Abstract
Abnormal systemic concentrations of proinflammatory cytokines/chemokines have been implicated in the development of long-term cardiovascular complications in type 1 diabetes (T1DM) and obesity. Whether leukocyte (WBC) gene expression of these proinflammatory mediators contributes to their increased systemic levels, however, remains unclear, especially in the pediatric patient populations. This study examines mRNA changes of 9 cytokines and chemokines in WBCs following ex vivo immunostimulation from 9 T1DM (13.4±0.5 yr, 4F/5M), 23 overweight (OW, 12.3±0.5 yr, 10F/13M, BMI% 97.1±0.5 and >90.0), and 21 healthy (CL, 13.8±0.7 yr, 9F/12M, BMI% 59.6±4.6 and <85.0) children. All subjects had been maintained in euglycemic conditions for at least 90 min before blood draws. Whole blood was then sampled and incubated with anti-T-cell receptor (TCR) antibody or heat-aggregated IgG (HAG) to stimulate T-cell and Fc receptors, respectively. After lysis of leukocytes, mRNA levels of 6 TNF superfamily cytokines (TNFSF2, 5, 6, 7, 9, 14) and 3 chemokines (CCL8, 20, and CXCL10) were measured using RT-PCR. Following TCR stimulation, T1DM displayed significantly greater mRNA responses than CL for TNFSF5, 7, 9, and CCL8, and CXCL10; TNFSF9, CCL8, and CXCL10 were also significantly higher in T1DM than OW; no difference was observed between OW and CL. Fc receptor (FcR) stimulation induced similar responses across groups. Therefore, leukocytes of T1DM children displayed exaggerated gene expression in response to ex vivo TCR induction of 5 key proinflammatory cytokines/chemokines. This elevated leukocyte gene expression may be one of the pathophysiological contributors to the development of vascular complications in T1DM.
Keywords: ex vivo, mRNA, cytokine, chemokine, type 1 diabetes, obesity
INTRODUCTION
Type 1 diabetes mellitus (T1DM) and obesity are major pediatric pathologies, increasing the risk of future cardiovascular morbidity and mortality through high incidences of micro- and macro-vascular complications [1],[2],[3],[4],[5]. While the complex link between diabetes, obesity, and cardiovascular complications is still mostly unclear, it is well established that altered inflammatory processes play a critical role [2],[6]. Acutely and/or chronically elevated pro-/anti-inflammatory mediators have been systematically associated with both diabetes and obesity [7],[8], correlating with the magnitude and duration of hyperglycemia and/or hyperlipidemia [9],[10],[11]. An exaggerated proinflammatory status leads to amplification of leukocyte (WBC) cytokine secretion, recruitment of additional immune cells, and enhanced WBC damage to contiguous tissues, the endothelium, and other components of the vascular wall [6],[8].
The biochemical details of altered WBC function in diabetes and obesity, however, remain nebulous, including lack of basic information on which WBC subtypes are functionally dysregulated, and whether WBC gene expression and secretion of inflammatory mediators is altered. In T1DM, for instance, several studies have focused on T-lymphocyte function, as activation of these cells plays a crucial role in early β-cells destruction [12]. Paradoxically, however, cellular proliferation and interleukin-2 (IL-2) and IL-4 secretion were actually reduced, not elevated, in isolated T-cells from T1DM adult patients [13],[14]. This discrepancy between in vivo and in vitro observations may be due to cell activation or deactivation associated with cell separation procedures, which can confound data interpretation [15]. To address this problem, our group developed an ex vivo incubation technique and has successfully utilized this technology on immune cells from healthy subjects and patients with various pathologies [16],[17], in which WBC subtypes are not separated but individually activated in whole blood via binding of specific surface receptors [16].
As children account for a growing proportion of all obese and T1DM patients, the age-specific understanding of underlying pathogenetic mechanisms should be the basis for effective cardiovascular prevention in this age group. However, as most pertinent studies were performed on adults, often metabolically and immunologically different from children [18],[19], information regarding pediatric populations is scarce [13],[14]. In this study, we therefore aimed to help define, in children with obesity and T1DM, the presence of possible alterations in key inflammatory responses of mRNA gene expression in specific WBC subtypes.
RESEARCH DESIGN AND METHODS
Experimental Objective
WBC activation was induced under ex vivo conditions via selective stimulation of the T-cell receptor (TCR, activating T-lymphocytes) or the Fc receptor (FcR, activating natural killer, or NK, and polymorphonuclear, or PMN, cells) [20]. mRNA expression was measured in 9 key modulators of inflammatory processes: 6 members of the tumor necrosis factor superfamily (TNFSF)—TNFSF2 (TNF-α), 5 (CD40 ligand), 6 (Fas ligand), 7 (CD70), 9 (4-1BB ligand), 14 (CD258)—and 3 chemokines—CCL8 (monocyte chemoattractant protein-2 or MCP-2), CCL20 (macrophage inflammatory protein-3α or MIP-3α), CXCL10 (interferon-γ inducible protein-10 or IP-10).
Subjects
All procedures were approved by the University of California, Irvine (UCI) Institutional Review Board; all participants and their guardians signed informed consent and assent forms. Nine T1DM (13.4±0.5 yr, 4F/5M, BMI% 68.9±10.3), 23 overweight (OW, 12.3±0.5 yr, 10F/13M, BMI% 97.1±0.5 and all >90%), and 21 healthy (CL, 13.8±0.7 yr, 9F/12M, BMI% 59.6±4.6 and all <85%) children were studied (Table 1). In T1DM children, diabetes duration ranged between 2 and 8 years. Therefore, the metabolic milieu of diabetic subjects had time to stabilize itself after the end of the “honeymoon phase”, but onset of the disease was still recent enough to exclude the confounding effect of tissue and vascular diabetic complications. Peripheral blood samples from all subjects were used for TCR stimulation, as described below. Heat-aggregated IgG (HAG)-mediated FcR stimulation experiments were performed on a subset of T1DM and CL subjects (T1DM, 13.3±0.6 yr, 2F/5M; CL, 14.4±0.8 yr, 9F/4M). This was due to the fact that methodological aspects of the the IgG-mediated FcR stimulation technique were still been optimized at the time of study initiation, and did not become available until after the first few subjects had already been studied. As diabetic and control subjects were studied in randomized order, this did not introduce selection bias. Data from an additional 3 subjects had to be dropped due to technical errors during assay procedures.
TABLE 1.
Demographic features of the 3 experimental groups: T1DM, OW, and CL
| Age (yrs) |
Gender (F/M) |
Height (cm) |
Weight (kg) |
BMI (%tile) |
Tanner | BP syst/diast (mmHg) |
n | |
|---|---|---|---|---|---|---|---|---|
| T1DM | 13.4 ± 0.5 | 4/5 | 157.5 ± 4.4 | 55.1 ± 5.0 | 68.9 ± 10.3 | 3.0 ± 0.4 | 109±4/61±2 | 9 |
| OW | 12.3 ± 0.5 | 10/13 | 155.4 ± 3.3 | *69.8 ± 4.5 | †97.1 ± 0.5 | 3.0 ± 0.2 | 115±2/63±1 | 23 |
| CL | 13.8 ± 0.7 | 9/12 | 157.8 ± 3.5 | 51.5 ± 3.3 | 59.6 ± 4.6 | 3.0 ± 0.3 | 108±3/60±2 | 21 |
Data are means ± SEM. BMI, body mass index. BP, blood pressure. Syst, systololic. Diast, diastolic.
significant difference versus CL
significant difference versus CL and T1DM.
Only subjects without any co-morbidity (including acute injury or low-grade infection) and on no medication (other than insulin for the T1DM group) were included. To guarantee that systemic inflammatory status in T1DM children was not related to the acute inflammatory events responsible for diabetes onset, only children diagnosed at least 2 years prior to study date were enrolled. To confirm a balanced composition of study groups with respect to maturational status, Tanner stage was then determined via a validated standard questionnaire [21].
Study Protocol
Subjects were admitted at the UCI Institute for Clinical Translational Science (ICTS) at ~7:30 am; vital signs were found to be within the normal ranges, and intravenous (I.V.) catheters were started at the median cubital veins of both arms for access to blood sampling and infusions of regular insulin and 20% dextrose (T1DM) or saline (OW, CL). To guarantee stable metabolic conditions, euglycemia was maintained in all subjects for at least 90 min following I.V. line insertion. While euglycemia was maintained spontaneously by OW and CL subjects (as confirmed by repeated blood glucose measurements), special procedures were required for the T1DM group as specified below.
Establishing and maintaining euglycemia in T1DM
During the early morning of the study, the participants checked their blood glucose on a home glucometer after awakening and called the study principal investigator for instructions regarding insulin administration. Subjects on a multiple-injection regimen administered only the rapid-acting component of their insulin regimen, if needed, based on their morning glucose reading. Subjects on insulin infusion pumps were instructed to maintain their basal infusion rate and bolus as needed.
After admission to the UCI ICTS and insertion of I.V. lines, insulin and dextrose infusions were started to achieve and maintain stable euglycemia (5.0–6.1 mmol/L) with baseline insulin infusion (~1 U/h) for 90 min. If diabetic subjects entered the study in the euglycemic range, the experimental clock was immediately started and euglycemia was maintained for 90 min. If participants entered the study at hyperglycemia (>6.1 mmol/L), blood glucose was brought to euglycemia (5.0–6.1 mmol/L) via tapered I.V. insulin infusion (9.0 nmol/h for every 5.6 mmol/L above euglycemia and gradually reduced as the blood glucose approached 6.1 mmol/L). Dextrose was only infused if plasma glucose concentration showed a trend towards dropping below 5.0 mmol/L. In all participants, therefore, after 90 min of euglycemia, peripheral whole blood samples were drawn into 1% heparinized tubes and immediately stored at 4°C until leukocyte stimulation.
Leukocyte Stimulation and RT-PCR Procedures
PCR primers and leukocyte stimulation
UniGene database in the GenBank provided the list of nucleotide sequences belonging to TNFSFs and chemokines genes. Primer Express (Applied Biosystems, Foster City, CA) and HYBsimulator (RNAture, Irvine, CA) generated the PCR primers for these genes [22]. Primer sequences were summarized in our previous publication [16]. Oligonucleotides were purchased from IDT (Coralville, IA). Twenty mg/mL human IgG (Sigma, St. Louis, MO) was incubated in PBS at 63°C for 15 min to yield heat-aggregated IgG (HAG) [23]. The used clones (IL26) of anti-human T-cell receptor (TCR) IgG1κ antibody (Ab) and mouse control IgG1κ (both 0.5 mg/mL) (BioLegend, San Diego, CA) were confirmed to be agonistic [16]. In the first set of 8-well strip microtubes, 1.2 μL each of 1:10 dilution of TCR or control IgG1κ was added in triplicate (total 6 wells). In the second set, 1.2 μL each of 1:2 dilution of HAG or PBS was added in triplicate (total 6 wells). These strips were stored at −80°C. On the same day as the clinical study, 60 μL of whole blood from each subject was added into 2 strips (total 12 wells) and incubated at 37°C for 2 (for TCR) or 4 (for HAG) h. Immediately thereafter, samples were stored at −80°C.
mRNA quantification
On assay day, frozen samples were simultaneously thawed and mRNA and cDNA were amplified, in triplicate, directly from whole blood using a validated technology developed from our laboratory [24]. Briefly, 96-well filterplates were placed over collection plates, washed with 150 μL 5 mM Tris pH 7.4, and then centrifuged at 120g for 1 min at 4°C. Fifty μL of blood samples from subjects were added to each well and 120g of centrifugation was employed for 2 min at 4°C, followed by washing of each well with 300 μL PBS once and then centrifugation at 2000g for 5 min at 4°C. Sixty μL stock lysis buffer, in addition to 0.5 mg/mL proteinase K (Pierce, Rockford, IL), 0.1 mg/mL E. coli tRNA (Sigma), 0.1 mg/ml salmon sperm DNA (5 Prime Eppendorf/Brinkmann, Westbury, NY), 1% 2-mercaptethanol (Bio-Rad, Hercules, CA), and a cocktail of 10 mM each of specific reverse primers were deposited onto the filterplates and incubated at 37°C for 10 min. The filterplates were then suspended over oligo(dT)-immobilized microplates (GenePlate, RNAture) [25], centrifuged at 2000g for 5 min at 4°C, and then incubated overnight at 4°C. Thereafter, the microplates were rinsed with 100 μL plain lysis buffer 3 times and then with 150 μL wash buffer (0.5 M NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA) 3 times at 4°C. Thirty μL 1x RT-buffer, 1.25 mM each of dNTP, 4 units rRNasin, and 80 units of MMLV reverse transcriptase (Promega, Madison, WI) (without primers) buffer was applied and then mixture was incubated at 37°C for 2 h to generate cDNAs. With the primer-primed cDNA already in the solution, and oligo(dT)-primed cDNA immobilized in the microplate [24], the SYBR Green PCR solution [26] was prepared by dilution of the cDNA in water by 4 folds. Four μL of this cDNA sample was aspirated into 384-well PCR plates with the addition of 5 μL iTaq SYBR master mix (Bio-Rad) and 1 μL oligonucleotide cocktail (15 μM each of forward and reverse primer). The PCR process was completed using PRISM 7900HT (Applied Biosystems), with one cycle of 95°C for 10 min, followed by 45 cycles of 95°C for 30 sec and 60°C for 1 min. 1x RT buffer was used as negative controls to identify the generation of primer dimers. The melting curve was assessed to ensure the PCR signals were derived from a single PCR product. The analytical software Sequence Detection System (Applied Biosystems) was used to obtain the Ct. ΔCt was calculated by subtracting Ct values from corresponding control samples, and fold increase was calculated by 2(−ΔCt) [27]. The interassay coefficients of variation (CV) for TCR stimulation assays were 10.7% vs. 4.7% vs. 4.3% (TNFSF2), 6.9% vs. 5.1% vs. 3.5% (TNFSF5), 10.6% vs. 4.3% vs. 5.1% (TNFSF6), 2.4% vs. 6.7% vs. 3.1% (TNFSF7), 6.1% vs. 4.7% vs. 3.4% (TNFSF9), 8.9% vs. 4.6% vs. 4.1% (TNFSF14), 10.7% vs. 4.7% vs. 6.1% (CCL8), 11.6% vs. 6.2% vs. 6.5% (CCL20), 11.8% vs. 8.8% vs. 8.1% (CXCL10), T1DM vs. OW vs. CL respectively; corresponding interassay CVs for FcR stimulation were 5.0% vs. 3.4% (TNFSF2), 1.8% vs. 1.3% (TNFSF5), 3.2% vs. 3.1% (TNFSF6), 1.5% vs. 1.8% (TNFSF7), 3.5% vs. 2.6% (TNFSF14), 5.9% vs. 4.4% (CCL8), 1.4×10−14% vs. 2.7×10−14 % (CCL20), T1DM vs CL respectively. More detailed description regarding the precision and interference of these stimulation techniques has also been described by our group in a previous report [24].
Leukocyte counts
2 cc blood samples were collected in sterile tubes coated with 3.6 mg K2-EDTA, inverted 60 times and mechanically mixed for 2 min. WBC count with automated differential was then obtained utilizing the VCS technology with a Beckman Coulter LH750 System (Beckman Coulter, Fullerton, CA).
Statistical Analysis
Immune responses were assessed as fold increases of mRNA levels following leukocyte stimulation versus unstimulated incubation. Since the observed mRNA fold increase data did not display normal distributions, a non-parametric method was used—Kruskal-Wallis (KW) test for overall significance followed by Wilcoxon rank-sum (WRS) test for paired comparisons with Bonferroni adjustment. As a confirmatory assessment, a χ2 test for overall and paired comparisons with Bonferroni correction between groups was subsequently also performed, with a fold increase >2.0 used as a cut point to define an elevated response (this more stringent analysis has been shown by our lab to yield 99% confidence in mRNA studies [28]). A consistent agreement between the two statistical procedures was observed; as such, unless otherwise specified, significance levels will be reported according to the KW and WRS results. WBC counts were analyzed using ANOVA. All statistical steps were verified by the UCI ICTS staff biostatistician.
RESULTS
TCR-induced leukocyte response
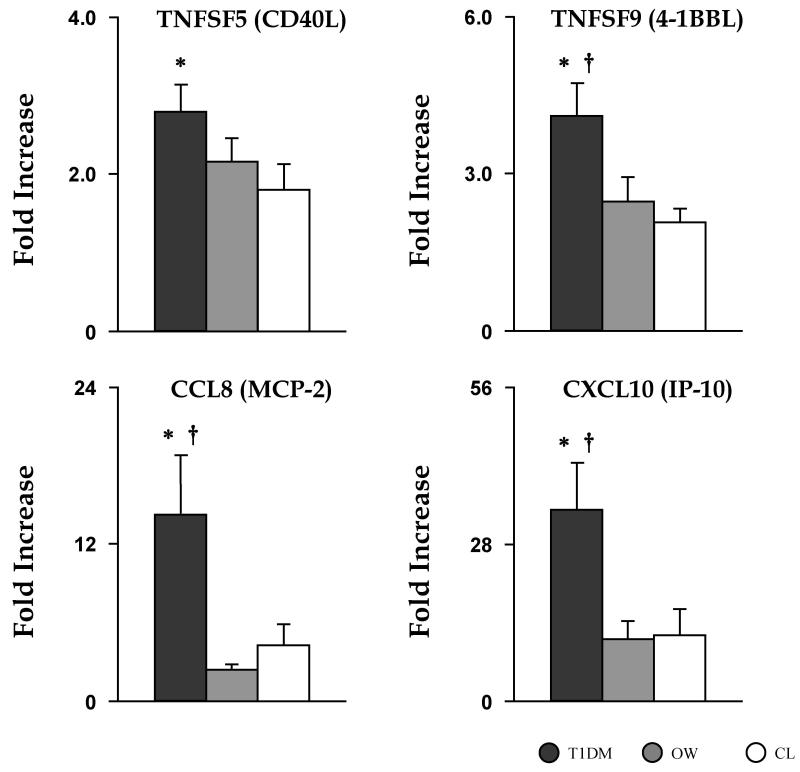
A TCR-mediated immune response was induced via incubation with specific anti-TCR Ab, while incubation with non-reactive IgG1κ served as the unstimulated baseline control. When compared to CL, T1DM displayed significantly elevated responses to TCR induction for TNFSF2 (4.09±0.59 vs. 2.47±0.42, p<0.02—significant by χ2 only), TNFSF5 (2.80±0.34 vs. 1.81±0.31, p<0.01), TNFSF7 (1.36±0.09 vs. 1.04±0.07, p<0.01), TNFSF9 (4.10±0.65 vs. 2.46±0.47, p<0.02), CCL8 (14.24±4.54 vs. 4.24±1.68, p<0.01), and CXCL10 (34.19±8.41 vs. 11.76±4.82, p<0.005). In addition, the mRNA fold increase in T1DM was significantly greater than in OW children for TNFSF9 (4.10±0.65 vs. 2.08±0.27, p<0.005), CCL8 (14.24±4.54 vs. 2.37±0.43, p<0.005), and CXCL10 (34.19±8.41 vs. 11.78±3.00, p<0.005). When compared to the CL group, OW children displayed no significant difference in the mRNA expression of tested inflammatory mediators, although TNFSF2 (4.16±0.55 vs. 2.47±0.42, p=0.021) approached statistical significance (Fig. 1).
Fig. 1. Elevated responses in gene expression of proinflammatory mediators following TCR stimulation for T1DM.
mRNA fold increases following 2 h ex vivo TCR stimulation of leukocytes in T1DM (dark grey bar), OW (grey bar), and CL (white bar) children. Data are mean ± SEM. * and † are significant differences between T1DM vs. CL and T1DM vs. OW, respectively.
FcR-induced leukocyte response
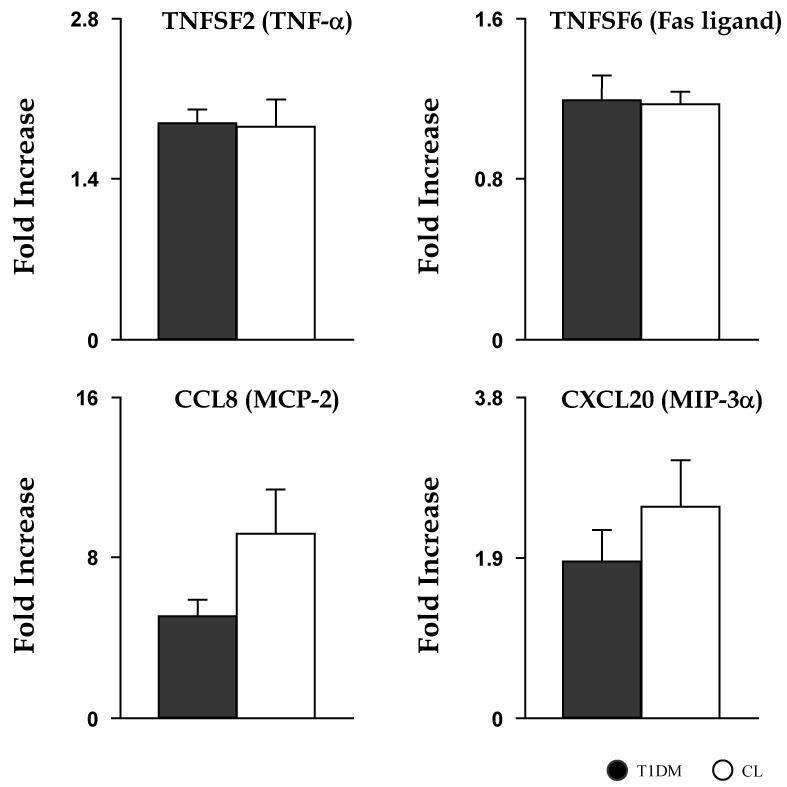
The generally exaggerated T1DM leukocyte mRNA responses to TCR stimulation, as compared to CL, suggested the possibility that additional aspects of leukocyte immunological activation may be altered in these subjects. In a subset of subjects from these two groups, leukocyte Fc receptors were stimulated using HAG in whole blood, subsequently measuring levels of mRNA expressions of a subset of inflammatory mediators (TNFSF2, TNFSF5, TNFSF6, TNFSF7, TNFSF14, CCL8, CCL20) (Fig. 2). Interestingly, and contrary to TCR stimulation experiments, no inflammatory mediator displayed greater mRNA expression in T1DM children; in fact, the only noted difference across groups was a modest and only marginally significant reduction in T1DM of a single variable (TNFSF5, 0.60±0.06 vs. 0.72±0.03, p<0.05), possibly due to the relatively small sample size of this portion of the protocol.
Fig. 2. Similar response in gene expression of proinflammatory mediators following HAG stimulation.
mRNA fold increases after 4 h ex vivo HAG stimulation of leukocytes in T1DM (dark grey bar) and CL (white bar) children. Data are mean ± SEM.
White blood cell counts
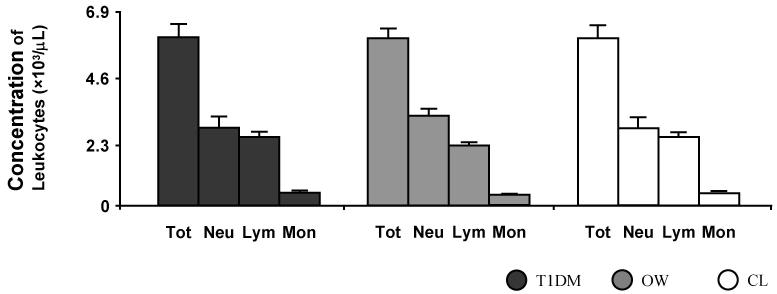
Since the elevated mRNA responses following TCR stimulation observed for T1DM could be attributed to higher numbers of leukocytes in the whole blood rather than intracellular activities, total leukocyte and major subtypes (neutrophils, lymphocytes, and monocytes) were quantified (Fig. 3). No difference was detected between the three groups for total WBCs (5.98±0.47, 5.98±0.31, and 5.78±0.31), neutrophils (2.76±0.40, 3.21±0.25, and 3.04±0.31), lymphocytes (2.24±0.17, 2.15±0.12, and 2.11±0.14), and monocytes (0.46±0.04, 0.43±0.03, and 0.45±0.03) (T1DM, OW, and CL, respectively; units for all total and subtype leukocyte counts results are ×103/μL), suggesting that the observed increased TCR response in T1DM is indeed due to exaggerated intracellular gene expression rather than to the presence of greater numbers of leukocytes.
Fig. 3. Similar blood concentrations of total leukocytes and major subtypes.
Baseline circulating levels of total white blood cell counts (Tot), neutrophils (Neu), lymphocytes (Lym), and monocytes (Mon) for the 3 experimental groups: T1DM (black bar), OW (grey bar), and CL (white bar) children. Data are mean ± SEM.
DISCUSSION
The main finding of this study is that a clear alteration in the pattern of mRNA expression of at least 5 key pro-inflammatory molecules can be detected from WBCs in response to TCR stimulation in children with T1DM compared to age-matched overweight and healthy controls. Conversely, FcR stimulation did not result in a significantly different effect in diabetic children. The novelty of this study was that mRNA expression was analyzed after ex vivo induction.
An intriguing observation is the selective upregulation of T-lymphocyte proinflammatory function in T1DM, while NK or PMN cells exhibited inflammatory characteristics similar to control subjects. Altered T-lymphocyte activity in T1DM was not unexpected, given the known pathogenetic role played by this WBC subtype in β-cell destruction at disease onset [12]. While in our study mRNA expression of several cytokines/chemokines from T1DM was in fact increased compared to both healthy and non-diabetic overweight children, however, reduced cell proliferations and secretion of some cytokines (IL-2, IL-4) was previously reported in T-lymphocytes from T1DM adults [13],[14]. It should be noted that this T-cell behavior was referred to as “paradoxical” in light of the simultaneous upregulation present in T1DM T-cell in vivo [13]. This apparent paradox can be explained in several ways. For instance, the reported reduced IL-2 and IL-4 secretions in vitro following application of TCR/CD3 agonist molecules does not exclude the possibility that expression/secretion of other cytokines/chemokines, including those reported in this study, may have been increased. Further, the above in vitro studies used isolated T-cells, whose function may have been altered during separation; after cell separation, these cells could not be exposed to extracellular molecular signaling from other WBCs [15], which may have compensated for intrinsic T-cell signaling deficits. Our ex vivo technique, while preserving the selectivity of T-lymphocyte stimulation via TCR activation, allows for full cell-to-cell communications, potentially explaining how T-cells, anergic in vitro, may be fully active in vivo [15]. Alternatively, the increased cytokine mRNA expression could derive from other WBC subtypes; even in this case, however, the initial trigger must have been of T-cell origin. Finally, it is possible that increased cytokine mRNA expression is age-dependent, as well-known metabolic differences occur between children and adults [18],[19]. In fact, we have recently reported profound differences between pre-pubertal boys and adults in the pattern of gene expression of peripheral blood mononuclear cells in response to intense exercise (expression of over 700 genes was altered in adults, but less than 100 in boys [29]).
It is debated whether, after the early inflammatory events causing T1DM onset, increased inflammation persists as an intrinsic feature of the disease or simply as a complication of uncontrolled hyperglycemia [30]. Our results suggest that in T1DM, an intrinsically altered pro-inflammatory cytokine signaling from T-lymphocytes may contributor to a sustained increase of systemic inflammation. Therefore, in patients in which hyperglycemia cannot be controlled sufficiently to limit systemic inflammation, tailored anti-inflammatory strategies may become a logical approach to reduce initial insulitis and/or downstream cardiovascular complications. We have in fact recently demonstrated that in a different pathological condition, also characterized by excessive inflammation (Crohn’s disease), an exaggerated WBC mRNA response to TCR stimulation could be pharmacologically suppressed [16] (TNF-α mRNA expression was completely blocked by 2 calcineurin inhibitors—cyclosporine A and tacrolimus) [16]. Application of these concepts to diabetes and obesity will require further investigation.
Interestingly, while the levels of WBC cytokine mRNA expression were not significantly greater in our OW group versus healthy controls, quantitatively, the data from the OW children were approximately half-way between healthy and T1DM children, suggesting that a milder level of WBC activation may also be present in the latter group. This is also consistent with the established concept that some degree of subclinical, chronic inflammation is present in obesity, (some large-scale studies reported elevated C-reactive protein and select proinflammatory cytokines [11],[31]). It should also be noted that discrepancies between RNA expression and systemic levels of peptides often exist, given the multiple post-transcriptional regulatory steps that are in place [32]. Correlations between mRNA and protein expression in human tissues, in fact, have yielded inconsistent results in the liver, lung, and prostate [33],[34],[35]. As for human WBCs, an overall positive association between mRNA and protein expression in ~71 genes is expected, particularly from isolated monocytes, with some variation across subjects and biological categories of gene ontology [36].
Another important consideration is that numerous tissues other than WBCs can contribute to systemic cytokine concentrations. A classic example is the proinflammatory cytokine IL-6, abundantly produced by lymphocytes, but whose total circulatory level is predominantly due to secretion by skeletal muscle (especially during exercise) [37] and, in the obese, by adipocytes [38]. The fact that leukocyte secretion of at least some cytokines only partially contributes to their systemic concentrations, however, should not detract from their crucial immunomodulatory role. The systemic effects of cytokines produced by tissues other than WBCs, in fact, require substantial protein production that may indiscriminately reach tissues and cells not involved in the original inflammatory process [38]. Conversely, leukocytes have the ability to circulate throughout the entire organism and aggregate in response to chemotactic signals [39], into defined areas in which high localized cytokine concentrations can be achieved for potent, selective, and targeted paracrine cell signaling with relatively low systemic spill over [40]. In this respect, cytokine-mediated inflammatory processes regulated by peripheral WBCs represent a much more phylogenetically sophisticated modulatory mechanism.
In summary, T1DM displayed an exaggerated WBC gene expression response of several key proinflammatory mediators when compared to age-matched overweight and healthy children following selective stimulation of the TCR receptor in the whole blood. Conversely, no difference across groups was observed following FcR stimulation, indicating that altered T-lymphocyte function rather than PMNs may be involved in the early stages of immune dysregulation in diabetes.
ACKNOWLEDGEMENTS
This project was supported by the extraordinary work of all UCI ICTS members, and by NIH grants M01-RR00827-28 and K-23 RR018661-01 and Juvenile Diabetes Research Foundation grant #11-2003-332.
REFERENCES
- [1].DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990–1999. Diabet Med. 2006;23:857–866. doi: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- [2].Daniels SR, Greer FR, the Committee on Nutrition Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122:198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- [3].The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- [4].The Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].The Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- [7].Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr Diabetes. 2006;7:16–24. doi: 10.1111/j.1399-543X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- [8].Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- [9].Galassetti PR, Iwanaga K, Pontello AM, Zaldivar FP, Flores RL, Larson JK. Effect of prior hyperglycemia on IL-6 responses to exercise in children with type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E833–839. doi: 10.1152/ajpendo.00445.2005. [DOI] [PubMed] [Google Scholar]
- [10].Rosa JS, Flores RL, Oliver SR, Pontello AM, Zaldivar FP, Galassetti PR. Sustained IL-1α, IL-4, and IL-6 elevations following correction of hyperglycemia in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:9–16. doi: 10.1111/j.1399-5448.2007.00243.x. [DOI] [PubMed] [Google Scholar]
- [11].Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- [12].Pinkse GGM, Tysma OHM, Bergen CAM, et al. Autoreactive CD8 T cells associated with β cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nervi S, Atlan-Gepner C, Fossat C, Vialettes B. Constitutive impaired TCR/CD3-mediated activation of T cells in IDDM patients co-exist with normal co-stimulation pathways. J Autoimmun. 1999;13:247–255. doi: 10.1006/jaut.1999.0313. [DOI] [PubMed] [Google Scholar]
- [14].De Maria R, Todaro M, Stassi G, et al. Defective T cell receptor/CD3 complex signaling in human type I diabetes. Eur J Immunol. 1994;24:999–1002. doi: 10.1002/eji.1830240433. [DOI] [PubMed] [Google Scholar]
- [15].Jenkins MK, Khoruts A, Ingulli E, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- [16].Mitsuhashi M, Targan SR. Ex vivo simulation of IgG Fc and T-cell receptor functions: an application to inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1061–1067. doi: 10.1002/ibd.20428. [DOI] [PubMed] [Google Scholar]
- [17].Mitsuhashi M, Ogura M, Endo K, et al. Ex vivo induction of mRNA in human whole blood as a new platform of drug and dietary supplement development. Pharm Res. 2008;25:1116–1124. doi: 10.1007/s11095-007-9510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Timmons BW, Tarnopolsky MA, Bar-Or O. Immune responses to strenuous exercise and carbohydrate intake in boys and men. Pediatr Res. 2004;56:227–234. doi: 10.1203/01.PDR.0000132852.29770.C5. [DOI] [PubMed] [Google Scholar]
- [19].Pietrobelli A, Malavolti M, Battistini NC, Fuiano N. Metabolic syndrome: a child is not a small adult. International Journal of Pediatric Obesity. 2008;3:67–71. doi: 10.1080/17477160801897133. [DOI] [PubMed] [Google Scholar]
- [20].López DH, Trevani AS, Salamone G, et al. Acidic pH increases the avidity of FcgammaR for immune complexes. Immunology. 1999;98:450–455. doi: 10.1046/j.1365-2567.1999.00884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- [22].Mitsuhashi M, Cooper A, Ogura M, Shinagawa T, Yano K, Hosokawa T. Oligonucleotide probe design - a new approach. Nature. 1994;367:759–761. doi: 10.1038/367759a0. [DOI] [PubMed] [Google Scholar]
- [23].Ostreiko KK, Tumanova IA, Sykulev YK. Production and characterization of heat-aggregated IgG complexes with pre-determined molecular masses: light-scattering study. Immunol Lett. 1987;15:311–316. doi: 10.1016/0165-2478(87)90134-9. [DOI] [PubMed] [Google Scholar]
- [24].Mitsuhashi M, Tomozawa S, Endo K, Shinagawa A. Quantification of mRNA in whole blood by assessing recovery of RNA and efficiency of cDNA synthesis. Clin Chem. 2006;52:634–642. doi: 10.1373/clinchem.2005.048983. [DOI] [PubMed] [Google Scholar]
- [25].Mitsuhashi M, Keller C, Akitaya T. Gene manipulation on plastic plates. Nature. 1992;357:519–520. doi: 10.1038/357519a0. [DOI] [PubMed] [Google Scholar]
- [26].Morrison TB, Weis JJ, Wittwer CT. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998;24:954–958. [PubMed] [Google Scholar]
- [27].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [28].Mitsuhashi M, Endo K, Obara K, et al. Quantification of drug-induced mRNA in human whole blood ex vivo. Clin Med Blood Disord. 2007;1:1–11. [Google Scholar]
- [29].Radom-Aizik S, Zaldivar FP, Leu S, Wilson LD, Cooper DM. Exercise-induced differential gene expression in PBMCs of early and late pubertal boys Med Sci Sports Exerc; ACSM Conference on Integrative Physiology of Exercise; 2006; p. S35. Abstract. [Google Scholar]
- [30].Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid Redox Signal. 2007;9:879–889. doi: 10.1089/ars.2007.1631. [DOI] [PubMed] [Google Scholar]
- [31].Shin JY, Kim SY, Jeung MJ, et al. Serum adiponectin, C-reactive protein and TNF-alpha levels in obese Korean children. J Pediatr Endocrinol Metab. 2008;21:23–29. doi: 10.1515/JPEM.2008.21.1.23. [DOI] [PubMed] [Google Scholar]
- [32].Halbeisen R, Galgano A, Scherrer T, Gerber A. Post-transcriptional gene regulation: from genome-wide studies to principles. Cell Mol Life Sci. 2008;65:798–813. doi: 10.1007/s00018-007-7447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:3–4. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- [34].Chen G, Gharib TG, Huang C-C, et al. Proteomic analysis of lung adenocarcinoma: identification of a highly expressed set of proteins in tumors. Clin Cancer Res. 2002;8:2298–2305. [PubMed] [Google Scholar]
- [35].Lichtinghagen R, Musholt PB, Lein M, et al. Different mRNA and protein expression of matrix metalloproteinases 2 and 9 and tissue inhibitor of metalloproteinases 1 in benign and malignant prostate tissue. Eur Urol. 2002;42:398–406. doi: 10.1016/s0302-2838(02)00324-x. [DOI] [PubMed] [Google Scholar]
- [36].Guo Y, Xiao P, Lei S, et al. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin. 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- [37].Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- [38].Guzik TJ, Mangalat D, Korbut R. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57:505–528. [PubMed] [Google Scholar]
- [39].Cahalan MD, Gutman GA. The sense of place in the immune system. Nat Immunol. 2006;7:329–332. doi: 10.1038/ni0406-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]