Abstract
Objective:
Among Finnish adolescent twins, we compared (a) a model that describes a direct impact of liability to tobacco use on cannabis and other illicit drug use with (b) a model that included a shared underlying liability for these substances. Furthermore, the extent to which genetic and environmental influences contribute to the covariation between liabilities to use these substances was examined.
Method:
Tobacco and illicit drug use were assessed at age 17.5 years. Twin data on 3,744 individuals were analyzed using standard biometrical methods. Two alternative multivariate models were fit and compared with Mx, a statistical program for genetic model fitting.
Results:
The multivariate model, including a direct impact of the initiation of tobacco use on illicit drug use, provided the best fit to the data. In this model, the total variation in the initiation of illicit drugs was decomposed to genetic factors (32%), common environmental factors (20%), unique environmental factors (8%), and a component due to initiation of smoking (40%). Most variation in the progression of illicit drug use was the result of initiation of smoking and illicit drug use (83%).
Conclusions:
Liability to initiate smoking directly affects illicit drug use in our best-fitting model. Our findings suggest that several common genetic influences may be related to tobacco use and illicit drugs but that a search for specific genes underlying illicit drug use is justified as well. Such specific genes may hold a key to understanding biological vulnerabilities that lead to illicit drug use, which could aid in the development of targeted interventions.
Smoking cigarettes is common among adolescents, of whom approximately 60% have ever smoked a cigarette and about 33% have smoked during the last month, according to recent surveys throughout Europe (Hibell et al., 2009). Although less prevalent than smoking, cannabis is the most commonly used and abused illicit drug in Western countries. Early onset of cannabis use has been shown to be a consistent, strong predictor of substance-related problems (Hawkins et al., 1992; Lynskey et al., 2003) and may result in adverse psychiatric effects, such as depression and psychotic illness. Adolescent users are especially vulnerable to these effects (Ferdinand et al., 2005; Fergusson et al., 2002; Rey et al., 2004). Therefore, more insight into the risk factors and mechanisms that may lead to initiation and continuation of cannabis use is needed for the development of effective prevention and treatment programs.
In adolescence, tobacco is often the first drug used (Li, 2003), followed by other drugs, such as cannabis. Because this sequence in substance use is found frequently, several studies have focused on tobacco use as a first “gateway” for cannabis use (e.g., Kandel et al., 2006). Similarly, cannabis use has been regarded as a second gateway to the subsequent use of other illicit drugs (Fergusson and Horwood, 2000; Fergusson et al., 2006). These gateway theories for tobacco, and particularly for cannabis use, have been a source of recent and lively debate (e.g., Anthony, 2002; Kandel et al., 2006; Kenkel and Mathios, 2002; Lynskey, 2002; Maccoun, 2006). Although most researchers emphasize the co-occurrence of use of various substances and agree with the normative sequence (Degenhardt et al., 2009)—beginning with licit drugs, such as alcohol and tobacco, followed by cannabis, then other illicit drugs—they differ in respect to how these phenomena can be explained. As a plausible alternative to the gateway theory, a common factor model, has been postulated (Morral et al., 2002), which may, for instance be reflected in an individual's propensity to associate with others who use drugs (Lynksey et al., 1998), or by a common genetic vulnerability underlying any substance use (Lynskey, 2002).
Recently, evidence has been found for a reverse gateway (i.e., cannabis use precedes cigarette smoking) that contradicts the gateway theory (Clough, 2005; Patton et al., 2005; Viveros et al., 2006). This would suggest that a common liability or common factor model, representing a common underlying factor, may explain the often observed co-occurrence of tobacco use and cannabis use, irrespective of the sequence of use of these substances.
In a previous study within our Finnish twin cohort, predictors for use of cannabis and other illicit drugs were investigated. In the final regression model, smoking initiation by age 12 years was the most powerful predictor among the individual-based analysis, with an odds ratio of 26 (p < .001). A similar association could be replicated within a matched case-control design, including discordant twin pairs only (odds ratio = 22, p < .001; Korhonen et al., 2008), in which one twin of a pair used drugs and the other twin did not. These findings provide evidence for a particularly strong temporal association between the onset of smoking at an early age and the subsequent onset of cannabis use. In the current study, we extend these findings by focusing on the underlying shared or specific genetic and environmental contributions to these outcomes.
Recently, Neale et al. (2006) described novel extensions to the modeling of the latent liability to initiate and progress the use of substances, including the development of first using one substance and then progressing to use of another substance. In their study, the authors described on a statistical level why previously existing models explaining the relation between initiation and progression of the use of one particular substance (or, univariate models) were difficult to extend to, for instance, multivariate models that included initiation and progression of the use of two different substances. They overcame this problem with their novel approach, which regarded the analysis of twin data on initiation and progression as a special case of missing data, as previously performed by Heath et al. (2002), wherein individuals who do not initiate are regarded as having missing data on progression measures. In addition, Neale et al. (2006) extended these models by jointly testing a series of causal, common, and contingent models. They illustrated this novel approach with several models, including one that is of particular relevance for our current study. In this multivariate model, reciprocal relations between both tobacco use and cannabis use initiation and progression were modeled. Therefore, it tests whether the liability to use one substance is a risk factor for use of another substance, or vice versa, in a sample of female adult twins. Their results showed a slightly better fit for the causal model, compared with a common liability model.
In the present study, we built on this novel approach to model genetically informative data, and analyzed the pattern of liability to initiate tobacco and cannabis use and the progress of use in an adolescent twin sample. Examining the genetic vulnerability to progress from initiation of cigarette smoking to cannabis use will provide insight into the potential mechanisms underlying this phenomenon. Within our large-scale adolescent twin study of boys and girls, we evaluated (a) whether a model in which the liability to initiate tobacco use directly affects the liability to initiate cannabis use or a model in which use of these substances are correlated because of shared liabilities, represented by shared genetic and environmental influences, better fits observed data; and (b) the extent to which genetic and environmental influences contribute to the covariation between the initiation and progression of tobacco and cannabis and other illicit drug use.
Method
Participants
The FinnTwin12 study—a collaborative research project of the Universities of Helsinki and Jyväskylä in Finland and Indiana University in the United States—was started in 1994 to examine genetic and environmental determinants of precursors of health-related behaviors in twins initially age 10 to 12 years (born in 1983–1987). The epidemiological investigation of five consecutive and complete birth cohorts of Finnish twin children (n = 5,600 twins and 5,000 parents from 2,800 families) included questionnaire assessments provided by twins, their parents, teachers, and classmates. The study protocol was approved by the institutional review board of Indiana University and the Ethical Committee of the University of Helsinki. The baseline was conducted late in the year before the twins reached age 12 years, with follow-ups at ages 14 and 17.5 years (Kaprio, 2006).
For this study, we used data collected from the third wave of the study, which was initiated in autumn of 2000 and completed in spring of 2005 (response rate: 92%; 4,236 questionnaires were returned from 4,594 mailed). Questionnaires were mailed out semi-annually to each half of a birth cohort, with the average age at mailing of 17.5 years. Among the 4,236 participants, 107 had missing data on essential study variables. Furthermore, we excluded those individuals who did not have data from their co-twin (n = 161). We also excluded participants whose zygosity could not be confirmed (n = 224). Thus, the twin sample of the current investigation included 3,744 individuals (1,872 twin pairs: 632 monozygotic [MZ], 287 males, and 345 females; 1,240 dizygotic [DZ], 322 males, 315 females, and 603 opposite sex).
Substance-use measures
We applied four main substance-use measures to describe variables that were modeled as latent liabilities: initiation and progression of cigarette smoking, and initiation and progression of cannabis use.
Smoking initiation.
Smoking initiation was assessed with the following question: “Have you ever smoked cigarettes (or experimented with at least one cigarette)?” (yes/no).
Smoking progression.
Those who had ever smoked were classified into (a) nondaily smoking (had smoked fewer than 51 cigarettes in their lifetime or smoked 51 or more cigarettes but who were not daily smokers) and (b) daily smoking (had smoked at least 51 cigarettes and who were daily smokers). Participants who never initiated cigarette smoking had missing data for progression. We used this limit of lifetime exposure because, in a national survey among Finnish adolescents, only those who had lifetime use of more than 50 cigarettes were considered as progressed beyond smoking experimentation (Rimpelä et al., 2007).
Initiation of cannabis and other illicit drug use.
Initiation was assessed by asking, “Have you ever tried or used drugs, such as hashish, something to sniff, or other drugs or substances that would make you feel intoxicated?” The options were the following: (a) I have never tried or used, (b) 1–3 times, (c) 4–9 times, (d) 10–19 times, and (e) 20 times or more. Our own unpublished interview data among a subsample of 1,852 intensively assessed twins at age 14 years shows that some 90% of reported illicit drug use was specifically cannabis use and less than 1% had ever used any substance other than tobacco, alcohol, or marijuana, which was in line with recent Finnish statistics (Virtanen and Sjöberg, 2006). However, because of the wording of our question, we chose to refer to “cannabis/other illicit drug use” or just “illicit drug use” throughout this article. To define the initiation of cannabis/other illicit drug use, we considered all who reported experimenting or using at least once in a lifetime as “initiated.”
Progression of cannabis/other illicit drug use.
Progression was defined as using four to nine times or more. A similar limit, such as marijuana use six or more times (Shelton et al., 2007), has been earlier used for progression of cannabis use among adolescents, indicating that these individuals have proceeded beyond the experimentation stage.
Statistical analyses
Twin data were analyzed using standard biometrical methods (Neale and Maes, 2006). We applied basic twin modeling, estimating genetic and environmental influences on our substance-use measures that are modeled as underlying normally distributed latent traits (i.e., liabilities to initiate or progress in substance use). More specifically, the basic twin model partitions variance in a trait into: genetic influences (A); common environmental influences shared by a twin pair (C); and unique environmental influences, also including measurement error (E). Such twin modeling is based on the knowledge that MZ twins are genetically identical, whereas DZ twins share on average 50% of their segregating genes. Thus, a greater similarity for MZ twins, compared with DZ twins, gives support to the hypothesis that genetic transmission is a component of importance, under the assumption that MZ and DZ share to the same extent their trait-relevant environmental experiences. The inclusion of multiple phenotypes (i.e., substance-use measures) in the multivariate model allows us to study whether overlap between various traits (liability to behaviors) is the result of shared genetic or environmental influences (Boomsma et al., 2002).
Descriptive analyses were conducted before model fitting. The MZ and DZ prevalences of smoking and illicit drug use initiation and progression were examined within males and females. Because of a low number of observations in illicit drug use progression, we pooled both sexes together into further analyses. However, we used different thresholds for men and women (reflecting different prevalence) for the traits in the model where needed. In further modeling, sex and age were included as covariates.
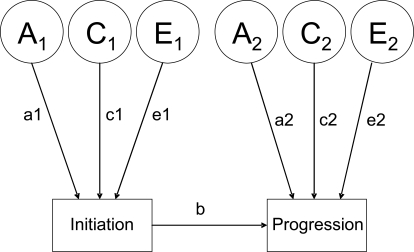
Next, conditional causal models were conducted separately for initiation and progression of smoking, as well as for initiation and progression of illicit drug use. Figure 1 illustrates the conditional causal model for initiation and progression of use of one substance only. It specifies a direct path from initiation to progression within each member of a twin pair. For example, A, C, and E influences on smoking initiation can also affect smoking progression. This is reflected in the path coefficient b in Figure 1. New A, C, or E influences could also affect progression of use. These specific influences on progression are reflected by path coefficients a2, c2, and e2. Two alternate multivariate Mx models were tested to explain the associations of stages in smoking and cannabis/other illicit drug use. We first tested the causal model, which hypothesizes that smoking cigarettes directly affects use of illicit drugs, such as cannabis. This model allows for the genetic and environmental influences on the initiation of smoking to influence the initiation of cannabis/other illicit drug use. It requires that all the influences are passed to the initiation of cannabis/other illicit drug use in a proportional way, according to the magnitude of the direct path between initiation of smoking and illicit drugs.
Figure 1.
Conditional causal model for initiation and progression of each substance use separately. A = additive genetic influences; C = common/shared environmental influences; E = unique/unshared environmental influences. a1 = path coefficient related to additive genetic influence on initiation of substance use; c1 = path coefficient related to common environmental influence on initiation of substance use; e1 = path coefficient related to shared environmental influence on initiation of substance use; a2 = path coefficient related to new additive genetic influence on progression of substance use; c2 = path coefficient related to new common environmental influence on progression of substance use; e2 = path coefficient related to new shared environmental influence on progression of substance use; b = path coefficient representing A, C, and E influences on the initiation of substance use that directly affect progression of substance use.
We used the Mx script provided by Neale et al. (2006), which we modified for an adolescent population. We assumed that, in this age group in Finland, initiation of illicit drug use before initiation of tobacco use is very rare. This assumption was supported by our observation within the same study (i.e., a subsample of 1,852 twins interviewed at age 14 years). By that age, 1.1% had ever used cannabis/other illicit drugs. Practically all of the participants were cigarette smokers. From these interview data, we concluded that the path from cannabis/other illicit drug use initiation to smoking initiation could be removed. We then tested the Cholesky decomposition as the second model to observe whether covariation between liabilities for behaviors—such as smoking initiation, onset of cannabis/other illicit drug use, as well as progression of smoking and progression of cannabis/other illicit drug use—could be accounted for by shared genetic or environmental correlations. Genetic or environmental correlation between two traits represents the extent to which the same genetic or environmental factors contribute to the observed correlation between those two traits (Neale and Maes, 2006). In general, the Cholesky decomposition is useful for estimating parameters that can be used to partition covariance between the traits into genetic and environmental components (Neale et al., 2006). In this correlated liability model, we allow for shared influences, but they may differentially affect initiation of smoking and initiation of cannabis/other illicit drug use. Model fitting is used to find the combination of components that best matches the observed pattern of familial resemblance in the data. Both models were modified additionally by adding covariate adjustments, including regression of sex, and quadratic and cubic effects of age on substance-use measures. These latter effects were added, because of the variation in age at the outcome assessment at age 17.5 years and because a graphical presentation of the data showed a nonlinear trend. Finally, to choose the most parsimonious model, the nested submodels may be compared with more saturated ones through chi-square difference tests, wherein a p value less than .05 means that the submodel is significantly worse than the less parsimonious model including more paths. When comparing models that are not nested submodels, such as models with the same number of parameters, the Akaike's Information Criteria (AIC) was used. Here, the lower AIC value—often a greater negative value—indicates the more parsimonious model (Neale and Maes, 2006).
Results
Descriptive statistics
The mean age among boys was 17.6 years (SD = 0.2, range: 17.2–19.3) and among girls was 17.6 years (SD = 0.3, range: 17.2–19.5). Within our sample, 67.7% of the MZ and 72.3% of the DZ twins had initiated smoking, whereas 12.9% of the MZ twins and 13.6% of the DZ twins had initiated cannabis use by the follow-up age of 17 years. Of those who had started smoking, 32.2% of the MZ twins and 37.4% of the DZ twins had progressed to daily smoking. Of those who had started using cannabis/other illicit drugs, 29.4% of the MZ twins and 37.7% of the DZ twins had progressed to using four times or more. Table 1 summarizes the number of subjects and the prevalence of each substance use for female and male MZ twins and DZ twins, as well as for opposite-sex twins. Some of the prevalences were ordered MZ < DZ, suggesting possible competitive sibling interaction effects, wherein MZ twins would be less likely to initiate smoking or illicit drug use—and once initiated, less likely to progress to regular use—than are DZ twins. Thus, we conducted a formal test of sibling interaction for each substance-use measure. However, no significant siblings interactions were found on smoking initiation (p = 1) or progression (p = 1) nor illicit drug use initiation (p = .33) or progression (p = .27). The number of discordant pairs was very small (<10) in progression of cannabis/other illicit drug use, particularly when broken by sex (not shown in tables).
Table 1.
The number of subjects and prevalence of each substance use measure for female and male monozygotic (MZ), dizygotic (DZ), and opposite-sex twins
| No. of subjects |
Prevalence (%) |
|||||
| Substance use measure | MZ | DZ | OS | MZ | DZ | OS |
| Smoking initiation | ||||||
| Male | 574 | 644 | 603 | 67.9 | 69.1 | 71.0 |
| Female | 690 | 630 | 603 | 67.5 | 73.0 | 76.1 |
| Smoking progression | ||||||
| Male | 386a | 442a | 428a | 33.9a | 37.1a | 39.5a |
| Female | 463a | 458a | 459a | 30.7a | 35.2a | 37.9a |
| Initiation of illicit drug use | ||||||
| Male | 574 | 644 | 603 | 11.7 | 11.3 | 12.9 |
| Female | 690 | 630 | 603 | 13.9 | 12.7 | 17.7 |
| Progression of illicit drug use | ||||||
| Male | 67a | 73a | 78a | 19.4a | 37.0a | 44.9a |
| Female | 96a | 80a | 107a | 36.5a | 32.5a | 36.4a |
Notes: MZ = monozygotic; DZ = dizygotic; OS = opposite-sex twins.
Among those who had initiated.
Two-stage models of smoking and illicit drug use
For smoking, the most parsimonious model (i.e., the one with the lowest AIC value), describing liability to initiate and progress smoking, was the “ACE” model (−2 log likelihood = 7,106.887, 6,366 df, AIC = −5,625.113), including genetic (A), common environmental (C), and unique environmental (E) influences. For illicit drugs, the most parsimonious two-stage model included an ACE model for initiation and an E model, including unique environmental influences only, for progression (−2 log likelihood = 3,280.099, 4,232 df, AIC = −5,183.901). This two-stage model had a better fit than the full model (−2 log likelihood = 3,280.141, 4,234 df, AIC = −5,187.859).
Shared and specific genetic and environmental influences on smoking and cannabis
We tested two different multivariate models, the causal model and the Cholesky decomposition, as described in the Statistical analyses section. Results of the most parsimonious causal and Cholesky decomposition models are given in Table 2. Figure 2 graphically presents the results of the causal model, whereas Figure 3 shows the results of the Cholesky decomposition. In these figures, the path coefficients are presented. To derive the A, C, and E components in Table 2, representing how much of the variance is explained by each factor, these path coefficients are squared. When comparing these models, the causal model provided a better fit (−2 log likelihood = 9,963.550, 10,601 df, AIC = −11,238.450), compared with the Cholesky model (−2 log likelihood = 9,978.852, 10,599 df, AIC = −11,219.148), based on the greater negative AIC value (Neale, 2006).
Table 2.
Standardized variance components and 95% confidence intervals between brackets from fitting two multivariate models to data on the initiation and progression of smoking and cannabis and other illicit drug use
| Smoking |
Cannabis use |
|||
| Variable | Initiation | Progression | Initiation | Progression |
| Causal model (−2 log likelihood = 9,963.550, df = 10,601, AIC = −11,238.450) | ||||
| Additive genetic | .58 | .43 | .32 | – |
| [.44, .72] | [.32, .55] | [.12, .53] | ||
| Common environment | .34 | – | .20 | – |
| [.21, .47] | [.02, .37] | |||
| Unique environment | .08 | .10 | .08 | .17 |
| [.05, .12] | [.05, .16] | [.03, .14] | [.07, .55] | |
| Initiation of smoking | na | .41 | .40 | |
| [.24, .57] | [.32, .48] | |||
| Initiation of cannabis | na | .06 | na | .83a |
| [.02, .13] | [.44, .93] | |||
| Cholesky factor model (−2 log likelihood = 9,978.852, df= 10,599, AIC = −11,219.148) | ||||
| Additive genetic | .61 | .13 | .59 | – |
| [.53, .69] | [.02, .24] | [.51, .70] | ||
| Common environment | .31 | .13 | .30 | – |
| [.24, .37] | [.02, .24] | [.19, .40] | ||
| Unique environment | .08 | .10 | .11 | .20 |
| [.06, .10] | [.07, .14] | [.08, .15] | [.09, .33] | |
| Initiation of smoking | na | .64 | na | na |
| [.56, .67] | ||||
| Initiation of cannabis | na | na | na | .80b |
| [.67, .89] | ||||
| Correlations | ||||
| Additive genetic | .57 | – | ||
| [.44, .65] | ||||
| Common environment | .82 | – | ||
| [.72, .95] | ||||
| Unique environment | .99 | – | ||
| [.89, 1.00] | ||||
Notes: AIC = Akaike's Information Criteria; na = not applicable.
Also includes path from initiation of smoking through initiation of cannabis/drug use (i.e., part of liability of cannabis/drug progression is attributable to the effect of liability to smoking initiation on cannabis/drug initiation);
also includes liabilities common to smoking initiation and initiation of cannabis/drug use.
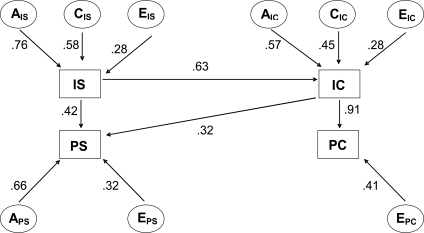
Figure 2.
Multivariate causal model of initiation and progression of smoking and initiation and progression of cannabis and other illicit drug use. IS = initiation of smoking, PS = progression of smoking; IC = initiation of cannabis use; PC = progression of cannabis use; AIS = additive genetic influences on initiation of smoking; CIS = common/shared environmental influences on initiation of smoking; EIS = unique/unshared environmental influences on initiation of smoking; APS = additive genetic influences on progression of smoking; EPS = unique environmental influences on progression of smoking; AIC = additive genetic influences on initiation of cannabis use; CIC = common environmental influences on initiation of cannabis use; EIC = unique environmental influences on initiation of cannabis use; EPC = unique environmental influences on progression of cannabis use. Numbers reflect the respective path coefficients.
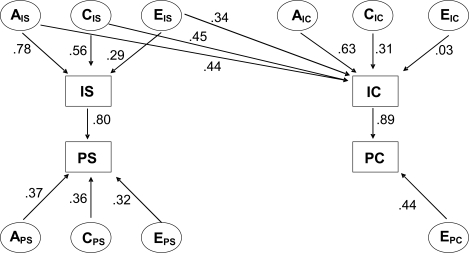
Figure 3.
Multivariate Cholesky model of initiation and progression of smoking and initiation and progression of cannabis and other illicit drug use. IS = initiation of smoking, PS = progression of smoking, IC = initiation of cannabis use; PC = progression of cannabis use; AIS = additive genetic influences on initiation of smoking; CIS = common/shared environmental influences on initiation of smoking; EIS = unique/unshared environmental influences on initiation of smoking; APS = additive genetic influences on progression of smoking; CPS = common environmental influences on progression of smoking; EPS = unique environmental influences on progression of smoking; AIC = additive genetic influences on initiation of cannabis use; CIC = common environmental influences on initiation of cannabis use; EIS = unique environmental influences on initiation of cannabis use; EPC = unique environmental influences on progression of cannabis use. Numbers reflect the respective path coefficients.
Multivariate causal model
Based on the results of the most parsimonious multivariate causal model (Figure 2), liability to initiate smoking indirectly affects liability to progress cannabis/other illicit drugs through initiation of the latter (indirect path coefficient .63 × .97 = .61), but no direct pathway (p = .27) was found from smoking initiation to progression of illicit drugs. From this latent construct reflecting liability to initiate smoking to progression of smoking, there is a direct pathway (path coefficient = .42) and also a pathway through initiation of cannabis/other illicit drug use (indirect path coefficient .63 × .32 = .20). There are also three more direct pathways suggested by the most parsimonious model: a pathway from initiation of cannabis/other illicit drug to progression (path coefficient = .97), a pathway from initiation of smoking to initiation of that illicit drug use (path coefficient = .63), and a pathway from initiation of illicit drug use to progression of smoking (path coefficient = .32).
Standardized estimates of the most parsimonious causal model are given in Table 2. The model decomposes the total variation in initiation of smoking into additive genetic factors (58%), common environmental factors (34%), and unique environmental factors (8%). The total variation in progression of smoking is decomposed to effects of additive genetic factors (43%), unique environmental factors (10%), influences shared with smoking initiation (41%), and influences on cannabis initiation (6%). The total variation in initiation of cannabis/other illicit drug use is decomposed into four components: (a) additive genetic factors (32%), (b) common environmental factors (20%), (c) unique environmental factors (8%), and (d) component due to initiation of smoking (40%). The total variation in cannabis/other illicit drug use progression is decomposed to unique environmental factors (17%) and paths due to initiation of cannabis/other illicit drug use and indirect effect of smoking initiation (83%).
Cholesky decomposition genetic factor model
Results of the most parsimonious Cholesky decomposition, which hypothesizes that tobacco and cannabis use are correlated as a result of shared underlying liability, are shown in Figure 3. Pathways from initiation to progression for both smoking (path coefficient = .80) and use of cannabis/other illicit drugs (coefficient = .89) were strong, suggesting that the total variance of the progression stage was explained for a moderate to large part by influences affecting the initiation stage.
Based on the standardized estimates shown in Table 2, the model decomposes the total variation of smoking initiation into components of additive genetic factors (61%), common environmental factors (31%), and unique environmental factors (8%). The total variation in smoking progression is decomposed into additive genetic factors (13%), common environmental factors (13%), unique environmental factors (10%), plus variation due to smoking initiation (64%).
The total variation in initiation of cannabis/other illicit drug use is decomposed into additive genetic factors (59%), common environmental factors (30%), and unique environmental factors (11%). The total variation in cannabis/other illicit drug use progression is decomposed into unique environmental factors (20%) and variation due to the direct effect of cannabis use initiation plus pathways common to initiation (80%).
Finally, additive genetic correlation between initiation of smoking and cannabis use was .57, whereas the common environmental correlation was .82 and the unique environmental correlation was .99 (Table 2).
Discussion
In this study among Finnish adolescent twins, we tested whether a so-called causal model, in which latent constructs reflected the liability to initiate smoking directly affects such a latent construct for initiation of cannabis use, could explain the association between initiation and progression of tobacco and cannabis/other illicit drug use. We compared this model with a Cholesky model, reflecting correlation between initiation of smoking and cannabis use resulting from shared underlying liability (i.e., shared genetic and environmental influences on use of these substances). We found some evidence for the causal model, because our comparative model fitting showed that this model had a slightly better fit than the Cholesky model, in line with previous work of Neale et al. (2006). Especially in our younger population of adolescents, it appears that initiation of illicit drug use does not precede smoking initiation, whereas early-onset smoking is a strong predictor of illicit drug use by age 17.5 years (Korhonen et al., 2008). Thus, the results of the present study, combined with our previous study, suggest that, in adolescence, smoking initiation may indeed have a direct impact on the propensity to initiate illicit drug use. Several mechanisms could be responsible for this strong association. For instance, Agrawal and Lynskey (2009) recently suggested, based on a longitudinal study of a large-scale U.S. adult sample, that the shared inhalation route of administration may explain the association often found between use of tobacco and cannabis. Shared environmental and social risk factors, including cultural norms, may also account for this association (Ellickson et al., 2004; Golub et al., 2005; Wagner et al., 2005). Yet, more research is still needed to understand this particularly strong association.
Because of the small difference in model fit, our results are not conclusive. Nevertheless, although the Cholesky model also fit the data adequately, we believe that the causal model better captures the complexity of the relationship between tobacco smoking and illicit drug use and may have a greater heuristic value. For instance, in the Cholesky model, once a person initiates the use of a specific substance, it can be related only to progression of use of that same substance. In contrast, our causal model showed associations across substances (i.e., an effect of tobacco initiation on illicit drug use initiation) and an effect of illicit drug use initiation on progression to daily smoking. This latter finding suggests that the propensity to initiate illicit drug use by age 17.5 years may have some additional health-threatening risks reflected by either more drug use or increased risk of daily smoking.
Our causal model also indicates that the factors accounting for inter-individual variation in liability to illicit drug use initiation are largely the same for progression. This is reflected by the moderate to large path coefficients between initiation of smoking and progression of smoking (.42) and between initiation of illicit drugs and progression of illicit drugs (.97) in Figure 2. This is consistent with results of a recent study (Shelton et al., 2007) showing a similarly high path coefficient between initiation of cannabis use and progression to using at least six times. Whether this may result in more regular illicit drug use has to be studied in the next follow-up of the present sample (ongoing in early adulthood), because at age 17.5 years, only 2.7% of all subjects had used illicit drugs 10 times or more.
When we consider the genetic and environmental influences on smoking and illicit drug use initiation and progression, it is striking to see that smoking initiation by late adolescence has a strong genetic component (58%). This finding is in line with several studies focusing on both adolescent and adult populations (Madden et al., 2004; Maes et al., 1999, 2006), although others have reported lower heritability for smoking onset in Dutch twins ages 12–25 years (Koopmans et al., 1999), in U.S. twins ages 17–18 years (Han et al., 1999), or in Finnish twins of the present cohort at ages 11–12 years (Rose et al., 2003). Similarly, we found a high heritability estimate for illicit drug use initiation and progression of use in our study. In a recent review, Agrawal and Lynskey (2006) concluded that, for cannabis use, a wide range of heritability estimates was found across studies, with 18% as the lowest estimate in an adolescent twin sample (McGue et al., 2000) and 72% as the highest estimate in a female adolescent mixed sibling, twin, and adoption sample (Rhee et al., 2003). Our estimates of common environmental influences and nonshared environmental influences for initiation of mostly cannabis use are similar to those of most of the studies reviewed by Agrawal and Lynskey (2006). Interestingly, our finding that new common environmental factors did not explain the progression of cannabis/other illicit drug use, whereas unique environmental factors did, at least to some extent, is in line with several studies that focused on cannabis abuse in the adult twin sample (Kendler and Prescott, 1998; Kendler et al., 2000; Tsuang et al., 1998). Yet, we could not examine abuse because of the very low prevalence of more regular use in our relatively young sample. Our measure of progression of use was defined as using at least four times, and is therefore clearly different from the more serious forms of actual abuse or prolonged use of cannabis yet similar genetic and unique environmental influences may apply. This hypothesis needs to be tested further, and may point to shared genetic and (unique) environmental influences on age-specific, risk-taking behavior regarding substance use in various age groups.
Also of interest is the path coefficient of .32 from the propensity to initiate cannabis use to the progression of smoking. This path actually indicates that cannabis use may increase the risk of progressed smoking behavior. This finding is in line with the recent work of Patton et al. (2005) and Agrawal et al. (2008), although these studies focused on different phenotypes (i.e., frequent cannabis use as determinant or nicotine dependence as outcome, respectively).
Several genetic and environmental influences present for smoking initiation also explain illicit drug use initiation. Approximately 40% of the genetic influence on cannabis/other illicit drug use initiation is shared with genetic influences on smoking initiation, which reflects some shared genetic vulnerability. Nonetheless, specific genetic influences explain almost one third of illicit drug use initiation. Therefore, future studies may look for genotypes that are specifically related to this kind of substance use. Similarly, 17% of variance in progression of illicit drug use is explained by unique environmental factors, which may point to peer influences not shared by twins within twin pairs. Especially because there does not seem to be an influence of new common or shared environmental influences on both progression to daily smoking and progression of illicit drug use, it is worthwhile to examine discordance in environmental factors within twin pairs (e.g., nonshared friends or peers, or other effects) to gain more insight into which of these factors relates to more progressed substance use. Twin designs are especially useful for such analyses, because they can control within-family factors. We did not find an overlap between unique environmental factors that accounted for both smoking and illicit drug use. This could be because all overlap among those factors was explained by the overlapping variation between initiation of use of tobacco or illicit drugs.
Strengths and limitations
Although previous studies of Agrawal et al. (2004) and Lynskey et al. (2003) already tested the gateway theory for cannabis use in adult samples, our study is the first to test and compare two alternative models on the theory of how tobacco use may affect cannabis use within one large adolescent twin sample. We applied a novel modeling technique—recently developed by Neale et al. (2006)—modified that model for an adolescent population, and were able to fit a causal model to the data. Fortunately, because of the rather narrow age range within our sample, there is little confounding of age effects. Additionally, our 95% confidence intervals of our estimated parameters were not too broad, which indicates reasonable discrimination. However, when interpreting the results of this study, several potential limitations should be considered. The assessments of initiation and progression of smoking and illicit drug use were obtained by self-report, and our findings are limited by the reliability of these data. Furthermore, smoking and use of illicit drugs were measured at a cross-sectional survey when the participants were, on average, age 17.5 years. A longitudinal design would have been more favorable and powerful to test our model. Unfortunately, the FinnTwin12 data included information on cannabis use only within the second follow-up survey at age 17.5 years, but we were able to use interview data from a subsample of the twins at age 14 years to rule out cannabis use preceding smoking initiation at an early age. Also, we did not have data on age of cannabis/other illicit drug use onset. Generally speaking, our models provide approximate estimates of the relative contributions of genetic and environmental factors to tobacco and illicit drug use. It must be noted that the genetic influences, estimated as A in our models, could also include gene–environment correlations. Finally, we could not differentiate progression of illicit drug use into several categories of severity (e.g., problematic use and abuse); therefore, our data did not permit advanced or other comprehensive multivariate modeling with multiple stages of illicit drug use. Future follow-up data of the present sample is needed to adequately test whether a pathway from daily smoking to frequent cannabis/other illicit drug use exists.
Conclusions and implications
By comparing two alternative models, our study provides further evidence that a causal model describes an association between smoking and later illicit drug use. However, it is difficult to discriminate between this causal model and the alternative model that describes an underlying common liability for tobacco and illicit drug use. Therefore, advancing an understanding in this area will necessitate reports from multiple studies to evaluate convergence across datasets. Our findings further suggest that several common genes may be related to tobacco use and illicit drugs but that a search for specific genes underlying illicit drug use is also justified. Such specific genes may hold a key to understanding biological vulnerabilities that lead to illicit drug use that could aid in the development of targeted interventions.
Footnotes
Data collection in FinnTwin12 was supported by National Institute on Alcohol Abuse and Alcoholism grants AA-09203, AA-12502, and AA-00145 awarded to Richard J. Rose and by Academy of Finland grants 100499, 204690, and 118555 awarded to Jaakko Kaprio. Jaakko Kaprio is also supported by the Academy of Finland Centre of Excellence in Complex Disease Genetics. Data analysis was also part of the GENOMEUTWIN project supported by the European Union Contract No. QLG2-CT-2002-01254, and was financially supported by NWO VIDI scheme grant 452-06-004 in The Netherlands awarded to Anja C. Huizink and Tellervo Korhonen.
References
- Agrawal A, Lynskey MT. The genetic epidemiology of cannabis use, abuse and dependence. Addiction. 2006;101:801–812. doi: 10.1111/j.1360-0443.2006.01399.x. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Tobacco and cannabis co-occurrence: Does route of administration matter? Drug and Alcohol Dependence. 2009;99:240–247. doi: 10.1016/j.drugalcdep.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT, Pergadia ML, Bucholz KK, Heath AC, Martin NG, Madden PA. Early cannabis use and DSM-IV nicotine dependence: A twin study. Addiction. 2008;103:1896–1904. doi: 10.1111/j.1360-0443.2008.02354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychological Medicine. 2004;34:1227–1237. doi: 10.1017/s0033291704002545. [DOI] [PubMed] [Google Scholar]
- Anthony JC. Death of the ‘stepping-stone’ hypothesis and the ‘gateway’ model? Comments on Morral et al. Addiction. 2002;97:1505–1507. doi: 10.1046/j.1360-0443.2002.00287.x. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews: Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Clough AR. Associations between tobacco and cannabis use in remote indigenous populations in Northern Australia. Addiction. 2005;100:346–353. doi: 10.1111/j.1360-0443.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Chiu WT, Conway K, Dierker L, Glantz M, Kalaydjian A, Merikangas K, Sampson N, Swendsen J, Kessler RC. Does the ‘gateway’ matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychological Medicine. 2009;39:157–167. doi: 10.1017/S0033291708003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellickson PL, Tucker JS, Klein DJ, Saner H. Antecedents and outcomes of marijuana use initiation during adolescence. Preventive Medicine. 2004;39:976–984. doi: 10.1016/j.ypmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Ferdinand RF, van der Ende J, Bongers I, Selten JP, Huizink A, Verhulst FC. Cannabis—psychosis pathway independent of other types of psychopathology. Schizophrenia Research. 2005;79:289–295. doi: 10.1016/j.schres.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Cannabis use and other illicit drug use: Testing the cannabis gateway hypothesis. Addiction. 2006;101:556–569. doi: 10.1111/j.1360-0443.2005.01322.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95:505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Swain-Campbell NR, Horwood LJ. Deviant peer affiliations, crime and substance use: A fixed effects regression analysis. Journal of Abnormal Child Psychology. 2002;30:419–430. doi: 10.1023/a:1015774125952. [DOI] [PubMed] [Google Scholar]
- Golub A, Johnson BD, Dunlap E. The growth in marijuana use among American youths during the 1990s and the extent of blunt smoking. Journal of Ethnicity in Substance Abuse. 2005;4:1–21. doi: 10.1300/J233v04n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: Implications for substance abuse prevention. Psychological Bulletin. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: Univariate and multivariate behavioral genetic analyses. Addicition. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2007 ESPAD Report: Substance Use Among Students in 35 European Countries. Stockholm, Sweden: The Swedish Council for Information on Alcohol and Other Drugs (CAN), European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Council of Europe, Co-operation Group to Combat Drug Abuse and Illicit Trafficking in Drugs (Pompidou Group); 2009. Retrieved from http://www.espad.org/documents/Espad/ESPAD_reports/2007/The_2007_ESPAD_Report-FULL_091006.pdf. [Google Scholar]
- Kandel DB, Yamaguchi K, Klein LC. Testing the gateway hypothesis. Addiction. 2006;101:470–472. doi: 10.1111/j.1360-0443.2006.01426.x. discussion 474–476. [DOI] [PubMed] [Google Scholar]
- Kaprio J. Social behaviors and health in twins: The Finn Twin Studies. New York: Cambridge University Press; 2006. [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. American Journal of Psychiatry. 1998;155:1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kenkel DS, Mathios AD. ‘Gateway effects’: Insights from economics are needed. Addiction. 2002;97:1505. doi: 10.1046/j.1360-0443.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Huizink AC, Dick DM, Pulkkinen L, Rose RJ, Kaprio J. Role of individual, peer and family factors in the use of cannabis and other illicit drugs: a longitudinal analysis among Finnish adolescent twins. Drug and Alcohol Dependence. 2008;97:33–43. doi: 10.1016/j.drugalcdep.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD. The genetics of smoking related behavior: A brief review. American Journal of the Medical Sciences. 2003;326:168–173. doi: 10.1097/00000441-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Lynskey M. An alternative model is feasible, but the gateway hypothesis has not been invalidated. Comments on Morral et al. Addiction. 2002;97:1505–1507. doi: 10.1046/j.1360-0443.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, Horwood LJ. The origins of the correlations between tobacco, alcohol, and cannabis use during adolescence. Journal of Child Psychology and Psychiatry. 1998;39:995–1005. [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. American Journal of Medical Genetics. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Maccoun RJ. Competing accounts of the gateway effect: The field thins, but still no clear winner. Addiction. 2006;101:473–474. doi: 10.1111/j.1360-0443.2006.01428.x. discussion 474–476. [DOI] [PubMed] [Google Scholar]
- Madden PA, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The epidemiology and genetics of smoking initiation and persistence: Crosscultural comparisons of twin study results. Twin Research. 2004;7:82–97. doi: 10.1375/13690520460741471. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Kendler KS, Martin NG, Heath AC, Eaves LJ. Genetic and cultural transmission of smoking initiation: An extended twin kinship model. Behavior Genetics. 2006;36:795–808. doi: 10.1007/s10519-006-9085-4. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, Eaves LJ. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: The Virginia Twin Study of Adolescent Behavioral Development. Journal of Studies on Alcohol. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Morral AR, McCaffrey DF, Paddock SM. Reassessing the marijuana gateway effect. Addiction. 2002;97:1493–1504. doi: 10.1046/j.1360-0443.2002.00280.x. [DOI] [PubMed] [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression: Applications to substance use and abuse. Behavior Genetics. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Norwell, MA: Kluwer Academic; 2006. [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Sawyer SM, Lynskey M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction. 2005;100:1518–1525. doi: 10.1111/j.1360-0443.2005.01220.x. [DOI] [PubMed] [Google Scholar]
- Rey JM, Martin A, Krabman P. Is the party over? Cannabis and juvenile psychiatric disorder: the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1194–1205. doi: 10.1097/01.chi.0000135623.12843.60. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rimpelä AR, Rainio S, Pere L, Lintonen TP. Use of tobacco products, alcohol use and exposure to drugs in 1977– 2005. Helsinki, Finland: Ministry of Social Affairs and Health; 2007. [Google Scholar]
- Rose RJ, Viken RJ, Dick DM, Bates JE, Pulkkinen L, Kaprio J. It does take a village: Nonfamilial environments and children's behavior. Psychological Science. 2003;14:273–277. doi: 10.1111/1529-1006.03434. [DOI] [PubMed] [Google Scholar]
- Shelton K, Lifford K, Fowler T, Rice F, Neale M, Harold G, … van den Bree M. The association between conduct problems and the initiation and progression of marijuana use during adolescence: A genetic analysis across time. Behavior Genetics. 2007;37:314–325. doi: 10.1007/s10519-006-9124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, … Eaves L. Co-occurrence of abuse of different drugs in men: The role of drug-specific and shared vulnerabilities. Archives of General Psychiatry. 1998;55:967–972. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- Virtanen A, Sjöberg S. Finland—Drug Situation 2005. 2005 National Report to the EMCDDA by the Finnish National Focal Point; 2006. [Google Scholar]
- Viveros MP, Marco EM, File SE. Nicotine and cannabi-noids: Parallels, contrasts and interactions. Neuroscience & Biobehavioral Reviews. 2006;30:1161–1181. doi: 10.1016/j.neubiorev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wagner FA, Velasco-Mondragon HE, Herrera-Vazquez M, Borges G, Lazcano-Ponce E. Early alcohol or tobacco onset and transition to other drug use among students in the state of Morelos, Mexico. Drug and Alcohol Dependence. 2005;77:93–96. doi: 10.1016/j.drugalcdep.2004.06.009. [DOI] [PubMed] [Google Scholar]