Abstract
The isoprenoid alcohol farnesol is an effective inducer of cell cycle arrest and apoptosis in a variety of carcinoma cell types. In addition, farnesol has been reported to inhibit tumorigenesis in several animal models suggesting that it functions as a chemopreventative and anti-tumor agent in vivo. A number of different biochemical and cellular processes have been implicated in the growth-inhibitory and apoptosis-inducing effects of farnesol. These include regulation of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and CTP:phosphocholine cytidylyltransferase α (CCTα), the rate limiting enzymes in the mevalonate pathway and phosphatidylcholine biosynthesis, respectively, and the generation of reactive oxygen species. In some cell types the action of farnesol is mediated through nuclear receptors, including activation of farnesoid X receptor (FXR) and peroxisome proliferator-activated receptors (PPARs). Recent studies have revealed that induction of endoplasmic reticulum (ER) stress and the subsequent activation of the unfolded protein response (UPR) play a critical role in the induction of apoptosis by farnesol in lung carcinoma cells. This induction was found to be dependent on the activation of the MEK1/2-ERK1/2 pathway. In addition, farnesol induces activation of the NF-κB signaling pathway and a number of NF-κB target genes. Optimal activation of NF-κB was reported to depend on the phosphorylation of p65/RelA by the MEK1/2-MSK1 signaling pathway. In a number of cells farnesol-induced apoptosis was found to be linked to activation of the apoptosome. This review provides an overview of the biochemical and cellular processes regulated by farnesol in relationship to its growth-inhibitory, apoptosis-promoting, and anti-tumor effects.
Keywords: farnesol, apoptosis, isoprenoid, chemoprevention, endoplasmic reticulum stress, unfolded protein response, CTP:phosphocholine cytidylyltransferase, nuclear receptor, cancer, NF-κB, apoptosome, MAP kinase
1. Introduction
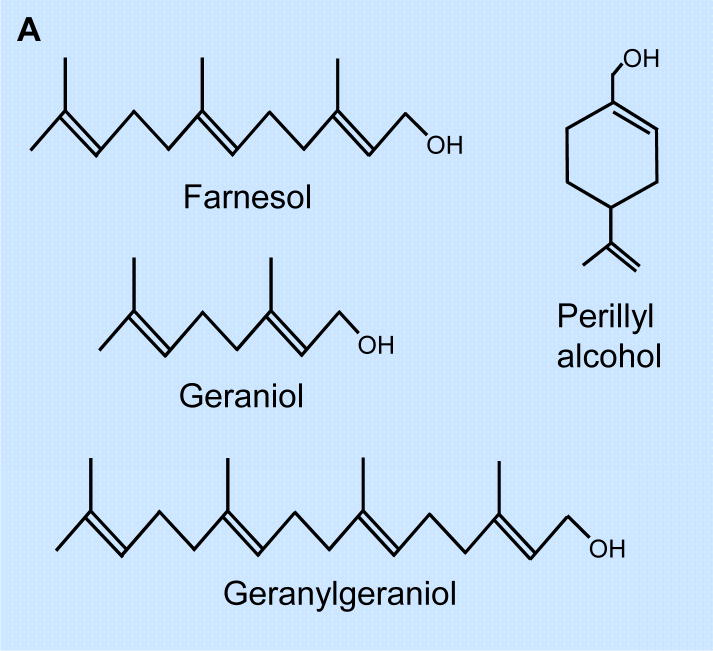
Isoprenoids are essential in the regulation of cell proliferation, apoptosis, differentiation, and lipid biosynthesis [1–9]. The isoprenoid pathway leads to the synthesis of farnesyl pyrophosphate (farnesyl-PP) and to geranylgeranyl pyrophosphate (geranylgeranyl-PP) which are involved in the prenylation of many proteins, and subsequently the biosynthesis of cholesterol, sterols, and other cholesterol derivatives [6,8–10]. The non-sterol isoprenoid farnesol is produced by dephosphorylation of farnesyl-PP, a metabolite of the cholesterol biosynthetic pathway. In addition to being produced endogenously, farnesol and the related isoprenoids (Fig. 1A), perillyl alcohol and geraniol, are natural compounds found in many fruits and aromatic plants, including citrus (perillyl alcohol, geraniol), sage, spearmint, nutmeg (perillyl alcohol), basil (geraniol), lemon grass (farnesol and geraniol), and chamomile (farnesol) [11–13]. This article reviews the current status of our knowledge of the effects of farnesol on mammalian cell proliferation, differentiation, apoptosis, and tumor suppression.
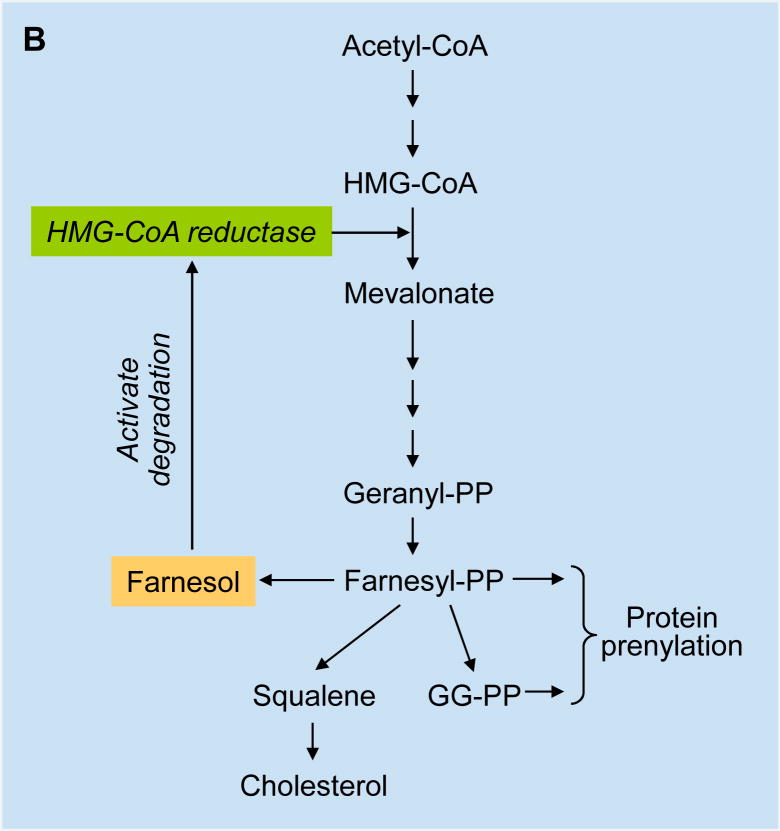
Fig. 1.
(A) Molecular structure of farnesol and farnesol-related isoprenoids, geraniol, geranylgeraniol, and perillyl alcohol. (B) Farnesol is found in many fruits and herbs and a catabolite of the mevalonate pathway. The mevalonate pathway starts with the formation of HMG-CoA that subsequently is converted into mevalonate by HMG-CoA reductase, the rate-limiting enzyme in this pathway. Mevalonate leads to the synthesis of farnesyl-PP, which is at the branch-point of several pathways. In addition to serving as precursor of cholesterol biosynthesis, it can be converted to geranylgeranyl-PP. Both farnesyl-PP and geranylgeranyl-PP are involved in the prenylation of a variety of proteins and can be metabolized to their alcohol derivatives. HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; farnesyl-PP, farnesyl pyrophosphate; geranyl-PP, geranyl pyrophosphate; GGPP, geranylgeranyl-pyrophosphate
2. Inhibition of cell proliferation and induction of apoptosis
2.1. In vitro cell systems
A number of studies have demonstrated that farnesol and related isoprenoids, including geraniol and perillyl alcohol, inhibit cell proliferation and induce apoptosis in a broad range of malignant cell types. Tumor cells were generally found to be considerably more sensitive to farnesol-induced growth inhibition than normal cells [2,14,15]. For example, in contrast to leukemic cells, human primary T lymphocytes or monocytes are rather resistant to farnesol-induced apoptosis. The mechanism underlying this differential sensitivity is not yet understood. Farnesol inhibits cell proliferation with IC50s that range from 25 to 250 μM. Leukemic cells appear among the most sensitive to the growth-inhibitory effects of farnesol [2,16,17]. Farnesol is usually more effective in inhibiting the proliferation of tumor cells than the related isoprenoids, nerolidol, geraniol, geranylgeraniol, and perillyl alcohol [11,16,18,19].
In most cell types, including lung adenocarcinoma, hepatoma, melanoma, lymphoblastic leukemia, colorectal carcinoma, oral squamous carcinoma, and pancreatic adenocarcinoma, farnesol, geraniol, and perillyl alcohol induce a GO/G1 cell cycle arrest [2,18–30]. In some cell types a transient accumulation in G2 has been observed. The GO/G1 cell cycle arrest in farnesol-treated human pancreatic adenocarcinoma cells was shown to be accompanied by a significant increase in the expression of the cyclin-dependent kinase (Cdk) inhibitors p21Cip1 and p27Kip1, and a reduction in the level of cyclin A, cyclin B1, and Cdk2 protein levels, while the expression of Cdk4 and Cdk6 was unaffected [18]. Cdk inhibitors play an important role in regulating the activity of Cdks and cell cycle progression [31]. An increased association of p21Cip1 and p27Kip1 with cyclin E/Cdk2 complexes was detected in farnesol-treated cells consistent with the observed reduction in Cdk2 activity and GO/G1 cell cycle arrest. As reported for farnesol, treatment with perillyl alcohol and geraniol also increased p21Cip1 in pancreatic carcinoma and non-small cell lung carcinoma [18,32]. In general, farnesol was more effective in enhancing p21Cip1 levels and inhibiting Cdk2 than geraniol and perillyl alcohol. The relative efficacies of these isoprenoids to enhance p21Cip1 levels correlated with their growth-inhibitory effects. Down-regulation of both p21Cip1 and p27Kip1 by corresponding siRNAs resulted in a considerable protection from the growth-inhibitory effect of these isoprenoids suggesting that inhibition of proliferation of pancreatic carcinoma cells is p21Cip1- and p27Kip1-dependent. Regulation of p21Cip1 and p27Kip1 protein expression and activity has been shown to be complex and controlled at the transcriptional and posttranscriptional level, including phosphorylation and protein stability. BCL2 and BCL-XL, which promote p27Kip1 protein stability [33,34], are down-regulated in farnesol-treated cells [19,28] and, therefore, do not appear to be involved in the increase in p27Kip1. The molecular mechanism by which these isoprenoids induce p21Cip1 and p27Kip1 has yet to be elucidated.
The presence of a sub-GO/G1 population suggested that in most cell types inhibition of cell growth by farnesol, geraniol, geranylgeraniol, or perillyl alcohol is accompanied by apoptosis. This was supported by the appearance of apoptotic bodies, increased annexin V binding, activation of various caspases, cleavage of poly-ADP-ribose polymerase (PARP), and DNA fragmentation [4,7,12,18–20,23–25,28,30,32,35–48]. In human lung adenocarcinoma H460 cells, the induction of apoptosis by farnesol was associated with activation of caspase-3, -4, and -9, while farnesol had little effect on caspase-8 [19]. Activation of caspases occurred within 4 hrs of farnesol treatment, a time course that correlated with that of PARP cleavage.
2.2. In vivo studies
Farnesol and other dietary isoprenoids have been shown to exhibit anti-tumor and -carcinogenesis effects in vivo [12,48]. Farnesol, perillyl alcohol, and geraniol greatly inhibited the growth of tumors in the Syrian Golden hamsters formed after subcutaneous injection of hamster pancreatic adenocarcinoma PC-1 cells [11,39]. Hamsters fed with a diet containing 20 g/kg diet geraniol or farnesol exhibited a complete inhibition of PC-1 pancreatic tumor growth. Moreover, farnesol and perillyl alcohol reduced the incidence of pancreatic cancer in hamsters treated with the carcinogen N-nitrosobis(2-oxopropyl)amine [39]. Hyperplastic pancreatic ductal neoplasms from perillyl alcohol- or farnesol-treated animals exhibited higher Bak protein expression, higher apoptotic rates, diminished expression of the antiapoptotic protein BCL-XL, and lower rates of DNA synthesis than controls [39]. The potency of farnesol to inhibit tumor growth of PC-1 cells was very similar to that of geraniol and significantly greater than that of perillyl alcohol. However, farnesol is about 7-times more potent in inhibiting the growth of cultured PC-1 cells than geraniol and perillyl alcohol. This difference in relative potency between these compounds in cultured cells versus in vivo tumors might be due to differences in their metabolism and pharmacokinetics [12]. However, relatively little is known about the metabolism of these compounds. Farnesol has been reported to be glucuronidated in human liver, kidney and intestine by uridine diphosphoglucuronosyltransferases UGT2B7 and UGT1A1 [49]. Substrate specificities of these enzymes might potentially explain differences in efficacy of isoprenoids in vitro and in vivo. A number of Phase I and Phase II enzymes have been reported to be increased in liver of farnesol-treated rats, including cytochrome P450 Cyp1A, Cyp2A1–3, Cyp2B1/2, Cyp3A1/2, and glutathione reductase [50]. Many of these enzymes can metabolize various drugs and carcinogens. Therefore, farnesol might alter the metabolism, efficacy, and/or toxicity of drugs, carcinogens, or isoprenoids.
Anti-tumorigenic effects of farnesol and geraniol have been also observed in an initiation-promotion hepatocarcinogenesis model in rats [7]. Farnesol inhibited the incidence, mean number, and size of preneoplastic hepatic lesions and was shown to be more effective than geraniol. Analysis of the BrdU-labeling index and the number of apoptotic cells indicated that this inhibition was at least in part due to an inhibition of proliferation and did not appear to involve increased apoptosis. It was concluded that farnesol may inhibit the initial phases of hepatocarcinogenesis possibly due to inhibition of the metabolic activation of the initiating agent diethylnitrosamine. Farnesol treatment has also a protective effect against Fe-nitrilotriacetic acid-induced oxidative damage in the kidney and early renal tumor promotion in a rat model [51]. Moreover, administration of farnesol was shown to significantly suppress carcinogen-induced formation of aberrant crypt foci and crypt multiplicity in the colon of rats treated with the carcinogen azozymethane [52,53]. Perillyl alcohol and geranylgeraniol had little effect on aberrant crypt formation under this protocol. A recent study reported that farnesol exhibits antigenotoxic effects against benzo(a)pyrene and reduces DNA strand breaks and the formation of DNA adducts in vivo [54]. These observations are consistent with the concept that farnesol functions as a chemopreventative agent in (colon) carcinogenesis and affects the initiation phase of tumorigenesis.
Until now, no clinical trials have been conducted with farnesol; however, several phase I and II trials have been reported with perillyl alcohol. Phase I clinical trials were conducted with patients with advanced malignancies [55–57] and solid tumors [58–60], while several phase II trials were reported with patients suffering from metastatic androgen-independent prostate cancer [61], advanced ovarian cancer [62], refractory metastatic breast cancer [63,64], and metastatic colorectal cancer [65]. In the regimens used in these studies, perillyl alcohol did not appear to have significant, clinical antitumor activity.
3. Mechanisms of farnesol-induced apoptosis
3.1. Effect of farnesol on HMG-CoA reductase
Initial studies indicated that the inhibition of cell proliferation and induction of apoptosis by farnesol might relate to its inhibitory effect on 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, an enzyme that converts HMG-CoA into mevalonate, the rate-limiting step in the isoprenoid biosynthetic pathway (Fig. 1B)[8,9,66]. Mevalonate leads to the synthesis of farnesyl-PP, which serves as a precursor of cholesterol biosynthesis, and can be converted to geranylgeranyl pyrophosphate (geranylgeranyl-PP) and farnesol. Farnesyl-PP and geranylgeranyl-PP are involved in the prenylation of a variety of proteins [6,10]. Both farnesyl-PP and farnesol have been demonstrated to accelerate the rapid and dose-dependent degradation of HMG-CoA reductase in vitro and in cultured cells [67–70]. Although, in several cell systems, the induction of apoptosis and the chemopreventative effects by farnesol and geraniol were shown to occur independently of HMG-CoA reductase [7,29], in lung carcinoma A549 cells the induction of apoptosis by farnesol is accompanied by a reduction in this enzyme [4]. The latter led to the suggestion that there might be a link between the two and that inhibition of HMG-CoA reductase might contribute to growth inhibition and the induction of apoptosis. This interpretation is consistent with other studies indicating a link between inhibition of HMG-CoA reductase, induction of apoptosis, suppression of tumor growth, and reduced cancer risk [71–77]. These effects could relate to an inhibition of prenylation of certain proto-oncogenes, such as members of the Ras family that play a key role in controlling cell proliferation. However, farnesol was reported not to inhibit the prenylation of Ha-Ras indicating that its growth-inhibition and induction of apoptosis appear to be unrelated to effects on prenylation [20]. Alternatively, because rapidly growing tumor cells require increased cholesterol biosynthesis, its growth-suppressing effects might relate to the inhibition of cholesterol biosynthesis. A recent study revealed yet another mechanism and demonstrated that inhibition of HMG-CoA reductase can induce endoplasmic reticulum (ER) stress, a major pathway leading to apoptosis [78]. As discussed below, the reduction in this enzyme may be a contributory factor in farnesol-induced ER stress [19].
3.2. Farnesol inhibits CDP-choline pathway
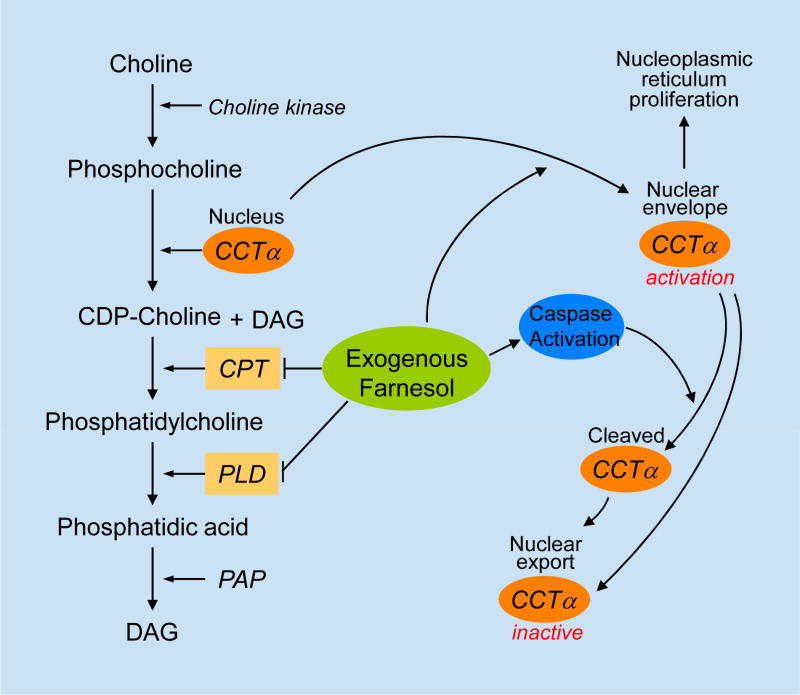
Several studies reported that, in various cell types, farnesol-induced apoptosis is associated with an inhibition of phosphatidylcholine (PC) synthesis (Fig. 2) [5,16,20,35,38,79,80]. PC plays a critical role in maintaining the physical structure of membranes and serves as a precursor of lipid several second messengers, including phosphatidic acid (PA), diacylglycerol (DAG), and fatty acids, which control a number of cellular processes, including proliferation and apoptosis [81–88]. The biosynthesis of CDP-choline is catalyzed by the rate-limiting enzyme CTP:phosphocholine cytidylyltransferase (CCTα) which has been shown to plays a critical role in embryonic development, cell proliferation, and apoptosis [82,87–90].
Fig. 2.
Effects of exogenous farnesol on CCTα. CCTα is the rate-limiting enzyme in the CDP-choline pathway leading to the biosynthesis of phosphatidylcholine, the major membrane lipid and precursor of lipid second messengers, including phosphatidic acid (PA) and diacylglycerol (DAG). Farnesol causes translocation of CCTα to the nuclear envelope resulting in nucleoplasmic reticulum proliferation and a transient increase in CCTα activity. CCTα is subsequently exported from the nucleus. Activation of caspases during farnesol-induced apoptosis results in CCTα cleavage; however, the export of CCTα occurs independently of caspases and may be due to loss of integrity of the nuclear envelope during apoptosis. CPT, CDP-choline:1,2-diacylglycerol cholinephosphotransferase; PLD, phospholipase D; PAP, phosphatidic acid phosphatase.
Several studies have demonstrated that farnesol functions as a potent modulator of the subcellular localization and activity of CCTα. In most cells, CCTα is localized primarily to the nucleus where it can interconvert between a soluble, inactive form that is dispersed throughout the nucleus, and a membrane-bound, active form [91–95]. Upon addition of farnesol, as well as DAG or fatty acids, CCTα rapidly translocates to the inner nuclear envelope (NE) [37,38,93]. A 50 amino acid, amphipathic α-helix (M domain) was identified to mediate the interaction with lipid bilayers thereby inducing a conformational change in CCTα that relieves the inhibitory constraint on the catalytic domain resulting in the transient activation of CCTα and increased PC synthesis. A C-terminal phosphorylation domain containing 16 serine phosphorylation sites, adjacent to the M domain negatively regulates the association of CCTα with the NE [37,96]. In addition to PC synthesis, CCTα is critical for the expansion of the nucleoplasmic reticulum (NR), an intranuclear membrane network that plays a role in intranuclear signaling. Association with the NE through the M domain, but not the catalytic activity of CCTα, is required for NR expansion [92,97].
Following the farnesol-induced translocation of CCTα to the NE, CCTα is exported from the nucleus to the cytoplasm causing a disruption of PC synthesis. Although activation of CCTα is accompanied by a decrease in the level of DAG, this reduction was found not to be a prerequisite for farnesol-induced apoptosis. Stable expression of CCTα in CCTα-deficient MT58 cells partially restored PC synthesis and delayed farnesol-induced apoptosis [38]. Oleate and dioctanoylglycerol (diC8) were shown to prevent farnesol-induced export of CCTα as well as apoptosis [16,30,35,38]. Oleate and diC8 preserve the nuclear localization of CCTα and maintain PC synthesis. These observations are consistent with the concept that the effects on CCTα and PC synthesis play a critical role in farnesol-induced apoptosis. Overexpression of choline/ethanolaminephosphotransferase 1 (CEPT1) in CHO cells restored PC biosynthesis in farnesol-treated CHO cells, but had little effect on the induction of apoptosis [5]. The latter may relate to different functions and/or subcellular localization of CCTα and CEPT1. Clearly, further studies are needed to understand the precise role of PC synthesis in farnesol-induced apoptosis and the mechanism by which various lipids, such as oleate and diC8, inhibit this apoptosis.
In addition to its translocation to the NE and nuclear export, CCTα has been reported to be cleaved by caspases activated during farnesol-induced apoptosis [37,38,95]. This proteolytic cleavage removes 28 amino acids at the N-terminus, including the NLS. However, inhibition of caspase activity or mutation of the caspase cleavage site in CCTα did not affect the translocation of CCTα to the membrane or CCTα activity nor did it affect the nuclear export of CCTα. It has been suggested that the release of CCTα from the nucleus may be due to loss of integrity of the nuclear envelope during apoptosis [37]. This is supported by observations showing that fluorescent-labeled dextran was no longer excluded from the nucleus of farnesol-treated cells undergoing apoptosis. Thus, these studies suggest that both the cleavage and nuclear release of CCTα is coupled to farnesol-induced apoptosis, but occur independently of each other (Fig. 2).
3.3. Farnesol induces endoplasmic reticulum stress
Farnesol inhibits the proliferation and induces apoptosis in a variety of human lung carcinoma cell types in a dosage- and time-dependent manner [4,19]. Of several isoprenoids analyzed, farnesol was found to be the most potent inducer of apoptosis in human lung adenocarcinoma H460 cells. Comparison of the gene expression profiles of vehicle- or farnesol-treated H460 cells revealed that farnesol enhances the expression of many genes known to be induced during endoplasmic reticulum (ER) stress, including activating transcription factor 3 (ATF3), DNA damage-inducible protein 3 (DDIT3, also named CHOP or GADD153), X-box binding protein 1 (XBP1), the chaperones BiP/GRP78 (immunoglobulin heavy chain-binding protein or glucose-regulated protein of MW 78 kD) and GRP94 (HSP90B1), and protein disulfide isomerase A4 (PDIA4), the homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1 (HERPUD1), and T cell death-associated protein (TDAG51 or PHLDA1) [19]. These observations suggested that farnesol-induced apoptosis in these cells is coupled to the activation of an ER stress response pathway and the unfolded protein response (UPR) (Fig. 3).
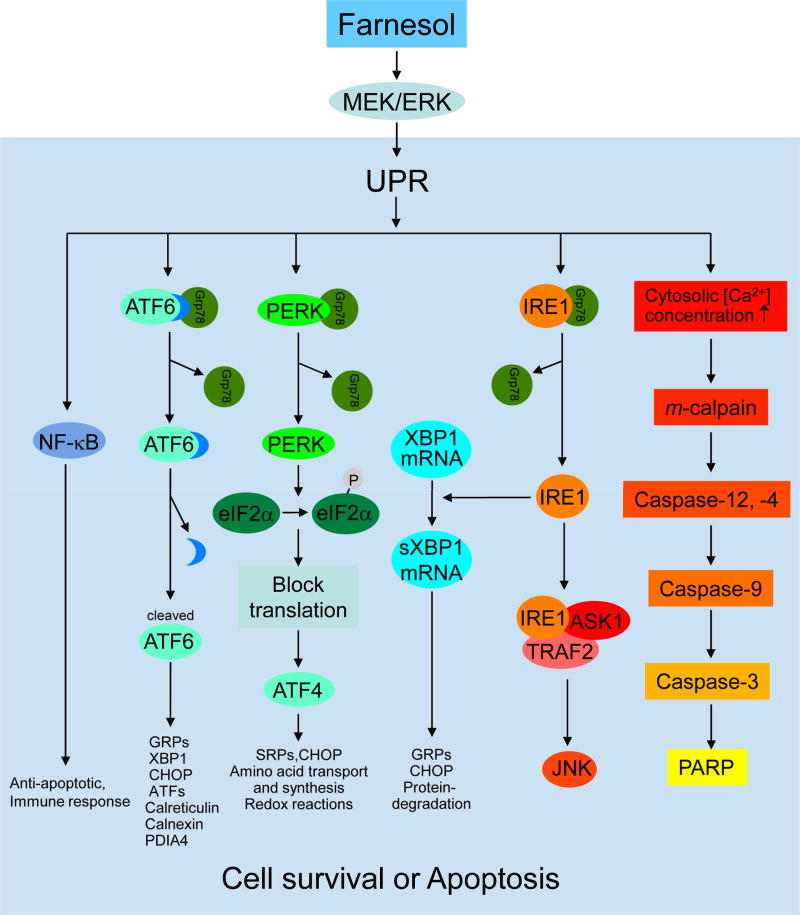
Fig. 3.
Farnesol induces ER stress and activation of the unfolded protein response. Activation of the MEK-ERK signaling pathway by farnesol is an early event that is critical in triggering the UPR. The UPR is initiated by the activation of several ER stress-sensor proteins, including PERK, IRE1, and ATF6. Dissociation of the ER chaperone BiP/GRP78 from UPR sensor protein complexes plays a critical role in their activation. Release of BiP leads to activation and nuclear translocation of ATF6. Activation of PERK results in the phosphorylation of eIF2α and subsequently to an attenuation of the rate of general mRNA translation and protein synthesis. However, it selectively enhances the translation of some mRNAs, including the transcription factor ATF4. Active IRE1 induces alternative splicing of XBP1 mRNA resulting in the synthesis of a potent transcriptional activator. ATF4, ATF6, and XBP1 enhance the transcription of several chaperones. Activation of these pathways lead to inhibition of newly synthesized protein, increased degradation of misfolded proteins, and amplification of the protein folding capacity with the intend to restore normal ER function and promote cell survival. If ER homeostasis cannot be restored, cells start to undergo apoptosis. The IRE1-TRAF2-ASK1-JNK cascade is an important pro-apoptotic signaling pathway in ER stress. GRPs, glucose response proteins; SRPs, stress response proteins; PARP, poly(ADP-ribose) polymerase
The UPR is initiated by the activation of several ER stress-sensor proteins, including PKR-like ER kinase (PERK), inositol-requiring enzyme 1 (IRE1), which functions as a type I ER transmembrane protein kinase, and activating transcription factor 6 (ATF6) [98–103]. Dissociation of the ER chaperone BiP/GRP78 from UPR sensor protein complexes plays a critical role in their activation which subsequently leads to inhibition of newly synthesized protein, increased degradation of misfolded proteins, and amplification of the protein folding capacity. These events are intended to restore normal ER function and promote cell survival. If ER homeostasis cannot be restored, cells are programmed to undergo apoptosis.
Evidence has been provided indicating that farnesol can induce activation of all three ER-stress sensor protein pathways [19]. Farnesol treatment was found to enhance phosphorylation of eukaryotic initiation factor 2α (eIF2α) in lung carcinoma H460 cells [19]. This phosphorylation is known to be mediated by PERK, which itself is activated after its release from BiP/GRP78 [98,100–102,104]. This subsequently leads to an attenuation of the rate of general mRNA translation and protein synthesis; however, phosphorylated eIF2α selectively enhances the translation of some mRNAs, including the transcription factor ATF4, which promotes the transcriptional activation of ATF3 and DDIT3, genes found to be induced in farnesol-treated H460 cells [19].
Induction of UPR signaling is supported by the observed splicing of a 26-base intron from X-box-binding protein 1 (XBP1) mRNA in farnesol-treated H460 cells [19]. The splicing of this intron has been reported to be mediated by IRE1, a Ser/Thr kinase containing a site-specific endoribonuclease domain, activated upon its dissociation from BiP/GRP78 [98,100–102,104]. The alternatively spliced XBP1 mRNA (XBP1s) generates a potent basic leucine zipper family transcriptional activator that heterodimerizes with NF-Y to enhance the transcription of several chaperones, thereby promoting the folding capacity in the ER [98,100–102,104,105]. Subsequently, XBP1s, in combination with other transcription factors, including ATF4 and ATF6, mediate the transcriptional activation of genes involved in restoring ER homeostasis. The observed activation of ER stress sensor proteins in farnesol-treated H460 cells and the induction of numerous UPR genes, encoding proteins involved in promoting protein-folding and ER biogenesis, including Bip/GRP78, GRP94, calreticulin, calnexin, and PDIA4, are consistent with the concept that farnesol induces ER stress and activates the UPR [19,106].
The precise mechanism by which farnesol induces ER stress has yet to be elucidated. This raised the question whether the induction of ER stress by farnesol is related to any of its other effects, such as inhibition of HMG-CoA reductase or the CDP-choline pathway [4,19]. In this regard it is interesting to note that a recent study provided evidence showing that inhibition of HMG-CoA reductase can lead to activation of the UPR [78]. One might speculate that the inhibition of HMG-CoA reductase by farnesol contributes to farnesol-induced ER stress at least in certain cell types.
Although, in lung carcinoma cells the induction of apoptosis by farnesol is related to UPR activation, in other cell types, including T-lymphoblastic leukemia MOLT4 cells, apoptosis is largely independent of the UPR [17]. This was indicated by findings showing that no splicing of XBP1 or phosphorylation of eIF2α was observed. This was supported by gene expression profile analysis which showed that the expression of various chaperone genes was not greatly enhanced. These observations suggest that the farnesol-induced apoptosis in MOLT4 cells occurs via a different mechanism. In contrast, several other pro- and anti-apoptotic genes were highly induced in farnesol-treated MOLT4 cells, including the cation transport regulator-like protein CHAC1. Interestingly, increased expression of this protein has been linked to apoptosis in human aortic endothelial cells [107]. The increased expression of CHAC1 might provide an alternative mechanism that contributes to the induction of apoptosis in farnesol-treated MOLT4 cells.
3.4. Induction of MAP-kinases by farnesol
In addition to c-Jun N-terminal kinase (JNK), treatment of H460 cells with farnesol results in activation of the extracellular signal-regulated kinase (ERK1/2) and mitogen-activated protein kinase (MAPK) p38 [19]. Inhibition of p38 MAPK had little effect on the farnesol-induced UPR; however, activation of the MEK-ERK signaling pathway was found to be an early event and critical in triggering farnesol-induced ER stress [19]. This was indicated by observations showing that inhibition of MEK1/2 by U0126 and knockdown of MEK1/2 expression by siRNA greatly reduced farnesol-induced splicing of XBP1 mRNA, eIF2α phosphorylation, activation of JNK1/2 and several caspases, the induction of several ER stress–related genes, and apoptosis. Although ERK1/2 activation is generally considered a prosurvival signal, it has been implicated in the induction of apoptosis in renal, neuronal, and hepatoma cells under a variety of conditions [108,109].
3.5. Activation of the apoptosome
Many apoptotic stimuli lead to the assembly of the apoptosome, a multisubunit protein complex, containing cytochrome c, apoptotic protease activating factor 1 (Apaf-1), and caspase 9, that serves as a platform for caspase activation [110]. Farnesol induces activation of caspases 3, 6, 7 and 9, but not caspase 8, suggesting that farnesol-induced apoptosis is mediated by the intrinsic, mitochondrial-dependent pathway and does not involve a caspase 8-dependent, extrinsic pathway [17,19,28,37,110]. Although the death receptor TNFRSF10B (DR5 or TRAILR2) is induced in farnesol-treated H460 cells, there is no evidence that this is involved in farnesol-induced apoptosis [15]. In mitochondrial-dependent apoptosis permeabilization of the mitochondrial membrane leads to the release of several pro-apoptotic proteins, including cytochrome c and apoptosis inducing factor (AIF). Interaction of cytochrome c with Apaf-1, triggers oligomerization of Apaf-1, and subsequently binding and activation of ‘initiator’ caspase 9, which then leads to the activation of executioner caspases. The collapse of the mitochondrial potential, release of cytochrome c, and activation of caspase 9 and several executioner caspases in farnesol-treated cells indicate that the induction of apoptosis in these cells is mediated through a mitochondrial-dependent pathway that involves activation of the apoptosome [17,19,28,30,37].
Members of the BCL2 family are critical regulators of the mitochondrial pathway of apoptosis by controlling the permeabilization of the outer mitochondrial membrane and the release of cytochrome c [111]. Induction of apoptosis by farnesol has been reported to be associated with increased expression of the pro-apoptotic protein Bak and a reduction in the anti-apoptotic proteins BCL2 and BCL-XL [28,39]. Exogenous expression of the anti-apoptotic protein BCL2 greatly inhibits the induction of apoptosis in MOLT4 cells by farnesol as indicated by the observed inhibition of the collapse of mitochondrial potential, release of cytochrome c, and caspase activation [17]. These observations are in agreement with the concept that at least in certain cell systems farnesol-induced apoptosis relies on mitochondrial-dependent formation and activation of the apoptosome. Farnesol has also been reported to inhibit the expression of survivin (Birc5a), a member of the inhibitors of apoptosis (IAP) family [28,30] which blocks apoptosis by interacting directly with several caspases [112]. Its down-regulation likely contributes to the pro-apoptotic effects of farnesol.
During ER stress, when cells are unable to re-store homeostasis mitochondrial-dependent and -independent apoptosis pathways can be activated. Ca2+ efflux from the ER into the cytoplasm leads to activation of Ca2+-dependent proteases, such as calpain, that are able to activate caspase-12 (in rodents) and subsequently induce the caspase cascade [98,100–102,104]. In addition, induction of DDIT3/CHOP represses the expression of the anti-apoptotic gene BCL2 thereby further promoting apoptosis while IRE1α by its interaction with TNF receptor-associated factor 2 (TRAF2) leads to apoptosis-signal-regulating kinase 1 (ASK1)-mediated activation of JNK [113,114]. The IRE1-TRAF2-ASK1-JNK cascade is an important pro-apoptotic signaling pathway in ER stress. The induction of DDIT3/CHOP, down-regulation of BCL2 expression, and the activation of JNK1/2 and the caspase cascade in farnesol-treated H460 cells are in agreement with the hypothesis that these UPR-induced events are part of pro-apoptotic signals of farnesol [19,106]. Findings showing that down-regulation of JNK1/2 expression by siRNAs inhibits caspase activation and the induction of apoptosis in farnesol-treated H460 cells are consistent with the concept that activation of IRE1-TRAF2-ASK1-JNK plays a critical role in farnesol-induced apoptosis [19].
3.6. Farnesol activates the NF-κB signaling pathway
Microarray analysis revealed that, in addition to the induction of UPR-related genes, treatment of lung carcinoma H460 cells with farnesol results in a greatly enhanced expression of many nuclear factor-κB (NF-κB) target genes, including IL-1, IL-6, IL-8, CXCL3, and COX-2, suggesting that farnesol treatment activates the NF-κB signaling pathway [106,115]. Such an association is consistent with reports showing a coupling between the UPR and the NF-κB signaling pathway in a number of pathologies [102,116,117] and in cells exposed to ER stress [101,102,106,117–120]
Recent studies have provided mechanistic insights into the molecular mechanisms of this relationship and shown that ER stress triggers several signals that act in synergy to reduce inhibitor of NF-κB (IκB) levels and activate NF-κB signaling [101,117]. Upon activation of the UPR, activated IRE1α binds TRAF2 and recruits IκB kinase (IKK), which subsequently phosphorylates IκB thereby promoting its degradation [99,121]. In addition, PERK-eIF2α-mediated attenuation of the rate of mRNA translation leads to a reduction in IκBα synthesis [122,123]. In H460 cells farnesol was found to induce activation of IRE1α-TRAF2 and PERK-eIF2α suggesting that both pathways are implicated in the observed reduction in IκBα protein and the activation of NF-κB [19,106].
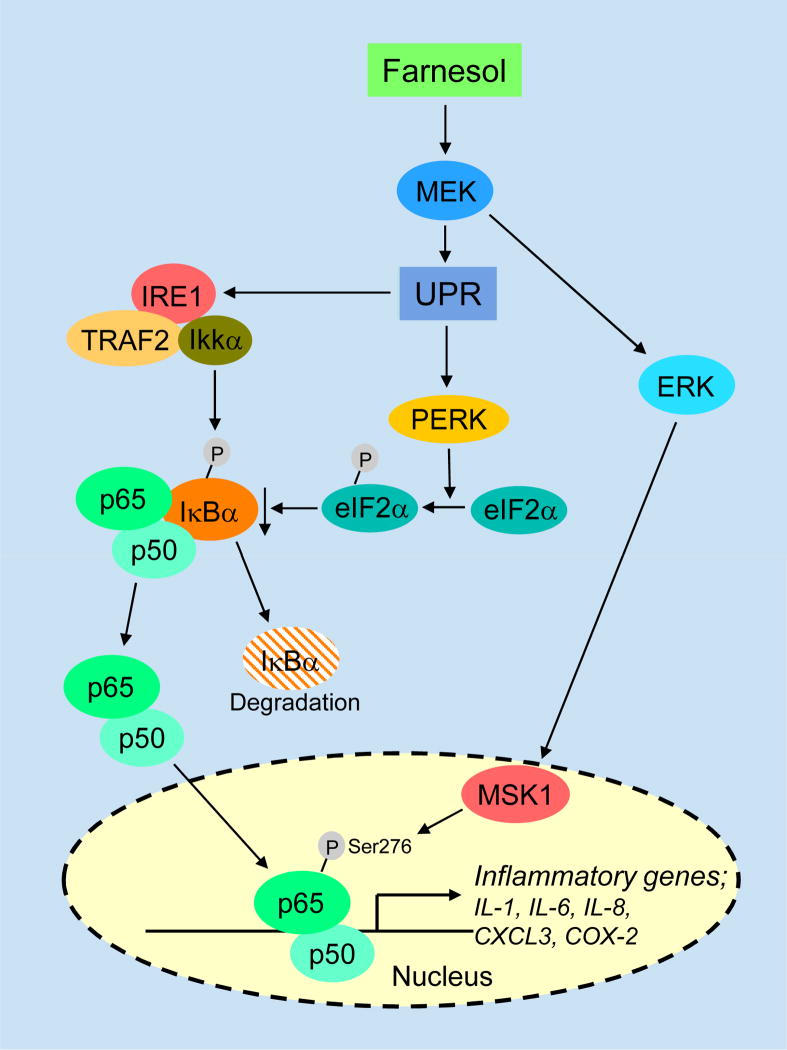
In addition to its nuclear translocation, post-translational modifications, including site-specific phosphorylation, are important for optimal transactivation activity of NF-κB [115,124,125]. Farnesol treatment induced phosphorylation of p65/RelA at Ser276 and Ser536 but only the S276A mutation significantly diminished the induction of NF-κB transactivation by farnesol suggesting that phosphorylation at Ser276 is required for optimal NF-κB activity [106]. Inhibition of MEK1/2 or mitogen- and stress-activated kinase-1 (MSK1), which acts down-stream of MEK1/2 and ERK1/2, by U0126 or H89, respectively, or knockdown of their expression by corresponding siRNAs significantly reduced the phosphorylation of p65 at Ser276 and inhibited the induction of several NF-κB target genes by farnesol. These observations indicated that this phosphorylation is mediated by the MEK1/2- ERK1/2-MSK1 pathway. Thus, MEK1/2-ERK1/2-dependent activation of the PERK-eIF2α and IRE1-TRAF2 signals as well as MEK1/2-ERK1/2-MSK1-dependent phosphorylation of p65/relA act synergistically in farnesol-induced NF-κB activation (Fig. 4). NF-κB activation in response to ER stress likely functions as a prosurvival signal [101,117].
Fig. 4.
Activation of the NF-κB signaling pathway by farnesol is related to ER stress. Upon activation of the UPR, activated IRE1α binds TRAF2 and recruits IKK, which subsequently phosphorylates IκB thereby promoting its degradation, while activation of the PERK-eIF2α pathway leads to attenuation of the rate of mRNA translation, including a reduction in IκBα synthesis. These events act synergistally to reduce IκBα and lead to the exposure of the nuclear localization signal of NF-κB, its translocation to the nucleus, and activation of NF-κB target genes. Activation of the MEK1/2-ERK1/2-MSK1 pathway by farnesol promotes the phosphorylation of p65/relA (Ser276) thereby enhancing NF-κB transcriptional activity.
3.7. Farnesol and generation of reactive oxygen species (ROS)
Farnesol has been reported to increase the level of ROS and to induce growth inhibition and cell death in fungi and yeast, including Aspergillus nidulans, Candida albicans, and Saccharomyces cerevisiae [126–131]. In addition to inhibiting germination in C. albicans, farnesol-induced apoptosis is accompanied by ROS accumulation, mitochondrial degradation, and increased levels of activated caspases [131]. In A. nidulans farnesol causes changes in the expression of several genes, including an apoptosis-inducing factor (AIF)-like mitochondrial oxidoreductase [130]. AIF-defective mutants exhibit an increased sensitivity to farnesol-induced apoptosis, which may be at least in part related to increased generation of ROS. In S. cerevisiae, the induction of cell death by farnesol was inhibited by the addition of antioxidants, such as α-tocopherol and N-acetyl cysteine, suggesting a link between cell death and ROS [126,128]. Studies have provided evidence suggesting a role for mitochondrial generation of oxygen radicals by the Rieske iron-sulfur component of complex III of the electron transport chain in farnesol-induced cell death [126,128]. Recently, a chemogenomic screen identified a number of genes that regulate the sensitivity of yeast to farnesol-induced cell death, including BCK1, encoding a mitogen-activated protein kinase kinase kinase (MAPKKK), and CLA4, encoding a p21-activated kinase [126]. BCK1 is known to be phosphorylated by protein kinase C1 (PKC1) and to phosphorylate the MAPKKs, MKK1 and MKK2. Treatment with farnesol induces relocalization of PKC1, BCK1, and MKK1 to the mitochondria while overexpression of active PKC1, BCK1, or MKK1 increases the resistance to farnesol and H2O2. These results suggest that the PKC pathway is critical in controlling the generation of ROS and farnesol sensitivity.
Whether ROS is a mediator of farnesol-induced cell death in mammalian cells needs to be studied further. Our preliminary results showed that farnesol can increase ROS production in a time- and dose-dependent manner in the human lung carcinoma H460 cells and T-lymphoblastic leukemia MOLT4 cells (J. H. Joo, unpublished data). However, inhibition of ROS generation by butylated hydroxyanisole (BHA), vitamin C, or N-acetyl-L-cysteine had little effect on farnesol-induced apoptosis suggesting that in these cells production of ROS is not a major contributory factor. Recent in vivo studies demonstrated that farnesol can significantly reduce oxidative stress, inflammation, and injury in lungs of rats exposed to intratracheal installation of cigarette smoke extract (CSE) [132]. This inhibition may contribute to the cancer preventative effects of farnesol.
4. Farnesol and nuclear receptors
4.1. Farnesol and the farnesoid X receptor (FXR)
Farnesol has been reported to activate the nuclear receptor, farnesoid X receptor (FXR, NR1H4) [133,134]. FXR is highly expressed in the liver, gut, kidney and adrenal cortex and regulates a variety of genes with roles in bile acid homeostasis, lipid and glucose metabolism [135,136]. FXR is activated by several conjugated and unconjugated bile acids, including lithocholic acid, chenodeoxycholic acid, and deoxycholic acid which bind FXR with high affinity [137–139]. A large variety of endogenous isoprenoids, retinoic acid, and several synthetic retinoids have been reported to weakly to moderately induce FXR activity; however, these agents do not function as direct ligands for FXR. In the case of farnesol, supraphysiological concentrations are required to activate FXR [133].
The role of FXR in growth regulation, apoptosis, and cancer is still controversial. Separate studies have established both positive and reciprocal correlations between FXR expression and cancer [140–144]. FXR agonists have been reported to inhibit aromatase expression and induce apoptosis in mammary carcinoma MCF7 and MDA-MB-468 cells [145] suggesting that FXR activation promotes apoptosis and may have protective effects against tumorigenesis. In contrast, the FXR antagonist guggulsterone promoted apoptosis in Barrett’s esophageal-derived cells in agreement with the concept that activated FXR functions as a negative regulator of apoptosis. Although farnesol is a weak activator of FXR, it is able to inhibit cell proliferation and induce apoptosis in a number cell types that do not express FXR suggesting that FXR activation is not a general mechanism in farnesol-induced apoptosis.
FXR has been recently implicated in the regulation of the proliferation of mammary carcinoma cells by farnesol [146]. Addition of low concentrations of farnesol had a mitogenic effect in estrogen receptor-positive, breast cancer MCF7 cells, but did not affect growth of estrogen-negative MDA-MB-231 cells. Farnesol-stimulated cell growth could be blocked by the addition of antiestrogens suggesting that the mitogenic effect of farnesol is dependent on the activation of the estrogen receptor. Farnesol treatment was found to reduce the level of estrogen receptor protein while enhancing the level of progesterone receptor (PGR) and FXR. Down-regulation of FXR expression by siRNA inhibited the increase in PGR by farnesol. Evidence was provided suggesting that farnesol may promote an interaction between estrogen receptor and FXR; however, the mechanism by which farnesol promotes this interaction and how this relates to growth stimulation has yet to be established.
4.2. Farnesol and PPARs
The peroxisome proliferator-activated receptors (PPARs) α, −β/δ, and −γ are nuclear receptors that are activated by a variety a fatty acids, arachidonic acid metabolites, and hypolipidemic agents [147]. PPARs have multiple functions in the regulation of cell proliferation, differentiation, cell survival, lipid metabolism, and energy homeostasis and have been implicated in several diseases [147–149].
Farnesyl phosphates and trans,trans-farnesol at micromolar concentrations have been shown to weakly, but significantly, activate PPAR-response elements (PPRE)-dependent transcriptional activation by PPARs, while cis,cis isomer of farnesol was inactive [1,28,150–153]. This was supported by data showing that farnesyl phosphates and farnesol significantly upregulated the expression of PPAR target genes, acyl-CoA oxidase (AOX) and carnitine palmitoyl transferase 1a (CPT1a), in rat hepatoma cell line H4IIEC3 and rat hepatocytes in vivo [154]. In addition, when administered to rats, farnesol was able to significantly lower serum triglycerides levels as has been reported for PPAR agonists. Farnesoic acid was found to be more active than farnesol and it was proposed that PPAR activation might involve a metabolite of farnesol. At high concentrations, both farnesol and farnesyl phosphates were able to weakly compete with rosiglitazone for PPARγ binding suggesting that they interact directly with PPARγ [152]. Whether these concentrations are in a range that is physiologically obtainable needs to be determined.
PPARs play an important role in the regulation of epidermal differentiation. For example, activation of PPARα by its respective ligands accelerates the development of the epidermal permeability barrier [155,156]. Farnesol enhances differentiation in normal human epidermal keratinocytes (NHEK) and adult murine epidermis through PPARα [1]. Differentiation of NHEK cells occurs in several stages. Commitment to irreversible growth arrest is an early event that is followed by a step-wise expression of different differentiation-specific genes [157]. Treatment of NHEK cells with farnesol causes inhibition of cell proliferation and induction of several differentiation marker genes, including the crosslinked envelope precursor involucrin and transglutaminase type I, an enzyme catalyzing the crosslinking between different crosslinked envelope proteins [1]. Because FXR expression was undetectable in NHEK, this effect does not involve FXR. Farnesol increased transcriptional activation of a reporter under the control of a 2.2 kb and 3.7 kb promoter region of the transglutaminase and involucrin genes, respectively. Deletion and point mutation analysis identified an AP-1 site in the involucrin promoter that was required for the farnesol-induced increase in promoter activity. This site was previously shown to be important for the induction of involucrin by PPARα activators. Subsequent analysis showed that farnesol was able to enhance PPRE-dependent transcriptional activation by PPARα. These data indicated that farnesol treatment leads to an increase in PPARα-mediated transcriptional activation. The increased activation was related to increased expression of PPARα in NHEK cells by farnesol.
Topical application of farnesol on normal murine skin also enhanced terminal differentiation in the epidermis as indicated by enhanced expression of the differentiation markers loricrin and profilaggrin [1]. This increase was not observed in PPARα−/− mice consistent with the conclusion that farnesol-induced induction of differentiation in epidermal keratinocytes is mediated through the PPARα signaling pathway. The mechanism by which farnesol enhances PPARα mediated transactivation has yet to be determined. It does not appear to involve a direct interaction of farnesol with PPARα. In addition to increased PPARα expression, an enhancement in the generation of endogenous ligands or activation of certain kinases that enhance PPARα activity may contribute to this effect of farnesol on PPARα activation.
4.3. Farnesol and the thyroid hormone receptor β (THRβ)
Treatment with micromolar concentrations of farnesol causes a significant decrease in the growth of MCF-7 cells and increases the expression of the thyroid hormone receptor β1 (THRβ1) at the mRNA and protein level [158]. Unexpectedly, the expression of several genes known to be induced by THRβ activation, including Spot 14 (S14), a nuclear protein involved in the regulation of fatty acid synthesis, glycerol-3-phosphate dehydrogenase (GPD1), and malic enzyme (ME) were down-regulated by farnesol. In addition, expression of the sodium iodide symporter gene (NIS), which previously was shown to be negatively regulated by activated THRs, was significantly enhanced by farnesol treatment [158]. However, in spite of the increased levels of THRβ1 protein in farnesol-treated cells, the binding of THRβ1 to THR-response element was reduced. The latter would result in an inhibition of THRβ mediated transcriptional activation and be consistent with the observed effects of farnesol on THR-mediated gene regulation. The molecular mechanism by which farnesol diminishes THRβ binding activity requires further study. Moreover, whether there is connection between the inhibition of THR-mediated transactivation by farnesol and its inhibition of proliferation of mammary cancer cells and its anti-tumor effects needs to be established. Study of such a link is complicated by the fact that the role of thyroid hormones and its receptors in mammary cancer is still controversial. Thyroid hormones have been reported to enhance the growth of mammary carcinoma cells [159], while THRβ has been reported to act as a potent suppressor of tumor invasiveness and metastasis [160].
5. Summary
The anti-tumor and chemopreventative effects of farnesol appear to involve several different mechanisms that can act at either the initiation or progression stage of tumorigenesis. Reduction of carcinogen-induced DNA strand breaks and of the formation of DNA adducts by farnesol suggests that its anti-tumorigenic effects may be a consequence of an inhibition at the initiation stage of tumorigenesis. Similarly, the protective effect of farnesol against cigarette smoke-induced lung injury and oxidative stress may relate to inhibition of cancer initiation and result in a reduced probability of lung cancer. The inhibition of cell growth and induction of apoptosis may be part of the mechanism by which farnesol exerts it anti-tumor effects at the progression stage. Induction of ER stress and activation of the UPR provides a major mechanism by which farnesol inhibits cell proliferation and promotes apoptosis in certain cell types. The ER stress response was found to depend on the activation of the MEK-ERK signaling pathway. Future studies are needed to determine the mechanism of this activation and how it relates to induction of ER stress. In addition, the rapid translocation of CCTα to the NE, the subsequent expansion of nucleoplasmic reticulum and transient CCTα activation provides an important mechanism through which farnesol mediates its action. The subsequent degradation of CCTα by caspases and its nuclear export result in decreased PC biosynthesis and is coupled to the induction of apoptosis by farnesol. It would be interesting to investigate whether a link exists between these events and the induction of ER stress. Farnesol has also been demonstrated to induce ROS and apoptosis in yeast; however, additional studies are required to establish whether ROS plays a role in the induction of apoptosis in mammalian cells. Alternative mechanisms of action of farnesol involve effects on several nuclear receptor-signaling pathways. Farnesol has a significant effect on epidermal keratinocyte differentiation and promotes growth arrest and differentiation through the activation of the PPARα signaling pathway. Farnesol treatment also induces activation of the NF-κB pathway and expression of inflammatory genes as part of the UPR. Although, farnesol inhibits cell growth and induces apoptosis in many cultured cells and have been reported to exhibit chemopreventative and anti-tumor effects in animal models, further studies are needed to determine whether it will be effective as a chemopreventative and therapeutic agent in human cancer.
Acknowledgments
The authors would like to thank Drs. Carl Bortner and Gary Zeruth (NIEHS) for their comments on the manuscript. This research was supported by the Intramural Research Program of the NIEHS, NIH (Z01-ES-101586).
Footnotes
Conflicts of interest statement
None declared.
References
- 1.Hanley K, Komuves LG, Ng DC, Schoonjans K, He SS, Lau P, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. Farnesol stimulates differentiation in epidermal keratinocytes via PPARalpha. J Biol Chem. 2000;275:11484–11491. doi: 10.1074/jbc.275.15.11484. [DOI] [PubMed] [Google Scholar]
- 2.Rioja A, Pizzey AR, Marson CM, Thomas NS. Preferential induction of apoptosis of leukaemic cells by farnesol. FEBS Lett. 2000;467:291–295. doi: 10.1016/s0014-5793(00)01168-6. [DOI] [PubMed] [Google Scholar]
- 3.Bifulco M. Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005;77:1740–1749. doi: 10.1016/j.lfs.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Miquel K, Pradines A, Favre G. Farnesol and geranylgeraniol induce actin cytoskeleton disorganization and apoptosis in A549 lung adenocarcinoma cells. Biochem Biophys Res Commun. 1996;225:869–876. doi: 10.1006/bbrc.1996.1265. [DOI] [PubMed] [Google Scholar]
- 5.Wright MM, Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. J Biol Chem. 2001;276:25254–25261. doi: 10.1074/jbc.M011552200. [DOI] [PubMed] [Google Scholar]
- 6.McTaggart SJ. Isoprenylated proteins. Cell Mol Life Sci. 2006;63:255–267. doi: 10.1007/s00018-005-5298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong TP, Heidor R, de Conti A, Dagli ML, Moreno FS. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis. 2006;27:1194–1203. doi: 10.1093/carcin/bgi291. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 9.Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- 10.Roskoski R., Jr Protein prenylation: a pivotal posttranslational process. Biochem Biophys Res Commun. 2003;303:1–7. doi: 10.1016/s0006-291x(03)00323-1. [DOI] [PubMed] [Google Scholar]
- 11.Burke YD, Stark MJ, Roach SL, Sen SE, Crowell PL. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids. 1997;32:151–156. doi: 10.1007/s11745-997-0019-y. [DOI] [PubMed] [Google Scholar]
- 12.Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- 13.McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adany I, Yazlovitskaya EM, Haug JS, Voziyan PA, Melnykovych G. Differences in sensitivity to farnesol toxicity between neoplastically- and non-neoplastically-derived cells in culture. Cancer Lett. 1994;79:175–179. doi: 10.1016/0304-3835(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 15.Yazlovitskaya EM, Melnykovych G. Selective farnesol toxicity and translocation of protein kinase C in neoplastic HeLa-S3K and non-neoplastic CF-3 cells. Cancer Lett. 1995;88:179–183. doi: 10.1016/0304-3835(94)03635-v. [DOI] [PubMed] [Google Scholar]
- 16.Melnykovych G, Haug JS, Goldner CM. Growth inhibition of leukemia cell line CEM-C1 by farnesol: effects of phosphatidylcholine and diacylglycerol. Biochem Biophys Res Commun. 1992;186:543–548. doi: 10.1016/s0006-291x(05)80842-3. [DOI] [PubMed] [Google Scholar]
- 17.Joo JH, Bortner C, Jetten AM. Induction of apoptosis in T-lymphoblastic leukemia MOLT4 cells by farnesol. Unpublished observations. [Google Scholar]
- 18.Wiseman DA, Werner SR, Crowell PL. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21(Cip1) and p27(Kip1) in human pancreatic adenocarcinoma cells. J Pharmacol Exp Ther. 2007;320:1163–1170. doi: 10.1124/jpet.106.111666. [DOI] [PubMed] [Google Scholar]
- 19.Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 2007;67:7929–7936. doi: 10.1158/0008-5472.CAN-07-0931. [DOI] [PubMed] [Google Scholar]
- 20.Miquel K, Pradines A, Terce F, Selmi S, Favre G. Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma A549 cells. J Biol Chem. 1998;273:26179–26186. doi: 10.1074/jbc.273.40.26179. [DOI] [PubMed] [Google Scholar]
- 21.He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668–674. doi: 10.1093/jn/127.5.668. [DOI] [PubMed] [Google Scholar]
- 22.Xu M, Floyd HS, Greth SM, Chang WC, Lohman K, Stoyanova R, Kucera GL, Kute TE, Willingham MC, Miller MS. Perillyl alcohol-mediated inhibition of lung cancer cell line proliferation: potential mechanisms for its chemotherapeutic effects. Toxicol Appl Pharmacol. 2004;195:232–246. doi: 10.1016/j.taap.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Yu SG, Hildebrandt LA, Elson CE. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J Nutr. 1995;125:2763–2767. doi: 10.1093/jn/125.11.2763. [DOI] [PubMed] [Google Scholar]
- 24.Sahin MB, Perman SM, Jenkins G, Clark SS. Perillyl alcohol selectively induces G0/G1 arrest and apoptosis in Bcr/Abl-transformed myeloid cell lines. Leukemia. 1999;13:1581–1591. doi: 10.1038/sj.leu.2401536. [DOI] [PubMed] [Google Scholar]
- 25.Elegbede JA, Flores R, Wang RC. Perillyl alcohol and perillaldehyde induced cell cycle arrest and cell death in BroTo and A549 cells cultured in vitro. Life Sci. 2003;73:2831–2840. doi: 10.1016/s0024-3205(03)00701-x. [DOI] [PubMed] [Google Scholar]
- 26.Shi W, Gould MN. Induction of cytostasis in mammary carcinoma cells treated with the anticancer agent perillyl alcohol. Carcinogenesis. 2002;23:131–142. doi: 10.1093/carcin/23.1.131. [DOI] [PubMed] [Google Scholar]
- 27.Rajesh D, Howard SP. Perillyl alcohol mediated radiosensitization via augmentation of the Fas pathway in prostate cancer cells. Prostate. 2003;57:14–23. doi: 10.1002/pros.10269. [DOI] [PubMed] [Google Scholar]
- 28.Au-Yeung KK, Liu PL, Chan C, Wu WY, Lee SS, Ko JK. Herbal isoprenols induce apoptosis in human colon cancer cells through transcriptional activation of PPARgamma. Cancer Invest. 2008;26:708–717. doi: 10.1080/07357900801898656. [DOI] [PubMed] [Google Scholar]
- 29.Duncan RE, Lau D, El-Sohemy A, Archer MC. Geraniol and beta-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem Pharmacol. 2004;68:1739–1747. doi: 10.1016/j.bcp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Scheper MA, Shirtliff ME, Meiller TF, Peters BM, Jabra-Rizk MA. Farnesol, a Fungal Quorum-Sensing Molecule Triggers Apoptosis in Human Oral Squamous Carcinoma Cells. Neoplasia. 2008;10:954–963. doi: 10.1593/neo.08444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Reviews Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 32.Yeruva L, Pierre KJ, Elegbede A, Wang RC, Carper SW. Perillyl alcohol and perillic acid induced cell cycle arrest and apoptosis in non small cell lung cancer cells. Cancer Lett. 2007;257:216–226. doi: 10.1016/j.canlet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Greider C, Chattopadhyay A, Parkhurst C, Yang E. BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene. 2002;21:7765–7775. doi: 10.1038/sj.onc.1205928. [DOI] [PubMed] [Google Scholar]
- 34.Janumyan Y, Cui Q, Yan L, Sansam CG, Valentin M, Yang E. G0 function of BCL2 and BCL-xL requires BAX, BAK, and p27 phosphorylation by Mirk, revealing a novel role of BAX and BAK in quiescence regulation. J Biol Chem. 2008;283:34108–34120. doi: 10.1074/jbc.M806294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor MM, Macdonald K, Morris AJ, McMaster CR. Enhanced apoptosis through farnesol inhibition of phospholipase D signal transduction. Febs J. 2005;272:5056–5063. doi: 10.1111/j.1742-4658.2005.04914.x. [DOI] [PubMed] [Google Scholar]
- 36.Ariazi EA, Satomi Y, Ellis MJ, Haag JD, Shi W, Sattler CA, Gould MN. Activation of the transforming growth factor beta signaling pathway and induction of cytostasis and apoptosis in mammary carcinomas treated with the anticancer agent perillyl alcohol. Cancer Res. 1999;59:1917–1928. [PubMed] [Google Scholar]
- 37.Lagace TA, Miller JR, Ridgway ND. Caspase processing and nuclear export of CTP:phosphocholine cytidylyltransferase alpha during farnesol-induced apoptosis. Mol Cell Biol. 2002;22:4851–4862. doi: 10.1128/MCB.22.13.4851-4862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagace TA, Ridgway ND. Induction of apoptosis by lipophilic activators of CTP:phosphocholine cytidylyltransferase alpha (CCTalpha) Biochem J. 2005;392:449–456. doi: 10.1042/BJ20051021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke YD, Ayoubi AS, Werner SR, McFarland BC, Heilman DK, Ruggeri BA, Crowell PL. Effects of the isoprenoids perillyl alcohol and farnesol on apoptosis biomarkers in pancreatic cancer chemoprevention. Anticancer Res. 2002;22:3127–3134. [PubMed] [Google Scholar]
- 40.Clark SS, Perman SM, Sahin MB, Jenkins GJ, Elegbede JA. Antileukemia activity of perillyl alcohol (POH): uncoupling apoptosis from G0/G1 arrest suggests that the primary effect of POH on Bcr/Abl-transformed cells is to induce growth arrest. Leukemia. 2002;16:213–222. doi: 10.1038/sj.leu.2402369. [DOI] [PubMed] [Google Scholar]
- 41.Unlu S, Mason CD, Schachter M, Hughes AD. Perillyl alcohol, an inhibitor of geranylgeranyl transferase, induces apoptosis of immortalized human vascular smooth muscle cells in vitro. J Cardiovasc Pharmacol. 2000;35:341–344. doi: 10.1097/00005344-200002000-00025. [DOI] [PubMed] [Google Scholar]
- 42.Ohizumi H, Masuda Y, Nakajo S, Sakai I, Ohsawa S, Nakaya K. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J Biochem (Tokyo) 1995;117:11–13. doi: 10.1093/oxfordjournals.jbchem.a124695. [DOI] [PubMed] [Google Scholar]
- 43.Stark WW, Jr, Blaskovich MA, Johnson BA, Qian Y, Vasudevan A, Pitt B, Hamilton AD, Sebti SM, Davies P. Inhibiting geranylgeranylation blocks growth and promotes apoptosis in pulmonary vascular smooth muscle cells. Am J Physiol. 1998;275:L55–63. doi: 10.1152/ajplung.1998.275.1.L55. [DOI] [PubMed] [Google Scholar]
- 44.Carnesecchi S, Schneider Y, Ceraline J, Duranton B, Gosse F, Seiler N, Raul F. Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Ther. 2001;298:197–200. [PubMed] [Google Scholar]
- 45.Haag JD, Gould MN. Mammary carcinoma regression induced by perillyl alcohol, a hydroxylated analog of limonene. Cancer Chemother Pharmacol. 1994;34:477–483. doi: 10.1007/BF00685658. [DOI] [PubMed] [Google Scholar]
- 46.Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;55:979–983. [PubMed] [Google Scholar]
- 47.Stayrook KR, McKinzie JH, Burke YD, Burke YA, Crowell PL. Induction of the apoptosis-promoting protein Bak by perillyl alcohol in pancreatic ductal adenocarcinoma relative to untransformed ductal epithelial cells. Carcinogenesis. 1997;18:1655–1658. doi: 10.1093/carcin/18.8.1655. [DOI] [PubMed] [Google Scholar]
- 48.Belanger JT. Perillyl alcohol: applications in oncology. Altern Med Rev. 1998;3:448–457. [PubMed] [Google Scholar]
- 49.Staines AG, Sindelar P, Coughtrie MW, Burchell B. Farnesol is glucuronidated in human liver, kidney and intestine in vitro, and is a novel substrate for UGT2B7 and UGT1A1. Biochem J. 2004;384:637–645. doi: 10.1042/BJ20040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horn TL, Long L, Cwik MJ, Morrissey RL, Kapetanovic IM, McCormick DL. Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chem Biol Interact. 2005;152:79–99. doi: 10.1016/j.cbi.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Jahangir T, Khan TH, Prasad L, Sultana S. Farnesol prevents Fe-NTA-mediated renal oxidative stress and early tumour promotion markers in rats. Hum Exp Toxicol. 2006;25:235–242. doi: 10.1191/0960327106ht616oa. [DOI] [PubMed] [Google Scholar]
- 52.Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect Prev. 2002;26:419–425. doi: 10.1016/s0361-090x(02)00119-8. [DOI] [PubMed] [Google Scholar]
- 53.Wargovich MJ, Jimenez A, McKee K, Steele VE, Velasco M, Woods J, Price R, Gray K, Kelloff GJ. Efficacy of potential chemopreventive agents on rat colon aberrant crypt formation and progression. Carcinogenesis. 2000;21:1149–1155. [PubMed] [Google Scholar]
- 54.Jahangir T, Sultana S. Benzo(a)pyrene-induced genotoxicity: attenuation by farnesol in a mouse model. J Enzyme Inhib Med Chem. 2008;23:888–894. doi: 10.1080/14756360701448768. [DOI] [PubMed] [Google Scholar]
- 55.Ripple GH, Gould MN, Stewart JA, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Pomplun M, Wilding G, Bailey HH. Phase I clinical trial of perillyl alcohol administered daily. Clin Cancer Res. 1998;4:1159–1164. [PubMed] [Google Scholar]
- 56.Ripple GH, Gould MN, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Binger K, Tutsch KD, Pomplun M, Wahamaki A, Marnocha R, Wilding G, Bailey HH. Phase I clinical and pharmacokinetic study of perillyl alcohol administered four times a day. Clin Cancer Res. 2000;6:390–396. [PubMed] [Google Scholar]
- 57.Bailey HH, Wilding G, Tutsch KD, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Marnocha R, Holstein SA, Stewart J, Lewis KA, Hohl RJ. A phase I trial of perillyl alcohol administered four times daily for 14 days out of 28 days. Cancer Chemother Pharmacol. 2004;54:368–376. doi: 10.1007/s00280-004-0788-z. [DOI] [PubMed] [Google Scholar]
- 58.Hudes GR, Szarka CE, Adams A, Ranganathan S, McCauley RA, Weiner LM, Langer CJ, Litwin S, Yeslow G, Halberr T, Qian M, Gallo JM. Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in patients with refractory solid malignancies. Clin Cancer Res. 2000;6:3071–3080. [PubMed] [Google Scholar]
- 59.Morgan-Meadows S, Dubey S, Gould M, Tutsch K, Marnocha R, Arzoomanin R, Alberti D, Binger K, Feierabend C, Volkman J, Ellingen S, Black S, Pomplun M, Wilding G, Bailey H. Phase I trial of perillyl alcohol administered four times daily continuously. Cancer Chemother Pharmacol. 2003;52:361–366. doi: 10.1007/s00280-003-0684-y. [DOI] [PubMed] [Google Scholar]
- 60.Azzoli CG, Miller VA, Ng KK, Krug LM, Spriggs DR, Tong WP, Riedel ER, Kris MG. A phase I trial of perillyl alcohol in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2003;51:493–498. doi: 10.1007/s00280-003-0599-7. [DOI] [PubMed] [Google Scholar]
- 61.Liu G, Oettel K, Bailey H, Ummersen LV, Tutsch K, Staab MJ, Horvath D, Alberti D, Arzoomanian R, Rezazadeh H, McGovern J, Robinson E, DeMets D, Wilding G. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Invest New Drugs. 2003;21:367–372. doi: 10.1023/a:1025437115182. [DOI] [PubMed] [Google Scholar]
- 62.Bailey HH, Levy D, Harris LS, Schink JC, Foss F, Beatty P, Wadler S. A phase II trial of daily perillyl alcohol in patients with advanced ovarian cancer: Eastern Cooperative Oncology Group Study E2E96. Gynecol Oncol. 2002;85:464–468. doi: 10.1006/gyno.2002.6647. [DOI] [PubMed] [Google Scholar]
- 63.Bailey HH, Attia S, Love RR, Fass T, Chappell R, Tutsch K, Harris L, Jumonville A, Hansen R, Shapiro GR, Stewart JA. Phase II trial of daily oral perillyl alcohol (NSC 641066) in treatment-refractory metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62:149–157. doi: 10.1007/s00280-007-0585-6. [DOI] [PubMed] [Google Scholar]
- 64.Stearns V, Coop A, Singh B, Gallagher A, Yamauchi H, Lieberman R, Pennanen M, Trock B, Hayes DF, Ellis MJ. A pilot surrogate end point biomarker trial of perillyl alcohol in breast neoplasia. Clin Cancer Res. 2004;10:7583–7591. doi: 10.1158/1078-0432.CCR-04-0295. [DOI] [PubMed] [Google Scholar]
- 65.Meadows SM, Mulkerin D, Berlin J, Bailey H, Kolesar J, Warren D, Thomas JP. Phase II trial of perillyl alcohol in patients with metastatic colorectal cancer. Int J Gastrointest Cancer. 2002;32:125–128. doi: 10.1385/IJGC:32:2-3:125. [DOI] [PubMed] [Google Scholar]
- 66.Schroepfer GJ., Jr Sterol biosynthesis. Annu Rev Biochem. 1981;50:585–621. doi: 10.1146/annurev.bi.50.070181.003101. [DOI] [PubMed] [Google Scholar]
- 67.Nakanishi M, Goldstein JL, Brown MS. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J Biol Chem. 1988;263:8929–8937. [PubMed] [Google Scholar]
- 68.Correll CC, Ng L, Edwards PA. Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3- methylglutaryl-coenzyme A reductase. J Biol Chem. 1994;269:17390–17393. [PubMed] [Google Scholar]
- 69.Meigs TE, Roseman DS, Simoni RD. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J Biol Chem. 1996;271:7916–7922. doi: 10.1074/jbc.271.14.7916. [DOI] [PubMed] [Google Scholar]
- 70.Meigs TE, Simoni RD. Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesyl pyrophosphatase. Arch Biochem Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 71.Brown AJ. Cholesterol, statins and cancer. Clin Exp Pharmacol Physiol. 2007;34:135–141. doi: 10.1111/j.1440-1681.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- 72.Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11–19. doi: 10.1016/j.canlet.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 73.Fritz G. HMG-CoA reductase inhibitors (statins) as anticancer drugs (review) Int J Oncol. 2005;27:1401–1409. [PubMed] [Google Scholar]
- 74.Houten SM, Frenkel J, Waterham HR. Isoprenoid biosynthesis in hereditary periodic fever syndromes and inflammation. Cell Mol Life Sci. 2003;60:1118–1134. doi: 10.1007/s00018-003-2296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 2004;229:567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- 76.Elson CE, Peffley DM, Hentosh P, Mo H. Isoprenoid-mediated inhibition of mevalonate synthesis: potential application to cancer. Proc Soc Exp Biol Med. 1999;221:294–311. doi: 10.1046/j.1525-1373.1999.d01-87.x. [DOI] [PubMed] [Google Scholar]
- 77.Sebti SM, Hamilton AD. Farnesyltransferase and geranylgeranyltransferase I inhibitors and cancer therapy: lessons from mechanism and bench-to-bedside translational studies. Oncogene. 2000;19:6584–6593. doi: 10.1038/sj.onc.1204146. [DOI] [PubMed] [Google Scholar]
- 78.Chen JC, Wu ML, Huang KC, Lin WW. HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc Res. 2008;80:138–150. doi: 10.1093/cvr/cvn160. [DOI] [PubMed] [Google Scholar]
- 79.Anthony ML, Zhao M, Brindle KM. Inhibition of phosphatidylcholine biosynthesis following induction of apoptosis in HL-60 cells. J Biol Chem. 1999;274:19686–19692. doi: 10.1074/jbc.274.28.19686. [DOI] [PubMed] [Google Scholar]
- 80.Voziyan PA, Goldner CM, Melnykovych G. Farnesol inhibits phosphatidylcholine biosynthesis in cultured cells by decreasing cholinephosphotransferase activity. Biochem J. 1993;295:757–762. doi: 10.1042/bj2950757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sciorra VA, Morris AJ. Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim Biophys Acta. 2002;1582:45–51. doi: 10.1016/s1388-1981(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 82.Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. doi: 10.1139/o03-073. [DOI] [PubMed] [Google Scholar]
- 83.Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Res. 2003;1:789–800. [PubMed] [Google Scholar]
- 84.Newton AC. Diacylglycerol’s affair with protein kinase C turns 25. Trends Pharmacol Sci. 2004;25:175–177. doi: 10.1016/j.tips.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta. 2002;1585:87–96. doi: 10.1016/s1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 86.Jackowski S, Fagone P. CTP: Phosphocholine cytidylyltransferase: paving the way from gene to membrane. J Biol Chem. 2005;280:853–856. doi: 10.1074/jbc.R400031200. [DOI] [PubMed] [Google Scholar]
- 87.Cui Z, Houweling M, Chen MH, Record M, Chap H, Vance DE, Terce F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J Biol Chem. 1996;271:14668–14671. doi: 10.1074/jbc.271.25.14668. [DOI] [PubMed] [Google Scholar]
- 88.Clement JM, Kent C. CTP:phosphocholine cytidylyltransferase: insights into regulatory mechanisms and novel functions. Biochem Biophys Res Commun. 1999;257:643–650. doi: 10.1006/bbrc.1999.0512. [DOI] [PubMed] [Google Scholar]
- 89.Wang L, Magdaleno S, Tabas I, Jackowski S. Early embryonic lethality in mice with targeted deletion of the CTP:phosphocholine cytidylyltransferase alpha gene (Pcyt1a) Mol Cell Biol. 2005;25:3357–3363. doi: 10.1128/MCB.25.8.3357-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y, Sweitzer TD, Weinhold PA, Kent C. Nuclear localization of soluble CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1993;268:5899–5904. [PubMed] [Google Scholar]
- 92.Gehrig K, Cornell RB, Ridgway ND. Expansion of the nucleoplasmic reticulum requires the coordinated activity of lamins and CTP:phosphocholine cytidylyltransferase alpha. Mol Biol Cell. 2008;19:237–247. doi: 10.1091/mbc.E07-02-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gehrig K, Morton CC, Ridgway ND. Nuclear export of the rate-limiting enzyme in phosphatidylcholine synthesis is mediated by its membrane binding domain. J Lipid Res. 2008 doi: 10.1194/jlr.M800632-JLR200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cornell RB, Northwood IC. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem Sci. 2000;25:441–447. doi: 10.1016/s0968-0004(00)01625-x. [DOI] [PubMed] [Google Scholar]
- 95.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell. 2002;13:3148–3161. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Kent C. Effects of altered phosphorylation sites on the properties of CTP:phosphocholine cytidylyltransferase. J Biol Chem. 1995;270:17843–17849. doi: 10.1074/jbc.270.30.17843. [DOI] [PubMed] [Google Scholar]
- 97.Lagace TA, Ridgway ND. The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum. Mol Cell Biol. 2005;16:1120–1130. doi: 10.1091/mbc.E04-10-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 99.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 102.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature Rev Drug Discovery. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 103.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nature Rev Mol Cell Biol. 2008:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 105.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 106.Joo JH, Jetten AM. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. J Biol Chem. 2008;283:16391–16399. doi: 10.1074/jbc.M800945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J Immunol. 2009;182:466–476. doi: 10.4049/jimmunol.182.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arai K, Lee SR, van Leyen K, Kurose H, Lo EH. Involvement of ERK MAP kinase in endoplasmic reticulum stress in SH-SY5Y human neuroblastoma cells. J Neurochem. 2004;89:232–239. doi: 10.1111/j.1471-4159.2004.02317.x. [DOI] [PubMed] [Google Scholar]
- 109.Zhuang S, Schnellmann RG. A death-promoting role for extracellular signal-regulated kinase. J Pharmacol Exp Ther. 2006;319:991–997. doi: 10.1124/jpet.106.107367. [DOI] [PubMed] [Google Scholar]
- 110.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nature Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 111.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nature Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 112.Li F, Brattain MG. Role of the Survivin gene in pathophysiology. Am J Pathol. 2006;169:1–11. doi: 10.2353/ajpath.2006.060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 114.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 115.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 116.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 117.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 119.Yoshida H. ER stress and diseases. Febs J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 121.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 124.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 125.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fairn GD, Macdonald K, McMaster CR. A chemogenomic screen in Saccharomyces cerevisiae uncovers a primary role for the mitochondria in farnesol toxicity and its regulation by the Pkc1 pathway. J Biol Chem. 2007;282:4868–4874. doi: 10.1074/jbc.M610575200. [DOI] [PubMed] [Google Scholar]
- 127.Machida K, Tanaka T. Farnesol-induced generation of reactive oxygen species dependent on mitochondrial transmembrane potential hyperpolarization mediated by F(0)F(1)-ATPase in yeast. FEBS Lett. 1999;462:108–112. doi: 10.1016/s0014-5793(99)01506-9. [DOI] [PubMed] [Google Scholar]
- 128.Machida K, Tanaka T, Fujita K, Taniguchi M. Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. J Bacteriol. 1998;180:4460–4465. doi: 10.1128/jb.180.17.4460-4465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Machida K, Tanaka T, Yano Y, Otani S, Taniguchi M. Farnesol-induced growth inhibition in Saccharomyces cerevisiae by a cell cycle mechanism. Microbiology. 1999;145:293–299. doi: 10.1099/13500872-145-2-293. [DOI] [PubMed] [Google Scholar]
- 130.Savoldi M, Malavazi I, Soriani FM, Capellaro JL, Kitamoto K, da Silva Ferreira ME, Goldman MH, Goldman GH. Farnesol induces the transcriptional accumulation of the Aspergillus nidulans Apoptosis-Inducing Factor (AIF)-like mitochondrial oxidoreductase. Mol Microbiol. 2008;70:44–59. doi: 10.1111/j.1365-2958.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- 131.Shirtlif fME, Krom BP, Meijering RA, Peters BM, Zhu J, Scheper MA, Harris ML, Jabra-Rizk MA. Farnesol-Induced Apoptosis in Candida albicans, Antimicrob. Agents Chemother. 2009 doi: 10.1128/AAC.01551-08. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qamar W, Sultana S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: An initial step in lung chemoprevention. Chem Biol Interact. 2008;176:79–87. doi: 10.1016/j.cbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 133.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 134.Seol W, Choi HS, Moore DD. Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 135.Laffitte BA, Kast HR, Nguyen CM, Zavacki AM, Moore DD, Edwards PA. Identification of the DNA binding specificity and potential target genes for the farnesoid X-activated receptor. J Biol Chem. 2000;275:10638–10647. doi: 10.1074/jbc.275.14.10638. [DOI] [PubMed] [Google Scholar]
- 136.Wang YD, Chen WD, Moore DD, Huang W. FXR: a metabolic regulator and cell protector. Cell Res. 2008;18:1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 137.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 138.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 139.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 140.Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589–9594. doi: 10.1158/0008-5472.CAN-08-1791. [DOI] [PubMed] [Google Scholar]
- 141.Maran RR, Thomas A, Roth M, Shen Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez F, Guo GL. FXR Deficiency in Mice Leads to Increased Intestinal Epithelial Cell Proliferation and Tumor Development. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.145409. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, Rubbia-Brandt L, Frossard JL, Dinjens WN, Rabinovitch PS, Hadengue A. Expression of the bile acid receptor FXR in Barrett’s esophagus and enhancement of apoptosis by guggulsterone in vitro. Molecular Cancer. 2006;5:48. doi: 10.1186/1476-4598-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Journe F, Durbecq V, Chaboteaux C, Rouas G, Laurent G, Nonclercq D, Sotiriou C, Body JJ, Larsimont D. Association between farnesoid X receptor expression and cell proliferation in estrogen receptor-positive luminal-like breast cancer from postmenopausal patients. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0094-2. In Press. [DOI] [PubMed] [Google Scholar]
- 145.Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, Bishop-Bailey D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res. 2006;66:10120–10126. doi: 10.1158/0008-5472.CAN-06-2399. [DOI] [PubMed] [Google Scholar]
- 146.Journe F, Laurent G, Chaboteaux C, Nonclercq D, Durbecq V, Larsimont D, Body JJ. Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. Breast Cancer Res Treat. 2008;107:49–61. doi: 10.1007/s10549-007-9535-6. [DOI] [PubMed] [Google Scholar]
- 147.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86:465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 148.Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 149.Kostadinova R, Wahli W, Michalik L. PPARs in diseases: control mechanisms of inflammation. Curr Med Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 150.O’Brien ML, Rangwala SM, Henry KW, Weinberger C, Crick DC, Waechter CJ, Feller DR, Noonan DJ. Convergence of three steroid receptor pathways in the mediation of nongenotoxic hepatocarcinogenesis. Carcinogenesis. 1996;17:185–190. doi: 10.1093/carcin/17.2.185. [DOI] [PubMed] [Google Scholar]
- 151.Inoue I, Itoh F, Aoyagi S, Tazawa S, Kusama H, Akahane M, Mastunaga T, Hayashi K, Awata T, Komoda T, Katayama S. Fibrate and statin synergistically increase the transcriptional activities of PPARalpha/RXRalpha and decrease the transactivation of NFkappaB. Biochem Biophys Res Commun. 2002;290:131–139. doi: 10.1006/bbrc.2001.6141. [DOI] [PubMed] [Google Scholar]
- 152.Liliom K, Tsukahara T, Tsukahara R, Zelman-Femiak M, Swiezewska E, Tigyi G. Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARs. Biochim Biophys Acta. 2006;1761:1506–1514. doi: 10.1016/j.bbalip.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takahashi N, Kawada T, Goto T, Yamamoto T, Taimatsu A, Matsui N, Kimura K, Saito M, Hosokawa M, Miyashita K, Fushiki T. Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;514:315–322. doi: 10.1016/s0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- 154.Duncan RE, Archer MC. Farnesol decreases serum triglycerides in rats: identification of mechanisms including up-regulation of PPARalpha and down-regulation of fatty acid synthase in hepatocytes. Lipids. 2008;43:619–627. doi: 10.1007/s11745-008-3192-3. [DOI] [PubMed] [Google Scholar]
- 155.Hanley K, Jiang Y, Crumrine D, Bass NM, Appel R, Elias PM, Williams ML, Feingold KR. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J Clin Invest. 1997;100:705–712. doi: 10.1172/JCI119583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanley K, Komuves LG, Bass NM, He SS, Jiang Y, Crumrine D, Appel R, Friedman M, Bettencourt J, Min K, Elias PM, Williams ML, Feingold KR. Fetal epidermal differentiation and barrier development In vivo is accelerated by nuclear hormone receptor activators. J Invest Dermatol. 1999;113:788–795. doi: 10.1046/j.1523-1747.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- 157.Jetten AM, Harvat BL. Epidermal differentiation and squamous metaplasia: from stem cell to cell death. J Dermatol. 1997;24:711–725. doi: 10.1111/j.1346-8138.1997.tb02523.x. [DOI] [PubMed] [Google Scholar]
- 158.Duncan RE, Archer MC. Farnesol induces thyroid hormone receptor (THR) beta1 but inhibits THR-mediated signaling in MCF-7 human breast cancer cells. Biochem Biophys Res Commun. 2006;343:239–243. doi: 10.1016/j.bbrc.2006.02.145. [DOI] [PubMed] [Google Scholar]
- 159.Hall LC, Salazar EP, Kane SR, Liu N. Effects of thyroid hormones on human breast cancer cell proliferation. J Steroid Biochem Mol Biol. 2008;109:57–66. doi: 10.1016/j.jsbmb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 160.Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]