Abstract
Adenylyl cyclase type 6 (AC6) and the ß1 adrenergic receptor (ß1AR) are pivotal proteins in transmembrane ßAR-signaling in cardiac myocytes. Increased expression of AC6 has beneficial effects on the heart, but increased ß1AR expression has marked deleterious effects. Why do these two elements of the ßAR pathway have such different effects? Using adenovirus-mediated gene transfer of the two transgenes in neonatal rat cardiac myocytes, we assessed cellular distribution and performed selected biochemical assays. ß1AR was found predominantly in the plasma membrane. In contrast, AC6 was found in the plasma membrane but also was associated with the nuclear envelope, sarcoplasmic reticulum, mitochondria, and cytoplasm. Increased ß1AR, but not AC6, increased follistatin expression, p38 phosphorylation, phosphatidylserine translocation to the PM, and apoptosis. In contrast, increased AC6, but not ß1AR, inhibited PHLPP2 activity, activated PI3K and Akt, and increased p70S6 kinase phosphorylation and Bcl-2 expression; apoptosis was unchanged. The distribution of AC6 to multiple cellular compartments appears to enable interactions with other proteins (eg, PHLPP2) and activates cardioprotective signaling (PI3K/Akt). In contrast, ß1AR, confined to the plasma membrane, increased phosphatidylserine translocation and apoptosis. These data provide a potential underlying mechanism for the beneficial vs deleterious effects of these two related ßAR-signaling elements.
Keywords: PI3K/Akt, PHLPP2, p38 MAP kinase, phosphatidylserine, follistatin, follistatin-like-1
1. Introduction
Sustained activation of the ß-adrenergic receptor (βAR)-stimulatory GTP-binding protein (Gαs)-adenylyl cyclase (AC) signaling pathway has deleterious effects on cardiac myocytes [1,2]. These deleterious effects also are associated with increased expression of ß1AR, Gαs, and protein kinase A (PKA) [3-7]. In contrast, increased expression of AC type 6 (AC6) has beneficial effects on cardiac myocytes. Cardiac-directed expression of AC6 is associated with increased left ventricular function in otherwise normal mice, attenuation of myocardial hypertrophy, reduced mortality in cardiomyopathy, and reduced cardiac myocyte apoptosis and increased survival in animal models of myocardial infarction and heart failure [8-14].
It is difficult to reconcile these data—which indicate directionally opposite effects of cardiac expression of β1AR vs AC6—with the commonly held belief that increased cardiac cAMP is uniformly bad for the heart. Perhaps βAR expression has deleterious effects unassociated with cAMP per se, or, alternatively, AC6 expression has beneficial effects independent of cAMP generation. This question provided the rationale for the present studies. That two related signaling proteins should have such differences when expressed in the heart in a variety of physiological and pathophysiogical settings, despite both contributing to cAMP generation, may reflect specific subcellular compartmentation of these two transgenes. In order to study intracellular interactions in a physiological context, we elected to use cultured cardiac myocytes and examine cellular compartmentation and effects on key signaling pathways after adenovirus-mediated expression of AC6 vs β1AR. Our hypothesis was that differences in transgene compartmentation would provide important insight into why expression of these two functionally related proteins has directionally opposite effects in vivo. Our studies revealed striking differences between β1AR and AC6 transgene intracellular location, and corresponding different effects on plasma membrane integrity and intracellular signaling.
2. Methods and Materials
2.1. Antibodies
Antibodies to Akt, phospho-Akt, GSK3, phospho-GSK3, p70S6K, and phospho-p70S6K, and an Akt kinase assay kit were purchased from Cell Signaling (Beverly, MA). Anti-AU1 antibody was purchased from Covance (Berkeley, CA). Anti-HA and anti-Activin A antibodies and protease inhibitor cocktail were obtained from Roche (Indianapolis, IN). Anti-voltage dependent anion selective channel protein (VDAC), anti-lamin A, anti-follistatin, and anti-follistatin-like-1 were obtained from Abcam (Cambridge, MA). Anti-caveolin-3 antibody was obtained from BD Transduction laboratories. Anti-protein disulphide-isomerase (PDI) antibody was obtained from Novus Biologicals (Littleton, CO). Anti-Bcl2, p-136-Bad, anti-Bax, and anti-P38 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
2.2. Cardiac myocyte culture and gene transfer
Neonatal rat cardiac myocytes were isolated as previously described; media contained 10% fetal bovine serum [15]. One day after plating, E1-deleted adenoviruses encoding murine AC6 (Ad.AC6, AU1 tagged) or human β1AR (Ad. β1AR, HA tagged, a gift of Dr. Brian Kobilka, Stanford University Medical School.) were added to the culture media. A null adenovirus (Ad.Null) or adenovirus encoding enhanced green fluorescence protein (Ad.EGFP) was used as control; all infections were performed using a vector concentration of 600 vp/cell.
2.3. Fluorescent-activated cell sorting (FACS)
Cardiac myocytes were trypsinized, resuspended in 500 μl of 1X Binding Buffer (Biovision), incubated on ice for 30 min and then stained with Annexin V-FITC and propidium iodide (PI) following the instruction provided by Biovision. Quantification of apoptosis was obtained by flow cytometric analysis using BD FACSCanto at Flow Cytometry Research Core Facility of the VA San Diego Healthcare System.
2.4. Immunoblotting analysis
Cardiac myocytes were lysed 36-40 hr after adenovirus incubation was initiated, and lysates subjected to SDS-PAGE and transferred to a PVDF membrane. The proteins on the membrane were then probed with their specific antibodies followed by HRP-conjugated secondary antibodies. The protein-antibody complexes were detected using enhanced ECL plus a western blotting detection system. Immunoblots for each protein were repeated three times and representative blots are presented.
2.5. Immunofluorescence staining and deconvolution analysis
Cardiac myocytes were incubated with adenovirus for 40 hr and then fixed with 10% formalin (15m, 23°C). Fixed cells were washed 4 times with phosphate-buffered saline (PBS). Cells were blocked with normal goat serum for 1 h and incubated (4°C, overnight) with anti-HA antibody (1:200; for detecting ß1AR transgene protein) or anti-AU1 antibody (1:300; for detecting AC6 transgene protein) or anti-Cav3 (1:100 for detecting caveolae or anti-PDI (1:1,000, for detecting SR) or anti-VDAC (1:200, for detecting mitochondria) or anti-lamin A (1: 200) for detecting nuclear envelope). The cells were washed 4 times with PBS and then incubated for 1 h with secondary antibodies that were conjugated with Alexia Fluo 488 or 594. To stain the nucleus, cells were washed and incubated with Hoechst dye (1:1000 dilution) for 20 m. Stained cells were imaged by fluorescence microscopy. Images were obtained with a 40× or 100 × lenses using a Delta Vision system and were subjected to deconvolution. Exposures were captured with a CoolSnap camera. To enable accurate comparisons, exposure times were identical for each fluorophore and kept within the linear range of the camera.
2.6. Statistical Analysis
Data represent means ± SE; group differences were d etected using one-way ANOVA, followed by Bonferroni t-testing. The null hypothesis was rejected when p < 0.05.
3. Results
3.1. Cellular Distribution of AC6 vs β1AR
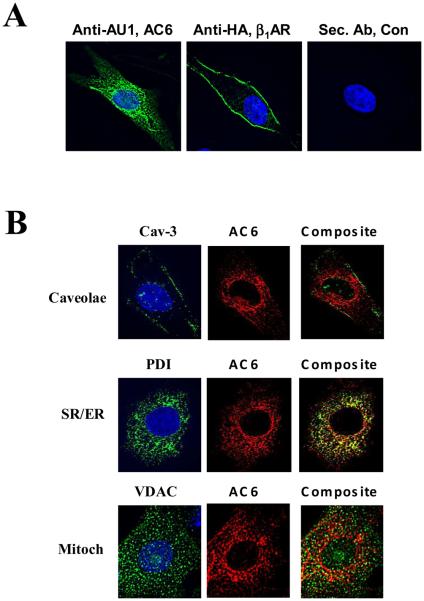
Cellular distribution was identified using anti-AU1 antibody for AC6 and anti-HA antibody for β1AR in immunofluorescence staining followed by deconvolution microscopic analysis. AC6 transgene was widely distributed throughout the cardiac myocyte, with the exception of the nucleus (Fig. 1A, left panel). In contrast, transgene β1AR protein was located predominantly in the plasma membrane and was minimally present or undetectable in other cellular compartments (Fig.1A, middle panel).
Figure 1. Location of AC6 and ß1AR transgene proteins.
A. Immunofluorescence staining and deconvolution analysis of cardiac myocytes after gene transfer of Ad.AC6 and Ad.ß1AR. Uninfected cardiac myocytes served as a control (Con). Anti-AU1 antibody was used to detect AC6 transgene (green), anti-HA for ß1AR transgene (green), and Hoechst dye was used to identify the nucleus (blue). AC6 transgene was evenly distributed in the plasma membrane and cytoplasm, but not within the nucleus. In contrast, ß1AR transgene was limited primarily to the plasma membrane (40X).
B. Double immunofluorescence staining of AC6 transgene by anti-AU1 antibody (red) with anti-caveolin 3 (Cav-3) antibody (green, for caveolae); with anti-voltage dependent anion selective channel protein (VDAC) antibody (green, for mitochondria); and with anti-protein disulphide-isomerase (PDI) antibody (green, for sarcoplasmic reticulum). AC6 transgene was localized in caveolae, mitochondria and SR.
To obtain more detailed information regarding the intracellular location of transgene AC6 protein, antibodies specific for a variety of cell organelles were used and images analyzed by deconvolution microcopy. AC6 co-localized with cavelion-3, VDAC and PDI, indicating AC6 transgene protein presence in caveolae, mitochondria, and SR (Figure 1B). AC6 transgene protein also co-localized with lamin A, indicating presence in the nuclear envelope (data not shown). The presence of transgene AC6 in these diverse cellular compartments enable protein:protein interactions unanticipated with endogenous AC6, which is limited predominantly to the inner plasma membrane.
3.2. Plasma membrane phospholipid and apoptosis
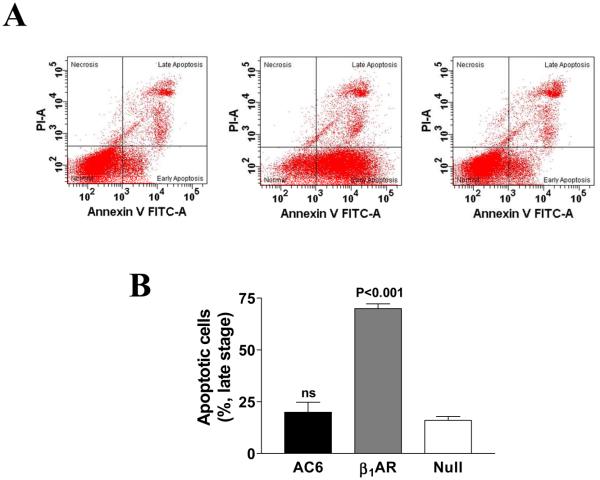
Relocation of phosphatidylserine (PS) to the outer PM, a marker for early stage apoptosis [16,17] was detected by Annexin V-FTIC staining, and late stage apoptosis was assessed by staining using both propidium iodide and Annexin V-FTIC. Increased AC6 expression was not associated with translocation of phosphatidylserine, and did not increase apoptosis (Fig. 2). In contrast, increased β1AR expression was associated with translocation of phosphatidylserine to the external PM, a population showing Annexin V positive and propidium iodide (PI) negative (Fig. 2A), suggestive of early apoptosis. Increased late stage apoptosis (Annexin V and PI positive) was detected 60-72 hr after Ad.β1AR gene transfer (3-fold increase vs. Ad.Null; p<0.001, Fig. 2B).
Figure 2. Flow cytometry analysis of cardiac myocyte apoptosis.
A. Cardiac myocytes incubated for 12h with Ad.AC6, Ad.β1AR, or Ad.Null were stained with Annexin V-FITC and propidium iodide (PI). Quantification of apoptotic cells was achieved by flow cytometric analysis. The quadrant lines were set based on Ad.Null control to separate normal cells (double negatives) from cell populations that were either PI positive, Annexin V positive, or both PI and Annexin V positive. Representative graphs from three independent experiments are shown. Gene transfer of β1AR, but not AC6, increased early stage myocyte apoptosis.
B. Late stage apoptosis. Cardiac myocytes incubated for 72h with Ad.AC6, Ad.β1AR, or Ad.Null were stained with Annexin V-FITC and propidium iodide (PI) and analyzed using flow cytometry. Bars in graph denotes mean±SD derived from three independent experiments. Comparisons were conducted using one-way ANOVA, followed by Bonferroni t-testing. Gene transfer of β1AR, but not AC6, increased late stage myocyte apoptosis.
3.3. Akt and PHLPP2
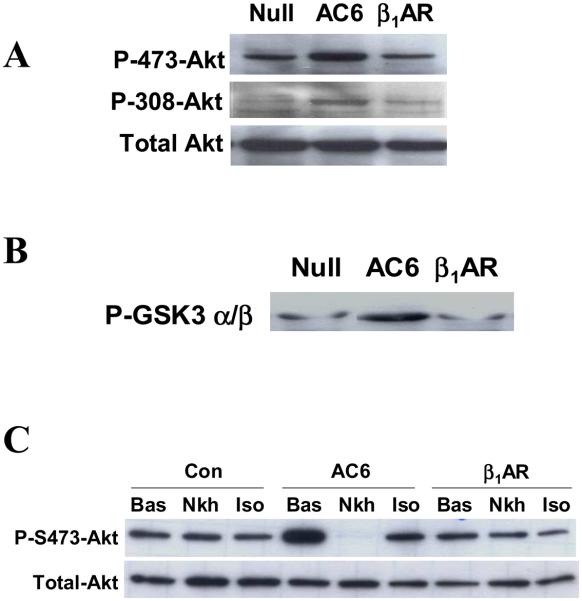
Gene transfer of both AC6 and β1AR did not alter expression and activation of PKA or EPAC, direct targets or cAMP (data not shown). However, increased expression of AC6 increased phosphorylation of Akt at Ser473 and Thr308 sites (Fig. 3A) and activated Akt, as indicated by GSK3 phosphorylation (Fig. 3B), confirming previous studies [18,19]. In contrast, β1AR expression did not increase Akt phosphorylation or activity (Fig. 3A and 3B). Figure 3C shows that Akt phosphorylation at Ser473 was rapidly reduced after isoproterenol or NKH477 (a water soluble forskolin analog that directly stimulates AC) in cardiac myocytes with increased AC6 as we previously reported [19], but this does not occur in cardiac myocytes with increased ß1AR expression.
Figure 3. Akt activation and dephosphorylation.
A. Immunodetection of Akt and phosphorylated Akt in homogenates of cardiac myocytes after incubation with Ad.AC6, Ad.ß1AR, or Ad.Null. Expression of AC6, but not ß1AR, was associated with increased phosphorylation of Akt at both Ser473 and Thr308 sites. There was no change in the amount of Akt.
B. Akt activity assay. Following incubation with Ad.AC6, Ad.ß1AR, or Ad.Null, an anti-Akt antibody was used in immunoprecipitation. Immunoprecipitated Akt then was incubated with GSK3α/β fusion protein in vitro. Phosphorylated GSK3 was detected by immunoblotting using anti-GSK3α/β antibody. AC6 expression, but not ß1AR, was associated with increased Akt activity.
C. Dephosphorylation of Akt. Stimulation with NKH477 (NKH; 10 μM, 10m) and isoproterenol (Iso; 10 μM, 10m) was associated with dephosphorylation of Akt at Ser473 in cardiac myocytes with increased AC6, but not with increased ß1AR. Results shown are representative of at least three experiments with similar results.
3.4. GSK3 and p70S6K
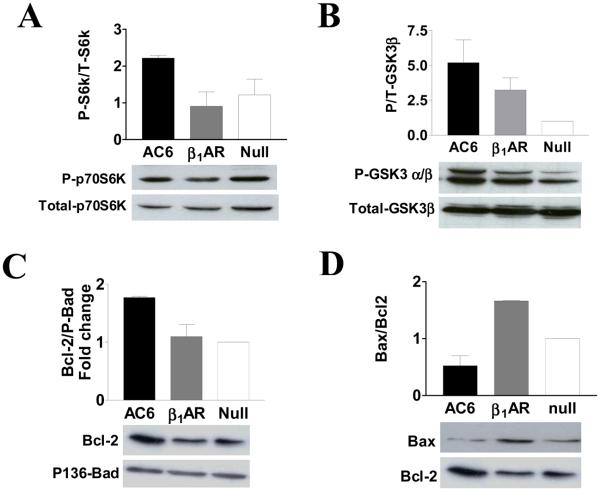
GSK3 and p70S6K are intracellular targets of Akt. Increased Akt activity associated with AC6 gene transfer was associated with increased phosphorylation of both p70S6K and GSK3 α/β proteins (Fig. 4A and 4B). However, increased β1AR expression inhibited p70S6K phosphorylation by 15% (Fig. 4A). Gene transfer of β1AR increased phosphorylation of GSK3 α/β subunits to a lesser degree than that seen after AC6 gene transfer (Fig. 4B), which indicates that signaling pathways other than Akt are activated by β1AR.
Figure 4. Alteration on Akt target and Bcl-2 family proteins.
A. Phosphorylated and total p70S6K were detected using their specific antibodies using immuoblotting. Increased Akt activity in Ad.AC6 infected cells was associated with increased phosphorylation of p70S6K protein. In contrast, ß1AR expression was associated with decreased phosphorylation of p70S6K protein.
B. Phosphorylated GSK3 α and β subunits and total GSK3 β protein were detected using their specific antibodies. Increased AC6 was associated with 5-fold increase in phosphorylation of GSK3 α and β subunits and ß1AR expression was associated with 2.5-fold increase in phosphorylation of these proteins.
C. Bcl-2 and phospho136 Bad proteins were detected using their specific antibodies. Increased AC6, but not ß1AR, was associated with a 2-fold increase in Bcl-2 expression.
D. Bax expression was detected using anti-Bax antibody and compared with Bcl-2 protein. Increased ß1AR protein was associated with an increased ratio of Bax/Bcl-2. Bars in graphs denote mean±SD derived from three independent experiments.
3.5. Bcl-2 family proteins
AC6 gene transfer increased the amount of Bcl-2 protein and reduced the ratio of Bax/Bcl-2 (Fig. 4C and 4D), which would be predicted to protect cardiac myocytes from programmed cell death. β1AR gene transfer, by contrast, was associated with increased the expression of Bax, and, consequently, an increased Bax/Bcl-2 ratio (Fig. 4D).
3.6. Follistatin and follistatin-like proteins
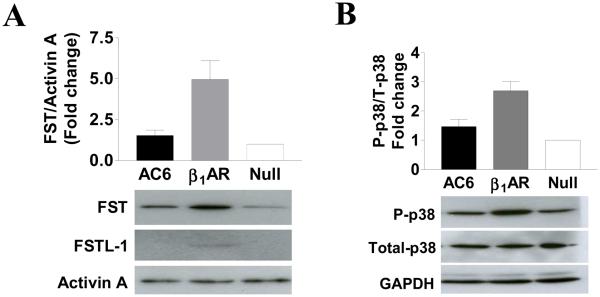
Gene transfer of β1AR, but not AC6, was associated with increased expression of follistatin (FST) protein and its related protein follistatin-like-1 (FSTL1, also known as TSC-36) without affecting activin A expression (Fig. 5A). Gene transfer of AC6 was not associated with increased expression of FST or FSTL1.
Figure 5. Follistatin and MAP kinase proteins.
A. Immunodetection of follistatin (FST), follistatin-like-1 (FSTL-1), and Activin A proteins. Expression of ß1AR, but not AC6, was associated with increased expression of FST and FSTL-1 proteins. Activin A levels were unchanged.
B. Immunodetection of p38, phosphorylated p38 and GAPDH proteins. Expression of ß1AR, but not AC6, was associated with increased phosphorylation of p38 without altering the amounts of total p38 or GAPDH.
Bars in graphs denote mean±SE derived from three independent experiments.
3.7. MAP kinase family proteins
β1AR and AC6 gene transfer did not affect the expression of ERK1, ERK2, JNK or phosphorylation of these proteins (data not shown). However ß1AR expression was associated with increased phosphorylation of p38 MAP kinase (Fig. 5B); AC6 expression had no such effect.
3.8. Summary of the findings
See Table.
Table.
Summary of Findings
| Ad.β1AR vs Control | Ad.AC6 vs Control | |
|---|---|---|
| Transgene location | PM | PM, NE, SR, MM, Cytoplasm |
| Follistatin family proteins | Increased 4-fold | No change |
| Phospho-p38 | Increased 3-fold | No change |
| Phosphatidylserine translocation |
To external PM, marked |
No change |
| PKA & EPAC activity/expression |
No change | No change |
| PHLPP2 activity | No change | Inhibited |
| Akt activity | No change | Increased 5-fold |
| p70S6 kinase phosphorylation | Decreased | Increased 2-fold |
| Bcl2 expression | No change | Increased 2-fold |
| Apoptosis | Increased 2-4 fold | No change |
PM, plasma membrane; NE, nuclear envelope; SR, sarcoplasmic reticulum; MM, mitochondrial membrane. The findings are summary of data from three or more experimental repeats.
4. DISCUSSION
In this study, using cultured cardiac myocytes, we determined the location of ß1AR vs AC6 protein after adenovirus-mediated gene transfer. Our hypothesis was that differences in intracellular compartmentation might explain why these two key members of the ßAR signaling pathway have such disparate effects when expressed in vivo. We found that transgene ß1AR was predominantly in the plasma membrane. In contrast, transgene AC6 was found in PM, but also was associated with the nuclear envelope, sarcoplasmic reticulum, mitochondrial membrane, and cytoplasm. Neither β1AR nor AC6 altered basal cAMP levels and PKA and EPAC expression and basal activity were unchanged. However, expression of β1AR, but not AC6, was associated with increased p38 phosphorylation, increased expression of follistatin family proteins, relocation of phosphatidylserine to the external plasma membrane, and increased apoptosis. In contrast, transgene AC6, but not β1AR, inhibited PHLPP2 activity (an Akt phosphatase), activated PI3K & Akt, increased p70S6 kinase phosphorylation, increased Bcl-2 expression (Table) and did not induce apoptosis. These findings suggest that cellular location of transgene may be an important contributing factor in the different effects observed following increased cardiac expression of these two elements of the ßAR signaling pathway.
4.1. Cellular location and function of transgene AC6
Endogenous AC activity is predominantly found in the plasma membrane, but small amounts of AC protein has also been identified in sarcoplasmic reticulum, the perinuclear region, and in the nuclear envelope in cardiac myocytes [20]. In nerve cells, endogenous AC is detected in endoplasmic reticulum and within the cytoplasm of terminal endings of nerve fibers [21]. Studies using cell homogenates before and after AC6 gene transfer, followed by sucrose gradient centrifugation showed that AC6 (endogenous and transgene) co-reside with caveolin proteins in the lipid raft fractions that represent caveolae [22-25]. Our studies using co-immunofluorescence staining confirm the existence of transgene AC6 in caveolae (Fig. 1). Other groups have shown that AC5 and AC8 are also localized to caveolae, but AC2, AC4 and AC7, are not [26], indicating that caveolae-localization of AC is isoforms specific. Indeed, it was recently demonstrated that the C1 and C2 domains of AC5 and AC6 appear to direct them to caveolae [26, 27].
Increased expression of AC6 did not alter the βAR content of caveolae [22] and did not influence β1AR and β2AR receptor-mediated signaling. Using the same adenovirus vectors to express AC6 and β1AR in cardiac myocytes, Ostrom et al showed that AC6 expression increases both β1AR and β2AR-mediated cAMP generation, and that β1AR-mediated response was increased 3-fold more than β2AR-mediated response, which correlates well with the fact that cardiac myocytes have 3-fold more β1AR than β2AR [28].
However, increased AC6 in subcellular domains may evoke signaling consequences not generally seen with endogenous AC6. For example, increased AC6 was associated with activation of PI3K/Akt signaling and suppression of PHLPP2 activity, events that are important for cardiac myocyte survival. It is noteworthy that increased ß1AR expression, in contrast to AC6, does not share this generalized distribution—indeed, transgene ß1AR distribution remains limited primarily to the plasma membrane. This property alone—differences in cellular transgene distribution—may underlay some of the differences observed after expression of these two transgenes, despite both of them being associated with cAMP generation. For example, differences in cellular distribution of transgene AC6 vs ß1AR provide an opportunity for AC6 to regulate intracellular events through interacting with intracellular proteins independently of cAMP and PKA activation.
4.2. Cellular location and function of transgene ß1AR
Transgene ß1AR was found predominantly in plasma membrane. An important finding in the present study was that increased expression of ß1AR alters plasma membrane lipoprotein distribution. Phosphatidylcholine (PS) is normally located in the inner plasma membrane. It can relocate to the outer membrane in response to stress induced apoptosis. Therefore, relocation of PS to the outer membrane is a marker for early stage apoptosis [16,17]. Cardiac myocytes with heterogeneous degrees of PS translocation, reflecting different expression levels of ß1AR (a common phenomenon of adenovirus mediated gene transfer), were detected as early as 12 hr after ß1AR gene transfer (Fig. 2). By 60-72 hr, increased late stage apoptotic cells were detected, which were both Annexin V and PI positives (Fig. 2B). However, there was another population with an extreme degree of PS translocation (very bright Annexin V signal) but that were not apoptotic or necrotic (PI negative, data not shown). The implication of this finding will require additional studies.
Despite the heterogeneous distribution of ß1AR on the plasma membrane, increased ß1AR increased phosphorylation of p38 (Fig. 5B) without altering total P38 or the expression and phosphorylation of other MAP kinase family proteins such as JNK, ERK1 and ERK2 (data not shown). Activation of p38 MAP kinase has been associated with stress-induced cardiac myocyte apoptosis [29-31], which is consistent with the findings in this study.
Increased expression of ß1AR is also associated with increased expression of follistatin and follistatin-like 1 without changing the level of activin A (Fig. 5A). Follistatin binds to activins, which are members of the TGFβ family implicated in diverse biological processes including cell proliferation, cell differentiation, fibrosis and the development of atherosclerosis [32,33]. Both FST and FSTL-1 are expressed in normal heart [34] and markedly increased in heart failure [35]. Since overexpression of FSTL-1 attenuates cardiac myocyte apoptosis induced by hypoxia/reperfusion injury [36], up-regulation of FSTL-1 induced by ß1AR expression might represent a compensation mechanism elicited by damaged cardiac myocytes.
5. Conclusion
Despite their shared importance in ßAR signaling and cAMP production, increased cardiac content of AC6 has favorable effects on heart function, while increased ß1AR content has deleterious effects. Our present studies show that AC6 gene transfer is associated with AC6 transgene distribution to multiple intracellular subdomains and promotes cell survival. In contrast, transgene ß1AR predominantly is associated with the plasma membrane and stimulates translocation of phosphatidylserine. Increased ß1AR is also associated with reduced p70S6K and Bcl2 expression and increases in proteins associated with heart failure (FST and FSTL1) and hypertrophy (phospho-p38), which may contribute to the deleterious effects of ß1AR expression on the heart.
Acknowledgements
This work was supported by grants from NIH (5P01HL066941, HL081741, HL088426–01), a Merit grant from the Department of Veterans Affairs, and Beginning Grant-In-Aid Awards from the American Heart Association (0765064Y and 0865147F). We thank Dr. Brian Kobilka for providing Ad.ß1AR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Communal C, Singh K, Pimentel DR, Colucci WS. Circulation. 2004;98:1329. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 2.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. J. Cell Physiol. 2001;89:257. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 3.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Circ. Res. 2001;89:997. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 4.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci WS, Bristow MR, Port DJ. J. Mol. Cell Cardiol. 2000;32:817. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7059. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelhardt S, Grimmer Y, Fan GH, Lohse MJ. Mol. Pharmacol. 2001;60:712. [PubMed] [Google Scholar]
- 7.Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, Vatner DE, Homcy CJ. J. Clin. Invest. 1995;95:1676. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao MH, Bayat H, Zhou JY, Roth DM, Drumm JD, Burhan J, Hammond HK. Cardiovasc. Res. 2002;56:197. doi: 10.1016/s0008-6363(02)00539-4. [DOI] [PubMed] [Google Scholar]
- 9.Lai NC, Roth DM, Gao MH, Fine S, Head BP, Zhu J, McKirnan MD, Kwong C, Dalton N, Urasawa K, Roth DA, Hammond HK. Circulation. 2000;102:2396. doi: 10.1161/01.cir.102.19.2396. [DOI] [PubMed] [Google Scholar]
- 10.Lai NC, Roth DM, Gao MH, Tang T, Dalton N, Lai YY, Spellman M, Clopton P, Hammond HK. Circulation. 2004;110:330. doi: 10.1161/01.CIR.0000136033.21777.4D. [DOI] [PubMed] [Google Scholar]
- 11.Lai NC, Tang T, Gao MH, Saito M, Takahashi T, Roth DM, Hammond HK. J. Am. Coll Cardiol. 2008;51:1490. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. Circulation. 1999;99:3099. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 13.Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, Hammond HK. Circulation. 2002;105:1989. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, Saito M, Lew WY, Clopton P, Hammond HK. Circulation. 2006;114:388. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 15.Gao MH, Ping P, Post S, Insel PA, Tang R, Hammond HK. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1038. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao XD, Tang AH, Chen Q, Jin HJ, Wu CH, Chen LY, Wang SQ. Biochem. Biophys. Res. Commun. 2003;310:405. doi: 10.1016/j.bbrc.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Monceau V, Belikova Y, Kratassiouk G, Charue D, Camors E, Communal C, Trouvé P, Russo-Marie F, Charlemagne D. Cardiovasc. Res. 2004;64:496. doi: 10.1016/j.cardiores.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, Pestonjamasp K, Feramisco JR, Hammond HK. J. Biol. Chem. 2008;283:33527. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao MH, Miyanohara A, Feramisco JR, Tang T. Biochem. Biophys. Res. Commun. 2009;384:193. doi: 10.1016/j.bbrc.2009.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto S, Kawamura K, James TN. Microsc. Res. Tech. 1998;40:479. doi: 10.1002/(SICI)1097-0029(19980301)40:6<479::AID-JEMT8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Drescher MJ, Kern RC, Hatfield JS, Drescher DG. Neurosci. Lett. 1995;196:145. doi: 10.1016/0304-3940(95)11764-n. [DOI] [PubMed] [Google Scholar]
- 22.Ostrom RS, Violin JD, Coleman S, Insel PA. Mol. Pharmacol. 2000;57:1075. [PubMed] [Google Scholar]
- 23.Ostrom RS, Liu X, Head BP, Gregorian C, Seasholtz TM, Insel PA. Mol. Pharmacol. 2002;62:983. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]
- 24.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. J. Biol. Chem. 2005;280:31036. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 25.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Biochem. Soc. Trans. 2005;33:1131. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 26.Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, Cooper DMF. J Biol Chem. 2005;280:6380. doi: 10.1074/jbc.M411987200. [DOI] [PubMed] [Google Scholar]
- 27.Thangavel M, Liu X, Sun SQ, Kaminsky J, Ostrom RS. Cell Signal. 2009;21:301. doi: 10.1016/j.cellsig.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. J Biol Chem. 2001;276:42063. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 29.Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. J. Cell Biol. 1997;139:115. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Huang S, Sah VP, Ross J, Jr., Brown JH, Han J, Chien KR. J Biol Chem. 1998;273:2161. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 31.Mao W, Fukuoka S, Iwai C, Liu J, Sharma VL, Sheu SS, Fu M, Liang C-S. Am. J. Physiol. Heart. Circ. Physiol. 2007;293:H1636. doi: 10.1152/ajpheart.01377.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kozaki K, Ouchi Y. J. Atheroscler. Thromb. 1998;5:36. doi: 10.5551/jat1994.5.36. [DOI] [PubMed] [Google Scholar]
- 33.Walsh K. Circ. J. 2009;73:13. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 34.an den Berg G, Somi S, Buffing AA, Moorman AF, van den Hoff MJ. Anat. Rec. (Hoboken) 2007;290:783. doi: 10.1002/ar.20559. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Endocrinology. 2008;149:5822. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 36.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. J. Biol..Chem. 2008;283:32802. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]