Abstract
Commonly used for research studies in the central nervous system, microdialysis has revealed a link between dysregulation of the excitatory neurotransmitter glutamate and ischemia and seizure, however limitations like slow temporal resolution have stalled the advancement of microdialysis as a diagnostic tool. We have developed and extensively characterized an enzyme-based microelectrode array technology for second-by-second in vivo amperometric measurements of glutamate in the mammalian CNS. The current studies demonstrated the ability of a human microelectrode array prototype (SG-2) to measure tonic and phasic glutamate neurotransmission in the putamen of unanesthetized non-human primates. We also showed that the SG-2 remains functional following sterilization. Ability to monitor dynamic changes in glutamate in real-time may assist in the development of clinical algorithms to potentially alert care-providers prior to onset of overt ischemia or seizure, or provide neurosurgeons with real-time measurements of rapid changes in extracellular glutamate which could help guide surgical procedures or aid in interventional strategies.
Keywords: Voltammetry, L-glutamate, Non-human primate, Putamen, Awake-behaving
Introduction
The gold standard for in vivo monitoring of neurochemistry has been microdialysis. In addition to the complexity of the procedure, the most limiting characteristic of microdialysis has been the time resolution of the method, which acts like a low-pass filter when sampling chemicals in the extracellular space. This is especially important when measuring glutamate, due to its rapid release and clearance processes. Researchers utilizing microdialysis techniques have explored many ways of improving the sampling of dialysates. One of the most popular techniques has been the coupling of microdialysis with on-line enzymatic conversion of analytes such as glutamate, followed by amperometric or fluorescence detection (Obrenovitch and Zilkha, 2001; Galvan et al., 2003; Zhang and Mao, 2005; Jin et al., 2008). Using similar principles, we have developed and extensively characterized a more streamlined approach using ceramic-based microelectrode arrays (MEAs) that have glutamate oxidase cross-linked to the platinum recording surfaces, coupled with real-time amperometric detection for sub-second (2 Hz) in vivo measurements of glutamate (Burmeister et al., 2002; Pomerleau et al., 2003). Based upon our prior work in rodents and non-human primates (NHP), we have now developed a human MEA prototype (Spencer-Gerhardt-2 (SG-2), ADTECH® Medical Instrument Corp., Racine, WI) for use in clinical research settings.
Monitoring of glutamate has been an area of interest for neurosurgeons and neuroscientists trying to understand a wide variety of pathological processes that are believed to have an excitotoxic component like epilepsy and traumatic brain injury (Hillered et al., 2005). The utility of MEAs for measuring fast transients in extracellular analytes like K+ during the first few minutes of cerebral injury has been demonstrated in rodents (Nilsson et al., 1993), but the temporal resolution of microdialysis has inhibited investigation for similar fast transients in humans (Reinert et al., 2002). Based upon recent MEA studies in our laboratory measuring second-by-second glutamate neurotransmission during rodent status epilepticus, we believe disruptions in the rapid release (TTX-sensitive) and clearance processes of glutamate neurotransmission during seizure are profound compared to slower changes in tonic glutamate (Stephens et al., unpublished data). The commercially available ISCUSflex clinical microdialysis analyzer (CMA Microdialysis, Solna, Sweden) has a 60 second sampling rate, but would not be able to capture fast transients in extracellular glutamate like those we have seen during seizure. In addition, 2 Hz sampling of the extracellular environment with MEAs improves the potential to correlate dynamic neurochemical changes with electrophysiological activity and behavior in real-time. Despite several research studies over the last 15 years reporting on the use of microdialysis in patients, no examples of clinical management guidelines based upon glutamate exist (Hillered et al., 2005), which may be partly related to the inability of the microdialysis method to measure second-by-second neurotransmission.
The size, structure and function of NHP brains have made them very desirable for neuroscientists aiming to closely approximate the human CNS in their investigations (for review see Bradberry, 2000). Despite the advantages over rodents, few studies report on glutamate measurements in the NHP brain (Graham et al., 1989; Kling et al., 1993; Yin et al., 1997; Enblad et al., 2001; Kodama et al., 2002; Galvan et al., 2003; Quintero et al., 2007; Zhao et al., 2007). Using the enzyme-based MEA recording technology, we have extensively investigated glutamate regulation in the mammalian CNS (Pomerleau et al., 2003; Nickell et al., 2005; Day et al., 2006; Nickell et al., 2007; Quintero et al., 2007; Stephens et al., 2009). Recently, we have begun adapting the basic-science MEA for potential clinical use. Our recording technology has a distinct advantage in temporal resolution compared to currently utilized cerebral microdialysis techniques. Our ability to monitor glutamate in real-time may advance the development of clinical treatment algorithms based upon glutamate fluctuations in the extracellular space to assist care-providers in determining the clinical course of patients with traumatic brain injury or sub-arachnoid hemorrhage, and may assist neurosurgeons during epilepsy surgery (see Stephens et al., 2008).
In these studies we used our human MEA prototype (SG-2) in awake NHPs to determine glutamate levels in the putamen. These studies contribute information to the literature about the NHP basal ganglia, which is known to be involved in a variety of pathological processes including Parkinson’s disease, Huntington’s diseases and psychiatric disorders (Kreitzer and Malenka, 2008), and evaluate the abilities of our SG-2 MEAs to target sub-cortical structures and accurately measure glutamate in a clinical setting. We also performed functional assessments of SG-2 MEAs post-sterilization, setting the stage for future studies in humans.
Materials and Methods
Animals
Three adult female rhesus monkeys (Macaca mulatta) obtained from a commercial supplier (Covance, Alice, TX) were used for these experiments (NHP 1 = 11 years old, 5.4 kg; NHP 2 = 19 y.o., 7.9 kg; NHP 3 = 21 y.o., 7.4 kg). All glutamate measurements were performed while the animals were awake during the ‘light phase’ of their 12 h light: 12 h dark maintenance schedule. All animals were individually housed throughout the study in the Laboratory Animal Facilities at the University of Kentucky which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALACI). Food and water were available ad libitum. All protocols used in this study were approved by the Institutional Animal Care and Use Committee.
MRI-guided Surgical Procedures
Similar surgical procedures have been recently described elsewhere (Xin et al., 2008). Briefly, the animal was positioned in a MRI-compatible stereotaxic head-frame under general anesthesia induced by Ketamine (150 mg/per animal, i.m.) followed by pentobarbital (50 mg/kg i.v.). Sets of three-dimensional anatomical T1-weighted images were collected on a 3T Siemens Trio clinical imager 2–3 weeks prior to the surgery. The target brain coordinates (dorsal putamen) were determined by the T1-weighted images (NHP 1, AP (from ear bars): +20 mm, ML: +11 mm, DV (from dura): −12 mm; NHP 2, AP: +18 mm, ML: +10.5 mm, DV: −15 mm; NHP 3, AP: +21.5 mm, ML: −11 mm, DV: −14.5 mm). Under sterile field conditions, a cannula was surgically implanted allowing chronic unilateral access to the putamen (left hemisphere: NHP 1 and 2; right hemisphere: NHP 3). The top part of the guide cannula (above the skull) was constructed from non-magnetic materials and surgically affixed to each animal’s skull using dental acrylic and nylon screws, and covered with a stainless steel cap (Figure 1). The lower part (below the skull) was a modified winged I.V. catheter (16g, JELCO™, Tampa, FL). The animals were anesthetized with isoflurane (1–3%) during surgical procedures and were allowed at least a 2 week recovery period before the first day of experimentation. Post operative T1-weighted images confirmed guide cannula placement (Figure 2). During experimentation, the SG-2 was manually lowered into the guide cannula. For glutamate measurements, the SG-2 MEA was first positioned 2 mm and then 4 mm past the tip of the guide cannula into the dorsal striatum.
Figure 1. Cannula and SG-2 MEA for real-time in vivo amperometric measurements of glutamate in NHPs.
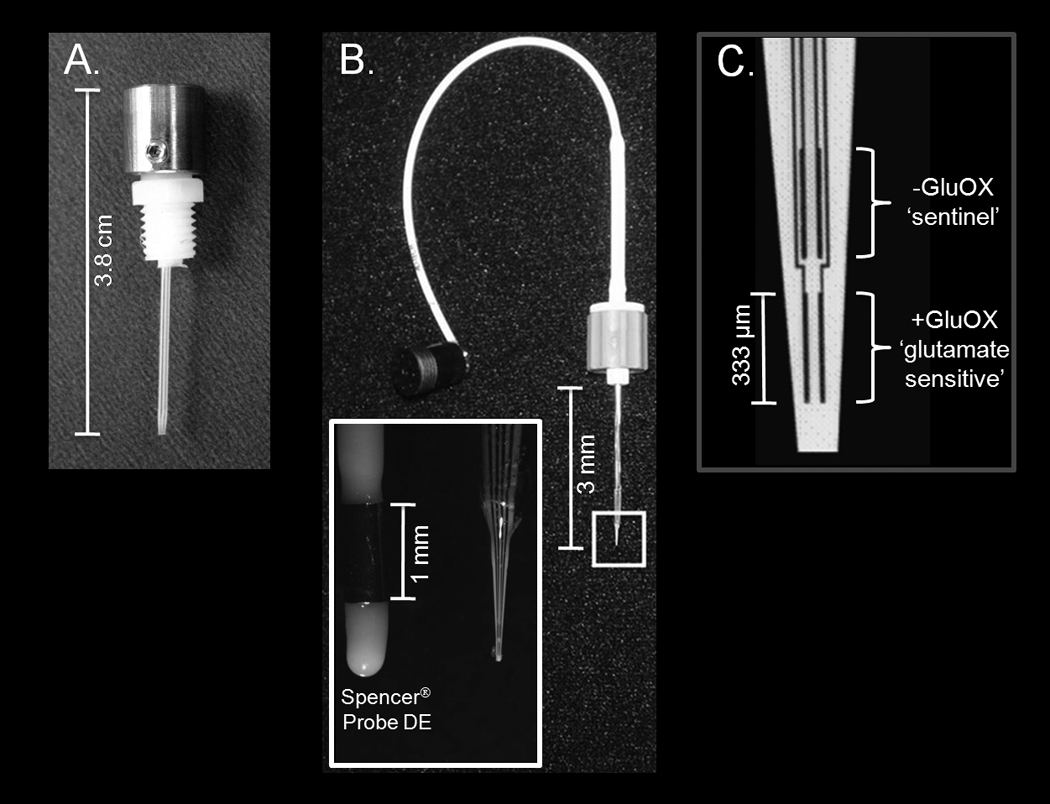
A) Guide cannula for access to the NHP putamen, with stainless steel cap; B) SG-2 MEA, inset: side-by-side comparison of SPENCER® Probe Depth Electrode and SG-2 MEA; C) magnification of electrode tip showing four platinum recording sites (15 × 333 µm each). The lower pair was prepared for the measurement of glutamate by application of glutamate oxidase (+GluOX). The upper ‘sentinel’ pair did not have glutamate oxidase (-GluOX). This MEA configuration allowed self-referenced glutamate recordings.
Figure 2. Sagittal and coronal MRIs confirming placement of guide cannula above the dorsal putamen.
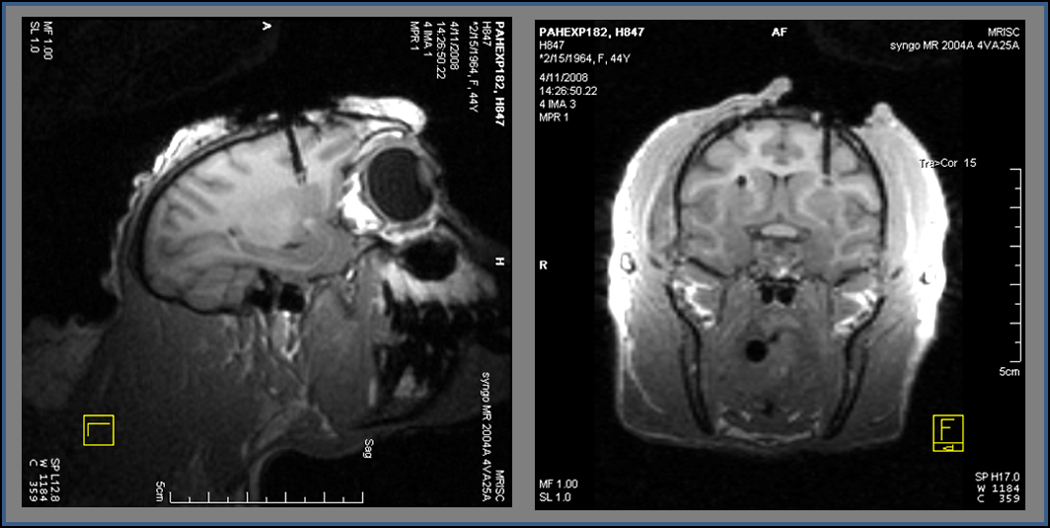
Three-dimension anatomical T1-weighted images were collected on a 3T Siemens Trio clinical imager to confirm correct placement of the guide cannula. Through the cannula, the SG-2 MEA was targeted to the dorsal putamen for amperometric glutamate measurements in NHPs.
Microelectrodes Spencer-Gerhardt-2 MEA
The SG-2 MEAs (Figure 1) were modeled after the clinically utilized SPENCER® Probe Depth Electrodes (ADTECH®, Racine, Wisconsin). The ceramic tip and four platinum recording sites configuration previously designed for basic science MEAs (each site = 15 × 333 µm, arranged in two pairs, stacked in a dorsal-ventral orientation, with 100 µm spacing between pairs, (Figure 1) was conserved in the SG-2 MEAs for comparisons of measures in rodents and primates (Day et al., 2006; Nickell et al., 2005; Nickell et al., 2007; Pomerleau et al., 2003; Quintero et al., 2007; Stephens et al., 2009). The fabrication of the SG-2 MEAs was modified from the basic science design to incorporate an extended flexible polyimide shaft for access to ventral brain regions in primates. Similar to the basic science MEA technology developed in our laboratory for glutamate measurements in freely-moving rodents (Rutherford et al., 2007), the SG-2 MEAs were connected to a miniature 4-channel low noise potentiostat (Quanteon, L.L.C., Nicholasville, KY) and the FAST-16 electrochemistry instrument (Quanteon, L.L.C.) via a locking AMS plug (Ginder Scientific, Ottawa, Ontario; Figure 2 and Figure 3). For these studies we used a non-invasive skin Ag/AgCl reference electrode (4 mm, shielded; World Precision Instruments, Inc., Sarasota, FL) which was modified to plug directly into the miniature potentiostat (Figure 3).
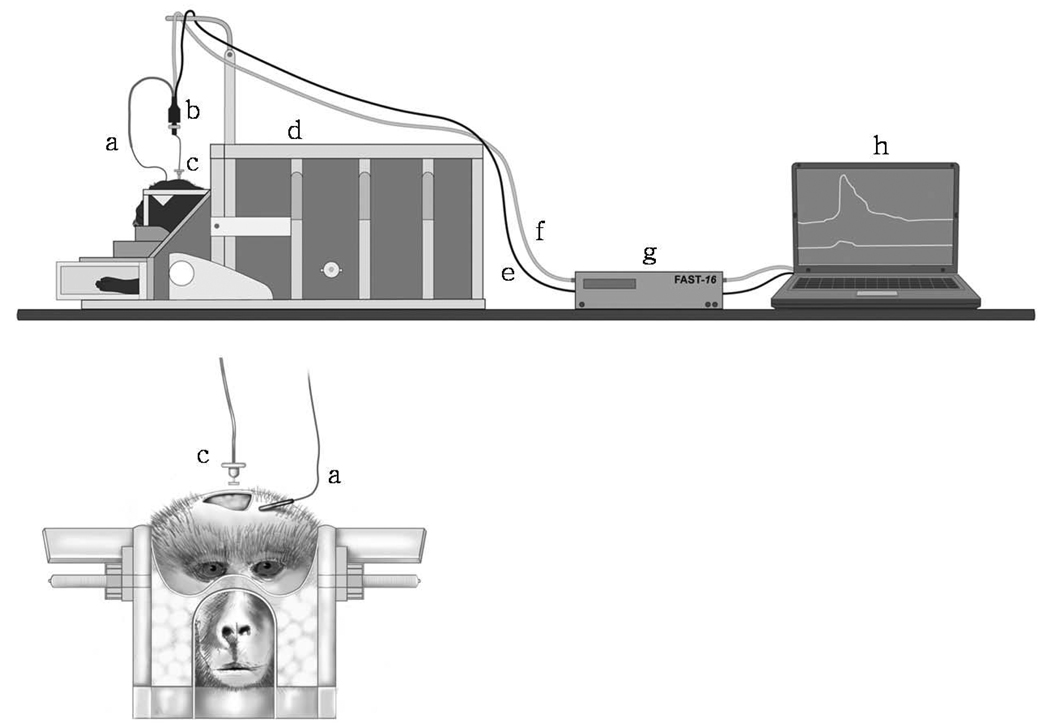
Figure 3. Illustrations of recording set-up for glutamate measurements in the putamen of awake NHPs.
a) Ag/AgCl reference electrode attached to the skin; b) miniature 4-channel low noise potentiostat; c) SG-2 MEA; d) MRI-compatible chair; e) grounding wire; f) connector; g) FAST16 electrochemistry instrument; h) laptop for visualization of glutamate measurements in real-time.
SG-2 preparation for sterilization studies
To maximize the sample size during the sterilization studies, all four platinum recording sites of the SG-2 MEAs were configured for the detection of glutamate by the application of enzyme (glutamate oxidase (GluOX)) and protein (bovine serum albumin (BSA)) solution (< 1 µL, 1% GluOX/ 1% BSA/ 0.125% glutaraldehyde) (Burmeister et al., 2002). These electrodes were not used for in vivo glutamate measurements as those MEAs were configured to have two glutamate-sensitive recording sites and two sentinel sites for self-referencing. Through enzymatic breakdown of glutamate, a reporter molecule of hydrogen peroxide is produced. The reporter molecule diffuses through the protein layer and is oxidized at the platinum recording surface (constant voltage amperometry, +0.7 V vs. Ag/AgCl reference electrode). To assess shelf-life, one batch of coated SG-2 MEAs were stored for approximately 11 months (n = 16) and one batch for one month (n = 16) at room temperature prior to electron beam sterilization at 25 Gy.
SG-2 preparation for in vivo glutamate measurements
The SG-2 MEA tips were dip-coated in Nafion® and dried at 175° C for 4 min. Nafion® repels anionic molecules such as 3-4-dihydroxyphenylacetic acid (DOPAC) and ascorbic acid that could interfere with in vivo electrochemical measurement of glutamate. SG-2 MEAs were then coated with the previously described GluOX/BSA/glutaraldehyde solution on the ventral pair of platinum recording sites. On the dorsal pair of sites a solution of just BSA/glutaraldehyde was applied. This coating process yielded one pair of ‘glutamate-sensitive’ (+GluOX) recording surfaces and one pair of background ‘sentinel’ (-GluOX) recording surfaces for self-referenced glutamate measurements (Figure 1) ( Burmeister et al., 2002; Pomerleau et al., 2003; Day et al., 2006). It should be emphasized that the MEA technology for recordings of L-glutamate are not specific for the detection of this analyte. Microdialysis methods employ analytical methods for separation and quantitation of multiple analytes in dialysates that are inherently more selective and sensitive. However, the MEAs employ the highly selective GluOX enzyme for the detection of L-glutamate, that when coupled with the self-referencing recording technology can rapidly measure changes in glutamate with a selectivity that rivals separation methods and with high sensitivity (Burmeister and Gerhardt, 2001). Thus, although the MEA technology is not devoid of the detection of interferents despite Nafion® application, the new self-referencing MEA technology is capable of identification and removal of interferents that would have been believed to be due to glutamate using other microelectrodes.
SG-2 in vitro calibration
To determine the effects of sterilization on MEA function, as well as to characterize the SG-2 MEAs on the day of experimentation, in vitro calibrations were performed (Burmeister et al., 2002; Pomerleau et al., 2003). The platinum recording sites were submerged in phosphate-buffered saline (0.5 M, pH 7.4, 37°C) and serial aliquots of glutamate (20 mM) added to yield final buffer glutamate concentrations of 20, 40 and 60 µM. The resulting increase in current (pA) from oxidation of hydrogen peroxide was measured by the FAST-16 electrochemistry instrument. The SG-2 MEAs exhibited typical linear increases in current following serial glutamate additions (r2 > 0.99). The slope of this line is referred to as the MEA sensitivity to glutamate (pA/µM). Of note is that unlike the previously published basic science calibration protocols utilizing a glass Ag/AgCl reference electrode, we submerged a Ag/AgCl skin reference electrode directly into the calibration beaker with SG-2 MEAs used for in vivo glutamate measurements. The same reference electrode was later used during the animal experiments. Post-sterilization calibrations were performed as usual with a glass Ag/AgCl reference electrode. The Ag/AgCl skin reference electrodes can be sterilized, which will assist in development of a completely sterile SG-2 MEA calibration procedure for clinical use. The SG-2 MEAs for in vivo use had an average glutamate sensitivity of 3.5 ± 0.4 nA/µM and a limit of detection (LOD = 3 times the signal-to-noise ratio) of 2.7 ± 0.8 µM (n = 6). These electrodes were also exposed to an ascorbic acid (500 µM) challenge and had an average selectivity ratio (glutamate:AA) of 20:1 (95% ascorbic acid blockade).
In vivo glutamate measurements
Animals were previously trained and extensively handled by the experimenters. Studies were performed in a non-human primate behavioral laboratory while the animals were seated in a MRI-compatible primate chair, which allowed free movement of the limbs and body but restrained the head (Figure 3) (Andersen et al., 2002). Under sterile conditions, the stainless steel cap was removed, and the guide wire was slowly pulled out. Then the SG-2 MEA was lowered into the putamen through the indwelling cannula. Constant voltage amperometry (+0.7 V vs. Ag/AgCl skin reference electrode) was performed using the FAST-16 electrochemistry instrument. Current from the four platinum recording sites was simultaneously recorded by the FAST software. Two depths in the putamen (2 mm apart) were targeted with the SG-2 MEA. The SG-2 MEA was lowered manually utilizing mm increments marked on the SG-2 MEA shaft. Glutamate was measured continuously (2 Hz) for around 40 minutes at each depth. Approximately four months after the first experiments, the studies were repeated to determine the reproducibility of the glutamate measurements. Time of day for experimentation and behavioral state of the animals was held constant across recordings, however different SG-2 MEAs were used for the follow-up glutamate measurements.
Data Analysis
Functional testing of sterilized SG-2 MEAs
In vitro calibrations were performed to determine the effects of sterilization on SG-2 MEA sensitivity to glutamate and limit of detection. Based upon empirical data, an MEA sensitivity to glutamate of 1 pA/µM or greater is considered functional. The percentage of SG-2 MEAs with at least one functional recording site was determined for each batch (1 and 11 months storage times). The average glutamate sensitivity and LOD for the functional sites was calculated. To determine if storage duration significantly affected MEA performance, a Student’s t-test was used to compare the sensitivity and LOD between the batches.
In vivo glutamate measurements
The background current produced by the oxidation of electroactive substances in the extracellular space and the charging current of the electrode surface (sentinel current) was subtracted from the current measured by the glutamate-sensitive recording sites. This is referred to as self-referencing (Figure 4 and Figure 5) (Burmeister and Gerhardt, 2001; Day et al., 2006). Following a thirty minute stabilization period, current (pA) obtained by self-referenced in vivo measurements at each depth in the putamen was divided by the SG-2 MEA glutamate sensitivity (pA/µM) determined during calibration and reported as concentration of basal glutamate (µM) (Quintero, et al. 2007). Combining data from the first and the follow-up experiments, we compared the basal glutamate levels in the dorsal putamen recording position to levels in the more ventral recording position with a Paired t-test (n = six each depth). We also compared the reproducibility of the basal glutamate levels between the first and follow-up recordings with a Paired t-test for repeated measures (n = 6).
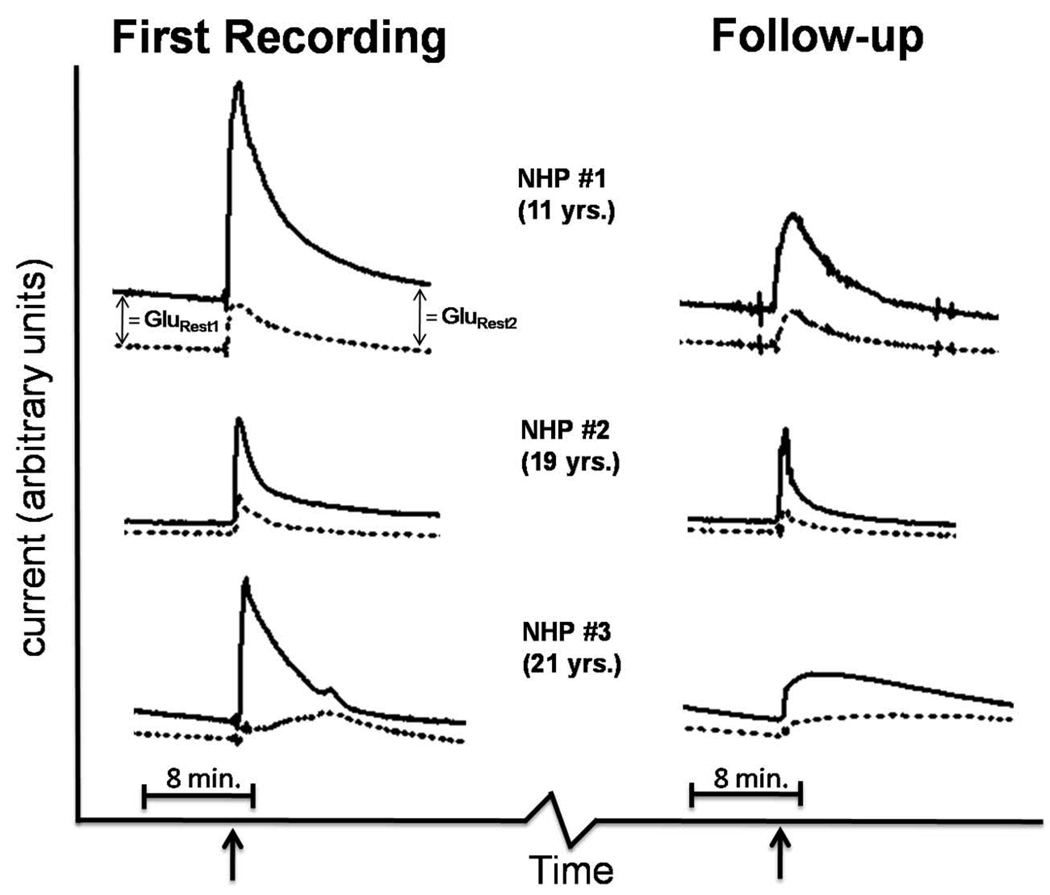
Figure 4. Basal glutamate measurements in the putamen of NHPs using SG-2 MEA human prototype.
Traces showing current measured on ‘glutamate-sensitive’ (black line) and sentinel (dashed line) recording sites. The first recordings and follow-ups were four months apart. Arrows indicate 2 mm ventral progression of the SG-2 MEA in the putamen. Self-referenced basal glutamate levels were determined by subtraction of current recorded by sentinel sites from current recorded by glutamate-sensitive sites. The resulting current value was divided by the MEA sensitivity to glutamate (pA/µM) determined during electrode calibration prior to each experiment. GluRest1 = basal glutamate at dorsal SG-2 MEA position. GluRest2 = basal glutamate at ventral SG-2 MEA position.
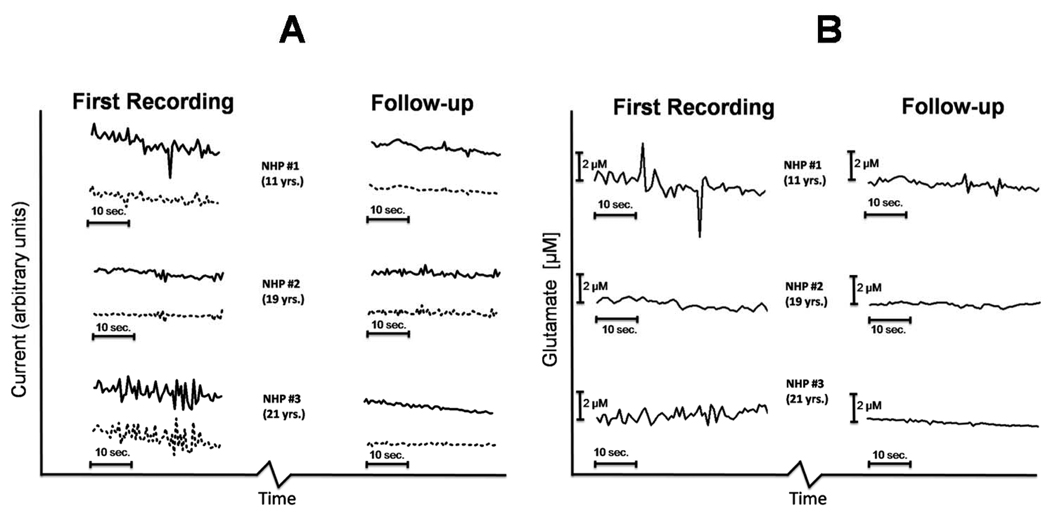
Figure 5. Dynamic Oscillations of extracellular glutamate in awake NHPs measured with real-time (2 Hz) amperometry.
A) Traces showing current measured on ‘glutamate-sensitive’ (black line) and sentinel (dashed line) recording sites. The first recordings and follow-ups were four months apart. B) Self-referenced glutamate levels were determined by subtraction of current recorded by sentinel sites from current recorded by glutamate-sensitive sites. The resulting current value was divided by the MEA sensitivity to glutamate (pA/µM) determined during electrode calibration prior to each experiment. Self-referencing increased the signal-to-noise ratio by mathematically removing noise of both electrical and mechanical origins.
Results
SG-2 MEAs remain functional post-sterilization
In the batch of SG-2 MEAs that had been coated with GluOX solution 11 months prior to sterilization, 10 out of 16 (63%) had at least one recording site that retained 1pA/µM glutamate sensitivity or greater. Of these 10 functional SG-2 MEAs, 8 (80%) actually had 3 or 4 recordings sites that remained sensitive to glutamate post-sterilization. The average sensitivity of the functional recording sites was 5.6 ± 0.7 pA/µM (n = 29), well above the threshold for in vivo use. The average LOD was 0.37 ± 0.09 µM. In the batch of SG-2 MEAs that had been coated with GluOX solution one month prior to sterilization, 14 out of 16 (88%) had at least one recording site with 1pA/µM glutamate sensitivity or greater. Again, almost all 14 of the functional SG-2 MEAs actually had multiple sites that retained the ability to record glutamate (12/14 (86%) had 3 or 4 functional sites). The average glutamate sensitivity of the functional recording sites was 7.5 ± 0.7 pA/µM (n = 47). The average LOD was 0.47 ± 0.14 µM. Though the mean sensitivity of the functional recording sites was greater in the batch prepared one month prior to sterilization, the difference was not significant (p = 0.06, (t = 1.9, df = 74)). Likewise, there was no significant difference in the LOD of the two batches (p = 0.58, t = 0.57, df = 74), indicating that enzyme-coated SG-2 MEAs have a good shelf life, and can withstand sterilization. It is important to explain that the LOD for SG-2 MEAs used during in vivo glutamate measurements was higher than the LOD in post-sterilization MEAs due to increased noise from the use of Ag/AgCl skin reference electrodes during calibration for in vivo experiments. The average glutamate sensitivities for all SG-2 MEAs were greater than 1pA/µM, which in the lowest acceptable sensitivity for glutamate detection based upon empirical data.
SG-2 MEAs can measure glutamate in the putamen of awake NHPs
The primary objective of these studies was to measure extracellular glutamate concentrations in the putamen of unanesthetized NHPs using the newly developed SG-2 MEA human prototype (Table 1). With self-referencing recording techniques (Figure 4) we were first able to determine basal glutamate levels at a dorsal position in the putamen (NHP 1 = 25.4 µM; NHP 2 = 6.9 µM; NHP 3 = 9.8 µM). As the SG-2 MEA was progressed 2 mm to a more ventral position in the putamen, we measured a robust increase in extracellular glutamate followed by clearance and stabilization of glutamate levels after approximately 15 min. Basal glutamate levels at the ventral depth were: NHP 1 = 34.2 µM; NHP 2 = 10.6 µM; NHP 3 = 11.5 µM. In the follow-up experiments conducted four months later, glutamate levels were as follows: dorsal: NHP 1 = 15.1 µM; NHP 2 = 4.9 µM; NHP 3 = 7.7 µM; ventral: NHP 1 = 12.6 µM; NHP 2 = 5.3 µM; NHP 3 = 5.6 µM). There was not a significant difference between basal glutamate measurements obtained in the dorsal recording position compared to the ventral position (p = 0.38 (t = 0.97, df = 5). The glutamate measurements recorded on the follow-up day of experimentation were significantly lower than the first measurements (p = 0.047 (t = 2.6, df = 5). No medical complications were reported throughout the duration of the study, which gave a preliminary indication of SG-2 MEA safety.
Table 1.
Basal Glutamate Measurements in the Putamen of Awake NHPs
| Animal | Deptha | First Recording (µM) |
Follow – up Recordingb (µM) |
|---|---|---|---|
| NHP 1 (11 y.o.) | 1 | 25.4 | 15.1 |
| 2 | 34.2 | 12.6 | |
| NHP 2 (19 y.o.) | 1 | 6.9 | 4.9 |
| 2 | 10.6 | 5.3 | |
| NHP 3 (21 y.o.) | 1 | 9.8 | 7.7 |
| 2 | 11.5 | 5.6 |
Measurements were not significantly different across depths (p=0.38).
Follow–up recordings (4 months later) were significantly lower compared to the first recordings(p=0.047).
Perhaps the most interesting findings from these studies were the rapid fluctuations of glutamate in the extracellular space of the putamen. We were able to capture dynamic fluctuations because of the fast temporal resolution (2 Hz) of the glutamate measurements obtained with the FAST16 instrument. We arbitrarily chose a 60 second window 10 minutes after the lowering of the SG-2 MEA to the ventral depth in the putamen to compare recordings from each animal (Figure 5). A qualitative assessment showed the youngest NHP had the most spontaneous activity with some of the bursts and rapid clearance events resulting in changes in extracellular glutamate of 2 µM or greater. This is remarkable because the youngest animal also had the highest basal glutamate levels. We can be sure these dynamic changes in extracellular glutamate are not artifacts because of our self-referenced recording technique. Self-referencing removes background noise and artifact whether electrical or due to movement of the animal. Though we do not have enough subjects in this study to make a statistical comparison across animals, we are very encouraged about the potential of this high-temporal resolution recording technique for use in monitoring glutamate in clinical applications, such as epilepsy surgery, where rapid changes in extracellular glutamate may guide surgical procedures or aid in interventional strategies.
Discussion
These studies demonstrated that we can obtain real-time measurements of extracellular glutamate in awake non-human primates using enzyme-based MEA technology coupled to amperometric recordings in a design that has been adapted for humans (SG-2). Through a chronically implanted access cannula, we obtained basal glutamate measurements at two different depths in the putamen and repeated the study four months later. The temporal resolution (2 Hz) of these measurements allowed us to record dynamic oscillations in extracellular glutamate, which to our knowledge has never been reported. We showed that sterilization did not significantly compromise the integrity and function of the enzyme/protein layers applied to SG-2 MEAs for the detection of glutamate. We also showed that the SG-2 MEAs have a shelf-life of at least one month, with several extending close to a year.
Glutamate measurements in the putamen
The basal glutamate measurements in our youngest subject agreed with a previous study from Galvan et al. (2003) that reported ~29 µM glutamate in the putamen of awake juvenile (3–4 kg) Rhesus monkeys. Glutamate levels appeared lower in the older animals. We have previously shown age-associated alterations in glutamate regulation in the striatum of rodents (Nickell et al., 2005; Nickell et al., 2007), but the current study was not designed to determine if a statistical difference in basal glutamate concentrations existed across age groups. Based upon our work, future studies of glutamate regulation in the striatum should also closely examine dynamic transients in the glutamate measurements. Only glutamate that comes into direct contact with a GluOX-coated platinum recording site is measured, therefore fast transient peaks and dips of glutamate concentration can tell us about neurotransmission resulting from dynamic release and clearance processes in the microenvironment around the MEA. Self-referencing greatly increased the signal-to-noise ratio of the MEA measurements, and allowed second-by-second fluctuations in extracellular glutamate to be isolated (Figure 5). The youngest NHP appeared to have larger magnitude peaks and dips in glutamate concentration compared to the older animals. This type of activity simply cannot be measured with microdialysis techniques, and potentially could provide a completely novel parameter to assess glutamatergic tone in vivo.
Due to the small size of the recording sites, the SG-2 MEAs can discriminate differences in extracellular glutamate in NHP brain areas separated by as little as 500 µm (Quintero et al., 2007). We measured basal glutamate in the putamen at two depths separated by a 2 mm dorsal-ventral progression of the SG-2 MEA. The measurements obtained at the two depths were not statistically different, but did highlight the ability of the SG-2 MEAs to target different brain layers and obtain a stable basal glutamate measurement much faster than the most recent report in the literature using microdialysis to measure glutamate in the NHP striatum (SG-2 MEA = 15–30 min. baseline vs. microdialysis = 60 min. baseline (Galvan et al., 2003)). Shortening the delay to stabilization of the glutamate measurements indicates that our technology could potentially sample from at least twice as many brain areas compared to microdialysis in a fixed amount of time.
The NHPs used in our studies were maintained in our animal care facility for four months after the first experiment day, and no adverse events were reported. This is a preliminary indication that the SG-MEAs can be safely used in vivo. Compared to the first day, basal glutamate measurements in the putamen were significantly lower after the four month maintenance period. We believe this is most likely due to presence of the chronic access cannula which may have initiated gliosis and served a substrate for extension of scarring into areas targeted by the SG-2 MEA. Histological evaluations of these tissues are needed to investigate this hypothesis and devise ways to carry-out longitudinal studies in the future. We have previously shown that our ceramic MEAs cause minimal damage and scarring in the surrounding tissue, even in chronic implants (Rutherford et al., 2007). In the future, we would like to utilize the methodology proposed by Kolachana et al. (1994) which via attachment of a ‘guide holder’ to the skull of rhesus monkeys, repeated studies can be performed in ‘fresh’ or ‘experienced’ brain parenchyma without the need of indwelling cannulae or multiple surgeries.
Sterilization of SG-2 MEAs
The commercially available ISCUSflex clinical microdialysis analyzer (CMA Microdialysis, Solna, Sweden) uses kinetic enzymatic conversion followed by colorimetry to measure glutamate in dialysate samples obtained once every 60 seconds. Our MEA recording technology bypasses the collection of samples and detects glutamate via enzymatic conversion at GluOX- coated recording sites on an indwelling MEA. Coupled to high speed amperometry, our MEAs are able to measure glutamate in vivo on a second-by-second time scale. Adapting our basic science MEA technology for potential clinical use necessitated the development of a sterilization protocol. With these studies we have determined that the performance of the SG-2 MEA remains well above the functional threshold for detection of glutamate following electron beam sterilization. Additionally, the SG-2 MEAs have a shelf-life of at least the duration of the study (11 months) which is very important to the logistics of distributing MEAs for clinical use. We have initiated development of a clinical calibration protocol using sterile reagents. Preliminary results indicate that there is no difference in the functional parameters (e.g. glutamate sensitivity) of MEAs when calibrated with sterile verses the non-sterile reagents (data not shown). We are currently performing a functional characterization of enzyme/protein coated SG-2 MEAs that have also been coated with Nafion® to determine the affects of sterilization on the anion repelling properties of Nafion®.
Advantages of amperometric recordings using enzyme-based SG-2 MEAs
Using enzyme-based amperometric MEAs for in vivo electrochemical detection of glutamate has several advantages over the current gold standard technique, microdialysis. To our knowledge, the fastest sampling rate for glutamate measurements obtained with microdialysis in laboratory setting is 1 second, but the exchange across the microdialysis membrane results in a delay of up to 12 seconds (see Lada et al. 1997). Also, Rossell et al. (2003) reported that based on their data in awake-behaving rats, glutamate release and clearance events were not adequately measured at with 1 Hz resolution because these events seemed to occur faster. We routinely measure glutamate at 2 Hz. As demonstrated by our prior studies of potassium-evoked glutamate release and uptake in NHPs (Quintero et al., 2007) and the present studies that show evidence for rapid glutamate transients, our temporal resolution is superior for monitoring the fast events associated with glutamatergic neurotransmission.
Microdialysis procedures can be laborious and time consuming, especially when incorporating adaptations to improve temporal resolution (Rossell et al., 2003). Major contributors to this are procedures for calibrating microdialysis probes and determining probe recovery rates (Hillered et al., 2005). Calibration of SG-2 MEAs is very straight-forward, and requires only 20 minutes to complete (Burmeister et al., 2002; Pomerleau et al., 2003). Microdialysis experimental protocols require tubing and pumps to flow artificial CSF through the microdialysis probe, which can introduce mechanical interference to measurements made by on-line detection methods that improve the temporal resolution (e.g. fluorometry). Even though this may be believed to be negligible (Galvan et al., 2003), our self-referencing technique allows for the removal of background noise, which in a clinical scenario could arise from a variety of sources including patient movement and the myriad other electrical devices. Also, the microdialysis procedure may have additional confounders like the use of room-temperature aCSF and air-saturated solutions (with markedly different O2 and CO2 saturations than the interstitial fluid) that can affect neurotransmission (Hillered et al., 2005). We avoid this by directly measuring glutamate in the extracellular space without the exchange of fluid.
Attached microelectrodes on microdialysis probes for dual electrophysiological and electrochemical recordings have been used to study a number of brain processes including epilepsy and TBI (During and Spencer, 1993; Obrenovitch and Zilkha, 1995; Fried et al., 1999; Alves et al., 2005). It has recently been reported that our commercial MEAs coated for electrochemical measurements can simultaneously measure electrophysiological currents in vivo (Zhanga et al., 2009). Combining information about the electrical activity of neurons and the resulting chemical environment in the extracellular space can provide a more comprehensive understanding of pathological processes and response to injury.
The spatial resolution of our MEAs (microns) allows us to obtain information from a more focal area of brain parenchyma than microdialysis probes, which are millimeters in length. The size of microdialysis probes not only lowers the spatial resolution of the measurements, but is also a substrate for damage to the surrounding tissue. It has been reported that microdialysis probes extensively injure brain parenchyma with damage extending up to 1.4 mm from the implant site (Clapp-Lilly et al. 1999; Borland et al. 2005). Chronic studies with our MEAs showed minimal gliosis around the implant site in the range of 50 to 100 microns, compared to 200–300 microns for microdialysis probes (Hascup et al., 2009).
Currently, a clear limitation of the SG-2 MEA is the ability to measure only one analyte at a time, whereas microdialysis allows for detection of several analytes. Though our standard MEAs have four platinum sites, arrays with up to 16 recording sites have been fabricated. To date, enzyme coatings have been developed to measure glutamate, choline, acetylcholine, lactate, and glucose (Burmeister et al., 2004; Burmeister et al., 2008). Electroactive compounds such as dopamine, norepinephrine, and O2 can also be measured (Burmeister et al., 2004). In the future we hope to be able to measure multiple analytes in vivo with a single MEA, and provide a real-time compositional analysis of the extracellular fluid.
Clinical applications of enzyme-based MEAs
Many studies with intracerebral microdialysis have shown that glutamate may be an important indicator of clinical course following brain injury. In acute focal ischemia imposed during the resection of brain tissue, patients have shown increased glutamate levels (Hillered et al., 1990; Kanthan et al., 1995) that are proportional to the extent of tissue resected, i.e. the duration of the ischemia (Kanthan et al., 1996). This has indicated a potential use for monitoring glutamate levels to predict intra-operative hypoxia (Mendelowitsch et al., 1998; Hutchinson et al., 2000), but study outcomes have not been consistently positive (Kett-White et al., 2002). The prospects for glutamate as a diagnostic tool have been better in subarachnoid hemorrhage (SAH). Increased glutamate levels have proceeded and predicted the deterioration of SAH patients (Unterberg et al., 2001; Sarrafzadeh et al., 2004). Monitoring glutamate levels, known to be very important to secondary injury cascades, for management of traumatic brain injury patients is also promising (for review see Hillered et al., 2005). Though glutamate, and other analytes such as lactate, glucose, and O2, show potential as markers of injury and clinical course, no medical guidelines are based upon cerebral monitoring with microdialysis (Hillered et al., 2005). For the reasons explained above, MEAs could monitor relevant compounds in the CNS faster than microdialysis, while causing less damage than microdialysis probes to already injured tissue. We believe this may give care-providers a more accurate analysis of the brain’s chemical profile, and facilitate the development of clinical algorithms based upon neurochemistry.
An especially novel application of MEAs may be in epilepsy surgery. Resection of diseased brain tissue has provided favorable outcomes for most patients undergoing surgery for seizures refractory to medication, however patients without a discrete focus of seizure activity or with foci in eloquent brain regions have remained difficult to treat. Inadequate delineation of the normal brain-epileptogenic zone interface has been the major contributor to poor surgical results in these patients, despite advances in multimodality image-guided surgery and brain function testing (Murphy et al., 2004). We believe MEAs could be used as a supplemental diagnostic to provide neurosurgeons with a real-time measurement of extracellular glutamate, which is known to be markedly elevated during seizure (During and Spencer, 1993) and remains elevated in epileptogenic brain regions (Cavus et al., 2005) . In addition to assisting with localization of foci, the ability to measure rapid fluctuations in extracellular glutamate as shown in the NHP putamen, may provide neurosurgeons and neuroscientists with a novel assessment of glutamate’s role in seizure initiation and tissue recruitment. This could possibly lead to the development of new medications and interventions for difficult to treat persistent epilepsy, and help validate animal models of seizure disorders.
Acknowledgements
Financial support was provided by USPHS NIH grants from AG13494 to Don Gash, NS39787 to Greg Gerhardt and NS050242 to Zhiming Zhang. Authors would like to thank Eric Forman and Alex Blandford for their assistance in animal handling, Dr. Peter Hardy for his help in MRI scans and Thomas Dolan for his medical illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves OL, Bullock R, Clausen T, Reinert M, Reeves TM. Concurrent monitoring of cerebral electrophysiology and metabolism after traumatic brain injury: an experimental and clinical study. J Neurotrauma. 2005;7:733–749. doi: 10.1089/neu.2005.22.733. [DOI] [PubMed] [Google Scholar]
- Andersen AH, Zhang Z, Barber T, Rayens WS, Zhang J, Grondin R, Hardy P, Gerhardt GA, Gash DM. Functional MRI studies in awake rhesus monkeys: methodological and analytical strategies. J Neurosci Methods. 2002;118(2):141–152. doi: 10.1016/s0165-0270(02)00123-1. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146(2):149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bradberry CW. Applications of microdialysis methodology in nonhuman primates: practice and rationale. Crit Rev Neurobiol. 2000;14(2):143–163. [PubMed] [Google Scholar]
- Burmeister JJ, Coates TD, Gerhardt GA. Multisite microelectrode arrays for measurements of multiple neurochemicals. Conf Proc IEEE Eng Med Biol Soc. 2004;7:5348–5351. doi: 10.1109/IEMBS.2004.1404493. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multisite microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73(5):1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Huettl P, Gash CR, Werner CE, Bruno JP, Gerhardt GA. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens Bioelectron. 2008;23(9):1382–1389. doi: 10.1016/j.bios.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Pomerleau F, Palmer M, Day BK, Huettl P, Gerhardt GA. Improved ceramic-based multisite microelectrode for rapid measurements of L-glutamate in the CNS. J Neurosci Methods. 2002;119(2):163–171. doi: 10.1016/s0165-0270(02)00172-3. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. J Neurosci Methods. 1999;90(2):129–142. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57(2):226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96(6):1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341(8861):1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Enblad P, Frykholm P, Valtysson J, Silander HC, Andersson J, Fasth KJ, Watanabe Y, Långström B, Hillered L, Persson L. Middle cerebral artery occlusion and reperfusion in primates monitored by microdialysis and sequential positron emission tomography. Stroke. 2001;32(7):1574–1580. doi: 10.1161/01.str.32.7.1574. [DOI] [PubMed] [Google Scholar]
- Fried I, Wilson CL, Maidment NT, Engel J, Jr, Behnke E, Fields TA, MacDonald KA, Morrow JW, Ackerson L. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. J Neurosurg. 1999;91(4):697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- Galvan A, Smith Y, Wichmann T. Continuous monitoring of intracerebral glutamate levels in awake monkeys using microdialysis and enzyme fluorometric detection. J Neurosci Methods. 2003;126(2):175–185. doi: 10.1016/s0165-0270(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Graham WC, Robertson RG, Sambrook MA, Crossman AR. Extracellular amino acid levels in the globus pallidus of the conscious primate studied by intracerebral microdialysis. Br J Pharmacol. 1989;98 Suppl:819P. [PubMed] [Google Scholar]
- Hascup ER, Af Bjerkén S, Hascup KN, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Histological studies of the effects of chronic implantation of ceramic-based microelectrode arrays and microdialysis probes in rat prefrontal cortex. Brain Res. 2009 Jul 3; doi: 10.1016/j.brainres.2009.06.084. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillered L, Persson L, Pontén U, Ungerstedt U. Neurometabolic monitoring of the ischaemic human brain using microdialysis. Acta Neurochir. (Wien) 1990;102(3–4):91–97. doi: 10.1007/BF01405420. [DOI] [PubMed] [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma. 2005;22(1):3–41. doi: 10.1089/neu.2005.22.3. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, Al-Rawi PG, O'Connell MT, Gupta AK, Pickard JD, Kirkpatrick PJ. Biochemical changes related to hypoxia during cerebral aneurysm surgery: combined microdialysis and tissue oxygen monitoring: case report. Neurosurgery. 2000;46(1):201–206. [PubMed] [Google Scholar]
- Jin G, Cheng Q, Feng J, Li F. On-line microdialysis coupled to analytical systems. J Chromatogr Sci. 2008;46(3):276–287. doi: 10.1093/chromsci/46.3.276. [DOI] [PubMed] [Google Scholar]
- Kanthan R, Shuaib A, Griebel R, Miyashita H. Intracerebral human microdialysis. In vivo study of an acute focal ischemic model of the human brain. Stroke. 1995;26(5):870–873. doi: 10.1161/01.str.26.5.870. [DOI] [PubMed] [Google Scholar]
- Kanthan R, Shuaib A, Griebel R, Miyashita H, Kalra J. Glucose-induced decrease in glutamate levels in ischemic human brain by in-vivo microdialysis. Neurosci Lett. 1996;209(3):207–209. doi: 10.1016/0304-3940(96)12642-2. [DOI] [PubMed] [Google Scholar]
- Kett-White R, Hutchinson PJ, al-Rawi PG, Gupta AK, O'Connell MT, Pickard JD, Kirkpatrick PJ. Extracellular lactate/pyruvate and glutamate changes in patients during per-operative episodes of cerebral ischaemia. Acta Neurochir Suppl. 2002;81:363–365. doi: 10.1007/978-3-7091-6738-0_92. [DOI] [PubMed] [Google Scholar]
- Kling AS, Tachiki K, Lloyd R. Neurochemical correlates of the Klüver-Bucy syndrome by in vivo microdialysis in monkey. Behav Brain Res. 1993;56(2):161–170. doi: 10.1016/0166-4328(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Kodama T, Hikosaka K, Watanabe M. Differential changes in glutamate concentration in the primate prefrontal cortex during spatial delayed alternation and sensory-guided tasks. Exp Brain Res. 2002;145(2):133–141. doi: 10.1007/s00221-002-1084-y. [DOI] [PubMed] [Google Scholar]
- Kolachana BS, Saunders RC, Weinberger DR. An improved methodology for routine in vivo microdialysis in non-human primates. J Neurosci Methods. 1994;55(1):1–6. doi: 10.1016/0165-0270(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lada MW, Vickroy TW, Kennedy RT. High temporal resolution monitoring of glutamate and aspartate in vivo using microdialysis on-line with capillary electrophoresis with laser-induced fluorescence detection. Anal Chem. 1997;69(22):4560–4565. doi: 10.1021/ac970518u. [DOI] [PubMed] [Google Scholar]
- Mendelowitsch A, Sekhar LN, Wright DC, Nadel A, Miyashita H, Richardson R, Kent M, Shuaib A. An increase in extracellular glutamate is a sensitive method of detecting ischaemic neuronal damage during cranial base and cerebrovascular surgery. An in vivo microdialysis study. Acta Neurochir. (Wien) 1998;140(4):349–355. doi: 10.1007/s007010050108. [DOI] [PubMed] [Google Scholar]
- Murphy MA, O'Brien TJ, Morris K, Cook MJ. Multimodality image-guided surgery for the treatment of medically refractory epilepsy. J Neurosurg. 2004;100(3):452–462. doi: 10.3171/jns.2004.100.3.0452. [DOI] [PubMed] [Google Scholar]
- Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats. J Neural Transm. 2005;112(1):87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28(11):1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Olsson Y, Sheardown MJ, Hansen AJ. Regional changes in interstitial K+ and Ca2+ levels following cortical compression contusion trauma in rats. J Cereb Blood Flow Metab. 1993;13(2):183–192. doi: 10.1038/jcbfm.1993.22. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Zilkha E. Microdialysis coupled to online enzymatic assays. Methods. 2001;23(1):63–71. doi: 10.1006/meth.2000.1106. [DOI] [PubMed] [Google Scholar]
- Pomerleau F, Day BK, Huettl P, Burmeister JJ, Gerhardt GA. Real time in vivo measures of L-glutamate in the rat central nervous system using ceramic-based multisite microelectrode arrays. Ann N Y Acad Sci. 2003;1003:454–457. doi: 10.1196/annals.1300.051. [DOI] [PubMed] [Google Scholar]
- Quintero JE, Day BK, Zhang Z, Grondin R, Stephens ML, Huettl P, Pomerleau F, Gash DM, Gerhardt GA. Amperometric measures of age-related changes in glutamate regulation in the cortex of rhesus monkeys. Exp Neurol. 2007;208(2):238–246. doi: 10.1016/j.expneurol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Reinert M, Khaldi A, Zauner A, Doppenberg E, Choi S, Bullock R. High level of extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. J Neurosurg. 2000;93(5):800–807. doi: 10.3171/jns.2000.93.5.0800. [DOI] [PubMed] [Google Scholar]
- Rossell S, Gonzalez LE, Hernández L. One-second time resolution brain microdialysis in fully awake rats. Protocol for the collection, separation and sorting of nanoliter dialysate volumes. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784(2):385–393. doi: 10.1016/s1570-0232(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Rutherford EC, Pomerleau F, Huettl P, Strömberg I, Gerhardt GA. Chronic second-by-second measures of L-glutamate in the central nervous system of freely moving rats. J Neurochem. 2007;102(3):712–722. doi: 10.1111/j.1471-4159.2007.04596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Haux D, Lüdemann L, Amthauer H, Plotkin M, Küchler I, Unterberg AW. Cerebral ischemia in aneurysmal subarachnoid hemorrhage: a correlative microdialysis-PET study. Stroke. 2004;35(3):638–643. doi: 10.1161/01.STR.0000116101.66624.F1. [DOI] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol. Aging. 2009 June 18; doi: 10.1016/j.neurobiolaging.2009.05.009. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens ML, Spencer DD, Cavus I, Hsiao MC, Song D, Courellis SH, Deadwyler SA, Hampson RE, Putz D, Quintero JE, Bensalem-Owen MK, Hascup KN, Rutherford EC, Day BK, Nickell JR, Pomerleau F, Huettl P, Burmeister JJ, Talauliker PM, Marmarelis VZ, Granacki JJ, Berger T, Gerhardt GA. Microelectrode-based Epilepsy Therapy: A Hybrid Neural Prosthesis Incorporating Seizure Prediction and Intervention with Biomemetic Maintenance of Normal Hippocampal Function. In: Soltesz I, Staley K, editors. Computational Neuroscience in Epilepsy. Academic Press; 2008. pp. 540–563. [Google Scholar]
- Ueda Y, Yokoyama H, Nakajima A, Tokumaru J, Doi T, Mitsuyama Y. Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus. Exp Brain Res. 2002;147(2):219–226. doi: 10.1007/s00221-002-1224-4. [DOI] [PubMed] [Google Scholar]
- Unterberg AW, Sakowitz OW, Sarrafzadeh AS, Benndorf G, Lanksch WR. Role of bedside microdialysis in the diagnosis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2001;94(5):740–749. doi: 10.3171/jns.2001.94.5.0740. [DOI] [PubMed] [Google Scholar]
- Xin T, Ai Y, Gerhardt G, Gash D, Zhang Z. Globus pallidus plays a critical role in neurotrophic factor induced functional improvements in hemiparkinsonian monkeys. Biochem Biophys Res Commun. 2008;370(3):434–439. doi: 10.1016/j.bbrc.2008.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin PB, Li BM, Ye WL, Mei ZT. A microdialysis study of excitatory amino acid levels of the monkeys' caudate nucleus during the delayed go/no-go task. Sheng Li Xue Bao. 1997;49(2):128–134. [PubMed] [Google Scholar]
- Zhang M, Mao L. Enzyme-based amperometric biosensors for continuous and on-line monitoring of cerebral extracellular microdialysate. Front Biosci. 2005;10:345–352. doi: 10.2741/1532. [DOI] [PubMed] [Google Scholar]
- Zhanga H, Lina SC, Nicolelis MAL. Acquiring local field potential information from amperometric neurochemical recordings. J Neurosci Methods. 2009;179:191–200. doi: 10.1016/j.jneumeth.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XD, Zhou XP, Liu HH, Li BM, Hu XW, Li FQ, You BM. Changes of amino acids neurotransmitters in striatum of hemi-parkinsonian rhesus monkey after high frequency stimulation of subthalamic nucleus. Zhonghua Wai Ke Za Zhi. 2007;45(24):1682–1684. [PubMed] [Google Scholar]