Abstract
Native Americans have some of the highest rates of marijuana and alcohol use and abuse, yet neurobiological measures associated with dependence on these substances in this population remain unknown. The present investigation evaluated the heritability of spectral characteristics of the electroencephalogram (EEG) and their correlation with marijuana and alcohol dependence in an American Indian community. Participants (n=626) were evaluated for marijuana (MJ) and alcohol (ALC) dependence, as well as other psychiatric disorders. EEGs were collected from six cortical sites and spectral power determined in five frequency bands (delta 1.0–4.0 Hz, theta 4.0–7.5 Hz, alpha 7.5–12.0 Hz, low beta 12.0–20.0 Hz and high beta/gamma 20–50 Hz). The estimated heritability (h2) of the EEG phenotypes was calculated using SOLAR, and ranged from 0.16 to 0.67. Stepwise linear regression was used to detect correlations between MJ and ALC dependence and the spectral characteristics of the EEG using a model that took into account: age, gender, Native American Heritage (NAH) and a lifetime diagnosis of antisocial personality and/or conduct disorder (ASPD/CD). Increases in spectral power in the delta frequency range, were significantly correlated with gender (p<0.001) and marijuana dependence (p<0.003). Gender, age, NAH and ASPD/CD were all significantly (p<0.001) correlated with theta, alpha and beta band power, whereas alcohol dependence (p<0.01), gender (p<0.001), and ASPD/CD (p<0.001) were all correlated with high beta/gamma band power. These data suggest that the traits of EEG delta and high beta /gamma activity are correlated with MJ dependence and alcohol dependence, respectively, in this community sample of Native Americans.
Keywords: Drug dependence, EEGs, marijuana dependence, alcohol dependence, Native Americans, ASPD
1. Introduction
Although tribes differ with regard to the use of alcohol and drugs, the United States Indian Health Service has cited substance abuse as one of the most urgent health problems facing Native Americans (Burns 1995; United States Indian Health Service 1977, 1982, 1997). It has been reported that several Native American communities have alcohol dependence rates that are 4–5 times higher than the general U.S. population (Gilder et al., 2004; Kinzie et al., 1992; Kunitz et al., 1999; Mitchell et al., 2003; Robin et al., 1998). Few studies have investigated illicit substance use involvement in Native American adults (Mitchell et al., 2003), however, some studies have investigated drug use in Native American adolescents. Marijuana is the most widely used illicit substance, especially in young adults, and represents a major public health concern (Grob and Dobkin de Rios, 1992; Stinson et al., 2005, 2006; United States Congress, 1990). Several studies have demonstrated that the use of marijuana is disproportionately higher among Native than non-Native Americans (Bachman et al., 1991; Beauvais, 1992, 1996; Blum et al., 1992; Federman et al., 1997; Gfellner, 1994; Stinson et al., 2006). The etiological factors responsible for the higher prevalence of marijuana use and use disorders in Native Americans are largely unknown, however, both genetic and environmental influences have been suggested to account for the variation in marijuana use in the general population (Kendler and Prescott, 1998; Kendler et al., 2000; Lynskey et al., 2002; Tsuang et al., 1996; van den Bree et al., 1998).
Some psychosocial factors have been suggested to account for increased prevalence of substance use in Native American adolescents, such as: life stress (King and Thayer, 1993), lower grades in school, antisocial behavior, the use of alcohol and other illicit substances (Novins and Mitchell, 1998). These factors may be a result of substance use and not necessarily pre-disposing factors. Beauvais and colleagues have emphasized that Indian culture or traditions do not appear to be causative in promoting the use of drugs or influencing the development of drug dependence (see Bates et al., 1997; Beauvais, 1992; Beauvais et al., 1985; Oetting et al., 1989).
In a population of SWC (Southwest California /Mission Indians) adults, consistent with the literature in populations of non-Indians, several risk factors have been found to be associated with alcohol and marijuana use disorders. Early age of onset of trying the drug, being male, and having an externalizing diagnosis (conduct disorder or antisocial personality disorder) are associated with both alcohol and marijuana dependence (Ehlers et al., 2006, 2007a). Whereas having an internalizing diagnosis (any affective or anxiety disorder) was not found to be associated with alcohol or marijuana abuse or dependence in SWC Indians (Gilder et al., 2004, 2006). Although substance dependence has been found to have a heritable component in SWC Indians, specific genetic factors and the genes that encode for them are as yet fully identified (Ehlers and Wilhelmsen, 2005, 2006; Ehlers et al., 2004b, 2007b, 2008a, 2008b; Wilhelmsen and Ehlers, 2005).
Electrophysiological measures are highly heritable phenotypes that may aid in linking brain function to the processes involved in the development of substance dependence in the general population (see Begleiter and Porjesz, 1999; Porjesz et al., 1998, 2005), as well as in SWC Indians (Criado and Ehlers, 2007; Ehlers et al., 2008a). Several features of the resting EEG have been shown to be genetically influenced, and EEG phenotypes based on frequency and amplitude characteristics that may be useful in genetic analyses have been suggested (Rangaswamy et al., 2002, 2004; Vogel, 1962). Evidence from twin studies confirm that a significant proportion of the variance in the EEG is genetically determined (Christian et al., 1996; Juel-Nielson and Harvald, 1958; Lykken et al., 1982; Stassen et al., 1987; van Beijsterveldt and Boomsma, 1994; Vogel, 1962). EEG patterns appear to remain highly stable over most of an adult’s lifespan (Vogel, 1970), with variation within an individual studied on two different occasions being not greater than that observed between monozygotic twins (Lykken et al., 1982).
Several important studies have begun to identify genes associated with certain EEG phenotypes. Low voltage alpha, in females, has been reported to be associated with a genetic variant that leads to low activity of the enzyme that metabolizes dopamine and norepinephrine, catechol-o-methyltransferase (COMT) (Enoch et al., 2003). Low voltage alpha has also been reported to be linked to the GABAergic system, as an association has been found between the exon 7 variant of the GABAB receptor gene and alpha voltage (Winterer et al., 2003a, 2003b). In particular, several genetic studies have demonstrated that bipolar EEG measures are highly heritable (Tang et al., 2007), and may be particularly useful endophenotypes for substance dependence. Porjesz and colleagues (2002) have also found significant associations between and the GABAergic system and bipolar measures of the human EEG. They found significant genetic linkage between the beta frequency of the EEG and a cluster of GABAA receptors genes on chromosome 4P. Additionally, this same GABAA receptor gene was found to be associated with a DSM-IV diagnosis of alcohol dependence (Edenberg et al., 2004). These genetic findings provide evidence that EEG measures are promising endophenotypes in the search for genes involved in alcohol dependence.
This report is part of a larger study exploring risk factors for substance dependence in an American Indian community (collectively called Mission Indians/ SWC Indians) (see Ehlers and Wilhelmsen, 2005, 2007; Ehlers et al., 2004a, 2004b, 2006, 2007a, 2007b, 2007c; Garcia-Andrade et al., 1996, 1997; Gilder et al., 2004; Wall et al., 2003). In this study, the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) was employed to evaluate a sample of 626 reservation-dwelling Indians from eight contiguous reservations. The specific goal of this report was to examine the heritability of bipolar EEG spectral phenotypes and to determine their associations with marijuana and alcohol dependence taking into consideration factors such as age, gender, Native American Heritage (NAH), and antisocial personality disorder and/or conduct disorder (ASPD/CD) in this Indian community.
2. Materials and Methods
2.1 Participants
Participants were recruited from eight geographically contiguous reservations, with a total population of about 3,000 individuals, using a combination of a venue-based method for sampling hard-to-reach populations (Kalton and Anderson, 1986; Muhib et al., 2001), as well as a respondent-driven procedure (Heckathorn, 1997) as previously described (Ehlers et al., 2004b; Gilder et al., 2004). The venues for recruitment included: tribal halls and culture centers, health clinics, tribal libraries, and stores on the reservations. A 10–25% rate of refusal was found depending on venue. Refusal rates were higher at tribal libraries and stores than health clinics and tribal halls/culture centers. Transportation from their home to The Scripps Research Institute was provided by the study.
To be included in the study, participants had to be an Indian indigenous to the catchment area, at least 1/16th Native American Heritage (NAH), between the age of 18 and 70 years, and be mobile enough to be transported from his or her home to The Scripps Research Institute (TSRI). Participants were excluded from electrophysiological analyses if they had a history of head trauma or were currently using medications that could bias the EEG recording. The protocol for the study was approved by the Institutional Review Board (IRB) of TSRI, and the Indian Health Council, a tribal review group overseeing health issues for the reservations where recruitment was undertaken.
2.2 Measures
Potential participants first met individually with research staff to have the study explained and give written informed consent. During a screening period, participants had blood pressure and pulse taken, and completed a questionnaire that was used to gather information on demographics, personal medical history, ethnicity, and drinking history (Schuckit, 1985). Participants were asked to refrain from alcohol and drug usage for 24 hours prior to the testing. No individuals with detectable breath alcohol levels were included in the study dataset (n=3). During the screening period, the study coordinator also noted whether the participant was agitated, tremulous, or diaphoretic and their data were eliminated from subsequent analyses. Each participant also completed an interview with the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) and the family history assessment module (FHAM) (Bucholz et al., 1994), which was used to make substance use disorder and psychiatric disorder diagnoses according to Diagnostic and Statistical Manual (DSM-III-R, American Psychiatric Association, 1987) criteria in the probands and their family members (American Psychiatric Association, 1987). The SSAGA is a semi- structured, poly-diagnostic psychiatric interview that has undergone both reliability and validity testing (Bucholz et al., 1994; Hesselbrock et al., 1999). It has been used in another Native American sample (Hesselbrock et al., 2000, 2003). Personnel from the Collaborative Study on the Genetics of Alcoholism (COGA) trained all interviewers. The SSAGA interview includes retrospective lifetime assessments of marijuana and alcohol use, abuse, and dependence. A research psychiatrist/addiction specialist made all best final diagnoses.
2.3 EEG Collection and Analyses
Six channels of bipolar EEG (F3-C3, C3-P3, P3-01 and F4-C4, C4-P4, P4-02, international 10–20 system) were obtained using an electrode cap (impedance < 5K ohms), as described. Bipolar recordings were obtained for comparison to previous studies in a wide range of ethnic groups (see Ehlers and Schuckit, 1990, 1991; Ehlers et al., 1999; Wall et al., 1993). A forehead ground electrode was used. An electrode placed left lateral infra-orbitally and referenced to the left earlobe was used to monitor both horizontal and vertical eye movement. Resting EEG was recorded in a temperature and noise controlled room while a participant was comfortably sitting on a chair. Participants were instructed to relax and keep their eyes closed, but to remain awake throughout the EEG recording. Ten to 15 minutes of EEG was collected on paper (Nihon Kohden, high-low pass filters 1–70 Hz) and also digitized for subsequent analyses. EEG records were monitored during all recordings for signs of drowsiness or artifact. Ten minutes of artifact-free, drowsiness-free EEG, as defined by Daly and Pedley (1990), was computer analyzed for each channel. Muscle and movement artifact are identified by a computer driven algorithm and verified by the user and epochs containing artifact are removed prior to processing. Time of recording with respect to the menstrual cycle was not controlled, as previous studies have demonstrated that the EEG variables under study are not sensitive to time during the cycle (see Ehlers et al., 1996).
Records were digitized at 128 Hz. The Fourier transform of consecutive four-second epochs was calculated and the power spectrum produced using an IBM compatible PC with software developed by Ehlers and Havstad (1982). Power density is calculated in microvolts squared per octave, a transformation that expands amplitudes at high frequencies and reduces them at low frequencies, producing a spectrum with less 1/f characteristics (Ehlers et al., 1989). A rectangular window is used. The transformed data were compressed into five frequency bands. Mean spectral power density (microvolts squared /octave) in the: delta 1.0–4.0 Hz, theta 4.0–7.5 Hz, alpha 7.5–12.0 Hz, low beta 12.0–20.0, and high beta/gamma 20–50 Hz frequency bands were calculated by summing the raw power spectral values within the band, multiplying by a scale factor derived from the calibration signal to produce the total power in the band in microvolts squared, and dividing by the width of the band in octaves. This width is the logarithm of the ratio of the maximum and minimum frequencies in the band, divided by the log of two. The details of the spectral analysis procedures have been previously described (Ehlers and Havstad, 1982; Ehlers et al., 1989).
2.4 Data Analyses
The data analyses were based on the overall aim that was to determine whether associations existed between heritable spectral characteristics of the EEG and MJ and ALC dependence. The focus was on five frequency bands (delta, theta, alpha, low beta, high beta/gamma). To reduce the number of dependent variables in our statistical model, a principal component analysis (PCA) was performed over the six bipolar electrode locations for each of the five frequency bands. For the delta and high beta/gamma bands, varimax rotation yielded three components (eigenvalues > 1, range 1.5–2.1). The electrode sites loading on the first factor were the two more posterior leads (P3-O1, P4-O2). The electrode sites loading on the second factor were the two left fronto-central-parietal leads (F3-C3, C3-P3) and the third factor was the two right fronto-central-parietal leads (F4-C4, C4-P4) (loadings ranged from 0.67 to 0.91). The three orthogonal factors explained 87% of the variance for each band. For the theta and low beta bands, varimax rotation yielded two components (eigenvalues > 1, range 2.4–2.6). The electrode sites loading on the first factor were the four fronto-central-parietal leads (F3-C3, F4-C4, C3-P3, C4-P4) and the electrode sites loading on the second factor were the two more posterior leads (P3-O1, P4-O2) (loadings ranged from 0.65 to 0.90). The two orthogonal factors explained between 85 to 91% of the variance. For the alpha band, varimax rotation also yielded two components (eigenvalues > 1, range 2.64–2.67). The electrode sites loading on the first factor were the two fronto-central leads (F3-C3, F4-C4) and the electrode sites loading on the second factor were the four more posterior leads (C3-P3, C4-P4, P3-O1, P4-O2) (loadings ranged from 0.64 to 0.93). The two orthogonal factors explained 87% of the variance. Mean power in each band was averaged across the electrode sites within each of the identified components generating a value for mean power (microvolts squared/octave) for each of the five bands (delta, theta, alpha, low beta, high beta/gamma) for each of the regions identified by the PCA for each participant. These values were the dependent variables in the statistical analyses.
The first aim of the study was to determine whether the EEG measures of interest, as described above, were heritable. To accomplish this aim the total additive genetic variance (heritability, h2) and its standard error were estimated for the EEG phenotypes using SOLAR http://solar.sfbrgenetics.org/ (Almasy and Blangero, 1998). SOLAR estimates heritability by parsing the observed variation in a trait into additive genetic and non-shared environmental influences. The total additive genetic heritability (h2) and its standard error were estimated, and the probability that h2 was greater than zero was determined using a Student's t-test. This was accomplished for each frequency band for each electrode location identified in the PCA and this information is provided in table 2. Participants’ age and sex were included as potential covariates and retained if they accounted for a significant proportion of the total variance.
Table 2.
Heritability of EEG principle components
| Frequency range | Electrode locations | h2 ± SE | p-value |
|---|---|---|---|
| Delta (1–4 Hz) | left fronto-central-parietal | 0.22 ± .12 | <.24 |
| bilateral parieto-occipital | 0.28 ± .19 | <.036 | |
| right fronto-central-parietal | 0.50 ± .13 | <.0001 | |
| Theta (4–7.5 Hz) | bilateral fronto-central-parietal | 0.31 ± .10 | <.0003 |
| bilateral parieto-occipital | 0.23 ± .09 | <.001 | |
| Alpha (7.5–12 Hz) | bilateral fronto-central | 0.62 ± .10 | <.000001 |
| bilateral centro-parieto-occipital | 0.67 ± .10 | <.000001 | |
| Low Beta (12–20 Hz) | bilateral fronto-central parietal | 0.51 ± .10 | <.000001 |
| bilateral parieto-occipital | 0.24 ± .10 | <.003 | |
| High Beta/Gamma (20–50 Hz) | left fronto-central-parietal | 0.16 ± .10 | <.03 |
| right fronto-central-parietal | 0.24 ± .10 | <.0008 | |
| bilateral parieto-occipital | 0.49 ± .13 | <.00001 |
One hundred and eighty-one pedigrees containing 1600 individuals were used in the heritability analyses. Of these, 416 individuals have both genotype and phenotype data and 236 additional individuals have only phenotypic data. Sixty-six families have only a single individual with phenotype data. These individuals were included within some analyses to the extent that they contribute information about trait means and variance and the impact of covariates. The family sizes for the remaining families ranged between 4 and 41 subjects (average 12.19±8.19). Eighty-one families were genetically informative. The data includes 142 parent-child, 260 sibling, 53 half sibling, 11 grandparent-grandchild, 235 avuncular, and 240 cousin relative pairs. Only sibling, half-sibling, avuncular and cousin pairs were included as being potentially genetically informative. Many individuals can be linked to a few large extended pedigrees with many founders and complex “loop” structures, which were “broken” to simplify the analysis.
The second aim of the study was to test for associations between marijuana and alcohol dependence and the identified EEG variables taking into account: age, gender, alcohol dependence, Native American Heritage (NAH) and a diagnosis of antisocial personality disorder and/or conduct (ASPD/CD) disorder in the analyses. This was accomplished by entering all the independent variables: Marijuana dependence, age, gender, alcohol dependence, NAH, ASPD/CD into a stepwise regression against the EEG dependent variables. In these analyses, the computer selected the order in which the independent variables were entered into the model.
Potential significant differences in demographic and length of abstinence variables between the MJ dependent and no MJ dependent groups and the ALC dependent and no ALC dependent groups were tested using Fisher's exact test for the dichotomous variables and analysis of variance (ANOVA) or analysis of co-variance (ANCOVA) for the continuous variables. Statistical significance was set at the 0.01 probability level. Statistical analyses were computed using SPSS version 11 (SPSS, Inc., Chicago, IL).
3. Results
Six hundred and twenty-six participants' records were available for these analyses. Two hundred four (204) (33%) of the participants met criteria for MJ dependence, 369 (60%) met criteria for Alcohol dependence, 224 (36%) had neither diagnosis, and 174 (28%) had dual diagnoses. An overall significant difference between participants with marijuana dependence and those without a marijuana dependence diagnosis was found for age, gender, education as seen in table 1. Those participants with a lifetime history of marijuana dependence were significantly (p<0.01) more likely to be younger, male, have one-third of one year less education, have a greater than 50% Native American Heritage, and to have a diagnosis of ASPD/CD. The participants with marijuana dependence did not differ from those without the diagnosis on employment, economic status, and marriage (see table 1). Those participants with a lifetime history of alcohol dependence were significantly (p<0.01) more likely to be male, have one-half of one year less education, have a greater than 50% Native American Heritage, and to have a diagnosis of ASPD/CD. The participants with alcohol dependence did not differ from those without the diagnosis on employment, economic status or marriage (see table 1).
Table 1.
Demographics
| MJ Dep | No MJ Dep | Alc Dep | No Alc Dep | |
|---|---|---|---|---|
| (n = 207) | (n = 419) | (n = 369) | (n = 257) | |
| Gender (n) | M = 113*, F = 94 | M = 146, F = 273 | M = 169+, F = 200 | M = 90, F = 167 |
| Married (n) | 32 | 76 | 63 | 45 |
| Employed (n) | 83 | 176 | 141 | 118 |
| Income ≥ $20,000 yr. | 108 | 214 | 178 | 144 |
| NAH ≥ 50% (n) | 99* | 155 | 171+ | 83 |
| Age (yrs) | 28 ± 0.6* | 31 ± 0.6 | 30.96 ± 0.573 | 29.98 ± 0.874 |
| Education (yrs) | 11.3 ± 0.1* | 11.6 ± 0.07 | 11.38 ± 0.081+ | 11.81 ± 0.092 |
| CD/ASPD (n) | 66* | 53 | 100+ | 19 |
p < 0.01 Marijuana Dependence (MJ Dep) vs. no Marijuana Dependence (no MJ Dep)
p < 0.01 Alcohol Dependence (Alc Dep) vs. no Alcohol Dependence (no Alc Dep)
Table 2 presents the heritability data for the EEG phenotypes used in the present study. All EEG phenotypes were significantly heritable (p<0.01) except delta in the left fronto-central-parietal and bilateral parieto-occipital regions and high beta/gamma in the left fronto-central-parietal lead. Heritability estimates ranged from 0.16– 0.67. The highest heritability was found for alpha activity in posterior regions.
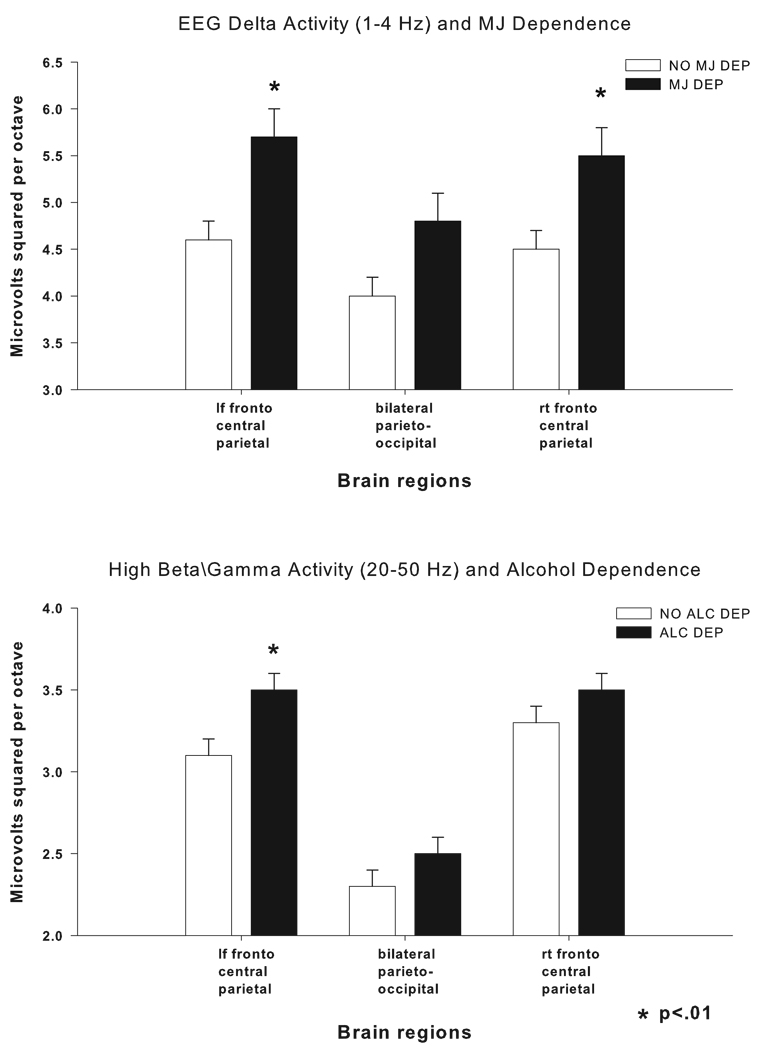
Stepwise linear regression revealed several significant correlations between the identified EEG variables and MJ dependence and ALC dependence as outlined in table 3. Marijuana dependence was found to be positively (p<0.01) correlated with power the delta frequency range (1–4 Hz) at the right and left fronto-central-parietal leads. In order to see whether length of abstinence was responsible for this EEG finding, participants with MJ dependence who reported not smoking during the month before the EEG evaluation were compared to those participants with MJ dependence who had smoked. Analysis of covariance (co-varying for age and gender) was used to compare the two groups on EEG delta activity and no significant differences were found between the groups.
Table 3.
Regression analyses of marijuana dependence, alcohol dependence, age, gender, Native American heritage and antisocial personality disorder/conduct disorder for EEG principal components.
| Frequency range | Electrode locations | Variable | Coefficient | p-value |
|---|---|---|---|---|
| Delta (1–4 Hz) | left fronto-central-parietal | Gender | 1.40 | 0.001 |
| MJ Dep | 1.28 | 0.003 | ||
| NAH | −0.81 | 0.044 | ||
| bilateral parieto-occipital | Gender | 1.29 | <0.001 | |
| NAH | −0.71 | 0.046 | ||
| MJ Dep | 0.90 | 0.018 | ||
| right fronto-central-parietal | NAH | −1.07 | 0.007 | |
| MJ Dep | 1.14 | 0.007 | ||
| Theta (4–7.5 Hz) | bilateral fronto-central-parietal | Gender | 2.38 | <0.001 |
| Age | −0.07 | <0.001 | ||
| NAH | −1.61 | <0.001 | ||
| CD/ASPD | 1.60 | 0.001 | ||
| bilateral parieto-occipital | Gender | 3.16 | <0.001 | |
| Age | −0.06 | 0.001 | ||
| NAH | −1.57 | <0.001 | ||
| CD/ASPD | 1.86 | 0.001 | ||
| Alpha (7.5–12 Hz) | bilateral fronto-central | Gender | 3.72 | 0.010 |
| Age | −0.09 | 0.028 | ||
| NAH | −4.49 | <0.001 | ||
| CD/ASPD | 5.08 | <0.001 | ||
| bilateral centro-parieto-occipital | Gender | 11.44 | <0.001 | |
| Age | −0.40 | <0.001 | ||
| NAH | −8.01 | 0.001 | ||
| CD/ASPD | 7.80 | 0.015 | ||
| Low Beta (12–20 Hz) | bilateral fronto-central parietal | Gender | 1.90 | <0.001 |
| NAH | −1.11 | 0.001 | ||
| CD/ASPD | 1.54 | <0.001 | ||
| bilateral parieto-occipital | Gender | 3.18 | <0.001 | |
| Age | −0.06 | 0.004 | ||
| NAH | −1.12 | 0.019 | ||
| CD/ASPD | 1.55 | 0.011 | ||
| High Beta/Gamma (20–50 Hz) | left fronto-central-parietal | Gender | 0.71 | <0.001 |
| Age | 0.02 | 0.020 | ||
| NAH | −0.46 | 0.019 | ||
| ALC Dep | 0.52 | 0.010 | ||
| right fronto-central-parietal | Gender | 0.63 | 0.004 | |
| CD/ASPD | 0.70 | 0.010 | ||
| bilateral parieto-occipital | Gender | 0.97 | <0.001 | |
| Age | −0.02 | 0.002 | ||
| CD/ASPD | 0.54 | 0.001 |
Alcohol dependence was found to have a different spectral profile than MJ dependence. ALC dependence was found to be positively (p<0.01) correlated with power in high beta/gamma (20–40 Hz) activity in the left fronto-central-parietal lead as seen in figure 1. The other variables that accounted for a significant part of the variance in our model were: age, gender, Native American Heritage (NAH) and a diagnosis of antisocial personality disorder and/or conduct (ASPD/CD) disorder. Being female was positively correlated with (p<0.01) spectral power in all frequency bands except delta in the right fronto-central-parietal leads. Age was negatively correlated with power in: theta, alpha, low beta, and high beta/gamma bands in all leads except bilateral fronto-central (low beta) and right fronto-central- parietal (high beta/gamma) regions. Having greater than 50% NAH was negatively correlated with (p<.01) spectral power in the delta, theta, alpha, low beta, and high beta/gamma frequency ranges except in the right fronto-central-parietal and bilateral parieto-occipital areas for high beta/gamma activity. ASPD/CD was positively correlated with (p<0.01) spectral power in theta, alpha, low beta, and high beta/gamma frequency bands except high beta/gamma activity in the right fronto-central-parietal lead (see table 3).
Figure 1.
In the upper graph mean (± S.E.) EEG Delta (1–4 Hz) power in three brain regions is displayed in the open bars for those participants with no marijuana dependence (no MJ Dep) and in the closed bars for participants with marijuana dependence (MJ Dep). In the lower graph EEG high beta/gamma (20–50 Hz) power in three brain regions is displayed in the open bars for those participants with no alcohol dependence (no Alc Dep) and in the closed bars for participants with alcohol dependence (Alc Dep). In both graphs EEG power is displayed in microvolts squared per octave. * indicates a significant positive correlation between the diagnosis and EEG power.
4. Discussion
Identifying biomarkers of marijuana and alcohol dependence in Native American populations is important because of the high burden of morbidity and mortality that substance use disorders pose to some Native American groups. As reported previously, Indians in this sample have high rates of marijuana dependence (44% in men and 25% in women) and alcohol dependence (65% in men 54% in women) (Ehlers et al., 2004b, 2007a, 2007b; Gilder et al., 2006, 2007). Our finding of high rates of DSM-III-R cannabis dependence is consistent with the findings of Mitchell et al., (2003) and Hesselbrock and colleagues (Hesselbrock et al., 2003, Malcolm et al., 2006) from two American Indian samples and one Alaska Native sample, respectively, as well as high rates of alcohol dependence noted in several other native communities (Kinzie et al., 1992; Kunitz et al., 1999; Mitchell et al., 2003; Robin et al., 1998). The identification of biomarkers that may represent endophenotypes in Indian populations may aid in genetic studies seeking to identify inherited factors that may contribute to the high rates of substance dependence in these populations.
One set of endophenotypes that may be particularly informative for the genetic analysis of substance dependence and other psychiatric disorders are human electrophysiological measures [e.g. EEG, event-related potentials (ERPs), event-related oscillations (EROs)] (see Begleiter and Porjesz, 2006). The EEG is a highly heritable, quantitative, biological measure that is less complex and presumably closer to gene function than diagnostic and psychological measures of substance dependence. Twin studies estimating the heritability of the spectral characteristics of the EEG have reported the average heritability for delta, theta, alpha and beta frequencies at 76%, 89%, 89% and 86% respectively (van Beijsterveldt et al., 1996). There have been several studies including a meta-analysis suggesting that heritability for the alpha band may be the highest (Smit et al 2005, 2006; van Beijsterveldt and van Baal, 2002; Zietsch et al., 2007).
In order for a phenotype to be useful in genetic analysis within a specific population it must be demonstrated to be heritable in the population of study. In a recent study, it has been demonstrated using a large data set of 1598 participants from 442 sibships from the Collaborative Study on the Genetics of Alcoholism (COGA), consisting of primarily Caucasian participants, that bipolar EEG power measures are highly heritable and may be particularly good endophenotypes to evaluate in relation to substance dependence (see Tang et al., 2007). In the study of Tang et al. (2007), a similar methodology for determining heritability to the present study of bipolar EEG power measures was employed (an additive genetic model using SOLAR) and very comparable results were reported. For example, Tang et al. (2007) reported heritability estimates from 0.22 to 0.64 and our range was from 0.16 to 0.67. In both studies, the highest heritabilities were observed in the alpha and beta frequencies, particularly in posterior areas. However, one difference between the two studies was that our study evaluated much fewer electrode locations and also estimated the heritability of EEG delta and high beta/gamma band activity and the Tang et al. (2007) study only reported data on theta, alpha and beta frequency bands. Taken together our study suggests that the EEG is significantly heritable in this Indian population and that heritability estimates for this population appear to be very similar to what has been found in the COGA population.
While it is important to demonstrate heritability of EEG phenotypes in the Indian population of study, it is also important to know if those phenotypes are actually significantly correlated or associated with substance dependence. In the present study, MJ dependence was found to be positively correlated with power in the delta frequency band, particularly in the frontal areas. A number of previous studies have linked changes in EEG spectral characteristics to marijuana dependence, but not all findings have been consistent. Changes in power in the frontal cortex was found in one set of studies that was initially conducted in psychiatric patients and subsequently in non-patients by Struve and colleagues (1989, 1994, 1999, 2003). Although both studies found changes in frontal areas, the EEG frequency characteristics of those changes were found to be different. In the studies by Struve and colleagues, they described quantitative EEG findings consisting of significant elevations of absolute and relative alpha power and alpha coherence, over the bilateral frontal cortex, as well as decreases in alpha frequency following “chronic heavy cumulative THC exposure”. This group also found increases in theta activity over the bilateral frontal cortex in a group with “very long duration use of MJ” (use for 15–24 yrs) (see Struve et al., 1998).
However, in another population sample, Herning et al. (2003) found decreases in EEG alpha and beta activity in posterior areas during early abstinence and 28 days following abstinence in marijuana abusers as compared to controls. The question is why did the three studies obtain different results? One difference between studies may be related to participant demographics. We have clearly demonstrated that EEG alpha activity differs substantially based on racial background and gender (see Ehlers and Phillips, 2003, 2007; Ehlers et al., 1999; 2004c, 2004d). In the present study all participants were Native American Indians and over half of the participants were women. Women were found to have higher voltage EEG’s than men did. This finding confirms data from our previous studies in Hispanics (Ehlers and Phillips, 2007; Ehlers et al., 2004c, 2004d) and those of others in mixed ethnic groups who have also noted that women in general have higher amplitudes in the resting EEG when compared to men (Veldhuizen et al., 1993; Wada et al., 1994).
In the present study Native American heritage was also found to be negatively correlated with EEG voltage in all five frequency bands. In our statistical model both gender and Native American heritage were investigated and not found to account for the EEG findings correlated with MJ dependence. However, in the study of Struve et al. (1999) neither the gender nor the racial background of the participants is given. Whereas in the study of Herning and colleagues (2003, 2008) the population was primarily male and African American and although gender was accounted for, African American Heritage was not. Thus, in order to compare the three studies more information about participant demographics and how they may have affected the results is needed.
Additional differences in the present study from previous EEG studies include the use of bipolar electrodes. Bipolar electrodes reduce signals that are common to the two leads that are being recorded from. Thus artifacts that may be common to the two leads (such as eye movements) will be rejected but also true signals in common to the two leads (such as alpha activity) will also be reduced in amplitude. Monopolar electrodes were used in the studies by Struve et al. (1999) and by Herning and colleagues (2003, 2008).
Another important difference between studies was the amount of time that participants were abstinent from MJ usage prior to the recording of the EEG. In the present study participants were recorded at a minimum of 24 hours of abstinence. In the study of Struve et al., (1999) participants were also required to be abstinent for a minimum of 24 hours, however, in the study by Herning et al., (2008) participants were recorded from within 72 hours of admission to a closed clinical research ward and a subset of the users were tested again after 28–30 days of monitored abstinence. Interestingly, Herning et al., (2008) found that especially for the marijuana users that endorsed using eight years or more that the EEG findings they reported were found to persist during the month of abstinence. In the present study we evaluated a subset of individuals who met lifetime diagnosis for MJ Dependence but reported not having used over the last month, and found no significant differences in EEG delta between those participants and those participants with a MJ dependence diagnosis who endorsed having used the drug over the last month.
Another difference between the studies is that the use of marijuana may, or may not, have caused a variety of persistent CNS changes in the study participants that were then reflected in the different EEG findings. Struve et al., (1998) have theorized that “casual” MJ users did not have an EEG profile associated with their use but that chronic users had “alpha hyperfrontality” and that use over 15 years ultimately led to increased EEG frontal theta activity. In our study increases in frontal delta activity were found in participants with MJ dependence. Since delta activity has been associated with mild cognitive impairment and memory decline with aging (Adler et al., 1999; Liddell et al., 2007) it is possible that the MJ dependent participants in our population (who had a mean age of 28) are displaying more signs of “aging” of their EEGs, however, this would have to be confirmed by follow-up studies using neuropsychological and neuroradiological indicators. In the study by Herning et al., (2008) they suggested that the reduced EEG power in the alpha and beta bands observed at posterior sites in the MJ using group could be related to cerebral perfusion deficits and/or alterations in thyroid function. Further studies will be necessary to determine if the EEG changes noted in the three different studies reflect persistent changes in identified brain systems.
Previous EEG studies have also primarily focused on identifying measures of the consequences of marijuana “use” rather than potentially heritable indices of risk for dependence on the drug. In our study, although EEG delta activity was found to be heritable particularly in the right fronto-central-parietal areas (h2 =0.50), 50% of the variance in this lead is “environmental” and therefore differences in EEG power in this lead between participants with and without MJ dependence may partially represent the effects of MJ exposure rather than a genetic factor or may indicate a gene-environment interaction. This may be particularly true in the left fronto-parietal area where heritability estimate were lower (h2 =0.22).
What is the evidence that specific EEG measures are associated or correlated with alcohol dependence? A number of laboratories have investigated this question and the findings between studies appear to be fairly consistent. Low voltage alpha has been associated with a subtype of alcohol dependence with anxiety disorders (Enoch et al., 1995, 1999). In the COGA study, increases in theta power and beta power have been reported to be associated with alcoholism (Rangaswamy et al., 2003, 2004). Beta power increases have also been reported by Coutin-Churchman et al. (2006) in a South American population of alcoholics. Costa and Bauer (1997) have also reported increases in beta power in alcohol-dependent and cocaine-dependent subjects, as well as higher beta power in relapsers compared with controls and abstainers (Bauer, 1994). Therefore, the present finding of a positive correlation of high beta/gamma activity and alcohol dependence, although combining beta and gamma activity into one band, is generally consistent with the findings of other studies that have reported increases in beta activity in alcohol dependent individuals. High beta/gamma activity was found to differ in heritability depending on the location with the more posterior leads having higher heritability (h2 =0.49) than the left fronto-central parietal (h2 =0.16) that was significantly correlated with alcohol dependence. These data suggest that environmental factors may play an important role in the correlation between high beta gamma activity and alcohol dependence.
Another factor that needs to be taken into consideration in evaluating EEG voltage and substance dependence is the presence of externalizing diagnoses (Bauer et al., 2001). A considerably large literature has clearly documented in the general population that substantial co-morbidity exists between antisocial behaviors and substance abuse (Bucholz, 1999; Compton et al., 2000; Dinwiddie and Reich, 1993; Fu et al., 2002; Goldstein et al., 2006; Holdcraft et al., 1998; Kessler et al., 1997; Regier et al., 1990; Simmons and Havens, 2006; Westermeyer and Thuras, 2005; Wong et al., 1999). In fact, it has been suggested that the relationship between antisocial behavior and substance abuse may be one of the best-documented findings in the psychopathology literature (see Waldman and Slutske, 2000). Both substance dependence and behavioral control disorders, such as conduct disorder and adult antisocial personality disorder (CD/ASPD), have been shown to have a significant genetic component to their etiology (Button et al., 2005, 2006; Cloninger et al., 1981; Ehlers et al., 2008b; Grove et al., 1990; Heath et al., 1997; Kendler et al, 1992, 2003; Prescott and Kendler, 1999; Slutske, 2001).
Behavioral genetics studies have the advantage in being one of the strongest methods for determining whether the co-morbidity among psychopathological conditions is due to common etiologies and or pathologies associated with the disorders or not. In a previous report, we demonstrated that ASPD/CD phenotypes are highly heritable and linked to several chromosomal regions in this Indian population (Ehlers et al., 2008b). In the present study, ASPD/CD was found to be positively correlated with EEG power in the theta, alpha, low beta and high beta/gamma frequency bands but not in the delta frequencies that were correlated with MJ dependence. Further studies evaluating whether there is overlap between genetic linkage for EEG phenotypes and ASPD/CD and substance dependence will be needed to determine whether common genetic factors may underlie these phenomena.
There are several aspects of the current study that are unique to the literature on the electrophysiology of marijuana dependence. Most studies have focused on “users” of marijuana as opposed to individuals with a DSM defined history of marijuana dependence. Some studies have not controlled for other psychiatric disorders in the populations they evaluated. Additionally, in most studies, sample sizes were small and had few women participants and as such, there was not enough power to detect differences in EEG as a function of gender. We have previously reported in a study that evaluated event-related potentials in this population of Native Americans; the women were found to have longer latency P450 responses to the stimuli that were used to generate late positive responses (happy and neutral faces) (Ehlers et al., 2008a). Women with MJ dependence were also found having longer P450 latencies than men did with MJ dependence suggesting that women may be more impacted by a marijuana dependence diagnosis.
The results of this study should be interpreted in the context of several limitations. First, the findings may not generalize to other Native Americans or represent all Indians within this population. Second, only retrospective and cross-sectional data on MJ, ALC and other drug use disorders were assessed. EEG high beta and gamma band activity were analyzed together within one frequency band which did not allow for the dissection of whether the gamma or the high beta activity was responsible for significant findings. Despite these limitations, this report represents an important step in an ongoing investigation to determine genetic and environmental factors associated with substance use and use disorders in this high risk and understudied ethnic group.
Acknowledgments
The authors thank Michelle Dixon, Linda Corey, Lilach Harris, Gina Stouffer, Philip Lau, Susan Lopez, and Vincent Wong for assistance in data collection and analyses, and Shirley Sanchez for editing the manuscript.
Role of Funding Source
Funding for this study was provided in part by the National Institute on Drug Abuse (NIDA)grant DA019333, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant AA010201, the National Center on Minority Health and Health Disparities (NCMHD), and the Stein Endowment Fund; the NIDA, NIAAA, NCMHD and Stein Endowment Fund had no further role in the design study, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Cindy Ehlers as the study PI, designed the study and interpreted the data. Evie Phillips is a registered EEG tech, and collected all study data and analyzed all spectral characteristics of the EEG. Ian Gizer did the heritability analyses and their interpretation and wrote the sections of the paper concerned those analyses. David Gilder did all best final diagnoses of all patients and wrote all clinical sections of the paper. Kirk Wilhelmsen did all the analyses of family relationships for the heritability analyses and did all final editing of the paper.
All authors have given intellectual contributions to the study and the paper and have approved the final manuscript.
Conflict of Interest
The authors have no commercial affiliation or consultant role that could be construed as a conflict of interest.
References
- Adler G, Bramesfeld A, Jajcevic A. Mild cognitive impairment in old-age depression is associated with increased EEG slow-wave power. Neuropsychobiology. 1999;40:218–222. doi: 10.1159/000026623. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am.J.Hum.Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnosis and statistical manual of mental disorders (DSM-III-R) Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- Bachman JG, Wallace JM, Jr, O'Malley PM, Johnston LD, Kurth CL, Neighbors HW. Racial/ethnic differences in smoking, drinking, and illicit drug use among American high school seniors, 1976–89. Am.J.Public Health. 1991;81:372–377. doi: 10.2105/ajph.81.3.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SC, Baeuvais F, Trimble J. American Indian adolescent alcohol involvement and ethnic identification. Subst.Use Misuse. 1997;32:2013–2031. doi: 10.3109/10826089709035617. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Electroencephalographic and autonomic predictors of relapse in alcohol-dependent patients. Alcohol Clin.Exp.Res. 1994;18:755–760. doi: 10.1111/j.1530-0277.1994.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Antisocial personality disorder and cocaine dependence: their effects on behavioral and electroencephalographic measures of time estimation. Drug Alcohol Depend. 2001;63:87–95. doi: 10.1016/s0376-8716(00)00195-2. [DOI] [PubMed] [Google Scholar]
- Beauvais F. Trends in Indian adolescent drug and alcohol use. Am.Indian Alsk.Native Ment.Health Res. 1992;5:1–12. doi: 10.5820/aian.0501.1990.1. [DOI] [PubMed] [Google Scholar]
- Beauvais F. Trends in drug use among American Indian students and dropouts, 1975 to 1994. Am.J.Public Health. 1996;86:1594–1598. doi: 10.2105/ajph.86.11.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais F, Oetting ER, Edwards RW. Trends in drug use of Indian adolescents living on reservations: 1975–1983. Am.J.Drug Alcohol Abuse. 1985;11:209–229. doi: 10.3109/00952998509016863. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin.Exp.Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int.J.Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Blum RW, Harmon B, Harris L, Bergeisen L, Resnick MD. American Indian--Alaska Native youth health. JAMA. 1992;267:1637–1644. doi: 10.1001/jama.267.12.1637. [DOI] [PubMed] [Google Scholar]
- Bucholz KK. Nosology and epidemiology of addictive disorders and their comorbidity. Psychiatr.Clin.North Am. 1999;22:221–240. doi: 10.1016/s0193-953x(05)70073-3. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J.Stud.Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Burns TR. How does IHS relate administratively to the high alcoholism mortality rate? Am.Indian Alsk.Native Ment.Health Res. 1995;6:31–45. doi: 10.5820/aian.0603.1995.31. [DOI] [PubMed] [Google Scholar]
- Button TM, Hewitt JK, Rhee SH, Young SE, Corley RP, Stallings MC. Examination of the causes of covariation between conduct disorder symptoms and vulnerability to drug dependence. Twin.Res Hum.Genet. 2006;9:38–45. doi: 10.1375/183242706776402993. [DOI] [PubMed] [Google Scholar]
- Button TM, Scourfield J, Martin N, Purcell S, McGuffin P. Family dysfunction interacts with genes in the causation of antisocial symptoms. Behav.Genet. 2005;35:115–120. doi: 10.1007/s10519-004-0826-y. [DOI] [PubMed] [Google Scholar]
- Christian JC, Morzorati S, Norton JA, Jr, Williams CJ, O'Connor S, Li TK. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33:584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch.Gen.Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Compton WM, III, Cottler LB, Ben Abdallah A, Phelps DL, Spitznagel EL, Horton JC. Substance dependence and other psychiatric disorders among drug dependent subjects: race and gender correlates. Am.J.Addict. 2000;9:113–125. doi: 10.1080/10550490050173181. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L. Quantitative electroencephalographic differences associated with alcohol, cocaine, heroin and dual-substance dependence. Drug Alcohol Depend. 1997;46:87–93. doi: 10.1016/s0376-8716(97)00058-6. [DOI] [PubMed] [Google Scholar]
- Coutin-Churchman P, Moreno R, Anez Y, Vergara F. Clinical correlates of quantitative EEG alterations in alcoholic patients. Clin.Neurophysiol. 2006;117:740–751. doi: 10.1016/j.clinph.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Criado J, Ehlers CL. Electrophysiological responses to affective stimuli in Southwest California Indians: relationship to alcohol dependence. J.Stud.Alcohol. 2007;68:813–823. doi: 10.15288/jsad.2007.68.813. [DOI] [PubMed] [Google Scholar]
- Daly DD, Pedley TA. Current practice of clinical electroencephalography. 2nd ed. New York, NY: Raven Press; 1990. [Google Scholar]
- Dinwiddie SH, Reich T. Attribution of antisocial symptoms in coexistent antisocial personality disorder and substance abuse. Compr.Psychiatry. 1993;34:235–242. doi: 10.1016/0010-440x(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am.J.Hum.Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Garcia-Andrade C, Wall TL, Cloutier D, Phillips E. Electroencephalographic responses to alcohol challenge in Native American Mission Indians. Biol.Psychiatry. 1999;45:776–787. doi: 10.1016/s0006-3223(98)00113-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Phillips E. P3 components of the event-related potential and marijuana dependence in Southwest California Indians. Addict.Biol. 2008a;13:130–142. doi: 10.1111/j.1369-1600.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Slutske WS, Lind PA, Wilhelmsen KC. Externalizing disorders in American Indians: comorbidity and a genome wide linkage analysis. Am.J Med.Genet.B Neuropsychiatr.Genet. 2008b;147B:690–698. doi: 10.1002/ajmg.b.30666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gilder DA, Wall TL, Phillips E, Feiler H, Wilhelmsen KC. Genomic screen for loci associated with alcohol dependence in Mission Indians. Am.J.Hum.Genet. 2004a;129B:110–115. doi: 10.1002/ajmg.b.30057. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral time series analysis. Psychopharmacology Bull. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Phillips E. EEG low voltage variants and alpha power in African American young adults: relation to family history of alcoholism. Alcohol Clin.Exp.Res. 2003;27:765–772. doi: 10.1097/01.ALC.0000065439.09492.67. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. Association of EEG alpha variants and alpha power with alcohol dependence in Mexican American young adults. Alcohol. 2007;41:13–20. doi: 10.1016/j.alcohol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol.Teratol. 2007c;29:153–163. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Parry BL. Electrophysiological findings during the menstrual cycle in women with and without late luteal phase dysphoric disorder: relationship to risk for alcoholism? Biol.Psychiatry. 1996;39:720–732. doi: 10.1016/0006-3223(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Schuckit MA. EEG alpha variants and alpha power in Hispanic American and white non-Hispanic American young adults with a family history of alcohol dependence. Alcohol. 2004c;33:99–106. doi: 10.1016/j.alcohol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Wall TL, Wilhelmsen K, Schuckit MA. EEG alpha and level of response to alcohol in Hispanic- and non-Hispanic-American young adults with a family history of alcoholism. J.Stud.Alcohol. 2004d;65:301–308. doi: 10.15288/jsa.2004.65.301. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. EEG fast frequency activity in the sons of alcoholics. Biol.Psychiatry. 1990;27:631–641. doi: 10.1016/0006-3223(90)90531-6. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. Evaluation of EEG alpha activity in sons of alcoholics. Neuropsychopharmacology. 1991;4:199–205. [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P. Age of first marijuana use and the occurrence of marijuana use disorders in Southwest California Indians. Pharmacol.Biochem.Behav. 2007a;86:290–296. doi: 10.1016/j.pbb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcohol Clin.Exp.Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Betancourt M, Gilder DA. The clinical course of alcoholism in 243 Mission Indians. Am.J.Psychiatry. 2004b;161:1204–1210. doi: 10.1176/appi.ajp.161.7.1204. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Corey L, Lau P, Gilder DA, Wilhelmsen K. Heritability of illicit drug use and transition to dependence in Southwest California Indians. Psych Genet. 2007b;17:171–176. doi: 10.1097/01.ypg.0000242201.56342.1a. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr.Clin.Neurophysiol. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic scan for alcohol craving in Mission Indians. Psychiatr.Genet. 2005;15:71–75. doi: 10.1097/00041444-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for loci associated with tobacco usage in Mission Indians. BMC.Med.Genet. 2006 Feb;10:7–9. doi: 10.1186/1471-2350-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmsen KC. Genomic screen for substance dependence and body mass index in Southwest California Indians. Genes Brain Behav. 2007;6:184–191. doi: 10.1111/j.1601-183X.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Rohrbaugh JW, Davis EZ, Harris CR, Ellingson RJ, Andreason P, Moore V, Varner JL, Brown GL, Eckardt MJ. Relationship of genetically transmitted alpha EEG traits to anxiety disorders and alcoholism. Am.J.Med.Genet. 1995;60:400–408. doi: 10.1002/ajmg.1320600510. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Robin RW, Ross J, Rohrbaugh JW, Goldman D. Association of low-voltage alpha EEG with a subtype of alcohol use disorders. Alcohol Clin.Exp.Res. 1999;23:1312–1319. [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr.Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Federman EB, Costello EJ, Angold A, Farmer EM, Erkanli A. Development of substance use and psychiatric comorbidity in an epidemiologic study of white and American Indian young adolescents the Great Smoky Mountains Study. Drug Alcohol Depend. 1997;44:69–78. doi: 10.1016/s0376-8716(96)01317-8. [DOI] [PubMed] [Google Scholar]
- Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, True WR, Jacob T, Tsuang MT, Eisen SA. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch.Gen.Psychiatry. 2002;59:1125–1132. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- Garcia-Andrade C, Wall TL, Ehlers CL. Alcohol expectancies in a Native American population. Alcohol Clin.Exp.Res. 1996;20:1438–1442. doi: 10.1111/j.1530-0277.1996.tb01146.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Andrade C, Wall TL, Ehlers CL. The firewater myth and response to alcohol in Mission Indians. Am.J.Psychiatry. 1997;154:983–988. doi: 10.1176/ajp.154.7.983. [DOI] [PubMed] [Google Scholar]
- Gfellner BM. A matched-group comparison of drug use and problem behavior among Candian Indian and white adolescents. J.Early Adolesc. 1994;14:24–28. [Google Scholar]
- Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from cannabis dependence in Southwest California Indians. J.Addict.Dis. 2007;26:23–30. doi: 10.1300/J069v26n04_04. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Dixon M, Corey L, Phillips E, Ehlers CL. Co-morbidity of select anxiety, affective, and psychotic disorders with cannabis dependence in Southwest California Indians. J.Addict.Dis. 2006;25:67–79. doi: 10.1300/J069v25n04_07. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Wall TL, Ehlers CL. Comorbidity of select anxiety and affective disorders with alcohol dependence in Southwest California Indians. Alcohol Clin.Exp.Res. 2004;28:1805–1813. doi: 10.1097/01.alc.0000148116.27875.b0. [DOI] [PubMed] [Google Scholar]
- Goldstein RB, Grant BF, Ruan WJ, Smith SM, Saha TD. Antisocial personality disorder with childhood- vs. adolescence-onset conduct disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J.Nerv.Ment.Dis. 2006;194:667–675. doi: 10.1097/01.nmd.0000235762.82264.a1. [DOI] [PubMed] [Google Scholar]
- Grob C, Dobkin de Rios M. Adolescent drug use in cross-cultural perspective. J.Drug Issues. 1992;22:121–138. [Google Scholar]
- Grove WM, Eckert ED, Heston L, Bouchard TJ, Jr, Segal N, Lykken DT. Heritability of substance abuse and antisocial behavior: a study of monozygotic twins reared apart. Biol.Psychiatry. 1990;27:1293–1304. doi: 10.1016/0006-3223(90)90500-2. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol.Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc.Problems. 1997;44:174–199. [Google Scholar]
- Herning RI, Better W, Cadet JL. EEG of chronic marijuana users during abstinence: relationship to years of marijuana use, cerebral blood flow and thyroid function. Clin.Neurophysiol. 2008;119:321–331. doi: 10.1016/j.clinph.2007.09.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning RI, Better W, Tate K, Cadet JL. EEG deficits in chronic marijuana abusers during monitored abstinence: preliminary findings. Ann.N.Y.Acad.Sci. 2003;993:75–78. doi: 10.1111/j.1749-6632.2003.tb07513.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock MN, Hesselbrock VM, Segal B, Schuckit MA, Bucholz K. Ethnicity and psychiatric comorbidity among alcohol-dependent persons who receive inpatient treatment: African Americans, Alaska Natives, Caucasians, and Hispanics. Alcohol Clin.Exp.Res. 2003;27:1368–1373. doi: 10.1097/01.ALC.0000080164.21934.F9. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM, Segal B, Hesselbrock MN. Alcohol dependence among Alaska Natives entering alcoholism treatment: a gender comparison. J.Stud.Alcohol. 2000;61:150–156. doi: 10.15288/jsa.2000.61.150. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG, McGue MK. Antisocial personality disorder and depression in relation to alcoholism: a community-based sample. J.Stud.Alcohol. 1998;59:222–226. doi: 10.15288/jsa.1998.59.222. [DOI] [PubMed] [Google Scholar]
- Juel-Nielsen N, Harvald B. The encephalogram in uniovular twins brought up apart. Acta Genet Statistica Medica (Basel) 1958;8:57–64. doi: 10.1159/000151054. [DOI] [PubMed] [Google Scholar]
- Kalton G, Anderson DW. Sampling rare populations. J. Royal Stat. Society. 1986;149:65–82. [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. JAMA. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a U.S. population-based sample of male twins. Arch.Gen.Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am.J.Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch.Gen.Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch.Gen.Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- King J, Thayer JF. Examining conceptual models for understanding drug use behavior among American Indian youth. In: De La Rosa M, editor. Drug abuse among minority youth: advances in research and methodology. Bethesda, MD: U. S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1993. pp. 129–143. [PubMed] [Google Scholar]
- Kinzie JD, Leung PK, Boehnlein J, Matsunaga D, Johnson R, Manson S, Shore JH, Heinz J, Williams M. Psychiatric epidemiology of an Indian village: A 19-year replication study. J Nerv.Ment.Dis. 1992;180:33–39. doi: 10.1097/00005053-199201000-00008. [DOI] [PubMed] [Google Scholar]
- Kunitz SJ, Gabriel KR, Levy JE, Henderson E, Lampert K, McCloskey J, Quintero G, Russell S, Vince A. Alcohol dependence and conduct disorder among Navajo Indians. J.Stud.Alcohol. 1999;60:159–167. doi: 10.15288/jsa.1999.60.159. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Paul RH, Arns M, Gordon N, Kukla M, Rowe D, Cooper N, Moyle J, Williams LM. Rates of decline distinguish Alzheimer's disease and mild cognitive impairment relative to normal aging: integrating cognition and brain function. J Integr.Neurosci. 2007;6:141–174. doi: 10.1142/s0219635207001374. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Tellegen A, Iacono WG. EEG spectra in twins: Evidence for a neglected mechanism of genetic determination. Physiol.Psychol. 1982;10:60–65. [Google Scholar]
- Lynskey MT, Heath AC, Nelson EC, Bucholz KK, Madden PA, Slutske WS, Statham DJ, Martin NG. Genetic and environmental contributions to cannabis dependence in a national young adult twin sample. Psychol.Med. 2002;32:195–207. doi: 10.1017/s0033291701005062. [DOI] [PubMed] [Google Scholar]
- Malcolm BP, Hesselbrock MN, Segal B. Multiple substance dependence and course of alcoholism among Alaska native men and women. Subst.Use Misuse. 2006;41:729–741. doi: 10.1080/10826080500391803. [DOI] [PubMed] [Google Scholar]
- Mitchell CM, Beals J, Novins DK, Spicer P. Drug use among two American Indian populations: prevalence of lifetime use and DSM-IV substance use disorders. Drug Alcohol Depend. 2003;69:29–41. doi: 10.1016/s0376-8716(02)00253-3. [DOI] [PubMed] [Google Scholar]
- Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, Smith PJ. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116 Suppl. 1:216–222. doi: 10.1093/phr/116.S1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novins DK, Mitchell CM. Factors associated with marijuana use among American Indian adolescents. Addiction. 1998;93:1693–1702. doi: 10.1046/j.1360-0443.1998.931116937.x. [DOI] [PubMed] [Google Scholar]
- Oetting ER, Swaim RC, Edwards RW, Beauvais F. Indian and Anglo adolescent alcohol use and emotional distress: path models. Am.J.Drug Alcohol Abuse. 1989;15:153–172. doi: 10.3109/00952998909092718. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van EP, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O'Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin.Exp.Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O'Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol.Psychol. 2002;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin.Neuropathol. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am.J.Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Rohrbaugh J, O'Connor SJ, Kuperman S, Reich T, Begleiter H. Beta power in the EEG of alcoholics. Biol.Psychiatry. 2002;52:831–842. doi: 10.1016/s0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Rohrbaugh J, O'Connor S, Kuperman S, Reich T, Begleiter H. Theta power in the EEG of alcoholics. Alcohol Clin.Exp.Res. 2003;27:607–615. doi: 10.1097/01.ALC.0000060523.95470.8F. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Reich T, Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. Int.J Psychophysiol. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Robin RW, Long JC, Rasmussen JK, Albaugh B, Goldman D. Relationship of binge drinking to alcohol dependence, other psychiatric disorders, and behavioral problems in an American Indian tribe. Alcohol Clin.Exp.Res. 1998;22:518–523. [PubMed] [Google Scholar]
- S.F.B.R. Sequential Oligogenic Linkage Analysis Routines. 2008 [On-line]. Available: http://solar.sfbrgenetics.org/
- Schuckit MA. Genetics and the risk for alcoholism. JAMA. 1985;254:2614–2617. [PubMed] [Google Scholar]
- Simmons LA, Havens JR. Comorbid substance and mental disorders among rural Americans: results from the national comorbidity survey. J Affect.Disord. 2006;99:265–271. doi: 10.1016/j.jad.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Slutske WS. The genetics of antisocial behavior. Curr.Psychiatry Rep. 2001;3:158–162. doi: 10.1007/s11920-001-0014-1. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Smit CM, Wright MJ, Hansell NK, Geffen GM, Martin NG. Genetic variation of individual alpha frequency (IAF) and alpha power in a large adolescent twin sample. Int.J Psychophysiol. 2006;61:235–243. doi: 10.1016/j.ijpsycho.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Bomben G, Propping P. Genetic aspects of the EEG: an investigation into the within-pair similarity of monozygotic and dizygotic twins with a new method of analysis. Electroencephalogr.Clin.Neurophysiol. 1987;66:489–501. doi: 10.1016/0013-4694(87)90095-2. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol.Med. 2006;36:1447–1460. doi: 10.1017/S0033291706008361. [DOI] [PubMed] [Google Scholar]
- Struve FA, Manno BR, Kemp P, Patrick G, Manno JE. Acute marihuana (THC) exposure produces a "transient" topographic quantitative EEG profile identical to the "persistent" profile seen in chronic heavy users. Clin.Electroencephalogr. 2003;34:75–83. doi: 10.1177/155005940303400206. [DOI] [PubMed] [Google Scholar]
- Struve FA, Patrick G, Straumanis JJ, Fitz-Gerald MJ, Manno J. Possible EEG sequelae of very long duration marihuana use: pilot findings from topographic quantitative EEG analyses of subjects with 15 to 24 years of cumulative daily exposure to THC. Clin.Electroencephalogr. 1998;29:31–36. doi: 10.1177/155005949802900110. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G. Persistent topographic quantitative EEG sequelae of chronic marihuana use: a replication study and initial discriminant function analysis. Clin.Electroencephalogr. 1994;25:63–75. doi: 10.1177/155005949402500207. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Leavitt J, Manno JE, Manno BR. Topographic quantitative EEG sequelae of chronic marihuana use: a replication using medically and psychiatrically screened normal subjects. Drug Alcohol Depend. 1999;56:167–179. doi: 10.1016/s0376-8716(99)00029-0. [DOI] [PubMed] [Google Scholar]
- Struve FA, Straumanis JJ, Patrick G, Price L. Topographic mapping of quantitative EEG variables in chronic heavy marihuana users: empirical findings with psychiatric patients. Clin.Electroencephalogr. 1989;20:6–23. doi: 10.1177/155005948902000106. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chorlian DB, Rangaswamy M, Porjesz B, Bauer L, Kuperman S, O'Connor S, Rohrbaugh J, Schuckit M, Stimus A, Begleiter H. Genetic influences on bipolar EEG power spectra. Int.J Psychophysiol. 2007;65:2–9. doi: 10.1016/j.ijpsycho.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am.J.Med.Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- United States Congress. Indian adolescent mental health; Congress of the United States, Office of Technology; Washington, D.C.. 1990. [Google Scholar]
- United States Indian Health Service. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Indian Health Service Division of Program Statistics; Alcoholism: A High Priority Health Problem. 1977
- United States Indian Health Service. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Indian Health Service, Division of Program Statistics; Analysis of Fiscal Year 1981. 1982
- United States Indian Health Service. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service, Indian Health Service, Division of Program Statistics; Trends in Indian health. 1997
- van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum.Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, De Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am.J Hum.Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol.Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Svikis DS, McGue M, Pickens RW. Genetic analysis of diagnostic systems of alcoholism in males. Biol.Psychiatry. 1998;43:139–145. doi: 10.1016/S0006-3223(97)00225-4. [DOI] [PubMed] [Google Scholar]
- Veldhuizen RJ, Jonkman EJ, Poortvliet DC. Sex differences in age regression parameters of healthy adults--normative data and practical implications. Electroencephalogr.Clin.Neurophysiol. 1993;86:377–384. doi: 10.1016/0013-4694(93)90133-g. [DOI] [PubMed] [Google Scholar]
- Vogel F. Erganzende Untersuchungen zur Genetik des menschlichen Niederspannungs-EEG. Deutsch Z Nervenheilk. 1962;185:105–111. [Google Scholar]
- Vogel F. The genetic basis of the normal human electroencephalogram (EEG) Humangenetik. 1970;10:91–114. doi: 10.1007/BF00295509. [DOI] [PubMed] [Google Scholar]
- Wada Y, Takizawa Y, Jiang ZY, Yamaguchi N. Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin.Electroencephalogr. 1994;25:81–85. doi: 10.1177/155005949402500209. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Slutske WS. Antisocial behavior and alcoholism: a behavioral genetic perspective on comorbidity. Clin.Psychol.Rev. 2000;20:255–287. doi: 10.1016/s0272-7358(99)00029-x. [DOI] [PubMed] [Google Scholar]
- Wall TL, Carr LG, Ehlers CL. Protective association of genetic variation in alcohol dehydrogenase with alcohol dependence in Native American Mission Indians. Am.J.Psychiatry. 2003;160:41–46. doi: 10.1176/appi.ajp.160.1.41. [DOI] [PubMed] [Google Scholar]
- Wall TL, Gallen CC, Ehlers CL. Effects of alcohol on the EEG in Asian men with genetic variations of ALDH2. Biol.Psychiatry. 1993;34:91–99. doi: 10.1016/0006-3223(93)90261-b. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Thuras P. Association of antisocial personality disorder and substance disorder morbidity in a clinical sample. Am.J Drug Alcohol Abuse. 2005;31:93–110. [PubMed] [Google Scholar]
- Wilhelmsen KC, Ehlers C. Heritability of substance dependence in a Native American population. Psychiatr.Genet. 2005;15:101–107. doi: 10.1097/00041444-200506000-00006. [DOI] [PubMed] [Google Scholar]
- Winterer G, Mahlberg R, Smolka MN, Samochowiec J, Ziller M, Rommelspacher HP, Herrmann WM, Schmidt LG, Sander T. Association analysis of exonic variants of the GABA(B)-receptor gene and alpha electroencephalogram voltage in normal subjects and alcohol-dependent patients. Behav.Genet. 2003a;33:7–15. doi: 10.1023/a:1021043315012. [DOI] [PubMed] [Google Scholar]
- Winterer G, Smolka M, Samochowiec J, Ziller M, Mahlberg R, Gallinat J, Rommelspacher HP, Herrmann WM, Sander T. Association of EEG coherence and an exonic GABA(B)R1 gene polymorphism. Am.J Med.Genet B Neuropsychiatr.Genet. 2003b;117B:51–56. doi: 10.1002/ajmg.b.10031. [DOI] [PubMed] [Google Scholar]
- Wong MM, Zucker RA, Puttler LI, Fitzgerald HE. Heterogeneity of risk aggregation for alcohol problems between early and middle childhood: nesting structure variations. Dev.Psychopathol. 1999;11:727–744. doi: 10.1017/s0954579499002291. [DOI] [PubMed] [Google Scholar]
- Zietsch BP, Hansen JL, Hansell NK, Geffen GM, Martin NG, Wright MJ. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol.Psychol. 2007;75:154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]