Abstract
Ewing sarcoma is the second most common type of bone cancer in children and young adults. In recent years, the mechanisms by which these tumors develop and maintain their vascular supply have been elucidated. Additional work has demonstrated that inhibition of angiogenic pathways or disruption of established vasculature can attenuate the growth of Ewing sarcoma mouse xenografts. Early clinical data suggest that these results may also extend to patients with Ewing sarcoma treated with anti-angiogenic or anti-vascular therapies. This review summarizes the available data supporting this approach.
Keywords: Angiogenesis, Ewing sarcoma, VEGF, bevacizumab, thrombospondin
Introduction
Ewing sarcoma is the second most common primary bone cancer affecting children and young adults. Despite improvements in outcome for patients with localized tumors1, 2, treatment for those who have metastatic disease remains unsatisfactory, with long-term overall survival (OS) below 30%, even with significant dose intensification of cytotoxic therapies.3, 4 Patients with relapsed disease also fare poorly, particularly if disease recurs early.5, 6 Improvements in outcome for patients with metastatic or relapsed disease will likely require novel therapeutic approaches.
One innovative strategy that has gained interest in recent years has been the use of angiogenesis inhibition and vascular disruption for patients with solid tumors. We performed an extensive review of the available medical literature related to angiogenesis and vascular supply in Ewing sarcoma. We utilized the US National Library of Medicine’s PubMed (www.pubmed.gov) search function to find relevant primary articles based on key search terms including VEGF, PDGF, thrombospondin, angiogenesis, angiogenic, microvessel, endothelial, vascular, vasculogenesis, bevacizumab, and metronomic. These search terms were searched together with “Ewing”, “Ewing’s”, or “bone cancer”. The “Related Articles” function of PubMed and references of relevant articles were utilized to expand the search and identify additional relevant articles.
This review summarizes the results of this search and will begin with a description of the mechanisms by which Ewing tumors develop and maintain their vascular supply. This will be followed by a discussion of the relationship between biomarkers of angiogenesis and features of Ewing sarcoma. The preclinical data supporting an anti-angiogenic or anti-vascular strategy in this disease will then be examined The review will conclude with an assessment of the few clinical studies to date that have treated patients with Ewing sarcoma using these approaches.
Mechanisms of Vascular Supply in Ewing Sarcoma
As with all solid tumors, Ewing sarcoma tumors require a viable vascular supply in order for tumor cells to grow beyond the limits of oxygen and nutrient diffusion into tissues. These tumors utilize three main strategies to develop and maintain their vascular supply: angiogenesis; vasculogenesis; and tumor cell vascular mimicry.7 These processes are summarized in the Figure. Vascular endothelial growth factor (VEGF) appears to play a crucial role in these processes across a wide range of tumor types.8 It is commonly understood that the tumor cells produce one or more VEGF isoforms which stimulate both proliferation and survival of the associated tumor endothelium via its receptors, VEGFR-1 and VEGFR-2. Endothelial cells in Ewing sarcoma tumors demonstrate a high proliferative rate, indicating active angiogenesis in these tumors.9 Several lines of evidence have highlighted the importance of VEGF in driving this process in Ewing sarcoma. For example, Ewing sarcoma cells deficient in expression of one VEGF isoform, VEGF165, produce smaller tumors with reduced microvessel density.10 Re-expression of VEGF165 in these cells restores microvessel density.
Figure 1.
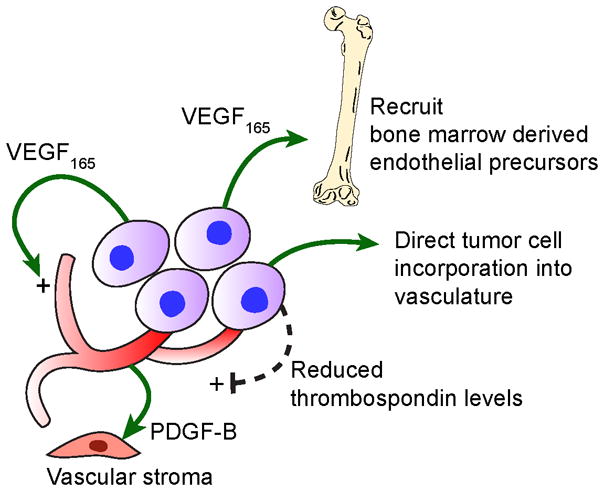
Schematic diagram indicating mechanisms that promote vascular development in Ewing sarcoma. VEGF165 directly stimulates tumor angiogenesis and also recruits bone marrow derived endothelial precursors. Tumor cells can be incorporated directly into the tumor vasculature. Down-regulation of thrombospondin by Ewing sarcoma cells also promotes angiogenesis. Endothelial-derived PDGF-B supports the vascular stroma surrounding the tumor vasculature.
However, VEGF165 also appears to be important in the recruitment of bone marrow derived cells into the vascular network of Ewing sarcoma, a process known as vasculogenesis. These bone marrow derived cells can differentiate into both tumor-associated endothelial cells and pericytes.11, 12 Studies have demonstrated that only CD34-positive bone marrow derived cells contribute to this process.12 VEGF165-deficient Ewing sarcoma cells recruited significantly fewer bone marrow derived cells into xenograft tumors compared to VEGF165-expressing cells.10, 13 Forced expression of another VEGF isoform, VEGF189, into VEGF165-deficient cells did not enhance bone marrow cell recruitment, indicating a specific role for VEGF165 in driving this process. Inhibiting VEGF receptor-2 attenuates the recruitment of bone marrow cells into growing tumors.14 While VEGF, and specifically VEGF165, stimulates vasculogenesis, other cytokines such as stromal cell-derived factor 1 (SDF-1) may also promote bone marrow derived cell recruitment into tumors and provide an alternate pathway supporting vascular development.15
Given the critical role of VEGF in both angiogenic and vasculogenic processes supporting tumor growth, interesting data have emerged supporting the hypothesis that the VEGF pathway may actually be a target of the EWS/ETS fusion oncoproteins characteristic of Ewing sarcoma. In one study, plasmids expressing EWS/FLI or EWS/ETV were transfected into RK13 cells and levels of VEGF expression recorded.16 Transfection of either EWS/FLI or EWS/ETV resulted in an increase in activation of the VEGF promoter. Follow-up experiments demonstrated that this effect does not appear to be due to direct DNA binding by either EWS/FLI or EWS/ETV and does not require the presence of the Hypoxia Response Element of the VEGF promoter.16
Another vascular network system in Ewing sarcoma may not be driven by VEGF. Ewing sarcoma cells appear capable of directly lining vascular spaces in a process known as vascular mimicry. In one study, pools of blood, known as blood lakes, were found in 92% of human Ewing sarcoma tumor samples.9 The cells lining these blood lakes were evaluated by immunohistochemistry (IHC) and found to be CD31and CD34 negative, but CD99 positive. Their appearance by electron microscopy was also characteristic of tumor and not endothelial cell origin. Further experiments demonstrated that these tumor cells expressed TFPI-1/2, VE-cadherin, and EphA2, proteins previously shown to be important in vascular mimicry. Follow-up preclinical studies showed that the Ewing sarcoma cell lines could form vascular structures to a variable extent when grown in a collagen matrix, however the formation of these structures was neither enhanced by VEGF nor inhibited by VEGF blocking antibody.9 Those cell lines with a greater propensity for forming vascular structures showed greater expression of genes identified in other malignancies as markers of vascular mimicry, including integrin α3, VE-cadherin, TFPI-1, EphA2, laminin5γ2, Tie-1, neuropilin, and endoglin. Perfusion and intravital microscopy studies in Ewing xenografts have demonstrated that these tumor cell-lined blood lakes appear to be functional, and in continuity with the systemic circulation.
Several parallel pathways have recently been implicated in the modulation of angiogenesis and vasculogenesis in Ewing sarcoma. The insulin-like growth factor (IGF) pathway, for example, may positively regulate VEGF expression. One study has demonstrated that treatment of Ewing sarcoma cells in vitro with IGF-1 increases expression of VEGF mRNA and protein.17 Moreover, treatment of Ewing sarcoma cells with either a monoclonal antibody directed against the IGF-1 receptor or transfection with antisense directed against the IGF-1 receptor resulted in significant attenuation in VEGF production.17 Additional work has shown that treatment of Ewing sarcoma cells or mouse xenografts with a small molecule inhibitor of the IGF-1 receptor attenuated VEGF production and tumor vascular mimicry.18 On the other hand, Ewing sarcoma cells may also stimulate angiogenesis through down-regulation of the endogenous anti-angiogenic protein, thrombospondin. In one study, NIH3T3 cells transfected with the Ewing sarcoma specific fusion oncogenes EWS/FLI1, EWS/ERG, and EWS/ETV1 showed reduced expression of thrombospondin 2.19 This effect may be mediated by binding of the fusion oncoprotein to the thrombospondin promoter. In Ewing sarcoma cell lines, thrombospondin 1 expression is repressed. With shRNA treatment intended to block the expression of EWS/FLI1, thrombospondin 1 expression is restored in these cells.19 These studies indicate that Ewing sarcoma cells rely on multiple mechanisms to initiate and maintain tumor vascular supply.
Finally, some consideration must be given to the tumor microenvironment that supports the vascular structures. Migration and proper application of pericytes and vascular mural cells fortifying endothelial tubes in developing tissues or tumors has been shown to selectively protect vessels against apoptosis when VEGF levels decline. PDGF-B secreted by the endothelium and signaling via PDGFR-β has been identified as a key mediator of this process.20, 21 Bone microvascular endothelial cells have been shown themselves to expressPDGFR-β in vitro and ligand-binding induces rapid phosphorylation and subsequent activation of Aktand ERK1/2 associated with an increase in endothelial cell division and survival.22 Indeed, functional PDGFR-β expression has also been demonstrated in Ewing sarcoma cell lines and Ewing sarcoma primary tumor specimens suggesting an important role for this signaling pathway in tumor development. However, the ligands PDGF-B or PDGF-D do not appear to be derived from the tumor cells and may derive in fact, from the vascular endothelium.23 Finally, in experimental Ewing sarcoma tumors which recur after exposure to VEGF blockade, vessels are characterized by significant increases in diameter and proliferation of vascular mural cells with increased expression of factors that promote endothelial integrity (angiopoietin-1; Ang-1) and PDGF-B.24 Thus, the PDGF pathway is likely to contribute to vascular stability and tumor proliferation in Ewing sarcoma.
Markers of Angiogenesis in Patients with Ewing Sarcoma
In order to consider the potential utility of anti-angiogenic and vascular targeting therapies, it would be helpful to understand the clinical impact of angiogenesis biomarkers in patients with Ewing sarcoma. Unfortunately, these data are relatively scarce and difficult to interpret. At least three groups have investigated tumor microvessel density (MVD) as a prognostic factor in Ewing sarcoma. One group demonstrated a significant association between elevated (MVD) and poor event-free and overall survival in 29 patients with Ewing sarcoma.25 In contrast, two other groups have reported that tumor MVD does not correlate with metastatic status, disease-free survival, or overall survival.26, 27
Other studies have focused on tumor and blood levels of VEGF, again with conflicting results. One group assessed VEGF expression by IHC in 40 patient tumor samples and found that patients with tumor VEGF expression above the median had improved relapse-free and OS compared to those with low VEGF expression.26 Another group studying 31 patient tumors concluded that those patients with tumors lacking VEGF expression had superior OS compared to patients with tumors staining positively for VEGF.16 As in most oncologic processes, mean serum VEGF levels in patients with Ewing sarcoma appear to be higher than those in healthy controls.28, 29 No study has evaluated the impact of serum VEGF levels on outcome specifically in patients with Ewing sarcoma. However, one study investigated this issue in a cohort of children with a range of solid tumors, including 5 patients with Ewing sarcoma.30 In this analysis, patients with higher serum VEGF levels had inferior outcomes compared to patients with lower serum VEGF levels.
Other biomarkers of angiogenesis have not been systematically studied in patients with Ewing sarcoma. These markers include soluble VEGF receptor, thrombospondin, placental growth factor, and circulating endothelial cells.31 Larger studies which specifically include patients with Ewing sarcoma will be required to further assess the utility of angiogenic biomarkers for prognostic and therapeutic guidance.
Preclinical Efficacy of Anti-Angiogenic and Vascular Targeting Strategies in Ewing Sarcoma
Several anti-angiogenic and vascular targeting strategies have been evaluated in preclinical models of Ewing sarcoma (Table 1). Most of these strategies have focused on blocking VEGF production or VEGF signaling activity via ligand neutralization or receptor inhibition. One group utilized small interfering RNA directed against VEGF to attenuate VEGF expression in Ewing sarcoma cell lines, and used these modified cells for subsequent in vivo studies.32 Attenuation of VEGF expression in this manner slowed the establishment of xenograft tumors, diminished their growth, and extended the length of time before mice died from tumor. Tumors from VEGF-attenuated cells showed diminished microvessel density and diminished osteolysis compared to control cells.32
Table 1.
Anti-angiogenic strategies evaluated in preclinical studies of Ewing sarcoma.
| Strategy | Specific Example | Preclinical Model | Ref |
|---|---|---|---|
| Reducing VEGF Expression | si-RNA against VEGF | TC71 xenograft | 32 |
| Binding VEGF | Bevacizumab | RD-ES and A673 xenografts | 34 |
| VEGF Trap | RD-ES, A673, and SK-NEP xenografts | 33, 34 | |
| Inhibiting VEGF Receptor(s) | Anti-VEGFR-2 monoclonal antibody | TC71 xenograft | 14 |
| Cedirinib (AZD2171) | PPTP panel | 35 | |
| Sunitinib (SU11248) | PPTP panel | 36 | |
| SU5416 | RD-ES and A673 xenografts | 34 | |
| SU6668 | RD-ES and A673 xenografts | 34 | |
| Augmenting Endogenous | Increasing thrombospondin expression | TC71 and TC667 xenografts | 19 |
| Anti-Angiogenic Pathways | Increasing VEGF165b expression | TC71 xenograft | 37 |
si-RNA = small interfering RNA; PPTP panel = panel of Ewing sarcoma cell lines and xenografts used by the Pediatric Preclinical Testing program
Bevacizumab is a monoclonal antibody directed against all human VEGF isoforms. While often used to study effects in mouse xenografts, it should be recognized that bevacizumab does not neutralize murine VEGF and thus leaves unopposed any local production of VEGF by host stroma. DC101 is a monoclonal antibody directed against murine VEGF receptor 2. VEGF-Trap (aflibercept) is a recombinant soluble VEGF decoy receptor that binds VEGF with a tenfold higher affinity than bevacizumab. VEGF-Trap has the additional properties of being active across species and of effectively neutralizing placental growth factor, which may play a role in vascular remodeling. In mouse Ewing sarcoma xenografts, treatment with DC101 resulted in significantly smaller tumors with reduced microvessel density compared to mice treated with immunoglobulin control.14 Bevacizumab and VEGF-Trap have also been evaluated preclinically and have been shown to retard the growth of Ewing sarcoma mouse xenografts and even transiently regress established tumors and lung metastases when compared to vehicle controls.33, 34 However, regressed tumors uniformly recur and xenograft tumors derived from one cell line appeared to be more sensitive to these therapies than those derived from another cell line. These observations suggest that anti-VEGF monotherapy is unlikely to be clinically effective and that tumor heterogeneity has the potential to influence clinical response.
Several small molecule inhibitors of the VEGF receptor each with additional “off-target” but potentially advantageous tyrosine kinase inhibitory activity have also been studied for their effects on Ewing sarcoma growth. Two early prototypes of these compounds, SU5416 and SU6668, attenuated tumor growth in Ewing sarcoma mouse xenografts.34 The Pediatric Preclinical Testing Program has now evaluated AZD2171 (cedirinib), an inhibitor of VEGF receptors 1–3, c-kit and PDGFR-β, as well as the even broader spectrum sunitinib, which inhibits VEGF receptors 1–3, c-kit and PDGFR-β, RET and Flt-3.35, 36 Both compounds resulted in little in vitro activity against a panel of Ewing sarcoma cell lines. In contrast, treatment with either compound in vivo resulted in significant prolongation of time to event (e.g tumor of the size requiring sacrifice) in mouse xenograft models. This pattern of in vivo activity and lack of in vitro activity supports an anti-angiogenic effect from these agents, rather than a direct tumor cell inhibitory effect. Interestingly, sunitinib was administered following the usual human dosing schedule of daily for 4 weeks followed by a 2-week rest period. The EW-5 Ewing sarcoma xenograft showed essentially complete inhibition of tumor growth during the 4-week treatment period followed by an abrupt increase in tumor size after discontinuation of treatment.36 The similarity of the findings between AZD2171, a relatively pure VEGF receptor tyrosine kinase antagonist, and sunitinib, with its broader inhibitory effects, suggests that the majority of anti-tumor activity observed with sunitinib was due to inhibition of VEGF receptor signaling. However, given that both compounds also inhibit c-kit, the contribution of c-kit inhibition to the observed anti-tumor activity observed in these experiments is unclear.
In addition to strategies directed specifically at VEGF, other groups have attempted to increase the expression of endogenous anti-angiogenic proteins. One group transfected Ewing sarcoma cells to over-express murine thrombospondin. Mouse xenografts derived from these transfected cells showed attenuated tumor growth in vivo with diminished microvessel density when compared to tumors derived from control cells.19 Another group investigated the endogenous anti-angiogenic isoform of VEGF known as VEGF165b in Ewing sarcoma.37 Tumors derived from Ewing sarcoma cells engineered to over-express VEGF165b also showed attenuated growth. These results suggest that augmentation of endogenous anti-angiogenic pathways may have a role against Ewing sarcoma.
The majority of xenograft experiments described above examined tumor suppression, or the ability of an agent to prevent tumor growth shortly after tumor inoculation. However, patients often present with bulky tumors with established vasculature. Another strategy reported recently has been to utilize a group of compounds known as vascular-disrupting agents, which typically disrupt existing blood vessels by binding to tubulin. In one study, researchers evaluated compound OXi4503/CA1P in preclinical models of Ewing sarcoma.38 Mouse xenografts demonstrated attenuated tumor growth in response to treatment with OXi4503/CA1P compared to vehicle control. Treatment with this agent resulted in increased areas of necrosis and reduced microvessel density compared to control. This compound did not reduce the proliferation rate of tumor cells or induce tumor cell apoptosis in these models, indicating that the effect was due to the effect on tumor vasculature. Treatment with OXi4503/CA1P and doxorubicin resulted in synergistic reductions in tumor growth, suggesting that this class of drugs may be best used in combination with chemotherapy for this disease.
Anti-angiogenic and Anti-vascular Therapies for Patients with Ewing Sarcoma
Preclinical studies have evaluated a range of different strategies and compounds to inhibit angiogenesis in laboratory models of Ewing sarcoma. Substantially fewer studies have been performed using these approaches in patients with Ewing sarcoma (Table 2). In most of these studies, patients with Ewing sarcoma have been treated within the context of phase I dose escalation trials evaluating new agents in patients with refractory solid tumors. Thus, the clinical evaluation of anti-angiogenic agents for Ewing sarcoma is at a very early stage of development. While suggestive of a potential role in this disease, these clinical results are nevertheless anecdotal and require more systematic evaluation in studies that specifically aim to evaluate the activity of antiangiogenic and antivascular stategies in patients with Ewing sarcoma.
Table 2.
Clinical experience with agents with anti-angiogenic effects in patients with Ewing sarcoma.
| Drug(s) | Number of Patients with Ewing sarcoma | Outcomes | Reference(s) |
|---|---|---|---|
| Bevacizumab | 5 | 3 patients with SD for at least 4 months | 39 |
| Bevacizumab/Doxorubicin | 4 | 2 patients with SD for at least 10 months | 40 |
| Sunitinib | 2 | Not reported | 43 |
| Sorafenib | 2 | Not reported | 44 |
| Metronomic Chemotherapy | 1 | SD for 7 months | 46 |
| 1 | SD for 15 weeks | 47 | |
| 2 | Not reported | 48 | |
| 36 | Not reported | Not reported | |
SD = stable disease; PR = partial response; CR = complete response
The first anti-angiogenic agent to earn US Food and Drug Administration (FDA) approval was bevacizumab. A Children’s Oncology Group (COG) phase I study investigated bevacizumab in children with refractory solid tumors.39 This study included 5 patients with relapsed or refractory Ewing sarcoma who were treated with bevacizumab every 2 weeks. No objective responses were reported. One patient had stable disease for 4 months and two patients had stable disease for 9 months of therapy. Another study treated four patients with Ewing sarcoma with bevacizumab and liposomal doxorubicin.40 Two of these patients had stable disease for at least 10 months. These results, while not definitive, suggest that bevacizumab may have a clinical role in inhibiting the growth of Ewing sarcoma.
In part based on these results, and in part based on clinical trial successes in adult colon breast and non-small cell lung carcinoma, bevacizumab is currently being evaluated in two ongoing trials for patients with Ewing sarcoma in combination with chemotherapy. This strategy is supported by growing evidence that VEGF blockade can transiently “normalize” tumor vasculature.41, 42 Nascent tumor vasculature is disorganized and hyperpermeable, with poorly applied pericytes and the absence of normal draining lymphatics. These abnormalities result in both increased interstitial fluid pressure (IFP), which impedes drug delivery, and poor intratumoral perfusion with resultant hypoxia and acidosis, which can impair cytotoxic drug efficacy. By restoring balance between angiogenic cytokines in the tumor microenvironment, it is believed that VEGF neutralization can induce vascular pruning and remodeling which transiently improves perfusion and reestablishes a gradient favoring drug transit into tumor parenchyma. The COG has initiated a randomized phase II trial of retrieval chemotherapy with or without the addition of bevacizumab for patients with first relapse of Ewing sarcoma. Patients on this study receive two cycles of therapy and undergo disease restaging. Patients with progressive disease are taken off study and patients with at least stable disease are eligible to receive an additional 10 cycles of this therapy. The primary outcome is progression-free survival and approximately 75 patients are expected to enroll. The European consortium, Innovative Therapies for Children with Cancer (ITCC) is pursuing a similar randomized phase II design of standard chemotherapy with and without bevacizumab for newly diagnosed patients with metastatic Ewing sarcoma. In addition to these ongoing studies, the St. Jude Children’s Research Hospital is planning a trial combining conventional chemotherapy with bevacizumab for patients with newly diagnosed Ewing sarcoma. The results of these trials are eagerly anticipated, but phase III randomized trials with larger numbers of patients will still be required if definitive efficacy is to be demonstrated.
Clinical evaluation of oral multi-targeted tyrosine kinase inhibitors in patients with Ewing sarcoma has been more limited. The COG is conducting phase I studies of sunitinib43, VEGF-Trap (aflibercept), and sorafenib44, which targets Raf kinase in addition to VEGFR and PDGFR. The Pediatric Oncology Branch of the NCI is conducting a phase I trial of AZD2171 (cedirinib). The Dana-Farber Cancer Institute is conducting a phase II study of sorafenib in adult patients with refractory sarcomas. All these trials include patients with Ewing sarcoma but they are ongoing and response data are not currently available.
Additionally, an anti-angiogenic strategy known as metronomic chemotherapy that involves the application of daily low-dose continuous chemotherapy has been used in patients with Ewing sarcoma. With this low-dose approach, apoptosis is induced in the less frequently dividing endothelial cell rather than the tumor cell and the release of bone marrow derived endothelial precursors is suppressed 45. At least three small studies of metronomic chemotherapy have included patients with Ewing sarcoma, with some indication of disease stabilization with this approach (Table 2).46–48 The COG has completed a pilot feasibility study of the addition of celecoxib and thrice-weekly vinblastine along with a conventional chemotherapy backbone in 36 patients with newly diagnosed metastatic Ewing sarcoma. The results of this study are anticipated shortly.
Finally, two other classes of biologic agents with anti-angiogenic properties are under initial investigation in patients with Ewing sarcoma. First, several monoclonal antibodies directed against the IGF-1R are being evaluated and early studies have demonstrated activity in patients with refractory Ewing sarcoma.49, 50 Second, rapamycin and related mTOR inhibitors have recently been studied for the treatment of a range of malignancies. These agents have anti-angiogenic properties which may contribute to their antineoplastic effects.51 One rapalogue, deforolimus, has shown particular promise in Ewing sarcoma. In phase I, one patient with Ewing sarcoma had a confirmed partial response52 and a phase II study of this agent included 50 patients with bone sarcoma and reported a 30% clinical benefit rate.53 Given that inhibition of the IGF-1R or mTOR pathways may directly inhibit the growth of Ewing sarcoma cells,54, 55 the contribution of the anti-angiogenic effects of these agents to the activity in Ewing sarcoma remains unclear.
Conclusions
Patients with metastatic and relapsed Ewing sarcoma require novel approaches to improve their outcomes. Ewing sarcoma tumors have multiple mechanisms by which they develop and maintain their vascular supply. These mechanisms allow these tumors to continue to grow in size and to metastasize. Numerous and robust preclinical studies have demonstrated that targeting these mechanisms provides a valid approach to inhibiting the growth of these tumors. Early clinical data suggest that anti-angiogenic and anti-vascular strategies may be beneficial in the treatment of patients with Ewing sarcoma. Additional studies are urgently needed to expand these findings, to further evaluate the role of angiogenesis biomarkers, and to determine how to optimize these strategies for use in the management of patients with Ewing sarcoma.
Acknowledgments
Support: Supported in part by the Campini Foundation (SGD), the Jamie Deutsch Foundation (JGB), and by NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130 (SGD). Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors acknowledge the assistance of Kathleen Jee with graphic design.
Footnotes
Financial Disclosures: None
References
- 1.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 2.Womer R, West DC, Krailo M, Dickman P, Pawel B. Randomized comparison of every-two-week v. every-three-week chemotherapy in Ewing sarcoma family tumors. Journal of Clinical Oncology. 2008;26:10504. [Google Scholar]
- 3.Miser JS, Goldsby RE, Chen Z, Krailo MD, Tarbell NJ, Link MP, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy--a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49(7):894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- 4.Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide--a Children’s Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22(14):2873–6. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Jurgens H, Ranft A, Dirksen U, Vieth V, Paulussen M, Franzius C, et al. Risks of recurrence and survival after relapse in patients with Ewing tumor. J Clin Oncol. 2007;25(18S):10012. [Google Scholar]
- 6.Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(3):334–8. doi: 10.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Schaft DW, Hillen F, Pauwels P, Kirschmann DA, Castermans K, Egbrink MG, et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 2005;65(24):11520–8. doi: 10.1158/0008-5472.CAN-05-2468. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Reddy K, Guan H, Kleinerman ES. VEGF(165), but not VEGF(189), stimulates vasculogenesis and bone marrow cell migration into Ewing’s sarcoma tumors in vivo. Mol Cancer Res. 2007;5(11):1125–32. doi: 10.1158/1541-7786.MCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 11.Reddy K, Cao Y, Zhou Z, Yu L, Jia SF, Kleinerman ES. VEGF165 expression in the tumor microenvironment influences the differentiation of bone marrow-derived pericytes that contribute to the Ewing’s sarcoma vasculature. Angiogenesis. 2008;11(3):257–67. doi: 10.1007/s10456-008-9109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy K, Zhou Z, Schadler K, Jia SF, Kleinerman ES. Bone marrow subsets differentiate into endothelial cells and pericytes contributing to Ewing’s tumor vessels. Mol Cancer Res. 2008;6(6):929–36. doi: 10.1158/1541-7786.MCR-07-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TH, Bolontrade MF, Worth LL, Guan H, Ellis LM, Kleinerman ES. Production of VEGF165 by Ewing’s sarcoma cells induces vasculogenesis and the incorporation of CD34+ stem cells into the expanding tumor vasculature. Int J Cancer. 2006;119(4):839–46. doi: 10.1002/ijc.21916. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Bolontrade MF, Reddy K, Duan X, Guan H, Yu L, et al. Suppression of Ewing’s sarcoma tumor growth, tumor vessel formation, and vasculogenesis following anti vascular endothelial growth factor receptor-2 therapy. Clin Cancer Res. 2007;13(16):4867–73. doi: 10.1158/1078-0432.CCR-07-0133. [DOI] [PubMed] [Google Scholar]
- 15.Reddy K, Zhou Z, Jia SF, Lee TH, Morales-Arias J, Cao Y, et al. Stromal cell-derived factor-1 stimulates vasculogenesis and enhances Ewing’s sarcoma tumor growth in the absence of vascular endothelial growth factor. Int J Cancer. 2008;123(4):831–7. doi: 10.1002/ijc.23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing’s sarcoma. Clin Cancer Res. 2004;10(4):1344–53. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 17.Strammiello R, Benini S, Manara MC, Perdichizzi S, Serra M, Spisni E, et al. Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing’s sarcoma cells. Horm Metab Res. 2003;35(11–12):675–84. doi: 10.1055/s-2004-814149. [DOI] [PubMed] [Google Scholar]
- 18.Manara MC, Landuzzi L, Nanni P, Nicoletti G, Zambelli D, Lollini PL, et al. Preclinical in vivo study of new insulin-like growth factor-I receptor--specific inhibitor in Ewing’s sarcoma. Clin Cancer Res. 2007;13(4):1322–30. doi: 10.1158/1078-0432.CCR-06-1518. [DOI] [PubMed] [Google Scholar]
- 19.Potikyan G, Savene RO, Gaulden JM, France KA, Zhou Z, Kleinerman ES, et al. EWS/FLI1 regulates tumor angiogenesis in Ewing’s sarcoma via suppression of thrombospondins. Cancer Res. 2007;67(14):6675–84. doi: 10.1158/0008-5472.CAN-06-4140. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125(9):1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 21.Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 22.Langley RR, Fan D, Tsan RZ, Rebhun R, He J, Kim SJ, et al. Activation of the platelet-derived growth factor-receptor enhances survival of murine bone endothelial cells. Cancer Res. 2004;64(11):3727–30. doi: 10.1158/0008-5472.CAN-03-3863. [DOI] [PubMed] [Google Scholar]
- 23.Uren A, Merchant MS, Sun CJ, Vitolo MI, Sun Y, Tsokos M, et al. Beta-platelet-derived growth factor receptor mediates motility and growth of Ewing’s sarcoma cells. Oncogene. 2003;22(15):2334–42. doi: 10.1038/sj.onc.1206330. [DOI] [PubMed] [Google Scholar]
- 24.Frischer JS, Huang J, Serur A, Kadenhe-Chiweshe A, McCrudden KW, O’Toole K, et al. Effects of potent VEGF blockade on experimental Wilms tumor and its persisting vasculature. Int J Oncol. 2004;25(3):549–53. [PubMed] [Google Scholar]
- 25.Simpson A, Grimer R, Mangham C, Cullinane C, Lewis I, Burchill S. MVD predicts disease-free and overall survival in tumours of the Ewing’s sarcoma family. Br J Cancer. 2002;86(Supplement 1):S95. [Google Scholar]
- 26.Kreuter M, Paulussen M, Boeckeler J, Gerss J, Buerger H, Liebscher C, et al. Clinical significance of Vascular Endothelial Growth Factor-A expression in Ewing’s sarcoma. Eur J Cancer. 2006;42(12):1904–11. doi: 10.1016/j.ejca.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 27.Mikulic D, Ilic I, Cepulic M, Giljevic JS, Orlic D, Zupancic B, et al. Angiogenesis and Ewing sarcoma--relationship to pulmonary metastasis and survival. J Pediatr Surg. 2006;41(3):524–9. doi: 10.1016/j.jpedsurg.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Holzer G, Obermair A, Koschat M, Preyer O, Kotz R, Trieb K. Concentration of vascular endothelial growth factor (VEGF) in the serum of patients with malignant bone tumors. Med Pediatr Oncol. 2001;36(6):601–4. doi: 10.1002/mpo.1136. [DOI] [PubMed] [Google Scholar]
- 29.Pavlakovic H, Von Schutz V, Rossler J, Koscielniak E, Havers W, Schweigerer L. Quantification of angiogenesis stimulators in children with solid malignancies. Int J Cancer. 2001;92(5):756–60. doi: 10.1002/1097-0215(20010601)92:5<756::aid-ijc1253>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Tabone MD, Landman-Parker J, Arcil B, Coudert MC, Gerota I, Benbunan M, et al. Are basic fibroblast growth factor and vascular endothelial growth factor prognostic indicators in pediatric patients with malignant solid tumors? Clin Cancer Res. 2001;7(3):538–43. [PubMed] [Google Scholar]
- 31.Shaked Y, Bocci G, Munoz R, Man S, Ebos JM, Hicklin DJ, et al. Cellular and molecular surrogate markers to monitor targeted and non-targeted antiangiogenic drug activity and determine optimal biologic dose. Curr Cancer Drug Targets. 2005;5(7):551–9. doi: 10.2174/156800905774574020. [DOI] [PubMed] [Google Scholar]
- 32.Guan H, Zhou Z, Wang H, Jia SF, Liu W, Kleinerman ES. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing’s sarcoma growth in a xenograft mouse model. Clin Cancer Res. 2005;11(7):2662–9. doi: 10.1158/1078-0432.CCR-04-1206. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci U S A. 2003;100(13):7785–90. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalal S, Berry AM, Cullinane CJ, Mangham DC, Grimer R, Lewis IJ, et al. Vascular endothelial growth factor: a therapeutic target for tumors of the Ewing’s sarcoma family. Clin Cancer Res. 2005;11(6):2364–78. doi: 10.1158/1078-0432.CCR-04-1201. [DOI] [PubMed] [Google Scholar]
- 35.Maris JM, Courtright J, Houghton PJ, Morton CL, Gorlick R, Kolb EA, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(3):581–7. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 36.Maris JM, Courtright J, Houghton PJ, Morton CL, Kolb EA, Lock R, et al. Initial testing (stage 1) of sunitinib by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;51(1):42–8. doi: 10.1002/pbc.21535. [DOI] [PubMed] [Google Scholar]
- 37.Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98(7):1250–7. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalal S, Burchill SA. Preclinical evaluation of vascular-disrupting agents in Ewing’s sarcoma family of tumours. Eur J Cancer. 2009 doi: 10.1016/j.ejca.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 39.Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2008;26(3):399–405. doi: 10.1200/JCO.2007.11.9230. [DOI] [PubMed] [Google Scholar]
- 40.Skubitz K, Haddad P. Combination of pegylated-liposomal doxorubicin (PLD) and bevacizumab (B) (PLD-B) in sarcoma (SAR) J Clin Oncol. 2007;25(18S):20506. [Google Scholar]
- 41.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 43.DuBois SG, Shusterman S, Ingle AM, Baruchel S, Stempak D, Sun J, et al. A pediatric phase I trial and pharmacokinetic (PK) study of sunitinib: A Children’s Oncology Group Phase I Consortium study. J Clin Oncol. 2008;26(May 20 Supplement):3561. [Google Scholar]
- 44.Widemann BC, Fox E, Adamson PC, Baruchel S, Kim A, Ingle AM, et al. Phase I study of sorafenib in children with refractory solid tumors: A Children’s Oncology Group Phase I Consortium trial. J Clin Oncol. 2009;27(15S):10012. doi: 10.1158/1078-0432.CCR-11-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4(6):423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 46.Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C, et al. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer. 2004;101(7):1664–71. doi: 10.1002/cncr.20544. [DOI] [PubMed] [Google Scholar]
- 47.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27(11):573–81. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 48.Stempak D, Gammon J, Halton J, Moghrabi A, Koren G, Baruchel S. A pilot pharmacokinetic and antiangiogenic biomarker study of celecoxib and low-dose metronomic vinblastine or cyclophosphamide in pediatric recurrent solid tumors. J Pediatr Hematol Oncol. 2006;28(11):720–8. doi: 10.1097/01.mph.0000243657.64056.c3. [DOI] [PubMed] [Google Scholar]
- 49.Olmos D, Okuno S, Schuetze S, Paccagnella M, Yin D, Gualberto A, et al. Safety, pharmacokinetics and preliminary activity of the anti-IGF-IR antibody CP-751,871 in patients with sarcoma. J Clin Oncol. 2008;26:10501. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolcher AW, Rothenberg ML, Rodon J, Delbeke D, Patnaik A, Nguyen L, et al. A phase I pharmacokinetic and pharmacodynamic study of AMG 479, a fully human monoclonal antibody against insulin-like growth factor type 1 receptor (IGF-1R), in advanced solid tumors. J Clin Oncol. 2007;25(18S):3002. [Google Scholar]
- 51.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8(2):128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 52.Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, et al. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26(3):361–7. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 53.Chawla S, Tolcher A, Staddon A, Schuetze S, D’Amato G, Blay J, et al. Updated results of a phase II trial of AP23573, a novel mTOR inhibitor, in patients (pts) with advanced soft tissue or bone sarcomas. J Clin Oncol. 2006;24(18S):9505. [Google Scholar]
- 54.Houghton PJ, Morton CL, Kolb EA, Gorlick R, Lock R, Carol H, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(4):799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 55.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1190–7. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]


