Abstract
Sleep fragmentation (SF) impairs the restorative/cognitive benefits of sleep via as yet unidentified alterations in neural physiology. Previously, we found that hippocampal synaptic plasticity and spatial learning are impaired in a rat model of SF which utilizes a treadmill to awaken the animals every 2 min, mimicking the frequency of awakenings observed in human sleep apnea patients. Here, we investigated the cellular mechanisms responsible for these effects, using whole-cell patch-clamp recordings. 24h of SF decreased the excitability of hippocampal CA1 pyramidal neurons via decreased input resistance, without alterations in other intrinsic membrane or action potential properties (when compared to cage controls, or to exercise controls that experienced the same total amount of treadmill movement as SF rats). Contrary to our initial prediction, the hyperpolarizing response to bath applied adenosine (30 µM) was reduced in the CA1 neurons of SF treated rats. Our initial prediction was based on evidence that sleep loss upregulates cortical adenosine A1 receptors; however, the present findings are consistent with a very recent report that hippocampal A1 receptors are not elevated by sleep loss. Thus, increased adenosinergic inhibition is unlikely to be responsible for reduced hippocampal long-term potentiation in SF rats. Instead, the reduced excitability of CA1 pyramidal neurons observed here may contribute to the loss of hippocampal long-term potentiation and hippocampus-dependent cognitive impairments associated with sleep disruption.
Keywords: Sleep interruption, input resistance, whole cell, A1 receptor
Sleep fragmentation (SF), a common symptom in several clinical disorders, can lead to impaired cognitive function, via mechanisms which are currently poorly understood [5]. The cognitive impairments experienced after SF have been shown to be as severe as those of total sleep deprivation even though total sleep time may not be significantly altered [5,30]. Specifically, unlike total sleep deprivation, it is the frequent arousals and restructuring of sleep caused by SF that are thought to underlie the neurocognitive impairments, rather than the loss of total sleep time. In fact, in clinical populations with severe sleep fragmentation (such as obstructive sleep apnea) total sleep time typically diminishes only slightly [10].
While many previous animal studies have investigated the neurophysiological and behavioral effects of total sleep deprivation or selective REM sleep deprivation, much less is known concerning the effects on the brain of SF. However, SF is potentially more clinically relevant, as humans are more likely to experience fragmented sleep than sleep deprivation [30]. Recently, our group developed an animal model of SF whereby rats were awakened once every 2 min via 30 s of slow movement on an automated treadmill [24,27,31]. This method of SF resulted in increased electrographic and behavioral measures of sleepiness [27] and impairments in cognitive tasks such as attentional set-shifting [24] and spatial learning in the Morris water maze [31,33], mimicking deficits observed in humans. In addition, 24 h of SF resulted in a decrease in hippocampal long-term potentiation (LTP), but no change in hippocampal long-term depression (LTD) in the CA1 region of the hippocampus [31]. The impairment of LTP is likely to be involved in the spatial learning deficits induced by SF. However other changes in the properties of hippocampal neurons are possible. For instance, changes in the excitability of hippocampal neurons have been associated with hippocampus-dependent trace eye-blink conditioning [28]. Moreover, previous work by McDermott and colleagues [25] demonstrated a decrease in input resistance and spike frequency adaptation in neurons from REM sleep deprived rats compared to controls suggesting that REM sleep deprivation decreases the intrinsic membrane excitability of CA1 pyramidal neurons. Here we specifically investigated postsynaptic mechanisms of CA1 pyramidal neurons since paired-pulse facilitation (PPF), a measure of presynaptic function, showed no differences between sleep fragmented and control rats [31].
Recent work by our laboratory and others has investigated adenosine (AD) as a biological signal mediating the sleepiness that accompanies prolonged wakefulness (reviewed in [4]). Indeed, recent data specifically indicate that a sleep interruption schedule, identical to the one utilized in this study, elevates AD levels in the basal forebrain and increases sleepiness [27], presumably via adenosine inhibition of cortically projecting, wake-promoting basal forebrain neurons [2,6]. Hippocampal AD levels are also directly related to sleep and activity since hippocampal extracellular AD levels are higher during active periods compared to inactive periods in rats [21]. AD has potent inhibitory pre- and postsynaptic effects on hippocampal neurons [14,19,20] and AD can inhibit LTP induction at Schaffer collateral-CA1 synapses [1], making increased adenosinergic tone a likely mechanism by which sleep loss might interfere with hippocampal plasticity and learning. In addition to increases in extracellular AD levels, prolonged sleep deprivation is associated with upregulation of AD A1 receptors in the basal forebrain [3] as well as in several neocortical areas [16] providing an additional level of homeostatic control of brain function by adenosine during sleep disruption. Thus, in addition to investigating the intrinsic excitability of hippocampal CA1 neurons we also investigated the sensitivity of these neurons to exogenous AD application to determine if SF increases the response to AD via an upregulation of AD A1 receptors.
Young, male Sprague-Dawley rats (Charles River, Wilmington, MA), 21–30 days old, were housed under a 12:12 light dark cycle (lights-on 0700 h-1900 h) with food and water available ad libitum. All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals. Experiments were designed to ensure minimal pain or discomfort to the rats. All procedures were approved by the institutional animal care and use committee of the VA Boston Healthcare System.
SF was induced in an identical manner to our previous study [31]. Rats lived in a modified treadmill cage (l × w × h = 51 × 16.5 × 30.5 cm) in which the floor is a horizontal belt programmed to move slowly at .02 m/s, a speed we previously determined to reliably produce awakenings. The treadmills ran at this speed for 30 sec, followed by no treadmill activity for 90 sec, producing 30 interruptions of sleep per hour continuously throughout the 24 hrs of sleep interruption. The sleep changes associated with this procedure were documented in detail in our previous studies [27,31]. Non-REM sleep episode duration, delta activity and REM sleep amount are strongly decreased during the 24 hr, resulting in increased sleepiness when assessed electrographically or behaviorally following the procedure. Food and water were available ad libitum in the treadmill.
Rats received two 1 hr daily training (habituating) sessions in the treadmill prior to the experiment (5 min on: 5 min off), in order to habituate the rat to the treadmill movement. To control for motor activity and the potential stress of being on the treadmill apparatus, an exercise control group was included. In the exercise group, rats obtained an equivalent amount of treadmill movement, but the duration of treadmill operation (10 min on/30 min off) allowed for longer periods of undisturbed sleep [24,27,31]. The cage control group was housed in the treadmill for a 24 hr period, but the treadmill device was not turned on.
For in vitro brain slice experiments, rats from the different experimental groups were deeply anesthetized with isoflurane and rapidly decapitated. Brains were quickly removed and horizontal slices (300 µm) containing hippocampus were cut using a vibroslicer in ice cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 1.8, KH2PO4 1.2, MgSO4 1.3, CaCl2 2, NaHCO3 25.6, D-glucose 10. Slices were placed in ACSF and oxygenated with 95% O2–5% CO2 at room temperature for at least 1 hr. Slices were tested in a recording chamber which was constantly perfused with oxygenated ACSF at 33°C. Patch pipettes (3–6 MOhm) were filled with solution containing (in mM) potassium methylsulfate 130, NaCl 5, MgCl2 1.3, HEPES 10, EGTA 0.1, Na2ATP 2, NaGTP 0.5, MgATP 4, Spermine 1 (pH 7.25 with KOH, 280 mOsm). Whole cell recordings were made from CA1 pyramidal neurons under visual control (using infra-red, differential interference microscopy). Series resistance was 8–20 MΩ. Recordings were discarded if series resistance was higher than 20 MΩ, if it changed by more than 20% during the experiment, or if the recordings were unstable. Signals were amplified using an Axoclamp 2A amplifier (Axon Instruments, St Louis, MO), digitized with a Digidata 1322A (Molecular Devices, Sunnyvale, CA) and recorded/analyzed on a PC using pClamp 9.0 software (Molecular Devices). Membrane voltage measurements were adjusted for a 9 mV liquid junction potential (calculated using pClamp 9.0).
To assess postsynaptic membrane properties a series of 1 sec long hyperpolarizing and depolarizing current pulses were applied through the recording pipette according to our standard techniques [7]. The size of the first (hyperpolarizing) pulse was adjusted so that the peak voltage-response reached −120 mV. Each subsequent current step was altered in the positive direction by one-fifth of this initial value so that after the sixth current pulse, the steps became depolarizing. Depolarizing sag was determined from the response to the first (largest) hyperpolarizing current step and defined as sag(%)=100 –((voltage difference at end of step/voltage difference at peak)*100). Input resistance (Rin) was calculated according to Ohms’ Law from the peak of the response to the smallest hyperpolarizing pulse. Action potential height and afterhyperpolarization amplitude were determined relative to the threshold voltage for the first action potential elicited during the smallest depolarizing current step. Action potential rise time and duration were also measured. The amplitude of slow afterhyperpolarizations following the offset of depolarizing pulses eliciting action potentials was determined in relation to the baseline period preceding the pulse. Initial firing rate was determined from the first depolarizing step.
In preliminary experiments, we determined that a brief (2 min) bath application of 30 µM AD led to a substantial but submaximal hyperpolarization, allowing us to observe up or downregulation of the postsynaptic response to AD. During drug (AD, 30 µM) application, small hyperpolarizing current pulses (equal in amplitude to the smallest hyperpolarizing pulses applied in the excitability analyses above) were applied every 15 s to monitor the effect of AD on membrane potential and input resistance. A total of 4 neurons (1 cage control and 3 exercise control) were excluded from the AD application analyses either because the recording was lost during the AD application, or because the membrane potential did not return to baseline after AD washout. During recordings, slices were filled with biocytin in order to allow later histological confirmation that the cells recorded were pyramidal neurons. Current-clamp recordings were acquired at 20 kHz and low-pass filtered at 10 kHz. Bridge balance was maintained during the experiment.
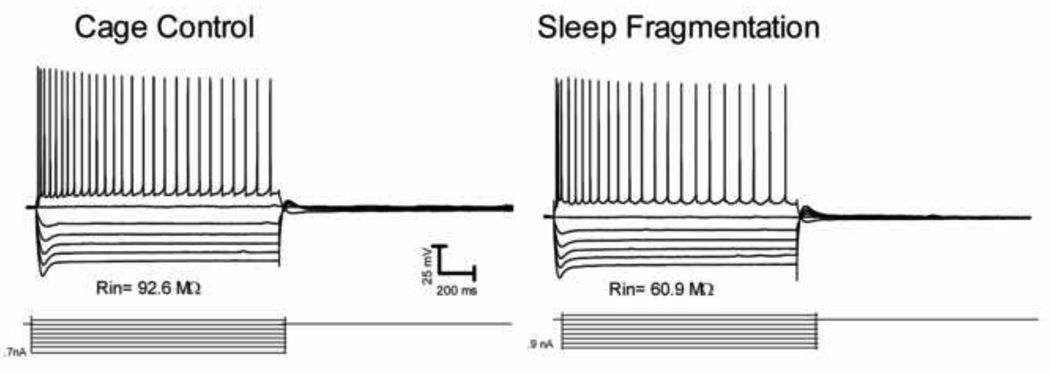
Our results showed that compared to CA1 pyramidal neurons recorded from cage control rats (n=10) and exercise control rats (n=7) pyramidal neurons from sleep fragmented rats (n=9) had no change in the action potential amplitude, width or firing rate during a series of depolarizing current injections (Table 1, Fig. 1). However, analysis of variance (ANOVA) revealed significant group differences on the measure of input resistance (Rin) F(2,23) = 4.280, p<0.05 and Tukey post-hoc analyses further indicated that the sleep fragmented group had significantly lower Rin compared to the control groups (Table 1, Fig. 1; p<0.05), while control groups were not significantly different from each other (Table 1). Depolarizing sag during hyperpolarizing current injections, due to activation of the hyperpolarization activated cation current Ih, was not significantly affected by either sleep fragmentation or exercise control (Note: current step values were calibrated to compensate for differences in input resistance between neurons, allowing us to determine depolarizing sag and action potential firing rate at equivalent levels of hyperpolarization or depolarization respectively – see methods above). The afterhyperpolarizations (AHP) following individual action potentials for the exercise control group (but not the sleep fragmentation group) were significantly smaller than the cage control group (p<0.05).
Table 1. Passive and active postsynaptic membrane properties of CA1 pyramidal neurons in the 3 different experimental groups.
Summary of intrinsic membrane properties and response to adenosine application of CA1 pyramidal neurons in sleep fragmented, cage control and exercise control rats. Hippocampal CA1 neurons from sleep fragmented rats had decreased input resistance (Rin) during hyperpolarizing current injection when compared to cage control and exercise control rats. Sleep fragmented rats had less hyperpolarization in response to AD application compared to cage control rats. However, exercise control rats also showed less hyperpolarization in response to AD application compared to cage control rats, at a level that was intermediate to the cage control and the sleep fragmentation groups.
| Property | Cage Control | Exercise Control | Sleep Fragmentation |
|---|---|---|---|
| (n=10) | (n=7) | (n=9 | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| RMP (mV) | −70.1 ± 1.3 | −68.5 ± 1.9 | −68.9 ± 0.6 |
| Rin (MΩ) | 107 ± 13.7 | 110 ± 11.1 | 69.0 ± 6.5*† |
| Membrane Time constant (ms) | 17.3 ± 1.4 | 15.3 ± 0.7 | 14.2 ± 1.3 |
| % Sag (Largest hyperpolarizing step) | 15.4 ± 1.8 | 10.8 ± 1.8 | 8.2 ± 5.4 |
| AP threshold (mV) | −55.1 ± 0.8 | −53.9 ± 0.9 | −54.3 ± 1.5 |
| AP amplitude (mV) | 79.4 ± 3.9 | 70.2 ± 3.1 | 75.7 ± 3.8 |
| AP width (ms) | 1.6 ± 0.2 | 2.0 ± 0.1 | 1.6 ± 0.1 |
| AHP (mV) | −9.0 ± 1.2 | −4.6 ± 1.2* | −8.3 ± 1.1 |
| sAHP (mV) | −7.3 ± 0.7 | −6.2 ± 0.5 | −6.8 ± 0.8 |
| Initial firing frequency (Hz) | 16.6 ± 1.9 | 13.6 ± 4.2 | 14.6 ± 1.8 |
| First dep. step | |||
| Max firing frequency (Hz) | 42.8 ± 6.2 | 39.3 ± 6.2 | 40.2 ± 3.8 |
| Property | Cage Control | Exercise Control | Sleep Fragmentation |
| before and during AD (30 µM) | (n=9) | (n=7) | (n=6) |
| application | Mean ± SEM | Mean ± SEM | Mean ± SEM |
| RMP pre AD (mV) | −69.7 ± 1.9 | −66.4 ± 1.5 | −68.8 ± 0.5 |
| RMP post AD (mV) | −74.9 ± 1.8 | −70.1 ± 1.5 | −71.6 ± 0.7 |
| AD hyperpolarization (mV) | −5.2 ± 0.4 | −3.6 ± 0.2* | −2.9 ± 0.3** |
Abbreviations: AD=adenosine, AP=action potential, AHP=afterhyperpolarization, RMP=Resting membrane potential, Rin=input resistance, sAHP=slow after hyperpolarization.
Asterisks indicates significant difference from cage control (* p<0.05, ** p<0.001)
Dagger indicates significant difference from exercise control († p<0.05)
Figure 1.
Sleep fragmented animals had reduced input resistance but were unchanged in other intrinsic membrane properties. Representative recordings for the depolarizing and hyperpolarizing current injections from rats in the cage control and sleep fragmented groups.
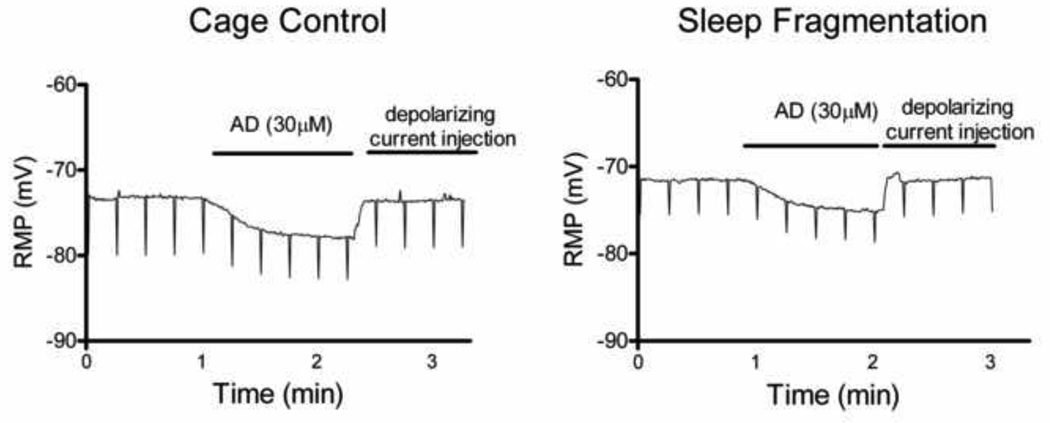
There was also a significant difference between groups in the voltage change to AD application (F(2,19) = 13.94, p<0.05, Table 1, Fig. 2). Opposite to our expectation of an increased response to AD in sleep disrupted animals (due to upregulation of A1 receptors), we found that sleep fragmented rats (n=9) had less hyperpolarization in response to AD application compared to cage control rats (n=7, p<0.001). Exercise control rats (n=7) also showed less hyperpolarization in response to AD application compared to cage control rats (p<0.05) although at a level that was intermediate to cage control and sleep fragmentation (Table 1).
Figure 2.
Sleep fragmented rats had a reduced hyperpolarizing response to adenosine (AD, 30 µM). Representative recordings from the AD application from rats in the cage control and sleep fragmented groups.
Here, we demonstrate that compared to control groups, 24 hours of sleep fragmentation significantly lowers the input resistance of CA1 pyramidal neurons without altering other intrinsic membrane or action potential properties. We also determined that, contrary to our expectations, the hyperpolarizing response to AD application was reduced after sleep fragmentation (and to a lesser degree after exercise control).
Our findings complement previous work on sleep loss and hippocampal function. Specifically, one previous study found that 72 hours of primarily REM sleep deprivation produced a 25 % decrease in the input resistance of neurons in the CA1 region of the hippocampus with a concomitant decrease in LTP generation [25]. Our sleep fragmentation paradigm results in reductions of REM sleep as well as a decrease in non-REM sleep episode duration [31]. Thus, the reduced excitability we observed here and the loss of LTP we observed in our previous study [31] may be related to the loss of REM sleep. However, the magnitude of the excitability changes we observed was larger (36% input resistance reduction) than that observed after selective REM sleep deprivation (25% reduction) suggesting that the reduction in NREM sleep episode duration may also have played a role.
Retention of NMDA receptor subunits (NR1 and NR2A) in the cytoplasm has been proposed as a mechanism for the LTP changes in the studies of McDermott and colleagues [9,26]. This work suggests that the loss of REM sleep results in postsynaptic molecular changes which ultimately alter hippocampal functioning. Combined, our present and previous findings [31] also support the idea that sleep loss-induced alterations in hippocampal CA1 region functioning are caused by postsynaptic mechanisms. A reduction in input resistance after sleep fragmentation might contribute to the impaired LTP induction we observed after sleep fragmentation [31] since a decrease in input resistance would result in reduced depolarization during tetanic stimulation and reduced Ca2+ influx leading to a decrease in the amount of LTP induced during high frequency stimulation. In addition, reduced input resistance would likely result in other changes such as an impaired ability to generate place fields and reduced/impaired hippocampal oscillations which could result in the observed impaired spatial learning in sleep fragmented animals [31,33].
We also tested the hypothesis here that increased AD receptor levels during sleep fragmentation might be one cause of impaired LTP. Previous studies showed that adenosine is a potent inhibitor of LTP [1]. We have shown that AD levels rise in the basal forebrain of rats during sleep fragmentation [27] and also in the cortex of cats during sleep deprivation [29], whereas other areas, such as the thalamus or preoptic area show a decrease or no change. This increase in AD causes an upregulation of adenosine A1 receptors in basal forebrain and neocortex [3,4,16,17] allowing a stronger inhibitory action of adenosine during prolonged periods of sleep deprivation. However, to our surprise the effect of adenosine was reduced and not increased in the sleep fragmentation group. In the sleep fragmentation group this reduction in adenosine hyperpolarization (55.6 % of cage control values) can likely be accounted for mainly by the reduced input resistance (64.4 % of cage control values) of the neurons in this group, according to Ohms Law (voltage proportional to resistance if current, i.e. number of open channels, is unchanged). However, other mechanisms, such as a desensitization of postsynaptic adenosine receptors are also possible [34]. A (less substantial) reduction in AD responsiveness also occurred in the exercise control group suggesting that some non-sleep related factors may also play a role in this reduced AD effect. Surprisingly, AHP amplitude but not input resistance was reduced in the exercise control group; at present we do not have an explanation for this effect.
Considering our results here, it seems unlikely that increased adenosinergic inhibition can account for the large decrease in LTP induction we have observed in sleep fragmented rats [31]. The unchanged resting membrane potential of CA1 neurons (current study) and unchanged paired-pulse facilitation of schaffer-collateral inputs [31] also argue against an upregulation of extracellular adenosine or adenosine A1 receptors since adenosine, acting on postsynaptic and presynaptic A1 receptors, is a potent modulator of these properties [13,14,20,32]. The conclusion that sleep fragmentation does not increase adenosinergic inhibition in the hippocampus is consistent with recent findings that 24 hour sleep deprivation does not significantly increase A1 receptor binding in the hippocampus of the rat [17]. Thus, despite diurnal fluctuations in adenosine levels in the hippocampus [21], A1 receptors in this structure do not appear to be especially sensitive to sleep disruption, in contrast to other regions such as the basal forebrain and neocortex [3,4,16,17]. Interestingly, in contrast to the basal forebrain, postsynaptic adenosine A1 receptors in the hippocampus are downregulated following prolonged adenosine exposure [34].
A reduction of synaptic NMDA receptors or an alteration of NR2A/NR2B subunit ratio, which has now been observed in several recent sleep deprivation studies [9,22,23,26] together with reduced excitability of CA1 pyramidal neurons, might be a more likely mechanism to account for the sleep fragmentation induced inhibition of LTP. However, alterations in NMDA receptors have not been shown so far for sleep fragmentation, which is likely to be a more clinically relevant model for sleep disorders compared to total or selective REM sleep deprivation. Further studies of the mechanisms by which sleep disruption lead to reduced excitability of hippocampal neurons and impaired LTP will be important in order to develop therapeutic strategies to target impaired neurobehavioral function associated with self-imposed or disease-induced sleep disturbance.
Acknowledgments
We thank John Franco for care of the animals. This research was supported by VA, NHBLI-P50 HL060292 (RES and RWM) and NHBLI-T32 HL07901 (JLT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: This is not an industry supported study. The authors have indicated no financial conflicts of interest.
References
- 1.Arai A, Kessler M, Lynch G. The effects of adenosine on the development of long-term potentiation. Neurosci. Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- 2.Arrigoni E, Chamberlin NL, Saper CB, McCarley RW. Adenosine inhibits basal forebrain cholinergic and non-cholinergic neurons in vitro. Neuroscience. 2006;140:403–413. doi: 10.1016/j.neuroscience.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–1899. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- 4.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog. Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH. Sleep Fragmentation. In: Lenfant C, editor. Sleep deprivation. New York: Marcel Dekker; 2005. pp. 103–117. [Google Scholar]
- 6.Brown RE, Franciosi S, McKenna JT, Winston S, Yanagawa Y, McCarley RW. Electrophysiological and pharmacological characterization of cortically projecting basal forebrain neurons in the mouse. Soc. Neurosci. Abs. 2008 384.16. [Google Scholar]
- 7.Brown RE, Winston S, Basheer R, Thakkar MM, McCarley RW. Electrophysiological characterization of neurons in the dorsolateral pontine rapid-eye-movement sleep induction zone of the rat: intrinsic membrane properties and responses to carbachol and orexins. Neuroscience. 2006;143:739–755. doi: 10.1016/j.neuroscience.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J. Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Hardy M, Ziang J, LaHoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem. Biophys. Res. Comm. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn MA, Karacan I, Kupfer DJ, Lemmi H, Miles LE, Orr WC, Phillips ER, Roth T, Sassin JF, Schmidt HS, Weitzman ED, Dement WC. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. J.A.M.A. 1982;247:997–1003. [PubMed] [Google Scholar]
- 11.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 12.Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- 13.Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J. Pharmacol. Exp. Ther. 1989;249:1–7. [PubMed] [Google Scholar]
- 14.Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J. Physiol. 1985;369:365–377. doi: 10.1113/jphysiol.1985.sp015907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Ann. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Elmenhorst D, Meyer PT, Winz OH, Matusch A, Ermert J, Coenen HH, Basheer R, Haas HL, Zilles K, Bauer A. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 2007;27:2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredholm BB, Lindström K. Autoradiographic comparison of the potency of several structurally unrelated adenosine receptor antagonists at adenosine A1 and A(2A) receptors. Eur. J. Pharmacol. 1999;380:197–202. doi: 10.1016/s0014-2999(99)00533-6. [DOI] [PubMed] [Google Scholar]
- 19.Gerber U, Greene RW, Haas HL, Stevens DR. Characterization of inhibition mediated by adenosine in the hippocampus of the rat in vitro. J. Physiol. 1989;417:567–578. doi: 10.1113/jphysiol.1989.sp017819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog. Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-l. [DOI] [PubMed] [Google Scholar]
- 21.Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RK. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neuroscience. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- 22.Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J. Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. NR2A at CA1 synapses is obligatory for the susceptibility of hippocampal plasticity to sleep loss. J. Neurosci. 2009;29:9026–9041. doi: 10.1523/JNEUROSCI.1215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 25.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J.Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J. Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer JR, Thomson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: An in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 30.Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25:268–276. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- 31.Tartar JL, Ward CP, McKenna JT, Thakkar MM, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur. J. Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson SM, Haas HL, Gaehwiler BH. Comparison of the actions of adenosine at preand postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward CP, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs spatial reference but not working memory in Fischer/Brown Norway rats. J. Sleep Res. 2009;18:238–244. doi: 10.1111/j.1365-2869.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. J. Neurosci. 2002;22:1248–1255. doi: 10.1523/JNEUROSCI.22-04-01248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]