Abstract
Collagen antibody induced arthritis is a robust murine model of arthritis that histologically recapitulates the inflammatory characteristics of rheumatoid arthritis including pannus formation and destruction of articular cartilage and bone. PECAM is a molecule expressed by both leukocytes and endothelial cells that has been shown to play a major role in the extravasation of leukocytes into sites of inflammation. Genetic deletion of many molecules will blunt the onset and progression of arthritis in murine models, as will administration of various anti-inflammatory therapies given prior to the onset of disease. However, patients seek medical attention when symptomatic, which means that the disease is well established. We investigated whether blocking PECAM interactions would inhibit progression of established disease in the collagen antibody induced arthritis model. We report that treatment of symptomatic mice with a PECAM-Fc chimera significantly reduced inflammation and virtually eliminated cartilage and bone destruction. The results suggest that therapies that block PECAM function may be beneficial in the treatment of established arthritis.
Keywords: Arthritis, rheumatoid arthritis, PECAM, inflammation, leukocyte, mouse model
Introduction
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by joint inflammation and progressive cartilage and bone erosion (Feldmann and Maini, 1999). Murine models of collagen induced arthritis (CIA) have been widely used to understand the development of erosive autoimmune arthritis (Van den Berg, 2002). Pathogenesis of both RA and CIA involve pannus formation and hyperplastic inflammatory tissue that invades the articular cartilage and bone of the joints (Zvaifler and Firestein, 1994). Leukocyte infiltration into the joint space and tissue is a key component of both RA and CIA (Tarrant and Patel, 2006). Cytokines generated by the leukocytes that have migrated into synovial tissue (specifically TNF-α, IL-1β and Il-6) enhance inflammation (Feldmann and Maini, 1999). These cytokines activate endothelial cells and leukocytes and cause upregulation of adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which are known to regulate adhesion that promotes subsequent transendothelial migration (TEM) of leukocytes across vascular endothelium (Harlan, 1985).
Active models of CIA involve immunizing susceptible strains of mice with heterologous type II collagen (Courtenay et al., 1980; Wooley et al., 1981) whereas passive models involve passive transfer of K/BxN sera (Kouskoff et al., 1996, 1997; Maccioni et al., 2002) or anti collagen type II antibodies (Terato et al., 1985). A recent commercially available arthritis induction protocol has provided a new murine RA model (Kachigian, 2006). In this collagen antibody induced arthritis model (CAIA) disease is triggered by systemic administration of a cocktail of monoclonal antibodies against various regions of type II collagen. Lipopolysaccharide (LPS) is administered following the antibody cocktail to provide an inflammatory stimulus that initiates disease (Terato et al., 1995). The pathogenic features of CAIA resemble those of RA and CIA, including pannus formation, leukocyte infiltration, synovitis and cartilage and bone destruction. Disease onset and progression are severe and rapid in CAIA with onset of symptoms within days of immunization.
Platelet endothelial cell adhesion molecule 1 (PECAM) is constitutively expressed on the surface of leukocytes and at the borders of endothelial cells and is known to play an important role in leukocyte transmigration out of the vasculature and into tissues (Muller et al., 1993; Vaporciyan et al., 1993; Bogen et al., 1994; Muller, 2003; Muller, 2009). PECAM on the leukocyte participates in a homophilic interaction with PECAM on the endothelial cell, and blocking this interaction inhibits leukocyte TEM both in vitro and in vivo (Muller et al., 1993; Liao et al., 1995; Liao et al., 1997; Liao et al., 1999). Blockade of PECAM using monoclonal antibodies and chimeric soluble PECAM fused to human IgG Fc (PECAM-Fc) significantly blocks monocyte and neutrophil emigration in several murine models of acute inflammation (Bogen et al., 1994; Liao et al., 1997; Reinke et al., 2007).
There is increasing evidence that blocking PECAM may also affect leukocyte emigration in in vivo models of chronic inflammation. Blocking PECAM has been shown to suppress inflammation in murine models of neuroinflammation (Kalinowska and Losy, 2006; Reinke et al., 2007) and experimental colitis (Rijcken et al., 2007).
Our laboratory has previously developed a soluble PECAM-Fc chimera that binds to PECAM in a homophilic manner and inhibits TEM both in vitro and in vivo (Muller, 1995; Liao et al., 1997). This construct is a better therapeutic agent than xenogeneic monoclonal antibodies, as it does not opsonize leukocytes (Liao et al., 1997), stimulate host production of neutralizing antibodies, or activate cells by high affinity binding of cell surface molecules. In this study we show that murine PECAM-Fc chimera (mPECAM-Fc) treatment ameliorates CAIA in DBA 1/J mice when given after the onset of disease. In addition to suppressing hind paw swelling, we show for the first time that PECAM-Fc chimera also reduced bone and cartilage destruction during the course of disease. These results show that PECAM plays an important role in the progression of CAIA and suggest that PECAM-Fc may have therapeutic value for the clinical treatment of RA.
Materials and Methods
Mice
Female DBA 1/J (6 wks old) were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed for 2 wks at Weill Medical College of Cornell University prior to experiments. Animals were used at 8 wks of age.
Isolation and purification of mPECAM-Fc from transgenic sera
Transgenic mice expressing high levels of mPECAM-Fc (Tg11) have been described previously (Liao et al., 1999). These mice constitutively secrete a fusion protein composed of the extracellular portion of murine PECAM and the Fc domain of human IgG1. The transgenic mPECAM-FC protein was purified from pooled Tg11 serum by affinity chromatography using a protein A sepharose column. The bound protein was eluted with 0.1 M glycine (pH 2.5) and neutralized with 1/10 vol of 1 M Tris-HCl. After dialysis in PBS, the purified protein was filter-sterilized and stored at −20°C until use. Human IgG1 was purified in a similar manner and used as a negative control.
The molecular size of the purified transgenic protein was verified by SDS-PAGE under non-reducing conditions. The gel was stained with Coomassie blue to verify the 230 kD mPECAM-Fc band and purity.
PECAM-Fc Quantification
Soluble chimeric PECAM-Fc protein was quantified by ELISA using purified human IgG1 as standards as described by Liao et al (Liao et al., 1995). In brief, 96-well polyvinyl microtiter dishes were coated with 25 µg/ml of purified goat anti-human Fc Ab (Pierce, Rockford, IL), nonspecific binding was blocked with PBS containing 0.1% OVA, and dilutions of the test sera (or purified chimera) were then incubated on the treated plates, which were then washed extensively. Bound chimera was detected with alkaline phosphatase-conjugated goat anti-human Fc polyclonal Ab (Pierce) and substrate (p-nitrophenyl phosphate) in Attophos substrate buffer (JBL Scientific, San Luis Obispo, CA). Fluorescence was quantified on a Cytofluor 3500 (PerSeptive Biosystems, Framingham, MA) using known quantities of human IgG1 as standards.
Induction of Arthritis
Commercially available Arthrogen-CIA Monoclonal Antibody Blend (Chemicon International), was used according to the protocol described (Kachigian, 2006). Briefly at day -3 mice received an intraperitoneal (i.p.) injection of 4 mg of Arthrogen monoclonal antibody cocktail under isofluorane anaesthesia. Control mice received an equal volume of PBS. On day 0 all mice received and i.p. injection of 50 µg of LPS. Mice were monitored daily for arthritic symptoms. By day 2 all mice receiving arthrogen showed hind paw redness and edema. At this point all mice were randomized into treatment and control groups. Mice received i.p. injections of 100 µg of mPECAM-Fc (or control human IgG1, to match the isotype of the human Fc portion of the chimera) every other day (experiment 1) or every third day (experiment 2) for 14–17 days after which mice were sacrificed by CO2 asphyxiation and analyzed.
Peripheral Leukocyte Counts
Blood was obtained from all mice in the middle and end of the experiment by retro-orbital bleeding. Approximately 20 ul of heparinized blood was diluted with 180 ul of Turks solution to lyse RBC’s. Leukocytes were visualized under a light microscope and counted using a hemacytometer.
Ankle Thickness Measurements
Hind paw swelling was monitored throughout the experiment by measurements with a dial thickness gauge (Mitutoyo, USA).
Histologic Analysis of Joints
Following sacrifice, the hind legs and forearms of every animal were severed and fixed in 10% (wt/vol) buffered formalin. The specimens were then decalcified embedded in paraffin. Sections were stained with hematoxylin and eosin for microscopic evaluation. The severity of arthritis in each joint was graded by an experienced pathologist blind to the identity of the specimens. A histopathology score was assigned to each slide based on the following criteria: 0= normal, no pathology; 1=mild inflammation, mild synovial hyperplasia; 2=moderate synovial hyperplasia and pannus formation; 3=severe synovial hyperplasia with no bone destruction; 4=focal bone destruction; 5=diffuse bone destruction. After scoring the results were unblinded and the score for all four paws was averaged for each mouse.
Statistical Analysis
Values were expressed as the mean +/− SEM. Statistical analysis between group I and group II was performed using Student’s t-test and Wilcoxon sum rank test. All analysis was performed using GraphPad Prism software.
Results
mPECAM-Fc treatment protects against inflammation
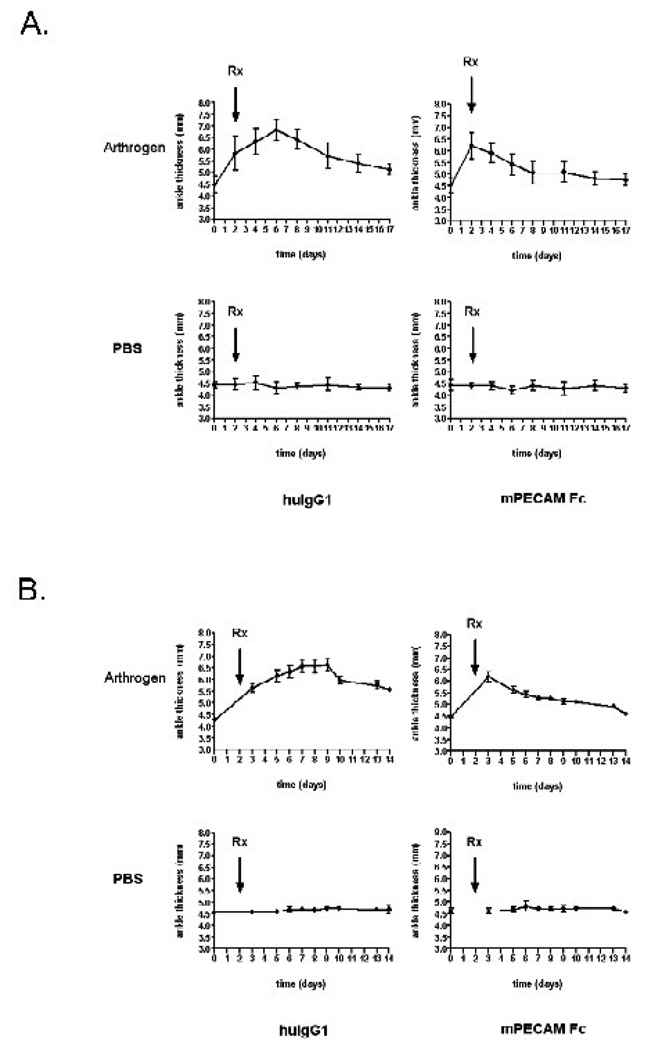
Figure 1a shows ankle thickness measurements throughout the course of CAIA. On Day -3, mice received either 4 mg of arthrogen antibodies or PBS. On Day 0 all mice received 50 µg of LPS. Several mice that had received arthrogen already showed mild ankle swelling at this point. By Day 2 almost all of the arthrogen treated mice were symptomatic and showed a significant increase in ankle thickness, but were otherwise healthy. The control mice appeared to be unaffected. At this point the arthrogen-treated and PBS-treated mice were randomized into two treatment groups. One group received 100 µg of mPECAM-Fc every other day; the other received 100 µg of human IgG1 every other day.
Figure 1. Soluble PECAM chimera reduces inflammation in collagen antibody induced arthritis.
Ankle thickness measurements (in mm) are shown throughout the time course of CAIA. On Day -3 (not shown), mice received either 4 mg of arthrogen antibody cocktail or PBS. The mice in the top panels received arthrogen (10 mice per group) whereas the mice in the bottom panels received PBS (5 mice per group). All mice received 50 µg of LPS on day 0. Starting at day 2 (arrow Rx,) all mice that received arthrogen or PBS were randomized into two treatment groups that received 100 µg of either human IgG1 (left column) or mPECAM-Fc (right column) intraperitoneally. A) Mice were treated with mPECAM-Fc or human IgG1 (huIgG1) every other day. The total area under the curve is significantly less for the arthrogen mice that received PECAM-Fc (12.49 arbitrary units) compared to arthrogen mice that received human IgG1 (23.35 arbitrary units). B) Mice were treated every third day. The total area under the curve is significantly less for the arthrogen mice that received PECAM-Fc (11.57 arbitrary units) compared to arthrogen mice that received human IgG1 (22.24 arbitrary units).
In the arthrogen-treated control group (treated with human IgG1 every other day) ankle thickness steadily increased between days 3 and 7. Although joint swelling began to decrease after day 7 (as is customary in this model (Kachigian, 2006), ankle thickness remained elevated above the initial background level until the end of the experiment (day 17). The progression of ankle swelling was significantly different in the mice treated with mPECAM-Fc every other day: Swelling decreased down to background levels from day 2 (the day they received mPECAM-Fc) through the end of the experiment. Therefore, it appeared that treating mice with mPECAM-Fc limited ankle joint inflammation. Control mice treated with either mPECAM-Fc or human IgG1 showed no change in ankle thickness throughout the time course.
Based on the success of this experiment, we repeated the experiment and administered mPECAM-Fc (or human IgG) to the mice every third day. A similar trend in ankle thickness was seen where mPECAM-Fc reduced the severity and progression of ankle thickness (Figure 1b). The area under the curve in both experiments for the mPECAM-Fc treated mice is half of the area for the arthritic mice treated with control IgG1.
mPECAM-Fc treatment reduced the severity and progression of CAIA
Since joint swelling represents mostly tissue edema, and this resolved (although not to baseline in the group treated with control IgG), we carried out histopathologic studies at the end of the experiments to determine whether there were any permanent changes in the joints as a result of the arthritis.
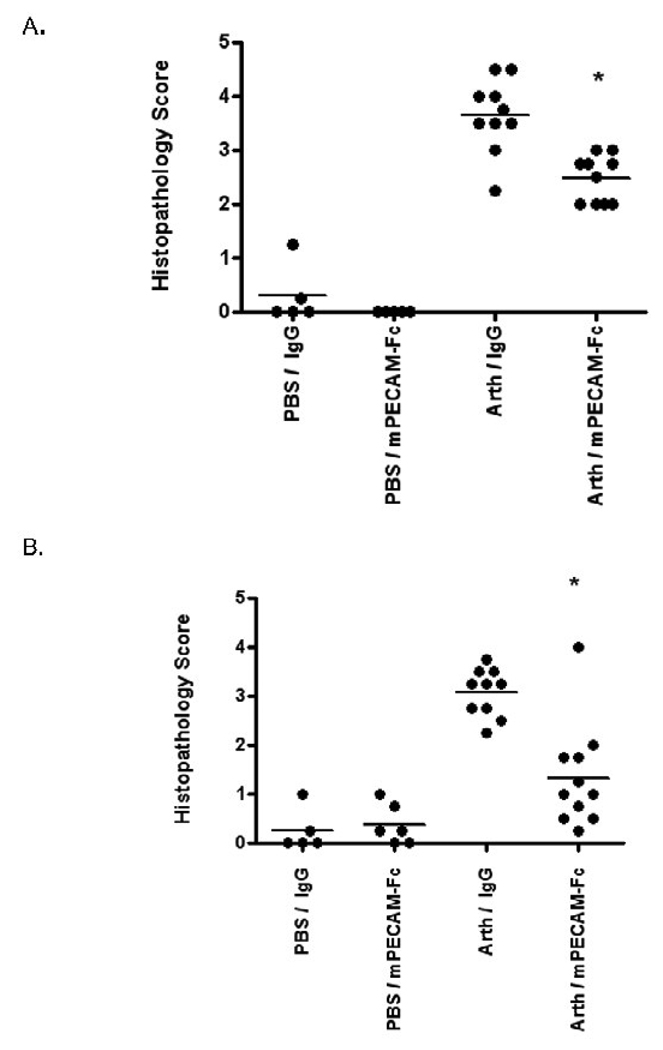
Each joint was assigned a histopathology score based on the severity of disease as described in Materials and Methods. The average histopathology score (assessing all four joints) for each mouse, as well as the mean for each treatment group, are shown in Figure 2. The full spectrum of chronic inflammatory changes was seen in affected joints, including severe erosion and invasion of articular cartilage by pannus and destruction of bone. Mice exposed to the arthrogen but treated with mPECAM-Fc showed a significantly lower histopathology score including much less bone destruction than arthrogen-exposed mice treated with human IgG1. This was true whether the mPECAM-Fc treatment was given every other day (Fig. 2A) or every third day (Fig. 2B). A score of 4 indicates focal bone destruction; a score of 5 indicates diffuse bone destruction. Although mPECAM-Fc treated mice showed evidence of disease (as compared to the PBS control groups), the treatment group showed significantly less histopathologic damage in terms of leukocyte infiltration, pannus formation and bone destruction than the arthritic mice treated with human IgG1.
Figure 2. Soluble PECAM chimera reduces pannus formation and joint destruction even when given after symptoms.
The histopathology score for mice in each treatment group is shown. The average combined score across all four joints (2 fore and 2 hind) is shown for each mouse as an individual dot. The mean value for each group is shown (bar). A) Mice were treated with mPECAM-Fc or human IgG1 every other day. B) Mice were treated every third day. In both experiments, arthrogen-treated mice (Arth) treated with mPECAM-Fc had a significantly lower mean histopathology score than arthrogen-treated mice treated with control IgG (* t-test, p<0.05).
mPECAM-Fc treated mice have normal numbers of circulating leukocytes
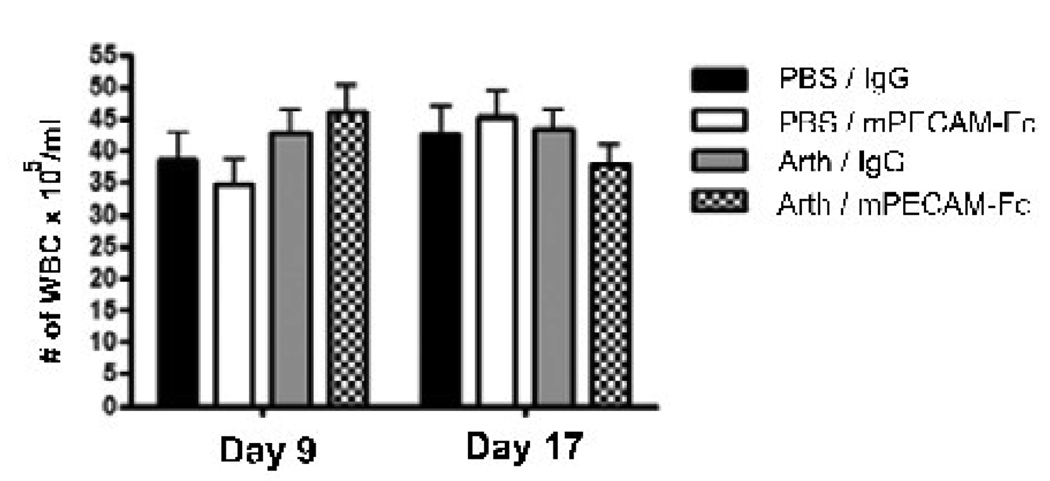
The number of circulating leukocytes in each animal at two different time points during the experiment is shown in Figure 3. The number of leukocytes present in peripheral blood for each animal was determined at the middle and end of arthritis progression. Comparison of the means shows that all four treatment groups had similar numbers of peripheral leukocytes throughout the time course of the experiment. These data show that mPECAM-Fc treatment does not decrease the total number of circulating leukocytes in the treated mice and that the decrease in joint infiltrating leukocytes seen in the treatment group is not due to a reduction in total number of leukocytes present in the animal.
Figure 3. Soluble PECAM chimera does not affect circulating leukocyte counts.
Peripheral leukocyte counts are shown for each treatment group at two timepoints (day 9 and day 17). Values are shown as the number of white blood cells per ml of blood. Data are shown as the average +/− SEM for each treatment group. There are no significant differences between the means across all treatments groups at both timepoints (p<0.01)
mPECAM-Fc ameliorates bone and cartilage destruction in arthritic mice
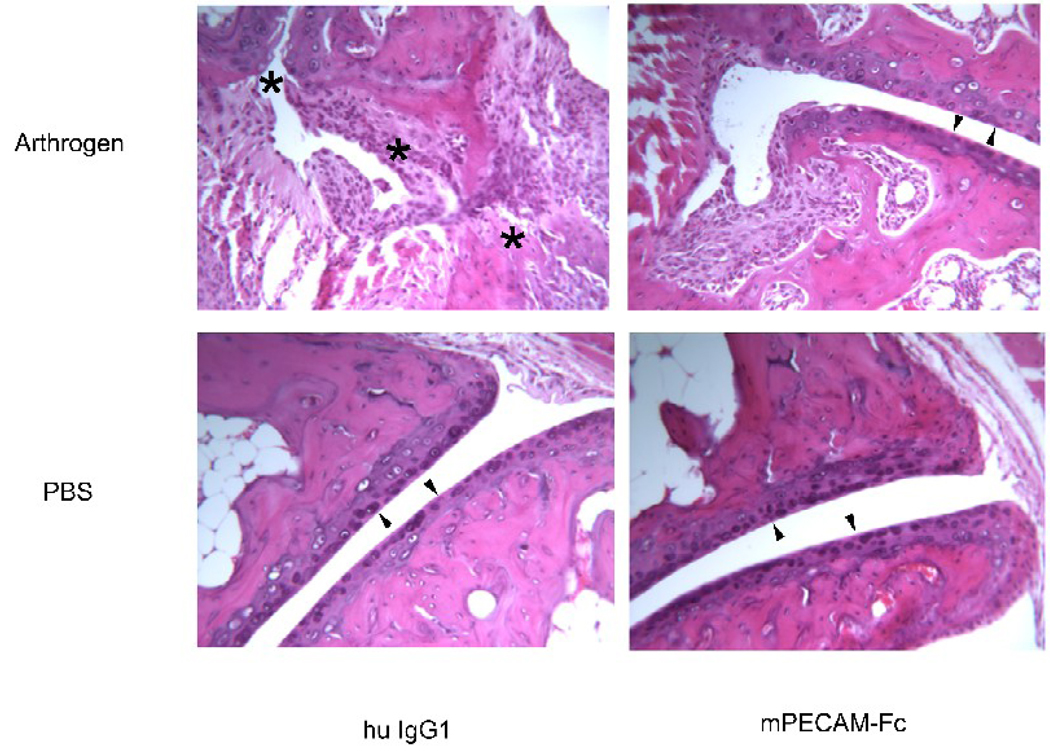
Representative joint histologic sections are shown in Figure 4. In the non-arthrogen treated animals no inflammatory cell infiltrates, synovitis, or joint destruction was observed. In the control arthritic mice treated with human IgG a massive inflammatory response was seen in the ankle joint. A large number of migrating leukocytes were present in the synovium. The synovial tissue showed significant pannus formation and extensive hyperplasia. The articular surface of the joint was almost completely destroyed and there was evidence of massive bone and cartilage erosion. In marked contrast, the mPECAM-Fc treated mice show few infiltrating leukocytes, limited synovial hyperplasia and much less bone and cartilage destruction.
Figure 4. Soluble PECAM chimera protects from bone destruction in CAIA.
Representative histopathology of hind joints. The same portion of the joint is shown in each panel. Minimal synovial hyperplasia or leukocyte infiltration is seen in the mice that did not receive arthrogen (bottom two panels). Note smooth articular cartilage surfaces (arrowheads). Mice that received arthrogen and were treated with control human IgG1 showed significant synovial hyperplasia, pannus formation and numerous infiltrating leukocytes (upper left panel). There was significant bone and cartilage destruction and disruption of the articular surface of the joint (asterisks along what used to be joint space). In contrast, mice that received arthrogen and were subsequently treated with PECAM-Fc showed markedly reduced leukocyte infiltration and synovial hyperplasia. Note preserved articular surface (arrowhead). There was also an absence of cartilage erosion or bone destruction, and the articular surfaces of the joint appear intact and smooth (upper right panel). Pictures are representative of all mice in each treatment group from all experiments. Original magnification = 125×.
Discussion
Our data show that treatment with a PECAM-blocking agent inhibits inflammation and progression of arthritis in the CAIA model. This model is a very robust one that mimicks the efferent phase of rheumatoid arthritis, where leukocytes are attracted by and respond to immune complexes in the joint. Affected joints display acute and chronic leukocyte infiltration, pannus formation, articular cartilage erosion and destruction of underlying bone. To our knowledge, this is the first report of a treatment that can prevent cartilage and bone destruction in a mouse model. In a previous study using the CIA model, treating arthritic mice with anti-PECAM antibody inhibited hindpaw swelling but failed to have an effect on joint destruction (Ishikawa et al., 2002). The discrepancies may relate to differences in the model. The chronic inflammatory changes and joint destruction that we observed were far more severe than the images shown in that study (Ishikawa et al., 2002), so the salutary effects of blocking PECAM function are likely to be more apparent in our model.
Just as important, the treatment was effective when given after the mice became symptomatic. This represents a more realistic clinical situation where patients present after the onset of symptoms. Mice were randomized into treatment groups after joint swelling was readily apparent. Those treated with control IgG developed progressive inflammation and joint destruction. In contrast, joint swelling in those treated with PECAM-Fc did not progress to the same severity and resolved more rapidly (Figure 1). The protection afforded by blockade of PECAM function was not limited to soft tissue edema. Histopathologic studies demonstrated markedly and significantly reduced inflammatory cell infiltrates in mice treated with mPECAM-Fc, which corresponded to a significant reduction in the irreversible changes associated with arthritis—cartilage and bone destruction (Figure 2 and Figure 4). Virtually no bone destruction was seen in any joint of affected mice treated with mPECAM-Fc.
Blockade of PECAM using monoclonal antibodies and PECAM-Fc significantly blocks monocyte and neutrophil emigration in several murine models of acute (Bogen et al., 1994; Liao et al., 1997; Reinke et al., 2007) and chronic inflammation (Kalinowska and Losy, 2006; Reinke et al., 2007; Rijcken et al., 2007). The antibody or PECAM-Fc interfere with the homophilic leukocyte PECAM-endothelial cell PECAM interactions that are required for leukocytes to cross blood vessels into sites of inflammation. The reductions in inflammatory cell infiltration and subsequent joint destruction in the mPECAM-Fc treated mice in this study are consistent with the known role of PECAM in leukocyte extravasation from blood into inflamed tissues, as seen in other models of inflammation.
However in studies where PECAM deficient (i.e. “PECAM knockout”) mice were used to examine models of acute and chronic inflammation, the results have been more varied and indicated both pro- and anti-inflammatory roles for PECAM. For example, PECAM deficient mice showed increased susceptibility to CIA and experimental autoimmune encephalitis (EAE) (Graesser et al., 2002; Tada et al., 2003; Wong et al., 2005) thereby indicating that in these studies the absence of PECAM accelerated disease progression. In other studies, PECAM deficiency had a protective effect on CIA and peritonitis. (Schenkel et al., 2004; Wong et al., 2005). The complexity of the response of PECAM deficient mice in models of chronic inflammation may be due in part to the fact that some studies focused on the role of T cells in the afferent arm of the response while others focused on the role of myeloid cells in the efferent arm. However, the recent finding that PECAM-1 controls leukocyte transmigration in all strains of mice tested except C57Bl/6 (Schenkel et al., 2006; Seidman et al., 2009) suggests that those studies employing PECAM-deficient mice in the C57Bl/6 strain may need to be reinterpreted (Graesser et al., 2002; Wong et al., 2005).
In summary, in the CAIA model of rheumatoid arthritis, which resembles the destructive (efferent) arm of the disease process, we find that blocking PECAM function using mPECAM-Fc significantly reduces inflammation and joint destruction even when given after the mice become symptomatic. Therefore PECAM may be an important therapeutic target for established inflammatory arthritis.
Acknowledgements
This work was supported by NIH grants R01 HL046489 and R37 HL064774 to W.A.M. and a predoctoral and postdoctoral fellowship (T32 AI07621) to B.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no financial conflicts relating to this work.
References
- Bogen S, Pak J, Garifallou M, Deng X, Muller WA. Monoclonal antibody to murine PECAM-1 [CD31] blocks acute inflammation in vivo. J Exp Med. 1994;179:1059–1064. doi: 10.1084/jem.179.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Maini RN. The role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatology. 1999;38 Suppl. 2:3–7. [PubMed] [Google Scholar]
- Graesser D, Solowiej A, Bruckner M, Osterweil E, Juedes A, Davis S, Ruddle NH, Engelhardt B, Madri JA. Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J Clin Invest. 2002;109:383–392. doi: 10.1172/JCI13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JM. Leukocyte-endothelial interactions. Blood. 1985;65:513–525. [PubMed] [Google Scholar]
- Ishikawa J, Okada Y, Bird IN, Jasani B, Spragg JH, Yamada T. Use of anti-platelet-endothelial cell adhesion molecule-1 antibody in the control of disease progression in established collagen-induced arthritis in DBA/1J mice. Jpn J Pharmacol. 2002;88:332–340. doi: 10.1254/jjp.88.332. [DOI] [PubMed] [Google Scholar]
- Kachigian LM. Collagen antibody-induced arthritis. Nature Protocols. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- Kalinowska A, Losy J. PECAM-1, a key player in neuroinflammation. Eur J Neurol. 2006;13:1284–1290. doi: 10.1111/j.1468-1331.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87:811–822. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. A new mouse model of rheumatoid arthritis: organ-specific disease provoked by systemic autoimmunity. Ryumachi. 1997;37:147. [PubMed] [Google Scholar]
- Liao F, Ali J, Greene T, Muller WA. Soluble domain 1 of platelet-endothelial cell adhesion molecule (PECAM) is sufficient to block transendothelial migration in vitro and in vivo. J Exp Med. 1997;185:1349–1357. doi: 10.1084/jem.185.7.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Huynh HK, Eiroa A, Greene T, Polizzi E, Muller WA. Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J Exp Med. 1995;182:1337–1343. doi: 10.1084/jem.182.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Schenkel AR, Muller WA. Transgenic mice expressing different levels of soluble platelet/endothelial cell adhesion molecule-IgG display distinct inflammatory phenotypes. J Immunol. 1999;163:5640–5648. [PubMed] [Google Scholar]
- Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, Marchal P, Duchatelle V, Degott C, van Regenmortel M, Benoist C, Mathis D. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. The role of PECAM-1 [CD31] in leukocyte emigration: Studies in vitro and in vivo. Journal of Leukocyte Biology. 1995;57:523–528. doi: 10.1002/jlb.57.4.523. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology. 2003;24:326–333. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke EK, Lee J, Zozulya A, Karman J, Muller WA, Sandor M, Fabry Z. Short-term sPECAM-Fc treatment ameliorates EAE while chronic use hastens onset of symptoms. J Neuroimmunol. 2007;186:86–93. doi: 10.1016/j.jneuroim.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijcken E, Mennigen RB, Schaefer SD, Laukoetter MG, Anthoni C, Spiegel HU, Bruewer M, Senninger N, Krieglstein CF. PECAM-1 (CD 31) mediates transendothelial leukocyte migration in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G446–G452. doi: 10.1152/ajpgi.00097.2007. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Chlipala E, Harbord MW, Muller WA. Different susceptibilities of PECAM-deficient mouse strains to spontaneous idiopathic pneumonitis. Exp Mol Pathol. 2006;81:23–30. doi: 10.1016/j.yexmp.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol. 2004;173:6403–6408. doi: 10.4049/jimmunol.173.10.6403. [DOI] [PubMed] [Google Scholar]
- Seidman MA, Chew TW, Schenkel AR, Muller WA. PECAM-independent thioglycollate peritonitis is associated with a locus on murine chromosome 2. PLoS ONE. 2009;4:e4316. doi: 10.1371/journal.pone.0004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Koarada S, Morito F, Ushiyama O, Haruta Y, Kanegae F, Ohta A, Ho A, Mak TW, Nagasawa K. Acceleration of the onset of collagen-induced arthritis by a deficiency of platelet endothelial cell adhesion molecule 1. Arthritis Rheum. 2003;48:3280–3290. doi: 10.1002/art.11268. [DOI] [PubMed] [Google Scholar]
- Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13:1–14. doi: 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Terato K, Harper DS, Griffiths MM, Hasty DL, Ye XJ, Cremer MA, Seyer JM. Collagen-induced arthritis in mice: synergistic effect of E. coli lipopolysaccharide bypasses epitope specificity in the induction of arthritis with monoclonal antibodies to type II collagen. Autoimmunity. 1995;22:137–147. doi: 10.3109/08916939508995311. [DOI] [PubMed] [Google Scholar]
- Terato K, Hasty KA, Cremer MA, Stuart JM, Townes AS, Kang AH. Collagen-induced arthritis in mice. Localization of an arthritogenic determinant to a fragment of the type II collagen molecule. J Exp Med. 1985;162:637–646. doi: 10.1084/jem.162.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg WB. Lessons from animal models of arthritis. Curr Rheumatol Rep. 2002;4:232–239. doi: 10.1007/s11926-002-0070-5. [DOI] [PubMed] [Google Scholar]
- Vaporciyan AA, Delisser HM, Yan H-C, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- Wong MX, Hayball JD, Hogarth PM, Jackson DE. The inhibitory co-receptor, PECAM-1 provides a protective effect in suppression of collagen-induced arthritis. J Clin Immunol. 2005;25:19–28. doi: 10.1007/s10875-005-0354-7. [DOI] [PubMed] [Google Scholar]
- Wooley PH, Luthra HS, Stuart JM, David CS. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]