Abstract
Objective:
Psychosocial interventions that are practical, transportable, and effective in promoting treatment adherence and efficacy are greatly needed in both research and clinical settings involving alcohol-dependence pharmacotherapy. In this article, we describe the development and preliminary evaluation of an integrative treatment blending motivational interviewing and compliance enhancement therapy (MI-CET) as a means of enhancing adherence and retention in an ongoing clinical trial.
Method:
Medication adherence, session attendance, and study completion rates were examined for 121 treatment-seeking, alcohol-dependent adults participating in a randomized clinical trial of citalopram (n = 81) versus placebo (n = 40). All participants received the manual-guided MI-CET intervention as an adjunct to pharmacotherapy. Preliminary adherence and retention data for this trial were compared with data from prior studies involving treatment for alcohol dependence with a selective serotonin reuptake inhibitor.
Results:
High rates of medication adherence (79% of citalopram and 91% of placebo completers took ≥80% of doses), session attendance (average of 90% for citalopram and 93% for placebo groups), and study completion (81% for citalopram and 88% for placebo groups) were obtained in the present study using MI-CET. These rates were at least comparable to or were, in some cases, 20%–30% higher than rates obtained in the comparison trials.
Conclusions:
These results suggest that MI-CET is feasible as a psychosocial adjunct to alcohol-dependence pharmacotherapy. Given its strengths as a clinical and research intervention (e.g., practicality, transportability), further evaluation of its efficacy is warranted.
Aconcomitant psychosocial therapy platform has become an essential component of any clinical trial designed to test the efficacy of a medication to treat alcohol dependence (Carroll et al., 2004). The ideal platform would be effective enough to yield high participant retention and study drug adherence rates but not so powerful that the effects of the medication cannot be detected when tested against placebo. The intervention should limit excessive variance by proscribing other uncontrolled therapy techniques, and, given the limited availability of therapists in medical settings, the adjunctive intervention should be time limited and manual guided so that clinicians with limited psychotherapy training can administer it effectively.
To attempt to achieve these goals, we combined motivational interviewing (MI) and compliance enhancement therapy (CET) to create motivational interviewing and compliance enhancement therapy (MI-CET), an adjunctive psychosocial intervention for a randomized clinical trial comparing alcohol-dependent patients' responses to citalopram or placebo. Before we describe the preliminary findings from the trial regarding the feasibility and efficacy of MI-CET, the literature on MI and CET will be reviewed briefly, followed by a discussion of the rationale for the integrated intervention.
Motivational interviewing
Miller (1983) developed MI as a brief therapy approach to enhance an individual's readiness to change heavy alcohol use and related behavioral problems. Consistent with the Ro-gerian client-centered therapy approach (Rogers, 1951), MI espouses an accepting and respectful attitude toward patients, often referred to as “MI spirit” (Britt et al., 2003; Miller, 1998). MI also uses a variety of explicit reflective listening and change-promoting techniques that make this approach a directive and time-efficient therapeutic intervention (Miller and Rollnick, 2002; Project MATCH, 1994).
MI has been used by a broad array of providers with variable educational and training backgrounds. MI has been found to be efficacious and effective as either a stand-alone intervention or as a prelude to a more intensive intervention in a variety of diagnostically and culturally diverse patient and community populations (e.g., Bennett et al., 2007; Carroll et al., 2006; Steinberg et al., 2004; Woodall et al., 2007). A notable success of MI is its ability to engage patients who may be ambivalent about participating in treatment and making changes in their problematic behaviors (Booth et al., 2004; Brown and Miller, 1993). The largest proportion of published research to date involves clinical trials designed to treat problematic alcohol use ranging from heavy drinking in college students to alcohol dependence in adult patients (e.g., Allsop et al., 1997; Carey et al., 2006; LaBrie et al., 2008; Sellman et al., 2001).
Hettema (2007) conducted the most recent and largest meta-analysis to date of 85 MI randomized clinical trials to examine MI's treatment effects across a variety of problematic behaviors that included excessive alcohol use. Consistent with prior studies (Bertholet et al., 2005; Hettema et al., 2005; Vasilaki et al., 2005), this meta-analysis found treatment effect sizes in the small to medium range (average effect size = .41) across these broad behavioral domains when compared with a control condition or a comparison intervention. The study also found that MI's effects appeared fairly rapidly within the first 3 months of treatment and diminished over time (Hettema, 2007; Vasilaki et al., 2005). Thus, given MI's modest and time-limited effects, we opted to combine it with another manual-guided platform, CET, in our randomized clinical trial aimed at producing high treatment engagement and supporting extended and clinically significant improvement in patients with mild to moderate alcohol dependence.
Compliance enhancement therapy
CET was developed by Carroll and O'Malley (1996) to serve as a lower-intensity, minimal impact comparison treatment for active interventions such as cognitive-behavioral therapy (CBT; Project MATCH, 1995a) and twelve-step facilitation therapy (Project MATCH, 1995b). It has been used in clinical trials testing a combination of pharmaco-therapy and psychosocial interventions in treating alcohol and other substance dependence. CET provides a pharma-cotherapy rationale and elements common to many types of psychotherapy (empathy, education, convincing rationale, and supportive therapeutic relationship) that are compatible with MI.
Adapted from Fawcett et al.'s (1987) Clinical Management, which was developed for psychiatrists to monitor an-tidepressant treatment, CET was designed for a broad range of clinicians (qualified nurses, psychologists, social workers, and counselors) to work in collaboration with a prescribing physician. The clinician's role is to monitor and increase the patient's medication compliance and to support abstinence or a reduction of problematic substance use.
To date, CET has been used as the concomitant psychosocial intervention in studies testing the effects of sertraline (Kranzler et al., 2006), naltrexone (Petrakis et al., 2005), and disulfiram (Petrakis et al., 2005) on alcohol dependence in patients with and without comorbid psychiatric disorders. Less frequently, CET has also been used as a rigorous control to determine the optimal psychotherapy combination with pharmacotherapy to treat alcohol and illicit substance dependence (Carroll et al., 1998). The medication compliance rates (56%–86%) and treatment completion rates (56%–78%) of the CET placebo conditions in these investigations suggest that there is room for improvement in both treatment retention and medication compliance, possibly by adding a low dosage of an active alcohol intervention such as MI.
Rationale for integrating motivational interviewing and compliance enhancement therapy as an adjunctive psychosocial intervention
Only a limited body of research is currently available on the effects of different behavioral therapies delivered as an adjunct to pharmacotherapy (Anton et al., 2006; O'Malley et al., 2003; Pettinati et al., 2000). The results of these studies provide support for the development of a brief psychosocial intervention that integrates an MI approach into a clinical or medical management model of counseling. Findings from two randomized, controlled trials contrasting adjunctive therapy approaches suggest that brief, medically oriented platforms are at least as efficacious as more intensive psychotherapy platforms in promoting adherence and retention.
O'Malley et al.'s (2003) head-to-head comparison of CBT and primary care management (PCM) as psychosocial adjuncts to open-label naltrexone treatment for alcohol dependence failed to reveal any differences between the interventions on medication adherence (71.3% in the PCM group vs. 67.3% in the CBT group) and participant retention (72% completion rate for the PCM group vs. 68% for the CBT group) in the trial. Similarly, findings from the multi-center COMBINE (Combining Medications and Behavioral Interventions) study (Anton et al., 2006) suggested that the addition of an intensive, 20-session, combined behavioral intervention to brief, nine-session medical management did not significantly improve the medication adherence and study completion rates associated with medical management counseling alone. Importantly, this study also demonstrated that the effects of naltrexone on alcohol use were statistically indistinguishable from placebo in those who received the combined behavioral intervention.
This nonsignificant medication effect suggests that a relatively intensive behavioral intervention such as CBT may not be the adjunctive behavioral treatment of choice when testing new medications for the treatment of alcohol dependence. That is, based on the COMBINE data, it appears that intensive CBT does not improve medication adherence or retention beyond that produced by the briefer, less complex medical management intervention and may even reduce the potential to detect medication effects.
Results from a study conducted by Pettinati et al. (2000) provide support for the integration of MI into a medically based counseling approach. This study compared CBT-based counseling with a novel intervention known by the acronym BRENDA, both delivered in conjunction with naltrexone pharmacotherapy. The BRENDA platform (i.e., Biopsycho-social evaluation, Report of evaluation to the patient, Empathy, identification and treatment planning based on individual Needs, Direct advice regarding change in drinking, and Assessment of the patient's response to advice) incorporates strategies to enhance motivation into a medical model of treatment (Volpicelli et al., 2001) to assist patients in reducing or discontinuing alcohol use and taking medication as prescribed. Analyses reported by Pettinati et al. (2000) suggest that patients who received BRENDA therapy had higher rates of both treatment completion (83.0% vs. 55.7%, p < .001) and medication adherence (77.0% vs. 60.8%, p < .01) than those who received the CBT-based addictions counseling. One significant limitation of this study, however, is that the comparison of CBT and BRENDA was made across consecutive trials rather than within the same trial.
In summary, available evidence suggests that brief adjunctive behavioral therapies involving a medical model of counseling are just as efficacious as more intensive interventions in terms of treatment retention and adherence. In some cases, these brief interventions may even produce superior retention and adherence (e.g., Pettinati et al., 2000) without washing out the effects of medication such that the efficacy of new pharmacotherapies cannot be detected. Although additional research is needed to determine the relative efficacy of different approaches to adjunctive behavioral therapy on crucial outcomes such as medication adherence and treatment completion, there is evidence to suggest that an integrative treatment approach infusing MI into a clinical management model of counseling is associated with high rates of adherence and retention (Pettinati et al., 2000).
Preliminary investigation of motivational interviewing-compliance enhancement therapy's feasibility and efficacy
To determine the feasibility and efficacy of MI-CET as an adjunct to alcohol-dependence pharmacotherapy in a clinical trial, we examined preliminary data on treatment engagement and retention as well as medication adherence for an ongoing trial in which MI-CET is being used as an adjunctive therapy platform. Because these analyses are primarily exploratory, consistent with the earliest stage of behavioral treatment development (i.e., Stage Ia, as described in the Stage Model of Behavioral Therapies Research outlined by Rounsaville et al., 2001), we did not conduct formal hypothesis testing. Instead, as an approximation of comparative feasibility and efficacy, we contrasted our adherence and retention data against those reported in previous alcohol-dependence pharmacotherapy trials that share key characteristics with the present study (e.g., same class of medication, use of a manual-guided intervention).
Method
Study overview
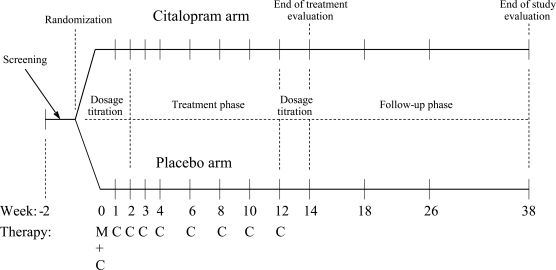
MI-CET was developed for use in an ongoing trial investigating pharmacogenetic factors in alcohol-dependence treatment with the selective serotonin reuptake inhibitor (SSRI) citalopram (ClinicalTrials.gov Identifier: NCT00249405). Briefly, this trial is a 12-week, double-blind, placebo-controlled study with 1-, 3-, and 6-month follow-up assessments (see Figure 1 for an overview of study design). Participants receive citalopram pharmacotherapy (titrated over the first 2 weeks to a maximum of 2.0 mg/unit of body mass index) or placebo in combination with MI-CET, which is provided on a weekly basis for the first 5 weeks of the 12-week treatment phase and every other week for the remainder of the active treatment period.
Figure 1.
Study design: Predicting alcoholics' treatment responses to a selective serotonin reuptake inhibitor; C = compliance enhancement therapy; M = motivational interviewing
Adherence to medication is assessed at each visit through the use of the Medication Event Monitoring System (MEMS; AARDEX, Union City, CA), an electronic method of recording adherence using a medication bottle cap that stores the time and date of bottle openings. In this study, the availability of MEMS data on a weekly basis to the MI-CET therapist allowed for immediate feedback and discussion related to adherence in the context of the therapy sessions.
Description of participants included in the preliminary data analysis
Of the 200 participants estimated to be randomized to receive either citalopram or placebo in combination with MI-CET as part of the study, we examined preliminary data from the first 121 consecutively randomized subjects who had finished the follow-up phase of the study (i.e., those who had completed the last follow-up visit or discontinued the study early). To preserve the integrity of the ongoing study, we chose not to break the medication blind for individuals who were still participating in the treatment phase of the trial. For this same reason, we also did not examine any of the alcohol data from the study and instead focused only on indicators of treatment completion and medication adherence.
To be included in the study, participants had to be between 21 and 65 years of age, meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994), criteria for alcohol dependence, and express a desire to discontinue or substantially reduce their alcohol consumption. Exclusion criteria were the following: (a) lifetime Axis I disorders other than a substance-use disorder, attention-deficit/hyperactivity disorder, or conduct disorder; (b) any drug dependence (excluding nicotine dependence) within the past 12 months or drug abuse within the past 6 months; (c) use of any psychotropic medications or investigational drugs within the 30 days before screening; (d) use of any pharmacotherapy for alcohol dependence within the 90 days before screening; (e) a seizure disorder or history of severe alcohol withdrawal; (f) pregnancy or planning to become pregnant during the period of participation; (g) hypersensitivity to SSRIs; or (h) pending legal charges or court-ordered treatment for alcohol or drug use. All participants gave informed consent to be included in the study.
The randomization procedures for the study involved a 2:1 ratio of citalopram to placebo group assignment, which is common in pharmacogenetic trials to allow for analysis of medication by genotype interactions. Thus, a larger portion of this sample of 121 received the active medication (n = 81; 67%) as opposed to the placebo (n = 40; 33%).
Of the 121 participants included in this preliminary analysis, the overall completion rate for the 12-week treatment phase of the study was 83% (n = 101 treatment completers), and the completion rate for the 6-month posttreatment follow-up period was 74% (n = 90 follow-up completers). Five participants in the placebo group and 15 participants in the citalopram group discontinued the study during the active treatment phase. Reasons for study discontinuation in the placebo group included subject's choice (n = 1), lost to follow-up (n = 1), lack of efficacy (n = 1), and required more intensive treatment (n = 2). In the citalopram group, reasons for discontinuation were adverse events (n = 3), lost to follow-up (n = 8), lack of efficacy (n = 1), and required more intensive treatment (n = 3). It should be noted that one participant in the citalopram group discontinued the medication because of adverse events but elected to continue in the study.
Description of motivational interviewing-compliance enhancement therapy
MI-CET consisted of nine individual, structured sessions designed to be delivered by trained clinical research staff, including psychologists, physicians, counselors, nurses, and research assistants. Session 1 consisted of 60 minutes of MI and 15 minutes of CET, and Sessions 2–9 consisted of 15–20 minutes of CET. For the first 5 weeks of the 12-week treatment phase of the study, MI-CET was delivered on a weekly basis, after which the therapy took place biweekly. This “dosage” combination of MI and CET was intended to produce a psychosocial intervention with a low-to-moderate intensity that could promote high treatment retention and medication adherence in both medication and placebo participants and allow detection of citalopram effects when tested against a placebo.
The first MI-CET session began with an overview of the study, including a discussion of both the counseling and the medication. The next portion of the session was a brief assessment of the participant's alcohol use (both current and past) along with a review of any prior efforts to quit or reduce drinking. A structured motivational interview comprised the largest portion of Session 1, consisting of MI techniques that were designed to bolster motivation and self-efficacy to reduce or discontinue alcohol use. Such techniques included a decisional balance exercise in which the pros and cons of continued alcohol use were discussed, an exploration of barriers to change, identification of factors that enhance confidence in the ability to reduce or discontinue drinking, and creation of a plan for change.
Personalized feedback also was provided to each participant, including an overview of the frequency of heavy drinking at baseline, severity of alcohol dependence (as measured on the Alcohol Dependence Scale; Skinner and Allen, 1982), liver function tests (gamma glutamyltransfer-ase [GGT], aspartate aminotransferase [AST], and alanine aminotransferase [ALT]), and mean corpuscular volume (MCV) ascertained from the complete blood count. The first session concluded with a review of instructions for taking the medication and problem-solving of any anticipated difficulty with medication adherence.
Sessions 2–9, which included only CET, followed a similar structure. Each session started with a brief (<5 minutes) check-in to assess both global and domain-specific (e.g., family, work) functioning since the last session. Next was an in-depth review of any alcohol use in the preceding 1- to 2-week period, including the amount, timing, and any consequences of drinking as well as identifiable precipitants. Medication adherence, side effects, and perceived effectiveness were then discussed. MEMS cap data, which had been downloaded, printed, and provided to the study therapists before the session, also were reviewed at this point. A key aspect of the medication discussion was the importance of medication adherence, with the therapist highlighting any linkages between adherence and changes in drinking (or lack thereof) that support the potential benefits of the medication.
The final portion of the CET sessions was a review of goals from the previous week (if not already discussed during the check-in or the alcohol-use assessment) and setting of new goals for the upcoming week. Importantly, the therapist's role in goal setting was to facilitate the participant's planning and problem solving by asking about strategies that s/he thinks may be helpful. Although the therapist may ask guiding questions (e.g., “What has worked for you in the past?”), no direct advice regarding strategies for change were supplied by the therapist, which differentiates MI-CET from other adjunctive psychosocial interventions such as BRENDA (Volpicelli et al., 2001) and Medical Management (Pettinati et al., 2005), which encourage direct recommendations by treatment providers.
Therapist training
The MI-CET therapists in this study were purposefully chosen to be diverse in terms of background and experience and included a physician, two doctoral-level clinical psychology fellows, predoctoral clinical psychology students, and a master's-level counselor. A clinical psychologist (S.W.B.) served as the primary MI-CET supervisor.
Initial MI-CET training involved approximately 8 hours of didactics in which the rationale and techniques for the intervention were presented and therapists engaged in role-plays to practice the skills. Therapists were given several opportunities to practice MI-CET through role-plays before seeing their first patient in the trial. At that point, the therapists transitioned to weekly individual or group supervision to review session audiotapes and receive feedback on their adherence to the treatment protocol and competence in delivering the intervention.
Development of treatment integrity measures
Treatment integrity refers to the extent to which therapists (a) adhere to the therapy protocol and (b) demonstrate competence in delivering the treatment (Waltz et al., 1993). Both the adherence and the competence components were assessed for each prescribed and proscribed task in each session based on a Likert-type rating scale of 1 to 7. The adherence rating was designed to capture the degree to which the intervention was used within each session (1 = not at all, 4 = somewhat, 7 = extensive), whereas competence captured the quality of the intervention (1 = very poor, 4 = adequate, 7 = excellent).
Prescribed task items were divided into three categories: general, MI, and CET. Proscribed task items included performing CBT (e.g., skill training, role playing, cognitive restructuring) and psychodynamic therapies (e.g., exploring conflicts about important relationships, making transference interpretations).
Based on the contents of our manual-guided MI-CET intervention, three session-specific treatment integrity measures were developed for Session 1, Sessions 2–8, and Session 9 (final session with MI-CET therapist). Depending on the number of session-specific tasks, each measure included 4–6 general items (e.g., assessment of alcohol use, presenting treatment rationale) and 7–10 CET items (e.g., discussion of medication compliance, relating alcohol outcomes to medication compliance).
The Session 1 treatment integrity evaluation form also included seven items assessing MI-specific tasks based on the five principles of MI (e.g., rolling with resistance, supporting self-efficacy). The CET items were taken directly from the CET (also referred to as clinical management) scale of the Yale Adherence and Competence Scales (Carroll et al., 2000). Although developed specifically for this study, our MI subscale was quite similar to the measure used to assess MI treatment integrity in the National Institute on Drug Abuse Clinical Trial Network 0004 (CTN 0004) protocol titled, Motivational Interviewing to Improve Treatment Engagement and Outcome in Individuals Seeking Treatment for Substance Abuse (Carroll et al., 2006), which was not available at the time the treatment integrity measures for our study were developed.
Using session-specific measures, an independent treatment integrity evaluator (G.Q.T.) rated therapist adherence and competence based on randomly selected audiotapes of approximately 20% of Sessions 1 and 10% of Sessions 2–9. The clinical supervisor (S.W.B) reviewed the independent evaluator's treatment integrity ratings with the study therapists and incorporated them into weekly clinical supervision.
Selection of comparison studies
Because this first study in which MI-CET was used was not designed to evaluate the efficacy of this behavioral intervention compared with another psychosocial platform, a preliminary assessment of feasibility and potential efficacy can be accomplished only by comparing rates of retention and adherence with historical “control” trials conducted by other groups. Arguably, the best available comparison trials would be those that also involved treatment with an SSRI, because of a presumably similar incidence and pattern of side effects. To obtain a more homogeneous sample of comparison studies to facilitate cross-study comparisons, we excluded investigations involving dual-diagnosis populations (i.e., individuals diagnosed as having both alcohol dependence and independent Axis I disorders such as anxiety disorders; e.g., Book et al., 2008; Brady et al., 2005) and combination pharmaco-therapies (Farren et al., 2009). However, we included studies involving participants with lifetime or current symptoms of major depressive disorder, because the distinction between independent depressive episodes and alcohol-induced depressive episodes is often unclear or not assessed.
Based on these criteria, we located 10 studies describing the outcome of pharmacotherapy with an SSRI for alcohol dependence (Angelone et al., 1998; Cornelius et al., 1997; Gual et al., 2003; Janiri et al., 1996; Kabel and Petty, 1996; Kranzler et al., 2006; Moak et al., 2003; Naranjo et al., 2005; Pettinati et al., 2001; Tiihonen et al., 1996). Of these 10 studies, only 3 provided enough information regarding treatment retention and medication compliance to serve as comparison studies (i.e., Kranzler et al., 2006; Moak et al., 2003; Pettinati et al., 2001). Specifically, in all of the seven excluded studies, data on medication adherence and session attendance were missing or incomplete.
Data analysis
Descriptive data (i.e., demographic and clinical characteristics) for the sample were compared by treatment condition using independent-samples t tests and chi-square tests. Rates of treatment completion were calculated only for the 12-week treatment phase of the study to facilitate comparisons with the results of prior investigations.
Treatment completers were defined as those subjects who were active participants in the study at the end of the 12-week treatment phase and who missed no more than two visits during the treatment phase. Session attendance was calculated by determining the percentage of completed visits for each randomized participant (i.e., number of sessions attended of nine total sessions that occurred during the active-treatment phase of the study) and averaging within each treatment group. Similarly, medication adherence, derived from MEMS cap data, was calculated by averaging within each treatment group the percentage of days the MEMS cap was opened out of the total number of days that the MEMS cap was in use (approximately the 12-week treatment phase for treatment completers).
Results
Demographic and clinical characteristics for the participants included in the preliminary analyses are depicted in Table 1. With the exception of a difference in highest level of education that was statistically significant but relatively small (i.e., less than 1 year), there were no statistically significant differences between the citalopram and placebo treatment groups on demographics such as age, marital status, income, sex, or racial composition. Likewise, the groups did not differ in the prevalence of cigarette smokers or the severity of alcohol dependence, as measured by the Alcohol Dependence Scale.
Table 1.
Demographic and clinical characteristics of the sample for the preliminary analyses
| Citalopram | Placebo | ||
| Variable | (n = 81) | (n = 40) | P |
| Age, M (SD) | 47.0 (8.0) | 48.2 (10.0) | .49 |
| Sex, male, no. (%) | 52 (64%) | 24 (60%) | .65 |
| Race, Caucasian, no. (%) | 72 (89%) | 38 (95%) | .27 |
| Education, years, M (SD) | 14.2 (2.0) | 15.0 (1.7) | .03 |
| Married, no. (%) | 49 (60%) | 20 (50%) | .27 |
| Household income,aM (SD) | 84.8 (57.3) | 78.3 (43.9) | .49 |
| Current smoker, no. (%) | 27 (33.3) | 16 (40.0) | .47 |
| ADS score, M (SD) | 13.9 (6.7) | 13.2 (5.8) | .60 |
Notes: ADS = Alcohol Dependence Scale.
In thousands of U.S. dollars.
MI-CET treatment integrity ratings were calculated for a random sample of sessions (n = 22 [18%] Session 1 ratings; n = 118 [14%] Sessions 2–9 ratings) for participants included in this preliminary data analysis. Mean adherence and competence scores were calculated separately for each category of ratings (i.e., general, MI, CET). Results indicated that the average adherence ratings ranged from “somewhat” to “considerable” across the three subscales (i.e., 5.8 for general, 6.4 for MI, and 3.9 for CET), and competence ratings ranged from “good” to “very good” (i.e., 5.9 for general, 6.3 for MI, and 5.1 for CET). An inspection of the data suggested that the lower mean adherence rating for CET (i.e., 3.9) was a result of several items on that scale representing interventions for which the optimal frequency and extensiveness of discussion is contingent on the participant's experiences with the medication (e.g., protocol-consistent assessment of side effects ranges from a brief initial probe if the individual is not having any side effects to extensive discussion of the nature, timing, and course of any side effect that is reported).
Thus, by using an adherence rating scale that captures only the absolute frequency/extensiveness of interventions, lower ratings are not necessarily indicative of poor adherence. Overall, however, the mean ratings of adherence and competence suggest that the intervention was delivered as intended, and therapists demonstrated a high degree of competence. At the same time, the validity of the adherence scales requires further examination and will likely be revised in future iterations of the treatment integrity measures.
Table 2 provides an overview of the design and results of the selected comparison trials involving SSRI pharmaco-therapy for alcohol dependence. Notably, all of the selected comparison trials involved pharmacotherapy with sertraline but each employed a unique approach to the concomitant psychosocial intervention. These approaches include twelve-step facilitation (e.g., Pettinati et al., 2001), CBT (e.g., Moak et al., 2003), and CET (Kranzler et al., 2006).
Table 2.
Summary of preliminary data and comparison studies of selective serotonin reuptake inhibitors for the treatment of alcohol dependence
| Authors | Medication | Behavioral intervention | Adherence component | Provider | Medication adherence | Session attendance | Treatment completion | Follow-up completion |
| Preliminary Data (first 121 randomized participants) | ||||||||
| Anthenelli etal. (ongoing, single-site) | Citalopram | MI-CET (weekly for 5 visits, bi-weekly for 4 visits) | Yes | Clinical psychology trainees, physician, masters-level counselor | Took ≥ 80% doses: A: 79% P: 91% (MEMS caps) | A: 8.1/9 (90%) P: 8.4/9 (93%) | A: 81% P: 88% (12-wk. tx. phase) | A: 72% P: 80% (6-month post-treatment) |
| Comparison Studies | ||||||||
| Kranzler et al. (2006)a (N=328, multisite) | Sertraline | CET (weekly for 4 visits, biweekly for 3 visits) | Yes | NR | Took ≥ 80% doses: A: 74%, 76% P: 74%, 77% (riboflavin) | NR | A: 56%, 59% P: 56%, 78% (10-wk. tx. phase) | NR |
| Moak et al. (2003) (N = 82, single-site) | Sertraline | CBT (from Project MATCH, weekly for 12 weeks) | No | NR | Took ≥ 75% doses: A: 79% P: 77% (riboflavin) | A: 10.5/12 (88%) P: 10.8/12 (90%) | A: 84% P: 67% (12-wk. tx. phase) | NR |
| Pettinati et al. (2001)a (N = 100, single-site) | Sertraline | Twelve-step facilitation (weekly, 14 weeks) + self-help referral | No | Counselor, unspecified credentials | A: 73%, 76% P: 79%, 81% (riboflavin) | A: 8.1/14 (58%), 8.3/14 (59%) P: 7.6/14 (54%), 8.6/14 (61%) | A: 63%, 65% P: 48%, 56% (14-wk. tx. phase) | NR |
Notes: MI-CET = motivational interviewing and compliance enhancement therapy; A = active medication; P = placebo; MEMS = Medication Event Monitoring System; wk. = week; tx. = treatment; NR = not reported; CBT = cognitive-behavioral therapy.
Multiple values for treatment adherence and retention data reflect subgrouping of participants by depressive symptoms.
Although the differences in study design, methods, medication(s) administered, participant characteristics, and retention data reported preclude a direct comparison of the adherence and retention data among all of these investigations and our preliminary data, the available evidence suggests that MI-CET compares favorably to other adjunctive behavioral treatments delivered in methodologically rigorous alcohol-dependence pharmacotherapy trials in terms of treatment engagement and retention. In fact, the 88% treatment completion rate for the placebo group in this trial is cause for optimism in light of the placebo completion rates for previous trials, which range from 47.8% to 78%.
MI-CET session attendance was similarly high, averaging 90% in the citalopram group and 93% in the placebo group. These figures are comparable to the CBT session attendance (88%–90%) reported by Moak et al. (2003) and considerably higher than the twelve-step facilitation session attendance (54%–61%) in the Pettinati et al. (2001) study. Importantly, there is some indication that the addition of brief MI to a CET platform may enhance treatment retention, because CET alone was associated with 56%–78% study completion (Kranzler et al., 2006) as compared with 81%–88% attained in MI-CET (see Table 2). Additionally, although none of the comparison studies reported data on completion of follow-up visits, rates of follow-up completion were high in our study, with 80% of the placebo group and 72% of the citalopram group remaining in the study through the 6-month follow-up.
In terms of medication adherence, it is somewhat difficult to compare across studies given the different methods of measuring, analyzing, and reporting adherence data. To match our preliminary medication-adherence data with the most similar of the SSRI comparison studies (i.e., Kranzler et al., 2006, which also used MEMS cap adherence data as well as CET therapy), we chose to report the percentage of treatment completers who took greater than or equal to 80% of the prescribed medication doses, which mirrors Kranzler et al.'s (2006) method of reporting adherence as well as the method used in other major alcohol-dependence pharmaco-therapy trials (e.g., COMBINE; Zweben et al., 2008). Additionally, predictive validity of the 80% adherence cutoff has been supported in terms of its relationship to better treatment outcomes with naltrexone (Baros et al., 2007). In our preliminary analyses, medication adherence in the active medication group was roughly comparable to that reported by Kranzler et al. (2006) (79% vs. 74%–76% taking ≥80% of doses). In the placebo group, our adherence rate appeared to be higher (i.e., 91% vs. 74%–77%).
Discussion
In this article, we describe the development of a novel, manual-guided intervention that combines motivational interviewing with compliance enhancement therapy (MI-CET) and present preliminary data on treatment engagement and retention as well as medication adherence. Although the study is ongoing, early results from our trial of citalopram are promising with respect to the feasibility and potential utility of MI-CET as a means of promoting medication adherence, treatment engagement, and treatment retention.
Because the study does not include a therapy comparison condition, we considered our findings in the context of past SSRI treatment trials involving alcohol-dependent participants, with or without lifetime depressive disorders. In this context, MI-CET retention and medication adherence data are at least comparable to those reported in previous trials and, in some cases, appear to be more favorable. When compared with a multicenter study that used CET only as the psycho-social intervention (i.e., Kranzler et al., 2006), treatment completion rates are 20%–30% higher for the current study in which MI-CET is being used, suggesting that the addition of MI may boost the effects of CET on retention. Similarly, medication adherence in this preliminary analysis was at least comparable to that obtained by Kranzler et al. (2006), with our placebo adherence rates appearing to be higher.
Treatment retention and adherence to pharmacotherapy are critical targets of psychosocial interventions for alcohol-and other substance-use disorders, as a preponderance of evidence suggests that both of these factors are linked to better treatment outcomes (Ernst et al., 2008; Oslin et al., 2008; Volpicelli et al., 1997). Additionally, high rates of study dropout and poor medication adherence threaten the validity of research findings on the efficacy of pharmacotherapy for alcohol and drug dependence and generally impede the process of identifying the most effective treatments for these debilitating disorders. Consequently, methods of promoting retention and adherence in a clinical trial through the use of an adjunctive therapy warrant additional research, and our preliminary findings with MI-CET suggest that it should be evaluated further for this purpose.
The results that we reported have several limitations. As mentioned previously, comparing MI-CET against “control” behavioral interventions used in previous trials of SSRI pharmacotherapy for alcohol dependence as opposed to comparing against a within-study control intervention limits the strength of the conclusions that can be drawn. Additionally, it should be noted that the participants in the present study were predominantly White and middle age, with mid- to upper-level household incomes and low to medium severity of alcohol dependence. Consequently, the results may not be generalizable to all individuals who receive pharmacotherapy for alcohol dependence, either in research or clinical settings. Despite these limitations, our careful assessment of treatment integrity through empirically validated procedures that were adapted for use in this study and consideration of the applications of MI-CET in both clinical and research settings in the earliest stages of treatment development are noteworthy strengths.
In conclusion, brief MI and clinical management methods of counseling require less training and expertise than more intensive interventions such as CBT. Consequently, MI-CET has the potential to be delivered competently by a range of treatment providers and transported across a variety of settings. These preliminary findings, as well as the available evidence regarding comparative efficacy of behavioral treatments delivered in combination with pharmacotherapy, suggest that MI-CET holds promise as an adjunct to alcohol-dependence pharmacotherapy in clinical trials. Further research will be necessary to provide a more complete evaluation of the efficacy of MI-CET in promoting medication adherence, treatment retention, and detection of medication effects when compared against a placebo.
Acknowledgments
The authors thank Anne Autry, M.D., Reene Cantwell, Kerri Dawson-Earles, B.S., Kelly Ickes, M.A., Julie Jansen, B.A., and Stephanie Nolting, M.Ed., for their assistance on this project and Kathleen Carroll, Ph.D., for serving as a consultant on the study and for reviewing a draft of this article.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant AA013957. The writing of this manuscript was supported, in part, by NIAAA grant AA013307 and by the Department of Veterans Affairs.
References
- Allsop S, Saunders B, Phillips M, Carr A. A trial of relapse prevention with severely dependent male problems drinkers. Addiction. 1997;92:61–74. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Angelone SM, Bellini L, Di Bella D, Catalano M. Effects of fluvoxamine and citalopram in maintaining abstinence in a sample of Italian detoxified alcoholics. Alcohol and Alcoholism. 1998;33:151–156. doi: 10.1093/oxfordjournals.alcalc.a008371. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. Journal of the American Medical Association. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baros AM, Latham PK, Moak DH, Voronin K, Anton RF. What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcoholism: Clinical and Experimental Research. 2007;31:596–603. doi: 10.1111/j.1530-0277.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: A randomized controlled trial. Nursing Research. 2007;56:18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Bertholet N, Daeppen J, Wietlisbach V, Fleming M, Burmand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: Systematic review and meta-analysis. Archives of Internal Medicine. 2005;165:986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- Book SW, Thomas SE, Randall PK, Randall CL. Parox-etine reduces social anxiety in individuals with a co-occurring alcohol use disorder. Journal of Anxiety Disorders. 2008;22:310–318. doi: 10.1016/j.janxdis.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Corsi KF, Mikulich-Giberson SK. Factors associated with methadone maintenance treatment retention among street recruited injection drug users. Drug and Alcohol Dependence. 2004;74:177–185. doi: 10.1016/j.drugalcdep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcoholism: Clinical and Experimental Research. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Britt E, Blampied NM, Hudson SM. Motivational interviewing: A review. Australian Psychologist. 2003;38:193–201. [Google Scholar]
- Brown JM, Miller WR. Impact of motivational interviewing on participation and outcome in residential alcoholism treatment. Psychology of Addictive Behaviors. 1993;7:211–218. [Google Scholar]
- Carey KB, Carey MP, Maisto SA, Henson JM. Brief motivational interventions for heavy college drinkers: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2006;74:943–954. doi: 10.1037/0022-006X.74.5.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, Martino S, Frankforter TL, Farentinos C. Motivational interviewing to improve treatment engagement and outcome in individuals seeking treatment for substance abuse: A multisite effectiveness study. Drug and Alcohol Dependence. 2006;81:301–312. doi: 10.1016/j.drugalcdep.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Kosten TR, Rounsaville BJ. Choosing a behavioral therapy platform for pharmacotherapy with substance abusers. Drug and Alcohol Dependence. 2004;75:123–134. doi: 10.1016/j.drugalcdep.2004.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Ball SA, McCance E, Rounsaville BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93:713–728. doi: 10.1046/j.1360-0443.1998.9357137.x. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Nich C, Sifrey R, Frankforter T, Nuro KF, Ball SA, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Carroll K, O'Malley S. Compliance enhancement: A manual for the psychopharmacotherapy of alcohol dependence. Unpublished manual. New Haven, CT: Yale University; 1996. [Google Scholar]
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Perel JM, Black A. Fluoxetine in depressed alcoholics: A double-blind, placebo-controlled trial. Archives of General Psychiatry. 1997;54:700–705. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- Ernst DB, Pettinati HM, Weiss RD, Donovan DM, Longabaugh R. An intervention for treating alcohol dependence: Relating elements of medical management to patient outcomes with implications for primary care. Annals of Family Medicine. 2008;6:435–440. doi: 10.1370/afm.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farren CK, Scimeca M, Wu R, O'Malley S. A double-blind, placebo-controlled study of sertraline with naltrexone for alcohol dependence. Drug and Alcohol Dependence. 2009;99:317–321. doi: 10.1016/j.drugalcdep.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management-imipramine/placebo administration manual: NIMH Treatment of Depression Collaborative Research Program. Psychopharmacology Bulletin. 1987;23:309–324. [PubMed] [Google Scholar]
- Gual A, Balcells M, Torres M, Madrigal M, Diez T, Serrano L. Sertraline for the prevention of relapse in detoxicated alcohol dependent patients with a comorbid depressive disorder: A randomized controlled trial. Alcohol and Alcoholism. 2003;38:619–625. doi: 10.1093/alcalc/agg124. [DOI] [PubMed] [Google Scholar]
- Hettema J. Meta-analysis of motivational interviewing across behavioral domains. Dissertation Abstract International: Section B. Sciences and Engineering. 2007;67(9-B):5406. [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidepressant doses on short-term outcome of detoxified alcoholics. International Clinical Psychopharmacology. 1996;11:109–117. [PubMed] [Google Scholar]
- Kabel DI, Petty F. A placebo-controlled, double-blind study of fluoxetine in severe alcohol dependence: Adjunctive pharmacotherapy during and after inpatient treatment. Alcoholism: Clinical and Experimental Research. 1996;20:780–784. doi: 10.1111/j.1530-0277.1996.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Hester R. Cognitive-behavioral coping skills therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. NIAAA Project MATCH Monograph Series (Vol. 3, NIH Publication No. 94–3724) Washington, DC: Government Printing Office; 1995. [Google Scholar]
- Kranzler HR, Mueller T, Cornelius J, Petinatti HM, Moak D, Martin PR, Hasin D. Sertraline treatment of co-occurring alcohol dependence and major depression. Journal of Clinical Psychopharma-cology. 2006;26:13–20. doi: 10.1097/01.jcp.0000194620.61868.35. [DOI] [PubMed] [Google Scholar]
- LaBrie JW, Huchting K, Tawalbeh S, Pederson ER, Thompson AD, Schelesky K, Neighbors C. A randomized motivational enhancement prevention group reduces drinking and alcohol consequences in first-year college women. Psychology of Addictive Behaviors. 2008;22:149–155. doi: 10.1037/0893-164X.22.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR. Motivational interviewing with problem drinkers. Behavioural Psychotherapy. 1983;11:147–172. [Google Scholar]
- Miller WR. Enhancing motivation for change. In: Miller WR, Heather N, editors. Treating addictive behaviors. 2nd ed. New York: Plenum Press; 1998. pp. 121–132. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. NIAAA Project MATCH Monograph Series (Vol. 2., NIH Publication No. 94–3723. Washington, DC: Government Printing Office; 1994. [Google Scholar]
- Moak DH, Anton RF, Latham PK, Voronin KE, Waid RL, Durazo-Arvizu R. Sertraline and cognitive behavioral therapy for depressed alcoholics: Results of a placebo-controlled trial. Journal of Clinical Psychopharmacology. 2003;23:553–562. doi: 10.1097/01.jcp.0000095346.32154.41. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Bremner KE, Lanctot KL. Effects of citalo-pram and a brief psycho-social intervention on alcohol intake, dependence, and problems. Addiction. 1995;90:87–99. doi: 10.1046/j.1360-0443.1995.9018712.x. [DOI] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Twelve step facilitation therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. NIAAA Project MATCH Monograph Series (Vol. 1, NIH Publication No. 94–3722) Washington, DC: Government Printing Office; 1995. [Google Scholar]
- O'Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O'Connor PG. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: A nested sequence of 3 randomized trials. Archives of Internal Medicine. 2003;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Pettinati HM, Kampman KM, Gariti P, Gelfand L, O'Brien CP. Placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psycho-social intervention. Alcoholism: Clinical and Experimental Research. 2008;32:1299–1308. doi: 10.1111/j.1530-0277.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry. 2005;60:777–783. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. Journal of Clinical Psychopharmacology. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Pierce JD, O'Brien CP. Improving naltrexone response: An intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. Journal of Addictive Disorders. 2000;19:71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Dundon W, Miller WR, Donovan D, Ernst DB, Rounsaville BJ. A structured approach to medical management: A psychosocial intervention to support pharma-cotherapy in the treatment of alcohol dependence. Journal of Studies on Alcohol. 2005;(Supplement No. 15):170–178. doi: 10.15288/jsas.2005.s15.170. [DOI] [PubMed] [Google Scholar]
- Rogers CR. Client-centered therapy. Boston, MA: Houghton Mifflin; 1951. [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from Stage I. Clinical Psychology: Science and Practice. 2001;8:133–142. [Google Scholar]
- Sellman JD, Sullivan PF, Dore GM, Adamson SJ, MacEwan I. A randomized controlled trial of motivational enhancement therapy (MET) for mild to moderate alcohol dependence. Journal of Studies on Alcohol. 2001;62:389–396. doi: 10.15288/jsa.2001.62.389. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: Measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Steinberg ML, Ziedonis DM, Krejci JA, Brandon TH. Motivational interviewing with personalized feedback: A brief intervention for motivating smokers with schizophrenia to seek treatment for tobacco dependence. Journal of Consulting and Clinical Psychology. 2004;72:723–728. doi: 10.1037/0022-006X.72.4.723. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rynänen O-P, Kauhanen J, Hakola HPA, Salaspuro M. Citalopram in the treatment of alcoholism: A double-blind placebo-controlled study. Pharmacopsychiatry. 1996;29:27–29. doi: 10.1055/s-2007-979538. [DOI] [PubMed] [Google Scholar]
- Vasilaki EI, Hoster SG, Cox M. The efficacy of motivational interviewing as a brief intervention for excessive drinking: A meta-analytic review. Alcohol and Alcoholism. 2005;41:328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Pettinati HM, McLellan AT, O'Brien CP. Combining medication and psychosocial treatments for addictions: The BRENDA Approach. New York: Guilford Press; 2001. [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O'Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Archives of General Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of psychotherapy protocol: assessment of adherence and competence. Journal of Consulting and Clinical Psychology. 1993;61:620–630. doi: 10.1037//0022-006x.61.4.620. [DOI] [PubMed] [Google Scholar]
- Woodall WG, Delaney HD, Kunitz SJ, Westerberg VS, Zhao H. A randomized trial of a DWI intervention program for first offenders: Intervention outcomes and interactions with antisocial personality disorder among a primarily American-Indian sample. Alcoholism: Clinical and Experimental Research. 2007;31:974–987. doi: 10.1111/j.1530-0277.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- Zweben A, Pettinati HM, Weiss RD, Youngblood M, Cox CE, Mattson ME, Ciraulo D. Relationship between medication adherence and treatment outcomes: The COMBINE Study. Alcoholism: Clinical and Experimental Research. 2008;32:1661–1669. doi: 10.1111/j.1530-0277.2008.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]