motivated sexual behaviors in the female rat.
Acknowledgements
We thank Joseph A. McQuail for his assistance on this project.
Role of Funding Source
This research was supported by a fellowship from NICHD/ORWH, 5 K12 HD 043489 awarded to Jessica A. Mong and NIH grant MH52716-010 awarded to Margaret M. McCarthy. The NIMH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare that, except for income received from the primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Apostolakis EM, Garai J, Clark JH, O'Malley BW. In vivo regulation of central nervous system progesterone receptors: Cocaine induces steroid-dependent behavior through dopamine transporter modulation of D5 receptor in rats. Mol. Endocrinol. 1996;10:1595–1604. doi: 10.1210/mend.10.12.8961269. [DOI] [PubMed] [Google Scholar]
- Auger AP. Ligand-independent activation of progestin receptors: Relevance for female sexual behavior. Reproduction. 2001;122:847–855. doi: 10.1530/rep.0.1220847. [DOI] [PubMed] [Google Scholar]
- Auger AP. Steroid receptor control of reproductive behavior. Horm. Behav. 2004;45:168–172. doi: 10.1016/j.yhbeh.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Auger AP, Moffatt CA, Blaustein JD. Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology. 1997;138:511–514. doi: 10.1210/endo.138.1.4986. [DOI] [PubMed] [Google Scholar]
- Bai W, Rowan BG, Allgood VE, O'Malley BW, Weigel NL. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J. Biol. Chem. 1997;272:10457–10463. doi: 10.1074/jbc.272.16.10457. [DOI] [PubMed] [Google Scholar]
- Bao SLX. Regulation of estrogen and phytoestrogen on the dopaminergic systems of amygdala in rats. Acta Physiologica Sinica. 2003;55:589–593. [PubMed] [Google Scholar]
- Beach FA. Importance of progesterone to induction of sexual receptivity in spayed female rats. Proc. Soc. Exp. Biol. Med. 1942;51:369–371. [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity and receptivity in female mammals. Horm. Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudrick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: A microdialysis study. Pharmacology, Biochemistry, & Behavior. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudrick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J. Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu. Rev. Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Denner LA, Weigel NL, Maxwell BL, Schader WT, O'Malley BW. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity of subsequent rebound hypersomnolence in the rat. J. Pharmacol. Exp. Ther. 1997;283:757–769. [PubMed] [Google Scholar]
- Ellingsen E, Ågmo A. Sexual-incentive motivation and paced sexual behavior in female rats after treatment with drugs modifying dopaminergic neurotransmission. Pharmacology, Biochemistry, & Behavior. 2004;77:431–445. doi: 10.1016/j.pbb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Balster RL. Rating the behavioral effects of amphetamine. Eur. J. Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: A review. Horm. Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Mating-induced increases in FOS protein in preoptic area and medial amygdala of cycling female rats. Brain Res. Bull. 1993;32:447–451. doi: 10.1016/0361-9230(93)90289-n. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: Pharmacological consequences and implication for neurotoxicy. Eur. J. Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fukui R, Svenningsson P, Matsuishi T, Higashi H, Nairn AC, Greengard P, Nishi A. Effect of methylphenidate on dopamine/DARPP signalling in adult, but not young, mice. J. Neurochem. 2003;87:1391–1401. doi: 10.1046/j.1471-4159.2003.02101.x. [DOI] [PubMed] [Google Scholar]
- Gray TS. Functional and anatomical relationships among the amygdala, basal forebrain, ventral striatum, and cortex.An integrative discussion. Ann. N. Y. Acad. Sci. 1999;877:439–444. doi: 10.1111/j.1749-6632.1999.tb09281.x. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Benson A. "Coffee, tea and me": moderate doses of caffeine affect sexual behavior in female rats. Pharmacol. Biochem. Behav. 2005;82:522–530. doi: 10.1016/j.pbb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Clark AS. Amphetamine modulation of paced mating behavior. Pharmacology, Biochemistry, & behavior. 2003;76:505–515. doi: 10.1016/j.pbb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Megroz AB, Clark AS. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004;999:40–52. doi: 10.1016/j.brainres.2003.10.056. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur. J. Neurosci. 2008;27:1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Hardy DF, Debold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiol. Behav. 1971;7:643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- Hosokawa N, Chiba A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007;1175:66–75. doi: 10.1016/j.brainres.2007.07.071. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulatioin in the female rat. Eur. J. Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Käll K, Nilsonne Å. Preference for sex on amphetamine: A marker for HIV risk behavior among male intravenous amphetamine users in Stockholm. AIDS Care. 1995;7:171–188. doi: 10.1080/09540129550126696. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Erskine MS. Glutaminergic stimulation of the medial amygdala induces steriod dependent c-fos expression within forebrain nuclei responsive to mating stimulation. Neuroscience. 2005;136:55–64. doi: 10.1016/j.neuroscience.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Mani SK. Ligand-independent activation of progestin receptors in sexual receptivity. Horm. Behav. 2001;40:183–190. doi: 10.1006/hbeh.2001.1687. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O'Malley BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol. Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JMC, Clark JH, Blaustein JD, O'Malley BW. Convergent pathways for steroid hormone-and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW. Requirement for DARPP-32 in progesterone-facilitate sexual receptivity in female rats and mice. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- Mansergh G, Purcell DM, Stall R, McFarlane M, Semann S, Valentine J, Valdiserri R. CDC consultation on methamphetamine use and sexual risk behavior for HIV/STD infection: Summary and suggestions. Public Health Rep. 2006;121:127–132. doi: 10.1177/003335490612100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masco DH, Carrer HF. Pathways conducting amygdaloid influence on feminine sexual behavior in the rat. Behav. Brain Res. 1984;11:205–212. doi: 10.1016/0166-4328(84)90212-2. [DOI] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Meredith JM, Moffatt CA, Auger AP, Snyder GL, Greengard P, Blaustein JD. Mating-related stimulation induces phosphorylation of dopamine-and cyclic AMP-regulated phosphoprotein-32 in progestin receptor-containing areas in the female brain. J. Neurosci. 1998;18:10189–10195. doi: 10.1523/JNEUROSCI.18-23-10189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav. Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Michanek A. Potention of D- and L-Amphetamine effects on copulatory behavior in female rats by treatment with a-adrenoreceptor blocking drugs. Arch. Int. Pharmacodyn. Ther. 1979;239:241–256. [PubMed] [Google Scholar]
- Michanek A, Meyerson BJ. A Comparative Study of Different Amphetamines of Copulatory Behavior and Stereotype Activity in the Female Rat. Psychopharmacology (Berl) 1977a;53:175–183. doi: 10.1007/BF00426489. [DOI] [PubMed] [Google Scholar]
- Michanek A, Meyerson BJ. The Effects of Different Amphetamines on Copulatory Behaviour and Stereotype Activity in the Female Rat, after Treatment with Monoamine Depletors and Synthesis Inhibitors. Arch. Int. Pharmacodyn. Ther. 1977b;229:301–312. [PubMed] [Google Scholar]
- Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacology, Biochemistry and behavior. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Ågmo A. Reproductive motivation. In: Phashler H, Gallistel R, editors. Steven's Handbook of Experimental Psychology. Learning, Motivation, and Emotion. New York: Wiley; 2002. pp. 709–736. [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increase dopamine transmisstion in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila G. What Can Animal Models Tell Us About Human Sexual Response? Annu. Rev. Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala. New York: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Polston EK, Erskine MS. Patterns of induction of the immediate-early genes c-fos and egr-1 in the female rat brain following differential amounts of mating stimulation. Neuroendocrinology. 1995;62:371–384. doi: 10.1159/000127027. [DOI] [PubMed] [Google Scholar]
- Polston EK, Heitz M, Barnes K, Erskine MS. NMDA-mediated activation of the medial amygdala initiates a downstream neuroendocrine memory responsible for pseudopregnancy in the female rat. J. Neurosci. 2001;21:4104–4110. doi: 10.1523/JNEUROSCI.21-11-04104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power RF, Mani SK, Codina J, Conneely OM, O'Malley BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Washton A, Domier CP, Reiber C. Drugs and sexual effects: Role of drug type and gender. J. Subst. Abuse Treat. 2002;22:103–108. doi: 10.1016/s0740-5472(01)00215-x. [DOI] [PubMed] [Google Scholar]
- Riviere GJ, Byrnes KA, Gentry WB, Owens SM. Spontaneous locomotor activity and pharmacokinetics of Intrvenous methamphetamine and its metabolite amphetamine in the rat. J. Pharmacol. Exp. Ther. 1999;291:1220–1226. [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J. Pharmacol. Exp. Ther. 1997;282:561–573. [PubMed] [Google Scholar]
- Semple SJ, Grant I, Patterson TL. Female methamphetamine users: Social characteristics and sexual risk behavior. Women Health. 2004a;40:35–50. doi: 10.1300/j013v40n03_03. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Grant I. The context of sexual risk behavior among heterosexual methamphetamine users. Addict. Behav. 2004b;29:807–810. doi: 10.1016/j.addbeh.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Whalen RE. Estrogen-progesterone induction of mating behavior in female rats. Horm. Behav. 1974;5:157–162. doi: 10.1016/0018-506x(74)90040-3. [DOI] [PubMed] [Google Scholar]