Abstract
The purpose of this study was to examine expression and function of estrogen receptor-related receptors (ERRs) in human glioma and astrocytoma cell lines. These estrogen receptor-negative cell lines expressed ERRα and ERRγ proteins to varying degree in a cell context dependent manner, with U87MG glioma cells expressing both orphan nuclear receptors. Cell proliferation assays were performed in the presence of ERR isoform-specific agonists and antagonists, and the calculated EC50 and IC50 values were consistent with previous reported values determined in other types of cancer cell lines. Induction of luciferase expression under the control of ERR isoform-specific promoters was also observed in these cells. These results indicate that ERRα and ERRγ are differentially expressed in these tumor cell lines and likely contribute to agonist-dependent ERR transcriptional activity.
Keywords: DY131, biochanin A, XCT790, 4-hydroxytamoxifen, AAB-TATA-Luc, 3xERE-TATA-Luc, T98G, U87MG, A-172 cells, ERRγ, ERRα
1. Introduction
In humans, gliomas form the vast majority of malignant brain tumors and are among the most lethal (Wrensch et al., 2006). Gliomas are tumors of glial cells, and among them astrocytomas represent about half of the central nervous system cancers (Trojan et al., 2007). Glioblastomas, the highest grade of gliomas, are highly invasive and exhibit spontaneous resistance to chemotherapeutic drugs, which is partly due to a lack of specificity of the drugs and to the inability to achieve their efficacious targeting concentrations. As a result, the various therapeutic means have thus far been inefficient in significantly improving patient survival. So new ways of treatment are constantly being investigated (Prados et al., 2006; Stupp et al., 2006).
It has been previously reported that a subset of estrogen receptor (ER)-negative breast cancers can respond to tamoxifen (Goldenberg and Froese, 1982, Gelmann, 1996), whereas some ERα-positive breast cancers are tamoxifen resistant (Ingle et al., 1991, Jaiyesimi et al., 1995). These and other findings led to the search for novel tamoxifen-binding nuclear receptors and resulted in the identification of the estrogen related receptors ERRα and ERRγ (Giguère et al., 1988, Heard et al., 2000). It was later found that the antiestrogen tamoxifen was a competitive inhibitor of ERRγ, while being inactive toward ERRα (Coward et al., 2001). A recent study has showed that tamoxifen can inhibit the proliferation of a series of malignant ER-negative glioma cells (Hui et al., 2004); however, no attempt was made to establish the presence of ERR in these gliomas.
ERRα, ERRβ, and ERRγ form a subfamily of orphan nuclear receptors that have significant amino acid homology with the estrogen receptors ERα and ERβ (Ariazi and Jordan, 2006). As with other nuclear receptors ERRs are organized into modular domains with a less characterized N-terminal domain, a highly conserved DNA binding domain (DBD), and a potential ligand binding domain (LBD). ERRs share high homology in their DBD, and are constitutively active without binding to natural estrogen (Giguère, Horard and Vanacker, 2003). Like ERs, all ERRs bind to the classic ERE motif (AGGTCANNNTGACCT), suggesting that ERRs may be involved in related ER-mediated signaling pathways through association with coregulatory proteins and regulation of target gene expression. ERRs also bind to ERE-related response elements with extended half-site core sequences (TNAAGGTCA; ERRE/SF-1RE; Razzaque et al., 2004), indicating that ERRs may also have their own independent regulatory pathways or functions distinct from ERs. Therefore, it is likely that ERRs may regulate a broad spectrum of genes in target cells.
Previous studies have demonstrated that ERRs are constitutively active and that this activity is controlled by coactivator concentration rather than small ligands (Greschik et al., 2002; Kallen et al., 2004). Indeed, it has been shown that a ligand is not required for the ERRγ LBD to adopt a transcriptionally active conformation and interact with the PPARγ coactivator-1 (PGC-1) (Hentschke et al., 2002). In addition, while ERRs share significant LBD homology with ERs, they have different responses to ER agonists and antagonists. For example, the ER agonist estradiol is inactive in ERR expressing cell lines (Giguère et al., 1988, Hong et al., 1999), and diethyl stilbestrol, a structurally unrelated ER agonist, exerts antagonistic properties on all three ERR subtypes (Tremblay et al 2001). While 4-hydroxytamoxifen (4-OHT) does not affect ERRα, it acts instead as an ERRβ and ERRγ antagonist (Coward P et al., 2001).
The tissue-specific distribution, expression levels, and function of various ERR subtypes has been reported previously (Giguère et al., 2008; Bookout et al., 2006). However, the relative quantity and/or repertoire of ERR subtypes in glioma and astrocytoma tumor lines has not been addressed to date. The objectives of this study were to examine the expression of ERRs in a number of human ERα-negative glioma and astrocytoma cell lines. Moreover, the effect of known isoform-specific agonists and antagonists of ERRα and ERRγ on cellular proliferation and ERR reporter activity was investigated. These results could lead to new therapeutic approaches for the treatment of malignant gliomas and astrocytomas.
2. Materials and Methods
2.1. Cell culture
All cell lines were purchased from American Type Culture Collection (Manassas, VA). T98G, U87MG, U138, U118MG, U373MG, and LN229 cells were cultured in phenol red-free minimal essential medium (α-MEM) (Invitrogen, Carlsbad, CA), while the A-172 and MCF-7 cells were maintained in phenol red-free DMEM (Invitrogen). Both media were supplemented with penicillin (25 U/ml), streptomycin (25 U/ml), L-glutamine (Invitrogen), pyruvic acid, nonessential amino acids, and 5% charcoal/dextran-treated fetal bovine serum (Hyclone, Logan, Utah). Cells were maintained at 37°C in a humidified incubator with 5% CO2.
2.2. Western blotting
Cells grown at approximately 70–80% confluence were lysed in lysis buffer (10 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 5 mM EDTA, pH 8.0, 1% Triton X-100) supplemented with a protease inhibitor cocktail (Complete EDTA-free protease inhibitor mixture tablets; Roche Molecular Biochemical, Indianapolis, IN). Insoluble material was removed by centrifugation (12,000 × g for 20 min at 4 °C) and the precleared lysates were aliquoted and stored at −70 °C until analysis. The protein concentration of the lysates was measured with the BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL) using bovine serum albumin as standard, and samples were normalized for protein content. An equal amount of 3X sample buffer was added, and samples (70 µg) were separated by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes using iBLOT (Invitrogen). The membranes were blocked with 5% milk in Tris-buffered saline/0.1% Tween-20, and then probed with primary antibodies at optimal dilutions, followed by horseradish peroxidase-conjugated secondary antibodies. The ECL Western blotting detection system (GE Healthcare, Piscataway, NJ) was used for visualization. The primary antibodies used were: rabbit polyclonal antibodies against ERRα and ERRγ (Affinity BioReagents, Golden, CO) and mouse monoclonal anti-β-actin (Abcam, Cambridge, MA).
2.3. MTS assay
The non-radioactive CellTiter 96® AQueous cell proliferation assay was performed according to the manufacturer’s protocol (Promega, Madison, WI). Briefly, cells were seeded in 96-well plates at a density of 3500 cells/well and cultured in complete phenol red-free medium for 24 h. Test compounds dissolved in dimethyl sulfoxide (DMSO) and mixed with culture medium were added to the cells in different concentrations. The compounds were as followed: DY131 (Tocris Bioscience, Ellisville, MO) [25, 50, 75, 100, 125, 150 and 175 nM]; 4-OHT (Sigma-Aldrich, St. Louis, MO) [0.1, 0.25, 0.5, 0.75, 1 and 2 µM]; 5,7-dihydroxy-4’-methoxyisoflavone (biochanin A) (Sigma-Aldrich) [25, 50, 75, 100, 125 and 250 nM]; and XCT790 (Sigma-Aldrich) [0.1, 0.5, 1, 2, 3 and 4 µM]. Control cultures were treated with DMSO. The maximum concentration of DMSO added to the medium was 0.5 % and was found not to be cytotoxic. After 48 h, 20 µl of 0.5 mg/ml MTS solution was added to each well, and the cultures were further incubated for 1 h. The absorbance was measured at 490 nm with a microplate reader (Thermo Scientific, USA). Change in growth rate was calculated as follows: [A490nm of treated cells/A490nm of control cells]. Three wells were used for each treatment, and the experiments were repeated three times. EC50 for agonists DY131 and biochanin A, and IC50 for antagonists 4-OHT and XCT790 were calculated using the Graphpad Prism software (La Jolla, CA).
2.4. Luciferase assays
For reporter assays, cells were approximately 70% confluent at the time of transfection in six-well plates. For each dish, 0.2 µg ERR reporter plasmid (AAB-TATA-Luc or 3X ERE-TATA-Luc; both kindly provided by Christina T. Teng, National Institute of Environmental Health Sciences, Research Triangle Park, NC) and 0.1 µg pCMVβ-gal vector (Clontech Laboratories, Mountain View, CA) were mixed and used for transient transfection using LipofectAMINE 2000. After overnight incubation, the medium was replaced with complete medium with or without test compounds. Twenty-four hours later, cells were harvested and aliquots of each lysate were assayed for luciferase and β-gal activities according to the manufacturer’s instructions (Promega). The luciferase activity was normalized to that of β-gal. These experiments were performed in three separate experiments each in triplicate.
2.5. Statistical analyses
All the experiments were performed in triplicate and repeated three times. Unless otherwise indicated, the data represent the means ± SE. Differences between mean values were compared statistically by Student’s t test and ANOVA. P < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Estrogen receptor-related receptor expression in glioblastoma and astrocytoma cell lines
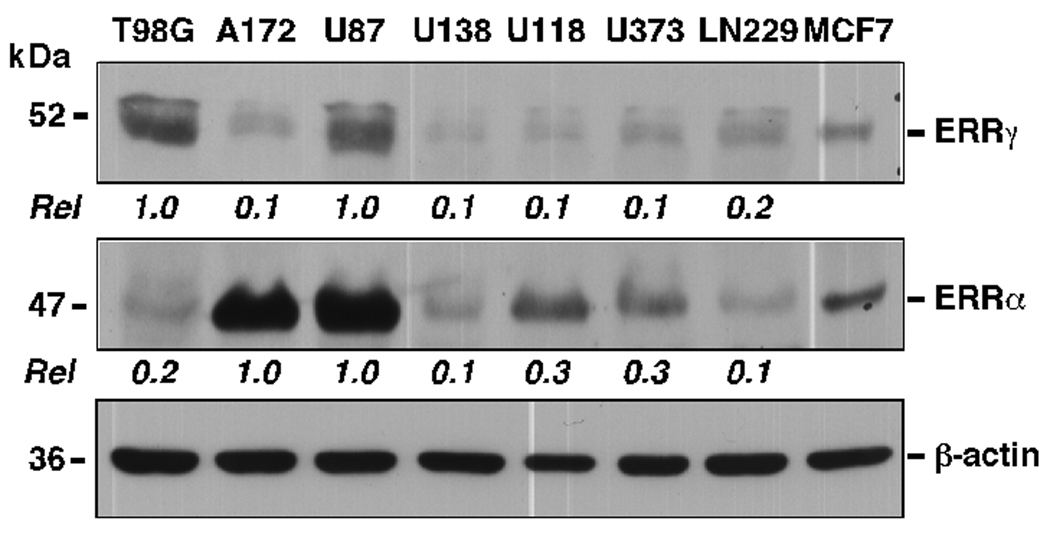
A comparative analysis of the relative expression levels of ERRs was performed in seven human ER-negative glioblastoma and astrocytoma cell lines using Western immunoblotting (Fig. 1). The breast cancer cell line MCF-7 was used as positive control as it expresses ERRα and ERRγ (Cheung et al., 2005). The results demonstrated cell-type specific expression of ERRα and ERRγ proteins. High constitutive levels of ERRα expression were observed in A-172 and U87MG cells, and significantly lower abundance in T98G, U138MG, and LN229 cells (Fig. 1). In contrast, the levels of ERRγ were the highest in T98G and U87MG cells, but almost undetectable in the other cell lines. These findings demonstrate that these ER-negative glioma and astrocytoma cell lines express a unique repertoire of ERR proteins, which could be reflective of distinct cellular functions.
FIG. 1.
Constitutive expression of cellular ERRα and ERRγ proteins. The human brain tumor cell lines T98G, A-172, U87MG, U138MG, U118MG, U373MG and LN229, and the MCF-7 breast cancer cells were lysed and processed for Western blot analysis. The relative units (Rel) show the average of triplicate experiments, where the densitometric quantitation of the signals corresponding to ERRα and ERRγ proteins were normalized to that of β-actin.
3.2. Role of ERRs in cell proliferation
The constitutive expression of ERRα and/or ERRγ in T98G, A-172 and U87MG cells make them suitable models for the study of the role of ERRs in astrocytoma and glioblastoma cell proliferation. The non-radioactive MTS cell proliferation assay can be substituted for [3H]thymidine incorporation. Consequently, compounds blocking cell proliferation will decrease the reduction of the soluble tetrazolium salt, MTS, to formazan product in intact cells.
The ERRγ agonist DY131, which has been shown to induce a ~3 to 4-fold increase in ERRγ transcriptional activity in CV-1 cells (Yu and Forman, 2005), and 4-OHT were used to characterize the role of endogenous ERR in cell proliferation. Treatment of T98G and U87MG cells with DY131 for 48 h increased cell proliferation in a dose-dependent manner, with a maximum at 0.175 µM and EC50 values of 0.054 ± 0.016 µM and 0.062 ± 0.001 µM in T98G and U87MG cells, respectively (Fig. 2A). Blocking the ERRγ activity with 4-OHT markedly reduced cell proliferation, with IC50 values of 1.58 ± 0.21 µM and 1.46 ± 0.16 µM in T98G and U87MG cells, respectively (P ≤ 0.01) (Fig. 2B). These values are consistent with the previously reported IC50 value of 4.2 ± 2.0 µM in MCF-7 human breast cancer cells (Davis et al., 2008). The A-172 cells did not respond to either DY131 or 4-OHT, consistent with their lack of ERRγ protein (see Fig. 1).
FIG. 2.
Effect of ERR agonists and antagonists on glioma cell proliferation. A-172, T98G and U87MG cells were incubated with the indicated concentrations of the ERRγ agonist, DY131 (A), ERRγ antagonist, 4-OHT (B), ERRα agonist, biochanin A (C), or ERRα antagonist, XCT790 (D) for 48 h followed by another 60 min incubation in the presence of 0.5 mg/ml MTS. Reduction of MTS was determined colorimetrically. (E) U87MG cells were incubated either with vehicle, 1 µM 4-OHT, 1.5 µM XCT790 or the combination 4-OHT plus XCT790 for 48 h followed by MTS treatment. Values are means ± SE from 3 independent experiments performed in triplicate. Statistical analysis by ANOVA, with aP < 0.05 and bP < 0.01 vs. vehicle-treated controls.
Three isoflavones (daidzein, biochanin A and genistein) and one flavone (6, 3’, 4’-trihydroxyflavone) have been identified as specific ERRα agonists, capable at inducing ERRα transcriptional activity in HeLa cells (Suetsugi et al., 2003). Of these, biochanin A was the most potent phytoestrogen tested. In contrast, the compound XCT790 has been found to inhibit ERRα transcriptional activity in the human breast cancer derived MCF-7 cell line, while promoting proteosomal degradation of this orphan nuclear receptor (Lanvin et al., 2007). Treatment of A-172 and U87MG cells with biochanin A significantly increased cell proliferation after 48 h, with a maximum at 0.250 µM and EC50 values of 0.046 ± 0.001 µM and 0.045 ± 0.002 µM in A-172 and U87MG cells, respectively (Fig. 2C). These values are 10-fold lower than the previously reported EC50 value of 0.46 µM in MCF-7 cells (Joung et al., 2003). The ERRα antagonist XCT790 produced a significant inhibition of cell proliferation in A-172 and U87MG cells, with IC50 values of 2.1 ± 0.19 µM and 3.7 ± 0.4 µM, respectively (Fig. 2D), in close agreement with the reported IC50 value of ~ 0.40 µM in various cell-based assays (Willy et al., 2004). The T98G cells did not respond to either biochanin A or XCT790, consistent with their lack of ERRα protein (see Fig. 1).
Because U87MG cells contain both ERRα and ERRγ, we evaluated the effect of the combination 4-OHT plus XCT790 on cell proliferation. When used at concentrations at or near IC50, both compounds acted in an additive fashion with more than 67 % inhibition as compared to the 24% and 29% inhibition when 4-OHT and XCT790 were added alone (Fig. 2E).
3.3. In vitro transcriptional activity of ERRs
To confirm that the endogenously expressed ERRs are transcriptionally active, a luciferase reporter construct containing either the 3xERE-TATA-Luc or AAB-TATA-Luc (Zhang and Teng, 2007) was transfected into T98G, A-172 and U87MG cells along with a plasmid encoding β-galactosidase for normalization (Fig. 3A). The AAB-TATA-Luc promoter activity was greater in T98G and U87MG cells than in A-172 cells, in agreement with an earlier report showing that ERRγ is specific for AAB elements (Zhang and Teng, 2007). Conversely, 3xERE-TATA-Luc promoter activity level was significantly higher in A-172 and U87MG cells when compared to T98G cells, consistent with a previous report showing that ERRα is specific for 3xERE element (Zhang and Teng, 2007).
FIG. 3.
Constitutive and ligand-induced ERR promoter activity in human brain tumor cell lines. (A) A-172, T98G and U87MG cells were transiently transfected with either the 3xERE-TATA-Luc or AAB-TATA-Luc plasmid together with β-galactosidase vector. Twenty-four hours later, the cells were incubated in medium supplemented with 5% charcoal/dextran-treated serum for 24 h. (B) T98G and U87MG cells were transfected with the AAB-TATA-Luc plasmid followed by a 24-h incubation either with DMSO, 175 nM DY131, or 2 µM 4-OHT. (C) A-172 and U87MG cells were transfected with the 3xERE-TATA-Luc plasmid, followed by a 24-h incubation either with vehicle (DMSO), 250 nM biochanin A, or 2 µM XCT790. Results are expressed as means ± SD of a single experiment performed in triplicate dishes. Results are representative of three separate experiments with similar results.
We examined the ability of ERRγ agonist and antagonist to modulate AAB-TATA-Luc promoter activity in T98G and U87MG cells. The ligand DY131 (175 nM) stimulated the promoter activity level by ~2.5-fold, while the antagonist 4-OHT (2 µM) blocked the constitutive ERRγ transcriptional activity (Fig. 3B). Moreover, the ability of ERRα agonist and antagonist to modulate the 3xERE-TATA-Luc promoter activity was examined in A-172 and U87MG cells. The promoter activity level was stimulated approximately 2-fold with biochanin A (250 nM), but markedly inhibited upon cell treatment with XCT790 (2 µM) (Fig. 3C).
It has been recently reported that the transcriptional activity of ectopically expressed ERRγ construct was dependent on AP-1/c-Jun complex, indicating a non-nuclear action of ERRγ (Riggins et al., 2008). Of interest, we sequenced the two promoters used in our study (3XERE-TATA-Luc and AAB-TATA-Luc), and could not find the consensus AP-1 sequence (TGAC/GTC/GA). However, several ‘AP-1 like’ sites were present especially in the AAB-TAT-Luc construct (Supplemental Fig. S1). Future experiments aimed at determining the role of these ‘AP-1 like’ sites in ERR transcriptional activity is warranted but these fall outside the scope of the current study.
Given that ERRα and ERRγ are differentially expressed both in normal and tumor tissues, it is tempting to speculate that some of the cellular processes involved in the control of gene expression may depend on the expression profile of ERR isoforms. On the other hand, protein dimerization modulates the transcriptional activities of ERRs. Although active as a monomer, ERRγ homodimer exhibits enhanced transcriptional activity, whereas its heterodimerization with ERRα results in impaired activities of both ERRα and ERRγ (Huppunen and Aarnisalo, 2004). Therefore, formation of ERRγ-ERRα heterodimers in U87MG cells may well direct some gene expression program distinct from that of ERRγ homodimers or ERRα homodimers in T98G and A-172 cells, respectively.
4. Conclusion and implications
There have been a number of clinical studies exploring the use of tamoxifen in the treatment of gliomas, both as monotherapy or in combination with DNA alkylating agents or radiotherapy (Parney and Chang, 2003; Robins et al., 2006). The results have been varied and, on the whole, disappointing as the use of high dose tamoxifen appears to have limited efficacy in a majority of the patients. However, there continues to be interest in the use of tamoxifen and other selective estrogen receptor modulators (SERMs) in the treatment of gliomas, even though these tumors are estrogen receptor negative. A recent study by Hui et al. (2004) demonstrated that both tamoxifen and CC-8490, a benzopyrone with SERM activity, displayed antiglioma activity in vitro and in vivo. The ability of tamoxifen to inhibit glioma cell proliferation and induce apoptosis has been associated with inhibition of protein kinase C (PKC) activity (Baltuch et al., 1995; Mastronardi et al., 1998) and the data from the study of Hui. et al. suggests that the antiglioma activity of SERMs can be enhanced through the inhibition of the NF-κ pathway (Hui et al., 2004).
It is of interest that the majority of the in vitro data reported by Hui et al. was obtained using the U87MG cell line and the in vivo xenograft model was also based upon implanted U87MG cells (Hui et al., 2004). The data from this study indicates that the U87MG cells express high levels of both ERRα and ERRγ (Fig. 1) and that these proteins are responsive to isoform-specific inhibitors (Fig. 2 and Fig. 3). This suggests that the SERM related antiglioma activity observed by Hui et al. (2004) might by due in part to the antagonist activity of these compounds at the level of ERRγ. In addition, the data from this study demonstrate that ERRα and ERRγ were differentially expressed at the protein levels in the three glioma and astrocytoma cell lines examined, and that the A172 and T98G cell lines contained only ERRα or ERRγ protein, respectively. Our results suggest that the variable response to tamoxifen in the clinical treatment of glioblastomas may reflect the variable expression of ERRα or ERRγ and that better outcomes may be obtained through the use of tamoxifen and an ERRα antagonist. The expression of ERRα and ERRγ in tumor biopsy samples is currently in progress and the results will be reported elsewhere.
Supplementary Material
Acknowledgments
The authors wish to thank Sarah S. Subaran, Research Resources Branch, Intramural Research Program of the National Institute on Aging, NIH for her expert technical assistance with the immunofluorescence assays. The work was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr. Top. Med. Chem. 2006;6:203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- Baltuch GH, Dooley NP, Villemure JG, Yong VW. Protein kinase C and growth regulation of malignant gliomas. Can. J. Neurol. Sci. 1995;22:264–271. doi: 10.1017/s0317167100039457. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J. Clin. Endocrinol. Metab. 2005;90:1830–1844. doi: 10.1210/jc.2004-1421. [DOI] [PubMed] [Google Scholar]
- Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DD, Díaz-Cruz ES, Landini S, Kim YW, Brueggemeier RW. Evaluation of synthetic isoflavones on cell proliferation, estrogen receptor binding affinity, and apoptosis in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008;108:23–31. doi: 10.1016/j.jsbmb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmann EP. Tamoxifen induction of apoptosis in estrogen receptor-negative cancers: new tricks for an old dog? J. Natl. Cancer Inst. 1996;88:224–226. doi: 10.1093/jnci/88.5.224. [DOI] [PubMed] [Google Scholar]
- Giguère V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Giguère V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Giguère V. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Goldenberg GJ, Froese EK. Drug and hormone sensitivity of estrogen receptor-positive and -negative human breast cancer cells in vitro. Cancer Res. 1982;42:5147–5151. [PubMed] [Google Scholar]
- Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, Renaud JP. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol. Cell. 2002;9:303–313. doi: 10.1016/s1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRγ, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol. Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Süsens U, Borgmeyer U. PGC-1 and PERC, coactivators of the estrogen receptor-related receptor γ. Biochem. Biophys. Res. Commun. 2002;299:872–879. doi: 10.1016/s0006-291x(02)02753-5. [DOI] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J. Biol. Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Horard B, Vanacker JM. Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J. Mol. Endocrinol. 2003;31:349–357. doi: 10.1677/jme.0.0310349. [DOI] [PubMed] [Google Scholar]
- Hui AM, Zhang W, Chen W, Xi D, Purow B, Friedman GC, Fine HA. Agents with selective estrogen receptor (ER) modulator activity induce apoptosis in vitro and in vivo in ER-negative glioma cells. Cancer Res. 2004;64:9115–9123. doi: 10.1158/0008-5472.CAN-04-2740. [DOI] [PubMed] [Google Scholar]
- Huppunen J, Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRγ. Biochem. Biophys. Res. Commun. 2004;314:964–970. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- Ingle JN, Twito DI, Schaid DJ, Cullinan SA, Krook JE, Mailliard JA, Tschetter LK, Long HJ, Gerstner JG, Windschitl HE, et al. Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer. 1991;67:886–891. doi: 10.1002/1097-0142(19910215)67:4<886::aid-cncr2820670405>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. J. Clin. Oncol. 1995;13:513–529. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- Joung KE, Kim YW, Sheen YY. Assessment of the estrogenicity of isoflavonoids, using MCF-7-ERE-Luc cells. Arch. Pharm. Res. 2003;26:756–762. doi: 10.1007/BF02976687. [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou Riou, Graham A, Strauss A, Geiser M, Fournier B. Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J. Biol. Chem. 2004;279:49330–49337. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]
- Lanvin O, Bianco S, Kersual N, Chalbos D, Vanacker JM. Potentiation of ICI182,780 (Fulvestrant)-induced estrogen receptor-α degradation by the estrogen receptor-related receptor-α inverse agonist XCT790. J. Biol. Chem. 2007;282:28328–28334. doi: 10.1074/jbc.M704295200. [DOI] [PubMed] [Google Scholar]
- Mastronardi L, Farah JO, Puzzilli F, Ruggeri A. Tamoxifen modulation of carboplatin cytotoxicity in human U-138 glioma cell line. Clin. Neurol. Neurosurg. 1998;100:89–93. doi: 10.1016/s0303-8467(98)00004-3. [DOI] [PubMed] [Google Scholar]
- Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003;9:149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, Mehta M, Fine HA, Wen PY, Cloughesy T, Chang S, Nicholas MK, Schiff D, Greenberg H, Junck L, Fink K, Hess K, Kuhn J North American Brain Tumor Consortium. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque MA, Masuda N, Maeda Y, Endo Y, Tsukamoto T, Osumi T. Estrogen receptor-related receptor-γ has an exceptionally broad specificity of DNA sequence recognition. Gene. 2004;340:275–282. doi: 10.1016/j.gene.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Riggins RB, Lan JP, Zhu Y, Klimach U, Zwart A, Cavalli LR, Haddad BR, Chen L, Gong T, Xuan J, Ethier SP, Clarke R. ERRγ mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68:8908–8917. doi: 10.1158/0008-5472.CAN-08-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins HI, Won M, Seiferheld WF, Schultz CJ, Choucair AK, Brachman DG, Demas WF, Metha MP. Phase 2 trial of radiation plus high-dose tamoxifen for glioblastoma multiforme: RTOG protocol BR-0021. Neuro Oncol. 2006;8:47–52. doi: 10.1215/S1522851705000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG European Organization for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Changing paradigms--an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol. Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguère V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR β. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J, Cloix JF, Ardourel MY, Chatel M, Anthony DD. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;148:795–811. doi: 10.1016/j.neuroscience.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Murray IR, Qian J, Busch BB, Stevens WC, Jr, Martin R, Mohan R, Zhou S, Ordentlich P, Wei P, Sapp DW, Horlick RA, Heyman RA, Schulman IG. Regulation of PPARγ coactivator 1α (PGC-1α) signaling by an estrogen-related receptor α (ERRα) ligand. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8912–8917. doi: 10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrensch M, Rice T, Miike R, McMillan A, Lamborn KR, Aldape K, Prados MD. Diagnostic, treatment, and demographic factors influencing survival in a population-based study of adult glioma patients in the San Francisco Bay Area. Neuro-oncol. 2006;8:12–26. doi: 10.1215/S1522851705000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DD, Forman BM. Identification of an agonist ligand for estrogen-related receptors ERRβ/γ. Bioorg. Med. Chem. Lett. 2005;15:1311–1313. doi: 10.1016/j.bmcl.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teng CT. Interplay between estrogen-related receptor α (ERRα) and γ (ERRγ) on the regulation of ERRα gene expression. Mol. Cell. Endocrinol. 2007;264:128–141. doi: 10.1016/j.mce.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.