Abstract
Sox2 has been variously implicated in maintenance of pluripotent stem cells or, alternatively, early stages of cell differentiation, depending on context. In the developing inner ear, Sox2 initially marks all cells in the nascent sensory epithelium and, in mouse, is required for sensory epithelium formation. Sox2 is eventually downregulated in hair cells but is maintained in support cells, the functional significance of which is unknown. Here we describe regulation and function of sox2 in the zebrafish inner ear. Expression of sox2 begins after the onset of sensory epithelium development and is regulated by Atoh1a/b, Fgf and Notch. Knockdown of sox2 does not prevent hair cell production, but the rate of accumulation is reduced due to sporadic death of differentiated hair cells. We next tested the capacity for hair cell regeneration following laser-ablation of mature brn3c:gfp-labeled hair cells. In control embryos, regeneration of lost hair cells begins by 12 hours post-ablation and involves transdifferentiation of support cells rather than asymmetric cell division. In contrast, regeneration does not occur in sox2-depleted embryos. These data show that zebrafish sox2 is required for hair cell survival, as well as for transdifferentiation of support cells into hair cells during regeneration.
Keywords: regeneration, hair cell, support cell, zebrafish, heat shock, atoh1a, Fgf, Notch, laser ablation
INTRODUCTION
The capacity for maintenance and regeneration are fundamental properties of many mature tissues and organ systems. Regeneration often involves reactivation of developmental regulatory factors that coordinate growth and differentiation of pluripotent progenitor cells or stem cells. In the inner ear, sensory epithelia comprise interspersed patterns of sensory hair cells and support cells that in most vertebrates are capable of self-renewal (Corwin and Oberholtzer, 1997; Ozeki et al., 2007; Edge and Chen, 2008). Hair cells are highly susceptible to a number of environmental insults that can trigger apoptosis. Lost hair cells can be regenerated from support cells through either of two processes: Support cells may directly transdifferentiate into hair cells or, alternatively, undergo asymmetric division to yield a hair cell and another support cell (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Adler and Raphael, 1996). Unfortunately, the capacity for regeneration has been lost in the mammalian cochlea (Ozeki et al., 2007; Corwin and Oberholtzer, 1997; Edge and Chen, 2008), accounting for progressive irreversible hearing loss in humans as we age. To some extent this may be due to elevated expression levels of the mitotic inhibitors p27(Kip1) and Ink4d in support cells (Chen and Segil, 1999; Lowenheim et al., 1999; Chen et al., 2003), thereby preventing regeneration through asymmetric cell division. However, it is not clear why cochlear support cells cannot undergo transdifferentiation.
A candidate for a regulator of maintenance and regeneration of hair cells is Sox2. Sox2 encodes a transcription factor well known for its role in maintaining pluripotent stem cell populations, as well as differentiation during early development. For example, Sox2 is required to maintain pluripotency in mouse embryonic stem cells (Avilion et al., 2003; Masui et al., 2007) whereas misexpression of Sox2 facilitates conversion of adult differentiated cell types into pluripotent stem cells (Takahahsi and Yamanaka, 2006; Yu et al., 2007). Sox2 is also one of the first regulators of early specification of neurectoderm during vertebrate gastrulation (Kishi et al., 2000; Graham et al., 2003). How Sox2 orchestrates the mutually exclusive activities of maintaining pluripotency vs. stimulating differentiation is not fully understood. In sensory epithelia of the inner ear, Sox2 is initially expressed in progenitors of both hair cells and support cells (Kiernan et al., 2005; Hume et al., 2007; Neves et al., 2007). It is eventually lost from hair cells after differentiation but is maintained in support cells. The role of Sox2 in support cells is unknown. In mouse, disruption of Sox2 blocks initial formation of the entire sensory epithelium, thereby obscuring its subsequent role in support cells, as well as its possible involvement in hair cell maintenance (Kiernan et al., 2005).
We have investigated the role of sox2 in zebrafish, taking advantage of the fact that it is not required for establishment of the sensory epithelium during early otic development. We find that knockdown of sox2 does not prevent the emergence of hair cells and support cells but does lead to subsequent sporadic cell death of hair cells, and possibly support cells as well. We further show that, in wild-type embryos, regeneration of hair cells following laser-ablation involves transdifferentiation of support cells but not cell division, and that knockdown of sox2 totally blocks the regeneration process. These findings suggest that sox2 is required to maintain support cells in a pluripotent state or, alternatively, sox2 facilitates a discrete aspect of support cell differentiation that provides the facultative ability to transdifferentiate under appropriate conditions. The data further indicate that sox2 is required for survival of at least some hair cells, either directly by regulating early stages of hair cell differentiation or indirectly by regulating essential non-autonomous functions of support cells.
MATERIAL AND METHODS
Strains and analysis of gene expression
The wild-type strain was derived from the AB line (Eugene, OR). hsp70:Gal4, UAS:NICD and brn3c:gfp lines were previously described (Scheer and Campos-Ortega, 1999; Xiao et al., 2005). In situ hybridization was performed at 67°C as described (Millimaki et al., 2007). Where indicated in the text, statistical significance was assessed using t-tests.
Misexpression
To generate heat shock vectors for misexpression, full length cDNAs of fgf8, atoh1a, or sox2 (Pujic et al., 2006) were ligated to hsp70 heat shock promoter (Shoji et al., 1998) with flanking I-SceI meganuclease sites (Thermes, 2002; Rembold et al., 2006). Recombinant plasmid (10–40 pg/nl) was coinjected with I-SceI meganuclease (NEB, 0.5 U/μl) into 1-cell stage embryos. Stable transgenic lines Tg(hsp70:fgf8a)x17, Tg(hsp70:atoh1a)x20 and Tg(hsp70:sox2)x21 were generated by raising injected embryos to adulthood and screening by in situ hybridization for overexpression of the transgene or PCR for germline transmission.
Morpholinos
Translation-blocking morpholino oligomers (MOs) were obtained from Gene Tools, Inc. Embryos were injected at the one-cell stage with MOs as follows: 5 ng sox2-MO, 5′-AACCGATTTTCTGAAAGTCTACCC-3′ (Pujic et al., 2006); 2.5 ng atoh1a-MO, 5′-ATCCATTCTGTTGGTTTGTGCTTTT-3′; 7.5 ng atoh1b-MO, 5′-TCATTGCTTGTGTAGAAATGCATAT-3′ (Millimaki et al., 2007). In all knockdown experiments, embryos were coinjected with 7.5 ng of p53-MO (Robu et al., 2007) to inhibit non-specific cell death sometimes caused by off-target effects of MOs. Under the conditions used here, co-injection of atoh1a-MO, atoh1b-MO and p53-MO (2.5, 7.5 and 7.5 ng, respectively) resulted in complete absence of hair cells through at least 48 hpf in more than 90% of morphants. Efficacy of sox2-MO was confirmed by showing that staining with Sox2 polyclonal antibody (Millipore, 1:100 dilution) was undetectable in the otic vesicles of sox2-morphants at 36 hpf, and staining in the brain was strongly reduced (data not shown). Uninjected embryos of comparable stage and genetic background were used as controls for knockdown experiments.
SU5402 and DAPT inhibitor treatment
SU5402 was dissolved in DMSO to prepare a 20 mM stock solution. DAPT was dissolved in DMSO to prepare a 10mM stock solution and was diluted 100× for incubations. Embryos were treated in their chorions with 110 μM SU5402 and/or 100 μM DAPT beginning at 26 hpf, and then fixed at 30 hpf to examine changes in sox2 expression.
Cell transplantation and laser-ablation
Ablations were performed using a MicroPoint laser system with either a 40× or 100× objective. Multi-cell ablations required sequential targeting of individual cells. For lineage-tracing experiments, donor embryos were injected with lineage tracer (lysine fixable rhodamine 10,000 MW dextran, mixed 1:4 with biotinylated dextran in 0.2 M KCl) at the one-cell stage. Labeled donor cells were transplanted to unlabeled host embryos at the blastula stage. After allowing chimeras to develop to the indicated stages, hair cells in close proximity to lineage-labeled support cells were laser ablated. During ablations, we frequently observed temporary photo-bleaching of GFP in non-targeted hair cells. GFP fluorescence typically recovered within two hours. Laser irradiation also caused varying degrees of photo-bleaching of rhodamine-dextran in nearby support cells. Although rhodamine-fluorescence was still readily detectable several hours later, fluorescence often continued to diminish with time as lineage label accumulated in vesicles and appeared to be secreted into the lumen of the otic vesicle. In some cases rhodamine fluorescence could no longer be detected by 24 hours post-ablation. In such cases, staining for biotinylated dextran usually permitted detection of lineage-labeled cells. In other experiments, embryos were examined for evidence of regeneration 17 hours post-ablation, prior to complete loss of rhodamine fluorescence. Loss of lineage-label was never observed in non-laser irradiated embryos.
BrdU incorporation
BrdU pulse labeling was performed as described by Gray et al. (2001). Dechorionated embryos were incubated in fish water containing 10mM BrdU and 10% DMSO for 30 min at 33°C. Embryos were rinsed and incubated twice in fish water for 15min at 33°C. Embryos were then fixed in MEMFA (see in situ hybridization), briefly rinsed, and incubated in 2N HCl for 1h at 37°C. Embryos were washed and stained with anti-BrdU (Beckton-Dickinson, 1:250).
Cell death assay
For acridine orange staining, dechorionated embryos were incubated in 7 ml of 1μg/ml acridine orange solution in fish water for 30 minutes. Embryos were then washed with fish water 3 times, 10 minutes each wash. Analysis was completed immediately.
RESULTS
Expression of sox2
Otic expression of sox2 begins at around 14 hpf in the nascent otic placode (Fig 1A). This is 4 hours after the onset of atoh1b, the main gene responsible for specifying the prosensory equivalence group (Millimaki et al., 2007). Expression of sox2 is contiguous along the medial edge of the otic placode with elevated expression in two domains marking the future utricular and saccular maculae. Expression is eventually restricted to the macular domains, which increase in size as the maculae expand within the otic vesicle (Fig 1B). Sectioning reveals that nascent hair cells at the periphery of the maculae still express sox2 but expression is lost as hair cells mature (Fig. 1C). Support cells maintain sox2 expression, as has been seen in mouse and chick (Fig 1C) (Hume et al., 2007; Neves et al., 2007). By 48 hpf, primordia of the cristae also begin to express sox2 (data not shown). Otic expression of sox2 continues through at least 72 hpf, the latest stage examined (data not shown).
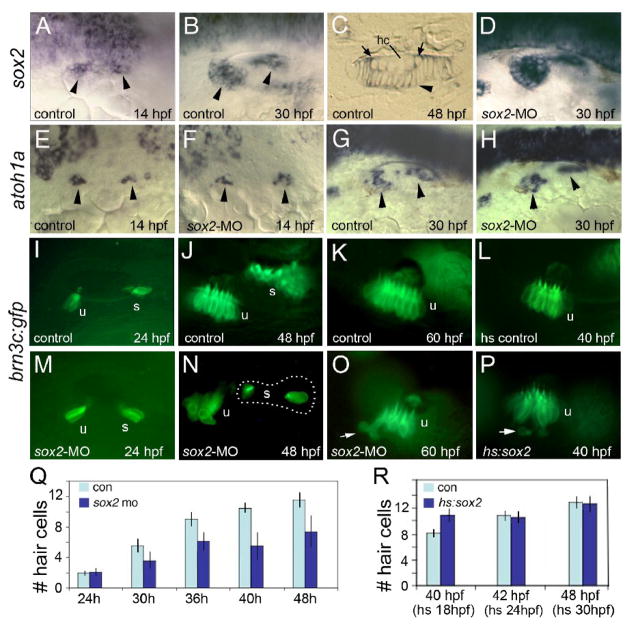
Figure 1. Sox2 is not required for hair cell development.
(A–C) sox2 expression in control embryos at 14 hpf (A), 30 hpf (B) and in a cross section of the utricular macula at 48 hpf (C). sox2 expression is lost from mature hair cells (hc) but is still detected in recently formed hair cells (arrows) and all surrounding support cells (arrowhead). (D) sox2 expression at 30 hpf in a sox2 morphant. (E–H) Expression of atoh1a in control embryos (E, G) and sox2 morphants (F, H) at the indicated times. Arrowheads mark macular expression domains. (I–P) brn3c:gfp expression in control embryos at 24 hpf (I), 48 hpf (J) and 60 hpf (K); expression in a control embryo heat shocked at 24 hpf and photographed at 40 hpf (L); expression in sox2 morphants at 24 hpf (M), 48 hpf (N) and 60 hpf (O); and expression in a hs:sox2 transgenic embryo heat shocked at 24 hpf and photographed at 40 hpf (P). Positions of the utricular (u) and saccular (s) maculae are indicated. Note the absence of hair cells in the middle of the saccular macula in the sox2 morphant (N). Arrows in (O, P) show hair cells being extruded from the utricular macula. All images show lateral views with anterior to the left and dorsal to the top. (Q) A time course showing the mean number of utricular hair cells in control embryos (con) and sox2 morphants (sox2 mo). Sox2 morphants exhibited a normal number of hair cells at 24 hpf (p = 0.88) but showed significantly fewer hair cells at later time points (p < 0.0001 for each time point). (R) Number of utricular hair cells in control embryos and hs:sox2/+ embryos subjected to heat shock at 18, 24 or 30 hpf, and counted at 40, 42 or 48 hpf, respectively. Transgenic embryos heat shocked at 18 hpf produced significantly more hair cells than normal (p < 0.0004), whereas the number of hair cells was not altered by heat shocking at 24 or 30 hpf (p = 0.78 or 0.73, respectively). Error bars in (Q, R) represent standard deviations, with n ≥ 15 for each time point.
Effects of knocking down sox2
We next assessed the consequences of knocking down sox2. Injection of translation-blocking morpholino oligomer (MO) to knockdown sox2 in zebrafish did not block early expression of atoh1a or atoh1b in the otic placode (Fig. 1F and data not shown). At later stages, the macular domains of atoh1a expression were nearly normal or slightly reduced in size (Fig 1H). The macular domain of sox2 expression appeared relatively normal in sox2 morphants, though the level of transcript was higher than normal (Fig. 1D). To determine whether knockdown of sox2 perturbs hair cell formation, we injected sox2-MO into transgenic embryos expressing brn3c:gfp, a marker of differentiated hair cells (Xiao et al., 2005). Tether cells, the first hair cells to differentiate during otic development (Riley et al., 1997), formed on time and appeared normal in sox2-depleted embryos (sox2 morphants) (Fig 1M). At later stages, additional hair cells continued to form but accumulated significantly more slowly than normal (p < 0.0001) (Fig. 1N, Q). Additionally, the saccule of sox2 morphants usually showed a notable gap between newly forming hair cells (anterior) and the initial tether cells (posterior) (Fig. 1N). Finally, hair cells appeared disorganized in sox2 morphants, and some hair cells appeared to be extruded into the underlying mesenchyme (Fig 1O). Such displacement has been previously associated with loss of cells undergoing apoptosis (Kwak et al., 2006). Thus, hair cell production is not blocked in sox2 morphants, but nevertheless occurs slowly and shows signs of irregular patterning. Such deficiencies could indicate faulty hair cell maturation or an increase in hair cell death or both.
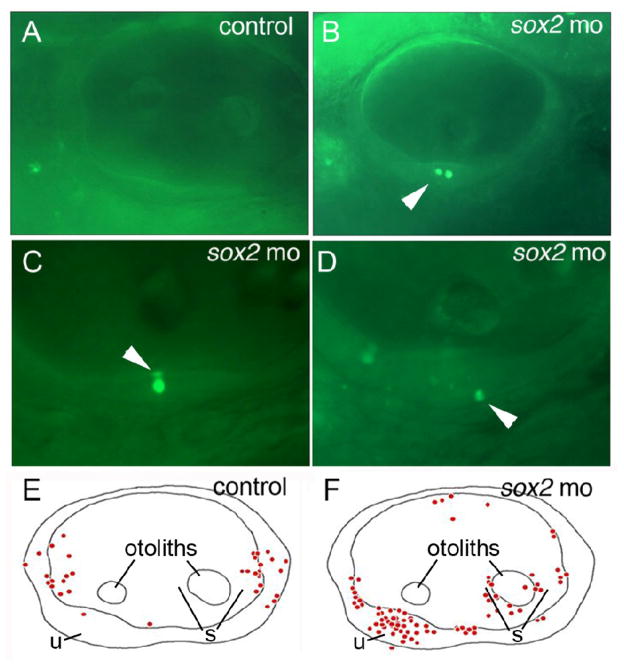
To test whether sox2-deficiency causes increased cell death, we stained sox2 morphants and control embryos with the vital dye acridine orange (AO) at 48 hpf. In sox2 morphants, AO- positive cells were observed in the otic vesicle in 31 of 33 specimens examined and, on average, 2.6 positive cells were seen per ear (Fig. 2B). The majority (66%) of AO-positive cells were seen within the developing maculae of sox2 morphants and marked both the apical and basal layers of the sensory epithelium, indicating the presence of dying hair cells and possibly support cells as well (Fig. 2C, D, F). In control embryos, only 20 of the 33 specimens exhibited AO-positive cells with an average of only 1 positive cell per ear examined. Moreover, only a single control specimen showed any AO-positive cells within the maculae (Fig 2A, E), a far lower incidence than was seen in sox2 morphants (p < 0.0001). Thus, cell death is normally quite rare in sensory epithelia but is common in sox2 morphants, confirming that sox2 directly or indirectly influences hair cell survival.
Figure 2. Loss of Sox2 results in macular death.
(A–D) AO-labeling of dying cells in a control embryo (A) and sox2 morphants (B–D). Morphants often contained multiple dying cells within sensory epithelia (B), and were observed in apical (C) or basal (D) regions of the maculae (arrowheads). (E, F) Schematic maps depicting the distribution of all AO-positive cells seen in otic vesicles of 33 control embryos (E) or 33 sox2 morphants (F) at 48 hpf. Positions of the utricular macula (u), saccular macula (s) and otoliths are indicated. No AO-positive cells were detected in the lateral wall of the otic vesicle. All images show lateral views with anterior to the left and dorsal to the top.
Effects of sox2 misexpression
Injection of sox2 mRNA caused severe patterning defects throughout the embryo, confounding interpretation of its effects in the inner ear (data not shown). We therefore generated a transgenic line to misexpress sox2 under the control of the heat shock-inducible promoter hsp70 (Shoji et al., 1998). Activation of hs:sox2 at 18hpf caused a 20–30% increase in the number of hair cells produced by 40 hpf (Fig 1R). The resulting maculae appeared somewhat disorganized and occasionally (≤ 10% of embryos) exhibited hair cells being ejected from the macula (Fig 1P). In contrast, activation of hs:sox2 at 24 hpf or later had no discernable effect (Fig. 1R). At no time did activation of hs:sox2 result in production of ectopic hair cells beyond the endogenous macular domains, indicating that, unlike atoh1a/b (Millimaki et al., 2007), sox2 is not sufficient to establish a prosensory equivalence group.
Co-misexpression of Sox2 and Atoh1a
Misexpression studies in mouse suggest that Sox2 and Atoh1 are mutually antagonistic with respect to cell fate specification in the cochlea (Dabdoub et al., 2008). We therefore tested whether hs:sox2 could block the ability of hs:atoh1a to stimulate hair cell production. Activation of hs:atoh1a at 24 hpf resulted in production of excess and ectopic hair cells throughout the ventromedial wall of the otic vesicle by 33–34 hpf (Fig. 3A). Co-activation of hs:atoh1a and hs:sox2 also led to formation of ectopic hair cells (Fig. 3B), similar to activation of hs:atoh1a alone. Thus, misexpression of sox2 does not antagonize atoh1a function sufficiently to block hair cell differentiation in zebrafish. However, the pattern of ectopic hair cells was less orderly following co-activation of hs:sox2 and hs:atoh1a (note the absence of straight rows of hair cells in Fig. 3B), suggesting that excess Sox2 weakly impairs the ability of Atoh1a to pattern the macula.
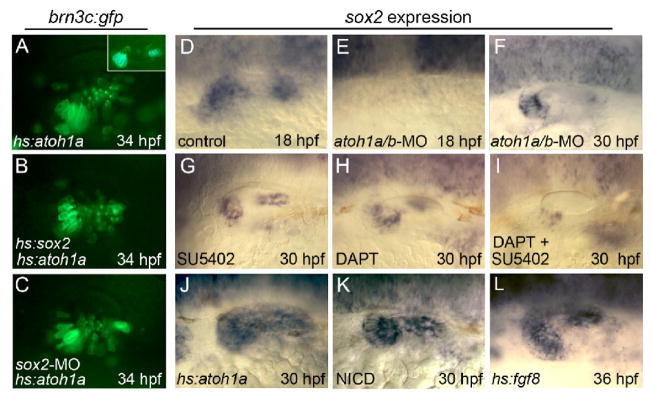
Figure 3. Relationship between Sox2 and upstream regulators of hair cell development.
(A–C) Expression of brn3c:gfp in hs:atoh1a/+ transgenic embryos (A, C) and a hs:atoh1a/+;hs:sox2/+ double transgenic embryo (B) heat shocked at 24 hpf and photographed at 34 hpf. The specimen in (C) was also injected with sox2-MO. The inset in (A) shows a heat-shocked brn3c:gfp/+ control embryo at 34 hpf. (D–L) sox2 expression in a control embryo (D), atoh1a/b morphants (E, F), wild-type embryos exposed to SU5402 (G), DAPT (H), or both DAPT and SU5402 (I) beginning at 26 hpf, a hs:atoh1a/+ embryo heat shocked at 24 hpf (J), a hs:gal4/+;UAS-NICD/+ embryo heat shocked at 24 hpf (K), and a hs:fgf8/+ embryo heat shocked at 30 hpf (L). sox2 expression is shown at 30 hpf, except (D, E, 18 hpf) and (L, 36 hpf). Expression in control embryos does not change appreciably between 30 and 36 hpf. All images show lateral views with anterior to the left and dorsal to the top.
Regulation of sox2 by Atoh1, Fgf and Notch
To better understand the role of sox2 in macular development, we examined its functional relationship to other genes known to regulate early steps in the process, Atoh1a/b, Notch, and Fgf (Millimaki et al., 2007). In atoh1a/b double morphants, which lack hair cells and support cells, sox2 expression was not detectable until 20 hpf, a delay of six hours (Fig 3E and data not shown). At 30 hpf, atoh1a/b double morphants continue to express sox2 in two macular domains, though both domains are smaller than normal (compare Figs. 3F and 1B). These data show that Atoh1a/b activity is required for initiation of sox2 expression at the correct time. To block Fgf signaling we incubated embryos with the pharmacological inhibitor SU5402. This does not block sox2 expression but reduces its level of expression (compare Figs. 3G and 1B). To block Notch signaling embryos were treated with DAPT, which blocks proteolytic processing necessary to activate Notch. This also reduced the level of sox2 expression (Fig. 3H). Treatment with both SU5402 and DAPT nearly eliminated sox2 expression (Fig. 3I), suggesting that these signals act in parallel to regulate sox2.
To further test their roles in sox2 regulation, we used heat shock lines to misexpress Atoh1a, Fgf8 or an activated intracellular domain of Notch (NICD) (Scheer and Campos-Ortega, 1999). Activation of hs:atoh1a at 24 hpf led to a dramatic expansion of the sox2 domain to cover the entire ventromedial wall of the otic vesicle by 30 hpf (Fig. 3J). This correlated with production of ectopic hair cells in the same domain several hours later (Fig. 3A). However, expansion of the domain of sox2 expression is not required for ectopic hair cell production, since activation of hs:atoh1 in sox2 morphants also led to overproduction of hair cells (Fig. 3C). Heat shock activation of NICD led to nearly as great an expansion in sox2 expression (Fig. 3K). Activation of hs:fgf8 caused a modest expansion of the macular domains of sox2, as well as a low level of ectopic expression in intervening tissue (compare Figs. 3L and 1B). Under the conditions used here, neither NICD nor Fgf8 were sufficient to stimulate ectopic hair cell formation. Thus, Atoh1a, Notch and Fgf activity are all able to activate ectopic expression of sox2, but this response is neither necessary nor sufficient for ectopic hair cell production.
Analysis of hair cell regeneration and the role of sox2
Regeneration of hair cells in the inner ear has not been previously examined in zebrafish embryos. To do so, we used a laser to ablate GFP-positive hair cells in brn3c:gfp/+ embryos and established a timeline for hair cell regeneration. We initially targeted only hair cells at the macular center to distinguish subsequent regeneration from normal developmental accumulation of hair cells along the periphery. When ablation was initiated at 48 hpf, the resulting gap in the macula was still easily discernable 12 hours later (Fig. 4A). By 24 hours post-ablation most gaps had been largely filled with new hair cells (Fig. 4B). Thus, substantial hair cell regeneration takes place between 12 and 24 hours post-ablation. Next, to assess the capacity for wholesale regeneration, we ablated all visible hair cells in the utricular macula at 30 hpf, taking care to examine embryos at 34 hpf to confirm that all hair cells had been killed. We then counted the number of hair cells present at 38 hpf (before there is discernable regeneration) and again at 50 hpf (after regeneration has occurred). In unablated controls, the number of hair cells increased by an average of 3.6 ± 0.9, representing normal hair cell production as the macula grows (Fig. 4E, F). In ablated ears, 6.4 ± 0.5 hair cells were produced in this time, representing both normal and regenerative hair cell production (Fig. 4E, F). We infer that the difference between control and laser-irradiated groups (2.8 hair cells/16 hours, p < 0.005) represents the number of hair cells produced through regeneration.
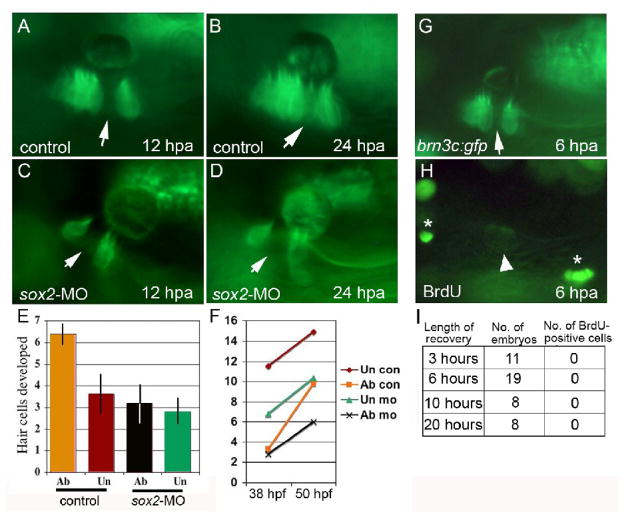
Figure 4. Hair cell regeneration requires sox2 but does not involve cell division.
(A–D) brn3c:gfp following ablation in a control embryo (A, B) and a sox2 morphant (C, D). Hair cells were ablated at 48 hpf, and ablated regions (arrows) were still evident at 12 hours post-ablation (hpa) (A, C) and 24 hpa (B, D). By 24 hpa, the gap filled in with newly formed hair cells in the control (B) but not in the sox2 morphant (D). (E, F) The number of hair cells produced following wholesale ablation of utricular hair cells. Ablation was conducted at 30 hpf, embryos were allowed to recover, and hair cells were counted at 38 hpf and again at 50 hpf. Typically 2 hair cells were produced during the recovery period. The number of hair cells produced between 38 and 50 hpf (E), and the total number of hair cells (F) are indicated for ablated (ab) and unablated (un) control embryos and sox2-morphants. Each time point shows the mean ± standard error of 3 or 4 experiments, with sample sizes of 19 to 23 embryos. (G–I) BrdU incorporation at various times following ablation initiated at 48 hpf. After 3, 6, 10 or 20 hours of recovery, embryos were incubated with BrdU for 3 hours and then fixed for processing. A specimen just before fixation at 6 hours post ablation (G) shows that the hair cell gap is still evident (arrow). After processing with anti-BrdU (H), dim GFP fluorescence is still detectable (arrowhead) and shows that no brightly labeled BrdU-positive cells (asterisks) are evident within the macula.
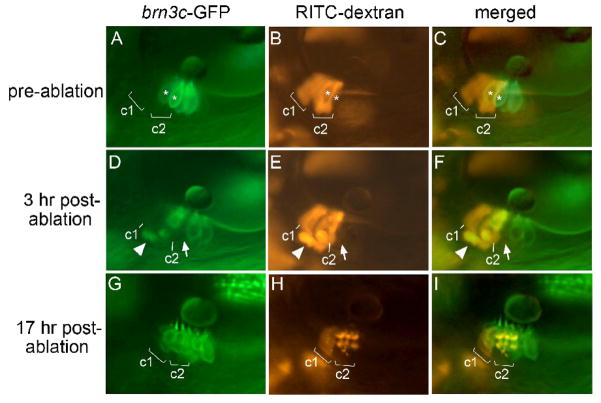
We next examined whether regeneration involves transdifferentiation or asymmetric cell division. To examine whether regeneration involves cell division, hair cells were ablated in the center of the utricular macula at 48 hpf, embryos were allowed to recover for 3, 6, 10 or 20 hours post-ablation, and then BrdU was added for a 3 hour pulse-label. We examined a total of 46 embryos, with at least 8 specimens per time point. Although BrdU-positive cells were detected in many regions of the embryo at each time point, no BrdU incorporation was detected in the macula in any specimen (Fig 4G–I). This indicates that regeneration seen within 24 hours post-ablation does not involve asymmetric cell division. To test whether regeneration involves transdifferentiation, we performed a lineage analysis in laser-irradiated brn3c:gfp embryos. Rhodamine-labeled cells were transplanted into unlabeled host embryos at the mid-blastula stage, and host embryos were screened at 36 hpf (n = 310 embryos) or 48 hpf (n = 280 embryos) to identify rare cases in which lineage-label was detected in support cells but few or no hair cells (Fig. 5A–C). Of 590 embryos (1180 ears) screened, 38 showed appropriate labeling patterns. In these specimens, hair cells near the lineage-labeled support cells were laser-ablated. Because laser-targeting sometimes causes photo-bleaching without killing hair cells, specimens were examined again 3 hours post-ablation to confirm that targeted hair cells had indeed been killed (Fig. 5D–F). By 17–24 hours post-ablation, 16 out of 38 specimens showed rhodamine-positive hair cells, with a corresponding disappearance of rhodamine-positive support cells (Fig. 5G–I). The remaining 22 specimens gave inconclusive results due to variable loss of lineage label (see Materials and Methods). These data show that support cells can transdifferentiate into hair cells within 17–24 hours post-ablation, thereby facilitating regeneration in zebrafish embryos.
Figure 5. Regeneration occurs through transdifferentiation.
(A–C) Lineage-labeled embryo at 48 hpf, just before laser-ablation, showing brn3c:gfp labeled hair cells in the utricular macula (A), two clusters (c1 and c2) of lineage-labeled cells (B) and an overlay showing both labels (C). Most lineage-labeled cells are support cells. Asterisks mark hair cells that were subsequently targeted for ablation. (D–F) The same specimen 3 hours post-ablation. A notable gap in the hair cell layer (arrow) marks the position previously occupied by one of the targeted hair cells. Accumulation of lineage-label plus GFP beneath the macula appears to show a fragmenting apoptotic hair cell being ejected from the macula (arrowhead). Labeled support cells are still evident in clusters c1 and c2. (G–I) The same specimen 17 hours post-ablation. Support cells in cluster c1 are still evident, though fluorescence intensity has decreased as described in Materials and Methods. In contrast, lineage-label is no longer visible in the support cell layer in cluster c2. Instead, lineage-labeled cells now occupy the hair cell layer and express brn3c:gfp. Much of the lineage label is concentrated in vesicles, as is typical at this stage following laser irradiation (see Materials and Methods). All images show lateral views with anterior to the left and dorsal to the top.
We next examined whether hair cell regeneration occurs in sox2 morphants. Ablation of hair cells in the macular center in sox2-morphants at 48 hpf produced gaps that remained unfilled at 72 hpf, 24 hours after ablation (Fig. 4C, D, n = 9). Similar results were obtained following wholesale ablation: In sox2-morphants in which all hair cells were ablated at 30 hpf, an average of 3.2+/− 0.9 hair cells were produced between 38 hpf and 50 hpf. In unablated sox2-morphants an average of 2.8 +/− 0.6 hair cells were produced (Fig 4E, F). Because there was no difference in the number of hair cells produced in ablated and unablated embryos (p = 0.75), we infer that no regeneration occurred by 50 hpf. Together these data suggest that sox2 is required for hair cell regeneration in zebrafish embryos.
DISCUSSION
We have shown a requirement for sox2 in maintenance and regeneration of hair cells in the zebrafish inner ear. It is possible that both functions are co-regulated in support cells or, alternatively, they could reflect independent functions in hair cells and support cells, respectively. Although sox2 is not required for overt hair cell formation, the sporadic cell death seen later could reflect faulty regulation of early hair cell differentiation. Alternatively, the requirement for hair cell survival could indicate that sox2 regulates an essential non-autonomous function in support cells. Analysis of mib mutants in zebrafish suggests that support cells are required for hair cell survival. In this background, the entire sensory equivalence group differentiates precociously as hair cells, all of which subsequently die by 36 hpf (Haddon et al., 1998). Deficiencies in support cell functions are clearly subtler in sox2 morphants, and hair cell death occurs only sporadically over a protracted period. Additionally, it is possible that support cells themselves die in sox2 morphants, though this is difficult to resolve without reliable support cell-specific markers.
The requirement for sox2 in regeneration clearly points to an essential function in support cells. We find that support cells directly transdifferentiate into hair cells following laser ablation in zebrafish, as has been observed in neonatal mice (Kelley et al., 1995). Maintenance of sox2 expression might allow support cells to retain developmental plasticity even as they differentiate enough to execute their essential functions. Alternatively, sox2 might regulate a discrete aspect of support cell differentiation that enables them to respond to macular damage by transdifferentiation into hair cells. The mechanism governing transdifferentiation is not well understood, but studies in chick suggest that Atoh1 is involved (Cafaro et al., 2007). In this case, downregulation of sox2 might be required for upregulation of Atoh1. It is also known that Atoh1-null cells can sometimes become hair cells when surrounded by wild-type cells, indicating the existence of an alternate hair cell pathway (Du et al., 2007). The status of sox2 in this pathway is unknown. It will be interesting to explore whether the loss of regenerative processes in the mammalian cochlea involve changes in Sox2 regulation. Support cells in mouse might lack the ability to reduce expression of Sox2 enough to allow Atoh1 activation. Alternatively, expression levels may be too low to maintain pluripotency. Cochlear support cells are highly specialized and differentiated, which could indicate a more stable commitment to these specific fates (Corwin and Oberholtzer, 1997). Expression of sox2 in the lateral line in zebrafish is also consistent with a role in regeneration, though this can apparently occur by transdifferentiation or asymmetric cell division (Woods et al., 2004; Hernandez et al., 2007; Ma et al., 2008).
The role of sox2 in patterning of the inner ear and sensory epithelium shows some interesting parallels between zebrafish and mouse, though there are clearly also some important differences. We have shown that zebrafish sox2 expression begins within the maculae downstream of atoh1a/b, and knockdown of sox2 does not block atoh1a/b expression. In contrast, mouse Sox2 is initially expressed throughout the ventral half of the otic vesicle well before formation of the sensory primordia (Kiernan et al., 2005). Moreover, Sox2 mutant mice produce no sensory cells and fail to express Atoh1. These observations have led to the suggestion that mouse Sox2 acts as a proneural gene to establish the prosensory equivalence group (Kiernan et al., 2005; Dabdoub et al., 2009). As a potential correlate, we detected a 20–30% increase in hair cell production following activation of hs:sox2 at18 hpf. This corresponds to a brief period in zebrafish when Notch activity stimulates atoh1a expression (Millimaki et al., 2007), suggesting that the pulse of sox2 misexpression may help mediate this effect. However, in contrast to Atoh1 (Woods et al., 2004; Millimaki et al., 2007), misexpression of Sox2 is not sufficient to activate formation of ectopic sensory epithelia in mouse or zebrafish, arguing against a simple prosensory role. An alternative explanation for the early requirement in mouse is that Sox2 initially acts as a regional specifier for the floor of the otic vesicle without which all ventral fates are lost. This would explain why the prosensory inductive signal Jag1 is not expressed in Sox2 mutants (Kiernan et al., 2005).
In a second phase of Sox2 function, zebrafish and mouse appear much more alike in their expression and regulation of Sox2. In both species, Sox2 is induced by Notch activity, and possibly Fgf signaling as well (Pirvola et al., 2002; Brooker et al., 2006; Kiernan et al., 2006; Hayashi et al., 2008) (Fig. 3). Interestingly, early expression of Atoh1 is co-induced by these same signals (Pirvola et al., 2002; Woods et al., 2004; Brooker et al., 2006; Kiernan et al., 2006; Millimaki et al., 2007; Hayashi et al., 2008). Subsequent mutual antagonism between Atoh1 and Sox2 (Dabdoub et al., 2009) could then reinforce cell fate diversification mediated by Notch-dependent lateral inhibition (Haddon et al., 1998; Riley et al., 1999; Brooker et al., 2006; Kiernan et al., 2006; Millimaki et al., 2007). Perturbing the balance of these activities might explain why in our studies misexpression of sox2 led to more chaotic arrangements of hair cells. However, unlike misexpression experiments in mouse (Dabdoub et al., 2009), we did not see a reduction in hair cell production following misexpression of sox2, arguing that Sox2 does not directly antagonize Atoh1 activity. It is possible that variation in the relative abundance or perdurance of misexpressed proteins influences how cells respond in different settings (Boer et al., 2007; Kopp et al., 2008).
Acknowledgments
This work was supported by NIH-NIDCD grant R01-DC03806.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neruosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boer B, Kopp J, Mallanna S, Desler M, Chakravathy H, Wilder PJ, Bernadt C, Rizzino A. Elevating the levels of Sox2 in embryonal carcinoma cells and embryonic stem cells inhibits the expression of Sox2:Oct-3/4 target genes. Nucl Acids Res. 2007;35:1773–1786. doi: 10.1093/nar/gkm059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy f, Abdala c, Liu F, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Oberholtzer JC. Fish n’ chicks: Model recipes for hair-cell regeneration? Neuron. 1997;19:951–954. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla c, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2009;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Jensen P, Goldowitz D, Hamre KM. Wild-type cells rescue genotypically Math1-null hair cells in the inner ears of chimeric mice. Dev Biol. 2007;305:430–438. doi: 10.1016/j.ydbio.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Edge A, Chen ZY. Hair cell regeneration. Curr Opin Neurobiol. 2008;18:377–382. doi: 10.1016/j.conb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khurdyakov J, Ellis P, Pevny L. Sox2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signaling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci. 2008;28:5991–5998. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the progenitor cell marker Sox2 and proliferation-dependent and –independent mechanisms of hair cell renewal. Dev Neurobiol. 2007;67:637–654. doi: 10.1002/dneu.20386. [DOI] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT, et al. Replacement of hair cells after laser microbeam irradiation in cultured organs of Corti from embryonic and neonatal mice. J Neurosci. 1995;15:3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required or sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genetics. 2006;2:e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi M, Mizuseki K, Sasai N, Yamazaki H, Shiota K, Nakanishi S, Sasai Y. Requirement of Sox2-mediated signaling for differentiation of early Xenopus neurectoderm. Development. 2000;127:791–800. doi: 10.1242/dev.127.4.791. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Ormsbee BD, Desler M, Rizzino A. Small increases in the level of Sox2 trigger the differentiation of mouse embryonic stem cells. Stem Cells. 2008;26:903–911. doi: 10.1634/stemcells.2007-0951. [DOI] [PubMed] [Google Scholar]
- Kwak SJ, Vemaraju S, Moorman SJ, Zeddles D, Popper AN, Riley BB. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev Dyn. 2006;235:3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness dN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of Corti. Proc Natl Acad Sci USA. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui S, Nakatake Y, toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, Ko MS, Niwa H. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–625. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Oshima K, Senn P, Kurihara H, Kaga K. Development and regeneration of hair cells. Acta Orolaryngol Suppl. 2007;559:38–44. doi: 10.1080/03655230701597200. [DOI] [PubMed] [Google Scholar]
- Privola U, Ylikoski J, Trokovic r, Hebert JM, McConnell SK, Partanen J. FGFR1 is required for the development of the auditory sensory epithelium. Neuron. 2002;35:671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pujic Z, Omori Y, Tsujikawa M, Thisse B, Thisse C, Malicki J. Reverse genetic analysis of neurogenesis in the zebrafish retina. Dev Biol. 2006;293:330–347. doi: 10.1016/j.ydbio.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Rembold M, Lahiri K, Foulkes NS, Wittbrodt J. Transgenesis in fish: efficient selection of transgenic fish by co-injection with a fluorescent reporter construct. Nature Protocols. 2006;1:1133–1139. doi: 10.1038/nprot.2006.165. [DOI] [PubMed] [Google Scholar]
- Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev Biol. 1997;191:191–201. doi: 10.1006/dbio.1997.8736. [DOI] [PubMed] [Google Scholar]
- Riley BB, Chiang MY, Farmer L, Heck R. The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JK, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genetics. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Shoji W, Yee CS, Kuwada JY. Zebrafish Semaphorin Z1a collapses specific growth cones and alters their pathway in vivo. Development. 1998;125:1275–1283. doi: 10.1242/dev.125.7.1275. [DOI] [PubMed] [Google Scholar]
- Takahahsi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thermes V. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev. 2002;118:91–98. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Woods C, Montacouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Xiao T, Roeser R, Staub W, Baier H. A GFP-based genetic screen reveals mutations that disrupt the architecture of the zebrafish retinotectal projection. Development. 2005;132:2995–2967. doi: 10.1242/dev.01861. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Sterwart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]