Abstract
Exacerbated inflammatory responses have been reported following traumatic injury to the aged brain. The present study was designed to investigate the involvement of the transcription factors belonging to the CCAAT/enhancer binding protein (C/EBP) family that regulate expression of many of the pro-inflammatory genes which show increased expression following injury to the aged brain. Controlled cortical impact injury was induced in adult (5–6 mo) and aged (22–24 mo) C57/BL6 mice. C/EBP mRNA and protein expression were analyzed in injured cortex at 1, 3, 7 days post injury. Expression of C/EBPα was reduced relative to baseline at day 1 in both adult and aged mice, whereas, it increased at day 3 and 7 days post-injury. No significant differences were observed between adult and aged brain. Up-regulation of C/EBPβ was observed 1 day following injury in both the adult and aged brain, but there were no major age-related differences in mRNA levels. However, there was higher C/EBPβ protein in the aged brain. C/EBPδ expression increased beginning 1 day post injury in both adult and aged brain. In this case, the increase in C/EBP δ expression was higher in the aged brain than in the adult at all time points studied. Expression of CCAAT/enhancer binding protein homologous protein (CHOP), a transcription factor involved in ER stress and protein unfolding responses, was also up-regulated in response to injury, but CHOP levels were significantly lower in the aged than the adult brain. Based on these results, we conclude that differential expression of C/EBP β, δ and CHOP might contribute to the hyper-inflammatory response and poor prognosis following traumatic brain injury in the elderly. In addition elevated C/EBPδ levels following TBI in the aged brain may play a role in the link between TBI and Alzheimer’s disease.
Keywords: Traumatic brain injury, inflammation, CCAAT enhancer binding proteins, transcription factors, C/EBPα, C/EBPβ, C/EBPδ, CHOP, aging
Introduction
Traumatic brain injury (TBI), according to the World Health Organization, will surpass many diseases as a major cause of death and disability by the year 2020 (Hyder et al., 2007). In the United States alone it directly affects an estimated 1.5 million annually. Of the TBIs suffered each year, ~50,000 deaths occur, and nearly 100,000 injuries lead to life-long disability with dramatic impacts on their own and their families’ lives and enormous socio-economic costs. TBI is a particularly serious problem in the geriatric population. In the last decade, there has been a 21 % increase in TBI events in individuals over the age of 65 (Adekoya et al., 2002). The number of cases will likely increase dramatically because, according to the United Nations’ estimates, the elderly (> 65 years of age), who currently account for 12 % of US population, will increase to 18 % of the population by 2025 (Martin et al., 2006). Age is also an important factor influencing prognosis after TBI. In elderly trauma patients outcomes are notoriously poor, recovery is incomplete and unsatisfactory, and the mortality and functional disability rates are twice those of younger patients (Hukkelhoven et al., 2003; Jacobs, 2003). Although this problem is increasingly recognized, the underlying mechanisms responsible for this age-associated vulnerability remain largely unknown.
Neuroinflammation contributes to the pathophysiology of brain trauma (Lucas et al., 2006; Morganti-Kossmann et al., 2002; Tansey et al., 2009). Following brain injury, an inflammatory process is initiated by the activation of resident microglia and astrocytes and infiltrating leukocytes that release cytokines and chemokines (Ghirnikar et al., 1998). Inflammation is considered to be a critically important determinant of outcome following acute injury to the CNS, potentially contributing to the development of secondary injury (Kleinig and Vink, 2009). Studies from our laboratory have demonstrated exacerbated inflammatory responses associated with increased number of dying neurons and a deficit in behavioral recovery following brain injury in aged rodents (Onyszchuk et al., 2008; Sandhir et al., 2004; Sandhir et al., 2008). In addition, we have also reported that the HIF-1 α neuroprotective pathway is compromised following injury to the aged brain, further contributing to poor prognosis (Anderson et al., 2009).
Transcription factors play a pivotal role in controlling inflammatory gene expression (Poli, 1998). CCAAT/enhancer binding proteins (C/EBPs) belong to the basic-leucine zipper DNA-binding protein family and have recently been implicated in inflammation in the central nervous system (Yi et al., 2007). C/EBPs regulate the expression of genes critical to glial activation (Aroa Ejarque-Ortiz et al., 2007), and C/EBP binding sites have been identified in the promoter regions of numerous cytokines and other pro-inflammatory genes (Wedel and Ziegler-Heitbrock, 1995). In brain C/EBPα, C/EBPβ, C/EBPδ and C/EBP homologous protein (CHOP) are the most abundant forms. In the recent years, C/EBPs have been implicated in the inflammation observed in neurodegenerative diseases (Aroa Ejarque-Ortiz et al., 2007; Cardinaux et al., 2000; Tengku-Muhammad et al., 2000) and following brain injury (Cortes-Canteli et al., 2004).
The substantial incidence, poor outcomes and accentuated inflammatory response following brain injury in the elderly suggests that there is a need for comprehensive understanding of the mechanisms that contribute to poor outcomes. The present study was therefore designed to determine whether increased/differential expression of C/EBP transcription factors may contribute to the hyper-inflammatory state observed following TBI in aged mice.
Materials and methods
Animals
C57BL/6 adult (28–32 g, 5–6 months) and aged (28–32 g, 21–24 months) male mice were obtained from the National Institute of Aging colonies. They were barrier raised, monitored for genetic purity and screened for bacterial and viral pathogens strictly according to the NIA guidelines. The animals were housed in the Laboratory Animal Resources (LAR) of the University of Kansas Medical Center. The mice were maintained on a 12-hour light dark cycle and were provided food and water without restriction. All the procedures followed protocols approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee (IACUC) and were accordance with all current NIH regulations.
Controlled Cortical Impact Injury
Adult and aged mice were randomly assigned to undergo moderate impact injury utilizing a model that causes non-penetrating localized deformation of the cortex. The impactor consisted of a microprocessor controller linear motor device (Linmot, Zurich, Switzerland) mounted on an adjustable manipulator (Kopf, Tujunga, CA, USA) that allowed precise positioning, and control of velocity and the level of cortical deformation as described previously (Anderson et al., 2009; Onyszchuk et al., 2007). A 3.0mm flat face tip with a strike velocity of 1.5 m/s, strike depth of 1.0 mm, and contact time of 85 ms was used for injury. Briefly, mice were anesthetized with isoflurane (induction, 2.5%; maintenance, 1.0%) and stabilized in a Cunningham stereotaxic frame (Stoelting, Wood Dale, IN, USA), and the body temperature maintained at 37 ± 1°C throughout the procedure. The skull was exposed after a midline incision and a 3.5mm diameter circular craniotomy was performed using a burr drill, lateral to the mid-sagittal suture, with the center having the coordinates: AP = 0, ML = +2.0 from bregma. After the dural surface was exposed, the position of the impactor and tip was adjusted perpendicular to the brain surface so that the tip contacted the dura. The injury center was 2.0 mm lateral to bregma and included motor (M1, M2) and sensory (S1FL, S1HL) cortical areas. The cortical impact was initiated through the interface of the impactor control software. After the impact, the scalp was sutured closed, anesthesia was discontinued and animals allowed to recover in a temperature-controlled environment. Age-matched uninjured mice were used as controls, because even a minor injury to the skull can cause an inflammatory response in the brain (Stokely and Orr, 2008).
Total RNA isolation
Brains were harvested at survival times of 0 (no injury), 1, 3, and 7 days post injury and stored in RNA Later (Ambion, Austin, TX, USA) for 24 hrs at 4°C. After 24 hrs in RNA Later, ipsilateral cortex was dissected into 5 mm wide rostral and caudal section from injury epicenter using a mouse brain matrix (Harvard Apparatus, Holliston, MA). The dissected cortex represents the same areas in the adult and injured brain because the injury parameters used in this study produce injuries with the same lesion volume in the adult and aged brain (Onyszchuk et al., 2008). Total RNA was extracted using Trizol Reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) using with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) according to manufacturer’s instructions. The quantity of RNA in each sample was determined by absorption at 260 nm (Biophotometer; Eppendorf AG, Hamburg, Germany). The purity of the preparation was checked by ratio of absorbance at 260 and 280 nm, the samples prepared had values of 1.8 or more. The extracted total RNA (2μg/sample) was separated on 1.5% agarose/formaldehyde gels to check for RNA integrity and quality and the samples with 28S and 18S ribosomal RNA ratios near 2.0 were considered acceptable.
Real-time PCR analysis
Gene expression analysis was conducted using quantitative real-time PCR. The following transcripts were analyzed: C/EBP α, β, δ and CHOP. Total RNA samples were treated with DNaseI to remove any contaminating genomic DNA using DNA-free Kit (Ambion, Inc. Austin, TX, USA). cDNAs were reverse transcribed by using 1 μg total RNA from each sample in a Taqman reverse transcription reaction (Applied Biosystems, Foster City, CA, USA) after priming with random hexamers. The expression of C/EBPs was analyzed by real-time PCR using SYBR Green dye. In brief, 10 ng cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq-DNA polymerase, dNTPs mixture dUTP and optimal buffer components; Applied Biosystems, Foster City, CA, USA) and subjected to PCR amplification (one cycle at 50°C for 2 min, one cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min) in a TaqMan 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA). PCR reactions were conducted in duplicate. Primer sequences used were designed either from the literature or designed using Primer Express software (Applied Biosystems) and based on GenBank accession numbers (Table 1). The primer alignment and specificity was assessed using the Blast database (NCBI). The resulting amplicon products were visualized on an agarose gel to further verify size and specificity of the RT-PCR reaction. The endpoint used in the real-time PCR quantification is defined as the PCR cycle number that crosses the signal threshold (Ct). The quantification of target gene expression was performed using the comparative Ct method (Sequence Detector User Bulletin no. 2; PE Biosystems, Foster City, CA), and reported as the fold difference relative to the housekeeping gene. To calculate the fold change (increase or decrease), the Ct of the housekeeping gene (GAPDH) was subtracted from the Ct of the target gene to yield the ΔCt; the change in expression of the normalized target gene as a result of an experimental sample was then expressed as 2−ΔΔCt, where ΔΔCt = ΔCt sample −ΔCt control (sample used as reference). This is based on the method described by Pfaffl et al. (2002).
Table 1.
Primer sequences used for Real-time RT-PCR
| Gene | Primer Sequence | Position | Amplicon (bp) | |
|---|---|---|---|---|
| C/EBPα (NM 007678) | sense | 5′-TGG-ACA-AGA-ACA-GCA-ACG-AGT-AC-3′ | 962–984 | 239 |
| antisense | 5′-GCA-GTT-GCC-CAT-GGC-CTT-GAC-3′ | 1180–1200 | ||
| C/EBP β (NM009883) | sense | 5′-GGT-TTC-GGG-ACT-TGA-TGC-A-3′ | 1183–1201 | 130 |
| antisense | 5′-CAA-CAA-CCC-CGC-AGG-AAC-3′ | 1295–1312 | ||
| C/EBP δ (NM007679) | sense | 5′-CCC-CAA-AGC-TAT-GTG-CCT-TTC-3′ | 1614–1634 | 96 |
| antisense | 5′-CCT-GGA-GGG-TTT-GTG-TTT-TCT-G -3′ | 1688–1709 | ||
| CHOP/Gadd153 (NM007837) | sense | 5′-CCA-CCA-CAC-CTG-AAA-GCA-GAA-3′ | 129–149 | 151 |
| antisense | 5′-AGG-TGC-CCC-CAA-TTT-CAT-CT-3′ | 260–279 | ||
| GAPDH (XM001478544) | sense | 5′-ATG-ACA-TCA-AGA-AGG-TGG-TG-3′ | 839–858 | 177 |
| antisense | 5′-CAT-ACC-AGG-AAA-TGA-GCT-TG-3′ | 996–1015 |
Immunoblotting
Ipsilateral cortices from control and injured brains were homogenized using cold T-PER extraction reagent (Pierce Biotechnology, Rockford, IL, USA) containing mammalian protein inhibitor cocktail (P8340; Sigma Aldrich, St. Louis, MO) and 0.1mM phenylmethylsulfonyl fluoride (Sigma Aldrich, St. Louis, MO, USA). The brain homogenate was centrifuged at 12,000 g (15 min, 4 ° C). The clear supernatant was collected and the total protein concentration measured using a bicinchoninic acid assay kit with bovine serum albumin as a standard (Pierce Biotechnology, Inc., IL, USA). Proteins were separated through 12 % SDS-PAGE, electrophoretically transferred to PVDF membranes and probed with affinity purified rabbit polyclonal antibodies raised against C/EBPα (sc-61), C/EBPβ (sc-150), C/EBPδ (sc-151), CHOP (sc-793) and affinity purified goat polyclonal antibody against GAPDH (sc-20357) from Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA. Bands were visualized by the addition of IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA, USA) and Alexa 680 (Invitrogen, Eugene, OR, USA)-conjugated secondary antibodies using an Odyssey Infrared Imaging System from LI-COR (Lincoln, NE). Relative band intensity was determined using Odyssey software version 2.0 (LI-COR).
GAPDH used as a reference gene for normalization of the data might not be a perfect control. Thal et al. (2008) have shown increased GAPDH mRNA following TBI in mice. However, the increase in GAPDH mRNA was statistically not significant. In addition, Rhinn et al. (2008) found GAPDH mRNA is the most efficient normalizing factors for gene expression studies in a murine model of TBI. However, no report is available on alterations at the protein level following TBI. GAPDH protein levels change following ischemia (Tanaka et al., 2002; Hwang et al., 2007). Nevertheless, it is still the most widely used control for normalization of the data and we have also not observed any significant differences in the GAPDH levels at various time points studied.
Statistical analysis
Values are presented as mean ± standard deviation of n determinations. Significant group differences were determined by a one-way analysis of variance (ANOVA), followed by a post-hoc analysis using the Student–Newman–Keul’s test. In all cases, a p values less than or equal to < 0.05 were considered significant.
Results
The aim of this study is to determine whether the hyper-inflammatory state observed in the aging brain following brain trauma involves higher/differential expression of C/EBP transcription factors. This was examined by quantization of mRNA and protein expression using real-time RT-PCR and immunoblot analysis.
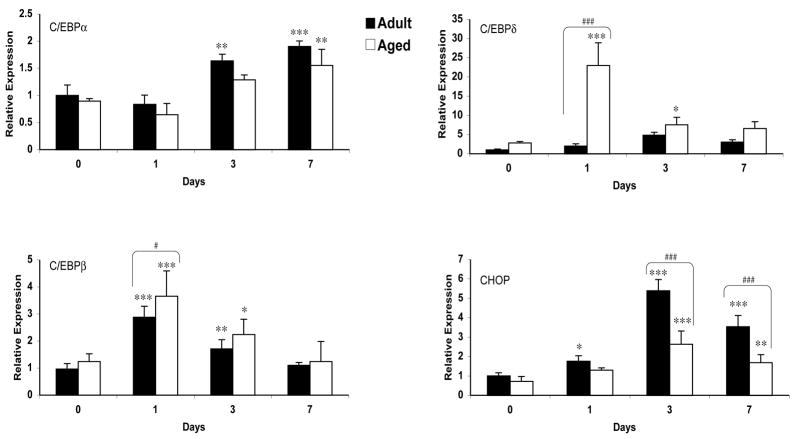
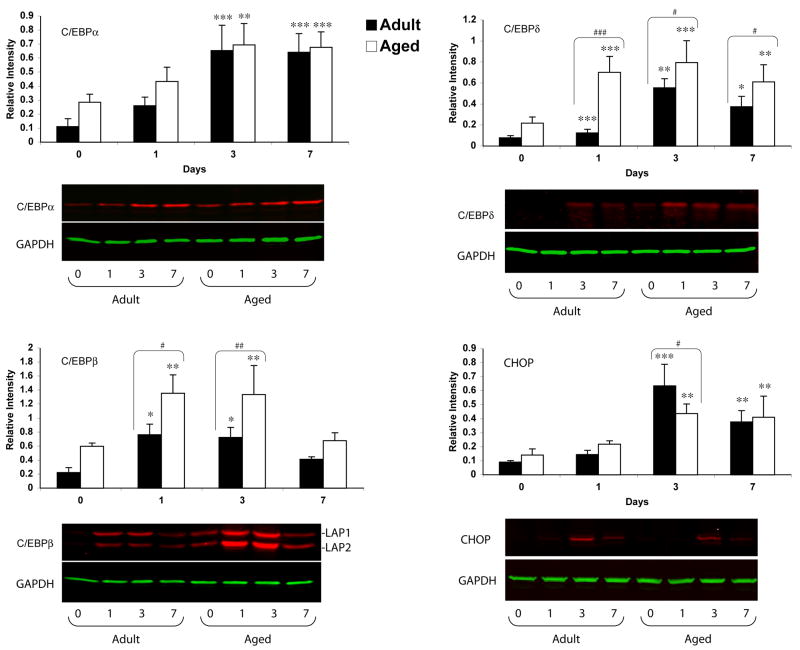
Basal CCAAT/enhancer binding protein (C/EBP), alpha C/EBPα mRNA expression was similar in the aged and adult brain. In response to injury, C/EBPα mRNA expression decreased on day 1 in both adult and aged brain, although this decrease was statistically not significant (Fig. 1). A significant increase in C/EBPα mRNA was observed on day 3 (p<0.01) and 7 (p<0.001) in the adult brain, whereas in the aged brain increase was significant only on day 7 (p<0.01). The increase in C/EBPα mRNA expression post-injury was 1.9-fold in the adult brain compared to 1.7-fold in the aged brain at day 7. However, no significant differences were observed between the adult and aged brain at any of the time points studied. Immunoblot analysis was performed to observe if mRNA expression translates into increased C/EBPα protein levels (Fig. 2). The results showed that C/EBPα protein levels increased in both adult and aged brain with a maximal increase at day 3 and 7 post injury (p<0.001).
Fig. 1.
Expression of c/EBP α, β, δ and CHOP mRNA in cerebral cortex of adult and aged mice 1, 3 and 7 days post-injury analyzed by real-time RT-PCR. The real time reactions were performed in duplicates for both the target gene and GAPDH used as a housekeeping control. The relative expression was calculated using delta delta CT method. Data are presented as mean ± SEM (n=5/group). Asterisk denotes statistical significance after students t-test where *p < 0.05 injured vs. control**p<0.01; ***p<0.001and #p<0.05 adult aged vs. adult; ##p<0.01; ###p<0.001.
Fig. 2.
Western blot analysis of c/EBP α, β, δ and CHOP protein extract from cerebral cortex of adult and aged mice 1, 3 and 7 days post-injury. Values represent relative levels of protein based on optical density normalized to GAPDH signal. Data are presented as mean ± SEM (n=3/group). The representative western blot for c/EBP α, β, δ and CHOP along with GAPDH used as loading control as shown below each graph. Asterisk denotes statistical significance after students t-test where *p < 0.05 injured vs. control;**p<0.01; ***p<0.001 and #p<0.05 adult aged vs. adult; ##p<0.01; ###p<0.001.
CCAAT/enhancer binding protein beta (C/EBPβ) is a member of C/EBP family that plays a critical role in inflammatory response in nervous system. We observed up-regulation of CEBPβ mRNA in both aged and adult brain following brain injury. In both cases, the maximum increase was observed after 24 hours of injury followed by a gradual decline (Fig. 1). The increase was 2.9 fold in both adult and aged brain after 24 hours of injury (p<0.001). The C/EBPβ protein levels were also up-regulated in response to injury in adult and aged brain (Fig. 2). The western blot analysis of C/EBPβ representing 38 (LAP1) and 35 (LAP2) kda isoforms were significantly elevated after 24 hours of injury and remained higher at 72 hours post injury. Interestingly, the C/EBPβ protein levels were significantly higher in the aged animals compared to those in adult animals (1.8 fold). The protein and mRNA levels returned to baseline 7 days after injury in both aged and adult brains.
Another member of C/EBP family, CCAAT/enhancer binding protein delta (C/EBPδ), is also widely expressed in nervous system, like C/EBPβ plays an important role in modulation of immune responses. The expression of C/EBPδ was analyzed in adult and aged animals after 1, 3, and 7 days post injury. We observed that C/EBPδ mRNA levels were elevated 24 hours after injury in the aged, whereas in the adult brain they peaked 3 days after injury. There was a significantly higher (12-fold) induction in the CEBPδ mRNA in aged brain than in the adult brain 24 hours after injury (Fig. 1). Immunoblot analysis also revealed significantly higher expression of CEBPδ protein following injury in the brain of aged mice at all the time points studied (Fig. 2).
The expression of the C/EBP homologous protein (CHOP), also known as growth arrest and DNA damage-inducible gene 153 (GADD153), was examined in the cortex of adult and aged brain following TBI. A significant increase in CHOP mRNA expression was observed in both adult and aged animals. Maximum increase was observed 3 days post injury in both adult and aged brain followed by decrease at day 7 post-injury. The induction in adult cortex was significantly higher (2.0 fold) than in the aged brain at 3 day time point (Fig. 1). The western blot analysis for CHOP protein correlated with the expression of mRNA, indicating a diminished ability of aged brain to express CHOP following injury (Fig. 2).
Both the temporal pattern and magnitude of expression of C/EBPs were different in response to injury. C/EBPβ and δ appeared earlier than C/EBPα and CHOP in both adult and aged brain following injury. C/EBPβ protein and both C/EBPδ mRNA and protein were expressed at higher levels following injury to the aged brain.
Discussion
CCAAT/enhancer binding proteins (C/EBPs) are key regulators of cell differentiation and are linked to processes such as proliferation, apoptosis, and gene expression in several organs (Nerlov, 2007). C/EBP transcription factors are involved in the expression and production of inflammatory cytokines (Cloutier et al., 2009), but little is known of their role in regulating inflammatory response following brain injury, especially in the aged brain.
We found that C/EBPα expression was down regulated 24 hours after injury and upregulated in both adult and aged brain 3 days after injury, but there were no significant differences between adult and aged mouse brain in the expression of C/EBPα at either the mRNA or protein level at any of the time points studied. C/EBPα has been shown to be expressed in activated microglial cells but not in astrocytes or neurons after brain injury (Walton et al., 1998). Increased expression of C/EBPα might play a critical role in activation and/or proliferation of microglia following brain injury. In addition, the down regulation of mRNA 24 hours after injury might be due to induction of CEBPβ as it has been reported that induction of C/EBPβ down regulates CEBPα (Yiangou et al., 2001).
C/EBPβ is an important member of C/EBP family that plays a role in cell differentiation and is a major mediator of inflammatory responses (Kalvakolanu and Roy, 2005). We observed up-regulation of C/EBPβ following brain injury in both adult and aged brain. The first appearance and the time at which maximal C/EBPβ expression occurred were similar in adult and aged mice. However, the aged injured brain demonstrated more C/EBPβ protein than the adult injured brain, suggesting that translational regulation of C/EBPβ may change with age. Rabeek et al (1998) have also found an increase in the amount of C/EBPδ protein without a significant change in mRNA levels in livers of aged mice treated with lipopolysaccharide, and this was correlated this with higher translation efficiency. C/EBPβ has been shown to induce the expression of several genes involved in inflammatory processes following brain injury (Cortes-Canteli et al., 2004), and reducing C/EBPβ provides protection from excitotoxic injury (Cortes-Canteli et al., 2008) and stroke (Kapadia et al., 2006). Nadeau et al. (2005) showed that the levels of C/EBPβ and its phophoprotein increased in neurons following axonal injury, which was suggested to be essential for the neuronal injury response by transcriptionally activating regeneration-associated gene expression. Recently, C/EBPβ has been shown to be upregulated in activated astrocytes and microglia, suggesting that it also participates in neurotoxic events associated with glial activation (Ejarque-Ortiz et al., 2007). We have previously reported exaggerated glial activation in aged brain following brain injury (Sandhir et al., 2008). In a study by Choi et al. (2009) retinoic acid has been shown to exert anti-inflammatory action in ischemia-induced cerebral injury through inhibition of C/EBPβ-mediated COX-2 induction.
We observed that both C/EBPδ mRNA and protein levels were higher in the aged brain than in the adult brain following injury. Rabek et al. (1998) observed higher C/EBPδ protein levels in the aged mouse liver after treatment with lipopolysacchride combined with a delay in recovery to baseline levels in the aged mice. Activated C/EBPδ regulates neurotoxic iNOS expression (Won et al., 2003), which is also selectively upregulated after injury to the aged brain (Sandhir et al., 2004). C/EBPδ mRNA is expressed in low levels and its expression is dramatically induced by LPS and cytokines, suggesting a role in acute phase and inflammatory response (Johnson, 1994). Curiously, C/EBPδ knockout mice do not exhibit any major phenotypes except enhanced contextual fear, whereas, C/EBPα and β knockouts exhibit perinatal lethality or rapid deterioration within months after birth (Sterneck et al., 1998). These findings suggest that C/EBPδ is not essential in normal physiological development, but may be important in response to pathological insults by playing a role in the modulation of proinflammatory responses and neuronal homeostasis in neurodegenerative diseases.
C/EBPδ is elevated in the brains of Alzheimer’s patients (Li et al., 2004) and is reported to be upregulated in cortex after 24 hours of TBI in rats (von Gertten et al., 2005) which is in agreement to our findings. The link between elevated C/EBPδ and Alzheimer’s disease is intriguing and may involve both brain injury and amyloid accumulation. Many studies have reported an association of traumatic brain injury and Alzheimer’s disease (Van Den Heuvel et al., 2007), but the role TBI may play in the mechanism of disease development or progression is not understood. However, TBI is known to increase expression of amyloid precursor protein (Loane et al., 2009). It is therefore possible that the increased expression of C/EBPδ in the aged brain following injury or in response to accumulation of beta amyloid may contribute to the pathogenesis of Alzheimer’s disease.
CHOP, a member of the CCAAT/enhancer-binding protein family of transcription factors (McCullough et al., 2001), is a highly inducible gene that is expressed following disruption of endoplasmic reticulum homeostasis that promotes apoptosis under conditions of ER stress (Oyadomari and Mori, 2004). We observed significantly higher expression of CHOP in adult brain following TBI compared to the aged brain. Increased expression of CHOP mRNA has been reported following transient ischemia (Paschen et al., 1998), whereas no significant change in CHOP mRNA was observed in brain trauma in mice (Paschen et al., 2004). Induction of CHOP in the present study suggests an increase in ER stress in both adult and aged animals following brain injury. CHOP mediates it action through induction of apoptosis through activation of caspase-12 (Szegezdi et al., 2003). However, lower induction in the aged animals suggests either that the ER stress/unfolded protein response or that CHOP induction is impaired in the aged brain. Naido et al. (2008) have reported impaired unfolded protein response in aging contributes to acceleration of neurodegenerative disorders. The difference in the CHOP response in adult and aged brain suggests that mechanism involved in cell death after brain injury might be different. In adult animals, higher induction of CHOP may increase apoptosis of cells through activation of caspase-12, whereas, in the aged animals lower induction of CHOP may result in cells dying of ER protein overload or caspase-12 independent mechanisms. In addition, higher expression of CHOP in the adult brain might also be beneficial as it has been reported to act as a dominant negative regulator of C/EBPs (Ubeda et al., 1999). Thus the role of CHOP in brain injury is complex and requires further investigation.
The results of our study show clear differences in temporal profile of C/EBPs suggesting that C/EBPβ and δ are part of the early inflammatory response while C/EBPα and CHOP appear later, which is in agreement with the findings of Tengku-Muhammad et al. (2000) showing differential regulation of C/EBPs by lipopolysaccharide and cytokines in murine macrophages.
These observations suggest that differential expression of C/EBPβ, δ and CHOP might be important factors contributing to the exaggerated inflammatory response observed in aged brain link between TBI and development of Alzheimer’s disease. Regulation of C/EBPs is complex. It is known that they can act as either transcriptional enhancers or repressors, that individual subtypes can heterodimerize with other members, potentially altering DNA binding specificities or transactivating capabilities. In addition, dominant negative isoforms of C/EBPβ occur naturally (Xiong et al., 2001). Further work will be required to understand how differential regulation of these transcription factors contributes to poor outcomes following injury the aged brain.
Acknowledgments
The authors acknowledge the assistance of Eugene Gregory in carrying out the work and Dr. Y.Y. He for his help with controlled cortical impact injury. The study was supported in part by the Steve Palermo Endowment and the National Institute of Health (R01 AG031140 and research core support P30 HD02528).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adekoya N, Thurman DJ, White DD, Webb KW. Surveillance for traumatic brain injury deaths--United States, 1989–1998. MMWR Surveill Summ. 2002;51:1–14. [PubMed] [Google Scholar]
- Anderson J, Sandhir R, Hamilton ES, Berman NE. Impaired Expression of Neuroprotective Molecules in the HIF-1-alpha Pathway following Traumatic Brain Injury in Aged Mice. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejarque-Ortiz Aroa, Medina Manel G, Tusell Josep M, Pérez-González Anna P, Serratosa Joan, Saura Josep. Upregulation of CCAAT/enhancer binding protein beta in activated astrocytes and microglia. Glia. 2007;55:178–188. doi: 10.1002/glia.20446. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–7. [PubMed] [Google Scholar]
- Choi BK, Kim JH, Jung JS, Lee YS, Han ME, Baek SY, Kim BS, Kim JB, Oh SO. Reduction of ischemia-induced cerebral injury by all-trans-retinoic acid. Exp Brain Res. 2009;193:581–9. doi: 10.1007/s00221-008-1660-x. [DOI] [PubMed] [Google Scholar]
- Cloutier A, Guindi C, Larivee P, Dubois CM, Amrani A, McDonald PP. Inflammatory Cytokine Production by Human Neutrophils Involves C/EBP Transcription Factors. J Immunol. 2009;182:563–571. doi: 10.4049/jimmunol.182.1.563. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Wagner M, Ansorge W, Perez-Castillo A. Microarray Analysis Supports a Role for CCAAT/Enhancer-binding Protein-{beta} in Brain Injury. J Biol Chem. 2004;279:14409–14417. doi: 10.1074/jbc.M313253200. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Luna-Medina R, Sanz-SanCristobal M, Alvarez-Barrientos A, Santos A, Perez-Castillo A. CCAAT/enhancer binding protein {beta} deficiency provides cerebral protection following excitotoxic injury. J Cell Sci. 2008;121:1224–1234. doi: 10.1242/jcs.025031. [DOI] [PubMed] [Google Scholar]
- Ejarque-Ortiz A, Medina MG, Tusell JM, Pérez-González AP, Serratosa J, Saura J. Upregulation of CCAAT/enhancer binding protein beta in activated astrocytes and microglia. Glia. 2007;55:178–188. doi: 10.1002/glia.20446. [DOI] [PubMed] [Google Scholar]
- Ghirnikar RS, Lee YL, Eng LF. Inflammation in traumatic brain injury: role of cytokines and chemokines. Neurochem Res. 1998;23:329–40. doi: 10.1023/a:1022453332560. [DOI] [PubMed] [Google Scholar]
- Hukkelhoven CW, Steyerberg EW, Rampen AJ, Farace E, Habbema JD, Marshall LF, Murray GD, Maas AI. Patient age and outcome following severe traumatic brain injury: an analysis of 5600 patients. J Neurosurg. 2003;99:666–73. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Kim DW, Choi JH, Lee IS, Won MH. Hyperoxidized peroxiredoxins and glyceraldehyde-3-phosphate dehydrogenase immunoreactivity and protein levels are changed in the gerbil hippocampal CA1 region after transient forebrain ischemia. Neurochem Res. 2007;32:1530–1538. doi: 10.1007/s11064-007-9345-6. [DOI] [PubMed] [Google Scholar]
- Jacobs DG. Special considerations in geriatric injury. Curr Opin Crit Care. 2003;9:535–9. doi: 10.1097/00075198-200312000-00012. [DOI] [PubMed] [Google Scholar]
- Johnson PF, Williams SC. CCAAT/Enhancer Binding (C/EBP) Proteins. In: Yaniv M, Tronche F, editors. Liver gene expression. Landes Company; Austin: 1994. pp. 231–258. [Google Scholar]
- Kalvakolanu DV, Roy SK. CCAAT/enhancer binding proteins and interferon signaling pathways. J Interferon Cytokine Res. 2005;25:757–69. doi: 10.1089/jir.2005.25.757. [DOI] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. J Neurochem. 2006;98:1718–1731. doi: 10.1111/j.1471-4159.2006.04056.x. [DOI] [PubMed] [Google Scholar]
- Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Current Opinion in Neurology. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- Li R, Strohmeyer R, Liang Z, Lue LF, Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol Aging. 2004;25:991–9. doi: 10.1016/j.neurobiolaging.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Pocivavsek A, Moussa CEH, Thompson R, Matsuoka Y, Faden AI, Rebeck GW, Burns MP. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med. 2009;15:377–379. doi: 10.1038/nm.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147(Suppl 1):S232–40. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz L-O, Aw T-Y, Holbrook NJ. Gadd153 Sensitizes Cells to Endoplasmic Reticulum Stress by Down-Regulating Bcl2 and Perturbing the Cellular Redox State. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8:101–5. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Hein P, Fernandes KJL, Peterson AC, Miller FD. A transcriptional role for C/EBP [beta] in the neuronal response to axonal injury. Molecular and Cellular Neuroscience. 2005;29:525–535. doi: 10.1016/j.mcn.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging Impairs the Unfolded Protein Response to Sleep Deprivation and Leads to Proapoptotic Signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends in Cell Biology. 2007;17:318–324. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Onyszchuk G, Al-Hafez B, He YY, Bilgen M, Berman NE, Brooks WM. A mouse model of sensorimotor controlled cortical impact: characterization using longitudinal magnetic resonance imaging, behavioral assessments and histology. J Neurosci Methods. 2007;160:187–96. doi: 10.1016/j.jneumeth.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyszchuk G, He YY, Berman NE, Brooks WM. Detrimental Effects of Aging on Outcome from Traumatic Brain Injury: A Behavioral, Magnetic Resonance Imaging, and Histological Study in Mice. J Neurotrauma. 2008;25:153–171. doi: 10.1089/neu.2007.0430. [DOI] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Paschen W, Gissel C, Linden T, Althausen S, Doutheil J. Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction. Molecular Brain Research. 1998;60:115–122. doi: 10.1016/s0169-328x(98)00180-6. [DOI] [PubMed] [Google Scholar]
- Paschen W, Yatsiv I, Shoham S, Shohami E. Brain trauma induces X-box protein 1 processing indicative of activation of the endoplasmic reticulum unfolded protein response. J Neurochem. 2004;88:983–992. doi: 10.1046/j.1471-4159.2003.02218.x. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–82. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Rabek JP, Scott S, Hsieh CC, Reisner PD, Papaconstantinou J. Regulation of LPS-mediated induction of C/EBP delta gene expression in livers of young and aged mice. Biochim Biophys Acta. 1998;1398:137–47. doi: 10.1016/s0167-4781(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Rhinn H, Marchand-Leroux C, Croci N, Plotkine M, Scherman D, Escriou V. Housekeeping while brain’s storming Validation of normalizing factors for gene expression studies in a murine model of traumatic brain injury. BMC Mol Biol. 2008;9:62. doi: 10.1186/1471-2199-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–80. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneck E, Paylor R, Jackson-Lewis V, Libbey M, Przedborski S, Tessarollo L, Crawley JN, Johnson PF. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein Î′. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokely ME, Orr EL. Acute effects of calvarial damage on dural mast cells, pial vascular permeability, and cerebral cortical histamine levels in rats and mice. J Neurotrauma. 2008;25:52–61. doi: 10.1089/neu.2007.0397. [DOI] [PubMed] [Google Scholar]
- Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-Stress-Mediated Apoptosis. Annals of the New York Academy of Sciences. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Mochizuki H, Suzuki A, Katsube N, Ishitani R, Mizuno Y, Urabe T. Induction of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression in rat brain after focal ischemia/reperfusion. J Cereb Blood Flow Metab. 2002;22:280–288. doi: 10.1097/00004647-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Kernie SG, Larry RS. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. Inflammation in Neurodegenerative Disease and Injury; pp. 131–136. [Google Scholar]
- Tengku-Muhammad TS, Hughes TR, Ranki H, Cryer A, Ramji DP. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- Thal SC, Wyschkon S, Pieter D, Engelhard K, Werner C. Selection of endogenous control genes for normalization of gene expression analysis after experimental brain trauma in mice. J Neurotrauma. 2008;25:785–794. doi: 10.1089/neu.2007.0497. [DOI] [PubMed] [Google Scholar]
- Ubeda M, Vallejo M, Habener JF. CHOP Enhancement of Gene Transcription by Interactions with Jun/Fos AP-1 Complex Proteins. Mol Cell Biol. 1999;19:7589–7599. doi: 10.1128/mcb.19.11.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Heuvel C, Thornton E, Vink R, John TW, Andrew IRM. Progress in Brain Research. Vol. 161. Elsevier; 2007. Traumatic brain injury and Alzheimer’s disease: a review; pp. 303–316. [DOI] [PubMed] [Google Scholar]
- von Gertten C, Flores Morales A, Holmin S, Mathiesen T, Nordqvist AC. Genomic responses in rat cerebral cortex after traumatic brain injury. BMC Neurosci. 2005;6:69. doi: 10.1186/1471-2202-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M, Saura J, Young D, MacGibbon G, Hansen W, Lawlor P, Sirimanne E, Gluckman P, Dragunow M. CCAAT-enhancer binding protein alpha is expressed in activated microglial cells after brain injury. Brain Res Mol Brain Res. 1998;61:11–22. doi: 10.1016/s0169-328x(98)00169-7. [DOI] [PubMed] [Google Scholar]
- Wedel A, Ziegler-Heitbrock HW. The C/EBP family of transcription factors. Immunobiology. 1995;193:171–85. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- Won J-S, Im Y-B, Key L, Singh I, Singh AK. The Involvement of Glucose Metabolism in the Regulation of Inducible Nitric Oxide Synthase Gene Expression in Glial Cells: Possible Role of Glucose-6-Phosphate Dehydrogenase and CCAAT/Enhancing Binding Protein. J Neurosci. 2003;23:7470–7478. doi: 10.1523/JNEUROSCI.23-20-07470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Hsieh C-C, Kurtz AJ, Rabek JP, Papaconstantinou J. Regulation of CCAAT/enhancer-binding protein-{beta} isoform synthesis by alternative translational initiation at multiple AUG start sites. Nucl Acids Res. 2001;29:3087–3098. doi: 10.1093/nar/29.14.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int. 2007;50:1014–27. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou M, Scott SG, Rabek JP, An MR, Xiong W, Papaconstantinou J. Effects of mercuric chloride on the regulation of expression of the acute phase response components [alpha]1-acid glycoprotein and C/EBP transcription factors. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2001;1518:47–56. doi: 10.1016/s0167-4781(01)00165-8. [DOI] [PubMed] [Google Scholar]