Abstract
The role of human medial temporal structures in fear conditioning has led to the suggestion that neurons in these structures might respond to painful stimuli. We have now tested the hypothesis that recordings from these structures will demonstrate potentials related to the selective activation of cutaneous nociceptors by a painful laser stimulus (laser evoked potential, LEP)(Kenton et al., 1980).
Recordings were carried out through electrodes implanted bilaterally in these structures for the investigation of intractable epilepsy. Reproducible LEPs were commonly recorded both bilaterally and unilaterally, while LEPs were recorded at contacts on the left (9/14, P=0.257) as commonly as on the right (5/14), independent of the hand stimulated. Along electrodes traversing the amygdala the majority of LEPs were recorded from dorsal contacts near the central nucleus of the amygdala and the nucleus basalis. Stimulus evoked changes in theta activity were observed at contacts on the right at which isolated early negative LEPs (N2*) responses could be recorded. Contacts at which LEPs could be recorded were as commonly located in medial temporal structures with evidence of seizure activity as on those without. These results demonstrate the presence of pain-related inputs to the medial temporal lobe where they may be involved in associative learning to produce anxiety and disability related to painful stimuli.
Keywords: Pain, amygdala, hippocampus, human, laser evoked potential, hippocampal theta
INTRODUCTION
The medial temporal lobe consists of the amygdala, the hippocampus, plus associated entorhinal, perirhinal and parahippocampal corticies, are structures well known to be involved in memory and emotional learning (LeDoux, 1992; Squire, 1992; Davis, 1992). The role of these structures in emotional learning and memory is particularly well characterized in classical fear conditioning, a form of plasticity which occurs when a neutral conditioned stimulus (CS), such as a light, is paired with an unconditioned aversive stimulus (US), such as a foot shock. After conditioning, the light CS itself acquires the ability to evoke a conditioned response, such as increased skeletal muscle tension and robust autonomic arousal. Although any stimulus which produces pain is intrinsically an unconditioned aversive stimulus, there is limited evidence of nociceptive processing in the primate medial temporal lobe, which could serve as the basis for pain-related emotional learning in man.
In rodents, there is good evidence that nociceptive pathways project to the amygdala through the pontine parabarachial nucleus (Bernard et al., 1992; Neugebauer et al., 2009). This nociceptive pathway is based upon anatomic evidence of connections from the spinal dorsal horn via the parabrachial nucleus to the central nucleus of the amygdala (Ma and Peschanski, 1988; Bernard and Besson, 1990; Saper, 1995). Noxious stimuli activate neurons in this nucleus (Bernard and Besson, 1990; Bernard et al., 1992; Slugg and Light, 1994), and may lead to synaptic plasticity such as increased responses to subsequent noxious stimuli (Neugebauer et al., 2000; Neugebauer et al., 2009)(see also (Shi and Davis, 1999; Lanuza et al., 2008). Therefore, a broad range of evidence supports nociceptive transmission to, and pain-related plasticity of, the amygdala in rodents.
In primates, anatomic studies have demonstrated that the spinothalamic tract (STT) projects to the amygdala, particularly the central nucleus (Newman et al., 1996). Imaging studies have shown that the amygdala or hippocampus or both show changes in blood flow and blood oxygen level dependent (BOLD) activity in response to a painful contact heat stimulus (Derbyshire et al., 1997; Becerra et al., 1999), or a painful cutaneous laser stimulus (Bornhovd et al., 2002; Bingel et al., 2002; Apkarian et al., 2005). Although these activations have been interpreted in terms of the affective and cognitive aspects of pain, there is no direct evidence of nociceptive inputs to these structures in primates, to our knowledge. We now test the hypothesis that recordings from the human amygdala and hippocampus demonstrate potentials related to selective activation of cutaneous nociceptors which is evoked by a painful laser stimulus (laser evoked potential, LEP)(Kenton et al., 1980). The results demonstrate that LEPs or theta rhythms evoked by a painful cutaneous laser can be recorded along electrodes implanted in the human amygdala and hippocampus for the investigation of medically intractable seizures.
METHODS
The protocol for these studies was reviewed and approved annually by the Institutional Review Board of Johns Hopkins Medicine. These studies were carried out after implantation of depth electrodes in the amygdala and hippocampus for investigation of medically intractable epilepsy in four subjects (Table 1). All subjects gave written informed consent for participation in these studies. All the techniques used in this study have been previously reported (18). Preoperative evaluation by a neurologist and neurosurgeon, which included standard somatic sensory testing, disclosed no neurological, medical, or psychiatric abnormality except epilepsy (Lenz et al., 1993)(Table 1).
Table 1.
Characteristics of Subjects, Laser side and VAS out of 10, Seizures, Complete list of Medications, Interval since last medications, and electrical results of depth monitoring for seizures. Under Wada test the types of memory impaired by injection of pentobarbital into the carotid artery leading to temporary loss of neurologic functions subserved by the hemisphere on the injected side. Abbreviations CPSz complex partial seizures, Lt left, MT medial temporal, MTS mesial temporal sclerosis, NA not available, and NAA n-acetyl aspartate, Rt right.
| ID # Sex, Age, handed | Laser Side & VAS | Aura | Seizure characteristics, MR finding | Memory impaired by side of injection (Wada test). | Medications; name (half life), mg/day. | Results of depth monitoring |
|---|---|---|---|---|---|---|
| 1, F, 48, Right | Rt & 3-6/10 | Anxiety or premoniti on of seizure. | PCSz ?GM, MRI normal, MRSpec ↓ MT NAA | Lt inject memory for words & objects Rt words | Lamotrigene (36), 700 | Left hippocampus amygdale onsets |
| 2, M, 39, Right | Lt & 3/10 | Déjà vu | Left onset, ictal bradycardia, MRI normal | NA | Carbamazepine (15) 1200, Topiramate (21) 200 | Bilateral hippocampus amygdale onsets |
| 3. F, 30, Right | Lt & 2-4/10 | Nil | CPSz, bilateral automatic movements Left MTS | Impaired objects, words, faces bilaterally | Levitiracetam 1500, Carbatrol (34) 600, Valproate (16) 2000 | Left amygdale hippocampus onsets |
| 4, M, 29, Left | Rt & 6/10 | Patient states that he ‘locks down’ | CPSz, left arm face automatisms Right MTS | Impaired objects, words, faces Lt > Right injection | Carbatrol (34) 1800 | Right mesial temporal onsets |
Surgical Technique
A single depth electrode was implanted in the amygdala and hippocampus on both sides by a stereotactic technique using the Leksell frame, for a total of 4 electrodes per subject. Targeting was carried out on T2 coronal MRI scans. MRI scans were not done postoperatively because of concerns regarding MRI induced heating of the metal contacts in the brain (Baker et al., 2004).
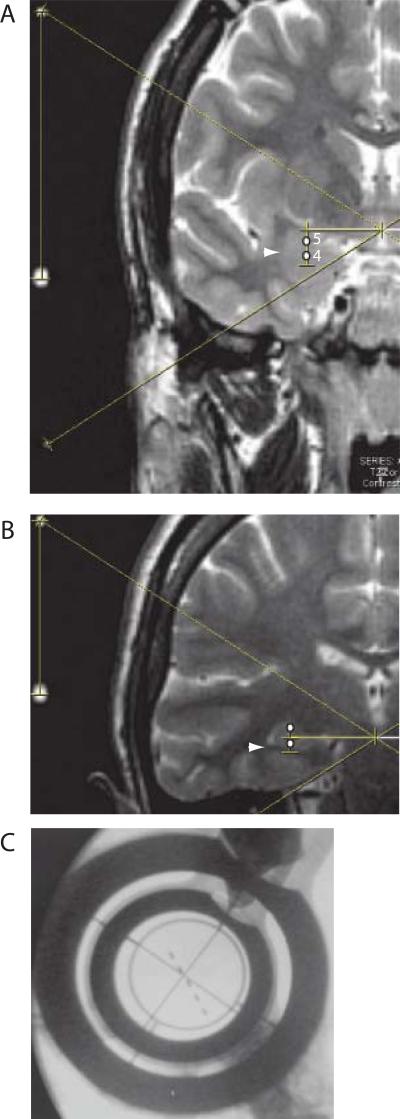
During implantation, twist drill holes (4mm diameter) were made at and anterior to the coronal suture 2.5 cm off the midline. Electrodes were composed of silastic cylinders (diameter 0.8 mm) with five cylindrical titanium contacts at the distal end of the electrode. The contacts were 2.5mm long, were evenly spaced over an electrode length of 2 cm, and were indicated by white dots in Figures 1A and 1B. The distal contact on the amygdala electrode was centered at 1.25 cm below the target in the amygdala which was located on the coronal plane having the maximal amygdala area on the MRI scan (globular structure indicated by the arrowhead in Figure 1A). In this plane, the stereotactic target in the amygdala was at midpoint of the maximal vertical cord between the superior aspect of the amygdala, and the surface of the medial temporal lobe inferiorly.
Figure 1.
Stereotactic technique for placing depth electrodes. Panel A is a T2 weighted coronal image through the amygdala in which the oblique targeting lines define the center of the stereotactic coordinate system, the horizontal line indicates the medial-lateral coordinate, and the vertical line indicates the vertical coordinate. Ventral and dorsal white dots indicate the location of electrodes 4 and 5 on the amygdala electrode in panel A (labeled), and panel B shows the location of the corresponding hippocampal electrode (unlabelled). Panel C is a fluoroscopic image showing the centering crosshairs aligned with the target. See methods section on ‘Surgical Technique’.
The hippocampal electrodes were centered 1.25 cm below the lower border of the body of the hippocampus in the first coronal slice posterior to the head of the hippocampus (Figure 1B). The white arrow in Figure 1B was pointed toward the inferior extent of the ventricle (white crescent shaped structure). In this figure, the hippocampus was indicated by the two white dots (contacts 4 and 5), and formed the deep, convex lateral surface of the ventricle. Therefore, the two dorsal contacts (4 and 5) were estimated to be in the dorsal aspect of the amygdala and the hippocampus ( Figure 1A and B).
The crosshairs in Figure 1C were used indicate the stereotactic target, and to orient the beam of the fluoroscope to be orthogonal both to the frame and to the plane defined by the midline of the brain. The intraoperative fluoroscopy was used to adjust the position of electrode so as to optimize the position of the electrode along the trajectory. The xray image during the targeting procedure (Figure 1C) was a lateral view with the patient facing to the left, so that the left upper corner of the panel was at the anterior, superior aspect of the skull, near the coronal suture. The errors of this technique were associated with the MRI, which has intrinsic errors, and of the intraoperative fluoroscopy, which has errors related both to procedural limitations and to resolution of the technique (see Figure 1C)(Bourgeois et al., 1999).
LEP recording and analysis
During the laser studies, the subject wore protective glasses and reclined in bed with their eyes open, quietly wakeful. Noxious cutaneous heat stimulation, that the subject expected could be painful, was delivered by Thulium YAG laser (wavelength 2 μm, duration 1 ms; Wavelight, Starnberg, Germany). Stimuli were applied to the dorsum of the left or right forearm at random. To avoid sensitization or fatigue of primary nociceptive afferents (Meyer et al., 1994), the laser beam was moved at random to a slightly different position for each stimulus.
During LEP recording, laser pulses were administered at an intensity which produced a pain rating of 3-4/10 in initial screening. This yielded energy levels in the 640 to 830 mJ over a laser beam of 6 mm diameter. A total of 80 laser pulses were applied during one run with an inter-run interval of 1–2 min, and with an interstimulus interval of 7 to 10 s. The subject was asked to rate the average pain intensity of the laser pulses in each run.
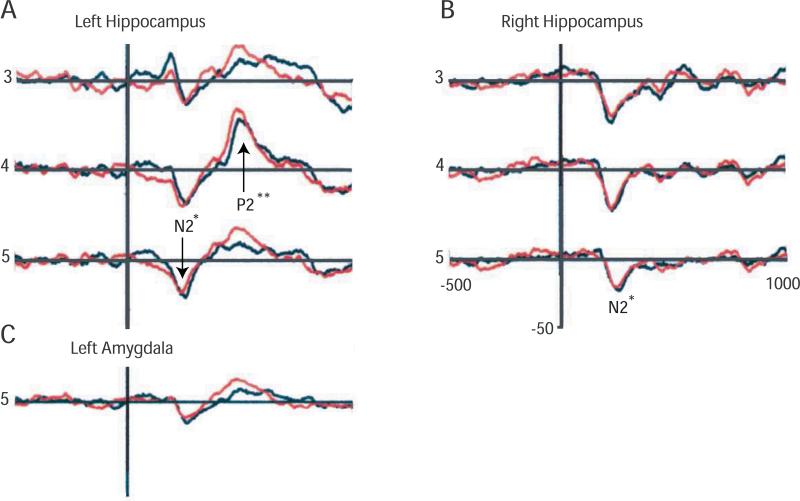
Local field potentials (LFPs) recorded from the electrodes were amplified (12A5, Astro-Med Grass, Inc., West Warwick, RI), band-pass filtered at 0.1-300 Hz, and digitized at 1000 Hz. Multi-channel LFP signals were re-montaged to an average reference of LFP recordings to minimize the influence of the location and activity of the reference electrode (Lehmann, 1987; Crone et al., 1998). LFPs were averaged relative to the onset of laser pulse for LEPs. As in our prior studies of the lateral temporal lobe (Ohara et al., 2004b), intracranial recordings of LEPs and non-phase locked activity were acquired in a 1.5 s post-stimulus window preceded by a 0.5 s pre-stimulus period, which served as the baseline. Responses to individual laser pulses were reviewed manually, and trials with artifacts or large baseline fluctuations were excluded from further analysis. Reproducibility of LEPs was confirmed by examination of the results from two separate recording runs, which were indicated by red and black tracings in Figure 2.
Figure 2.
Reproducible LEPs and estimated electrode locations for subject 4. LEPs are shown for the left (A) and right (B) hippocampus and for the left amygdala (C). The reproducibility of these potentials is shown by the overlay of tracings from two separate runs, one colored black and the other red. The peaks are indicated by the position (latency) of the corresponding label (N2* or P2**) along the horizontal axis, and by an arrow in A4, A5, and B5. The contact at which the potential was recorded is indicated to the left of each potential. The voltage (mV) and time scales (ms), and the polarity of the potentials, indicated in panel B, apply to all three panels.
As shown in Figure 2A, the early and late latency peaks were negative and positive (see scale in Figure 2B), congruent with the polarity of LEPs at the vertex (Tarkka and Treede, 1993; Kunde and Treede, 1993; Beydoun et al., 1993; Kitamura et al., 1995; Chen and Bromm, 1995; Lenz et al., 1998a; Lenz et al., 1998b). In this paper, we make the usual assumption that the earliest reproducible peak of latency corresponding approximately to the vertex N2 is designated N2* (see table 1 in (Lenz et al., 1998a)). Similarly, the following reproducible positive peak of vertex P2 latency is designated P2**. Peak latencies and amplitudes were measured from the reproducible averaged waveforms. Peak amplitudes were measured from the baseline value, which was defined as the pre-stimulus average. Peak latencies were measured at the time of the peak amplitude for each component and peak amplitude was regarded as significant when it was more than 2 standard deviations above the pre-stimulus level. Non-significant peaks were assigned a value of zero.
Time-frequency analysis
Several investigators have demonstrated a large inter-individual variability in the frequency composition of event-related, non-phase locked activity in EEG or local field potentials (Klimesch et al., 1990; Klimesch et al., 1997a; Klimesch et al., 1997b; Klimesch et al., 1998; Pfurtscheller, 1999). To analyze this activity across time and frequency (Figure 3), we used a Matching Pursuit (MP) algorithm based approach (Mallat and Zhang, 1993; Zygierewicz et al., 2005). This procedure combined features of a short time Fourier transform and wavelet analysis and was particularly well suited for analysis of transient non-stationary signals. We used a similar approach to previous studies of event related activity (Zygierewicz et al., 2005). The MP algorithm iteratively decomposed a given signal into a linear combination of elementary waveforms. The optimal time-frequency resolution was obtained when these waveforms were sine modulated Gaussians, known as Gabor atoms. The time–frequency energy density distributions were obtained by calculating the Wigner-Ville distribution of individual atoms, and then taking the weighted sum.
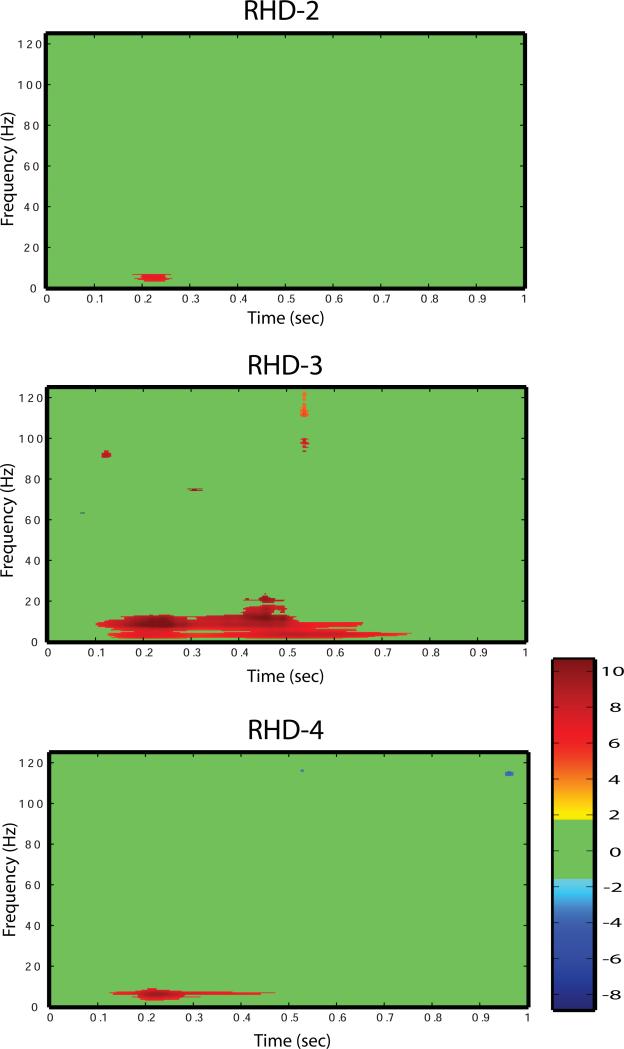
Figure 3.
Time-frequency map of hippocampal changes in energy for the local field potential response to the laser stimulus. The horizontal scale is time following the laser stimulus (s), the vertical scale is frequency (Hz), and the color scale is change in energy relative to baseline in logarithmic scale. The color dimension indicates significant increases (hot colors) or decreases in energy (cold colors) in (dB) after laser stimulation relative to baseline. The green background represents time-frequency regions with no significant change relative to baseline. For example, a pixel with the maximum temperature color of 10 dB represents a significant increase in energy which is 10 dB greater than baseline for a given time and frequency.
Using this approach, we estimated LFP energy of the response to the laser stimulus. The signal energy was computed for each trial, and significant changes in energy in time-frequency plane were identified using statistical testing. To test for the significant changes in the energy, we used Welch's t-test to compare each point in time and frequency after the onset of stimulation with the prestimulus energy, including a correction for multiple comparisons. In this study, the false discovery rate correction was applied to avoid possible false rejections (Snedecor and Cochran, 1967) (Benjamini and Yekutieli, 2001) (Durka et al., 2004). This correction assured a maximal false discovery rate of 5%.
RESULTS
This study was carried out in four subjects with medically intractable complex partial seizures, but not tonic clonic seizures (see Table 1). Scalp monitoring suggested the possibility of temporal lobe seizures in all subjects which were further investigated by implantation of depth electrodes in the hippocampus and amygdala. Seizure monitoring was carried out over a one week period starting the day after implantation. No subject took medications other than anti-epileptic drugs and these were discontinued for 36 hours after the implantation of the electrodes. Therefore, all subjects had substantial blood levels of these drugs at all time points relevant to this study (Levy et al., 2002).
LEP presence, latency and amplitude
Figure 2 showed reproducible LEPs recorded from electrodes implanted in subject 4. The first positive potential in Figure 2A (contact 3) was not reproducible, and so was not analyzed further. All other LEPs recorded on the left (Figure 2A and 2C) showed a reproducible negative wave (N2*) which was followed by a reproducible positive wave (P2**), as indicated by the calibration bar in Figure 2B. The location of the (N2P2*) maximum for the left amygdala (Figure 2C) was at contact 5, the only contact along that electrode with a reproducible LEP wave.
LEPs were recorded in all but one subject (number 3), consistent with evidence that painful stimuli may not lead to BOLD activation of a given forebrain structure in a proportion of individuals studied (Davis et al., 1998). Results in this subject were useful in relating LEPs to clinical and imaging characteristics of this group of subjects (Table 3). There was no significant difference in the incidence of N2* potentials or N2P2* potentials for hippocampal versus the amygdala electrodes (Table 3). There were too few contacts with isolated P2* potentials for statistical analysis.
Table 3.
Electrodes at which N2, P2, and N2P2, or ERD were recorded. The presence of these potentials or event related desynchronization (ERD) at a particular electrode is indicated by the number of the electrode from 1 to 5. Italics indicate a potential or ERD on the right side, regular text indicates the left side. Bolding indicates the maximum potential for an electrode. Underlining indicates that an isolated N2 or P2 potential was recorded at the underlined contact.
| Subject/electrode | Stimulus | N2 | P2 | N2P2 | Theta | MTS | Onset |
|---|---|---|---|---|---|---|---|
| 1/hippocampus | Right | 4, 5. | N, ↓L MT NAA |
L | |||
| 2/hippocampus | Left | 5. | 5. | 5. | N | Bil | |
| 3/hippocampus | Left | L | L | ||||
| 4/hippocampus | Right | 3, 4, 5. 3, 4, 5. |
3, 4, 5. | 3, 4, 5. | Theta ↑ 2, 3 & 4. | R | R |
| 1/amygdala | Right | 5. | 5 | 5. | N, ↓L MT NAA |
L | |
| 2/amygdala | Left | 5, 5. | 5 | 5. | Theta ↑ & beta ↓ 5. | N | Bil |
| 3/amygdala | Left | L | L | ||||
| 4/ amygdala | Right | 5. | 5, 4. | 5. | R | R |
Among 14 contacts, reproducible LEPs including N2*, P2**, or N2P2* were commonly recorded both bilaterally (4/14) and unilaterally (9/14, P=0.115, Fischer). The number of contacts at which LEPs recorded on the left (9/14, P=0.257) was not significantly different than the right (5/14), independent of the hand stimulated. Neither was the incidence different on the side contralateral (8/14) versus that ipsilateral to the stimulus (5/14, P=0.71, Fisher). Therefore, sites which received input arising from nociceptors werecommonly located bilaterally, and unilateral potentials were equally common on the left versus the right, but did not reflect preferential input from contralateral peripheral structures.
Latencies and amplitudes of LEPs were shown in Table 2. The N2* potential latencies and amplitudes were not different between the amygdala and hippocampus (P=0.9 or P=0.17, t test). Latencies and amplitudes of P2 potentials were not different between the amygdala and the hippocampus (P=0.44 and P=0.41). The latencies and amplitudes of left versus right N2* potentials overall were not significantly different (P>0.05).
Table 2.
Mean (Standard Deviation) of Latencies and amplitudes of reproducible LEP N2 and P2 potentials recorded from contacts along electrodes targeting the amygdala and hippocampus bilaterally. All results were averaged across all contacts in subjects 1, 2, and 4, unless otherwise noted in the left column. The left hand was stimulated in some subjects (1, 4), and the right was stimulated in the others (2, 3)(Tables 1 and 3).
| N2* latency | N2* amplitude | P2** latency | P2** amplitude | |
|---|---|---|---|---|
| Overall | 261ms (70), n=13 | −50μV (19). | 494ms (47), n=7 | 54μV (18). |
| Amygdala left | 248 (59), n=3 | −56 (19). | 506 (64), n=3 | 48 (23). |
| Right: Subject 2 | 220, n=1 | −30. | ||
| Hippocampus left | 260 (74), n=6 | −45 (20). | 478 (24), n=4 | 57 (31). |
| Right; Subject 4 | 210-232, n=3 | −25 to −35. |
The N2P2* amplitude at contacts on electrodes traversing the amygdala (72 ± 4μV) was not different from that for the hippocampus (67 ± 55 μV). The amplitude of the N2P2* was not different among electrodes contralateral to the stimulus (82±47) versus those ipsilateral (31±5 P=0.06, t-test electrodes). The N2* and P2** latencies in Table 2 were consistent with those recorded from intracranial electrodes over the temporal cortex (Lenz et al., 1998a).
Locations of recording sites for LEPs and LEP maxima
In Figure 2, the maxima for the left hippocampal and right hippocampal leads were at contact 4 while smaller potentials were observed at contacts 3 and 5. Two other electrodes had a reproducible potential at contact 4 while no such potential was seen at either contact 3 or 5 (Table 3). This is strong evidence of a local generator at contact 4, which was the lower of the two electrodes (white dots) shown in Figures 1A and 1B.
In the other five electrodes with reproducible potentials, maxima were located at contact 5 in both the absence of reproducible potentials at the ventrally adjacent electrode, and the absence of a dorsally adjacent electrode. The presence of a single reproducible LEP without any potentials at an adjacent electrode, makes the possibility of a remote generator unlikely in the case of these five electrodes. However, a local generator is a possibility which cannot be proven to explain the results from these electrodes (Table 3).
Maxima in the amygdala were found at contact 4 once and contact 5 on 4 electrodes (Table 3). The most dorsal contacts (number 5) was just below the upper border of the light grey globular structure, indicated by the white arrowhead in Figure 1A. This upper border was either at the upper or the lower aspect of nucleus basalis depending upon the atlas used (Schaltenbrand and Bailey, 1959)(Mai et al., 2007), while both atlases agree that the nucleus basalis was just dorsal to the central nucleus of the amygdala. Therefore, on amygdala electrodes contacts 4 or 5 or both may be in the central nucleus or nucleus basalis.
Maxima in the hippocampus were found at contact 4 (three times) or 5 (once) which were approximately located in the dorsal or ventral aspect of the hippocampus, as shown in Figure 1. The likelihood of a local hippocampal generator was high, based upon the results of the electrodes for which the contacts on either side of contact 4 had absent or smaller potentials than at contact 4.
Non-phase locked activity
As in the case of LEPs, non-phase locked changes in local field potentials at the time of the laser stimulus were found in a minority of contacts and electrodes. These non-phase locked changes in local field potentials always included the theta range (Figure 2). The latencies of these changes were short at onset (105ms ± 25, mean ± standard deviation) and offset (451 ± 246). The time course of changes overlapped with but was substantially longer than the duration of the N2* potentials recorded at two of these four contacts (Subject 2, electrodes 4 and 5). Therefore, in these two cases the non-phase locked changes are not explained by the frequency composition of the N2* potential.
Among contacts at which N2* potentials were recorded, contacts on the left were as common (9/13) as those on the right (4/13, P=0.11 Fisher). The recording of N2* potentials on the right occurred at 4 out of 40 electrodes; non-phase locked potentials were recorded at 3 out of these 4 electrodes, and at one adjacent electrode. This degree of coincidence of LEPs with non-phase locked activity at the same contacts was greater than expected at random (P=0.0075, Binomial).
Relationship of LEPs to Clinical Variables
The subjects in this study all had temporal lobe epilepsy as determined by clinical and scalp EEG criteria. Two subjects had evidence of mesial temporal sclerosis, scarring of the medial temporal lobe, which is common in patients with temporal lobe epilepsy (Williamson et al., 1993). Mesial temporal sclerosis might be predicted to impair physiologic processes, such as the response to the laser stimulus. However, the presence of LEPs was not different between electrodes located in structures with medial temporal sclerosis (5/20) versus those without (9/60, P=1 Fisher). If a decrease in N-acetyl aspartate on magnetic resonance spectroscopy was included as a criterion for mesial temporal sclerosis then LEPs were still not different on that side (8/30) versus the other (6/50, P=1 Fisher 2). Finally, if we consider only hippocampal electrodes, then the proportion of LEPs recorded on the side with mesial temporal sclerosis (3/10) was not different from the side without mesial temporal sclerosis (6/30, P=0.665 Fisher). Therefore, the present results suggested that the presence of mesial temporal sclerosis did not impair recordings of LEPs.
Pathologic electrical activity such as inter-ictal spikes or seizure activity onsets might impair physiologic processes such as LEPs. However, in these results the number of contacts at which LEPs were recorded on the side of seizure onset (9/50) was not significantly different from that on the other side (5/30, P=1 Fisher). Therefore, differences in the side on which LEPs were recorded could not be explained by the presence of mesial temporal sclerosis or electrical seizure related activity.
The average pain ratings across runs of laser stimuli (see methods) across all four patients ranged from 2.3 to 4/10 for average intensity and 3 to 5/10 for average unpleasantness. Analysis across patients showed that these pain ratings were not correlated with either numbers of contacts with reproducible LEPs, or with LEP amplitude.
DISCUSSION
We have tested the hypothesis that the primate amygdala and hippocampus receive inputs arising from nociceptors. The results showed that LEP N2*, P2** and N2P2* were recorded from electrodes implanted in the amygdala and hippocampus. The latencies of these potentials were similar to those recorded from scalp electrodes over the vertex and subdural electrodes over the sylvian fissure (present Table 2, and Table 1 in (Lenz et al., 1998a)). The amygdala and hippocampal responses resulted from the cutaneous application of a laser stimulus which produced a pure pain sensation by selective activation of nociceptors (Kenton et al., 1980). Therefore, it is very likely that these LEPs were related both to nociceptive transmission and to the sensation of pain.
Along electrodes through the amygdala, the majority of LEPs were recorded dorsally at approximately the level of the central nucleus of amygdala and the adjacent nucleus basalis (Schaltenbrand and Bailey, 1959; Mai et al., 2007). Non-phase locked theta rhythms and isolated N2* potentials were recorded at the same contacts on the right. In the absence of studies with an innocuous stimulus, we cannot be certain that these results applied uniquely to noxious stimuli. Nevertheless, these results strongly suggested the presence of pain-related inputs to the medial temporal lobe where they may be involved in avoidance behaviors or disability related to painful stimuli, including aversive conditioning.
Nociceptive Mechanisms to the Medial Temporal lobe
In rodents and cats, there is strong evidence that nociceptive pathways project to the amygdala through the pontine parabarachial nucleus and the thalamic posterior intralaminar nucleus (Lanuza et al., 2008; Neugebauer et al., 2009). Physiologic studies demonstrate that dorsal horn lamina I nociceptive specific neurons project to the PB nucleus (McMahon and Wall, 1985; Hylden et al., 1986; Hayashi and Tabata, 1989)(Light et al. 1987)(Cechetto et al., 1985; Blomqvist et al., 1989; Berkley and Scofield, 1990). Neurons in the parabrachial nucleus and the central nucleus of the amygdala respond to noxious stimuli (Slugg and Light, 1994)(Hayashi and Tabata 1990)(Bernard et al., 1992)(Bernard and Besson 1988).(Light et al. 1987)(Cechetto et al., 1985; Blomqvist et al., 1989; Berkley and Scofield, 1990). Neurons in the parabrachial nucleus and the central nucleus of the amygdala respond to noxious stimuli (Slugg and Light, 1994)(Hayashi and Tabata 1990)(Bernard et al., 1992)(Bernard and Besson 1988).
Neurons in the rodent septal nuclei, which project to the hippocampus, respond to noxious stimuli (Dutar et al. 1985). The receptive fields of these neurons in the amygdala and septal nuclei are often large and bilateral, consistent with the bilateral responses which were observed in our study. The presence of such neurons is consistent with imaging evidence that the hippocampus is involved in all the procedural phases of fear conditioning, and that hippocampal BOLD activations occur in response to conditioned stimuli used in fear conditioning (see Introduction)(Knight et al., 2004; Delgado et al., 2008). Hippocampal activity is often proposed to mediate the role of the environment or the context of fear conditioning. In fact, hippocampal activations may be better related to context than to the US (Marschner et al., 2008).
There is some evidence that neurons in the rodent nucleus basalis also respond to noxious stimuli (Zhang et al., 2002). Electrical stimulation of the nucleus basalis can condition somatic or auditory stimuli in a cholinergic dependent fashion, and so lead to changes in the cortical responses evoked by these stimuli (Verdier and Dykes, 2001; Miasnikov et al., 2001). Stimulation of nucleus basalis can also lead to changes in heart rate, respiratory rate, and in ongoing cortical theta and gamma local field potential signals (Miasnikov et al., 2001). Therefore, the LEP, local field potential, and behavioral activation which occurs during pain-related attention tasks could be the result of nociceptive activation of the nucleus basalis (Wenk, 1997; Ohara et al., 2004a; Ohara et al., 2004c). This suggestion may be consistent with evidence that the nucleus basalis and the central nucleus of amygdala are jointly involved in attentional modulation of behavior in classical conditioning paradigms (Kapp et al., 1994; Holland and Gallagher, 1999).
In the present study, increases in theta LFP were recorded in response to the painful laser from electrode contacts in the right amygdala and hippocampus. A previous study in rats has demonstrated hippocampal theta lasting 7 s following immersion of the tail in hot water. In addition, depression of the CA1 population spike was observed for up to 1 hour (Khanna and Sinclair, 1989). Increased theta has been observed up to one hour following injection of formalin into the hind paw, and may be associated with pain related motor behavior (Tai et al., 2006). Hippocampal theta has been tied to behavioral states and information processing functions in many species including humans (Bland, 1986; Buzsaki, 2002). Therefore, the present results suggested that the LEPS and the theta rhythms in local field potentials may be related to the cognitive aspect of pain.
The relation of theta frequency activity and LEPs to pain is consistent with the observation that injection of morphine into the rat amygdala bilaterally leads to anti-nociception (Yaksh and Rudy, 1978). In addition, manipulations which decrease the breakdown of endogenously released opioids in the amygdala bilaterally lead to anti-nociception (al-Rodhan et al., 1990). Finally, injections of lidocaine or a NMDA blocker into the hippocampus has an analgesic effect (McKenna and Melzack, 1992; McKenna and Melzack, 2001). These results strongly support the importance of the amygdala and hippocampus in rodent pain processing, which is consistent with the present human study.
Comparisons with Human Imaging Studies
In the present study, LEPs (N2*, P2**, N2P2*) were commonly recorded unilaterally and bilaterally; unilateral potentials were equally as common on the left as on the right. In imaging studies, symmetrical responses to painful stimuli were found to be increases or decreases in BOLD signal in the amygdala (Derbyshire et al., 1997) (Becerra et al., 1999), or in the hippocampus and perihippocampal gyrus (Bornhovd et al., 2002; Bingel et al., 2002) on either side, following stimulation of either hand. Other studies have found asymmetrical activations in the amygdala, (Schneider et al., 2001)), and the hippocampus (Derbyshire et al., 1997) (Schneider et al., 2001)) or the perihippocampal gyrus (Derbyshire et al., 1997). Therefore, the present results are generally consistent with the imaging studies. However, the interpretation of many of these activations will be dependent upon the conditions compared, such as warm minus threshold painful heat stimuli (Derbyshire et al., 1997), or different conditioning and cognitive conditions (Ploghaus et al., 2001) (Phelps et al., 2001).
These responses of neurons in the amygdala to peripheral stimuli may change after peripheral injury. The effects of such injury may include facilitation of responses which are dependent upon mechanisms involving receptors for glutamate and corticotrophin releasing factor (Fu and Neugebauer, 2008; Palazzo et al., 2008). The potentiating effect of nerve injury upon the synapse from the parabrachial nucleus to the central nucleus of the amygdala may be lateralized. In a spinal nerve model of neuropathic pain, changes in responses of neurons in the central nucleus to stimulation of the parabrachial nucleus were observed predominantly contralateral to the side of the nerve lesion (Ikeda et al., 2007). These results point to the differences in the response of neurons in the amygdala to acute pain versus pain related to peripheral injury.
The role of the amygdala in fear conditioning is well established (LeDoux, 1996). The present results demonstrated that aversive painful stimuli led to potentials in the amygdala and hippocampus. The modulation of these potentials by cognitive and behavioral behaviors may provide a useful probe into human aversive conditioning. Finally, the relationship between amygdala and aversive conditioning may be an important contributor to pain-related avoidance behaviors and resulting pain related disability (Grotle et al., 2004; Leeuw et al., 2007).
Acknowledgement
This work was supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke (NS38493 and NS40059 to FAL). We thank L.H. Rowland and J. Winberry for excellent technical assistance.
Reference List
- al-Rodhan N, Chipkin R, Yaksh TL. The antinociceptive effects of SCH-32615, a neutral endopeptidase enkephalinase inhibitor, microinjected into the periaqueductal, ventral medulla and amygdala. Brain Res. 1990;520:123–130. doi: 10.1016/0006-8993(90)91697-f. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European Journal of Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baker KB, Tkach JA, Nyenhuis JA, Phillips M, Shellock FG, Gonzalez-Martinez J, Rezai AR. Evaluation of specific absorption rate as a dosimeter of MRI-related implant heating. J Magn Reson Imaging. 2004;20:315–320. doi: 10.1002/jmri.20103. [DOI] [PubMed] [Google Scholar]
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG, Borsook D. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn Res Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Yekutieli Y. The control of the false discovery rate under dependency. Ann Stat. 2001:1165–1188. [Google Scholar]
- Berkley KJ, Scofield SL. Relays from the spinal cord and solitary nucleus through the parabrachial nucleus to the forebrain in the cat. Brain Res. 1990;529:333–338. doi: 10.1016/0006-8993(90)90847-5. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Besson JM. The spinotrigeminopontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. [DOI] [PubMed] [Google Scholar]
- Beydoun A, Morrow TJ, Shen JF, Casey KL. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol. 1993;88:173–181. doi: 10.1016/0168-5597(93)90002-7. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Ma W, Berkley KJ. Spinal input to the parabrachial nucleus in the cat. Brain Res. 1989;480:29–36. doi: 10.1016/0006-8993(89)91563-1. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Bourgeois G, Magnin M, Morel A, Sartoretti S, Huisman T, Tuncdogan E, Meier D, Jeanmonod D. Accuracy of MRI-guided stereotactic thalamic functional neurosurgery. Neuroradiology. 1999;41:636–645. doi: 10.1007/s002340050816. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Chen ACN, Bromm B. Pain-related generators of laser-evoked brain potentials: brain mapping and dipole modeling. In: Bromm B, Desmedt JE, editors. Pain and the Brain: From Nociception to Cognition. Raven Press, Ltd.; New York: 1995. pp. 245–266. [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 12):2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ. MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol. 1998;80:1533–1546. doi: 10.1152/jn.1998.80.3.1533. Functional. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. 1992. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire SWG, Jones AKP, Gyulai F, Clark S, Townsend D, Firestone L. Pain processing during three levels of noxious stimulation produces different pattern of central activity. Pain. 1997;73:431–445. doi: 10.1016/S0304-3959(97)00138-3. [DOI] [PubMed] [Google Scholar]
- Durka PJ, Zygierewicz J, Klekowicz H, Ginter J, Blinowska KJ. On the statistical significance of event-related EEG desynchronization and synchronization in the time-frequency plane. IEEE Trans Biomed Eng. 2004;51:1167–1175. doi: 10.1109/TBME.2004.827341. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotle M, Vollestad NK, Veierod MB, Brox JI. Fear-avoidance beliefs and distress in relation to disability in acute and chronic low back pain. Pain. 2004;112:343–352. doi: 10.1016/j.pain.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Tabata T. Physiological properties of sensory trigeminal neurons projecting to mesencephalic parabrachial area in the cat. J Neurophysiol. 1989;61:1153–1160. doi: 10.1152/jn.1989.61.6.1153. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Hayashi H, Dubner R, Bennett GJ. Physiology and morphology of the lamina I spinomesencephalic projection. J Comp Neurol. 1986;247:505–515. doi: 10.1002/cne.902470410. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Takahashi Y, Inoue K, Kato F. NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain. 2007;127:161–172. doi: 10.1016/j.pain.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Supple WF, Jr., Whalen PJ. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kenton B, Coger R, Crue B, Pinsky J, Friedman Y, Carmon A. Peripheral fiber correlates to noxious thermal stimulation in humans. Neurosci Lett. 1980;17:301–306. doi: 10.1016/0304-3940(80)90040-3. 1980. [DOI] [PubMed] [Google Scholar]
- Khanna S, Sinclair JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain. 1989;39:337–343. doi: 10.1016/0304-3959(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Kakigi R, Hoshiyama M, Koyama S, Shimojo M. Watanabe S Pain-related somatosensory evoked magnetic fields. Electroencephalography and Clinical Neurophysiology. 1995;95:463–474. doi: 10.1016/0013-4694(95)00139-5. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neurosci Lett. 1997a;238:9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Russegger H. Event-related desynchronization in the alpha band and the processing of semantic information. Brain Res Cogn Brain Res. 1997b;6:83–94. doi: 10.1016/s0926-6410(97)00018-9. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J. Induced alpha band power changes in the human EEG and attention. Neurosci Lett. 1998;244:73–76. doi: 10.1016/s0304-3940(98)00122-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Pfurtscheller G, Mohl W, Schimke H. Event-related desynchronization, ERD-mapping and hemispheric differences for words and numbers. Int J Psychophysiol. 1990;8:297–308. doi: 10.1016/0167-8760(90)90020-e. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Kunde V, Treede RD. Topography of middle-latency somatosensory evoked potentials following painful laser stimuli and non-painful electrical stimuli. Electroencephalography and Clinical Neurophysiology. 1993;88:280–289. doi: 10.1016/0168-5597(93)90052-q. [DOI] [PubMed] [Google Scholar]
- Lanuza E, Moncho-Bogani J, LeDoux JE. Unconditioned stimulus pathways to the amygdala: effects of lesions of the posterior intralaminar thalamus on foot-shock-induced c-Fos expression in the subdivisions of the lateral amygdala. Neurosci. 2008;155:959–968. doi: 10.1016/j.neuroscience.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. Emotion. In: Mountcastle VB, editor. Handbook of Physiology. Section 1: The Nervous System. Volume: The Higher Functions. The American Physiologic Society; Bethesda: 1996. [Google Scholar]
- LeDoux JE. Emotion and the amygdala. In: Aggleton JP, editor. The Amygdala. Wiley-Liss; New York: 1992. pp. 339–351. [Google Scholar]
- Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Principles of spatial analysis. In: Gevins AS, Remond A, editors. Methods of analysis of brain electrical and magnetic signals. Handbook of electroencephalography and clinical neurophysiology, revised series. Vol. 1. Elsevier; Amsterdam: 1987. pp. 309–354. [Google Scholar]
- Lenz FA, Rios M, Chau D, Krauss GL, Zirh TA, Lesser RP. Painful stimuli evoke potentials recorded from the parasylvian cortex in humans. J Neurophysiol. 1998;80:2077–2088. doi: 10.1152/jn.1998.80.4.2077. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Rios M, Zirh A, Chau D, Krauss G, Lesser RP. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J Neurophysiol. 1998b;79:2231–2234. doi: 10.1152/jn.1998.79.4.2231. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol. 1993;70:200–212. doi: 10.1152/jn.1993.70.1.200. [DOI] [PubMed] [Google Scholar]
- Levy RH, Mattson RH, Melega W, Perucca E. Antiepilpetic Drugs. Lippincott, Williams and Wilkins; NY NY: 2002. [Google Scholar]
- Ma W, Peschanski M. Spinal and trigeminal projections to the parabrachial nucleus in the rat: electron-microscopic evidence of a spino-ponto-amygdalian somatosensory pathway. Somatosens Res. 1998;5:247–257. doi: 10.3109/07367228809144629. 1988. [DOI] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the human brain. Academic Press; NY NY: 2007. [Google Scholar]
- Mallat S, Zhang Z. Matching pursuit with time-frequency dictionaries. IEEE Trans Signal Proc. 1993;41:3397–3415. [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–9036. doi: 10.1523/JNEUROSCI.1651-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. Analgesia produced by lidocaine microinjection into the dentate gyrus. Pain. 1992;49:105–112. doi: 10.1016/0304-3959(92)90195-H. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Melzack R. NMDA receptors in the hippocampal dentate gyrus with AP5 produces analgesia in the formalin pain test. Exp Neurol. 2001;172:92–99. doi: 10.1006/exnr.2001.7777. Blocking. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wall PD. Electrophysiological mapping of brainstem projections of spinal cord lamina I cells in the rat. Brain Res. 1985;333:19–26. doi: 10.1016/0006-8993(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Campbell JN, Raja SN. Peripheral neural mechanisms of nociception. In: Wall PD, Melzack R, editors. Textbook of Pain. Churchill Livingstone; Edinburgh: 1994. pp. 13–44. [Google Scholar]
- Miasnikov AA, McLin D, III, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. Neurorep. 2001;12:1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365:640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clin Neurophysiol. 2004a;115:1641–1652. doi: 10.1016/j.clinph.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Treede RD, Lenz FA. Cutaneous painful laser stimuli evoke responses recorded directly from primary somatosensory cortex in awake humans. J Neurophysiol. 2004b;91:2734–2746. doi: 10.1152/jn.00912.2003. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Vogel H, Treede RD, Lenz FA. Attention to pain is processed at multiple cortical sites in man. Exp Brain Res. 2004c;156:513–517. doi: 10.1007/s00221-004-1885-2. [DOI] [PubMed] [Google Scholar]
- Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V. Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharm. 2008;55:537–545. doi: 10.1016/j.neuropharm.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Quantification of ERD and ERS in the time domain. In: Pfurtscheller G, Lopes da Silva FH, editors. Event-related desynchronization. Elsevier; Amsterdam: 1999. pp. 89–105. [Google Scholar]
- Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The spinoparabrachial pathway: shedding new light on an old path. J Comp Neurol. 1995;353:477–479. doi: 10.1002/cne.903530402. [DOI] [PubMed] [Google Scholar]
- Schaltenbrand G, Bailey P. Introduction to stereotaxis with an atlas of the human brain. Thieme; Stuttgart: 1959. [Google Scholar]
- Schneider F, Habel U, Holthusen H, Kessler C, Posse S, Muller-Gartner HW, Arndt JO. Subjective ratings of pain correlate with subcortical-limbic blood flow: an fMRI study. Neuropsychobiology. 2001;43:175–185. doi: 10.1159/000054887. [DOI] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci. 1999;19:420–430. doi: 10.1523/JNEUROSCI.19-01-00420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slugg RM, Light AR. Spinal cord and trigeminal projections to the pontine parabrachial region in the rat as demonstrated with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1994;339:49–61. doi: 10.1002/cne.903390106. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames: 1967. [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tai SK, Huang FD, Moochhala S. Khanna S Hippocampal theta state in relation to formalin nociception. Pain. 2006;121:29–42. doi: 10.1016/j.pain.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol. 1993;10:513–519. doi: 10.1097/00004691-199310000-00009. [DOI] [PubMed] [Google Scholar]
- Verdier D, Dykes RW. Long-term cholinergic enhancement of evoked potentials in rat hindlimb somatosensory cortex displays characteristics of long-term potentiation. Exp Brain Res. 2001;137:71–82. doi: 10.1007/s002210000646. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Narcotic analgestics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299–359. doi: 10.1016/0304-3959(77)90145-2. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Mei J, Lu SG, Zhao ZQ. Age-related alterations in responses of nucleus basalis magnocellularis neurons to peripheral nociceptive stimuli. Brain Res. 2002;948:47–55. doi: 10.1016/s0006-8993(02)02947-5. [DOI] [PubMed] [Google Scholar]
- Zygierewicz J, Durka PJ, Klekowicz H, Franaszczuk PJ, Crone NE. Computationally efficient approaches to calculating significant ERD/ERS changes in the time-frequency plane. J Neurosci Methods. 2005;145:267–276. doi: 10.1016/j.jneumeth.2005.01.013. [DOI] [PubMed] [Google Scholar]